Abstract
Glaciers have shaped past and present habitats for Pacific salmon (Oncorhynchus spp.) in North America. During the last glacial maximum, approximately 45% of the current North American range of Pacific salmon was covered in ice. Currently, most salmon habitat occurs in watersheds in which glacier ice is present and retreating. This synthesis examines the multiple ways that glacier retreat can influence aquatic ecosystems through the lens of Pacific salmon life cycles. We predict that the coming decades will result in areas in which salmon populations will be challenged by diminished water flows and elevated water temperatures, areas in which salmon productivity will be enhanced as downstream habitat suitability increases, and areas in which new river and lake habitat will be formed that can be colonized by anadromous salmon. Effective conservation and management of salmon habitat and populations should consider the impacts of glacier retreat and other sources of ecosystem change.
Keywords: climate change, glaciers, Oncorhynchus, Pacific salmon, watershed
Glaciers are retreating rapidly across Pacific salmon (Oncorhynchus spp.) landscapes, driven in large part by anthropogenic climate change (figure 1; Marzeion et al. 2014). In western North America, glaciers are predicted to lose up to 80% of their ice volume by 2100 (Radić et al. 2013) and have already lost up to 3% per year between 2006 and 2016 (Zemp et al. 2019). This rapid contemporary ice loss follows longer-term glacier retreat; most glaciers in North America have been retreating since the 1600s–1800 s Little Ice Age maxima (Menounos et al. 2009).
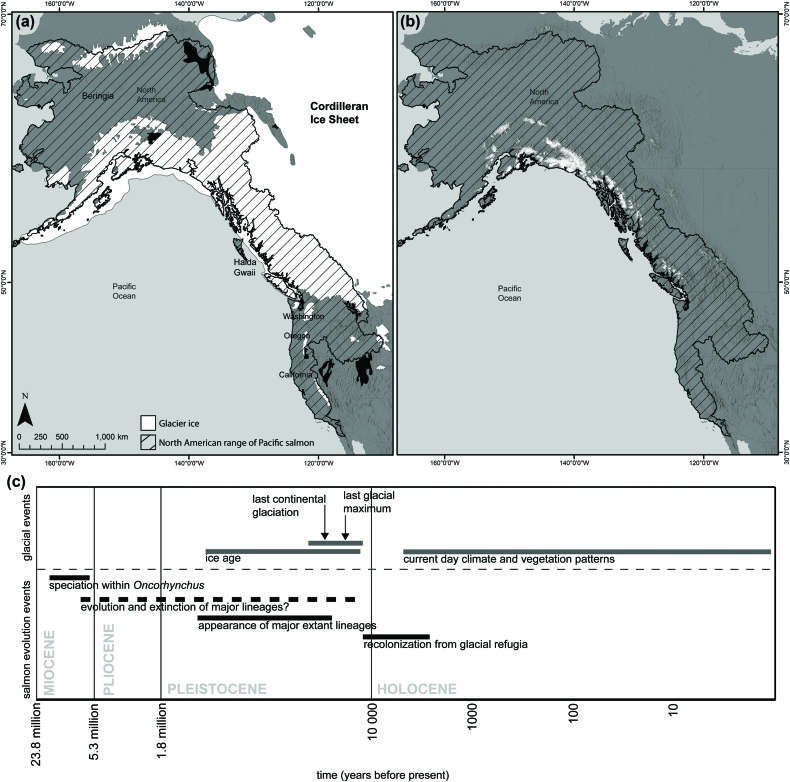
Figure 1.
The North American range of Pacific salmon, ice extents (historic and present), and the evolution of Pacific salmon in North America. (a) Approximate ice extent from the Cordilleran Ice Sheet around 16,000 years ago (Dyke 2004), including regions of ice-free refugia (i.e., Haida Gwaii, Beringia, and parts of the Washington, Oregon and California coasts). The core range of current-day salmon is shown in black-hatched lines (www.stateofthesalmon.org/resources/sosdb.php). We acknowledge that there are some peripheral populations beyond this hatched region (e.g., Mackenzie River). (b) Approximate current-day ice extent from the Randolph Glacier Inventory 6.0 (Pfeffer et al. 2014) overlapping with the North American range of Pacific salmon. Note that there are small, high-elevation glaciers present in conterminous United States. (c) Timeline of major glacial changes and evolutionary history of Pacific salmon (from 23.8 million years ago to the present), adapted from Waples et al. 2008.
Glacier retreat can increase or decrease wild Pacific salmon productivity by modifying downstream habitat conditions and by creating new habitat. Changes in glacier runoff (i.e., all water discharged from the glacier terminus) have important downstream effects on hydrology, sediment transport, water temperature, and biogeochemical fluxes, which alter conditions for salmon in freshwater and nearshore marine habitats (O’Neel et al. 2015, Milner et al. 2017). For example, a significant decrease of glacier contribution to total watershed runoff generally increases downstream water temperature, which could be either beneficial or stressful to salmon. In cold rivers (below 5 degrees Celsius [°C]), increases in water temperature could increase juvenile salmon growth potential (Fellman et al. 2014), whereas in warm rivers (more than 15°C), increases in water temperature could increase stress and mortality rates of adult salmon as they migrate upstream (Martins et al. 2012).
Glacier retreat can also directly create new habitat for salmon. For example, in Glacier Bay, Alaska, tidewater glacier retreat created new river systems that were colonized by pink salmon (Oncorhynchus gorbuscha) within 30 years of formation (Milner et al. 2011). Therefore, glacier change can affect salmon ecosystems through a variety of mechanisms (Moore et al. 2009, O’Neel et al. 2015, Milner et al. 2017). Overall, the net effects of glacier retreat on salmon will likely depend on the phase of glacier retreat, the life-history traits of salmon species, and a suite of local environmental, geographic, and ecological characteristics of watersheds.
Understanding how glacier retreat will affect Pacific salmon will help inform the management and conservation of these economically and culturally important species. There is growing understanding of the pathways by which glacier retreat alters aquatic environments (O’Neel et al. 2015, Milner et al. 2017) and a large body of research on how environmental variables influence salmon across their life cycle (Quinn 2018). By integrating these two fields of study, we offer a conceptual synthesis of how glacier retreat may affect Pacific salmon populations in North America and how these effects may vary by species and watershed context. Specifically, we review the historical interaction of glaciation and Pacific salmon in North America over geological time scales, propose a conceptual model for the evolution of salmon watersheds in response to glacier retreat, quantify the current status of glaciers in salmon watersheds, propose research frontiers, and highlight implications of glacier loss for salmon management and policy.
Glaciation and Pacific salmon watersheds over geological time scales
To provide context for the response of Pacific salmon to contemporary glacier retreat, we briefly review Pacific salmon and glacier dynamics over geological time scales. Over time, the advance and retreat of glaciers are controlled by the difference between rates of ice accumulation (via snowfall on the glacier) and ice ablation (via melting, sublimation, and glacier calving). Such advance and retreat of glaciers have been driven by shifts in global and local climate patterns (Menounos et al. 2009), with rapid glacier retreat occurring in the recent decades because of climate change (Zemp et al. 2019). For example, with recent glacier ice-loss rates being up to 3% per year, most of today's glacier volume in western Canada and conterminous United States will vanish by the second half of this century (Zemp et al. 2019).
Pacific salmon evolved over millions of years during the Miocene epoch, a time of warmer global temperature and relatively little glacier coverage (figure 1; Stearley 1992). The Miocene radiation, 6 million–20 million years ago, resulted in the species of anadromous Pacific salmon in North America (hereafter, salmon) that are present today (table 1; Waples et al. 2008). In this article, we focus on six species: Chinook salmon (Oncorhynchus tshawytscha), chum salmon (Oncorhynchus keta), coho salmon (Oncorhynchus kisutch), pink salmon, sockeye salmon (Oncorhynchus nerka), and steelhead trout (anadromous Oncorhynchus mykiss; table 1) because of their ecological and economic importance and the extensive body of related scientific research. During the late Pliocene and early Pleistocene, 1.8 million years ago–17,000 years ago, glaciers repeatedly advanced and retreated, reworking the surface of the northwestern North American landscape. These repeated ice sheet expansions covered large portions of Alaska and British Columbia. Salmon survived in ice-free refugia, including Beringia and along the coasts of British Columbia (e.g., Haida Gwaii), Washington, Oregon, and California (figure 1; Smith et al. 2001). On the basis of the maximum spatial extent of ice (Dyke 2004), we estimate that approximately 45% of the current North American range of salmon was covered by ice at some point in the past (figure 1).
Table 1.
Trends in Pacific salmon life cycles across species.
| Spawning | Freshwater rearing | |||||
|---|---|---|---|---|---|---|
| Species | Years to maturity | Winters at sea | Spawning location | Spawning timing | Length of freshwater rearing | Rearing location |
| Chinook | 3–8 | 1–5 | Medium to large rivers, sometimes downstream of lakes | Summer–fall | Depends whether ocean or stream typec | Rivers, estuaries |
| Chum | 3–5 | 1–4 | Lower reaches of riversa | Late summer–fall | None, but sometimes stay in streams for a few days per weeks | None |
| Coho | 4–5 | 1–2 | Often in smaller tributaries | Late summer–winter | Weeks–2 years | Small streams, off-main-channel habitats, beaver ponds, lake margins, estuaries. |
| Pink | 2 | 1 | Rivers, generally close to ocean | Late summer–fall | None, but sometimes stay in streams for a few days per weeks | None |
| Sockeye | 3–6 | 1–4 | Rivers, creeks, lake beachesb | Late summer–fall | Weeks–2 years | Usually lakesb |
| Steelhead | 1–12 | 1–5 | Small to medium rivers | Late winter–spring | 1–5 years | High gradient reaches |
Note: There is enormous variation in salmon life cycles within species. We use this table as a simplifying construct to compare different species. Information for this table was predominantly obtained from Quinn (2018).
Some chum salmon populations are long-distance migrants.
There are also ocean type, populations that migrate to sea in their first year of life, and river type, populations that rear in rivers for a year before going to sea.
Ocean type migrate downstream right after emergence (few months in river); stream type spend a full year in the river. Ocean type are almost exclusively south of 56 degrees.
The late Pleistocene and Holocene brought the onset of deglaciation (beginning around 17,000 years ago; figure 1; Booth et al. 2003), which shifted and increased the spatial distribution of freshwater habitats available to salmon (Smith et al. 2001, Waples et al. 2008). During this time, rapid glacier retreat opened major river valleys, and the land rebounded as a result of postglacier isostatic adjustment, and the sea level rose (Beechie et al. 2001, Waples et al. 2009). Deglaciation led to a range of landscape disturbances, from high-magnitude catastrophic glacier lake outburst floods (e.g., Lake Missoula; Benito and O’Connor 2003) to low-magnitude events, such as landslides and annual floods. Retreating glaciers left behind landscape features such as characteristic deep and wide U-shaped valley bottoms that set the stage for the development of high-quality salmon habitat (Benda et al. 1992, Beechie et al. 2001). Therefore, much of the current range of salmon bears the legacy of glaciers past.
Historical landscape disturbances, such as those associated with glacier dynamics, are thought to have shaped many of the life histories and traits of salmon that we see today (Waples et al. 2008, 2009). For example, some salmon species (e.g., Chinook and sockeye salmon and steelhead trout) have considerable diversity in age at maturity that can buffer populations against freshwater disturbances (Waples et al. 2008, 2009), such as glacier outburst floods that might eradicate a generation of spawning adults or rearing juveniles. Although most salmon return to spawn in their natal stream, some fraction of the population disperses or strays, enabling salmon to colonize new habitats (Pess et al. 2014), such as watersheds opened following glacier retreat (Milner et al. 2011). In addition, for species with freshwater juvenile rearing (e.g., Chinook and coho salmon and steelhead trout), dispersal within freshwater may further enable salmon to take advantage of newly opened and shifting habitat conditions (Reeves et al. 1995). Overall, a low but substantial level of dispersal may maximize population resilience in dynamic landscapes by enabling salmon to both maintain local adaptations but also enable metapopulation processes (Yeakel et al. 2018). Therefore, current salmon life histories and traits reflect adaptations to dynamic landscapes, such as those with a legacy of glacier disturbances (Waples et al. 2008, 2009).
Salmon life cycles are complex and vary across and within species (table 1). Salmon migrate a range of distances in freshwater, from river deltas to more than 1000 kilometers upriver, to spawn in diverse habitats that include the mainstem river, river side channels, small headwater streams, groundwater-fed sloughs, and littoral zones of lakes (Quinn 2018). Subsequently, salmon dig depressions (known as redds) in which they deposit their eggs in sediments that are generally pebbles to small cobbles (around 5–80 millimeters in diameter; Kondolf and Wolman 1993) that enable sufficient subsurface flow past the eggs, thus providing oxygen and removing nitrogenous wastes. Steelhead trout are iteroparous (i.e., can undergo multiple reproductive cycles) and generally spawn in the spring (Kendall et al. 2015), whereas the other species of Pacific salmon are semelparous (i.e., undergo a single reproductive episode before death) and spawn in the summer to fall (Quinn 2018). Depending on the species and population, juvenile salmon may migrate immediately to the ocean (e.g., chum and pink salmon) or stay in freshwater for months to several years (e.g., Chinook, coho, and sockeye salmon and steelhead trout; table 1). During this freshwater phase, juvenile salmon may rear in the main river channel or off-main channel habitats (e.g., Chinook salmon and steelhead trout), ponds (e.g., coho salmon), or lakes (e.g., sockeye salmon). Once young salmon migrate to the marine environment, survival can be strongly influenced by food web interactions and ocean conditions (Beamish et al. 2016). Therefore, life-history variation between and within each salmon species will influence how they respond to glacier retreat.
The evolution of salmon habitat across phases of glacier retreat
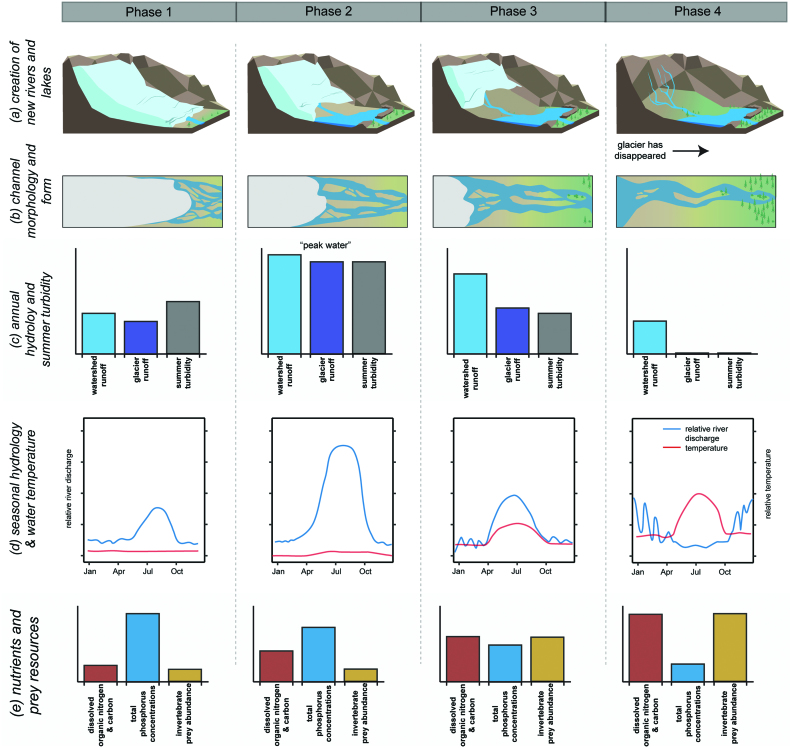
In the present article, we describe a conceptual model for the evolution of salmon habitat during the process of glacier retreat (figure 2). Our model describes general biophysical phases as glaciers retreat from the coast to higher elevations. Because of the overriding effect of local topography and climate on the rate of glacier retreat, we structure the model across four phases corresponding to glaciers covering distinct components of the salmon landscape rather than by discrete time periods. Therefore, different watersheds or regions can be in different phases of glacier retreat. Phase 1 refers to the beginning of glacier retreat from the coast, when new lakes and streams are being formed, but most of the watershed remains covered by ice. During phase 2, the glaciers continue to retreat up valley, further exposing new rivers and proglacial lakes, as well as a mosaic of lateral valley-bottom and hillslope tributary habitats. Phase 3 begins when the glaciers have retreated to higher elevations, above the extent of accessible salmon habitat, with glacier runoff still influencing downstream river evolution. Phase 4 considers the continued evolution of salmon habitats after the glaciers have disappeared. We suggest that these four generalized phases of glacier retreat are characterized by a unique set of processes that influence the biological and physical characteristics of downstream ecosystems (figure 2; Milner et al. 2001). We consider how these biophysical changes will affect salmon life stages across species and their habitats. Specifically, we assess the following watershed changes associated with glacier retreat: river and lake creation, channel morphology and form, annual and seasonal hydrology, water temperature, turbidity, and nutrients and prey resources.
Figure 2.
Predicted effects of glacier retreat (phases 1–4) on (a) river and lake habitats, (b) channel morphology and form, (c) total annual watershed runoff, glacier runoff, and summer turbidity, (d) seasonal hydrology and temperature relationships, and (e), predicted stream water organic matter and nutrient concentrations and prey availability.
Phase 1: Ice-dominated watersheds. As glaciers begin to retreat from the coast, freshwater habitats emerge (figure 2). However, during this initial phase of glacier retreat, much of the watershed is under ice. River systems are beheaded by glaciers and have relatively low quantity of salmon habitat because of the high glacier coverage. In addition, new rivers can be quite inhospitable to salmon because of high sediment loads, channel instability, and frigid temperatures but do represent new habitat that salmon can colonize (Milner et al. 2011). As glaciers retreat, they leave behind large unconsolidated glacial deposits in a vegetation-free landscape carved by new proglacial streams. Because of the high sediment load, low bank cohesion, and high specific discharge (i.e., discharge per unit watershed area), young proglacial streams are typically braided, wide, and shallow, with shifting and dynamic stream channels (figure 2; Milner and Petts 1994). Young proglacial lakes may also form in these landscapes, such as those dammed by glacial moraines. Moraine-controlled lakes commonly breach and may drain completely if their dams of unconsolidated sediments erode (Carrivick and Heckmann 2017). Consequently, new habitats created during the early phase of glacier retreat are often ephemeral, and those that persist are initially highly unstable.
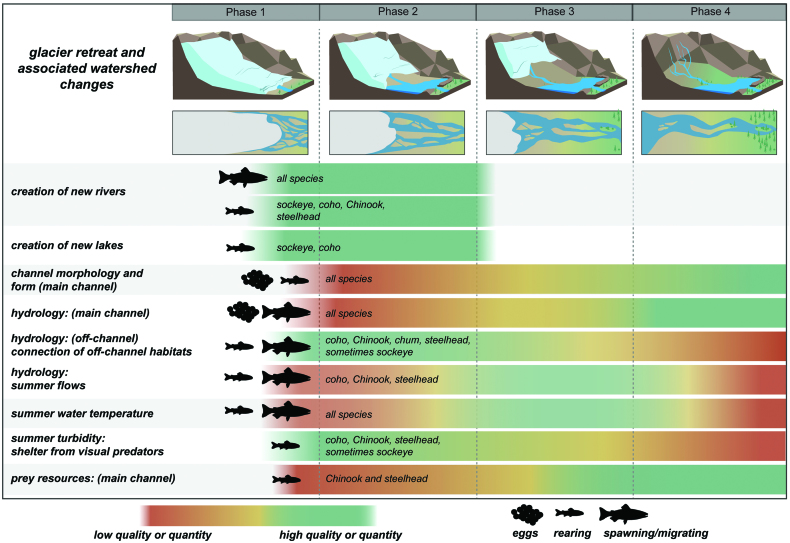
Unstable habitats pose many challenges to salmon spawning, egg incubation, and rearing. For example, all species of salmon construct redds in stream sediments in which eggs incubate for several months over the winter prior to emergence (Quinn 2018). During incubation in these young glacier streams, high sediment mobility can lead to streambed scour, entraining or destroying developing embryos (Jensen et al. 2009), or channel avulsions that can lead to dewatering of stream reaches resulting in the desiccation of eggs. High channel instability and widely fluctuating flow regimes can limit food resources for juvenile salmon that rear months to years in freshwater, such as Chinook and coho salmon and steelhead trout (table 1, figure 3), by reducing the abundance and diversity of aquatic macroinvertebrates (figure 3; Death and Winterbourn 1995). Within-season channel movement may also strand juveniles in abandoned stream channels. Therefore, channel instability of young proglacial streams can initially limit successful salmon reproduction and juvenile rearing during early phases of glacier retreat (Murphy et al. 1989), particularly for stream-rearing species (figure 3).
Figure 3.
Predictions of how glacier retreat and its associated watershed changes can affect salmon species across life phases during the different phases of glacier retreat.
Cold temperatures in young streams may present obstacles to salmon success when the landscape is dominated by glacier ice. In heavily glacierized watersheds (e.g., more than 40% glacier coverage), river temperatures remain very cold (below 5°C) throughout the year because discharge is dominated by glacier meltwater (figure 2; Fellman et al. 2014). For example, in a comparative study of watersheds that ranged from 0% to 65% glacier coverage, average summer water temperature was approximately 1 °C colder for every 10% increase in glacier coverage (Fellman et al. 2014). Water temperature plays a critical role in regulating the metabolism and development of embryos and juvenile salmon (Brett 1971). Cold water temperatures in heavily glacierized streams challenge the thermal performance of salmon egg and juvenile development (Brett 1971). However, cold water temperatures do not necessarily preclude salmon embryo and juvenile survival and growth (Adelfio et al. 2018). For example, recent work by Campbell and colleagues (2019) suggests that coho salmon in a cold stream (4 °C) grew at the same rate as those in a warmer stream (10°C–12 °C) that was closer to the assumed optimum temperature for the species (Richter and Kolmes 2005). Therefore, local adaptation or physiological compensation may potentially allow salmon to persist in what are presumed to be less-than-ideal thermal conditions. Regardless, when acting in combination with other features of young proglacial streams, such as channel instability and low prey abundance, cold temperatures in young streams can limit salmon productivity (Murray and McPhail 1988).
Despite these limitations, salmon can colonize highly dynamic and very cold rivers. Salmon straying combined with high population growth rate potential (approximately 20% to 100% per generation; Pess et al. 2014) can lead to rapid population expansion in newly deglaciated habitat. Estimated salmon stray rates range from approximately 1% to 20%, depending on the study, species, life histories, and environmental factors (Keefer and Caudill 2014). The amount of straying is also positively correlated to proximity; most straying individuals return to within 30 kilometers of their origin (Pess et al. 2014). Accordingly, salmon colonization is affected by four key factors that vary across species: the size and distance of nearby (donor) populations, species’ presence in the same or nearby watersheds, the suitability of newly available habitat for the species, and the presence of life-history variants in the donor population that facilitate colonization of newly opened habitats (Pess et al. 2014). For example, in Stonefly Creek, Alaska, pink salmon were the first salmon to establish populations following deglaciation and river habitat creation (Milner et al. 2011), likely because pink salmon tend not to have extended juvenile rearing phases, stray at high rates, and are in high abundance in this region. Therefore, different species of salmon will likely colonize river habitats created by recent deglaciation at different rates given their life-history traits and location of the deglaciated area (table 1).
Phase 2: Rivers and lakes fed by ice. During phase 2 of glacier retreat, substantial portions of the valley floor are revealed and reorganized by physical processes that affect the geomorphic evolution of potential salmon habitat (figure 2). During this phase, there is an increase in the diversity of habitats formed and therefore expanded opportunities for different salmon life histories. However, continued fluxes of water and sediments from the upstream glacier may limit salmon and prey productivity in mainstem river channels. Glaciers are effective agents of erosion and carve characteristically broad U-shaped valleys with large volumes of excavated sediment (Montgomery 2002). The valley bottoms left behind are typically organized into three linked hydrogeomorphic domains: canyons, gravel-bedded floodplains, and lakes (Hauer et al. 2016). Canyons occur where valleys are narrowly constrained by shallow resistant bedrock. Gravel-bedded floodplains occur where valleys are broad and may be filled with glacier sediment (i.e., silt, clay, sand, and gravel) of a range of sizes. Lakes form by dammed bedrock, landslide formations, or moraines (Hauer et al. 2016). These hydrogeomorphic domains can alternate and reappear as glaciers retreat up valley, depending on watershed-specific lithography and topography (Hauer et al. 2016). Streams confined by canyons have low physical complexity and, when combined with high river discharge, may even create salmon migration barriers or offer limited opportunities for salmon habitat development during glacier retreat (Murphy et al. 1989, Bellmore and Baxter 2014, Hauer et al. 2016).
In contrast, broad, unconfined floodplains provide opportunities for the development of complex and diverse river habitats (Bellmore and Baxter 2014, Hauer et al. 2016). Riverbanks still have low cohesion because terrestrial vegetation remains in early successional stages. Increased stream discharge and high sediment loads can maintain unstable braided channels (figure 2). Glacier runoff is typically at its highest, known as peak water, during this phase of glacier retreat before declining in later phases as glacier melt declines (figure 2; Jansson et al. 2003, Huss and Hock 2018). This peak in glacier runoff typically leads to watershed runoff also being at its maximum during this phase of glacier retreat (figure 2). High summer air temperature can also intensify runoff from the glacier, resulting in increases in seasonal glacier meltwater that decreases downstream water temperature (Fellman et al. 2014). Because of this high glacier and watershed runoff, glacier-fed rivers during this phase can have high sediment loads. Included in these high sediment loads are suspended sediments that are formed as the glacier grinds against rock resulting in fine silt or glacier flour. In watersheds dominated by glaciers, more than 500 milligrams per liter of glacier flour are typical and up to 2000 milligrams per liter occur frequently (Gurnell et al. 1987), primarily during the summer months. For example, more than 29 ⋅ 106 tons of suspended sediment are deposited annually into Cook Inlet, Alaska, from the heavily glacierized Susitna River watershed (Brabets et al. 1999). Comparatively, the Kenai River watershed, which is less glacierized and contains large lakes that trap sediment, is about one-20th the size of the Susitna River watershed but deposits about one-300th the amount of suspended sediment (approximately 1 ⋅ 104 tons) into the Cook Inlet (Brabets et al. 1999).
High turbidity can act in concert with cold temperature and channel instability to limit food resources and growth of juvenile salmon (Milner et al. 2001, Brown and Milner 2012). The high turbidity can limit visual foraging success by juvenile salmon such as Chinook and coho salmon and steelhead trout (Lloyd et al. 1987). However, juvenile salmon may shift to forage on benthic prey or move to more productive off-channel habitats that are typically lower in turbidity levels (Tippets and Moyle 1978). Furthermore, some degree of glacier-derived turbidity may benefit juvenile salmon during rearing and outmigration by sheltering them from visual predators (figure 3; Gregory and Levings 1998).
Side channels and other off-main-channel habitats may be particularly important salmon habitat in unconstrained floodplains during this phase of glacier retreat. As braided streams cut new paths across broad floodplains, their abandoned channels remain as preferential flow paths for clearwater side channels often fed by groundwater (Lorenz and Eiler 1989, Curran et al. 2011, Hauer et al. 2016). In combination with other lateral habitats, such as precipitation-fed tributaries, side channels can provide important habitats for some salmon species to spawn (e.g., Chinook, chum, and coho salmon) or rear (e.g., coho and sockeye salmon, steelhead trout; table 1, figure 3) because they are often warmer, less turbid, have higher prey production, and have lower velocities than the mainstem channel dominated by glacier meltwater (Murphy et al. 1989, Curran et al. 2011, Rine et al. 2016). For example, in the heavily glacierized Taku River in Alaska and British Columbia, where sockeye salmon rear within the river rather than in lakes, juvenile Chinook, coho and sockeye salmon were found at extremely low densities or not at all in the mainstem during the summer, but instead were found rearing in tributaries or side channel habitats (Murphy et al. 1989). Typically, in heavily glacierized watersheds, groundwater-fed valley margin habitats can receive disproportionately high use by salmon for spawning and rearing (Lorenz and Eiler 1989, Murphy et al. 1989, Curran et al. 2011). Therefore, during this phase of glacier retreat there is a mosaic of habitat conditions produced that salmon can use across their life phases.
As glaciers retreat, they leave behind moraines that can result in the formation of ice marginal or proglacial lakes (figure 2). Immediately after formation, these unstable moraine-dammed lakes are typically cold and have shallow euphotic zones because of high levels of suspended glacier flour (Lloyd et al. 1987), rendering them relatively unproductive. Regardless, sockeye salmon may spawn and rear in young proglacial lakes, even those with actively calving glaciers and high turbidity (Ramstad et al. 2004, Barouillet et al. 2019). Other salmon species do not typically spawn in lakes; however, newly created lakes can provide rearing habitat for juvenile coho salmon (Milner et al. 2011). Therefore, the creation of lakes from glacier retreat can directly increase salmon spawning and rearing habitat, particularly for sockeye salmon (figure 3).
Glacier-created lakes can also have substantial downstream effects on salmon habitats and their suitability (Dorava and Milner 2000). Proglacial lakes modify downstream conditions by attenuating peak flows, sustaining base flow through the drier summer months, settling bedload and suspended sediment, and increasing stream temperature (Dorava and Milner 2000). Consequently, stream channel stability is much higher below proglacial lakes, and turbidity and thermal regimes are more hospitable to salmon reproduction and juvenile rearing (Dorava and Milner 2000, Schoen et al. 2017). For example, Chinook salmon in many regions spawn extensively downstream of large lake systems presumably because of the suitability of these habitats for egg incubation because of lake moderation of flow, temperature, and sediment transport (table 1; Roni and Quinn 1995, Brabets et al. 1999, Schoen et al. 2017). Therefore, lakes can be a key mediating factor that influences the downstream effects of glacier retreat.
Phase 3: High-elevation glaciers with downstream effects. As glaciers recede, they retreat up valley to steeper terrain that is inaccessible to salmon. Therefore, during this phase, there is no creation of additional accessible river habitat to salmon, but glacier retreat affects salmon habitat via downstream effects and continual river evolution despite lower levels of watershed and glacier runoff than the previous peak-water phase, phase 2 (figure 2). Decreased summer river discharge and lower sediment transport lead to increased stabilization of downstream mainstem channels and floodplains. Riparian forests have typically matured to the point of stabilizing stream banks, corresponding with a more general transition from strict physical control of the deglaciated landscape to a period of increasing biotic influence (figure 2; Milner et al. 2007). Riparian forests also begin to influence habitat quality as wood is recruited to stream channels. Wood accumulations trap suitable-size spawning gravel (Buffington et al. 2004) and causes local hydraulic forcing that sorts sediment and scours pools, thus increasing the size and number of areas available for juvenile rearing, particularly for Chinook and coho salmon (Mossop and Bradford 2004). Increases in channel stability may also improve conditions for species that spawn in the mainstem, such as Chinook salmon and steelhead trout.
Overall, at this later phase of glacier retreat, the proportional contribution of glacier meltwater to total watershed runoff will be lower, and therefore, downstream water temperatures will be warmer (figure 2). However, glacier runoff from relatively small high-elevation glaciers can still play an important role in regulating downstream water temperature. During periods of warm weather, glaciers will provide more cold meltwater and thus decrease the climate sensitivity of downstream river water temperatures (Jansson et al. 2003). For example, during late summer months, glacier runoff can contribute up to 25% of total watershed runoff even in watersheds that are only 1% glacierized (Huss and Hock 2018). Given that summer water temperatures have been shown to decrease by approximately 1 °C for every 10% increase in glacier coverage (Fellman et al. 2014), in regions in which water temperatures may otherwise reach high levels (e.g., more than 15 °C), high-elevation glaciers may be an important source of cold water by stabilizing or buffering stream temperatures.
Increased water temperature during this phase of glacier retreat will generally increase the development and growth rates of salmon, but the overall effects on salmon populations are complex. Warmer temperatures can accelerate embryo development, potentially resulting in smaller (Beacham and Murray 1990) and less well developed fish (Fuhrman et al. 2018) that emerge earlier (Adelfio et al. 2018). Increased temperatures can also increase juvenile growth rates (Bailey et al. 2018), which could lead to increased marine survival because of escaping size-selected mortality (Ward and Slaney 1988). However, warmer freshwater temperatures could also induce juvenile salmon to complete freshwater rearing in fewer years, which could decrease marine survival (Cline et al. 2019). Therefore, salmon responses to changing temperatures are complex and will depend on how effects cascade over their life cycles.
Total fluxes and concentrations of suspended sediments are predicted to be lower in phase 3 systems than in the previous phase of glacier retreat (figure 2; Milner et al. 2017). Increased water clarity could increase prey production and foraging success of mainstem-feeding juvenile salmon (Milner et al. 2001). With stream temperatures increasing in phase 3, prey species for juvenile salmon may shift from cold-water adapted macroinvertebrates, such as a restricted set of chironomids, to a more diverse benthic invertebrate assemblage (Milner et al. 2008). Terrestrial invertebrates associated with riparian vegetation may also increase the diversity of prey available for juvenile salmon (Wipfli and Baxter 2010). Downstream lake habitats will also likely have greater juvenile salmon growth potential with increases in summer temperature and light penetration, which is particularly important for increasing the prey available to lake-rearing species, such as sockeye salmon. However, lower turbidity could also increase predation rates on rearing and outmigrating salmon (figure 3).
Continued river system evolution and decreased contributions from glacier melt during phase 3 will also shift downstream river physiochemistry (figure 2). For example, watersheds in southeast Alaska with less than 10% glacier coverage typically have substantially higher summer dissolved organic carbon concentration, suggesting that terrestrial ecosystem processes play an increasingly important role in determining stream water nutrient concentrations as glaciers recede (Hood and Berner 2009). Large watersheds with high-elevation glaciers may contain a wide range of aquatic habitats, including clearwater, brownwater, and glacier-fed tributaries that feed into the mainstem (Schoen et al. 2017). These complex habitats offer diverse habitat mosaics and food webs that can support different species and life histories of salmon.
Phase 4: Watersheds without permanent ice. The complete loss of glaciers during phase 4 eliminates the effects of glacier meltwater on downstream salmon habitat (figure 2). Most notably, the loss of high-elevation glaciers eliminates an important source of stored water that would otherwise be released as cold meltwater during the summer season, increasing the risk of detrimental low summer flows, which can further exacerbate sensitivity to warm air temperature (Fellman et al. 2014). Therefore, the loss of glaciers results in a fundamental change in seasonal patterns of hydrology and temperature (figure 2).
The effects of these shifts in hydrology and temperature on salmon will likely vary with environmental context. For example, in warm regions with low summer precipitation, warm summer water temperature and low stream flows could negatively affect salmon by decreasing survival of migratory adult salmon (figure 3; Eliason et al. 2011, Martins et al. 2012) or by restricting juvenile rearing across habitats (Sloat and Osterback 2013) or seasons (Munsch et al. 2019) because of reduced or changed stream flow patterns. In temperate coastal regions, the loss of glacier ice and shifts in precipitation from snow to rainfall can cause higher streamflow stochasticity that may increase winter flooding risks to salmon (Sloat et al. 2018). In addition, low summer stream flows and warm temperatures could result in increased frequency of hypoxic events in streams with high salmon abundance (Sergeant et al. 2017). Finally, as glaciers are removed from watersheds, there may be microclimatic effects, such as local temperature and rainfall pattern changes, influencing salmon habitat on a smaller scale (Oerlemans 2010). Generally, the loss of glaciers and their meltwater may pose challenges in some regions for salmon in terms of habitat quality and quantity, but such impacts will be strongly influenced by local context and local adaptations.
The effects of glaciers on salmon ecosystems are evident for centuries or millennia after glaciers have disappeared. For example, thousands of years ago, glaciers shaped large lakes and linked river systems in watersheds of southwest Alaska. Presently, these watersheds are now devoid of glaciers but are thriving and dynamic salmon ecosystems (Hilborn et al. 2003, Brennan et al. 2019). Gravel-bedded river floodplains are another prominent relict feature that can represent highly productive ecosystems (Hauer et al. 2016). Comparative studies of the evolution of deglaciated landscapes suggest an increase in salmon habitat quality as watershed stability increases, but a gradual decrease in salmon habitat quantity over time with continued channel incision and decreased lateral groundwater-fed habitats over thousands of years (Benda et al. 1992). Therefore, glaciers have a long-lasting legacy of influence on salmon ecosystems.
Contemporary glaciers in salmon watersheds
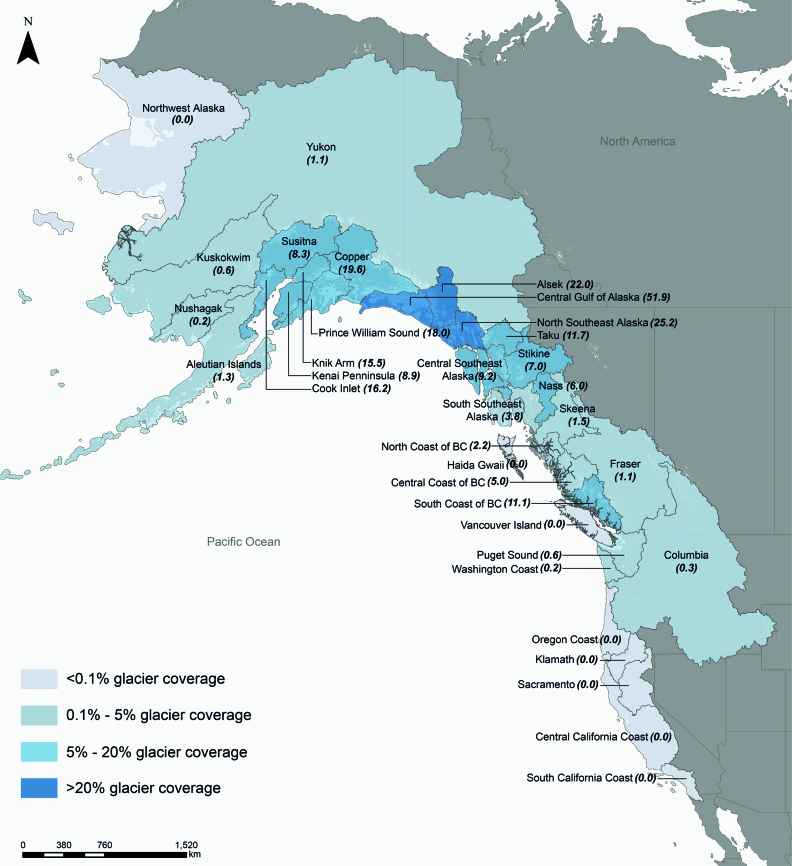
North America's major salmon watersheds currently have varying degrees of glacier coverage that roughly correspond to different phases of the conceptual model (figure 2). We obtained glacier data from the Randolph Glacier Inventory v6.0 (Pfeffer et al. 2014), a global inventory of glacier outlines, throughout the current North American range of salmon to quantify the percentage of glacier cover in regions that are either major salmon watersheds or aggregate coastal regions that contain numerous smaller watersheds (figure 4). For example, we estimated glacier coverage in the 50,000 square kilometers Susitna River watershed draining into Cook Inlet, Alaska, as well as the Central Gulf of Alaska Region, which contains an aggregate of small coastal watersheds, some completely covered by glacier ice. Therefore, this analysis of glacier coverage is on the scale of larger watersheds and drainage regions. Collectively, these watersheds and regions cover the North American range of salmon (figures 1 and 4).
Figure 4.
Map showing the percent glacier cover for watersheds or regions between California and Alaska. The numbers in parentheses following the watershed or region names refer to percent cover of glaciers in the watershed or region.
Salmon watersheds and regions have vastly different extents of current glacier coverage. For instance, 9% of watersheds or regions (3 of 34 watersheds or regions) have high glacier coverage (more than 20% of watershed area), particularly in south-central Alaska (figure 4). About 32% of watersheds or regions (11 of 34) have significant glacier coverage (5% to 20%), such as in southeast and south-central Alaska and along the British Columbia coast. Watersheds or regions with high to significant glacier coverage are likely to contain habitats that are in the earlier phases (phase 1 and 2) of our conceptual model of glacier retreat (figure 2). Similarly, 35% of watersheds or regions (12 of 34) have low glacier coverage (0.1% to 5%), spanning from the Columbia River to the Yukon River and Alaska. These watersheds or regions are generally expected to exhibit characteristics of phase 3 of our conceptual model. Finally, 24% of watersheds or regions (8 of 34) have minimal or no glacier coverage (less than 0.1%), corresponding to phase 4 of our conceptual model. These watersheds or regions primarily occur at the southern and northern range extent for salmon, such as the Klamath and Sacramento watersheds and northwest Alaska, respectively (figure 4). Collectively, 85% of watersheds or regions (29 of 34) that we consider have at least some glacier coverage (figure 4). Therefore, most of North American salmon watersheds or regions are being influenced by contemporary glacier retreat.
Larger watersheds or regions included in our analysis contain many smaller watersheds within them that all have varying degrees of glacier coverage. For example, our analysis indicates that the large Susitna River watershed has significant glacier coverage (figure 4). However, the Susitna River watershed also contains many small subwatersheds, some that have no glacier coverage (i.e., phase 4; figure 2) and other catchments that have high glacier coverage and are better represented by phase 1. Therefore, watersheds or regions contain many smaller subwatersheds at different phases of glacier retreat.
Research frontiers
In this section, we describe research frontiers associated with glacier retreat and salmon habitat.
Mediating factors and context dependency
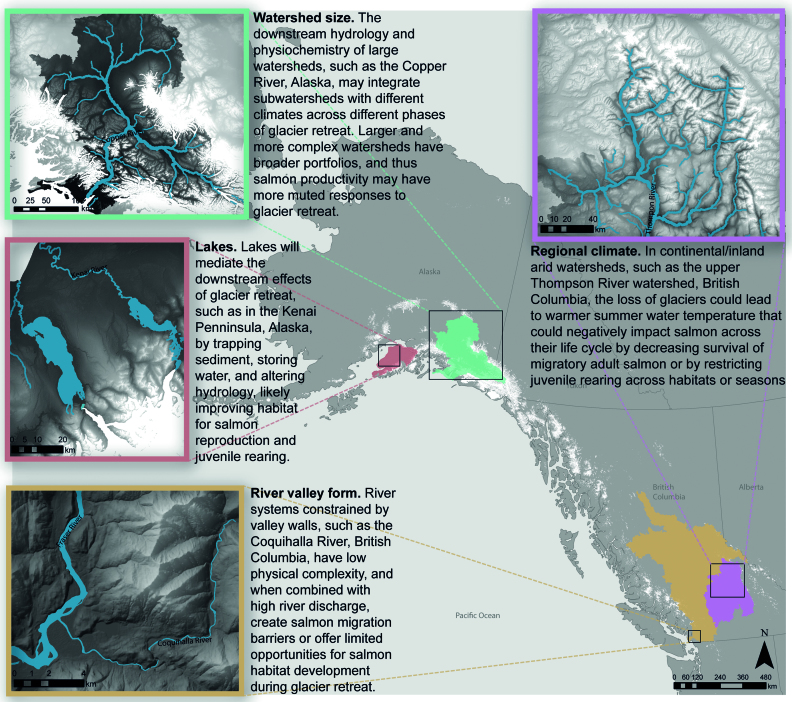
The effects of glacier retreat on salmon and their habitat will likely be context dependent and influenced by mediating factors such as lakes, watershed size, river valley form, and geographic location (figure 5). As was discussed above, lakes trap sediments, store water, and alter hydrology; through these processes, lakes mediate the downstream effects of glacier retreat (Dorava and Milner 2000, Schoen et al. 2017). Watershed size and complexity may also be key mediating factors. For example, the downstream hydrology and physiochemistry of large watersheds such as the Copper River in Alaska integrates subwatersheds with different climates across the phases of glacier retreat (figure 5). Larger and more complex watersheds have broader portfolios of glacier recession, climate variability, and habitat types and may therefore have more muted responses (Moore et al. 2015, Chezik et al. 2017). In contrast, in watersheds that have linear topology (few tributaries) or are smaller, runoff from a single glacier may be the main driver of downstream hydrology (figure 5). The location of the watershed and its associated climate will be another key mediating factor. Coastal watersheds with more moderate climates, higher mean annual precipitation may be less at risk of warm summer water temperature and low summer flows following glacier loss. Generally, there is a need for long-term studies that address how landscape features modulate the impacts of glacier retreat.
Figure 5.
Map highlighting the mediating factors such as watershed size, presence of lakes, river valley form, and regional climate influencing the effects of glacier retreat on salmon and their habitat. Watershed boundaries (various colors), and rivers and lakes (blue) data were obtained from the National Hydro Database (www.usgs.gov/products) for Alaska and the Freshwater Atlas (www2.gov.bc.ca/gov/content/data/geographic-data-services/topographic-data/freshwater) for British Columbia. Glacier outlines (white), were obtained from the Randolph Glacier Inventory v6.0 (Pfeffer et al. 2014).
Plastic and evolutionary responses to glacier retreat
Salmon have remarkable capacity for both plastic and adaptive responses to rapid environmental change (Crozier and Hutchings 2014). Accordingly, rapid evolution and phenotypic plasticity will likely mediate the population-level responses of salmon to glacier retreat. For example, although adult Chinook salmon may be physiologically sensitive to warm water temperature (Muñoz et al. 2015), adaptive or plastic changes in migration phenology could drastically reduce their exposure to periods of warm water (Mantua et al. 2015). For instance, an ecoevolutionary model predicted that evolution may shift sockeye salmon migration timing by approximately 10 days over the next century in a warming river, which could increase the probability of population persistence (Reed et al. 2011). However, there is great uncertainty in such predictions, and these remain important key research frontiers. For example, can salmon evolve at a rate that keeps pace with climate-driven habitat change (Reed et al. 2011)? What are the limits and cues of their adaptive plasticity (Crozier and Hutchings 2014)?
Fish community responses to glacier retreat
Glacier retreat will not only affect anadromous Pacific salmon, but also other fishes. For example, Dolly Varden (Salvelinus malma) and their close relative, bull trout (S. confluentus), are char with extremely flexible and diverse life histories that may be particularly well suited for utilizing glacier-fed rivers. With both migratory and resident life histories, these fish can use coastal ocean habitats and migrate among river basins (Brenkman and Corbett 2005). Furthermore, these species are particularly adapted to cold waters, such as those found in rivers that are heavily glacierized (e.g., phase 2; figure 2). For example, Milner et al. (2008) discovered that Dolly Varden were the first fish to colonize a new river following glacier retreat. Cutthroat trout (Oncorhynchus clarkii) also have flexible and diverse life histories, including frequent movements within and between watersheds (Trotter 1989, Saiget et al. 2007). Therefore, the behavior and life histories of cutthroat trout may allow them to capitalize on opportunities offered by glacier retreat. Another fish species that may be particularly affected by glacier retreat are eulachon (Thaleichthys pacificus) that are found in many large river systems in the region with high glacial influence (Moody and Pitcher 2010). Eulachon are remarkably lipid-rich anadromous smelt whose late-winter or early-spring migration to rivers is of critical importance to wildlife and human harvesters. These fish migrate and broadcast spawn in the lower reaches of rivers during the late winter and early spring, and their planktonic larvae drift out to estuaries shortly thereafter (Moody and Pitcher 2010). Although these elusive fish remain relatively understudied, their spawning timing and survival may be extremely sensitive to changes in the hydrology and sediments of the lower reaches of rivers that could be modified greatly by rapid glacial retreat. Other fishes, such as sculpins (Cottus aleuticus) and stickleback (Pungitius pungitius), although not of direct cultural importance, may also colonize glacier rivers and play roles in their food webs (Milner et al. 2011). These shifts in other components of the fish communities may play important roles in the ecology of salmon watersheds through the phases of glacier retreat.
Ocean and estuary conditions
Glacier retreat from either marine- or land-terminating glaciers can affect ocean and estuary conditions, by changing the amount and timing of sediment, nutrients, and freshwater inputs (O’Neel et al. 2015). On an ocean-basin scale, increased variability in glacier runoff patterns is expected to affect the strength of the Alaska Coastal Current (ACC), the dominant coastal circulation pattern in the Central Gulf of Alaska, and therefore the cross-shelf transport of organisms and nutrients (O’Neel et al. 2015). Glacier meltwater is also an important source of bioavailable carbon and nutrients such as phosphorus and iron to downstream habitats (Fellman et al. 2010, Schroth et al. 2011), and transport terrestrial and riverine organic matter and nutrients downstream, where they are incorporated into estuarine food webs (Arimitsu et al. 2018). However, relatively little is known about how changes in magnitude and timing of freshwater, organic matter, and nutrient fluxes from glacier rivers will affect estuaries and oceans. Therefore, glacier retreat could have profound effects on the ocean basin as well as for coastal ocean populations (O’Neel et al. 2015).
Outmigrating juvenile salmon in estuaries and coastal oceans may also be affected by glacier retreat via different potential pathways of impact. For example, substantial changes in the ACC could affect primary production or the spatiotemporal overlap of salmon with their prey. For example, interannual variability in the abundance of juvenile chum salmon, sampled in July via surface trawl in southeast Alaska, was positively correlated to freshwater runoff in the spring (Kohan et al. 2017). This relationship was attributed to primary production resulting from stronger water column stratification. Changes in environmental conditions arising from shifts in glacier runoff could also structure the distribution of salmon and other marine organisms, as in the heavily glacierized Glacier Bay, Alaska, where variation in community structure was associated with turbidity, water temperature, stratification, and distribution of icebergs from calving glaciers (Arimitsu et al. 2016). Similarly, a study of Greenland fjords found that rising subsurface meltwater plumes from marine-terminating glaciers bring nutrient-rich water to the surface that sustains high phytoplankton productivity during the summer (Meire et al. 2017). Changes in turbidity in nearshore marine habitats could also affect the vulnerability of juvenile salmon to predators and the feeding success of smolts during their outmigration (Gregory and Levings 1998, De Robertis et al. 2003). Indeed, estuaries can function as both transitional and rearing habitat for all species of juvenile salmon during smolt outmigration to the ocean (Weitkamp et al. 2014). Overall, the net effect of glacier retreat on the productivity of estuaries and the ocean for salmon remains relatively unknown.
Multiple stressors and glacier retreat
Contemporary glacier retreat in salmon ecosystems is occurring in concert with a host of additional anthropogenic stressors, such as ocean acidification, habitat loss, warming ocean and freshwater temperatures, shifting precipitation regimes, and hatchery influences. For example, in many regions, glacial meltwater contributions to runoff will decrease as air temperatures increase (Bliss et al. 2014, Huss and Hock 2018), processes that could act additively or multiplicatively to rapidly increase water temperatures. Furthermore, some climate precipitation models also predict drier summers in the study region (Mote and Salathé 2010), which, when combined with losses of summer glacier meltwater, could collectively decrease low summer flows. However, precipitation climate models are generally highly uncertain and spatially variable (Mote and Salathé 2010). Shifts in water temperatures could also lead to an increase in invasive or exotic species that could reduce or negatively affect salmon (Lawrence et al. 2014). Therefore, the impacts of glacier retreat on salmon should be considered through the lens of cumulative effects. It is also likely that stressors will influence the response of salmon to glacier retreat. For example, if the capacity of the ocean to support thriving salmon populations is compromised by climate change, then salmon may be slower to colonize new habitats. Alternatively, losses of salmon genetic diversity, because of—for example—habitat loss, overharvest, or hatcheries, may compromise their capacity for rapid evolution (McClure et al. 2008). Therefore, it is unknown how these multiple processes will interact and affect salmon and their ecosystems, because glacier retreat is only one of these many ongoing stressors.
Salmon management in an era of rapid glacier retreat
Glacier loss may pose challenges and opportunities for effective management and conservation of salmon and their habitats. Salmon productivity will likely shift across space and time depending on a watershed's various phases of glacier retreat. Over the coming decades, we predict that there will be areas in which salmon populations will be disadvantaged because of glacier retreat and associated loss of predictable water flows and increased water temperature (Mantua et al. 2010), such as some watersheds or regions in phase 4 of glacier retreat (figures 2 and 4); areas in which glacier retreat will enhance salmon productivity as downstream habitat suitability increases (Milner et al. 2008, Fellman et al. 2014), such as watersheds or regions in phases 2 and 3 of glacier retreat (figures 2 and 4); and areas of completely new habitat that can be colonized by significant numbers of salmon as glaciers retreat and river and lake habitats form (some watersheds or regions in phase 1 of glacier retreat; figures 2 and 4; Milner et al. 2011). Therefore, glacier retreat, as well as other drivers of global change, will shift salmon production capacity and challenge current management systems. Below we highlight key challenges to salmon management associated with glacier retreat.
Predictive population models, management plans, forecasts, and sustainable harvest rates will need to be revisited and revised as salmon productivity shifts within and between watersheds with glacier retreat. Relationships between salmon returns and environmental conditions may shift as past relationships are pushed beyond their historically enumerated bounds and as other processes become dominant drivers. In other words, glacier retreat may expedite nonstationarity in relationships between environmental factors and salmon (Litzow et al. 2018). As glacier retreat shifts salmon productivity, it would be beneficial to frequently revisit management goals such as escapement targets and sustainable fishing levels. It is also possible that temporary decreases in harvest levels during the expansion phase of salmon colonization may expedite the establishment of thriving salmon populations. For instance, in areas in which glacier retreat enhances salmon production, new salmon harvest opportunities may be created, such as on the Kenai Peninsula, south-central Alaska, where the establishment of sockeye salmon populations supported a commercial fishery (Milner 1997). In addition, complex and differential responses to glacier retreat within regions may differentially shift the productivity of particular locations or populations of salmon. Such response diversity, if untracked, may exacerbate risks of accidental mixed-stock overharvest. Terminal or carefully managed fisheries may be more robust to such shifts in productivity. Alternatively, in regions in which glacier loss will degrade salmon habitat, such as in some of the southern portion of salmon's range, where glaciers have or are nearly retreated from the landscape, fisheries may need to be managed more conservatively. For example, in British Columbia's warming Fraser River, salmon managers restrict fisheries in years in which the river becomes too warm for migrating sockeye (Martins et al. 2011). Effective monitoring will enable adaptive management responses to the shifting landscape of salmon.
Salmon restoration activities should be designed and undertaken with a forward-looking outlook (Beechie et al. 2013) that considers how landscapes may change because of glacier retreat. It may be tempting to employ major engineering and infrastructure approaches to mitigate the effects of lost glaciers. In the European Alps, it has been proposed that new reservoirs could be constructed to mitigate projected changes in seasonal water availability from melting glaciers by offsetting up to 65% of the expected summer runoff changes from presently glacierized basins (Farinotti et al. 2016). However, such engineering approaches would take massive financial investment, would mitigate only one of the important potential pathways of connection between glaciers and salmon, and would likely pose major risks to salmon. Therefore, we suggest that, in most cases, such engineering approaches to mitigating lost glaciers for salmon may not be appropriate. Instead, process-based restoration will likely be more effective in this era of rapid global change. Process-based restoration enables the fundamental processes that generate and maintain habitat and will therefore be more robust to a changing world. This approach to restoration should include targeting the root cause of ecosystem change, tailoring restoration actions to the local potential, matching the scale of the restorative action to the scale of the biological or physical process, and being explicit about outcomes and recovery time frames (Beechie et al. 2010). Typical actions such as restoring floodplain connectivity, protecting river floodplains from encroaching human infrastructure (Johnson et al. 2019), maintaining or restoring wetlands and beaver ponds (Weber et al. 2017), decreasing human water withdrawals to maintain stream flow regimes, and reaggrading incised channels are most likely to ameliorate stream flow and temperature changes and increase habitat diversity and population resilience (Beechie et al. 2013). Such process-based restoration and habitat protection would represent substantial investment, but large-scale analyses have suggested that such approaches may be cost effective and could provide multiple benefits (Johnson et al. 2019).
Proactive protection of future salmon habitat is also likely a wise investment. Regions with high glacier coverage (e.g., the Central Gulf of Alaska; figure 4) might have substantial gains in salmon habitat and associated returns over the next century, and salmon are already growing in importance to commercial fishing portfolios (Beaudreau et al. 2019). However, glacier retreat may also expose substantial mineral deposits, and choices will need to be made about fostering salmon production versus extracting mineral resources. Environmental decision-making is often based on current estimates of risks to important species, such as salmon. For example, mines in the transboundary region of British Columbia and Alaska have been recently approved in part because they are in heavily glacierized areas and, at present, have a presumed low value for salmon (Canadian Environmental Assessment Agency 2018). Such environmental decision-making fails to incorporate the risk of lost future salmon production. Meanwhile, tools such as intrinsic potential models can be used to quantify the potential value of future salmon habitat (Bidlack et al. 2014). There is a need for proactive decision-making and conservation that incorporates the potential values and benefits of future salmon habitat.
The future states of resources will always be extremely difficult to forecast (Schindler and Hilborn 2015). Preserving the genetic diversity and evolutionary potential of salmon will be of foundational importance in enabling the adaptive capacity of salmon systems (Schindler et al. 2008, Waples et al. 2008). Protecting diverse and connected salmon watersheds is also essential for supporting sustainable fisheries (Hilborn et al. 2003). The story of glaciers and salmon goes back millions of years. In the present phase, there is a key role for management and conservation that are robust to rapid glacier retreat and an uncertain future of salmon stocks.
Acknowledgments
We gratefully acknowledge funding from the Gordon and Betty Moore Foundation for the Salmon Science Network Initiative, which provided the opportunity to hold a working group of scientists from Canada, United States, and United Kingdom in November 2017. Kara Pitman was supported by the National Science and Engineering Research Council and Association of Canadian Universities for Northern Studies. We thank three anonymous reviewers for insightful comments.
Author Biographical
Kara J. Pitman (kpitman@sfu.ca) and Jonathan W. Moore are affiliated with the Earth2Oceans Research Group at Simon Fraser University, in Burnaby, Canada. Matthew R. Sloat is affiliated with the Wild Salmon Center, in Portland, Oregon. Anne H. Beaudreau is affiliated with the College of Fisheries and Ocean Sciences at the University of Alaska Fairbanks, in Juneau, Alaska. Allison L. Bidlack is affiliated with the Alaska Coastal Rainforest Center at the University of Alaska Southeast, in Juneau, Alaska. Richard E. Brenner is affiliated with the Alaska Department of Fish and Game, in Juneau, Alaska. Eran W. Hood is affiliated with the Environmental Science Program at the University of Alaska Southeast, in Juneau, Alaska. George R. Pess is affiliated with the National Marine Fisheries Service, National Oceanic and Atmospheric Administration, in Seattle, Washington. Nathan J. Mantua is affiliated with the Southwest Fisheries Science Center at the National Oceanic and Atmospheric Administration, in Santa Cruz, California. Alexander M. Milner is affiliated with the School of Geography, Earth, and Environmental Sciences at the University of Birmingham, in Birmingham, United Kingdom, and with the Institute of Arctic Biology at the University of Alaska, in Fairbanks, Alaska. Valentina Radić is affiliated with the Department of Earth, Ocean, and Atmospheric Sciences at the University of British Columbia, in Vancouver, British Columbia, Canada. Gordon H. Reeves is affiliated with the US Department of Agriculture's Forest Service, in Corvallis, Oregon. Daniel E. Schindler is affiliated with the School of Aquatic and Fishery Sciences at the University of Washington, in Seattle, Washington. Diane C. Whited is affiliated with the Flathead Lake Biological Station, at the University of Montana, in Polson, Montana.
References cited
- Adelfio LA, Wondzell SM, Mantua NJ, Reeves GH. 2018. Warm winters reduce landscape-scale variability in the duration of egg incubation for coho salmon (Oncorhynchus kisutch) on the Copper River Delta, Alaska. Canadian Journal of Fisheries and Aquatic Sciences 76: 1362–1375. [Google Scholar]
- Arimitsu ML, Hobson KA, Webber DN, Piatt JF, Hood EW, Fellman JB. 2018. Tracing biogeochemical subsidies from glacier runoff into Alaska's coastal marine food webs. Global Change Biology 24: 387–398. [DOI] [PubMed] [Google Scholar]
- Arimitsu ML, Piatt JF, Mueter F. 2016. Influence of glacier runoff on ecosystem structure in Gulf of Alaska fjords. Marine Ecology Progress Series 560: 19–40. [Google Scholar]
- Bailey CJ, Braun DC, McCubbing D, Reynolds JD, Ward B, Davies TD, Moore JW. 2018. The roles of extrinsic and intrinsic factors in the freshwater life-history dynamics of a migratory salmonid. Ecosphere 9: e02397. [Google Scholar]
- Barouillet C, Cumming BF, Laird KR, Perrin CJ, Selbie DT. 2019. Influence of glacial flour on the primary and secondary production of sockeye salmon nursery lakes: A comparative modern and paleolimnological study. Canadian Journal of Fisheries and Aquatic Sciences 76: 2303–2314. [Google Scholar]
- Beacham TD, Murray CB. 1990. Temperature, egg size, and development of embryos and alevins of five species of Pacific salmon: A comparative analysis. Transactions of the American Fisheries Society 119: 927–945. [Google Scholar]
- Beamish RJ, Neville CM, Sweeting RM, Beacham TD, Wade J, Li L. 2016. Early ocean life history of Harrison River sockeye salmon and their contribution to the biodiversity of sockeye salmon in the Fraser River, British Columbia, Canada. Transactions of the American Fisheries Society 145: 348–362. [Google Scholar]
- Beaudreau AH, Ward EJ, Brenner RE, Shelton AO, Watson JT, Womack JC, Anderson SC, Haynie AC, Marshall KN, Williams BC. 2019. Thirty years of change and the future of Alaskan fisheries: Shifts in fishing participation and diversification in response to environmental, regulatory and economic pressures. Fish and Fisheries 20: 601–619. [Google Scholar]
- Beechie T et al.. 2013. Restoring salmon habitat for a changing climate. River Research and Applications 29: 939–960. [Google Scholar]
- Beechie TJ, Collins BD, Pess GR. 2001. Holocene and recent geomorphic processes, land use, and salmonid habitat in two north Puget Sound river basins. Geomorphic Processes and Riverine Habitat Water Science and Application 4: 37–54. [Google Scholar]
- Beechie TJ, Sear DA, Olden JD, Pess GR, Buffington JM, Moir H, Roni P, Pollock MM. 2010. Process-based principles for restoring river ecosystems. BioScience 60: 209–222. [Google Scholar]
- Bellmore JR, Baxter CV. 2014. Effects of geomorphic process domains on river ecosystems: A comparison of floodplain and confined valley segments. River Research and Applications 30: 617–630. [Google Scholar]
- Benda L, Beechie TJ, Wissmar RC, Johnson A. 1992. Morphology and evolution of salmonid habitats in a recently deglaciated river basin, Washington state, USA. Canadian Journal of Fisheries and Aquatic Sciences 49: 1246–1256. [Google Scholar]
- Benito G, O’Connor JE. 2003. Number and size of last-glacial Missoula floods in the Columbia River valley between the Pasco Basin, Washington, and Portland, Oregon. GSA Bulletin 115: 624–638. [Google Scholar]
- Bidlack AL, Benda LE, Miewald T, Reeves GH, McMahan G. 2014. Identifying suitable habitat for Chinook salmon across a large, glaciated watershed. Transactions of the American Fisheries Society 143: 689–699. [Google Scholar]
- Bliss A, Hock R, Radić V. 2014. Global response of glacier runoff to twenty-first century climate change. Journal of Geophysical Research: Earth Surface 119: 717–730. [Google Scholar]
- Booth DB, Troost KG, Clague JJ, Waitt RB. 2003. The Cordilleran Ice Sheet. Developments in Quaternary Sciences 1: 17–43. [Google Scholar]
- Brabets TP, Nelson GL, Dorava JM, Milner AM. 1999. Water-Quality Assessment of the Cook Inlet Basin, Alaska: Environmental Setting. US Geological Survey. Water-Resources Investigations Report no. 99–4025.
- Brenkman SJ, Corbett SC. 2005. Extent of anadromy in bull trout and implications for conservation of a threatened species. North American Journal of Fisheries Management 25: 1073–1081. [Google Scholar]
- Brennan SR, Schindler DE, Cline TJ, Walsworth TE, Buck G, Fernandez DP. 2019. Shifting habitat mosaics and fish production across river basins. Science 364: 783–786. [DOI] [PubMed] [Google Scholar]
- Brett JR. 1971. Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerka). American Zoologist 11: 99–113. [Google Scholar]
- Brown LE, Milner AM. 2012. Rapid loss of glacial ice reveals stream community assembly processes. Global Change Biology 18: 2195–2204. [Google Scholar]
- Buffington JM, Montgomery DR, Greenberg HM. 2004. Basin-scale availability of salmonid spawning gravel as influenced by channel type and hydraulic roughness in mountain catchments. Canadian Journal of Fisheries and Aquatic Sciences 61: 2085–2096. [Google Scholar]
- Campbell EY, Dunham JB, Reeves GH, Wondzell SM. 2019. Phenology of hatching, emergence, and end-of-season body size in young-of-year coho salmon in thermally contrasting streams draining the Copper River Delta, Alaska. Canadian Journal of Fisheries and Aquatic Sciences 76: 185–191. [Google Scholar]
- Canadian Environmental Assessment Agency 2018. Red mountain underground gold project. Draft environmental assessment report.
- Carrivick JL, Heckmann T. 2017. Short-term geomorphological evolution of proglacial systems. Geomorphology 287: 3–28. [Google Scholar]
- Chezik KA, Anderson SC, Moore JW. 2017. River networks dampen long-term hydrological signals of climate change. Geophysical Research Letters 44: 7256–7264. [Google Scholar]
- Cline TJ, Ohlberger J, Schindler DE. 2019. Effects of warming climate and competition in the ocean for life-histories of Pacific salmon. Nature Ecology and Evolution 3: 935–942. [DOI] [PubMed] [Google Scholar]
- Crozier LG, Hutchings JA. 2014. Plastic and evolutionary responses to climate change in fish. Evolutionary Applications 7: 68–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JH, McTeague ML, Burril SE, Zimmerman CE. 2011. Distribution, Persistence, and Hydrologic Characteristics of Salmon Spawning Habitats in Clearwater Side Channels of the Matanuska River, Southcentral Alaska. US Geological Survey. Scientific Investigations Report no. 2011–5012.
- De Robertis A, Ryer CH, Veloza A, Brodeur RD. 2003. Differential effects of turbidity on prey consumption of piscivorous and planktivorous fish. Canadian Journal of Fisheries and Aquatic Sciences 60: 1517–1526. [Google Scholar]
- Death RG, Winterbourn MJ. 1995. Diversity patterns in stream benthic invertebrate communities: The influence of habitat stability. Ecology 76: 1446–1460. [Google Scholar]
- Dorava J M, Milner AM. 2000. Role of lake regulation on glacier-fed rivers in enhancing salmon productivity: The Cook Inlet watershed, south-central Alaska, USA. Hydrological Processes 14: 3149–3159. [Google Scholar]
- Dyke AS. 2004. An outline of North American deglaciation with emphasis on central and northern Canada. Developments in Quaternary Sciences 2: 373–424. [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP. 2011. Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112. [DOI] [PubMed] [Google Scholar]
- Farinotti D, Pistocchi A, Huss M. 2016. From dwindling ice to headwater lakes: Could dams replace glaciers in the European Alps? Environmental Research Letters 11: 054022. [Google Scholar]
- Fellman JB, Spencer RGM, Hernes PJ, Edwards RT, D’Amore DV, Hood E. 2010. The impact of glacier runoff on the biodegradability and biochemical composition of terrigenous dissolved organic matter in near-shore marine ecosystems. Marine Chemistry 121: 112–122. [Google Scholar]
- Fellman JB, Nagorski S, Pyare S, Vermilyea AW, Scott D, Hood E. 2014. Stream temperature response to variable glacier coverage in coastal watersheds of southeast Alaska. Hydrological Processes 28: 2062–2073. [Google Scholar]
- Fuhrman AE, Larsen DA, Steel EA, Young G, Beckman BR. 2018. Chinook salmon emergence phenotypes: Describing the relationships between temperature, emergence timing and condition factor in a reaction norm framework. Ecology of Freshwater Fish 27: 350–362. [Google Scholar]
- Gregory RS, Levings CD. 1998. Turbidity reduces predation on migrating juvenile Pacific salmon. Transactions of the American Fisheries Society 127: 275–285. [Google Scholar]
- Gurnell AM, Clark MJ, Clark MJ. 1987. Glacio-Fluvial Sediment Transfer: An Alpine Perspective. Wiley. [Google Scholar]
- Hauer FR, Locke H, Dreitz VJ, Hebblewhite M, Lowe WH, Muhlfeld CC, Nelson CR, Proctor MF, Rood SB. 2016. Gravel-bed river floodplains are the ecological nexus of glaciated mountain landscapes. Science Advances 2: e1600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilborn R, Quinn T P, Schindler D E, Rogers D E. 2003. Biocomplexity and fisheries sustainability. Proceedings of the National Academy of Sciences 100: 6564–6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood E, Berner L.. 2009. Effects of changing glacial coverage on the physical and biogeochemical properties of coastal streams in southeastern Alaska. Journal of Geophysical Research 114: G03001. [Google Scholar]
- Huss M, Hock R. 2018. Global-scale hydrological response to future glacier mass loss. Nature Climate Change 8: 135–140. [Google Scholar]
- Jansson P, Hock R, Schneider T. 2003. The concept of glacier storage: A review. Journal of Hydrology 282: 116–129. [Google Scholar]
- Jensen DW, Steel EA, Fullerton AH, Pess GR. 2009. Impact of fine sediment on egg-to-fry survival of Pacific salmon: A meta-analysis of published studies. Reviews in Fisheries Science 17: 348–359. [Google Scholar]
- Johnson KA, Wing OEJ, Bates PD, Fargione J, Kroeger T, Larson WD, Sampson CC, Smith AM. 2019. A benefit-cost analysis of floodplain land acquisition for US flood damage reduction. Nature Sustainability 3: 56–62. [Google Scholar]
- Keefer ML, Caudill CC. 2014. Homing and straying by anadromous salmonids: A review of mechanisms and rates. Reviews in Fish Biology and Fisheries 24: 333–368. [Google Scholar]
- Kendall NW, McMillan JR, Sloat MR, Buehrens TW, Quinn TP, Pess GR, Kuzishchin KV, McClure MM, Zabel RW. 2015. Anadromy and residency in steelhead and rainbow trout (Oncorhynchus mykiss): A review of the processes and patterns. Canadian Journal of Fisheries and Aquatic Sciences 72: 319–342. [Google Scholar]
- Kohan ML, Mueter FJ, Orsi JA, McPhee MV. 2017. Variation in size, condition, and abundance of juvenile chum salmon (Oncorhynchus keta) in relation to marine factors in southeast Alaska. Deep Sea Research Part II: Topical Studies in Oceanography (art. 2017.09.005). doi:10.1016/j.dsr2.2017.09.005 [Google Scholar]
- Kondolf GM, Wolman MG.. 1993. The sizes of salmonid spawning gravels. Water Resources Research 29: 2275–2285. [Google Scholar]
- Lawrence DJ, Stewart-Koster B, Olden JD, Ruesch AS, Torgersen CE, Lawler JJ, Butcher DP, Crown JK. 2014. The interactive effects of climate change, riparian management, and a nonnative predator on stream-rearing salmon. Ecological Applications 24: 895–912. [DOI] [PubMed] [Google Scholar]
- Litzow MA, Ciannelli L, Puerta P, Wettstein JJ, Rykaczewski RR, Opiekun M. 2018. Non-stationary climate–salmon relationships in the Gulf of Alaska. Proceedings of the Royal Society B 285: 20181855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DS, Koenings JP, Laperriere JD. 1987. Effects of turbidity in fresh waters of Alaska. North American Journal of Fisheries Management 7: 18–33. [Google Scholar]
- Lorenz MJ, Eiler JH. 1989. Spawning habitat and redd characteristics of Sockeye salmon in the glacial Taku River, British Columbia and Alaska. Transactions of the American Fisheries Society 118: 495–502. [Google Scholar]
- Mantua N, Tohver I, Hamlet A. 2010. Climate change impacts on streamflow extremes and summertime stream temperature and their possible consequences for freshwater salmon habitat in Washington State. Climatic Change 102: 187–223. [Google Scholar]
- Mantua NJ, Crozier LG, Reed TE, Schindler DE, Waples RS. 2015. Response of chinook salmon to climate change. Nature Climate Change 5: 613–615. [Google Scholar]
- Martins EG, Hinch SG, Patterson DA, Hague MJ, Cooke SJ, Miller KM, Lapointe MF, English KK, Farrell AP. 2011. Effects of river temperature and climate warming on stock-specific survival of adult migrating Fraser River sockeye salmon (Oncorhynchus nerka). Global Change Biology 17: 99–114. [Google Scholar]
- Martins EG, Hinch SG, Patterson DA, Hague MJ, Cooke SJ, Miller KM, Robichaud D, English KK, Farrell AP. 2012. High river temperature reduces survival of sockeye salmon (Oncorhynchus nerka) approaching spawning grounds and exacerbates female mortality. Canadian Journal of Fisheries and Aquatic Sciences 69: 330–342. [Google Scholar]
- Marzeion B, Cogley GJ, Richter K, Parkes D. 2014. Attribution of global glacier mass loss to anthropogenic and natural causes. Science 345: 919–921. [DOI] [PubMed] [Google Scholar]
- McClure MM et al.. 2008. Evolutionary consequences of habitat loss for Pacific anadromous salmonids. Evolutionary Applications 1: 300–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meire L, Mortensen J, Meire P, Juul‐Pedersen T, Sejr MK, Rysgaard S, Nygaard R, Huybrechts P, Meysman FJR. 2017. Marine-terminating glaciers sustain high productivity in Greenland fjords. Global Change Biology 23: 5344–5357. [DOI] [PubMed] [Google Scholar]
- Menounos B, Osborn G, Clague JJ, Luckman BH. 2009. Latest Pleistocene and Holocene glacier fluctuations in western Canada. Quaternary Science Reviews 28: 2049–2074. [Google Scholar]
- Milner AM. 1997. Glacier recession and freshwater ecosystems in coastal Alaska. Pages 303–330 in Oswood M, Milner AM, eds. Freshwaters of Alaska. Springer. [Google Scholar]
- Milner AM, Brittain JE, Castella E, Petts GE. 2001. Trends of macroinvertebrate community structure in glacier-fed rivers in relation to environmental conditions: A synthesis. Freshwater Biology 46: 1833–1847. [Google Scholar]
- Milner AM, Fastie CL, Chapin FS, Engstrom DR, Sharman LC. 2007. Interactions and links among ecosystems during landscape evolution. BioScience 57: 237–247. [Google Scholar]
- Milner AM et al.. 2017. Glacier shrinkage driving global changes in downstream systems. Proceedings of the National Academy of Sciences 114: 9770–9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner AM, Petts GE. 1994. Glacial rivers: Physical habitat and ecology. Freshwater Biology 32: 295–307. [Google Scholar]
- Milner AM, Robertson AL, Brown LE, Sønderland SH, McDermott M, Veal AJ. 2011. Evolution of a stream ecosystem in recently deglaciated terrain. Ecology 92: 1924–1935. [DOI] [PubMed] [Google Scholar]
- Milner AM, Robertson AL, Monaghan KA, Veal AJ, Flory EA. 2008. Colonization and development of an Alaskan stream community over 28 years. Frontiers in Ecology and the Environment 6: 413–419. [Google Scholar]
- Montgomery DR. 2002. Valley formation by fluvial and glacial erosion. Geology 30: 1047–1050. [Google Scholar]
- Moody MF, Pitcher TJ. 2010. Eulachon (Thaleichthys pacificus): Past and Present. Fisheries Centre, University of British Columbia, Canada. Fisheries Centre research report 2010, vol. 18, no. 2.
- Moore JW et al.. 2015. Emergent stability in a large, free-flowing watershed. Ecology 96: 340–347. [DOI] [PubMed] [Google Scholar]
- Moore RD, Fleming SW, Menounos B, Wheate R, Fountain A, Stahl K, Holm K, Jakob M. 2009. Glacier change in western North America: Influences on hydrology, geomorphic hazards and water quality. Hydrological Processes 23: 42–61. [Google Scholar]
- Mossop B, Bradford MJ. 2004. Importance of large woody debris for juvenile chinook salmon habitat in small boreal forest streams in the upper Yukon River basin, Canada. Canadian Journal of Forest Research 34: 1955–1966. [Google Scholar]
- Mote PW, Salathé EP. 2010. Future climate in the Pacific Northwest. Climatic Change 102: 29–50. [Google Scholar]
- Muñoz NJ, Farrell AP, Heath JW, Neff BD. 2015. Adaptive potential of a Pacific salmon challenged by climate change. Nature Climate Change 5: 163–166. [Google Scholar]
- Munsch SH, Greene CM, Johnson RC, Satterthwaite WH, Imaki H, Brandes PL. 2019. Warm, dry winters truncate timing and size distribution of seaward‐migrating salmon across a large, regulated watershed. Ecological Applications (art. e01880). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ML, Heifetz J, Thedinga JF, Johnson SW, Koski KV. 1989. Habitat use by juvenile Pacific Salmon (Oncorhynchus) in the glacial Taku River, Southeast Alaska. Canadian Journal of Fisheries and Aquatic Sciences 46: 1677–1685. [Google Scholar]
- Murray CB, McPhail JD. 1988. Effect of incubation temperature on the development of five species of Pacific salmon (Oncorhynchus) embryos and alevins. Canadian Journal of Zoology 66: 266–273. [Google Scholar]
- Oerlemans J. 2010. The Microclimate of Valley Glaciers. Utrecht University. [Google Scholar]
- O’Neel S et al.. 2015. Icefield-to-ocean links across the northern Pacific coastal temperate rainforest ecosystem. BioScience 65: 499–512. [Google Scholar]
- Pess GR, Quinn TP, Gephard SR, Saunders R. 2014. Re-colonization of Atlantic and Pacific rivers by anadromous fishes: Linkages between life history and the benefits of barrier removal. Reviews in Fish Biology and Fisheries 24: 881–900. [Google Scholar]
- Pfeffer WT et al.. 2014. The Randolph Glacier Inventory: A globally complete inventory of glaciers. Journal of Glaciology 60: 537–552. [Google Scholar]
- Quinn T. 2018. The Behavior and Ecology of Pacific Salmon and Trout. University of Washington Press. [Google Scholar]
- Radić V, Bliss A, Beedlow AC, Hock R, Miles E, Cogley JG. 2013. Regional and global projections of twenty-first century glacier mass changes in response to climate scenarios from global climate models. Climate Dynamics 42: 37–58. [Google Scholar]
- Ramstad KM, Woody CA, Sage GK, Allendorf FW. 2004. Founding events influence genetic population structure of sockeye salmon (Oncorhynchus nerka) in Lake Clark, Alaska. Molecular Ecology 13: 277–290. [DOI] [PubMed] [Google Scholar]
- Reed TE, Schindler DE, Hague MJ, Patterson DA, Meir E, Waples RS, Hinch SG. 2011. Time to Evolve? Potential evolutionary responses of Fraser River sockeye salmon to climate change and effects on persistence. PLOS ONE 6 (art. e20380). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves GH, Benda LE, Burnett KM, Bisson PA, Sedell JR. 1995. A disturbance-based ecosystem approach to maintaining and restoring freshwater habitats of evolutionarily significant units of anadromous salmonids in the Pacific Northwest. American Fisheries Society Symposium 17: 334–349. [Google Scholar]
- Richter A, Kolmes SA. 2005. Maximum temperature limits for Chinook, coho, and chum salmon, and steelhead trout in the Pacific Northwest. Reviews in Fisheries Science 13: 23–49. [Google Scholar]
- Rine KM, Wipfli MS, Schoen ER, Nightengale TL, Stricker CA. 2016. Trophic pathways supporting juvenile Chinook and coho salmon in the glacial Susitna River, Alaska: Patterns of freshwater, marine, and terrestrial food resource use across a seasonally dynamic habitat mosaic. Canadian Journal of Fisheries and Aquatic Sciences 73: 1626–1641. [Google Scholar]
- Roni P, Quinn TP. 1995. Geographic variation in size and age of North American Chinook salmon. North American Journal of Fisheries Management 15: 325–345. [Google Scholar]
- Saiget DA, Sloat MR, Reeves GH. 2007. Spawning and movement behavior of migratory coastal cutthroat trout on the western Copper River delta, Alaska. North American Journal of Fisheries Management 27: 1029–1040. [Google Scholar]
- Schindler DE, Augerot X, Fleishman E, Mantua NJ, Riddell B, Ruckelshaus M, Seeb J, Webster M. 2008. Climate change, ecosystem impacts, and management for Pacific salmon. Fisheries 33: 502–506. [Google Scholar]
- Schindler DE, Hilborn R. 2015. Prediction, precaution, and policy under global change. Science 347: 953–954. [DOI] [PubMed] [Google Scholar]
- Schoen ER et al.. 2017. Future of Pacific salmon in the face of environmental change: Lessons from one of the world's remaining productive salmon regions. Fisheries 42: 538–553. [Google Scholar]
- Schroth AW, Crusius J, Chever F, Bostick BC, Rouxel OJ. 2011. Glacial influence on the geochemistry of riverine iron fluxes to the Gulf of Alaska and effects of glaciation. Geophysical Research Letters 38: L16605. [Google Scholar]
- Sergeant CJ, Bellmore RJ, McConnell C, Moore JW. 2017. High salmon density and low discharge create periodic hypoxia in coastal rivers. Ecosphere 8: e01846. [Google Scholar]
- Sloat MR, Osterback A-MK. 2013. Maximum stream temperature and the occurrence, abundance, and behavior of steelhead trout (Oncorhynchus mykiss) in a southern California stream. Canadian Journal of Fisheries and Aquatic Sciences 70: 64–73. [Google Scholar]
- Sloat MR, Reeves GH, Christiansen KR. 2018. Stream network geomorphology mediates predicted vulnerability of anadromous fish habitat to hydrologic change in southeast Alaska. Global Change Biology 23: 604–620. [DOI] [PubMed] [Google Scholar]
- Smith CT, Nelson RJ, Wood CC, Koop BF. 2001. Glacial biogeography of North American coho salmon (Oncorhynchus kisutch). Molecular Ecology 10: 2775–2785. [DOI] [PubMed] [Google Scholar]
- Stearley RF. 1992. Historical ecology of salmoninae, with special reference to Oncorhynchus. Pages 622–658 in Mayden RL, ed. Systematics, historical ecology, and North American Freshwater Fishes. Stanford University Press. [Google Scholar]
- Tippets WE, Moyle PB. 1978. Epibenthic feeding by rainbow trout (Salmo gairdneri) in the McCloud River, California. The Journal of Animal Ecology 47: 549. [Google Scholar]
- Trotter PC. 1989. Coastal cutthroat: A life history compendium. Transactions of the American Fisheries Society 118: 463–473. [Google Scholar]
- Waples RS, Beechie T, Pess GR. 2009. Evolutionary history, habitat disturbance regimes, and anthropogenic changes: What do these mean for resilience of Pacific salmon populations? Ecology and Society 14: 3. [Google Scholar]
- Waples RS, Pess GR, Beechie T. 2008. Evolutionary history of Pacific salmon in dynamic environments. Evolutionary Applications 1: 189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BR, Slaney PA. 1988. Life history and smolt-to-adult survival of Keogh River steelhead trout (Salmo gairdneri) and the relationship to smolt size. Canadian Journal of Fisheries and Aquatic Sciences 45: 1110–1122. [Google Scholar]
- Weber N, Bouwes N, Pollock MM, Volk C, Wheaton JM, Wathen G, Wirtz J, Jordan CE. 2017. Alteration of stream temperature by natural and artificial beaver dams. PLOS ONE 12 (art. e0176313). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitkamp LA, Goulette G, Hawkes J, O’Malley M, Lipsky C. 2014. Juvenile salmon in estuaries: Comparisons between North American Atlantic and Pacific salmon populations. Reviews in Fish Biology and Fisheries 24: 713–736. [Google Scholar]
- Wipfli MS, Baxter CV. 2010. Linking ecosystems, food webs, and fish production: Subsidies in salmonid watersheds. Fisheries 35: 373–387. [Google Scholar]
- Yeakel JD, Gibert JP, Gross T, Westley PAH, Moore JW. 2018. Eco-evolutionary dynamics, density-dependent dispersal and collective behaviour: Implications for salmon metapopulation robustness. Philosophical Transactions of the Royal Society 373: 20170018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemp M et al.. 2019. Global glacier mass changes and their contributions to sea-level rise from 1961 to 2016. Nature 568: 382–386. [DOI] [PubMed] [Google Scholar]