Abstract
In recent years, there has been a wider use of chemotherapy in the pre-operative setting for breast cancer (i.e., as neo-adjuvant chemotherapy). Most clinicians would agree that neo-adjuvant chemotherapy is justified for patients with inflammatory breast cancer, locally advanced breast cancer, or patients with large tumors and small breasts who are keen to undergo breast-conserving surgery. However, in the USA and many other western countries, neo-adjuvant chemotherapy is now used for greater numbers of breast cancer patients who do not fall within these categories. Yet, randomized trials have consistently shown that there are no differences in overall survival (OS) between breast cancer patients treated with neo-adjuvant chemotherapy versus adjuvant chemotherapy. However, neo-adjuvant chemotherapy may increase the risk of loco-regional recurrence after breast-conserving surgery, perhaps because of an increased risk of leaving behind residual tumor foci. Moreover, the effects of neo-adjuvant chemotherapy on the primary tumor does not appear to be a suitable way for assessing the potential overall benefits of systemic therapy regimens on distant micrometastases and risk of death. Yet, based on the results of the KATHERINE and CREATE-X trials, one might argue that neo-adjuvant chemotherapy should be recommended for patients with HER-2-positive and triple-negative tumors to identify the subsets of patients who do not achieve pathologic complete response (PCR). Patients with HER-2-positive tumors who do not achieve PCR may benefit from additional treatment with T-DM1, and those with triple-negative tumors who do not achieve PCR may benefit from additional treatment with capecitabine.
Keywords: Breast cancer, Neo-adjuvant chemotherapy, Breast-conserving surgery
Traditionally, for high-risk patients with primary breast cancer, chemotherapy has been administered after surgery (i.e., as adjuvant chemotherapy). However, in recent years, there has been a burgeoning interest in the use of chemotherapy in the pre-operative setting for breast cancer (i.e., as neo-adjuvant chemotherapy). Most clinicians would agree that neo-adjuvant chemotherapy is justified for patients with inflammatory breast cancer, locally advanced breast cancer, or patients with large tumors and small breasts who are keen to undergo breast-conserving surgery. However, in the USA and many other western countries, neo-adjuvant chemotherapy is now used for greater numbers of breast cancer patients who do not fall within these categories. Indeed, the US National Cancer Database indicates an increase in overall neo-adjuvant chemotherapy usage from 15.7% in 2010 to 26% in 2015 [1]. More recent data are not yet available, but most likely will show that the use of neo-adjuvant chemotherapy continues to increase.
Yet, randomized trials have consistently shown that there are no differences in overall survival (OS) between breast cancer patients treated with neo-adjuvant chemotherapy versus adjuvant chemotherapy [2–5]. Most of the increased usage of neo-adjuvant chemotherapy in recent years has been attributed to its wider usage in early stage (clinical stage I/II) disease, while rates of usage for stage III or locally advanced disease have been stable. This trend remained significant despite adjusting for demographic and clinical factors that may have changed over time [6]. In this article, we argue that the dramatic increase in neo-adjuvant chemotherapy usage for primary breast cancer is not entirely justified.
Although neo-adjuvant chemotherapy does not appear to have an effect on OS, it may increase the risk of loco-regional recurrence. Mauri et al. reported a meta-analysis of nine trials that randomized patients to neo-adjuvant versus adjuvant chemotherapy and found that neo-adjuvant chemotherapy was associated with statistically significant increased risk of loco-regional disease recurrence [2]. Another meta-analysis by the Early Breast Cancer Trials Cooperative Group (EBCTCG) that assessed neo-adjuvant chemotherapy for women enrolled across 10 randomized clinical trials also showed higher loco-regional recurrences (LRR) after breast-conserving surgery (BCS) for early stage tumors downsized with neo-adjuvant chemotherapy. The 15-year local recurrence rate was 21.4% for neo-adjuvant chemotherapy versus 15.9% for adjuvant chemotherapy (a 5.5% increase (95% CI 2.4–8.6), rate ratio 1.37 (95% CI 1.17–1.61); p = 0.0001) [3].
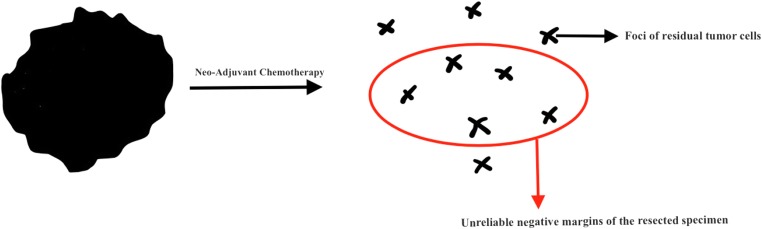
Vaidya et al. argued that after neo-adjuvant chemotherapy, breast-conserving surgery is problematic due to the obliteration of the discrete lump with scattered tumor foci left behind in the tumor bed, and hence the greater likelihood of leaving behind residual tumor foci after resection (Fig. 1) [7]. A “negative margin” after lumpectomy may fail to account for occult residual tumor deposits beyond the clear margins of resection, and this could explain the increased risk of loco-regional recurrences among patients treated with breast-conserving surgery following neo-adjuvant chemotherapy (Fig. 1). Although this increased LRR following neo-adjuvant chemotherapy has not been shown to have any effect on the OS in the short term, longer follow-up of patients enrolled in these trials is required before any definite conclusions can be drawn. Indeed, the EBCTCG meta-analysis indicates that 4 local recurrences lead to one extra breast cancer death, but it takes at least 15 years of follow-up to demonstrate this effect [3].
Fig. 1.
Following neo-adjuvant chemotherapy, negative margins are obtained after lumpectomy fails to account for residual tumor foci
Patients with locally advanced cancers are generally offered neo-adjuvant chemotherapy to downstage the tumors and facilitate surgical resection. However, extending the use of neo-adjuvant chemotherapy therapy to treat greater numbers of patients with early stage breast cancer with hopes of increasing breast conservation rates is not entirely justifiable. A retrospective analysis by Kantor et al. evaluating clinical stages I–III breast cancer cases reports increased numbers of patients undergoing bilateral mastectomy following neo-adjuvant chemotherapy rather than BCS [8]. The largest disparity was reported for cT1 tumors, with 32% of these patients opting for bilateral mastectomy after neo-adjuvant chemotherapy, even though they were candidates for BCS [8]. This suggests that a wider use of neo-adjuvant chemotherapy may not necessarily increase the rates of breast-conserving surgery and that decisions regarding local therapy are driven by multiple other factors. Kantor et al. report that many patients cited their desire for “peace of mind” as the basis for choosing bilateral mastectomy after neo-adjuvant chemotherapy, and many expressed the hope that bilateral mastectomy might prevent future need for chemotherapy [8].
Hypothetically, the response of the primary tumor to a particular neo-adjuvant chemotherapy regimen should serve as an indicator of that regimen’s efficacy in reducing the overall risk of recurrence and death from breast cancer. Clinicians could then potentially tailor drug therapies based upon the neo-adjuvant chemotherapy effects on the primary tumor and assume that the response of the primary tumor was an indicator of the drug regimen’s overall effect on distant micrometastases as well. The National Surgical Adjuvant Breast and Bowel Project (NSABP)-27 trial tested this hypothesis. That trial randomized patients with primary breast cancer to Adriamycin and Cyclophosphamide (AC) followed by surgery versus AC followed by Docetaxol (T) followed by surgery, versus AC followed by surgery followed by T. Pathologic complete response (PCR) rates increased for patients treated with AC followed by T prior to surgery when compared to pre-operative AC alone. Yet, even though PCR rates increased with the addition of pre-operative T, the overall risk of recurrence and death was identical in the two groups [9]. This trial therefore suggests that response of the primary tumor to neo-adjuvant chemotherapy regimens is perhaps not a good surrogate for its effects on micrometastases and overall outcomes. The effects of neo-adjuvant chemotherapy on the primary tumor is therefore not a suitable way for assessing the potential overall benefits of systemic therapy regimens on distant micrometastases, distant recurrences, and risk of death.
Long-term data from NSABP-18 and NSABP-27 trials have shown that patients who achieve PCR have superior OS as compared to those who do not achieve PCR [9]. However, neo-adjuvant chemotherapy simply serves as a means of identifying subsets of patients with a good prognosis (i.e., those who achieve PCR), but it has never been shown to improve overall survival. It is also noteworthy that most patients will not achieve PCR with neo-adjuvant chemotherapy. Moreover, there is significant heterogeneity in the outcomes among non-PCR patients, which is perhaps due to differences in prognosis and chemosensitivity between the primary tumor and micrometastasis [9, 10].
Yet, recent trials suggest that there is perhaps a role for neo-adjuvant chemotherapy in the management of specific subsets of patients with early breast cancer. The multicenter, multinational KATHERINE trial randomized 1486 patients with HER-2-positive early breast cancer, who had residual disease after neoadjuvant chemotherapy plus trastuzumab therapy, to receive adjuvant trastuzumab emtansine (T-DM1) versus adjuvant trastuzumab. The risk of recurrence or death was 50% lower in the adjuvant T-DM1 group [11]. Similarly, the CREATE-X trial randomized 910 patients, enrolled from several institutions across Japan and South Korea, with HER2-negative residual invasive breast cancer after neoadjuvant chemotherapy, to receive adjuvant systemic treatment with capecitabine versus not. The addition of adjuvant capecitabine was effective in prolonging DFS and OS, especially among patients with triple-negative breast cancer who did not achieve PCR [12].
Based on the results of these two recent trials, one might argue that neo-adjuvant chemotherapy should be recommended for patients with HER-2-positve and triple-negative tumors, to identify the high-risk subsets of patients who do not achieve PCR. Patients with HER-2-positive tumors who do not achieve PCR may benefit from additional treatment with T-DM1, and those with triple-negative tumors who do not achieve PCR may benefit from additional treatment with capecitabine. Yet, one would hope that we might eventually find better ways (rather than subjecting large numbers of patients to neo-adjuvant chemotherapy) to identify high-risk subsets that may benefit from additional treatment with TDM-1 and capecitabine.
In conclusion, neo-adjuvant chemotherapy has not met its promise in the management of breast cancer. Currently, the role of preoperative systemic therapy in inflammatory and locally advanced stages of breast cancer are widely accepted as a standard course of management, as it enables better local control of the primary tumor. However, the increased use of neo-adjuvant chemotherapy for the majority of patients with early stage disease cannot be entirely justified. Recent trials do indicate, however, that the use of neo-adjuvant chemotherapy in patients with triple-negative breast cancer and HER-2-positive breast cancer may serve to identify high-risk subsets of patients (i.e., those who do not achieve PCR), who may benefit from additional adjuvant systemic therapy regimens (i.e., capecitabine and TDM-1, respectively).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER-2-positive subtypes. Ann Surg Oncol. 2018;25(8):2241–2248. doi: 10.1245/s10434-018-6531-5. [DOI] [PubMed] [Google Scholar]
- 2.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. doi: 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18.JNCI. Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 5.Ragaz J, Baird R, Rebbeck P, et al. Preoperative (neoadjuvant) versus postoperative adjuvant chemotherapy for stage I-II breast cancer. Long-term analysis of British Columbia randomized trial. Proc Am Soc Clin Oncol. 1997;16:142a. [Google Scholar]
- 6.Puig CA, Hoskin TL, Day CN, Habermann EB, Boughey JC. National trends in the use of neoadjuvant chemotherapy for hormone receptor-negative breast cancer: a national cancer data base study. Annals of Surgical Oncology. 2016;24:1242–1250. doi: 10.1245/s10434-016-5733-y. [DOI] [PubMed] [Google Scholar]
- 7.Vaidya JS, Massarut S, Vaidya HJ, Alexander EC, Richards T, Caris JA, Sirohi B, Tobias JS. Rethinking neoadjuvant chemotherapy for breast cancer. BMJ. 2018;360:j5913. doi: 10.1136/bmj.j5913. [DOI] [PubMed] [Google Scholar]
- 8.Kantor O, Ajmani G, Wang CH, Datta A, Yao K. The shifting paradigm for breast cancer surgery in patients undergoing neoadjuvant chemotherapy. Ann Surg Oncol. 2018;25(1):164–172. doi: 10.1245/s10434-017-6217-4. [DOI] [PubMed] [Google Scholar]
- 9.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 10.Pusztai L, Foldi J, Dhawan A, DiGiovanna M, Mamounas EP. Changing frameworks in treatment sequencing of triple-negative and HER2-positive, early-stage breast cancers. Lancet Oncol. 2019;20(7):e390–e396. doi: 10.1016/S1470-2045(19)30158-5. [DOI] [PubMed] [Google Scholar]
- 11.KATHERINE Investigators Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 12.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]