Abstract
Dermatofibrosarcoma protuberans (DFSP) is a slow growing dermal tumor with a very low metastatic potential but with significant subclinical extension and capacity for local destruction with local recurrence rates ranging from 0 to 50%. Controversy exists regarding margin width and excision techniques, with some advocating Mohs surgery and others wide excision. We reviewed the excision technique along with the recurrence rates at a tertiary care center in eastern India. This study is a retrospective review of patients with DFSP from June 2011 to September 2018. Patients had initial wide excision using 2–3 cm margins with primary closure or reconstructive procedure; re-excision was done for positive margins. Pathologic analysis included en face sectioning. We evaluated margin width, number of excisions, reconstruction methods, radiation, and outcomes. A total of 31 patients with DFSP (15 males, 16 females), median age 41 years (range 14–82), were treated. Locations were extremities (13), trunk (12), and head and neck (06). The median number of excisions to achieve negative margins was 1 (range 1–3). Closure techniques included primary closure (13; 42%), tissue flaps (13; 42%), and skin grafting (05; 16%). There were 11 patients who received postoperative radiation, 4 for positive margins after maximal surgical excision. At a median follow-up of 24 months (range 1–72), 2 patients (6.5%) recurred locally, and 1 patient (3.2%) had lung metastasis. Using a standardized surgical approach including meticulous pathologic evaluation of margins, low recurrence rate (10%) was achieved with adequate margins (2–3 cm).
Keywords: Dermatofibrosarcoma protuberans, DFSP, Fibrosarcoma, Soft tissue sarcoma
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare low-grade cutaneous malignancy first described in 1890 by Taylor [1]. Darier [2] established DFSP as a clinicopathological entity in 1924 and Hoffman [3] was credited for establishing the term in 1925. Expert pathologic assessment with immunohistochemical testing is essential to differentiate DFSP from other superficial soft tissue neoplasms after biopsy or excision.
There are no known risk factors for the development of DFSP. Effective management of this tumor requires a careful appreciation of tumor biology and the nature of infiltrative growth into surrounding tissues. Morphologic studies of DFSP have revealed highly irregular borders with fingerlike extensions into surrounding and deep tissues [4]. Although DFSP rarely metastasizes, there is a probability for local recurrence that may be associated with significant morbidity [5]. As such, aggressive surgical resection with widely negative margins is essential to proper management. Radiotherapy may be indicated in special circumstances, including recurrent DFSP or when complete microscopic tumor clearance is not possible. DFSP patients require long-term follow-up with expert oncology practitioners, and ideally, a multidisciplinary team.
DFSP has an annual incidence of 0.8 to 4.2 cases per million [6]. It represents 0.1% of all malignancies [7] and 18% of cutaneous soft tissue sarcomas [8]. There is a slight male preponderance to the disease [6], and African Americans have an incidence that is almost double that reported among Caucasians [9]. But there are some studies showing the female preponderance to the disease also [10]. DFSP is most commonly seen in the third to fifth decades of life but can also present in infancy or in the elderly.
Being one of the tertiary care centers in eastern India, a vast majority of patient presenting to our institution, are with the recurrent/residual disease after single or multiple excisions outside. The aim of present study was to evaluate the management of DFSP and its recurrence rates at a tertiary care center.
Material and Methods
This was a retrospective study conducted in the Department of Plastic and Micro-vascular reconstructive surgery at tertiary care oncology center in eastern India. All patients registered with the department from June 2011 to September 2018 and having histopathological diagnosis of DFSP were included in the study. Data was collected from the hospital database. Medical records, operative notes, and detailed histopathology reports were reviewed for all the patients. Patients underwent MRI or CT scan when indicated. Treatment plan of all patients was discussed in a dedicated sarcoma multi-disciplinary team meeting. Patients who had sarcomatous changes on final histopathology reports underwent metastatic work-up after the primary surgery. We evaluated clinicopathologic factors, wide excision margin width, reconstruction methods employed, use of postoperative radiation, and outcomes including local recurrence rates.
Treatment
Excision Technique
The surgeons and pathologists at the institution followed a standard protocol for the excision and evaluation of DFSP. Intraoperatively, prior to defining the excision margins, DFSP lesions were examined clinically and extent of the lesion was carefully marked out. An excision was defined using minimum measured (predominantly 2–3 cm, depending upon the size and location of tumor) margins, and the specimen was excised down to and including underlying fascia and appropriately oriented for the pathologist. Specimen was sent for frozen section analysis for margin status. If positive margins were found on frozen section then re-excision of margins were performed during same procedure. If positive margins were found on permanent pathologic analysis, re-excisions were performed in the same fashion, if technically feasible, until all negative margins were achieved. Surgical defects were closed primarily; if feasible and if primary closure was not possible then defects were closed by either using skin graft or flap (pedicled or free flap) depending upon the size and location of the defect.
Pathologic Evaluation
Tissue from all peripheral margins underwent meticulous pathologic analysis of en face 2-mm tangential sections using routine hematoxylin and eosin staining. Immunohistochemistry (IHC) using anti-CD34 anti-body staining was performed as deemed necessary by the pathologist. A negative margin was defined as no evidence of DFSP by hematoxylin and eosin staining and/or IHC at the inked tangential margin. The transformed sarcomatous variant of DFSP was diagnosed when any percentage of histologic section demonstrated sarcomatous changes.
Statistical Analysis
This study analyzed clinico-pathologic factors and characteristics of resected specimens. Descriptive statistical analysis was performed. The median and range is reported for continuous variables while proportions were reported for the categorical variables.
Results
A total of 31 patients with DFSP, treated at our institution, were identified from the records. A summary of patient demographics and clinico-pathological characteristics for the entire population is presented in Table 1. Of the 31 patients, 51.6% were female. Median age at the time of presentation to our institution was 41 years (range 14–82). Twenty patients (64.5%) presented to us with recurrent/residual lesion after one or more prior resection, while 11 patients (35.5%) had their primary resection at our center. Patients who were referred to us after prior resection underwent re-resection of the previous scar site with adequate margin if prior resection margins were close/positive or if the status of margins were unreported in reports from other hospitals/laboratories.
Table 1.
Patient demographics and clinico-pathological features
| Characteristics | N (%) |
|---|---|
| Total patients | 31 (100%) |
| Primary | 11 (35.5%) |
| Recurrent | 20 (64.5%) |
| Total procedures | 31 |
| Male: female | 15:16 |
| Median age (range) | 41 (14–82) |
| Location | |
| Extremity | 13 (42%) |
| Trunk | 12 (39%) |
| Head and neck | 06 (19%) |
Wide Excision
A summary of operative results is presented in Table 2. Overall, 87% of patients had negative margins with one or more excisions. Median number of excisions required to achieve negative margins was 1 (range 1–3). Overall, 78% of patients (24 patients) required only 1 resection to achieve negative margins. Two patients (6.4%) required 2 resections to achieve negative margins while 1 patient (3.2%) required 3 resections to achieve negative margins. Re-excision was done with a minimum of 1-cm margin. It was difficult to properly define the margins in patients who required re-excisions owing to fibrosis and tissue shifting after primary closure or reconstructive procedures. Patients, who did not undergo re-excision, were a patient of 82 years with lesion in upper extremity, two which had undergone a free flap and had a deep margin positive, and a woman with recurrent lesion in the inguinal region with co-morbidity precluding further surgery. All those who did not undergo re-excision underwent radiation. This subgroup of patients has been disease free for 30 months. Of these 4 patients, 2 had classical DFSP, and 2 had DFSP with sarcomatous changes (DFSP-S).
Table 2.
Summary of operative results
| N (%) | |
|---|---|
| Type of excision, wide excision | 31 (100%) |
| Closure technique | |
| Primary closure | 13 (42%) |
| Tissue flap alone | 10 (32%) |
| Skin grafting alone | 05 (16%) |
| Tissue flap with skin grafting | 03 (10%) |
| Number of excisions required | |
| 1 excision | 24 (78%) |
| 2 excisions | 02 (6.4%) |
| 3 excisions | 01 (3.2%) |
Method of Closure of the Excision Defect
Primary closure was possible for 13 patients (42%) of all resections. Flap closure technique was performed in 10 patients (32%). While a skin graft was performed in 5 patients (16%). There were an additional 3 patients who had a skin graft and tissue flap in combination.
Reconstruction was based on the size, location, and whether the lesion had converted to a sarcoma or had a high risk of doing so. Primary closure was possible in the trunk and extremities (n = 10) and head and neck (n = 1). Whereas split thickness skin grafts were used in 4 patients (trunk = 2 and one each in head and neck and extremity)
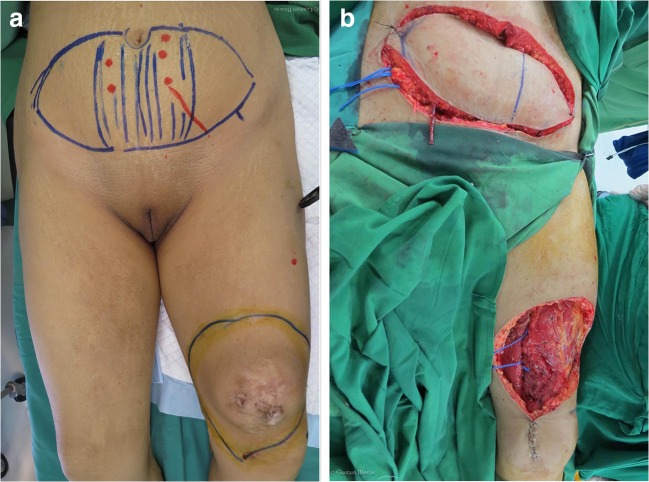
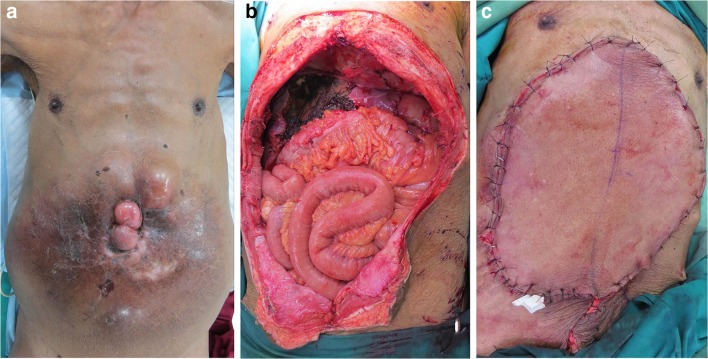
Flap closure was done in 12 patients, with perforator pedicled flaps (n = 4), local flaps (n = 3), and microvascular free flaps (n = 5) being the chosen option. The microvascular procedures necessitated resurfacing extensive defects with the choice based on size of flap volume and donor aesthetics. Free deep inferior epigastric perforator (DIEP) flap was the preferred choice in a young woman with DFSP of the knee, (Fig. 1) whereas to avoid any scar in exposed part a anterolateral thigh (ALT) flap was chosen for a man with a large recurrent tumor (40 cm) of the abdominal wall with involvement of the liver and stomach, who underwent wide excision of the abdominal wall with liver resection and subtotal gastrectomy with reconstruction of the abdominal wall using mesh and ALT flap (Fig. 2).
Fig. 1.
Photographic operative images of a patient with DFSP of thigh. Pre-excision marking of primary site and DIEP flap donor site (a). Post-excision images showing recipient bed and DIEP raised flap (b)
Fig. 2.
Photographic operative images of a patient with a large fungating DFSP of the abdominal wall (a). Wide resection (b) with 2-cm margins was performed with ALT free flap reconstruction (c)
Postoperative wound complications occurred in 5 patients (16%). These included minor wound dehiscence, infection, hematoma, and a failed skin graft. The most common complication was infection, and this was generally treated with oral antibiotics and dressings. No flap failures were seen.
Adjuvant Radiation
There were 11 patients (35.5%) who received adjuvant radiation, and 7 of these patients (64%) had DFSP-S. Two patients (18%) had DFSP-S with persistently positive margins that could not be re-excised. Also, 2 more patients (18%) received radiation for DFSP with persistently positive margins. One patient of DFSP-S could not receive the radiotherapy as he refused for the same. The usual dose was 52–60 Gy delivered in 20–30 fractions over 4–6 weeks. All patients started the planned radiotherapy within 6 weeks of their final surgery. Radiotherapy was delivered using megavoltage radiotherapy with CT-based planning. Of the 9 patients who received radiotherapy at our center, 5 patients were treated with 3-dimensional conformal radiotherapy, 3 patients received intensity modulated radiotherapy, and 1 patient received electron radiotherapy. Two patients received radiotherapy at other centers.
Recurrence Rate
At a median follow-up of 30 months (range 7–78), 2 patients (6.5%) recurred locally. One of these 2 patients recurred at 11 months. He had 40-cm tumor of abdominal wall with sarcomatous changes at the first instance but could not receive radiotherapy as he refused for the same. He is surviving with the disease at present. Another patient had DFSP of the neck region, and was resected with a negative margin. She recurred 25 months later and undergone additional resection with negative margins and is free of disease at present. One more patient with DFSP-S of the scapular region had wide resection with negative margins at first instance along with postoperative radiotherapy developed pulmonary metastasis at 11 months. She underwent resection of the metastatic lung nodule and is disease free at present.
Discussion
DFSP is a rare dermal tumor with low metastatic risk but with a wide range of local recurrence rates reported in the literature. In this study, we present one experience of 31 patients from India to date with DFSP treated with wide excision and a standardized pathologic margin analysis. There are many published series with conflicting results regarding the recurrence rates and optimal excision margins for DFSP treated with wide excision. Monnier et al. in 2005 published a retrospective review of DFSP in a French population. In 66 patients with DFSP followed for a median of 9.6 years, they reported a statistically significant difference in recurrence rates based on margin width: 47% recurrence with margins less than 3 cm as compared with 7% margins from 3 to 5 cm [6]. Similar findings were reported by Stojadinovic et al. in a series of 33 patients analyzing DFSPs. They reported 9% local recurrence at a median follow-up of 82 months; all of these patients had a wide resection of less than 2 cm [11]. In 2009, Heuvel et al. analyzed 38 patients treated with 2–3-cm margins of excision, and at a median follow-up of 89 months, they reported the local recurrence rate of 7% [12]. In 2000, Bowne et al. reviewed the results of 159 patients treated for DFSP, and on univariate analysis, they identified 5 unfavorable prognostic factors including: age [50 years, very close (< 1 mm) margins, DFSP-S, high mitotic rate, and increased cellularity [13]]. On multivariate analysis, margins of < 1 mm and DFSP-S were found to be the only 2 independent prognostic factors predicting local recurrence.
The anatomic distribution of these tumors varies by study, but DFSP most commonly arises in the trunk (42%), upper extremities (23%), lower extremities (18%), and head and neck (13%) [9]. Infrequent sites of disease include the breast, vulva, and penis [14]. In the present study, we reported the similar anatomic distribution. Owing to the wide range of potential primary sites, a dermal-based soft tissue tumor in any location should raise the possibility of DFSP.
In the current series, we reported a recurrence rate close to 10% at a median follow-up of 30 months (range; 7–78 months) for 31 patients with DFSP including patients with sarcomatous transformation. This was accomplished with a single wide excision in the majority of patients, with 10% of the patients requiring more than 1 excision to achieve negative margins. Our current practices is to use 2 or 3 cm margins based on location on the body and the size of the lesion and send the excised specimen for frozen section to evaluate for margins status. The surgical strategy should be tailored according to the anatomic site, patient’s attitude, and need for and type of reconstruction. We emphasize the importance of obtaining negative margins to reduce the chances of recurrence. We recognize that with additional follow-up, there may be more patients who present with recurrence, but with a median follow-up of 24 months, this represents one of the largest known series from this region of the world in the literature.
DFSP-S has received special attention due to its malignant potential and its tendency for hematogenous dissemination. It represents 7 to 16% of diagnosed DFSPs [15]. It is distinguished from classic DFSP by increased cellularity, cytologic atypia, mitotic activity, and rare expression of CD34 [16]. DFSP-S has been reported to have a higher rate of local recurrence, distant metastases, and death when compared with DFSP [17]. In 2015, Hoesly PM et al. reported the 5 year recurrence-free survival rate of 42% for patients with DFSP-S [18]. Voth H et al in 2011 reviewed their results and reported local recurrence rates of 36% and metastatic rates of 13%, which are significantly higher than classic DFSP [19]. Bowne and associates [13] reported worse outcomes for patients with DFSP-S, but Fields [20] found no association with disease-free survival and DFSP-S using an updated database from the same institution.
The role of adjuvant radiation therapy for the treatment of DFSP with positive margins and with sarcomatous changes is well established. In 2005, Dagan et al. evaluated adjuvant radiation therapy with a dose of 59–65 Gy in 9 patients with DFSP and 1 patient with DFSP-S [21]. In this series, 60% had positive or close margins. Of the 10 patients, 9 remained disease-free after the treatment while 1 patient had local recurrence and eventually died of his disease. In 2009, Heuvel et al. presented their experience in patients with positive margins or close excision limited by anatomic constraints and received adjuvant radiation therapy with 50–70 Gy. Of their patients, 8 received radiation; only 1 patient with multiple previous recurrences developed local recurrence for an 82.5% local control rate after adjuvant radiation therapy [12]. Similar to this study, our current treatment strategy is to use radiation therapy for patients with persistently positive margins that cannot be further excised because of anatomic limitations. At our institution, patients having DFSP-S are also the candidate for adjuvant radiotherapy if they have anyone of the following features including tumor size > 5 cm, recurrent tumor, or with high-grade sarcomatous changes.
In our study, those with persistently positive margins (n = 4) or for sarcomatous changes (n = 7) were considered for radiation. None of these patients had local recurrence. One of these patient with DFSP-S of the scapular region developed pulmonary metastasis at 11 months.
Conclusion
In conclusion, using a standardized surgical approach including a gross and microscopic pathologic evaluation of the entire peripheral and deep margin of resection, low recurrence rates can be achieved with wide excision with negative margins. Intraoperative frozen section should be sent to analyze the margin status. Available reconstructive methods should be used to avoid compromising the extent of excision. Based on the results obtained in our series, we recommend wide excision with negative margins and en face pathologic margin analysis with adjuvant radiotherapy whenever indicated for all patients of DFSP.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Taylor R. Sarcomatous tumor resembling in some respects keloid. Arch Dermatol. 1890;8:384–387. [Google Scholar]
- 2.Darier J. Dermatofibromes progressifs et recidivants ou fibrosarcomes de la peu. Ann Dermatol Syphiligr. 1924;5:545–562. [Google Scholar]
- 3.Hoffman E. Uber das knollentreibende fibrosarkom der haut (Dermatofibrsar- koma protuberans) Dermat Ztschr. 1925;43:1–28. doi: 10.1159/000250699. [DOI] [Google Scholar]
- 4.McGregor JK. Role of surgery in the management of dermatofibrosarcoma protu- berans. Ann Surg. 1961;154:255–258. doi: 10.1097/00000658-196108000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratner D, Thomas CO, Johnson TM, Sondak VK, Hamilton TA, Nelson BR, Swanson NA, Garcia C, Clark RE, Grande DJ. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. Results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37(4):600–613. doi: 10.1016/S0190-9622(97)70179-8. [DOI] [PubMed] [Google Scholar]
- 6.Monnier D, Vidal C, Martin L, Danzon A, Pelletier F, Puzenat E, Algros MP, Blanc D, Laurent R, Humbert PH, Aubin F. Dermatofibrosarcoma protuberans: a population-based cancer registry descriptive study of 66 consecutive cases diagnosed between 1982 and 2002. J Eur Acad Dermatol Venereol. 2006;20(10):1237–1242. doi: 10.1111/j.1468-3083.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- 7.Rutgers EJ, Kroon BB, Albus-Lutter CE, Gortzak E. Dermatofibrosarcoma protuberans: treatment and prognosis. Eur J Surg Oncol. 1992;18(3):241–248. [PubMed] [Google Scholar]
- 8.Rouhani P, Fletcher CD, Devesa SS, Toro JR. Cutaneous soft tissue sarcoma inci- dence patterns in the U.S. : an analysis of 12,114 cases. Cancer. 2008;113(3):616–627. doi: 10.1002/cncr.23571. [DOI] [PubMed] [Google Scholar]
- 9.Criscione VD, Weinstock MA. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J Am Acad Dermatol. 2007;56(6):968–973. doi: 10.1016/j.jaad.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS. Incidence and survival of primary dermatofibrosarcoma protuberans in the United States. Dermatol Surg. 2016;42:S24–S31. doi: 10.1097/DSS.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 11.Stojadinovic A, Karpoff HM, Antonescu CR, Shah JP, Singh B, Spiro RH, Dumornay W, Shaha AR. Dermatofibrosarcoma protuberans of the head and neck. Ann Surg Oncol. 2000;7:696–704. doi: 10.1007/s10434-000-0696-3. [DOI] [PubMed] [Google Scholar]
- 12.Heuvel ST, Suurmeijer A, Pras E, Van Ginkel RJ, Hoekstra HJ. Dermatofibrosarcoma protuberans: Recurrence is related to the adequacy of surgical margins. Eur J Surg Oncol. 2009;36:89–94. doi: 10.1016/j.ejso.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Bowne WB, Antonescu CR, Leung DH, Katz SC, Hawkins WG, Woodruff JM, Brennan MF, Lewis JJ. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88:2711–2720. doi: 10.1002/1097-0142(20000615)88:12<2711::AID-CNCR9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.Zlatnik MG, Dinh TV, Lucci JA, 3rd, et al. Dermatofibrosarcoma protuberans of the vulva: report of two new cases and review of the literature. J Low Genit Tract Dis. 1999;3(2):135–138. doi: 10.1046/j.1526-0976.1999.08100.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldblum JR. CD34 positivity in fibrosarcomas which arise in dermatofibrosarcoma protuberans. Arch Pathol Lab Med. 1995;119(3):238–241. [PubMed] [Google Scholar]
- 16.Kuzel P, Mahmood MN, Metelitsa AI, Salopek TG. A clinicopathologic review of a case series of dermatofibrosarcoma protuberans with fibrosarcomatous differentiation. J Cutan Med Surg. 2015;19(1):28–34. doi: 10.2310/7750.2014.13192. [DOI] [PubMed] [Google Scholar]
- 17.Liang CA, Jambusaria-Pahlajani A, Karia PS, Elenitsas R, Zhang PD, Schmults CD. A systematic review of outcome data for dermatofibrosarcoma protuberans with and without fibrosar- comatous change. J Am Acad Dermatol. 2014;71(4):781–786. doi: 10.1016/j.jaad.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Hoesly PM, Lowe GC, Lohse CM, Brewer JD, Lehman JS. Prognostic impact of fibrosarcomatous transformation in dermatofibrosarcoma protuberans: a cohort study. J Am Acad Dermatol. 2015;72(3):419–425. doi: 10.1016/j.jaad.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Voth H, Landsberg J, Hinz T, Wenzel J, Bieber T, Reinhard G, Höller T, Wendtner CM, Schmid-Wendtner MH. Management of dermatofibrosarcoma protu- berans with fibrosarcomatous transformation: an evidence-based review of the literature. J Eur Acad Dermatol Venereol. 2011;25(12):1385–1391. doi: 10.1111/j.1468-3083.2011.04141.x. [DOI] [PubMed] [Google Scholar]
- 20.Fields RC, Hameed M, Qin LX, Moraco N, Jia X, Maki RG, Singer S, Brennan MF. Dermatofibrosarcoma protuberans (DFSP): predictors of recurrence and the use of systemic therapy. Ann Surg Oncol. 2011;18(2):328–336. doi: 10.1245/s10434-010-1316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dagan R, Morris CG, Zlotecki RA, Scarborough MT. Menden- hall W. Radiotherapy in the treatment of dermatofibrosarcoma protuberans. Am J Clin Oncol. 2005;28:537–539. doi: 10.1097/01.coc.0000171278.69291.64. [DOI] [PubMed] [Google Scholar]