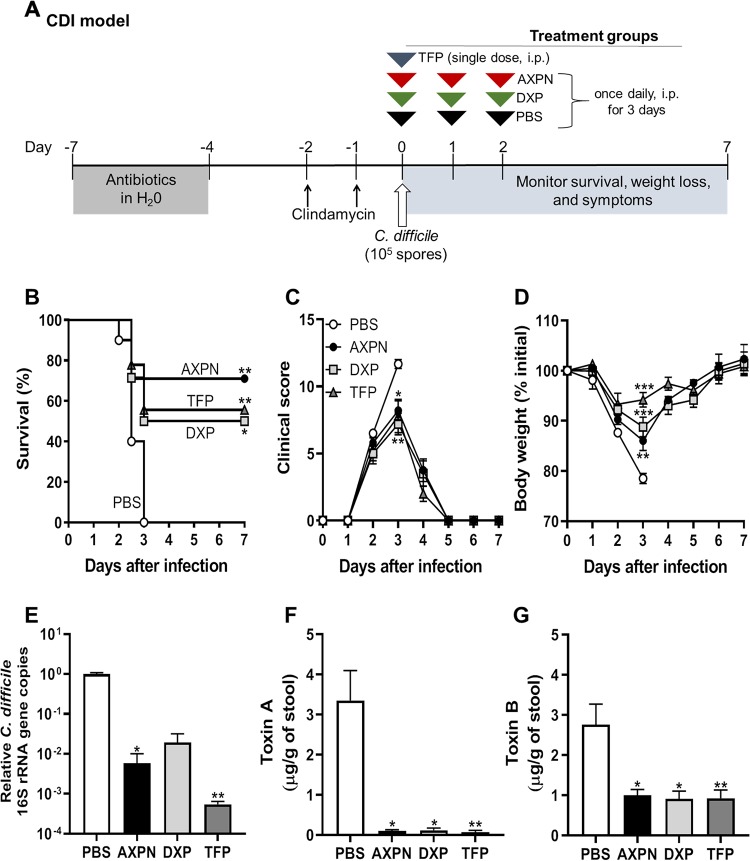
FIG 1.
Model of lead drugs protecting against lethal C. difficile infection (CDI). (A) Experimental design for CDI model. C57BL/6 mice were pretreated with antibiotics by administration of an antibiotic cocktail in the drinking water for 3 to 4 days, followed by injections of clindamycin for 1 to 2 days at 24-h intervals. Mice were then orally infected with C. difficile spores and administered lead drugs or the vehicle control PBS, starting at the time of infection. (B) Mice were monitored for survival (n = 9 or 10 mice/group). *, P < 0.05; **, P < 0.01 (determined by log rank survival statistics). (C and D) Development of clinical symptoms (C) and weight loss (D) were assessed at the indicated time points (mean ± SE, n = 9 or 10 mice/time point). *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared to results for PBS controls. (E) At 30 h postinfection, the bacterial burden was determined by qPCR analysis of the quantities of 16S rRNA gene copies of C. difficile relative to the total number of 16S rRNA gene copies in the cecal contents. Expression levels are shown in comparison to that of the PBS-treated group on a log10 scale. (F and G) Toxin levels were measured in the cecal contents by ELISA at 30 h postinfection. Data are the mean ± SE of the results of two independent experiments (n = 5 to 8 mice/group). *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared to results for the PBS control.