Introduction
An accessory pathway (AP) between the left atrial appendage (LAA) and left ventricle (LV) is rare and only 14 cases have been reported. Its features include the potential of causing ventricular fibrillation (VF) owing to a short effective refractory period (ERP) of the AP and the coexistence of multiple APs or an AP between the right atrial appendage and right ventricle, and it can cause difficulty in performing endocardial ablation.1, 2, 3, 4, 5, 6, 7 We report a case of a 6-year-old boy with multiple APs including an LAA-LV AP, giant LAA, and LV noncompaction (LVNC). Little has been reported on LAA-LV APs associated with a giant LAA.
Case report
A 6-year-old boy who had Klinefelter syndrome presented with Wolff-Parkinson-White syndrome during a school cardiac screening. He had no known history of palpitations, but the assessment was limited owing to presence of a developmental delay. However, the Holter electrocardiography (ECG) exhibited a paroxysmal supraventricular tachycardia (SVT) at 279 beats per minute. The echocardiogram revealed an LVNC with an ejection fraction of 69% and no mitral regurgitation. We decided to perform catheter ablation because of the possibility of sudden cardiac death and his difficulty in clearly expressing his symptoms.
The catheter ablation was performed under general anesthesia when he was 6 years old and weighed 24 kg. The electrode catheters were positioned in the right atrium, His bundle, right ventricular apex, and coronary sinus (CS). During the electrophysiological study, incremental atrial pacing revealed that there were 3 different delta wave morphologies suggesting multiple pathways (Supplementary Figure), and the ERPs during single atrial extrastimulation were 320 ms for the right AP, 300 ms for the left anterior AP, and 250 ms for the posterior AP. Those electrophysiological study findings suggested the possibility of rapid conduction during atrial fibrillation (AF) over the AP, resulting in VF. A shortest preexcited R-R interval of ≤250 ms or AP-ERP of ≤250 ms are risk factors for VF. Although atrial refractoriness was reached at baseline and under an isoproterenol infusion, no AF was induced. A ventricular single extrastimulus did not demonstrate any decremental retrograde conduction and ventricular constant pacing at 100 beats per minute from the right ventricular apex revealed that the earliest atrial activation site was on the left side. No paroxysmal SVT or atrial echo beats were induced. The multiple APs were ablated with a 4 mm nonirrigated radiofrequency (RF) catheter (NAVISTAR; Biosense Webster, Irvine, CA). One of the APs was a left anterior AP and was attempted to be ablated via transseptal and retrograde approaches. Another AP was located on the posterior septum and was ablated from both sides of the atrioventricular annulus. The last AP was ablated from a posterolateral site via an inferior vena cava approach. However, the 3 APs could not be eliminated after 32 RF energy applications, because the APs were presumed to be broad or associated with a structural abnormality. The ablation of the left anterior AP resulted in transient success after repetitive RF applications at a site slightly distant from the mitral annulus, but it recurred in a few minutes after the application. During the first session, we made the decision to terminate the case and leave the lab without achieving success.
A second session was performed at the age of 8 years when he weighed 31 kg. He did not have any antiarrhythmic drugs before the second session because he did not experience any syncopal episodes, and no SVTs were detected by a 24-hour ECG 2 months after the first session. Assuming that the APs were associated with some structural abnormality, we performed computed tomography (CT), which revealed a giant LAA, 12.9 mL/m2 in size (Figure 1) (normal: 6.32 ± 2.67 mL/m2in size8). The ECG exhibited ventricular preexcitation of all beats and a different QRS morphology was observed during the premature atrial contractions (PAC) of the second and fifth beats (Figure 2). That finding suggested that both the right- and left-sided APs remained during the second session. The morphology of the delta waves during sinus rhythm suggested a right-sided AP, whereas those during the PACs suggested a left anterior AP. During the second session, in addition to the catheters in the first session, a 2F EP Star steerable catheter (8 poles; Japan Lifeline, Tokyo, Japan) and 6F Inquiry Luma-cath (10 poles; St. Jude Medical, Irvine, CA) were positioned in the distal CS (Figure 1B). The ERP during single atrial extrastimulation was 370 ms for the right-sided AP, 320 ms for the left anterior AP, and 310 ms for the posterior AP. Though atrial refractoriness was reached, no AF was induced. The earliest ventricular activation site during sinus rhythm was recorded by the distal electrode of the CS catheter, which was near the LAA according to the angiography of the left atrium. Though we could not ablate the APs along the mitral annulus during the first session, we successfully ablated the AP using a 4 mm nonirrigated catheter (NAVISTAR; Biosense Webster) in the temperature control mode (maximum 55°C and 40 watts) at the base of the LAA, where the earliest ventricular activation preceding the delta wave on the surface ECG by 14 ms was recorded during sinus rhythm (Figure 3). No ST changes occurred during the delivery of the RF energy, and the distance between the successful ablation site and mitral annulus was 9 mm. Then we were able to successfully ablate the 2 other APs near the CS ostium and on the right posterolateral side of the tricuspid annulus. The posterior septal AP was successfully ablated at the CS ostium at the earliest ventricular site via an inferior vena cava approach after approaching it from both sides of the atrioventricular annulus. However, the right posterolateral AP was successfully ablated with 2 applications at almost the same location. The first application led to a slight change in the delta wave morphology and the second application accomplished the disappearance of the delta wave. That success was attributed to the identification of the appropriate AP sites and small number of RF applications delivered. The patient did not have any further recurrences of the delta waves for 2 years.
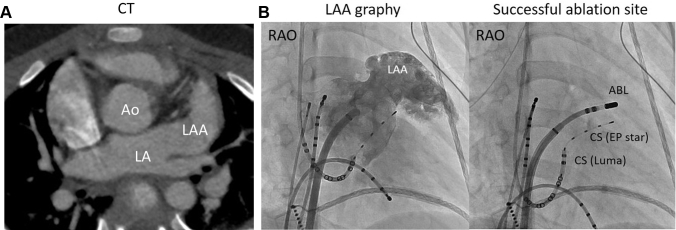
Figure 1.
A: Computed tomography (CT) exhibiting a giant left atrial appendage (LAA) 12.9 mL/m2 (normal 6.32 ±2.67 mL/m2) in size. Ao = aorta; LA = left atrium. B: LAA angiography and fluorography of the successful ablation site. The ablation catheter (ABL) (EP Star steerable catheter; Japan Lifeline, Tokyo, Japan; Inquiry Luma-cath; St. Jude Medical, Irvine, CA) was positioned at the base of the LAA. CS = coronary sinus; RAO = right anterior oblique.
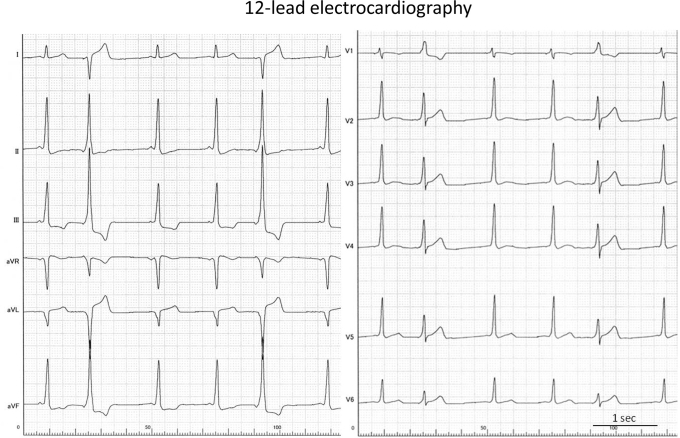
Figure 2.
A 12-lead electrocardiogram exhibiting ventricular preexcitation in all beats and a different QRS morphology during premature atrial contractions in the second and fifth beats, suggesting a left-sided accessory pathway.
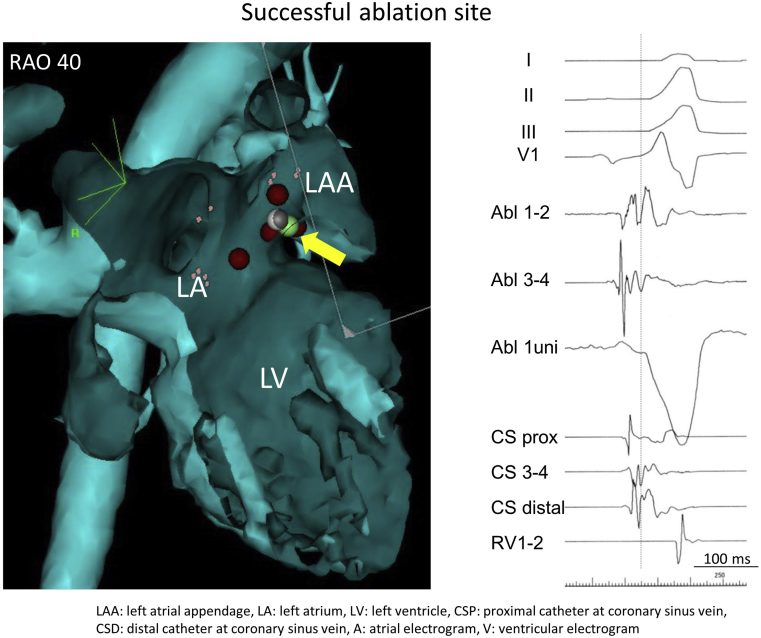
Figure 3.
Successful ablation site on the CARTO mapping system (right anterior oblique [RAO] projection) and intracardiac electrography. The green circle is the successful ablation site, which is 9 mm away from the mitral annulus and precedes the delta wave by 14 ms on the surface electrocardiogram during sinus rhythm. LA = left atrium; LAA = left atrial appendage; LV = left ventricle.
Discussion
A rare AP between a giant LAA and the LV associated with multiple APs was successfully ablated. Though there has been a report about a giant right appendage associated with Wolff-Parkinson-White syndrome,9 little has been reported on LAA-LV APs associated with giant LAAs. Giant LAAs are caused by congenital dysplasia of the atrial muscle or are secondary to mitral valve disease and lead to thromboembolisms, AF, atrial tachycardia,10 and compression of the coronary artery,11 and are also associated with LVNCs.12 LVNCs are associated with APs (15%–17%13) and their location has varied in each case.14,15 Defects of the atrioventricular annulus fibrosus, characterized by persistence of trabeculations during embryogenesis, are thought to allow for the development of an AP. The erroneous development of an embryonic left atrium and AV canal could cause this rare condition.
The ablation of LAA-LV APs is difficult because of (1) their rarity, (2) broad APs, (3) the tip of the LAA being associated with multiple APs, and (4) the close vicinity to the coronary artery.1,5 Though multiple APs make predicting the AP location confusing, an LAA-LV AP should be suspected when a giant LAA exists or the 12-lead ECG exhibits a preexcited QRS that originates from the left lateral wall. The placement of a CS catheter in the anterior interventricular vein could help us diagnose this AP.1,5 In our case, it was hard to diagnose the LAA-LV AP owing to multiple APs, including right-sided APs. However, the preexcited QRS wave during a PAC, giant LAA, and electrogram in the anterior intraventricular vein helped us to diagnose this AP. Fortunately, the AP was located at the base of the LAA, so the AP was successfully ablated by a 4-mm-tip nonirrigated catheter. A giant LAA might be associated with a broad AP, which should be ablated over a broad area based on the anatomy obtained from angiography or a CT. If the AP is located at the tip of the appendage, an irrigation catheter or surgical resection would be required; however, if the AP is located at the base of the LAA it would be possible to ablate the AP while being careful not to damage the coronary artery.
Conclusion
We reported a case of a 6-year-old boy with multiple APs including an LAA-LV AP, giant LAA, and LVNC. An LAA-LV AP should be suspected in tough cases with left-sided APs, and a CT scan would help to diagnose it.
Key Teaching Points.
-
•
Accessory pathways (AP) in the left atrial appendage are associated with multiple APs or a giant left atrial appendage.
-
•
This AP should be suspected in tough cases with left-sided APs.
-
•
A giant atrial appendage can help us to diagnose this rare AP.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2019.11.006.
Appendix. Supplementary data
A 12-lead ECG during the 1st session. The left panel shows WPW syndrome type B ECG during the sinus rhythm. Right panel: During the electrophysiological study, incremental atrial pacing revealed that there were three different delta wave morphologies suggesting multiple pathways, and the effective refractory periods during single atrial extra-stimulation were 320 ms for the right AP, 300 ms for the left anterior AP, and 250 ms for the posterior AP.
References
- 1.Benhayon D., Sinisterra S., Young M.-L. Wolff-Parkinson-White syndrome due to a left atrial appendage–to–left ventricular connection: a case of a successful pathway elimination from inside of the left atrial appendage. HeartRhythm Case Rep. 2018;4:519–522. doi: 10.1016/j.hrcr.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jagadheesan K.S., Rangasamy S., Selvaraj R.J. A deadly mix - rheumatic mitral stenosis, preexcited atrial fibrillation, left atrial appendage thrombus and left atrial appendage accessory pathway. Indian Pacing Electrophysiol J. 2017;17:183–185. doi: 10.1016/j.ipej.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mollazadeh R., Eslami M. Radiofrequency ablation of left atrial appendage accessory pathway. Europace. 2016;18:867. doi: 10.1093/europace/euv353. [DOI] [PubMed] [Google Scholar]
- 4.Long D.Y., Dong J.Z., Sang C.H. Ablation of left-sided accessory pathways with atrial insertion away from the mitral annulus using an electroanatomical mapping system. J Cardiovasc Electrophysiol. 2013;24:788–792. doi: 10.1111/jce.12122. [DOI] [PubMed] [Google Scholar]
- 5.Mah D., Miyake C., Clegg R. Epicardial left atrial appendage and biatrial appendage accessory pathways. Heart Rhythm. 2010;7:1740–1745. doi: 10.1016/j.hrthm.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Di Biase L., Schweikert R.A., Saliba W.I. Left atrial appendage tip: an unusual site of successful ablation after failed endocardial and epicardial mapping and ablation. J Cardiovasc Electrophysiol. 2010;21:203–206. doi: 10.1111/j.1540-8167.2009.01561.x. [DOI] [PubMed] [Google Scholar]
- 7.Servatius H., Rostock T., Hoffmann B.A., Willems S. Catheter ablation of an atrioventricular bypass tract connecting a funnel-shaped bilobular left atrial appendage with the ventricular free wall. Heart Rhythm. 2009;6:1075–1076. doi: 10.1016/j.hrthm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Boucebci S., Pambrun T., Velasco S., Duboe P.O., Ingrand P., Tasu J.P. Assessment of normal left atrial appendage anatomy and function over gender and ages by dynamic cardiac CT. Eur Radiol. 2016;26:1512–1520. doi: 10.1007/s00330-015-3962-2. [DOI] [PubMed] [Google Scholar]
- 9.Shah K., Walsh K. Giant right atrial diverticulum: an unusual cause of Wolff-Parkinson-White syndrome. Br Heart J. 1992;68:58–59. doi: 10.1136/hrt.68.7.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang B., Li H., Zhang L. Congenital left atrial appendage aneurysm: a rare case report and literature review. Medicine (Baltimore) 2018;97:e9344. doi: 10.1097/MD.0000000000009344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tandon R., Arisha M.J., Nanda N.C. Incremental benefit of three-dimensional transthoracic echocardiography in the assessment of left atrial appendage aneurysm leading to severe extrinsic compression of a coronary artery. Echocardiography. 2018;35:685–691. doi: 10.1111/echo.13901. [DOI] [PubMed] [Google Scholar]
- 12.Crean A.M., Provost Y., Paul N. Biventricular non-compaction and giant left atrial appendage. Eur Heart J. 2007;28:1318. doi: 10.1093/eurheartj/ehl459. [DOI] [PubMed] [Google Scholar]
- 13.Ichida F., Hamamichi Y., Miyawaki T. Clinical features of isolated noncompaction of the ventricular myocardium. J Am Coll Cardiol. 1999;34:233–240. doi: 10.1016/s0735-1097(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 14.Nihei K., Shinomiya N., Kabayama H. Wolff-Parkinson-White (WPW) syndrome in isolated noncompaction of the ventricular myocardium (INVM) Circ J. 2004;68:82–84. doi: 10.1253/circj.68.82. [DOI] [PubMed] [Google Scholar]
- 15.Steffel J., Kobza R., Namdar M. Electrophysiological findings in patients with isolated left ventricular non-compaction. Europace. 2009;11:1193–1200. doi: 10.1093/europace/eup187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A 12-lead ECG during the 1st session. The left panel shows WPW syndrome type B ECG during the sinus rhythm. Right panel: During the electrophysiological study, incremental atrial pacing revealed that there were three different delta wave morphologies suggesting multiple pathways, and the effective refractory periods during single atrial extra-stimulation were 320 ms for the right AP, 300 ms for the left anterior AP, and 250 ms for the posterior AP.