Introduction
The major weakness of transvenous cardiac pacing derives from the fact that energy is delivered to the heart muscle from the pacing device through transvenous lead (TL). The TL has been shown to be susceptible to degradation over time, and in the case of infection the event may be life-threatening. Dominant right ventricular (RV) pacing may also lead to left ventricular (LV) dysfunction.
The introduction of biventricular cardiac resynchronization pacing in the mid-1990s introduced new challenges to the TL. With lead dislodgement rates within the first year of implantation being reported to be between 2% and 12%, it is important to find a position that is anatomically accessible and stable in the long term1 and avoid phrenic stimulation, which is reported to occur in 2% to 37%.2
Recently, leadless RV pacing devices have been shown to be both safe and effective.3 In the 2 cases reported here, the Micra Transcatheter Pacemaker System (Medtronic plc, Minneapolis, MN) was used for RV pacing. The pacemaker is packaged in a hermetically sealed capsule that has all the functions and features of a single-chamber pacemaker.
A leadless technology employing ultrasound to energize a piezo transmitter for endocardial LV pacing in cardiac resynchronization therapy (CRT) has also been shown to be safe and effective.4 The WiSE CRT device (EBR Systems, Sunnyvale, CA) consists of a receiving electrode implanted in the left ventricle; an ultrasound transmitter, which is implanted surgically in an acoustic window (typically in the fifth or sixth intercostal space) after screening by a physician to keep continuous communication between the LV electrode and transmitter unaffected by the patient’s position or inhale/exhale; and a battery pack. The intensity and duration of the acoustic energy from the transmitter can be programmed to optimize energy consumption with regard to individual LV pacing threshold. The transmitter is trained to adapt the amplitude and width of the pacing pulse generated by the co-implant. The transmitter is connected to the battery pack, which is implanted subcutaneously.
We report on the implantation of a completely leadless cardiac resynchronization system combining 2 novel technologies: namely, the Micra device and the WiSE CRT wireless endocardial pacing system. To our knowledge, this is the first report of patients with a completely leadless approach to biventricular pacing.
Case report
Patient selection
Both patients were previously implanted with the Micra device. They qualified for inclusion in the trial after being diagnosed with atrial fibrillation (AF) requiring RV pacing. The patients subsequently developed heart failure with low LV ejection fraction (EF) and wide QRS complex, qualifying them for CRT.
Implant procedure
The Micra pacing device is introduced through the right femoral vein utilizing a proprietary introducer sheath, targeting the ventricular septum (which is safer and offers more physiological activation). Device fixation in the myocardium is achieved via 4 flexible nitinol tines.
Implantation of the wireless LV pacing system is a 2-stage process requiring 2 procedures carried out on consecutive days. First, the battery pack and transmitter are implanted. The transmitter is implanted in the fourth to sixth intercostal spaces lateral to the left parasternal border, which provides the bone- and lung-free acoustic window required for transmission of the acoustic energy to the receiving electrode.
Placement of the LV electrode was performed the day following generator and transmitter implant using a retrograde transaortic approach. An activated clotting time of 200–250 seconds was maintained by heparin infusion. A 12F steerable delivery catheter was used to access the left ventricle, through which an 8F catheter preloaded with the electrode is delivered. After the procedure, double antiplatelet therapy would be administered for the first 3 months, and aspirin for 6 months. In patients with oral anticoagulation therapy owing to AF, additional antiplatelet therapy is not required.
Case 1
The first patient was a 70-year-old man with arterial hypertension and type II diabetes referred with long-standing persistent AF with fast ventricular response. After implantation of the Micra device, ablation of the atrioventricular node was performed 5 months after implantation to control his heart rate. However, the patient developed heart failure with progression of LV systolic dysfunction, with an LV EF of 33%. The patient became symptomatic and was classified as having NYHA class III heart failure with dependent RV pacing and paced QRS duration of 198 ms (Supplementary Table S1). The WiSE LV system was implanted without complications.
Case 2
The second patient was a 75-year-old woman, who had previously undergone mitral and tricuspid annuloplasty with bilateral maze procedure for persistent AF. Unfortunately, the AF continued and the patient suffered episodes of complete atrioventricular block. The Micra device was implanted 1 year after cardiac surgery. Owing to the progression of LV dysfunction classified by LV EF 25%, we implanted the WiSE LV system (Supplementary Table S1).
Results
Case 1
WiSE procedure details
In both patients we used a 2-step approach. The transmitter and battery were implanted under general anesthesia with 40 minutes’ procedure time. The transmitter was implanted into the seventh intercostal space, with the position verified by echocardiography with an excellent acoustic window, and the battery was placed into a pocket at the lateral side of the chest and the cable connection between the 2 elements was tunneled under the skin.
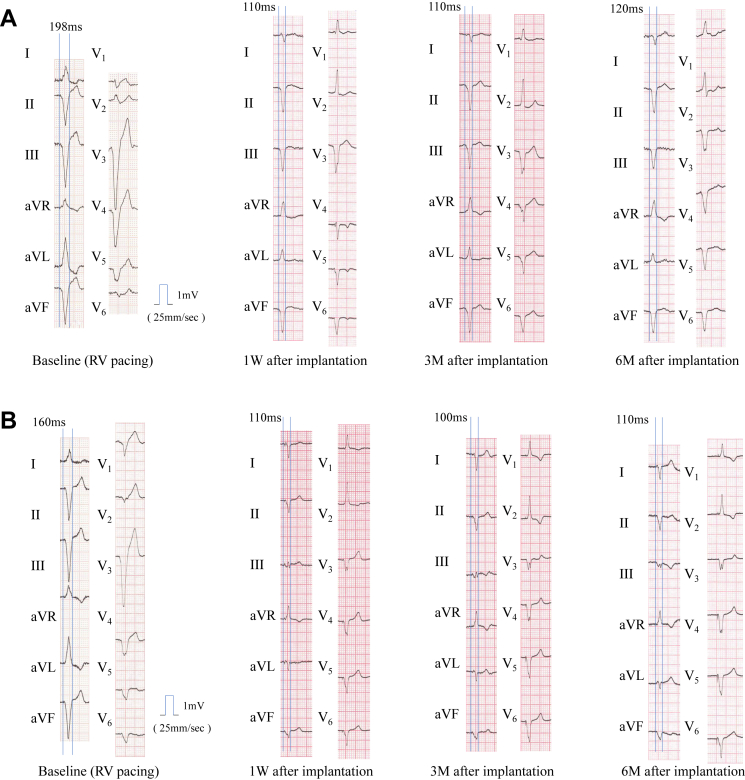
Implantation of the LV electrode was performed 2 weeks after transmitter and battery implantation. Two different sites in the LV lateral wall were tested guided by both fluoroscopy and intracardiac echocardiography. The optimal site in the LV lateral wall during biventricular pacing resulted in shortening of QRS complex width from 198 ms to 120 ms, which was the original QRS complex width during RV pacing only. The total procedure time was 16 minutes and fluoroscopy time was 5 minutes (Supplementary Table S2).
Clinical course after implantation
One week after implantation, the QRS width remained abbreviated and the patient’s heart failure symptoms had improved to NYHA Ⅱ. At 3 months, the pacing threshold of the LV electrode gradually improved from 2.5 V at 0.8 ms pulse width to 1.0 V at 0.8 ms. The patient was symptomatically stable and the QRS width remained the same.
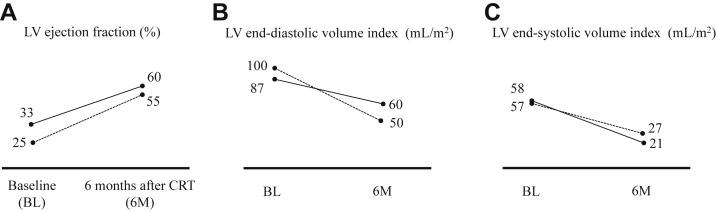
At a 6-month follow-up, the patient reported symptomatic improvement with increased exercise tolerance (including the ability to climb stairs daily). The QRS width on electrocardiography was 120 ms and transthoracic echocardiography showed improvement of both end-systolic and end-diastolic volume index and LV EF normalized (Figures 1 and 2A, Supplementary Table S3).
Figure 1.
Echocardiographic parameters before and 6 months after cardiac resynchronization therapy (CRT) with WiSE (EBR Systems, Sunnyvale, CA) CRT system. A: Low left ventricular (LV) ejection fraction (EF) during right ventricular apical pacing with leadless pacemaker normalized after CRT with WiSE CRT system. B,C: Both LV end-diastolic and end-systolic volume index clearly decreased compared with baseline (BL). Data are shown by solid line in patient 1 and dotted line in patient 2. 6M = 6 months.
Figure 2.
A: QRS width of baseline and after cardiac resynchronization therapy (CRT) with WiSE (EBR Systems, Sunnyvale, CA) CRT system in patient 1. 1 W = 1 week; 3 M = 3 months; 6 M = 6 months. B: QRS width of baseline and after CRT with WiSE CRT system in patient 2.
Case 2
WiSE procedure details
The WiSE transmitter was also implanted under general anesthesia. Total procedure time was 55 minutes. The LV electrode was placed 2 weeks after implantation. A total of 2 sites were tested, with the best site found in the LV lateral base. Total procedure time was 25 minutes, with fluoroscopy time of 6 minutes (Supplementary Table S2).
Clinical course after implantation.
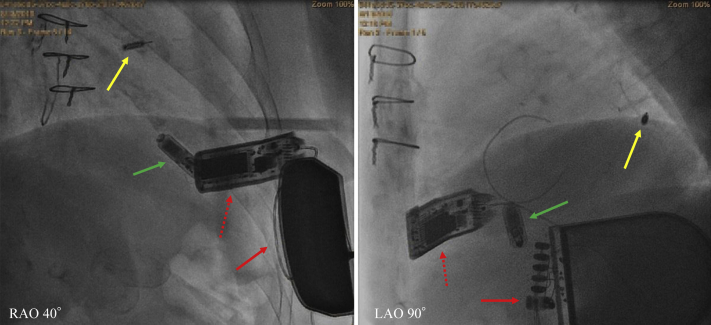
One week after the LV receiver placement, the patient’s symptoms of heart failure disappeared. The pacing threshold of the LV electrode showed no change from 1.0 V at 0.8 ms pulse width, the parameter at LV electrode placement. Three months after placement, QRS width and patient’s status were stable. At 6-month follow-up the patient had become completely asymptomatic and reported a large increase in both exercise tolerance and quality of life (including the ability to cycle). The QRS width and echocardiography indicated improvement of LV dysfunction. (Figures 1 and 2B, Supplementary Table S3). Chest radiograph at 6 months is shown in Figure 3.
Figure 3.
Fluoroscopy at 6 months in patient 2. Yellow arrow shows the WiSE (EBR Systems, Sunnyvale, CA) left ventricular electrode deployed in the basal lateral endocardium and green arrow points to the Micra leadless pacemaker (Medtronic plc, Minneapolis, MN) in the right ventricular apex. Subcutaneously implanted transmitter (dotted red arrow) and battery pack (solid red arrow) are connected with tunneled cable. LAO = left anterior oblique; RAO = right anterior oblique.
Discussion
Rationale for leadless pacing
In these 2 cases we were able to successfully instigate effective synchronized RV and LV pacing employing the Micra RV pacing and WiSE CRT devices. Both patients developed nonischemic cardiomyopathy and heart failure with left bundle branch block pattern, which is typically seen under RV apical pacing, and were referred for a CRT therapy with the WiSE CRT LV electrode system as the only option to take advantage of the currently implanted leadless device.
Physiological RV septal pacing and upgrade for biventricular pacing
The native conduction system without electrical block demonstrates the best mechanical cardiac synchronization. Once patients with existing RV pacing device develop heart failure with low EF, the typical practice would be to upgrade the device to CRT with additional coronary sinus pacing lead. Long-term results of upgrading to CRT from RV pacing compared with de novo CRT implantation have been shown to be favorable.5 QRS abbreviation with direct His-bundle pacing in patients with both ischemic and nonischemic cardiomyopathy is feasible. Vijayaraman and colleagues6 reported that physiologic pacing was achieved in over 90% of all candidates using left bundle branch area pacing, but there remain concerns about the hemodynamic effect and long-term advantage for heart failure.
Endocardial vs epicardial LV pacing
One of the advantages of a wireless electrode placed in the LV is the potential for more physiological endocardial pacing activation sequence. A recent animal study has shown LV endocardial pacing to be beneficial.7 This has been hypothesized and modeled to be as a result of the ability to engage the Purkinje network, leading to reduced activation times and increased synchronicity. Indeed, studies conducted in patients previously implanted with a CRT device showed that pacing from a specific endocardial site was superior to conventional epicardial pacing CRT when measuring hemodynamic response acutely.8 Also, a single case observation of a CRT nonresponder showed a major change in symptoms and ventricular function, which was hypothesized to have been due to a more physiological activation sequence.9 Moreover, the SELECT-LV study4 showed the efficacy of leadless LV endocardial pacing even for nonresponders to LV epicardial pacing or those who were excluded as candidates for existing transvenous CRT device implantation owing to anatomical limitations of the coronary sinus vein. Though an LV endocardial pacing site for CRT may have appeal, it has historically been achieved through placing a lead in the left ventricle through an atrial transseptal, ventricular transseptal, or ventricular apical approach. These approaches bring both technical challenges and requirement for life-long anticoagulation therapy owing to the possibility of thrombus formation on the lead in the left ventricle. Outcomes of reported clinical trials were positive in smaller cohorts, but a single larger study reported no significant improvement in terms of response rate.10 This may be due to the fact that superior LV endocardial pacing has been found to be highly site specific.8 The wireless LV electrode delivery system allows different areas of the left ventricle to be mapped for preferential pacing positions before implantation. Guidance for optimal site selection has recently been proposed from a study at 3 sites11 and has the potential to further improve implant success.
Conclusion
A completely leadless configuration of biventricular pacing was found to be safe and effective in these 2 patients. Leadless biventricular pacing utilizing a wireless LV electrode may be particularly attractive for patients with leadless pacemakers who develop iatrogenic dyssynchrony-related LV dysfunction. In these 2 cases we demonstrated high level of synchronization between leadless RV and wireless LV devices.
Key Teaching Points.
-
•
This is the first report describing totally leadless cardiac resynchronization therapy (CRT) with the combination of a self-contained leadless right ventricular (RV) pacemaker and a wireless ultrasonic left ventricular (LV) endocardial pacing system. The feasibility of totally leadless CRT was demonstrated in humans.
-
•
LV endocardium pacing is more physiological than coronary sinus epicardial pacing. An LV endocardium electrode also enables us to choose the optimal pacing site without anatomical limitation.
-
•
CRT with the leadless left ventricular endocardial pacing system is presently the only option to take advantages of leadless pacing devices upon upgrading from a single-chamber RV leadless pacemaker.
Footnotes
Financial Disclosure: This paper was supported by Ministry of Health Czech Republic, DRO (NNH, 00023884).
Conflict of Interest: Dr Reddy reports serving as a consultant to EBR Systems, Inc, and Medtronic plc. Dr Reddy also has conflicts of interest with other companies not directly related to this manuscript; a comprehensive list is provided in the Supplementary Appendix. The other authors report no conflicts.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2019.12.002.
Appendix. Supplementary data
References
- 1.Biffi M., Bertini M., Ziacchi M., Diemberger I., Martignani C., Boriani G. Left ventricular lead stabilization to retain cardiac resynchronization therapy at long term: when is it advisable? Europace. 2014;16:533–540. doi: 10.1093/europace/eut300. [DOI] [PubMed] [Google Scholar]
- 2.Moubarak G., Bouzeman A., Ollitrault J., Anselme F., Cazeau S. Phrenic nerve stimulation in cardiac resynchronization therapy. J Interv Card Electrophysiol. 2014;41:15–21. doi: 10.1007/s10840-014-9917-8. [DOI] [PubMed] [Google Scholar]
- 3.Duray G., Ritter P., El-Chami M. Long-term performance of a transcatheter pacing system: 12-month results from the Micra Transcatheter Pacing Study. Heart Rhythm. 2017;14:702–709. doi: 10.1016/j.hrthm.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Reddy V., Miller M., Neuzil P. Original investigation: cardiac resynchronization therapy with wireless left ventricular endocardial pacing. The SELECT-LV Study. J Am Coll Cardiol. 2017;69:2119–2129. doi: 10.1016/j.jacc.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 5.Foley P.W., Muhyaldeen S.A., Chalil S., Smith R.E., Sanderson J.E., Leyva F. Long-term effects of upgrading from right ventricular pacing to cardiac resynchronization therapy in patients with heart failure. Europace. 2009;11:495–501. doi: 10.1093/europace/eup037. [DOI] [PubMed] [Google Scholar]
- 6.Vijayaraman P., Subzposh F.A., Naperkowski A. Prospective evaluation of feasibility, electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm. 2019;16:1774–1782. doi: 10.1016/j.hrthm.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 7.van Deursen C., van Geldorp I.E., Rademakers L.M. Left ventricular endocardial pacing improves resynchronization therapy in canine left bundle-branch hearts. Circ Arrhythm Electrophysiol. 2009;2:580–587. doi: 10.1161/CIRCEP.108.846022. [DOI] [PubMed] [Google Scholar]
- 8.Shetty A.K., Sohal M., Chen Z. A comparison of left ventricular endocardial, multisite, and multipolar epicardial cardiac resynchronization: an acute haemodynamic and electroanatomical study. Europace. 2014;16:873–879. doi: 10.1093/europace/eut420. [DOI] [PubMed] [Google Scholar]
- 9.Auricchio A., Delnoy P., Regoli F., Seifert M., Markou T., Butter C. First-in-man implantation of leadless ultrasound-based cardiac stimulation pacing system: novel endocardial left ventricular resynchronization therapy in heart failure patients. Europace. 2013;15:1191–1197. doi: 10.1093/europace/eut124. [DOI] [PubMed] [Google Scholar]
- 10.Phillimore Gamble J., Herring N., Ginks M. Endocardial left ventricular pacing for cardiac resynchronization: systematic review and meta-analysis. Europace. 2018;20:73–81. doi: 10.1093/europace/euw381. [DOI] [PubMed] [Google Scholar]
- 11.Sieniewicz B.J., Behar J.M., Gould J. Guidance for optimal site selection of a leadless LV endocardial electrode improves acute hemodynamic response and chronic remodeling. JACC Clin Electrophysiol. 2018;4:860–868. doi: 10.1016/j.jacep.2018.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.