Abstract
Background:
Nicotine-containing electronic cigarette (e-cig) use has become widespread. However, understanding the biological impact of e-cigs compared to smoking on the lung is needed. There are major gaps in knowledge for chronic effects and for an etiology to recent acute lung toxicity among vapers leading to death.
Methods:
We conducted bronchoscopies in a cross-sectional study of 73 subjects (42 never-smokers, 15 e-cig users and 16 smokers). Using bronchoalveolar lavage and brushings, we examined lung inflammation by cell counts, cytokines, genome-wide gene expression and DNA methylation.
Results:
There were statistically significant differences among never-smokers, e-cig users, and smokers for inflammatory cell counts and cytokines (FDRq<0.1). The e-cig users had values intermediate between smokers and never-smokers, with levels for most of the biomarkers more similar to never-smokers. For differential gene expression and DNA methylation, e-cig users also more like never-smokers; many of these genes corresponded to smoking-related pathways, including those for xenobiotic metabolism, aryl hydrocarbon receptor signaling and oxidative stress. Differentially methylated genes were correlated with changes in gene expression, providing evidence for biological effects of the methylation associations.
Conclusions:
These data indicate that e-cigs are associated with less toxicity than cigarettes for smoking-related pathways. What is unknown may be unique effects for e-cigs not measured herein, and a comparison of smokers completely switching to e-cigs compared to former-smokers. Clinical trials for smokers switching to e-cigs who undergo serial bronchoscopy and larger cross-sectional studies of former smokers with and without e-cig use, and for e-cigs who relapse back to smoking, are needed.
Keywords: Electronic cigarettes, inflammation, DNA methylation, gene expression, lung
INTRODUCTION
Electronic cigarettes (e-cigs) are widely used by smokers, former smokers and never-smoking youth (1). Recent data indicate that e-cig use might be better than nicotine replacement therapy for smoking cessation (2,3) but conclusive evidence is yet available on the effectiveness of e-cigs and safety for long-term smoking cessation. Also, a systemic review study of 38 studies reported that e-cigs were associated with significantly reduced smoking cessation (4).
However, possible toxic effects of e-cigs are unclear, and the risk/benefit balance of use is different for never-smokers than for smokers. While it is suspected that adverse chronic effects of e-cigs are less than continued smoking, there is little direct data for effects in target organs, particularly the lung. Importantly, as of November 20 2019, 2,290 cases of acute lung injury including 47 deaths from 49 states was identified to be associated with e-cigarette product use or vaping across the nation (5-8).Many of these cases appear to be related to vaping cannabinoid oils (e.g., a different formulation than what is in nicotine e-cigs), but there are some reported cases in nicotine e-cig users. Thus, studies for the effects of nicotine-containing e-cigs are needed, particularly in the target organ such as the human lung.
E-cigs delivering nicotine by heating liquids contain flavors, propylene glycol (PG) and vegetable glycerin (VG). Although PG and VG are “generally regarded as safe” by the Food and Drug Administration (FDA) when used in foods and cosmetic products (https://www.atsdr.cdc.gov/toxprofiles/tp189-c1.pdf), their safety when inhaled as heated e-aerosols is unknown. Concerns revolve around e-aerosol constituents (e.g., volatile organic compounds) and in vivo and in vitro effects on inflammation, innate immune function, oxidative stress, cytotoxicity and genotoxicity (9,10). In humans, urinary and blood carcinogen biomarkers are substantially lower among e-cig users compared to smokers (9,11-13), while sputum and exhaled air studies show increased inflammation with e-cig use (9,14,15). Changes in lung proteomics, proteases, and gene expression have been shown to be associated with e-cig use in subjects undergoing bronchoscopy (16-18). Our research group found that there were changes in lung inflammasomes with e-cig use using the same study set as reported herein (19). The FDA has deemed regulatory authority over e-cigs, but it currently does not regulate the marketing of e-cigs. To address the need for data regarding effects of e-cigs on the lung and to inform policy determination, we conducted a cross-sectional bronchoscopy study of never-smokers, exclusive e-cig users, and cigarette smokers to assess group differences, examining inflammatory infiltrates, cytokines, genome-wide DNA methylation and gene expression in the lung. Given the recent epidemic of acute lung injury and deaths of some cases may be related to solely nicotine-containing e-cigs, this study is of great importance.
METHODS
Participants and Study Design
Healthy adults, age 21-30, willing to undergo bronchoscopy, were recruited from local print and television media (details regarding recruitment including inclusion and exclusion criteria are in the supplementary methods). While subjects were excluded who reported regular marijuana use, urinary carboxy-tetrahydrocannabinol was assessed for later confirmation. The bronchoscopy included a bronchoalveolar lavage (BAL) and bronchial epithelial brushing of grossly normal airway epithelium from the main bronchus. This study was approved by the Ohio State University Institution Review Board (the IRB approval number: 2015C0088) (ClinicalTrials.gov: NCT02596685).
Carboxy-Tetrahydrocannabinol (THC)
Gas Chromatography-Mass Spectrometry (GC-MS) was used to assess the presence of carboxy-Tetrahydrocannabinol by Mayo Clinic Laboratories (https://www.mayocliniclabs.com/test-catalog/Performance/8898) indicating recent marijuana use.
BAL Cell Counts and Inflammatory Cytokines
Automated cell counts from BAL were obtained by the Countess® Automated Cell Counter (Invitrogen, Carlsbad, CA). Differential counting was performed on Diff-Quik stained cytospins under light microscopy by a clinical histopathologist blinded to participant smoking status. BAL fluid samples were analyzed from supernatant using a V-PLEX Plus Proinflam Combo 10 panel that includes tobacco smoking associated proinflammatory cytokines (IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and TNF-α) (Meso Scale Discovery, Rockville, MD).
Whole Transcriptome Array and Genome-wide DNA methylation
Total RNA was extracted from the bronchial brushing using an Allprep DNA/RNA kit (Qiagen) and assayed for gene expression using the GeneChip® Human Transcriptome Array 2.0 (Affymetrix Inc, Santa Clara, CA). The raw data (CEL files) were imported into the Partek Genomics Suite™ 6.6 (St. Louis, MO) for log2 transformation and quantile normalization. Analysis of covariance (ANOVA) was used to remove potential batch effects.
A subset of subjects (32 out of 72) were analyzed for genome-wide methylation from bronchial brushings using the Infinium MethylationEPIC BeadChip (Illumina, San Diego, CA), following DNA extraction (Allprep DNA/RNA kit; Qiagen) and bisulfite treatment (EZ DNA Methylation kit, Zymo Research, Irvine, CA). Files were imported into Partek and normalized by Subset-quantile Within Array Normalization (20). CpGs were classified by genomic location based on the Illumina annotation file. For modeling purposes, M-values were derived from Beta-values by logit-transformation. GRCh37/hg19 (Human Genome version 19) was used as a reference genome. Excluded probes were in the Y chromosome to avoid gender bias, SNP-associated, off-target or had a detection P >0.05 (21,22).
Statistical Analysis
Cells and Cytokines:
Non-parametric Mann-Whitney and Kruskal-Wallis tests were used to assess differences for cell counts among groups because the data could not be transformed to resemble normality. Three subjects with significant red blood cell contamination in their BALs were excluded from cell counts analyses. Cytokines were log10 transformed to follow Gaussian distributions. One-way ANOVA was used to compare the cytokines for the groups. Parametric data were summarized as mean (standard deviation) and non-parametric as median (range). Statistical tests were two-sided. False discovery rate (FDR) (23) adjusted q<0.1 was considered statistically significant.
Differential DNA methylation and gene expression:
ANCOVA adjusted for gender was used to compare the three groups. An FDR q<0.1 (corresponding to raw P<6.13E-05 for DNA methylation and raw P<7.31E-03 for gene expression) was considered statistically significant. To correlate gene expression and DNA methylation, Spearman correlations were calculated for pairs of expression (transcripts) with cis methylation (CpG sites located within 1.5kb upstream or downstream of the corresponding transcripts) in 32 matched samples. For identification of patterns in DNA methylation and gene expression, unsupervised analysis including unsupervised hierarchical clustering (24) and Principal Component Analysis (25) were performed. For heatmaps, the Euclidian distance among groups was calculated by the average linkage.
Ingenuity Pathway Analysis (IPA)
Differentially methylated or expressed genes were classified by IPA (Ingenuity® Systems, www.ingenuity.com). The IPA comparison analysis tool was used to compare two datasets, taking into account the canonical pathway rank according to the calculated p-value across all observations and reporting it hierarchically. The score [score= -log10(p-value)] is a measure of the probability of finding identified genes in a set of a list of biological functions stored in the IPA knowledge base by chance alone.
RESULTS
Characteristics of study subjects
There were 73 participants: 16 current smokers, 15 e-cig users and 42 never-smokers (Table 1). The average age was 26 (range 21-30) and 47% were women. Smokers averaged 16 cigs/day (range 10-20) and had smoked for a mean of 6.6 years (range 0.6-13). All but one e-cig user (a cartridge-type e-cig) vaped flavored tank system e-cigs. All but three e-cig users were former smokers; the others were never-smokers. E-cig users had a mean duration of e-cig use of 2.7 years (range: 0.5-4), and their mean years of smoking, when smoking was 7.5 years (range 1-15). Mean daily use of e-cigs was 163 puffs per day (range 20-600), comparable to other studies (26,27). Demography, smoking/e-cig history, and THC testing results of individual e-cig subjects are provided in Supplementary Table 1.
Table 1.
Characteristics of study participants
| Cross-Sectional study (n=73) | Never-smokers (n=42) | E-cig users (n=15) | Smokers (n=16) | P-value2 |
|---|---|---|---|---|
| Age, years, average (range) | 25 (21-30) | 27 (21-30) | 26 (21-30) | 0.19 |
| Gender | 0.03 | |||
| Females, N (%) | 25 (60%) | 5 (33%) | 4 (25%) | |
| Race | 0.86 | |||
| White, N (%) | 31 (74%) | 12 (80%) | 14 (88%) | |
| Black or African American, N (%) | 3 (7%) | 1 (7%) | 1 (6%) | |
| Asian, N (%) | 7 (17%) | 1 (7%) | 1 (6%) | |
| More than one race, N (%) | 1 (2%) | 1 (7%) | 0 (0%) | |
| Smoking | ||||
| Former, N (%) | 0 (0%) | 12 (80%) | 0 (0%) | <0.0001 |
| Current, N (%) | 0 (0%) | 0 (0%) | 16 (100%) | |
| Never, N (%) | - | 3 (20%) | 0 (0%) | |
| Years of smoking, average (range) | - | 7.5 (1-15)1 | 6.6 (0.6-13) | 0.69 |
| Cigarettes per day, average (range) | - | 12.6 (0.7-20)1 | 16 (10-20) | 0.24 |
| Years since last cigarettes, average (range) | - | 2.1 (0.4-4.1)1 | ||
| Electronic cigarette (e-cig) use | - | |||
| Years of e-cig use, average (range) | - | 2.6 (0.5-4) | ||
| Puffs per day, average (range) | - | 163.3 (20-600) | ||
| E-liquid (ml) per day, average (range) | - | 8.3 (2-20) | ||
| Nicotine (mg/ml), average (range) | - | 10.7 (1.5-36) |
prior smoking e-cig users
Kruskal–Wallis (among three groups) or Mann–Whitney test (between two groups) for continuous variables, Fisher's exact test for categorical variables
Altered BAL inflammatory cells and cytokines in bronchoalveolar lavage fluids
There were statistically significant overall differences among the three groups for inflammatory infiltrates (FDR q<0.1) (Table 2). Compared to never-smokers, smokers had higher total cell counts (raw P=0.004), total cell concentrations (raw P=0.0003), macrophage cell counts (raw P<0.0001) and neutrophil cell counts (raw P=0.01). Lymphocyte cell counts were lower (raw P=0.02). E-cig users’ counts were intermediate between those of smokers and never-smokers except for percent of macrophages and neutrophil counts which were the same for never-smokers and e-cig users. Median cell concentration for the e-cig users was lower than for smokers (306 × 106/L, 434 × 106/L, respectively, raw P=0.05) and higher than the never-smokers (238 × 106/L, raw P=0.22). Macrophage counts for e-cig users were lower than for smokers (raw P=0.02) and higher than for never-smokers (raw P=0.13).
Table 2.
Inflammatory cell counts and cytokines in bronchoalveolar lavage fluids of never-smokers, e-cig users, and smokers
| Never-smoker (n=40) | E-cig user (n=13) | Smoker (n=16) | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | (Range) | Median | (Range) | Median | (Range) | Overall | Never vs. E-Cig | Never vs. Smokers | E-Cig vs. Smokers | |
| BAL Cells* | ||||||||||
| Instilled saline (mL) | 100 | (100-140) | 100 | (60-120) | 100 | (100-140) | 0.6 | 0.66 | 0.45 | 0.35 |
| Recovery (mL) | 57 | (40-71) | 55 | (28-65) | 46 | (31-71) | 0.02* | 0.27 | 0.004* | 0.2 |
| Total cell yield (× 106) | 13 | (8-34) | 15 | (5-28) | 23 | (11-64) | 0.01* | 0.38 | 0.004* | 0.11 |
| Cell concentration (x106/L) | 238 | (129-763) | 306 | (128-818) | 434 | (204-1689) | 0.001* | 0.22 | 0.0003* | 0.05 |
| Macrophages (x106/L) | 201 | (97-694) | 265 | (111-760) | 411 | (161-1588) | 0.0002* | 0.13 | <0.0001* | 0.02 |
| (%) | 89 | (61-100) | 89 | (69-100) | 95 | (79-100) | 0.08 | 0.62 | 0.03* | 0.14 |
| Lymphocytes (x106/L) | 22 | (0-159) | 13 | (0-70) | 10 | (0-59) | 0.07 | 0.54 | 0.02* | 0.16 |
| (%) | 9 | (0-37) | 4 | (0-27) | 1 | (0-19) | 0.003* | 0.31 | 0.0009* | 0.03 |
| Neutrophils (x106/L) | 3 | (0-31) | 3 | (0-30) | 10 | (0-118) | 0.03* | 0.18 | 0.01 | 0.43 |
| (%) | 1 | (0-9) | 2 | (0-8) | 3 | (0-9) | 0.16 | 0.19 | 0.09 | 0.73 |
| Eosinophils (x106/L) | 0 | (0-8) | 0 | (0-17 | 0 | (0-13) | 0.53 | 0.4 | 0.35 | 1 |
| (%) | 0 | (0-2) | 0 | (0-3) | 0 | (0-1) | 0.64 | 0.41 | 0.52 | 0.79 |
| Never-smoker (n=42) | E-cig user (n=15) | Smoker (n=16) | P-value | |||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Overall | Never vs. E-Cig | Never vs. Smokers | E-Cig vs. Smokers | |
| Cytokines (pg/ml) |
||||||||||
| IL-1ß | 0.82 | (0.39) | 1.51 | (1.48) | 6.08 | (5.37) | <0.0001* | 0.005* | <0.0001* | <0.0001* |
| IL-2 | 0.38 | (0.12) | 0.35 | (0.09) | 0.31 | (0.15) | 0.01* | 0.38 | 0.005* | 0.10 |
| IL-4 | 0.04 | (0.009) | 0.04 | (0.008) | 0.04 | (0.01) | 0.56 | 0.28 | 0.62 | 0.66 |
| IL-6 | 0.99 | (0.56) | 1.56 | (1.02) | 4.21 | (4.93) | <0.0001* | 0.02* | <0.0001* | 0.07 |
| IL-8 | 28.37 | (37.2) | 66.09 | (115.71) | 88.52 | (114.52) | 0.008* | 0.10 | 0.001* | 0.30 |
| IL-10 | 0.09 | (0.02) | 0.09 | (0.02) | 0.09 | (0.029) | 0.27 | 0.42 | 0.13 | 0.53 |
| IL-13 | 1.80 | (1.78) | 1.59 | (0.96) | 1.36 | (0.42) | 0.74 | 0.78 | 0.45 | 0.65 |
| IL-12p70 | 0.14 | (0.04) | 0.13 | (0.03) | 0.12 | (0.04) | 0.11 | 0.20 | 0.05 | 0.59 |
| IFN-γ | 0.91 | (0.27) | 0.74 | (0.31) | 0.65 | (0.33) | 0.005* | 0.02* | 0.0008* | 0.54 |
| TNF-α | 0.45 | (0.17) | 0.49 | (0.20) | 0.52 | (0.23) | 0.44 | 0.49 | 0.22 | 0.68 |
Significant P-values at the 0.05 level are bolded. Significant P-values after correction for multiple testing by adjusted-FDR at the 0.1 level were indicated by asterisks.
BAL with red blood cell contamination (2 never-smokers and 2 e-cig users) were removed for inflammatory cell counts which altered total cell counts.
SD Standard Deviation
Median, range, mean, and SD were presented as raw values.
For all but two of the 10 inflammatory cytokines measured, the e-cig users’ values were intermediate between those of the smokers and never-smokers; for the two, the three groups did not differ. For five of the cytokines, the differences reached statistical significance, with overall significant P-values reaching a threshold for FDR q<0.1 (Table 2). There were significant differences (P-values reaching a threshold for FDR q<0.1) between e-cig users and never-smokers for IL-1β, IL-6 and IFN-γ and between e-cig users and smokers for IL-1β. There was considerable overlap in values for individuals in the groups. Time since last cigarette, cigarettes per day when previously smoking, and gender were not significantly correlated with cell counts or cytokines.
Differential gene expression and methylation in bronchial epithelial cells
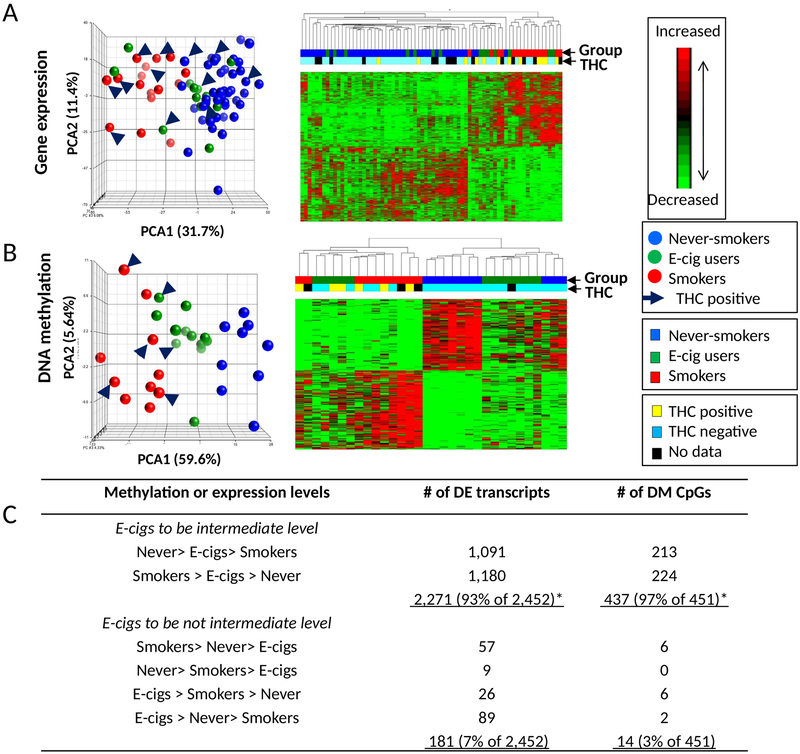
There were 2,452 differentially expressed transcripts (DETs), corresponding to 2,093 unique genes across the groups (Supplementary Table 2). Unsupervised principal component analysis (PCA) and hierarchical clustering are shown in Figure 1. The first principal component accounted for 31.7% of overall variation in gene expression. The expression profiles of never-smokers were closely clustered and separated from smokers, while the e-cig users and never-smokers were more similar to each other. E-cig users’ gene expression were intermediate between smokers and never-smokers for 93% of the 2,452 DETs (33% expected, P chi-square<0.0001) (Figure 1C; Supplementary Table 1). There were 181 transcripts that were related specifically to e-cig use (higher or lower than both smokers and never-smokers); the top 10 transcripts were MUC5B (4 transcripts), MIC5AC, ZNF445, REEP1, ABHK4, LINC00589, and TMPRSS3 (Supplementary Table 2).
Figure 1. Unsupervised clustering analysis of gene expression and DNA methylation from brushings of lung epithelial cells.
(A) 2,821 differentially expressed transcripts among 42 never-smokers, 14 e-cig users, and 16 smokers. (B) 517 differentially methylated CpGs among 10 never-smokers, 12 electronic cigarette users (e-cig users), and 10 smokers. Principal component analysis (PCA)(left) are plotted using the first 3 principal components. Data from never-smokers are shown in blue, data from e-cig users are in green, and smokers are in red. Subjects with carboxy-Tetrahydrocannabinol (THC) positive are indicated by the arrow. Unsupervised hierarchical clustering (right) of log2 transformed expression (A, rows) and M-values (B, rows) are shown. The blocks on the top of the heatmap represent each sample. The characteristics of the subjects including tobacco and THC status were color coded. For log2 transformed expression and M-values, red represents higher expression and higher methylation, green represents lower expression and lower methylation, respectively. (C) Numbers of differentially expressed transcripts or methylated CpGs in comparisons among never-smokers, e-cig users, and smokers. * Significantly higher number of differential signatures than by chance alone
A subset of subjects (10 never-smokers, 12 e-cig users, and 10 smokers) were assessed for differential DNA methylation (DGM). There were 451 differentially methylated CpGs at FDR q<0.1, corresponding to 273 unique genes and including 144 intergenic methylation loci (Supplementary Table 3). PCA and hierarchical clustering are shown in Figure 1B. The first principal component accounted for 59.6% of the overall variation in methylation. There was clustering by group with e-cig users falling between the smokers and never-smokers. Of the 451 differentially methylated CpGs, for 97%, the e-cig users were intermediate between smokers and never-smokers (33% expected, P chi-square<0.0001) (Figure 1C). There were 14 CpGs relating specifically relating to e-cig use (higher or lower than smokers and never-smokers) (lower levels: RHBDL2, TTC16, ZNF815, and 3 intergenic CpGs; higher levels for AMZ1, KRT12, NOX5/MIR548H4 co-localized, NRF1, and 4 intergenic CpGs).
There were no patterns on PCA and heatmap by THC status for all three groups combined, and for smokers alone, indicating that the DETs and DGM were shown to be independent of THC status (Figure 1).
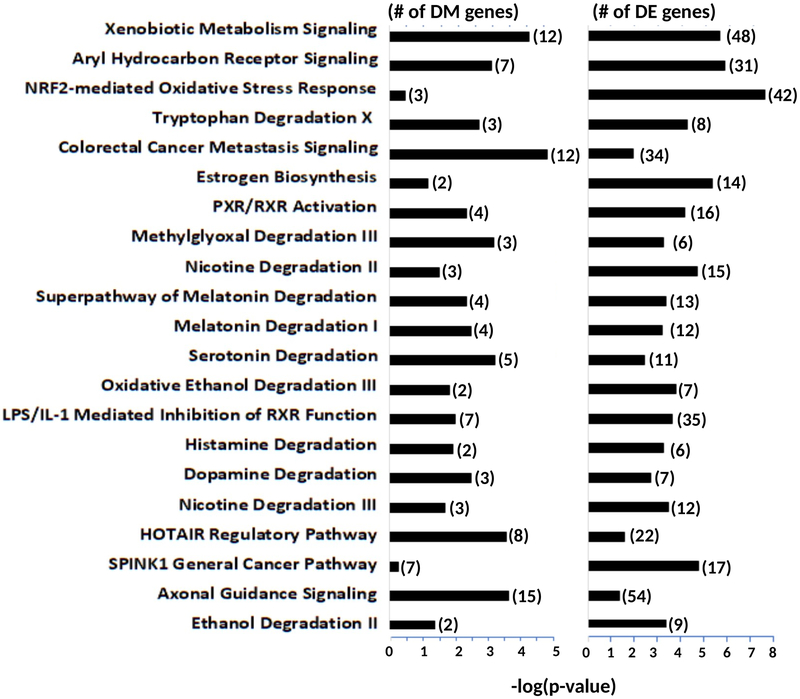
In IPA, the top 20 common canonical pathways for DETs included smoking and/or lung cancer-related pathways such as xenobiotic metabolism signaling, NRF2-mediated oxidative stress response, aryl hydrocarbon receptor signaling, PXR/RXR activation, and LPS/IL-1 mediated inhibition of RXR function (Figure 2). Of the top 20 common canonical pathways for DGM, xenobiotic metabolism signaling and colorectal cancer metastasis signaling were the most common pathways, followed by HOTAIR Regulatory Pathway and Axonal Guidance Signaling.
Figure 2. The common canonical pathways between differentially methylated genes and differentially expressed genes from brushings of lung epithelial cells among never-smokers, electronic cigarette users, and smokers.
The top 20 common canonical pathways based on the score (−log[p-value]) by Ingenuity Pathway Analysis with its annotation are shown with numbers of differentially methylated and expressed genes (next to bars). DM Differentially methylated; DE Differentially expressed
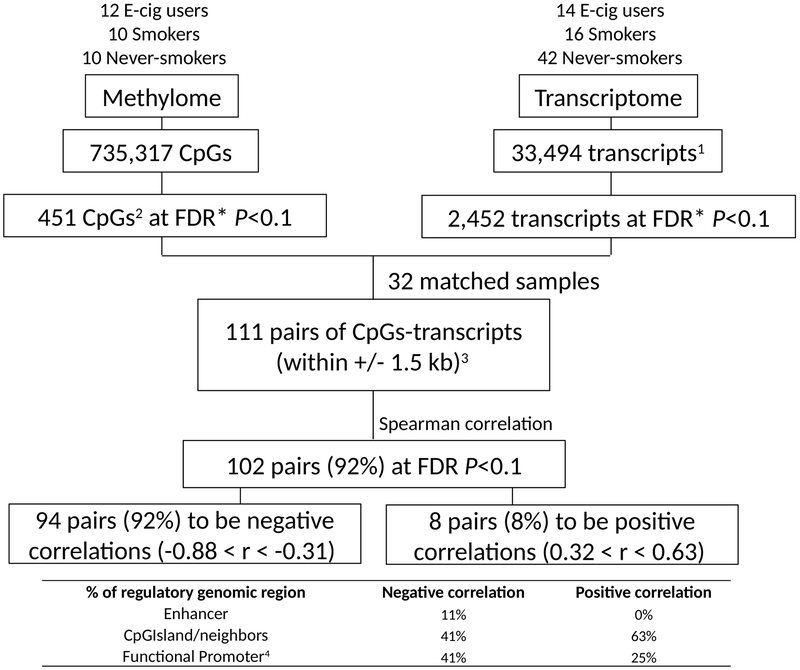
Correlation of differential gene methylation expression
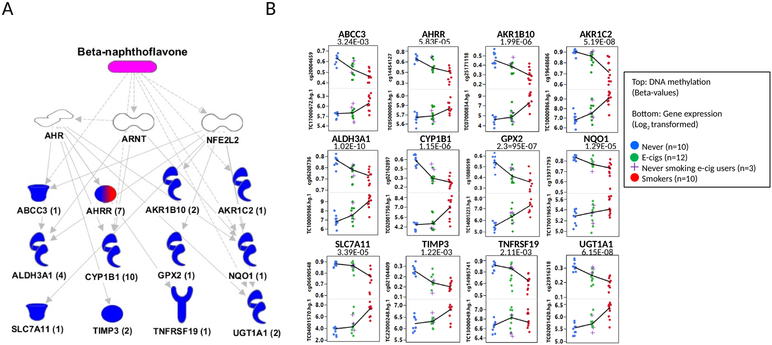
Among the 111 DGM CpG and DETs, within +/− 1.5 kb, that were statistically significant in both assays and present on both platforms, 102 (92%) were significantly correlated at FDR q<0.1, corresponding to 56 unique genes; 94 were negative correlations (down regulation) and 8 were positive (up regulation) (Figure 3 and Supplementary Table 4). Negatively correlated CpGs were more frequently enriched in promoters, while positively correlated CpGs were more frequently found in non-promoter enhancers (Figure 3). IPA analysis of the 56 unique genes showed the greatest enrichment for beta-naphthoflavone (Figure 4A). The mechanistic networks for beta-naphthoflavone that are related to smoking included the aryl hydrocarbon receptor (AHR), the aryl hydrocarbon receptor nuclear translocator (ARNT), and nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) (Figure 4A). The DET genes regulated by beta-naphthoflavone included 12 genes (ABCC3, AHRR, AKR1B10, AKR1C1, ALDH3A1, CYP1B1, GPX2, NQO1, SLC7A11, TIMP3, TNFRSF19, and UGT1A1) (Figure 4A-B), where all except one (TNFRSF19) were hypomethylated, with highest expression in smokers, lowest in never-smokers, and e-cig users intermediate. The most represented disease was cancer, encompassing 51 genes (91%, 51/56), which included 27 (53%, 27/51) involved in respiratory tumors (Supplementary Table 4).
Figure 3. The integration scheme between DNA methylation and gene expression from brushings of lung epithelial cells.
*Adjusted for gender; 1 Annotated transcripts with gene symbols; 2 Including 273 probes associated with genes; 3 32 paired sample analysis; 4 Functional promoters [within 1500 base pairs (bps) of a transcription start site (TSS) (TSS1500); within 200 bps of a TSS (TSS200); 5’ untranslated regions (5’UTR); first exon (1stExon)]
Figure 4. The IPA’s upstream analysis for significantly correlated genes between DNA methylation and gene expression from brushings of lung epithelial cells.
(A) Among significantly correlated 102 pairs identified at FDR q<0.1, 12 unique genes from 33 pairs are shown to be regulated by beta-naphthoflavone (pink) involving mechanistic pathways under AHR, ARNT, and NFE2L2 (white). Thirteen correlated genes were colored. A numbers of pairs for each gene were shown in parentheses. The different shapes represent the functional classes of proteins (http://ingenuity.force.com/ipa/IPATutorials?id=kA250000000TN2wCAG). Blue colored genes was confirmed to be negatively correlated. A mixed colored gene with red and blue was shown to have both negative and positive correlation. (B) The box plots are shown for DNA methylation (top) and gene expression (bottom) for never-smokers (blue), e-cig users (green), and smokers (red). If there is more than a pair for a gene, the most statistically significant pair is shown. Medians are connected by Lines. Raw correlated p-values are indicated on the top of boxes.
DISCUSSION
This study is the first to investigate inflammatory biomarkers, gene methylation and gene expression among smokers, e-cig users, and never-smokers in lung samples, building upon the knowledge of known differences between smokers and never-smokers (9,28,29). E-cigs have the potential to foster smoking cessation (2) and substantially reduce exposure to combustible tobacco toxicants, but the relative effect on the lung is unclear (9,30). In this cross-sectional study, using two methods of lung sampling (BAL for inflammatory cells and cytokines, and lung epithelial cell brushings for gene expression and methylation), we found that almost all of the biomarkers in the e-cig users were intermediate between current and never-smokers, occurring substantially more than by chance alone. These cross-sectional findings suggest that smoking effects on the lung may be at least partially reversible in smokers switching to e-cigs, findings which need to be substantiated in longitudinal studies including randomized trials. There may be some effects specific to e-cigs; we found expression for some genes and DNA methylation of some loci where values for e-cig users were higher or lower than both never-smokers’ and non-smokers’ (e.g., MUC5B and other important lung proteins). The canonical pathways that differed most among the three groups are well-known to be affected by smoking, including xenobiotic metabolism signaling, NRF2-mediated oxidative stress response, AHR signaling, PXR/RXR activation, and LPS/IL-1 mediated inhibition of RXR function (31-33). Importantly, the DGM were correlated with DET, corroborating biological impact of the DGM, especially in smoking-related pathways.
Inflammation is considered to play an important role in lung carcinogenesis and COPD; it is known that inflammatory biomarkers are higher among smokers than never-smokers. (29,34-36) The e-cig users in this study had higher inflammatory infiltrates than never-smokers, and lower than for smokers. Compared to never-smokers, there were significant differences for e-cig users for IL-1β, IL-6 and IFN-γ, associated with lung cancer (34-36); also for IL-2, IL-6, IL-6 and IFN- γ, associated with COPD (37,38). E-cig users were significantly different from smokers for IL-1β.
There were distinct patterns for DNA methylation and gene expression distinguishing smokers, e-cig users and never-smokers, where e-cig users’ levels also were intermediate between smokers and never-smokers (97% and 92%, respectively versus 33% by chance alone). Among the top genes included those known to be affected by smoking such as those involved in AHR and ARNT signaling pathways, and CYPs and other xeno-metabolizing enzymes (i.e., ALDH3A1 and CYP1B1), increasing DNA damage in a dose-dependent manner and associated with lung tumorigenesis in experimental animals and humans (39-44). In this study, compared to smokers, e-cig users had lower expression levels of AHR and xenobiotic metabolizing enzymes consistent with the a priori hypothesis of lower responses with lower carcinogen exposure. Other important cancer and COPD pathways lower in expression in e-cig users compared to smokers included the NRF2 oxidative stress response pathway, involved in the protection of cells from oxidative stress from cigarette smoke (45,46), and a regulator of innate immunity (47). Also found were effects on the PXR and the RXR pathways that affect xenobiotic metabolism through cytochrome P450s (48). The RXR are nuclear receptors for retinoids that affect the regulation of growth and differentiation in normal and tumor cells, including lung cancer and precursors to lung cancer (49).
Staudt, et al (2018) measured gene expression in healthy smokers exposed to a nicotine containing e-cig use for one day and conducted serial bronchoscopies (one week before and on the day of use) (17).They reported differential gene expression for 72 genes after the short term e-cig exposure. Among these genes, 11 genes (15%) were also identified in our study with similar directions in changes of expression (ATAD2, HCAR3, IP6K3, LYPD3, MKI67, MT1X, MT2A, PPP1R16B, RND3, SGK1, and ZBTB16). Separately, Gosh, et al. (2018), conducted a proteomic analysis of smokers, e-cig users, and never-smokers, and reported changes in CYP1B1 and MUC5AC levels specific to e-cig users (16). Our data somewhat differed; these genes and MUCL1 were intermediate for the e-cig users for gene expression, and also we found that MUC5B levels on gene expression were higher than both never-smokers and smokers. Further, their group revealed increased neutrophil elastates and matrix metalloprotease (MMP) levels as well as activities in e-cig users’ lung, resulting in disrupting the protease-antiprotease balance (18). Specifically, they observed higher protein levels for MMP-2 and MMP-9 among e-cig users compared to never-smokers. However, our study for transcription levels found no statistical differences for both genes, but MMP7 was shown to be intermediate between never-smokers and smokers.
There are some limitations that should be considered in the interpretation of these study results. While this study focused on critically important measures of biological effects on inflammation, gene expression and DNA methylation, there may be other biomarkers of exposure and effect which were not included. Additional studies are needed to explore those. This study also has small numbers, and while sufficiently powered to demonstrate the reported differences for this study, it may be that additional effects would be found with a larger study, e.g., gender differences. Further, because use of e-cigs is relatively recent, the study participants had used them for a relatively short period of time. These findings may not extrapolate to longer use; as use continues in the population, it will be important to examine a population with longer exposure. In an observational study such as this one, there was no control for the type of e-cig use. There may be differences based on the characteristics of the e-cig. In addition, the cross-sectional design precludes any temporal assessments of biomarker changes and it is not possible to ascertain causality. Further, study participants were volunteers whose characteristics may not be generalizable to the general population of smokers and e-cig users, especially those older than 21-30 years. Another limitation is that the study results may be affected by unknown confounders, perhaps relating to characteristics of the study participants use of cigarettes (e.g., depth in inhalation, brand of cigarette) or of e-cigs (e.g., characteristics related to the choice to use e-cigs as well as frequency and duration of use). A further limitation of the study is that we did not include former smokers who did not use e-cigs. Thus, we do not know if the observed differences between smokers and e-cig users reflect smoking cessation by any means or changes specific to the e-cigs. Finally, we were limited by the study size; a larger study would allow for greater stability of estimates.
This study has important strengths. It is the first to describe inflammatory cells, cytokines, gene methylation and with gene expression in current e-cig users, directly examining the lung as the target organ. We studied subjects with a narrow age range (21-30) to avoid age-related effects on lung physiology, and in order to represent typical e-cig users. Further, we investigated multiple biomarkers of effect to understand use at the biological and mechanistic level, providing a comprehensive description of e-cig use, and demonstrating consistency among a large group of biomarkers sampled in different ways from the lung.
There has been a recent and significant epidemic for vapers of cannabinoid oils and nicotine-containing e-cig users suffering acute lung injury and deaths (https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html).The reported illnesses and pathology differed, where some investigators believe that the etiology is related to altered lipid homeostasis (7,50) or analogies to smoking-related damage and chemical insult (5).If the acute lung injury is occurring in nicotine e-cig users (there may be false denials of vaping oils or mis-diagnosis), then our data indicate that the latter hypothesis for smoking-related damage is not correct. However, we have not measured markers of lipid homeostasis and other markers of lung integrity such as surfactant, which needs further study.
In summary, we compared lung inflammation, DNA methylation, and gene expression for never-smokers, smokers and e-cig users using bronchoscopy. The results were very consistent among the various biomarker methods and different lung sampling techniques. E-cig users were found to be intermediate between smokers and never-smokers for biomarkers of inflammation and for gene methylation and expression, including known smoking-related pathways. The e-cig levels were more closely related to never-smokers. While these findings are cross-sectional and therefore cannot be extrapolated with regards to temporality or causality, our findings are consistent with the hypothesis that e-cigs may be less harmful than smoking, at least for the smoking-related biomarkers measured herein, and there may be some unique effects of e-cigs. The findings are also consistent with the hypothesis that e-cig use has harmful effects compared to never smoking. The results may also be affected by study subjects who modify their tank-based e-cigs. Further studies, including longitudinal studies and randomized trials, are needed that also include long-term, never-smoking e-cig users and former smokers who quit using methods other than e-cigs. Understanding the biological impact of e-cig use, particularly on a target organ such as the lung, is critically important because of the high prevalence of use of these devices.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by funding from the National Cancer Institute of the National Institutes of Health (NIH) (P30 CA016058), the Food and Drug Administration Center for Tobacco Products (CTP) (P50CA180908 and R21HL147401), the National Center For Advancing Translational Sciences (UL1TR001070) and from Pelotonia Intramural Research Funds and the Prevent Cancer Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA. We thank the Genomics Shared Resource for performing the GeneChip® Human Transcriptome Array 2.0, Center for Clinical and Translational Science for measuring the inflammatory cytokines, and Department of Pathology for BAL differential cell counts at The Ohio State University (Columbus, OH). We also thank the Genomics Shared Resource at Roswell Park Cancer Institute (Buffalo, NY) for conducting the Illumina Infinium MethylationEPIC BeadChip. We acknowledge the support of the Bioinformatics Shared Resource and the Biostatistics Shared Resource at The Ohio State University (Columbus, OH). We also thank the study participants, the staff and nurses of the OSU Clinical Research Center, and Mrs. Sahar Kamel for assisting in recruiting participants.
Abbreviations list
- E-cigs
electronic cigarettes
- PG
propylene glycol
- BAL
bronchoalveolar lavage
- VG
vegetable glycerine
- FDA
Food and Drug Administration
- ANOVA
Analysis of covariance
- PCA
Principal Component Analysis
- IPA
Ingenuity Pathway Analysis
- DETs
differentially expressed transcripts
- DGM
differential gene methylation
- AHR
aryl hydrocarbon receptor
- ARNT
aryl hydrocarbon receptor nuclear translocator
- NFE2L2
erythroid-derived 2-like 2
Footnotes
CONFLICTS OF INTEREST
PGS has served as an expert witness and consultant in tobacco company litigation on behalf of plaintiffs. The other authors declare that they have no potential conflicts of interest.
REFERENCES
- 1.National Academies of Sciences E, and Medicine. Public health consequences of e-cigarettes. Washington, DC: The National Academies Press; 2018. doi 10.17226/24952. [DOI] [PubMed] [Google Scholar]
- 2.Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A Pragmatic Trial of E-Cigarettes, Incentives, and Drugs for Smoking Cessation. The New England journal of medicine 2018;378(24):2302–10 doi 10.1056/NEJMsa1715757. [DOI] [PubMed] [Google Scholar]
- 3.Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. The New England journal of medicine 2019;380(7):629–37 doi 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- 4.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med 2016;4(2):116–28 doi 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butt YM, Smith ML, Tazelaar HD, Vaszar LT, Swanson KL, Cecchini MJ, et al. Pathology of Vaping-Associated Lung Injury. The New England journal of medicine 2019. doi 10.1056/NEJMc1913069. [DOI] [PubMed] [Google Scholar]
- 6.Davidson K BA, Heetderks P, et al. Outbreak of Electronic-Cigarette–Associated Acute Lipoid Pneumonia — North Carolina, July–August 2019. MMWR Morb Mortal Wkly Rep 2019;68:784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddock SD, Cirulis MM, Callahan SJ, Keenan LM, Pirozzi CS, Raman SM, et al. Pulmonary Lipid-Laden Macrophages and Vaping. The New England journal of medicine 2019. doi 10.1056/NEJMc1912038. [DOI] [PubMed] [Google Scholar]
- 8.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde M, et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Preliminary Report. The New England journal of medicine 2019. doi 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 9.Shields PG, Berman M, Brasky TM, Freudenheim JL, Mathe E, McElroy JP, et al. A Review of Pulmonary Toxicity of Electronic Cigarettes in the Context of Smoking: A Focus on Inflammation. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2017;26(8):1175–91 doi 10.1158/1055-9965.EPI-17-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husari A, Shihadeh A, Talih S, Hashem Y, El Sabban M, Zaatari G. Acute Exposure to Electronic and Combustible Cigarette Aerosols: Effects in an Animal Model and in Human Alveolar Cells. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 2016;18(5):613–9 doi 10.1093/ntr/ntv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw Open 2018;1(8):e185937 doi 10.1001/jamanetworkopen.2018.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landmesser A, Scherer M, Pluym N, Sarkar M, Edmiston J, Niessner R, et al. Biomarkers of Exposure Specific to E-vapor Products Based on Stable-Isotope Labeled Ingredients. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 2019;21(3):314–22 doi 10.1093/ntr/nty204. [DOI] [PubMed] [Google Scholar]
- 13.Lorkiewicz P, Riggs DW, Keith RJ, Conklin DJ, Xie Z, Sutaria S, et al. Comparison of Urinary Biomarkers of Exposure in Humans Using Electronic Cigarettes, Combustible Cigarettes, and Smokeless Tobacco. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 2019;21(9):1228–38 doi 10.1093/ntr/nty089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reidel B, Radicioni G, Clapp P, Ford AA, Abdelwahab S, Rebuli ME, et al. E-Cigarette Use Causes a Unique Innate Immune Response in the Lung Involving Increased Neutrophilic Activation and Altered Mucin Secretion. American journal of respiratory and critical care medicine 2017. doi 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polosa R, Cibella F, Caponnetto P, Maglia M, Prosperini U, Russo C, et al. Health impact of E-cigarettes: a prospective 3.5-year study of regular daily users who have never smoked. Scientific reports 2017;7(1):13825 doi 10.1038/s41598-017-14043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh A, Coakley RC, Mascenik T, Rowell TR, Davis ES, Rogers K, et al. Chronic E-cigarette Exposure Alters the Human Bronchial Epithelial Proteome. American journal of respiratory and critical care medicine 2018. doi 10.1164/rccm.201710-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staudt MR, Salit J, Kaner RJ, Hollmann C, Crystal RG. Altered lung biology of healthy never-smokers following acute inhalation of E-cigarettes. Respiratory research 2018;19(1):78 doi 10.1186/s12931-018-0778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR Jr., Alexis NE, et al. Chronic E-Cigarette Use Increases Neutrophil Elastase and Matrix Metalloprotease Levels in the Lung. American journal of respiratory and critical care medicine 2019. doi 10.1164/rccm.201903-0615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai M, Song MA, McAndrew C, Brasky TM, Freudenheim JL, Mathe E, et al. Electronic vs Combustible Cigarette Effects on Inflammasome Component Release into Human Lung. American journal of respiratory and critical care medicine 2019. doi 10.1164/rccm.201808-1467LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maksimovic J, Gordon L, Oshlack A. SWAN: Subset-quantile within array normalization for illumina infinium HumanMethylation450 BeadChips. Genome biology 2012;13(6):R44 doi 10.1186/gb-2012-13-6-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran S, Arribas C, Esteller M. Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics 2016;8(3):389–99 doi 10.2217/epi.15.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCartney DL, Walker RM, Morris SW, McIntosh AM, Porteous DJ, Evans KL. Identification of polymorphic and off-target probe binding sites on the Illumina Infinium MethylationEPIC BeadChip. Genomics data 2016;9:22–4 doi 10.1016/j.gdata.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B 1995;57:289–300. [Google Scholar]
- 24.Quackenbush J Computational analysis of microarray data. Nature reviews Genetics 2001;2(6):418–27 doi 10.1038/35076576. [DOI] [PubMed] [Google Scholar]
- 25.Abdi H, Lynne WJ. Principal component analysis. Wiley Interdisciplinary Review 2010. doi 10.1002/wics.101 [DOI] [Google Scholar]
- 26.Etter JF. Electronic cigarettes: a survey of users. BMC Public Health 2010;10:231 doi 10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One 2015;10(2):e0117222 doi 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhavani S, Tsai CL, Perusich S, Hesselbacher S, Coxson H, Pandit L, et al. Clinical and Immunological Factors in Emphysema Progression. Five-Year Prospective Longitudinal Exacerbation Study of Chronic Obstructive Pulmonary Disease (LES-COPD). American journal of respiratory and critical care medicine 2015;192(10):1171–8 doi 10.1164/rccm.201504-0736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner DR, Fanidi A, Grankvist K, Muller DC, Brennan P, Manjer J, et al. Inflammatory Cytokines and Lung Cancer Risk in 3 Prospective Studies. Am J Epidemiol 2017;185(2):86–95 doi 10.1093/aje/kww159. [DOI] [PubMed] [Google Scholar]
- 30.National Academies of Sciences E, and Medicine. Public health consequences of e-cigarettes. Washington, DC: The National Academies Press; 2018. doi 10.17226/24952. [DOI] [PubMed] [Google Scholar]
- 31.Strulovici-Barel Y, Omberg L, O'Mahony M, Gordon C, Hollmann C, Tilley AE, et al. Threshold of Biologic Responses of the Small Airway Epithelium to Low Levels of Tobacco Smoke. American journal of respiratory and critical care medicine 2010;182(12):1524–32 doi 10.1164/rccm.201002-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitney DH, Elashoff MR, Porta-Smith K, Gower AC, Vachani A, Ferguson JS, et al. Derivation of a bronchial genomic classifier for lung cancer in a prospective study of patients undergoing diagnostic bronchoscopy. BMC Med Genomics 2015;8:18 doi 10.1186/s12920-015-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vachani A, Whitney DH, Parsons EC, Lenburg M, Ferguson JS, Silvestri GA, et al. Clinical Utility of a Bronchial Genomic Classifier in Patients With Suspected Lung Cancer. Chest 2016;150(1):210–8 doi 10.1016/j.chest.2016.02.636. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England journal of medicine 2017;377(12):1119–31 doi 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 35.Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther 2014;141(2):125–39 doi 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 36.DeCotiis C, Hu Y, Greenberg AK, Huie M, Tsay JC, Pass H, et al. Inflammatory cytokines and non-small cell lung cancer in a CT-scan screening cohort: Background review of the literature. Cancer Biomark 2016;16(2):219–33 doi 10.3233/CBM-150559. [DOI] [PubMed] [Google Scholar]
- 37.Bradford E, Jacobson S, Varasteh J, Comellas AP, Woodruff P, O'Neal W, et al. The value of blood cytokines and chemokines in assessing COPD. Respiratory research 2017;18(1):180 doi 10.1186/s12931-017-0662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds CJ, Quigley K, Cheng X, Suresh A, Tahir S, Ahmed-Jushuf F, et al. Lung Defense through IL-8 Carries a Cost of Chronic Lung Remodeling and Impaired Function. American journal of respiratory cell and molecular biology 2018;59(5):557–71 doi 10.1165/rcmb.2018-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang JT, Chang H, Chen PH, Lin SL, Lin P. Requirement of aryl hydrocarbon receptor overexpression for CYP1B1 up-regulation and cell growth in human lung adenocarcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research 2007;13(1):38–45 doi 10.1158/1078-0432.CCR-06-1166. [DOI] [PubMed] [Google Scholar]
- 40.Helmig S, Seelinger JU, Philipp-Gehlhaar M, Dohrel J, Schneider J. Cyp1B1 mRNA expression in correlation to cotinine levels with respect to the Cyp1B1 L432V gene polymorphism. Eur J Epidemiol 2010;25(12):867–73 doi 10.1007/s10654-010-9505-x. [DOI] [PubMed] [Google Scholar]
- 41.Shiizaki K, Kawanishi M, Yagi T. Modulation of benzo[a]pyrene-DNA adduct formation by CYP1 inducer and inhibitor. Genes Environ 2017;39:14 doi 10.1186/s41021-017-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y, Li MD. Establishment of a Strong Link Between Smoking and Cancer Pathogenesis through DNA Methylation Analysis. Scientific reports 2017;7(1):1811 doi 10.1038/s41598-017-01856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao X, Jia M, Zhang Y, Breitling LP, Brenner H. DNA methylation changes of whole blood cells in response to active smoking exposure in adults: a systematic review of DNA methylation studies. Clin Epigenetics 2015;7:113 doi 10.1186/s13148-015-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsay JJ, Tchou-Wong KM, Greenberg AK, Pass H, Rom WN. Aryl hydrocarbon receptor and lung cancer. Anticancer Res 2013;33(4):1247–56. [PMC free article] [PubMed] [Google Scholar]
- 45.Kitamura H, Motohashi H. NRF2 addiction in cancer cells. Cancer Sci 2018;109(4):900–11 doi 10.1111/cas.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho HY, Kleeberger SR. Association of Nrf2 with airway pathogenesis: lessons learned from genetic mouse models. Arch Toxicol 2015;89(11):1931–57 doi 10.1007/s00204-015-1557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Battino M, Giampieri F, Pistollato F, Sureda A, de Oliveira MR, Pittala V, et al. Nrf2 as regulator of innate immunity: A molecular Swiss army knife! Biotechnol Adv 2018;36(2):358–70 doi 10.1016/j.biotechadv.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Smutny T, Mani S, Pavek P. Post-translational and post-transcriptional modifications of pregnane X receptor (PXR) in regulation of the cytochrome P450 superfamily. Curr Drug Metab 2013;14(10):1059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SM, Lee JY, Choi JE, Lee SY, Park JY, Kim DS. Epigenetic inactivation of retinoid X receptor genes in non-small cell lung cancer and the relationship with clinicopathologic features. Cancer Genet Cytogenet 2010;197(1):39–45 doi 10.1016/j.cancergencyto.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Madison MC, Landers CT, Gu BH, Chang CY, Tung HY, You R, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest 2019;129(10):4290–304 doi 10.1172/jci128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.