Abstract
Purpose
Colorectal cancer survivorship has improved significantly over the last 20 years; however, few studies have evaluated depression among older colorectal cancer survivors, especially using a population-based sample. The aim of this study was to identify correlates for positive depression screen among colorectal cancer survivors who underwent potentially curative surgery.
Methods
Using the 1998–2007 Surveillance, Epidemiology, and End-Result registry and the Medicare Health Outcome Survey linked dataset, we identified patients over 65 with pathology confirmed and resected colorectal cancer enrolled in Medicare. Using univariate and multiple variable analyses, we identified characteristics of patients with and without positive depression screen.
Results
Resected colorectal cancer patients (1785) (median age 77, 50.8 % female) were identified in the dataset with 278 (15.6 %) screening positive for symptoms of depression. Median time from diagnosis to survey was 62 months. On univariate analysis, larger tumor size, advanced cancer stage, and extent of resection were not correlates of depressive symptoms (all p > 0.05). After adjusting for confounders, income less than US$30,000 per year (OR 1.50, 1.02–2.22, 95 % CI, p = 0.042), non-white race (OR 1.51, 1.05–2.17, 95 % CI, p = 0.027), two or more comorbidities (OR 1.78, 1.25–2.52, 95 % CI, p = 0.001), and impairment in activities of daily living (OR 5.28, 3.67–7.60, 95 % CI, p < 0.001) were identified as independent correlates of depressive symptoms in colorectal cancer survivors.
Conclusions
In the current study, socioeconomic status and features of physical health rather than tumor characteristics were associated with symptoms of depression among long-term colorectal cancer survivors.
Keywords: Colorectal cancer, Elderly, Quality of life, Cancer survivor, Depression, Surgery
Introduction
Colorectal cancer (CRC) is the fourth most prevalent cancer and the second most common cause of all cancer deaths in the USA [1]. Advancements in screening, surgical technique, and multimodal therapy have improved the 5-year survival rate for colon and rectal cancer over the past 20 years [2–5]. With these improvements in survivorship of CRC patients and an aging population, the focus has shifted to improving the physical and mental health of cancer survivors in addition to decreasing disease-related morbidity and mortality. In the elderly population, depression can go unrecognized and untreated due to comorbid conditions, cognitive impairment, normal physiologic changes of aging, and difficulty in differentiating somatic complaints (body aches and malaise) and affective complaints (sadness, guilt) [6, 7]. Unfortunately, few studies have investigated factors that influence the mental health of colorectal cancer survivors [8, 9].
Previous studies indicate that 13.7 % of cancer patients have major depressive disorder [10]. Depression is correlated with worse HRQOL among cancer and non-cancer patients, [11, 12] and previous studies of cancer survivors indicate that depression is a major determinant of worse HRQOL [13]. Importantly, we have limited understanding of depression among elderly cancer survivors. To date, few studies have investigated depression among patients with CRC [14]. We have limited understanding of who is at higher risk for positive depression screen among CRC survivors. As reported in prior population-based studies, depression may have a higher prevalence among patients with lower socioeconomic status [15]. With improvements in survivorship, understanding the factors associated with depression will be an important aspect of caring for CRC patients throughout their cancer care continuum.
The Surveillance, Epidemiology, and End Results-Medicare Health Outcomes Survey (SEER-MHOS) linked dataset provides a unique opportunity to investigate symptoms of depression among cancer survivors. This dataset provides population-level insight into the lives of cancer survivors with a particular focus on the elderly patient who struggles with multiple confounding issues including comorbidities, social isolation, and impairments in activities of daily living. In addition, the dataset allows for robust analysis of sociodemographic and tumor-specific characteristics. Using the SEER-MHOS dataset, the current study characterizes the prevalence of depressive symptoms among a population-based sample of older (over 65) colorectal cancer survivors who have undergone resection. The secondary aim of the study was to identify correlates of depressive symptoms among CRC survivors. We hypothesized that a lower socioeconomic status and poor health status, as determined by comorbidities and activities of daily living, are associated with increased risk of depression in colorectal cancer survivors after surgery.
Methods
Data source
We performed a retrospective cohort study using the SEER-MHOS linked dataset from 1998 to 2007, linked dataset released by the National Cancer Institute [16]. The Wake Forest Baptist Health institutional review board approved this study. This report is in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [17].
The SEER-MHOS linked dataset is a unique and robust dataset that provides population-based insight into the HRQOL of older US adults with cancer [16]. Both the SEER registry and the MHOS have a long history of excellent data integrity [16, 18–22]. Since 1973, the National Institutes of Health has funded the SEER registry. SEER is the major source for cancer statistics in the USA and represents 28 % of the US population [23]. The SEER dataset contains detailed records on cancer diagnosis, number of prior cancers, extent of disease, lymph node status, tumor size, grade, site-specific surgery, radiation therapy, and survivorship. MHOS is the first patient-reported outcomes measure used in Medicare managed care [24]. Since 1998, MHOS has been administered annually (with a two year follow-up survey) targeting a random sample of Medicare Advantage enrollees (over 150,000 per year). MHOS achieves a response rate around 70 % per survey cohort [25]. Data generated from MHOS are used by the Centers for Medicare and Medicaid Services (CMS) to guide quality improvement activities [24]. The MHOS dataset contains patient demographics, socioeconomic data, and self-reported chronic health conditions. Quality of life measures include the Short Form-36 (SF-36) from 1998 to 2005 then the Veterans Affairs-12 (VR-12) from 2006 to 2007. Physical and mental component scores are then derived from these measures [26]. SEER-MHOS is publically available.
Inclusion criteria
Using the SEER-MHOS linked dataset from 1998 to 2007, we identified all patients over 65 years old with histologically confirmed colon or rectal adenocarcinoma who completed a MHOS survey after diagnosis. All study participants had undergone surgical resection.
Exclusion criteria
Patients with the following were excluded: patients with prior cancer diagnosis, unable to determine response to survey depression questions, missing follow-up or incomplete dates, disabled or institutionalized patients, or death within 6 months of completing survey. Figure 1 details the study cohort selection. Since the MHOS captures responses from Medicare Advantage patients only, we excluded patients under 65 and those with disability given unknown factors that would make them eligible for Medicare. In addition, we excluded patients who died within 6 months of their diagnosis to ensure a study cohort of cancer survivors.
Fig. 1.
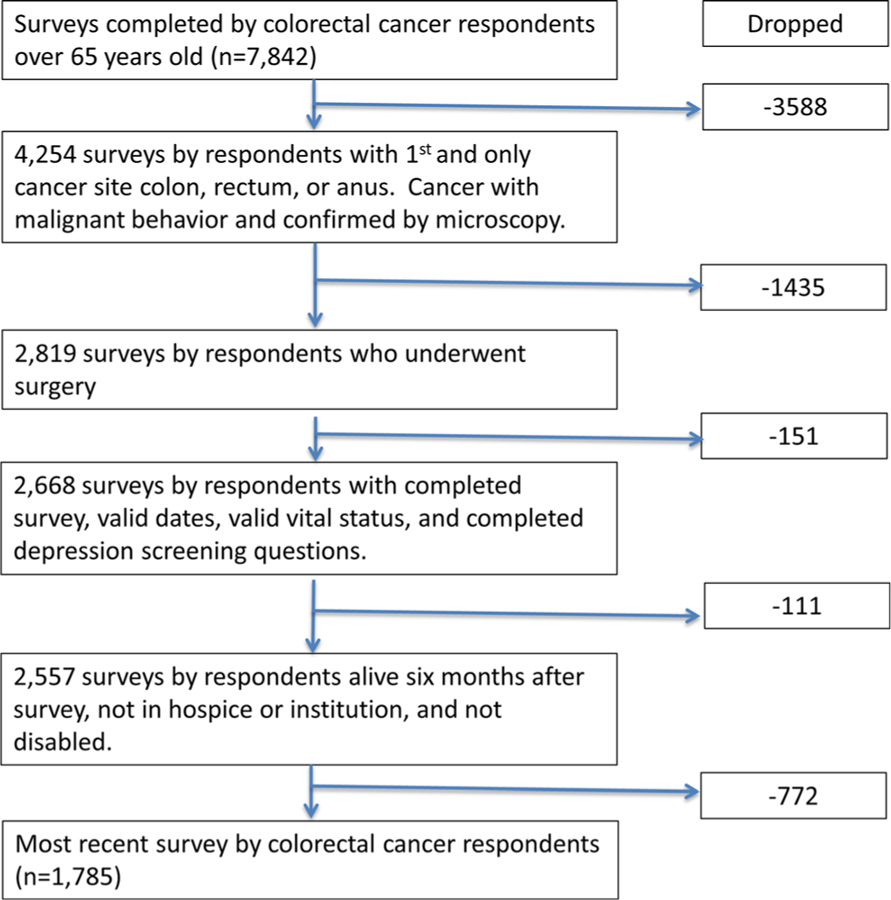
Sample selection of colorectal cancer patients who underwent resection
Study variables and definitions
Patient demographics, socioeconomic data, tumor characteristics, and HRQOL data were collected from SEER-MHOS. Demographic and socioeconomic data included age, race, sex, education, income, marriage status, and homeownership. Tumor and treatment characteristics included tumor size, stage, tumor location, type of surgery, and radiation therapy. Number of comorbidities was based on totaling responses to nine self-reported comorbidity questions in the MHOS (hypertension, coronary artery disease, congestive heart failure, myocardial infarction, other heart condition, stroke, chronic obstructive pulmonary disease, diabetes, and gastrointestinal disorder). Activities of daily living were derived from the MHOS. As in prior studies, positive depression screen was defined as an affirmative answer to one of the three questions of diagnostic interview schedule questions and a VR-12 mental component score less than 42 [27, 28].
Statistical analysis
Demographic and tumor characteristics were summarized for both the entire cohort and by positive depression screen. Differences in means and frequencies were assessed by ANOVA or chi-squared tests as appropriate. Univariate logistic regression was used to predict positive depression screen, and variables with p values less than 0.10 were retained in a multiple variable model. Covariates for the multiple variable model included gender, race, education, income, home ownership, marital status, cancer site, more than two comorbidities, impairment in more than two activities of daily living, cancer stage, type of surgery, radiation therapy, age at survey completion, tumor size, and number of months from CRC diagnosis to survey. All analysis was performed in SAS version 9.4 (Cary, NC) and a 0.05 significance level was used throughout the analysis. All counts less than 11 were reported as Bless than 11^ to protect respondent identity.
Results
Patient characteristics
There were 1785 patients with histologically confirmed and resected colorectal cancer (73.3 % colon and 26.7 % rectal cancer) identified. Patient characteristics are outlined in Table 1. The majority of patients were white (74.9 %, n = 1337). Few patients had metastatic disease at time of diagnosis (4.6 %, n = 74). The mean age was 78 years old (SD ±7 years). Mean time from diagnosis to survey was 62.3 months (SD ±50.0 months) with 95.1 % (n = 1697) of patients alive 12 months after completion of survey. Among colon cancer patients, type of resection was as follows: partial colectomy (41.4 %, n = 542), hemicolectomy (56 %, n = 733), and total colectomy (2.6 %, n = 34). Among rectal cancer patients, type of resection was as follows: segmental (74.0 %, n = 352), abdominoperineal resection (21.4 %, n = 102), pelvic exenteration (less than 11), and pull-through with sphincter preservation (4.2 %, n = 20).
Table 1.
Sociodemographic and cancer characteristics of colorectal cancer survivors, age 65 years and older (N = 1785)
| Variable | All patients n = 1785 |
|---|---|
| Age, years, mean (SD) | 78 (7) |
| Race, no. (%) | |
| White | 1337 (74.9) |
| Asian or Pacific Islander | 158 (8.9) |
| Black or African American | 122 (6.8) |
| Hispanic | 124 (7.0) |
| American Indian or Alaskan Native | 11 (0.6) |
| Another race or multirace | 33 (1.9) |
| Type of cancer, no. (%) | |
| Colon | 1309 (73.3) |
| Rectum | 476 (26.7) |
| SEER cancer stage, no. (%), missing =177 | |
| Localized | 820 (51.0) |
| Regional | 703 (43.7) |
| Distant | 74 (4.6) |
| Unstaged | 11 (0.7) |
| AJCC 7th Edition overall stage, no. (%), missing =375 | |
| 0 | 37 (2.6) |
| I | 357 (25.3) |
| IIa | 259 (18.4) |
| IIb | 49 (3.5) |
| IIc | 295 (20.9) |
| IIIa | 39 (2.8) |
| IIIb | 133 (9.4) |
| IIIc | 184 (13.1) |
| IV | 57 (4.0) |
| Tumor Size, mm, mean (SD), mean (SD), missing = 435 | 43.2 (20.8) |
| Number of months from diagnosis to survey, months, mean (SD) | 62.3 (50.0) |
| Alive 12 months after survey, no. (%) | 1697 (95.1) |
| Positive depression screen, no. (%) | 278 (15.6) |
Prevalence and correlates of depression
Two hundred seventy-eight (15.6 %) patients screened positive for depression. The prevalence of positive depression screen decreased over the study period from a high of 22.3 % in cohort 3 (2000) to a low of 8.6 % in cohort 7 (2004) (p = 0.04). For CRC survivors surveyed within 6 months of diagnosis, positive depression screen was 22.9 % (33 of 144); between 7 and 24 months it was 15.1 % (52 of 345); and in more than 24 months, it was 14.9 % (191 of 1276). Increasing time from diagnosis was associated with a decrease in the prevalence of positive depression screen (p = 0.04). Patient and tumor characteristics were compared between patients with and without positive depression screen (Table 2). Positive depression screen was not associated with tumor characteristics, specifically tumor type, tumor size, and higher overall cancer stage (all p > 0.05). Positive depression screen was not associated with extent of resection in either colon (p = 0.690) or rectal cancer patients (p = 0.460)
Table 2.
Comparison of resected colorectal cancer survivors with and without positive depression screen, age 65 years and older (N = 1785)
| Variable | No depression n = 1507 |
Depression n = 278 |
p value |
|---|---|---|---|
| Age, years, mean (SD) | 77.5 (6.7) | 78.0 (7.2) | 0.237 |
| Race, white, no. (%), missing = 0 | 1150 (76.3) | 187 (67.3) | 0.001 |
| Sex, female, no. (%), missing = 0 | 736 (48.8) | 171 (61.5) | <0.001 |
| High school or higher education, no. (%), missing = 43 | 1028 (69.9) | 167 (61.4) | 0.005 |
| Income less than $30,000, n (%), missing = 392 | 711 (60.6) | 169 (77.2) | <0.001 |
| Homeowner, n (%), missing = 140 | 1163 (83.3) | 186 (74.7) | 0.001 |
| Married, n (%), missing = 56 | 824 (56.6) | 117 (43.0) | <0.001 |
| 2 or more comorbiditiesa, n (%) | 343 (22.8) | 111 (39.9) | <0.001 |
| Impairment in two or more ADLs, n (%) | 182 (12.1) | 127 (46.7) | <0.001 |
| Type of Cancer, Colon, n (%) | 1099 (72.9) | 210 (75.5) | 0.370 |
| Radiation therapy, n (%), missing = 21 | 154 (10.4) | 20 (7.2) | 0.108 |
| SEER cancer stage, n (%), missing = 177 | 0.619 | ||
| Localized | 696 (51.1) | 124 (50.4) | |
| Regional | 597 (43.8) | 106 (43.1) | |
| Distant | 59 (4.3) | 15 (6.1) | |
| Unstaged | <11 | <11 | |
| Tumor size, mm, mean (SD), missing = 435 | 43.1 (20.8) | 43.7 (20.7) | 0.698 |
ADLs activities of daily living
Comorbidities are defined by nine independent survey questions in MHOS
Univariate analysis
Univariate analysis was performed to identify correlates of positive depression screen in resected CRC survivors (Table 3). Patients reporting male sex, higher education, home ownership, and were married were less likely to be associated with positive depression screen (all p < 0.05). Non-white race (OR 1.57, 1.19–2.09 95 % CI, p < 0.002) and income less than US$30,000 (OR 2.20, 1.57–3.08 95 % CI, p < 0.001) were associated with positive depression screen. Patients who reported multiple comorbidities were more likely to have a positive depression screen (OR 2.26, 1.72–2.95 95 % CI, p < 0.001). Similarly, impairment in activities of daily living (ADLs) was significantly associated with depressive symptoms (OR 6.12, 4.62–8.12 95 % CI, p < 0.001).
Table 3.
Univariate analysis for predictors of positive depression screen in resected colorectal cancer survivors, age 65 years and older
| Variable | Odds ratio | Lower 95 %CI | Upper 95 % CI | P value |
|---|---|---|---|---|
| Age, per 10 years | 1.12 | 0.93 | 1.35 | 0.237 |
| Sex, male | 0.60 | 0.46 | 0.78 | <0.001 |
| Race, non-white | 1.57 | 1.19 | 2.09 | 0.002 |
| High School or higher education | 0.68 | 0.52 | 0.89 | 0.006 |
| Income less than $30,000 | 2.20 | 1.57 | 3.08 | <0.001 |
| Homeowner | 0.59 | 0.43 | 0.81 | 0.001 |
| Married | 0.58 | 0.45 | 0.75 | <0.001 |
| 2 or more comorbidities * | 2.26 | 1.72 | 2.95 | <0.001 |
| Impairment in two or more ADLs** | 6.12 | 4.62 | 8.12 | <0.001 |
| SEER cancer state | ||||
| Localized | 0.70 | 0.39 | 1.27 | 0.244 |
| Regional | 0.70 | 0.38 | 1.28 | 0.243 |
| Unstaged | 0.39 | 0.05 | 3.32 | 0.391 |
| Distant | Reference | |||
| Tumor Size, mm | 1.00 | 0.99 | 1.01 | 0.910 |
| Radiation therapy | 0.67 | 0.42 | 1.094 | 0.110 |
| Time from diagnosis to survey, per month | 1.00 | 1.00 | 1.00 | 0.910 |
ADLs activities of daily living
Comorbidities are defined by nine independent survey questions in MHOS
Multiple variable analysis
Multiple variable analyses were then performed to identify independent predictors of positive depression screen among resected CRC survivors (Table 4). Patients with multiple comorbidities (OR 1.78, 1.25–2.52 95 % CI, p = 0.001) and impairment in ADLs (OR 5.28, 3.67–7.60 95 % CI, p < 0.001) were independently associated with positive depression screen. Socioeconomic factors independently associated with positive depression screen were non-white race (OR 1.51, 1.05–2.17 95 % CI, p = 0.03) and income less than US$30, 000 per year (OR 1.50, 1.02–2.22 95 % CI, p = 0.04).
Table 4.
Multiple variable analysis for predictors of positive depression screen in resected colorectal cancer survivors, age 65 years and older
| Variable | Odds ratio | Lower 95 %CI | Upper 95 % CI | p value |
|---|---|---|---|---|
| Gender, male | 0.59 | 0.41 | 0.85 | 0.004 |
| Race, non-white | 1.51 | 1.05 | 2.17 | 0.027 |
| High School or higher education | 0.97 | 0.68 | 1.38 | 0.850 |
| Income less than $30,000 | 1.50 | 1.02 | 2.22 | 0.042 |
| Married | 0.80 | 0.55 | 1.16 | 0.197 |
| Homeowner | 0.74 | 0.50 | 1.11 | 0.143 |
| Two or more comorbiditiesa | 1.78 | 1.25 | 2.52 | 0.001 |
| Impairment in two or more ADLs | 5.28 | 3.67 | 7.60 | <0.001 |
| Time from diagnosis to survey | 1.00 | 1.00 | 1.00 | 0.991 |
| Age, per 10 years | 0.82 | 0.63 | 1.06 | 0.123 |
ADLs activities of daily living
Comorbidities are defined by nine independent survey questions in MHOS
Discussion
This is the first population-based cohort study to investigate the association between socioeconomic factors and mental health among elderly CRC survivors who underwent resection. We determined that 15 % of colorectal cancer survivors who underwent resection screened positive for depression. Previous reports indicate that the prevalence of depression in cancer survivors varies by type of cancer and time since diagnosis [29, 30]. The prevalence of major depression can range from 0 to 38 % and depression spectrum syndromes can range from 0 to 58 % [30]. Prior single institution studies show that the prevalence of depression among colon cancer survivors ranges from 13 to 25 % [30]. The current study demonstrated a significant difference in the prevalence of depression from early to late after diagnosis. This is consistent with prior reports that report that the prevalence of depression among breast and gynecologic cancer survivors decreases over time after diagnosis [29].
The current study reports on the prevalence of positive depression screening among older colorectal cancer survivors. The true prevalence of major depressive disorder is not available in this study cohort. Diagnosis or misdiagnosis of depression among elderly patients is challenging and dependent on the specific assessment or instrument used for the study. In addition, comorbidities, such as dementia, or medication side effects further complicate detecting depression among older age patients [31]. The challenges providers face identifying patients with distress and depression are well recognized. New requirements by the American College of Surgeons Commission on Cancer focus on these challenges and require institutions to have psychosocial services for patients and a process to integrate and monitor on-site psychosocial distress screening [32].
In the current study, the strongest predictors of positive depression screen were not tumor-specific factors but higher number of comorbidities and impairment in activities of daily living. Previous studies investigating the quality of life for cancer patients have indicated that the number of comorbidities in cancer patients has a negative impact on physical and mental health for an individual [33–35]. In a study of 2552 breast cancer survivors, Schoormans et al. reported that comorbidities, specifically cardiovascular disease, negatively impacted HRQOL [35]. These data would indicate that the cancer alone does not completely account for the poor mental health of CRC survivors. Disability resulting from comorbidities and advanced age contribute to increased prevalence of depression. This is likely secondary to burden of the disease, decreased mobility, limited social interaction, challenges of managing the disease, multiple medications, and complications of treatment interventions. Prevalence of positive depression screen did decrease with time from diagnosis. This may represent adaptation and adjustment to cancer diagnosis or reflect treatment of depression.
In the current study, lower income was an independent predictor of depression among CRC survivors. Prior studies have found a similar association between income and quality of life in cancer survivors [36, 37]. Ell et al. reported that the economic stress of poverty leads to difficulties with coverage of medications and services, thus impacting one’s health-related quality of life [37]. Krupski et al. studied prostate cancer survivors and noted that income was a predictor of quality of life [36]. Although the current study identified an association between income and depression in CRC survivors, we were not able to adjust for potential financial hardship secondary to the CRC diagnosis.
Burden of disease, specifically tumor size and overall stage, were not significant predictors of depression in CRC survivors. With a median follow-up of 5 years and the majority still alive 1 year after their survey, the study cohort are truly cancer survivors. Therefore, we would not anticipate larger tumor or advanced stage to have a significant impact on quality of life or mental health. Previous studies have not found a uniform correlation between tumor size and quality of life in cancer patients. Poulakis et al. was able to demonstrate a statistically significant difference in HRQOL for renal cancer patients based on tumor size [38], while another study by Earlam et al. was not able to demonstrate a significant difference in HRQOL based on tumor size in CRC patients [39]. Using positive depression screen as a measure for mental health, our results are consistent with the findings by Earlam et al. [39]. In addition, the extent of resection (segmental colectomy vs. APR) was not associated with depression after surgery for CRC survivors. This is consistent with previous studies that demonstrated minimal difference in quality of life based on the type of operation for rectal cancer patients [40, 41].
With the large cohort and extended follow-up, the current study was able to adjust for multiple confounders, such as comorbidities. However, the current study has several limitations. First, this is a population-based study using SEER registry data [22]. We did not have comprehensive clinical data typically available in prospective trials and thus were unable to adjust for type and duration of chemotherapy, perioperative complications, and presence or absence of colostomy. Similarly, we were not able to adjust for disease recurrence or disease progression. The data obtained from SEER-MHOS were from different registries from various locations in the US; therefore, the data may show variations on treatment options and socioeconomic status based on the healthcare availability and location specific to the institution. Lastly, our data reported positive depression screen for CRC patients postsurgery. We did not know the patient’s depression status before their diagnosis of CRC; therefore, we were not able to characterize the development of depression over time for an individual patient.
Conclusion
In conclusion, 15 % of older colorectal cancer survivors have depressive symptoms but these symptoms will decrease with time after diagnosis. The major drivers of depressive symptoms are socioeconomic factors, specifically non-white race and low income, in addition to comorbidities and impairments in activities of daily living. These findings highlight the importance of psychosocial support for the colorectal cancer survivor early after their diagnosis but also need for long-term care of the whole patient including preexisting conditions and prevention of treatment-associated disability.
Acknowledgments
Funding source None.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.SEER. SEER Stat Fact Sheets: colon and rectum cancer. Natl Cancer Inst 2015. http://perma.cc/HXD2-SDSK.
- 2.Siegel R, Jemal A. Colorectal cancer facts and figures 2011–2013. Am Cancer Soc 2013:1–27. [Google Scholar]
- 3.Levin B, DA L, McFarland B, RA S, D B, KS A, et al. (2008) Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US multi-society task force on colorectal cancer, and the American College of Radiology. CA Cancer J Clin 58:130–160. doi: 10.3322/CA.2007.0018 [DOI] [PubMed] [Google Scholar]
- 4.Cukier M, Smith AJ, Milot L, Chu W, Chung H, Fenech D, et al. (2012) Neoadjuvant chemoradiotherapy and multivisceral resection for primary locally advanced adherent colon cancer: a single institution experience. Eur J Surg Oncol 38:677–682. doi: 10.1016/j.ejso.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Taylor WE, Donohue JH, Gunderson LL, Nelson H, Nagorney DM, Devine RM, et al. (2002) The mayo clinic experience with multimodality treatment of locally advanced or recurrent colon cancer. Ann Surg Oncol 9:177–185. doi: 10.1007/BF02557371 [DOI] [PubMed] [Google Scholar]
- 6.Weinberger MI, Bruce ML, Roth AJ, Breitbart W, Nelson CJ (2011) Depression and barriers to mental health care in older cancer patients. Int J Geriatr Psychiatry 26:21–26. doi: 10.1002/gps.2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parpa E, Tsilika E, Gennimata V, Mystakidou K (2015) Elderly cancer patients’ psychopathology: a systematic review: aging and mental health. Arch Gerontol Geriatr 60:9–15. doi: 10.1016/j.archger.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 8.Pinheiro LC, Wheeler SB, Chen RC, Mayer DK, Lyons JC, Reeve BB. The effects of cancer and racial disparities in health-related quality of life among older Americans: a case-control, population-based study. Cancer 2014:1–9. doi: 10.1002/cncr.29205. [DOI] [PubMed] [Google Scholar]
- 9.Marventano S, Forjaz M, Grosso G, Mistretta A, Giorgianni G, Platania A, et al. (2013) Health related quality of life in colorectal cancer patients: state of the art. BMC Surg 13(Suppl 2):S15. doi: 10.1186/1471-2482-13-S2-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao G, Okoro CA, Li J, White A, Dhingra S, Li C (2014) Current depression among adult cancer survivors: findings from the 2010 behavioral risk factor surveillance system. Cancer Epidemiol 38: 757–764. doi: 10.1016/j.canep.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly EJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Gaynes BN, Warden D, et al. (2010) Health-related quality of life in depression: a STAR*D report. Ann Clin Psychiatry 22:43–55 [PubMed] [Google Scholar]
- 12.Brown LF, Kroenke K, Theobald DE, Wu J, Tu W (2010) The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology 19:734–741. doi: 10.1002/pon.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeve BB, Stover AM, Jensen RE, Chen RC, Taylor KL, Clauser SB, et al. (2012) Impact of diagnosis and treatment of clinically localized prostate cancer on health-related quality of life for older Americans: a population-based study. Cancer 118:5679–5687. doi: 10.1002/cncr.27578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medeiros M, Oshima CTF, Forones NM (2010) Depression and anxiety in colorectal cancer patients. J Gastrointest Cancer 41: 179–184. doi: 10.1007/s12029-010-9132-5 [DOI] [PubMed] [Google Scholar]
- 15.Rojas-García A, Ruiz-Perez I, Rodríguez-Barranco M, Gonçalves Bradley DC, Pastor-Moreno G, Ricci-Cabello I (2015) Healthcare interventions for depression in low socioeconomic status populations: a systematic review and meta-analysis. Clin Psychol Rev 38: 65–78. doi: 10.1016/j.cpr.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 16.Ambs A, Warren JL, Bellizzi KM, Topor M, Haffer SC, Clauser SB (2008) Overview of the SEER–Medicare health outcomes survey linked dataset. Health Care Financ Rev 29:5–21 [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. (2007) Annals of internal medicine academia and clinic the strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting. Ann Intern Med 147:573–578 [DOI] [PubMed] [Google Scholar]
- 18.Surveillance Epidemiology and End Results (SEER) program: an overview. NIH Publ No 05–4772 2005.
- 19.Haffer SC (2003) Using multiple survey vendors to collect health outcomes information: how accurate are the data? Health Qual Life Outcomes 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care 2002;40:IV – 55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 21.SEER Brochure – NIH Publication No. 05–4772. 2005.
- 22.Nathan H, Pawlik TM (2008) Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol 15:415–423. doi: 10.1245/s10434-007-9658-3 [DOI] [PubMed] [Google Scholar]
- 23.Howlader N, Noone A, Krapcho M, Garshell J, Neyman N, Altekruse S, et al. SEER Cancer Statistics Review, 1975–2010. Natl Cancer Inst 2013. http://seer.cancer.gov/csr/1975_2010/.
- 24.Jones N, Jones SL, Miller NA (2004) The Medicare health outcomes survey program: overview, context, and near-term prospects. Health Qual Life Outcomes 2:33. doi: 10.1186/1477-7525-2-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Medicare and Medicaid Services. Medicare Health Outcomes Survey (HOS) response rates 2014. http://perma.cc/RYG8-RTL4 (accessed January 1, 2015).
- 26.National Cancer Institute. Brief description of the SEER-MHOS Level Analysis File 2014. http://perma.cc/V4Z6-CFNM (accessed January 1, 2015).
- 27.Rost K, Burnam MA, Smith GR (1993) Development of screeners for depressive disorders and substance disorder history. Med Care 31:189–200 [DOI] [PubMed] [Google Scholar]
- 28.Group Health Services Advisory. Report on the health status of managed care smokers and nonsmokers. 2007.
- 29.Stafford L, Judd F, Gibson P, Komiti A, Mann GB, Quinn M (2015) Anxiety and depression symptoms in the 2 years following diagnosis of breast or gynaecologic cancer: prevalence, course and determinants of outcome. Support Care Cancer. doi: 10.1007/s00520-014-2571-y [DOI] [PubMed] [Google Scholar]
- 30.Massie MJ (2004) Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr 10021:57–71. doi: 10.1093/jncimonographs/lgh014 [DOI] [PubMed] [Google Scholar]
- 31.Damián J, Pastor-Barriuso R, Valderrama-Gama E, de Pedro-Cuesta J. Association of detected depression and undetected depressive symptoms with long-term mortality in a cohort of institutionalised older people. Epidemiol Psychiatr Sci 2016:1–10. doi: 10.1017/S2045796015001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cancer Program Standards 2012: Ensuring patient-centered care. Comm Cancer 2012. https://www.facs.org/~/media/files/qualityprograms/cancer/coc/programstandards2012.ashx (accessed September 3, 2016).
- 33.Reeve BB, Stover AM, Jensen RE, Chen RC, Taylor KL, Clauser SB, et al. (2012) Impact of diagnosis and treatment of clinically localized prostate cancer on health-related quality of life for older Americans: a population-based study. Cancer 118:5679–5687. doi: 10.1002/cncr.27578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith AW, Reeve BB, Bellizzi KM, Harlan LC, Klabunde CN, Amsellem M, et al. (2008) Cancer, comorbidities, and health-related quality of life of older adults. Health Care Finance Rev 29: 41–56 [PMC free article] [PubMed] [Google Scholar]
- 35.Schoormans D, Czene K, Hall P, Brandberg Y (2015) The impact of co-morbidity on health-related quality of life in breast cancer survivors and controls. Acta Oncol (Madr) 54:727–734. doi: 10.3109/0284186X.2014.998277 [DOI] [PubMed] [Google Scholar]
- 36.Krupski TL, Fink A, Kwan L, Maliski S, Connor SE, Clerkin B, et al. (2005) Health-related quality-of-life in low-income, uninsured men with prostate cancer. J Health Care Poor Underserved 16:375–390. doi: 10.1353/hpu.2005.0037 [DOI] [PubMed] [Google Scholar]
- 37.Ell K, Xie B, Wells A, Nedjat-Haiem F, Lee P-J, Vourlekis B (2008) Economic stress among low-income women with cancer: effects on quality of life. Cancer 112:616–625. doi: 10.1002/cncr.23203 [DOI] [PubMed] [Google Scholar]
- 38.Poulakis V, Witzsch U, de Vries R, Moeckel M, Becht E (2003) Quality of life after surgery for localized renal cell carcinoma: comparison between radical nephrectomy and nephron-sparing surgery. Urology 62:814–820. doi: 10.1016/S0090-4295(03)00687-3 [DOI] [PubMed] [Google Scholar]
- 39.Earlam S, Glover C, Fordy C, Burke D, Allen-Mersh TG (1996) Relation between tumor size, quality of life, and survival in patients with colorectal liver metastases. J Clin Oncol 14:171–175 [DOI] [PubMed] [Google Scholar]
- 40.Vironen JH, Kairaluoma M, Aalto A-M, Kellokumpu IH (2006) Impact of functional results on quality of life after rectal cancer surgery. Dis Colon rectum 49:568–578. doi: 10.1007/s10350-006-0513-6 [DOI] [PubMed] [Google Scholar]
- 41.Andersson J, Angenete E, Gellerstedt M, Angerås U, Jess P, Rosenberg J, et al. (2013) Health-related quality of life after laparoscopic and open surgery for rectal cancer in a randomized trial. Br J Surg 100:941–949. doi: 10.1002/bjs.9144 [DOI] [PMC free article] [PubMed] [Google Scholar]


