Abstract
Background
Iron deficiency and iron deficiency anaemia (IDA) are common in young children. It has been suggested that the lack of iron may have deleterious effects on children's psychomotor development and cognitive function. To evaluate the benefits of iron therapy on psychomotor development and cognitive function in children with IDA, a Cochrane review was carried out in 2001. This is an update of that review.
Objectives
To determine the effects of iron therapy on psychomotor development and cognitive function in iron deficient anaemic children less than three years of age.
Search methods
We searched the following databases in April 2013: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, PsycINFO, LILACS, ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform (ICTRP). We also searched the reference lists of review articles and reports, and ran citation searches in the Science Citation Index for relevant studies identified by the primary search. We also contacted key authors.
Selection criteria
Studies were included if children less than three years of age with evidence of IDA were randomly allocated to iron or iron plus vitamin C versus a placebo or vitamin C alone, and assessment of developmental status or cognitive function was carried out using standardised tests by observers blind to treatment allocation.
Data collection and analysis
Two review authors independently screened titles and abstracts retrieved from the searches and assessed full‐text copies of all potentially relevant studies against the inclusion criteria. The same review authors independently extracted data and assessed the risk of bias of the eligible studies. Data were analysed separately depending on whether assessments were performed within one month of beginning iron therapy or after one month.
Main results
We identified one eligible study in the update search that had not been included in the original review. In total, we included eight trials.
Six trials, including 225 children with IDA, examined the effects of iron therapy on measures of psychomotor development and cognitive function within 30 days of commencement of therapy. We could pool data from five trials. The pooled difference in pre‐ to post‐treatment change in Bayley Scale Psychomotor Development Index (PDI) between iron and placebo groups was ‐1.25 (95% confidence interval (CI) ‐4.56 to 2.06, P value = 0.65; I2 = 33% for heterogeneity, random‐effects meta‐analysis; low quality evidence) and in Bayley Scale Mental Development Index (MDI) was 1.04 (95% CI ‐1.30 to 3.39, P value = 0.79; I2 = 31% for heterogeneity, random‐effects meta‐analysis; low quality evidence).
Two studies, including 160 randomised children with IDA, examined the effects of iron therapy on measures of psychomotor development and cognitive function more than 30 days after commencement of therapy. One of the studies reported the mean number of skills gained after two months of iron therapy using the Denver Developmental Screening Test. The intervention group gained 0.8 (95% CI ‐0.18 to 1.78, P value = 0.11, moderate quality of evidence) more skills on average than the control group. The other study reported that the difference in pre‐ to post‐treatment change in Bayley Scale PDI between iron‐treated and placebo groups after four months was 18.40 (95% CI 10.16 to 26.64, P value < 0.0001; moderate quality evidence) and in Bayley Scale MDI was 18.80 (95% CI 10.17 to 27.43, P value < 0.0001; moderate quality evidence).
Authors' conclusions
There is no convincing evidence that iron treatment of young children with IDA has an effect on psychomotor development or cognitive function within 30 days after commencement of therapy. The effect of longer‐term treatment remains unclear. There is an urgent need for further large randomised controlled trials with long‐term follow‐up.
Keywords: Child, Preschool; Humans; Infant; Infant, Newborn; Anemia, Iron‐Deficiency; Anemia, Iron‐Deficiency/drug therapy; Anemia, Iron‐Deficiency/psychology; Cognition; Cognition/drug effects; Iron Compounds; Iron Compounds/therapeutic use; Psychomotor Performance; Psychomotor Performance/drug effects; Randomized Controlled Trials as Topic
Plain language summary
Iron therapy for improving physical and intellectual development in children under the age of three who are anaemic due to a lack of iron
It has been suggested that a lack of iron in young children negatively affects their physical and intellectual development and therefore those who are anaemic should be treated. To assess the effects of iron therapy on psychomotor development and cognitive function in very young iron deficient anaemic children, we searched eight electronic databases in April 2013 for relevant studies. We looked for studies where children less than three years of age with iron deficiency anaemia were randomly assigned either to receive iron or iron plus vitamin C versus a placebo ('dummy pill') or vitamin C alone, and their developmental status or cognitive function was independently assessed by someone who did not know if they had received any iron. To make sure we had not missed any studies, we also searched reference lists, ran citation searches and contacted key experts in this area.
Two review authors independently read through all the titles and abstracts retrieved from the searches to see if the studies were relevant. Where necessary, we looked at the full‐text version of the paper to check if it matched the inclusion criteria for our review. The same review authors independently extracted data from the studies for analysis and assessed the risk of bias in each of the studies. We analysed the data separately depending on whether assessments were performed within one month of beginning iron therapy or after one month.
We found eight studies to include in the review. Six studies (involving 225 children) looked at the effects of iron therapy within 30 days of starting it and we were able to combine the results of five of them. Iron therapy did not appear to improve scores on either the Bayley Scale Psychomotor Development Index (PDI) or the Bayley Scale Mental Development Index (MDI). Two studies (160 children) considered the effects of iron therapy more than 30 days after starting it. One study found no benefit of iron therapy on the acquistion of skills as measured by the Denver Developmental Screening Test. The other study, however, found that scores on the Bayley scales PDI and MDI were significantly higher for the group receiving iron therapy.
This review concludes that there is no convincing evidence that iron given by mouth or by injection will improve physical or intellectual development in young children who are anaemic because of a lack of iron within one month after start of treatment. It is unclear whether longer‐term treatment is beneficial for physical and intellectual development in these children. Large randomised controlled trials with long‐term follow‐up are needed in the future.
Summary of findings
Summary of findings for the main comparison. Iron therapy compared with placebo for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia.
| Iron therapy compared with placebo for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia | ||||||
|
Patient or population: children less than 3 years of age with iron deficiency anaemia Settings: communities or hospitals Intervention: iron therapy Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Iron therapy | |||||
|
Bayley Scale PDI within 30 days of commencement of therapy |
The mean change in pre‐ to post‐treatment test scores ranged across control groups from 0.2 to 11 | The mean change in pre‐ to post‐treatment test scores in the intervention groups was 1.25 lower (95% CI ‐4.56 to 2.06) | ‐ | 162 (5 studies) |
++OO low1 | ‐ |
|
Bayley Scale MDI within 30 days of commencement of therapy |
The mean change in pre‐ to post ‐treatment test scores ranged across control groups from 4.1 to 13.58 | The mean change in pre‐ to post‐treatment test scores in the intervention groups was 1.04 higher (95% CI ‐1.30 to 3.39) | ‐ | 164 (5 studies) |
++OO low1 | ‐ |
|
Bayley Scale PDI more than 30 days of commencement of therapy |
The mean change in pre‐ to post‐treatment test scores in the control group was 5.1 | The mean change in pre‐ to post‐treatment test scores in the intervention group was 18.4 higher (95% CI 10.16 to 26.64) | ‐ | 47 (1 study) |
+++O2 moderate |
‐ |
|
Bayley Scale MDI more than 30 days of commencement of therapy |
The mean change in pre‐ to post‐treatment test scores in the control group was 0.5 | The mean change in pre‐ to post‐treatment test scores in the intervention group was 18.8 higher (95% CI 10.17 to 27.43) | ‐ | 47 (1 study) |
+++O2 moderate |
‐ |
|
Denver Developmental Screening Test more than 30 days of commencement of therapy |
The mean change in pre‐ to post‐treatment test scores in the control group was 3.2 | The mean change in pre‐ to post‐treatment test scores in the intervention group was 0.8 higher (95% CI ‐0.18 to 1.78) | ‐ | 97 (1 study) |
+++O2 moderate |
‐ |
| CI: confidence interval; MDI: Mental Development Index; PDI: Psychomotor Development Index. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The funnel plot indicated small study effects. Number of children was less than 400.
2 Number of children was less than 400.
Background
Description of the condition
Iron is important in the production of haemoglobin (Hb) and myoglobin and as co‐factor in the metabolism of some neurotransmitters including dopamine. In addition, it has been suggested that iron is required for the myelin production in the developing brain (Larkin 1990). Animal studies, mainly in rats, have suggested that severe iron deficiency can lead to a deficit of brain iron, that iron deficiency may be associated with alterations in motor activity and behaviour and that these deficits may persist (Dallman 1975; Felt 1996). In the clinical literature, children with severe iron deficiency are often described as irritable and apathetic with a poor appetite. Most, but not all, of the large number of observational studies that have examined the association between iron deficiency anaemia (IDA) and psychomotor development have found lower scores on tests of psychomotor development in young children with IDA (Lansdown 1995).
The reported prevalence of IDA in toddlers varies in different populations, depending in part on the case definition used, with estimates ranging from around 10% in Western societies to around 50% in less developed societies (De Maeyer 1985). IDA is generally defined as an Hb level of less than 110 g/L (with a microcytic, hypochromic picture after the exclusion of haemoglobinopathies). Children may also be classified as iron deficient without anaemia on the basis of decreased serum ferritin (< 10 μg/L), raised erythrocyte protoporphyrin (> 2.5 μg/g Hb), a decreased mean corpuscular volume (MCV) (< 72 fL) or some combination of these since earlier stages of iron deficiency may lead to decreased Hb synthesis before the appearance of frank anaemia. The choice of a particular cut‐off point is essentially arbitrary as there is no clear evidence to determine at which level iron deficiency begins to have significant deleterious effects (Logan 1997).
Description of the intervention
Iron therapy can be administered to children less than three years of age with IDA orally (ferrous sulphate, ferrous fumarate, sodium iron ethylenediaminetetra‐acetate and ferrous gluconate), intravenously (iron dextran, iron sucrose and ferric carboxymaltose) or intramuscularly (iron dextran) (Goddard 2011).
How the intervention might work
A number of possible mechanisms have been suggested for a postulated effect of iron deficiency on children's psychomotor development (Lozoff 1986; Roncagliolo 1998; Walter 1994). Lack of iron might interfere with the metabolism of neurotransmitters, which either might affect psychomotor function directly or might affect scores on tests of psychomotor function indirectly through effects on behaviour. Iron is important in the synthesis of Hb and myoglobin, and deficiency might adversely affect oxygen transport or storage, which might have effects on either the central nervous system or motor function. Finally, lack of iron at a critical stage of development of the nervous system might lead to interference with myelination. Iron therapy acts by replenishing the iron stores in the body. Since iron is essential to neurotransmitters, Hb and myelination, iron therapy may improve psychomotor development and cognitive function in iron deficient anaemic children.
The timing of any therapeutic response to iron therapy will depend on what mechanisms underlie the relationship between iron and psychomotor function. Many early studies were designed to look for rapid effects of iron, partly because clinicians treating children and adults with severe IDA often reported dramatic improvements in mood soon after beginning treatment (Lee 1993). Such rapid effects (i.e. within days of beginning treatment) would be consistent with the effects of iron being attributable to direct effects on the central nervous system, perhaps as a result of effects on metabolism of neurotransmitters. However, although children with IDA show raised levels of Hb soon after commencing treatment with iron, the full effect on Hb synthesis develops over weeks (Lee 1993). If an effect of iron on psychomotor function were mediated via effects of iron deficiency on oxygen transport or storage, then it might be expected that the therapeutic effects of iron would take some weeks to manifest in changes in scores on tests of psychomotor function. If this latter mechanism were involved, the inclusion of results of assessments of outcome carried out before sufficient time has elapsed would introduce bias. For this reason, in this review, studies were analysed separately depending on how long after institution of treatment the outcomes were measured (with an arbitrary cut‐off 30 days after beginning of treatment).
Why it is important to do this review
If lack of iron is associated with adverse effects on children's psychomotor development and these effects could be reversed by administration of iron, there would be a strong argument for either population supplementation with iron or screening of young children for IDA. It is widely acknowledged that the age period from birth to three years old is critical for psychomotor development and cognitive development, thus children under the age of three years would be the most sensitive population for this review. Consequently, we considered only children under the age of three years in this review. Safety outcomes are important to a Cochrane review. However, the purpose of this review is to assess the effect of iron therapy specifically on psychomotor development and cognitive development. The assessment of harm of iron therapy is beyond the scope of this review.
This is an update of a Cochrane review originally published in 2001 (Martin 2001). The original review concluded that there is no convincing evidence that iron therapy of iron deficient anaemic children under three years of age improves psychomotor development or cognitive function within 30 days of beginning treatment, and the effect of longer‐term treatment remains unclear.
Objectives
To assess the effect of iron therapy given to children less than three years of age with IDA on psychomotor development and cognitive function.
Methods
Criteria for considering studies for this review
Types of studies
Random allocation of participants to treatment and placebo arms.
Assessment of psychomotor development or cognitive function by assessors blind to treatment allocation.
Types of participants
Children up to three years of age with evidence of IDA based on Hb level, Hb plus ferritin or other author‐defined evidence of IDA.
Types of interventions
Iron (oral, intramuscular or intravenous) versus placebo or iron plus vitamin C versus placebo and vitamin C.
Types of outcome measures
Primary outcomes
Psychomotor development
Cognitive development
We had no secondary outcomes.
We accepted only standardised measures. Safety outcomes measures addressing adverse effects of iron therapy were ineligible. Outcome data were analysed separately depending on whether the testing was performed within 30 days of entry to the study or more than 30 days after entry to the study.
Search methods for identification of studies
We ran the searches for the original review in 2007 (Appendix 1). When we updated the searches in April 2013, we corrected a syntax error in the original MEDLINE and EMBASE searches, and added search terms for intramuscular injections and alternative forms of iron (Appendix 2). We ran the revised searches for all years and deduplicated against the records found in 2007 using EndNote. We applied no language limits.
Electronic searches
We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL), 2013, Issue 3, last searched 23 April 2013.
Ovid MEDLINE, 1948 to April week 2 2013, last searched 22 April 2013.
EMBASE (Ovid), 1980 to 2013 week 16, last searched 22 April 2013.
CINAHL (EBSCOhost), 1937 to current, last searched 23 April 2013.
PsycINFO (Ovid), 1806 to April week 3 2013, last searched 23 April 2013.
PaycINFO (EBSCOhost), 1837 to current, last searched 2 February 2011.
BIOSIS (Web of Knowledge), 1969 to 3 February 2011, last searched 3 February 2011.
LILACS, last searched 23 April 2013.
ClinicalTrials.gov, last searched 23 April 2013.
WHO International Clinical Trials Registry Platform (ICTRP), last searched 23 April 2013.
Searching other resources
We scrutinised the references of identified trials and important review articles for possible trials missed by the electronic searches. In addition, we performed forward citation searches on trials from the primary search within the Science Citation Index (24 April 2013). Finally, we contacted a number of key authors requesting other trials.
Data collection and analysis
Selection of studies
Two review authors (BW and TG) independently read titles and abstracts of studies identified on searches of electronic databases to determine whether they might meet the inclusion criteria, and then assessed full‐text copies of those possibly eligible articles. We resolved differences of opinion about suitability for inclusion by discussion. A third review author (SZ) made provision for arbitration.
Data extraction and management
Data including year of publication, randomisation procedures, allocation concealment, blinding, setting, characteristics of participants and interventions, outcomes as well as main results, were extracted independently by two review authors (BW and TG), using a pilot‐tested data extraction form. We resolved any disagreements by discussion. Where data were not available in the published trial reports, study authors were asked to supply missing information. One review author (BW) entered data for meta‐analysis into Review Manager (RevMan) software (RevMan 2011) and a second review author (TG) checked the entries.
Assessment of risk of bias in included studies
Two review authors (BW and TG) independently assessed the risk of bias in each included study following the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). We resolved any disagreements through consultation with a third review author (SZ). In order for a trial to be included in the review, we required blind assessment of outcome. We assessed risk of bias based on the following six domains with ratings of 'Yes' (low risk of bias); 'No' (high risk of bias) and 'Unclear' (uncertain risk of bias):
Sequence generation
Description: the method used to generate the allocation sequence was described in sufficient detail so as to enable an assessment to be made as to whether it should have produced comparable groups.
Review authors' judgement: was the allocation concealment sequence adequately generated?
Allocation concealment
Description: the method used to conceal allocation sequence was described in sufficient detail to assess whether intervention schedules could have been foreseen in advance of, or during, recruitment.
Review authors' judgement: was allocation adequately concealed?
Blinding of participants and personnel
Description: any measures used to blind participants and personnel were described in sufficient detail so as to assess possible knowledge of which intervention a given participant might have received.
Review authors' judgement: was participants and personnel knowledge of the allocated intervention adequately prevented during the study?
Blinding of outcome assessment
Description: any measures used to blind outcome assessor were described in sufficient detail so as to assess possible knowledge of which intervention a given participant might have received.
Review authors' judgment: was outcome assessor knowledge of the allocated intervention adequately prevented during the study?
Incomplete outcome data
Description: data on attrition were reported as well the numbers involved (compared with total randomised), and reasons for attrition were reported or obtained from investigators.
Review authors' judgement: were incomplete data dealt with adequately by the study authors?
Selective outcome reporting
Description: attempts were made to assess the possibility of selective outcome reporting by investigators, to include comparing published results to outcomes detailed in the study protocol or published methods, considering whether primary outcomes were stated a priori and considering whether or not commonly used outcomes reported in similar studies were reported.
Review authors' judgement: were reports of the study free of suggestion of selective outcome reporting?
Measures of treatment effect
As eligible measures of psychomotor development or cognitive function are continuous variables, we calculated mean difference (MD) with 95% confidence interval (CI). We used change values in pre‐ to post‐test scores on tests of psychomotor development or cognitive function to undertake meta‐analyses.
Unit of analysis issues
The unit of analysis was individual children under the age of three with IDA. We identified no cluster‐randomised trials, cross‐over trials or studies with multiple treatment groups for inclusion in this review. See Table 2 for other methods to be used in future updates of the review.
1. Methods to be used in future updates, where applicable.
|
Unit of analysis issues |
Cluster‐randomised trials Effect estimates and their standard errors from correct analyses of cluster‐randomized trials will be meta‐analysed using the generic inverse‐variance method in RevMan (RevMan 2011). For cluster‐randomised trials analysed by incorrect statistical methods (not taking the clustering into account), we plan to adjust their sample sizes using the methods, described in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial or other sources. If we use ICC from other sources, sensitivity analyses will be conducted to investigate the effect of variation in the ICC. Cross‐over trials For cross‐over trials, we will extract and analyse data from the first period only. Studies with multiple treatment groups For factorial studies, we will include all comparisons that differ only in the presence or absence of iron. For other studies, multiple eligible intervention groups will be combined. |
|
Assessment of reporting biases |
Funnel plots can investigate the relationship between effect size and standard error. Asymmetry may be attributable to publication bias, but might also reflect true heterogeneity. If we find such a relationship between trial size and effect size, we will examine clinical variation (such as intensity of intervention, underlying risk) of the studies. In current version of this review, no such analysis was undertaken due to the small number of included studies. |
|
Data synthesis |
If a high level of heterogeneity is detected (I2 ≥ 75%), we will combine studies by narrative summary rather than in a meta‐analysis, and explore the sources of heterogeneity by conducting predefined subgroup analyses. |
|
Subgroup analysis and investigation of heterogeneity |
If significant heterogeneity is identified within a meta‐analysis and there is a sufficient number of included studies (> 10), 3 possible sources will be investigated separately:
|
Dealing with missing data
We used available‐case analysis. We sought unclear or missing information by contacting the authors of the individual trials. The standard deviations (SD) for change values in pre‐ to post ‐test scores were not reported in most eligible studies; however, it was possible to impute SDs for these differences according to the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where appropriate data were available, we checked the plausibility of these estimates by calculating the implied correlation between pre‐ and post‐treatment test scores. See Table 3 for methods used to impute SDs for change values (where P values were treated as if resulting from paired or unpaired t‐tests as appropriate).
2. Methods used to impute standard deviations for changes values.
| Study | Group | Outcome measure | P value | Imputation | Imputed SDs | Implied correlation | Plausibility of imputation |
| Driva 19851 | Intervention | PDI | > 0.05 | Impossible | 10.94 | 0.69 | Plausible |
| MDI | < 0.01 | Possible | 10.94 | 0.78 | Plausible | ||
| Control | PDI | > 0.05 | Impossible | 10.94 | 0.82 | Plausible | |
| MDI | > 0.05 | Impossible | 10.94 | 0.79 | Plausible | ||
| Honig 19782 | Intervention | PDI | 0.10 | Possible | 21.22 | ‐0.26 | Plausible |
| MDI | 0.01 | Possible | 15.14 | ‐0.27 | Plausible | ||
| Control | PDI | > 0.05 | Impossible | 21.22 | 0.20 | Plausible | |
| MDI | > 0.05 | Impossible | 15.14 | 0.57 | Plausible | ||
| Kimmons [pers comm]3 | Intervention | PDI | 0.36 | Possible | 9.73 | 0.79 | Plausible |
| MDI | 0.27 | Possible | 3.90 | 0.97 | Plausible | ||
| Control | PDI | 0.36 | Possible | 9.73 | 0.79 | Plausible | |
| MDI | 0.27 | Possible | 3.90 | 0.97 | Plausible | ||
| Lozoff 19824 | Intervention | PDI | ‐ | Impossible | 7.4 | 0.92 | Plausible |
| MDI | ‐ | possible | 7.4 | 0.93 | Plausible | ||
| Control | PDI | ‐ | Impossible | 7.4 | 0.94 | Plausible | |
| MDI | ‐ | possible | 7.4 | 0.998 | Plausible | ||
| Lozoff 19875 | Intervention | PDI | > 0.80 | Impossible | ‐ | ‐ | ‐ |
| MDI | > 0.69 | Impossible | ‐ | ‐ | ‐ | ||
| Control | PDI | > 0.80 | Impossible | ‐ | ‐ | ‐ | |
| MDI | > 0.69 | Impossible | ‐ | ‐ | ‐ | ||
| Idjradinata 19936 | Intervention | PDI | < 0.001 | Possible | 17.91 | ‐0.59 | Plausible |
| MDI | < 0.001 | Possible | 25.09 | ‐1.84 | Implausible | ||
| Control | PDI | < 0.001 | Possible | 17.91 | ‐0.51 | Plausible | |
| MDI | ‐ | Impossible | 25.09 | ‐1.70 | Implausible |
MDI: Mental Development Index; PDI: Psychomotor Development Index; SD: standard deviation.
1 P values resulted from paired t‐tests (for within group changes). The SD for the change MDI in the intervention group (10.94) was used for the SDs of the change in PDI in both groups and the change MDI in the control group in the analyses, which were impossible to be imputed.
2 P values resulted from paired t‐tests (for within group changes). The SD for the change PDI in the intervention group (21.22) and for the change in MDI (15.14) were used for the SDs of the change in PDI and MDI in the control group, respectively, which were impossible to be imputed.
3 P values derived from a repeated measures analysis of variance were treated as if resulting from unpaired t‐tests (for the between group differences in changes).
4 Here the SDs for the change in MDI were imputed from the reported power of the study to detect differences of specified sizes in MDI given the observed SDs of the changes (although not giving the values of these SDs), rather than from P values. The SDs for the change MDI were used for the SDs of the change in PDI in the analyses, which were impossible to be imputed.
5 P values derived from a repeated measures analysis of variance were treated as if resulting from unpaired t‐tests (for the between group differences in changes).
6 P values were derived from a repeated measures analysis of variance. For PDI, P values were treated as if resulting from unpaired t‐tests (for the between group differences in changes). For MDI, P values were treated as if resulting from paired t‐tests (for within group changes). The SD for the change MDI in the intervention group (25.09) was used for the SDs of the change in MDI in the control group, which was impossible to be imputed. However, the estimated SDs for change in MDI would imply correlations between pre‐ and post‐test scores substantially in excess of ‐1, which is impossible. It was, therefore, decided instead to estimate SDs from these reported scores and standard errors, following methods described by Follmann (Follmann 1992), assuming a correlation of 0 between pre‐ and post‐treatment test scores. This provided an estimate of the SD for the change in MDI of 15.26 for the control group and 14.90 for the intervention group and of the SD for the change in PDI of 14.21 for the intervention group and 14.58 for the control group.
Assessment of heterogeneity
We evaluated heterogeneity using the Chi2 test, with significance set at P value = 0.10. We measured the quantity of heterogeneity by the I2 statistic. It shows the percentage of the variability in effect estimates that is due to heterogeneity rather than to chance. I2 values of 50% or greater denoted significant heterogeneity. Values over 75% indicated a high level of heterogeneity (Higgins 2003).
Assessment of reporting biases
We assessed selective reporting of outcomes. See the 'Risk of bias' tables in the Characteristics of included studies tables for the judgements on the risk of selective reporting for each study. We drew funnel plots to investigate any relationships between effect size and study precision (Higgins 2011). No statistical tests were used to detect publication bias. See Table 2 for other methods to be used in future updates of the review.
Data synthesis
In this review, data from assessments carried out within 30 days of commencement of treatment were analysed separately from data from assessments carried out later. Data were combined using random‐effects methods. Sensitivity analyses were conducted in which fixed‐effect methods were used (see Sensitivity analysis). We adopted the inverse variance (IV) method for synthesis both in fixed‐effect models and random‐effects models, as all eligible outcome measures were continuous data. We used RevMan 5 software (RevMan 2011) to undertake heterogeneity test and meta‐analysis. See Table 2 for other methods to be used in future updates of the review.
Subgroup analysis and investigation of heterogeneity
As only eight studies were included in this review, no subgroup analyses were performed for investigation of heterogeneity. See Table 2 for other methods to be used in future updates of the review.
Sensitivity analysis
Meta‐analyses were repeated using fixed‐effect methods. Only two studies reported SDs for changes in pre‐ to post‐test scores (Aukett 1986; Walter 1989). As the SDs for the other five trials had to be imputed, we decided to conduct a sensitivity analysis in which post‐intervention outcome measures were pooled in a meta‐analysis. Five studies (Driva 1985; Honig 1978; Idjradinata 1993; Kimmons [pers comm]; Lozoff 1982) reported post‐test Mental Development Index (MDI) and Psychomotor Development Index (PDI) scores on the Bayley Scales, of which four (Driva 1985; Honig 1978; Idjradinata 1993; Lozoff 1982) reported the SDs for these scores. For the study by Kimmons [pers comm], we decided to use the population SDs (15 points) for the Bayley scale in the sensitivity analysis (Bayley 1993).
Results
Description of studies
Results of the search
Figure 1 shows the selection of eligible studies. In total, we retrieved 7211 records from electronic databases and other sources. We removed 1007 records as duplicates, and 6194 records did not meet the inclusion criteria. Finally, 10 records (eight studies) were included in this systematic review.
1.

Selection of eligible studies: a flow diagram
Included studies
Although no new eligible study published after 2001 was identified, one study published in 1985 was determined as eligible and missed by the initial review (Driva 1985), and then included in this update. In total, eight trials (Aukett 1986; Driva 1985; Honig 1978; Idjradinata 1993; Kimmons [pers comm]; Lozoff 1982; Lozoff 1987; Walter 1989) were included with data from two studies reported in two papers each (Honig 1978; Walter 1989). The Characteristics of included studies tables provides details of each study.
Publication year
These studies were published between 1978 and 1993.
Location of studies
The trials were conducted in the USA (Honig 1978), Costa Rica (Lozoff 1987), Chile (Walter 1989), Indonesia (Idjradinata 1993), Greece (Driva 1985), Guatemala (Lozoff 1982) and the UK (Aukett 1986; Kimmons [pers comm]).
Participants
Included studies were relatively small, with 385 (range 24 to 110) iron‐deficient children randomised. In three studies (Honig 1978; Idjradinata 1993; Kimmons [pers comm]), children were recruited from paediatric clinics; in four (Aukett 1986; Lozoff 1982; Lozoff 1987; Walter 1989), community samples were recruited, and in one study (Driva 1985), children were recruited from an orphanage. All the children in included studies were less than 26 months old.
Interventions
The iron was given in oral form in four trials (Aukett 1986; Idjradinata 1993; Lozoff 1982; Walter 1989), intramuscularly in three (Driva 1985; Honig 1978; Kimmons [pers comm]), and both intramuscularly and orally in one (Lozoff 1987). Iron was compared to placebo in seven studies (Driva 1985; Honig 1978; Idjradinata 1993; Kimmons [pers comm]; Lozoff 1982; Lozoff 1987; Walter 1989), and iron plus vitamin C was compared to vitamin C alone in one study (Aukett 1986).
Outcome measures
All studies other than Aukett 1986, which used the Denver Developmental Screening Test, assessed development using the Bayley Scales of Infant Development administered by trained personnel. The Bayley Scale consists of two, age‐standardised subscales, MDI and PDI. The Denver Developmental Screening Test is a screening test for a limited numbers of psychomotor skills. All carried out pre‐treatment assessments. Outcome assessments were carried out between five and 11 days after commencement of therapy in six studies (Driva 1985; Honig 1978; Kimmons [pers comm]; Lozoff 1982; Lozoff 1987; Walter 1989), and after two (Aukett 1986), and four months (Idjradinata 1993), in the others.
Excluded studies
We excluded eight studies (see Characteristics of excluded studies tables). We excluded five studies due to lack of a placebo control (Deinard 1986; Linos 1990; Lozoff 1996; Oski 1983; Walter 1983), and the other three due to ineligible participants (Lozoff 2003; Moffatt 1994; Stoltzfus 2001).
Risk of bias in included studies
In general, the trials included in this review were at unclear risk of bias. The 'Risk of bias' tables in the Characteristics of included studies tables give acomprehensive description of the risk of bias for each study. Figure 2 provides review authors' judgements about each risk of bias item presented as percentages across all included studies. Figure 3 provides review authors' judgements about each risk of bias item for each included study.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Random sequence generation
We judged two studies to be 'low risk of bias' (Idjradinata 1993; Kimmons [pers comm]), and the other six studies to be 'unclear' as information about generation of random sequence was not reported (Aukett 1986; Driva 1985; Honig 1978; Lozoff 1982; Lozoff 1987; Walter 1989).
Allocation concealment
We judged five studies to be 'low risk of bias' (Aukett 1986; Honig 1978; Kimmons [pers comm]; Lozoff 1982; Lozoff 1987), while the remaining three trials were 'unclear' due to insufficient reporting (Driva 1985;Idjradinata 1993;Walter 1989).
Blinding of participants and personnel
The risk of bias for blinding of participants and personnel was judged 'low risk of bias' in six studies (Aukett 1986; Honig 1978; Idjradinata 1993; Lozoff 1982; Lozoff 1987; Walter 1989), and 'unclear' in two studies as not enough information on blinding of personnel was provided (Driva 1985; Kimmons [pers comm]). Although the trials gave no information on blinding of participants, this was unlikely to cause bias as children under the age of three might not fully comprehend the treatment or the trial itself.
Blinding of outcome assessment
Blinding of outcome assessment was an inclusion criterion for the review and was reported in all included studies.
Incomplete outcome data
Four trials reported complete follow‐up (Driva 1985;Honig 1978; Lozoff 1987; Walter 1989). For the other four trials, the attrition rate ranged from 0% to 20% in the intervention group (11.1% in Aukett 1986, 4.0% in Idjradinata 1993, 19.0% in Kimmons [pers comm], 20.0% for PDI and 0% for MDI in Lozoff 1982) and 0% to 19.0% in the control group (12.5% in Aukett 1986, 8.0% in Idjradinata 1993, 19.0% in Kimmons [pers comm], 9.2% for MDI and 0% for PDI in Lozoff 1982). None of the studies reported important differences in follow‐up between intervention and control groups.
Selective outcome reporting
All the included studies were free of suggestion of selective outcome reporting.
Effects of interventions
See: Table 1
Short‐term effects of iron therapy
We included six trials (Driva 1985; Honig 1978; Kimmons [pers comm]; Lozoff 1982; Lozoff 1987; Walter 1989) reported in eight papers (Driva 1985; Honig 1978; Kimmons [pers comm]; Lozoff 1982; Lozoff 1987; Walter 1989), which examined the effects of iron therapy on measures of psychomotor development within 30 days of commencement of therapy. The trials involveed 225 children with IDA. As described above, data from one study could not be included in the meta‐analysis (Lozoff 1987). All studies included measures of development before and between 5 to 11 days after commencement of therapy and used the Bayley Scales of Infant Development in the assessment of outcome.
The pooled difference in pre‐post treatment change in the Bayley Scale PDI between iron‐treated and placebo groups was ‐1.25 (95% CI ‐4.56 to 2.06, P value = 0.65; I2 = 33% for heterogeneity, random‐effects meta‐analysis, Figure 4) and in the Bayley Scale MDI was 1.04 (95% CI ‐1.30 to 3.39, P value = 0.79; I2 = 31% for heterogeneity, random‐effects meta‐analysis, Figure 5). Funnel plots for both outcomes indicated small study effects (Figure 6; Figure 7). The quality of evidence for both outcomes was low (Table 1).
4.
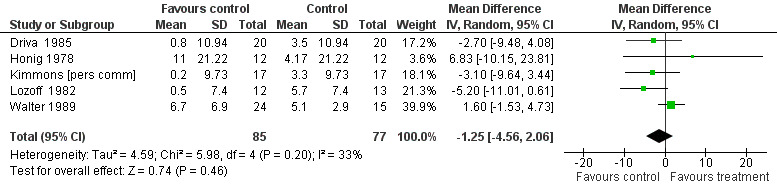
Forest plot of comparison: 1 iron treatment versus placebo in children with iron deficiency anaemia (IDA), outcome: 1.1 Tests of psychomotor development performed 5 to 30 days after study entry: change in Bayley Scale Psychomotor Development Index (PDI)
5.
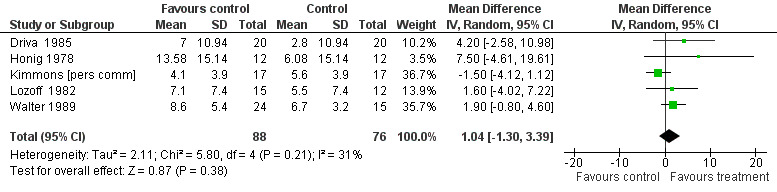
Forest plot of comparison: 1 iron treatment versus placebo in children with iron deficiency anaemia (IDA), outcome: 1.2 Tests of mental development performed 5 to 30 days after study entry: change in Bayley Scale Mental Development Index (MDI)
6.

Funnel plot of comparison: 1 iron treatment versus placebo in children with iron deficiency anaemia (IDA), outcome: 1.1 Tests of psychomotor development performed 5 to 30 days after study entry: change in Bayley Scale Psychomotor Development Index (PDI)
7.

Funnel plot of comparison: 1 iron treatment versus placebo in children with iron deficiency anaemia (IDA), outcome: 1.2 Tests of mental development performed 5 to 30 days after study entry: change in Bayley Scale Mental Development Index (MDI)
Sensitivity analysis showed compatible results between fixed‐effect methods and random‐effects methods. We pooled data from four trials reporting the post‐treatment difference between iron‐treated and placebo groups. The pooled difference in the post‐treatment Bayley Scale PDI was ‐1.62 (95% CI ‐9.79 to 6.55, P value = 0.70; I2 = 51% for heterogeneity, random‐effects meta‐analysis) and in the Bayley Scale MDI was ‐0.01 (95% CI ‐9.17 to 9.15, P value = 0.97; I2 = 62% for heterogeneity, random‐effects meta‐analysis).
The trial that was excluded from the meta‐analysis (Lozoff 1987), included 19 children randomised to receive intramuscular iron, 16 oral iron and 17 oral placebo. The authors compared both groups receiving iron with those only receiving placebo, and found that neither intramuscular iron nor oral iron treatments differed from placebo treatment in effects on Bayley Scale scores.
Long‐term effects of iron therapy
Two studies (Aukett 1986; Idjradinata 1993), including 160 randomised children with IDA, examined the effects of iron therapy on measures of psychomotor development more than 30 days after commencement of therapy. The use of very different outcome measures precluded the combination of results from these two studies: the Bayley Scale assesses developmental skills across a wide range of different aspects of development, while the Denver Developmental Screening Test was devised as a screening test for developmental problems rather than as an assessment tool for psychomotor development and only tests for a limited number of psychomotor skills. Therefore, we reported the results of these two studies separately.
Aukett 1986 assessed changes in children's development by administering 24 items taken from the Denver Developmental Screening Test at the beginning of the study and after two months of iron or placebo treatment. The results were reported as the mean number of skills gained over the period. On average, the intervention group gained 0.8 (95% CI ‐0.18 to 1.78, P value = 0.11) more skills than the control group. The quality of evidence for this outcomes was moderate (Table 1).
Idjradinata 1993 assessed the children's development using the Bayley Scales at the beginning of the study and after four months of iron or placebo treatment. The difference in pre‐ to post‐treatment change in Bayley Scale PDI between iron‐treated and placebo‐treated groups was 18.40 (95% CI 10.16 to 26.64, P value < 0.0001) and in Bayley Scale MDI was 18.80 (95% CI 10.17 to 27.43, P value < 0.0001). The post‐treatment difference between iron‐treated and placebo‐treated groups in Bayley Scale PDI was 14.50 (95% CI 8.82 to 20.18, P value < 0.00001) and in Bayley Scale MDI was 15.20 (95% CI 8.96 to 21.44, P value < 0.00001). The quality of evidence in both outcomes was moderate (Table 1).
Discussion
Summary of main results
The results of the studies included in this review provide no convincing evidence that administration of iron improves children's scores on tests of psychomotor development within six to 11 days of receiving intramuscular or commencing oral iron supplements (Table 1). The relatively small numbers of children included in these studies means, however, that the CIs around the effects of treatment are wide and the results could be compatible with moderate positive or adverse effects of short‐term iron therapy. Five other possible explanations have been advanced to explain this finding (AHRQ 2006; Sachdev 2004). First, prevention of neurodevelopmental consequences of IDA may require acting to prevent iron deficiency rather than detection and treatment of existing iron deficiency. Second, the duration of iron treatment may have been too short to correct the iron deficiency; all six trials that examined the effects of iron therapy within 30 days of commencement of therapy intervened for less than 11 days. Third, structural brain changes caused by iron deficiency or IDA may be irreversible in younger children below three years of age. Fourth, it is also arguable that changes in Bayley Scale scores, the outcome measures employed in these studies, may well be an insensitive measure of real changes, particularly of attributes such as attention or mood. Fifth, prevention of neurodevelopmental consequences may require screening and early treatment of multiple nutritional deficiencies, rather than IDA alone.
On the basis of the data included here, the effects of longer periods of iron therapy on psychomotor development in children with IDA remain unclear (Table 1). The larger of the two included studies (Aukett 1986), unfortunately employed a test designed to be used as a screening tool on a single occasion as the outcome measure, not a tool for the assessment of psychomotor development or for changes in psychomotor development. This measure may well be an insensitive instrument for the detection of changes in psychomotor development. Accepting these limitations, the data from this study did not suggest a substantial beneficial effect on psychomotor development of two months of iron therapy for children with IDA. On the contrary, Idjradinata 1993 reported extremely large effects, greater than one population SD of the tests, of four months of iron therapy in these children. Given the high prevalence of IDA in most populations, if an effect of this magnitude on children's psychomotor development could be achieved by the treatment of IDA, this intervention would have an enormous impact. However, given the inconsistency in the results of these two trials and the small numbers of children studied, the data should be interpreted with caution.
Overall, the evidence that lack of iron affects the psychomotor development of young children with IDA is unclear. Given the high prevalence of iron deficiency, there is an urgent need for large‐scale randomised controlled trials of iron therapy in young children with IDA.
Overall completeness and applicability of evidence
This review aimed to determine the effects of iron therapy on psychomotor development and cognitive function in children with IDA less than three years of age, but data were only available for children aged less than 26 months of age, and failed to include those aged 26 to 36 months. In terms of administration routes of iron therapy, only oral and intramuscular routes were addressed and the effects of iron therapy by the intravenous route were not investigated in this review. This means that the results of this review cannot be directly extrapolated to children aged 26 to 36 months or to iron therapy administrated intravenously.
Quality of the evidence
For changes in Bayley Scale PDI and MDI within 30 days of commencement of therapy, 162 children from five studies and 164 children from five studies were included, respectively. Neither serious methodological limitations of nor statistical heterogeneity across studies were found. The quality of the evidence for both outcomes was low due to possible publication bias and imprecision of results.
For changes in Bayley Scale PDI and MDI more than 30 days of commencement of therapy, one study with 46 children was included (Idjradinata 1993). No serious methodological limitations of the study existed. The quality of evidence for both outcomes was moderate due to imprecision of results.
For changes in Denver Developmental Screening Test, more than 30 days after commencement of therapy, only one study with 97 children was included (Aukett 1986). No serious methodological limitations of the study were identified and the quality of evidence was moderate due to imprecision of results.
Potential biases in the review process
Three limitations merit consideration. First, a comprehensive search strategy was developed and a large body of the published literature was scrutinised. However, we did not handsearch key journals and grey literature websites for this update. A potential publication bias should be taken into account in those outcomes for which no funnel plots were used. Second, one eligible study was not included in the meta‐analysis due to unavailable data (Lozoff 1987). However, the finding of this study that there was no evidence of effects of iron therapy on psychomotor development or cognitive function within seven days of commencement of therapy was consistent with the meta‐analysis. Third, SDs for changes in pre‐ to post‐test scores were imputed in five studies. Sensitivity analysis using post‐treatment measures, however, demonstrated the robustness of the results. All review authors had no conflicts of interest in the review process.
Agreements and disagreements with other studies or reviews
The findings of this updated review do not differ from those of the original review, which was first published 2001. One systematic review of randomised controlled trials evaluated the effect of iron supplementation on mental and motor development in children (less than 18 years old, including both anaemic and non‐anaemic) (Sachdev 2004). It found that there was no convincing evidence (Bayley Scale MDI, MD 0.95, 95% CI ‐0.56 to 2.46) that iron treatment had an effect on mental development in children below 27 months of age or on motor development. This has provided support to the result of our review to some degree. Another recently published systematic review of randomised controlled trials assessed the effect of iron supplementation in non‐anaemic children less than three years of age on mental development (Szajewska 2010). It found limited available evidence suggesting a possible positive effect on the Bayley Scale PDI (MD 4.21, 95% CI 2.31 to 6.12) and no effect on the MDI (MD 1.66, 95% CI ‐0.14 to 3.47). It is proposed that the inconsistency between Szajewska 2010 and our review is caused by different types of participants (iron deficient anaemic versus non‐anaemic) or different precisions of results, or both.
Authors' conclusions
Implications for practice.
Most clinicians seeing a child with IDA would elect either to treat with elemental iron or to attempt to improve the child's intake of dietary iron. However, clinicians cannot be sure if treatment of IDA to remedy the anaemia will result in positive changes to psychomotor development and cognitive function.
Implications for research.
There is an urgent need for large randomised controlled trials of iron therapy in young children less than three years old with IDA with long‐term follow‐up, and Bayley Scales of Infant Development should be used to assess development both at and more than one month after commencement of iron therapy. Meanwhile, appropriate methodologies in design and implementation should be used and clearly reported in these studies, including adequate allocation sequence generation; allocation concealment; and blinding of participants, personnel and outcome assessors.
What's new
| Date | Event | Description |
|---|---|---|
| 30 January 2013 | New citation required but conclusions have not changed | New study included. |
| 24 October 2012 | New search has been performed | Updated search. 'Risk of bias' tables and a 'Summary of findings' table added. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 13 September 2010 | Amended | Converted to new review format. |
| 26 February 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The review authors gratefully acknowledge the important assistance provided by Margaret Anderson and Joanne Abbott in the development of the search strategy. The review authors also thank Geraldine Macdonald, Laura MacDonald and Jane Dennis for co‐ordination and editorial feedback in the update of this review. We also acknowledge the authors of the original review who have not been involved in the update: Susanna Martins, Stewart Logan and Ruth Gilbert.
Appendices
Appendix 1. Search strategies 2007
MEDLINE 1966 to present
1 Anemia, Iron‐Deficiency/ (3474)
2 an#emi$.tw. (18,875)
3 (iron adj3 deficien$).tw. (10,598)
4 or/1‐3 (28,559)
5 child, preschool/ or infant/ (770,144)
6 (baby or babies or infant$ or preschool$ or pre‐school$ or toddler$ or girl$ or boy$ or child$).tw. (884,110)
7 or/5‐6 (1,248,065)
8 Dietary Supplements/ (12,350)
9 (iron adj3 (supplement$ or therap$)).tw. (4485)
10 iron.tw. (76,623)
11 Iron/ (56,146)
12 or/8‐11 (106,526)
13 4 and 7 and 12 (3243)
14 randomized controlled trial.pt. (233,672)
15 controlled clinical trial.pt. (74,707)
16 randomized controlled trials.sh. (48,151)
17 random allocation.sh. (57,661)
18 double blind method.sh. (90,848)
19 single blind method.sh. (10,848)
20 or/14‐19 (396,309)
21 (animals not humans).sh. (3,077,794)
22 20 not 21 (372,349)
23 clinical trial.pt. (434,900)
24 exp Clinical Trials/ (190,060)
25 (clin$ adj25 trial$).ti,ab. (128,925)
26 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. (90,129)
27 placebos.sh. (26,065)
28 placebo$.ti,ab. (101,593)
29 random$.ti,ab. (368,011)
30 research design.sh. (47,102)
31 or/23‐30 (839,228)
32 31 not 21 (779,398)
33 32 not 22 (424,737)
34 comparative study.sh. (0)
35 exp Evaluation Studies/ (592,383)
36 follow up studies.sh. (336,398)
37 prospective studies.sh. (219,516)
38 (control$ or prospectiv$ or volunteer$).ti,ab. (1,763,635)
39 or/34‐38 (2,548,511)
40 39 not 21 (2,032,351)
41 40 not (22 or 33) (1,545,989)
42 22 or 33 or 41 (2,343,075)
43 13 and 42 (1092)
CENTRAL May 2007
#1 MeSH descriptor: [Anemia, Iron‐Deficiency] explode all trees #2 an?emi* OR iron near/3 deficien* #3 #1 or #2 #4 MeSH descriptor: [Infant] explode all trees #5 child preschool near "MESH check words" #6 (baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*) #7 #4 or #5 or #6 #8 MeSH descriptor: [Dietary Supplements] this term only #9 iron near/3 supplement* #10 iron near/3 therap* #11 iron #12 MeSH descriptor: [Iron] this term only #13 #8 or #9 or #10 or #11 or #12 #14 #3 and #7 and #13
CINAHL ‐ Cumulative Index to Nursing & Allied Health Literature, 1982 to May week 1 2007
1 Anemia, Iron Deficiency/ (757)
2 an#emi$.tw. (495)
3 (iron adj3 deficien$).tw. (617)
4 or/1‐3 (1435)
5 child, preschool/ or infant/ (60,016)
6 (baby or babies or infant$ or preschool$ or pre‐school$ or toddler$ or girl$ or boy$ or child$).tw. (104,587)
7 or/5‐6 (127,455)
8 Dietary Supplements/ (1104)
9 (iron adj3 (supplement$ or therap$)).tw. (384)
10 iron.tw. (2066)
11 IRON/ (1184)
12 or/8‐11 (3511)
13 4 and 7 and 12 (370)
14 randomi$.mp. [mp=title, subject heading word, abstract, instrumentation] (26,401)
15 clin$.mp. [mp=title, subject heading word, abstract, instrumentation] (208,923)
16 trial$.mp. [mp=title, subject heading word, abstract, instrumentation] (54,940)
17 (clin$ adj3 trial$).mp. [mp=title, subject heading word, abstract, instrumentation] (39,249)
18 singl$.mp. [mp=title, subject heading word, abstract, instrumentation] (21,294)
19 doubl$.mp. [mp=title, subject heading word, abstract, instrumentation] (13,887)
20 tripl$.mp. [mp=title, subject heading word, abstract, instrumentation] (1185)
21 trebl$.mp. [mp=title, subject heading word, abstract, instrumentation] (17)
22 mask$.mp. [mp=title, subject heading word, abstract, instrumentation] (2220)
23 blind$.mp. [mp=title, subject heading word, abstract, instrumentation] (16,974)
24 (18 or 19 or 20 or 21) and (22 or 23) (12,672)
25 crossover.mp. [mp=title, subject heading word, abstract, instrumentation] (3869)
26 random$.mp. [mp=title, subject heading word, abstract, instrumentation] (56,592)
27 allocate$.mp. [mp=title, subject heading word, abstract, instrumentation] (2289)
28 assign$.mp. [mp=title, subject heading word, abstract, instrumentation] (22,677)
29 (random$ adj3 (allocate$ or assign$)).mp. (18,139)
30 Random Assignment/ (15,190)
31 exp Clinical Trials/ (43,840)
32 exp Meta Analysis/ (5259)
33 29 or 25 or 24 or 17 or 14 or 30 or 31 or 32 (65,563)
34 13 and 33 (98)
EMBASE 1980 to 2007 week 19
1 Iron Deficiency Anemia/ (4788)
2 an#emi$.tw. (13,101)
3 (iron adj3 deficien$).tw. (7522)
4 or/1‐3 (21,031)
5 Preschool Child/ (95,548)
6 Infant/ (157,383)
7 (baby or babies or infant$ or preschool$ or pre‐school$ or toddler$ or girl$ or boy$ or child$).tw. (599,782)
8 or/5‐7 (670,044)
9 diet supplementation/ (25,066)
10 (iron adj3 (supplement$ or therap$)).tw. (3667)
11 iron.tw. (62,538)
12 Iron/ (39,708)
13 or/9‐12 (99,486)
14 4 and 8 and 13 (2244)
15 clin$.tw. (1,319,956)
16 trial$.tw. (294,775)
17 (clin$ adj3 trial$).tw. (103,306)
18 singl$.tw. (548,489)
19 doubl$.tw. (231,554)
20 trebl$.tw. (214)
21 tripl$.tw. (35,994)
22 blind$.tw. (123,512)
23 mask$.tw. (27,831)
24 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (85,120)
25 randomi$.tw. (187,558)
26 random$.tw. (335,411)
27 allocat$.tw. (29,580)
28 assign$.tw. (94,268)
29 (random$ adj3 (allocat$ or assign$)).tw. (45,687)
30 crossover.tw. (24,127)
31 30 or 29 or 25 or 24 or 17 (328,908)
32 exp Randomized Controlled Trial/ (118,046)
33 exp Double Blind Procedure/ (63,899)
34 exp Crossover Procedure/ (18,621)
35 exp Single Blind Procedure/ (6576)
36 exp RANDOMIZATION/ (22,296)
37 32 or 33 or 34 or 35 or 36 or 31 (372,982)
38 14 and 37 (291)
LILACS (MAY 2007)
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Palavras] and ((anemi$ OR anaemi$ OR (iron deficien$)) AND (baby or babies or infant$ or preschool$ or toddler$ or girl$ or boy$ or child$)) [Palavras] and ((iron supplement$) OR (iron therap$) OR iron) [Palavras]
PsycINFO
#17 (((baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*)) and ((( an?emi* )or( iron near3 deficien* )) or ("Anemia‐" in MJ,MN)) and (("Dietary‐Supplements" in MJ,MN) or ("Iron‐" in MJ,MN) or (iron) or (( iron near 3 supplement* )or( iron near3 therap* )))) and ((random* or trial*) or ("Clinical‐Trials" in MJ,MN))(11 records)
#16 ((random* or trial*) or ("Clinical‐Trials" in MJ,MN)) and ((((baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*)) and ((( an?emi* )or( iron near3 deficien* )) or ("Anemia‐" in MJ,MN)) and (("Dietary‐Supplements" in MJ,MN) or ("Iron‐" in MJ,MN) or (iron) or (( iron near 3 supplement* )or( iron near3 therap* )))) and (( develop* )or( behavio?r* )))(8 records)
#15 (random* or trial*) or ("Clinical‐Trials" in MJ,MN)(122,212 records)
#14 random* or trial*(122,212 records)
#13 "Clinical‐Trials" in MJ,MN(1436 records)
#12 (((baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*)) and ((( an?emi* )or( iron near3 deficien* )) or ("Anemia‐" in MJ,MN)) and (("Dietary‐Supplements" in MJ,MN) or ("Iron‐" in MJ,MN) or (iron) or (( iron near 3 supplement* )or( iron near3 therap* )))) and (( develop* )or( behavio?r* ))(90 records)
#11 ( develop* )or( behavio?r* )(1,077,624 records)
#10 ((baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*)) and ((( an?emi* )or( iron near3 deficien* )) or ("Anemia‐" in MJ,MN)) and (("Dietary‐Supplements" in MJ,MN) or ("Iron‐" in MJ,MN) or (iron) or (( iron near 3 supplement* )or( iron near3 therap* )))(107 records)
#9 ("Dietary‐Supplements" in MJ,MN) or ("Iron‐" in MJ,MN) or (iron) or (( iron near 3 supplement* )or( iron near3 therap* ))(1266 records)
#8 "Iron‐" in MJ,MN(238 records)
#7 iron(946 records)
#6 ( iron near 3 supplement* )or( iron near3 therap* )(21 records)
#5 "Dietary‐Supplements" in MJ,MN(334 records)
#4 (baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*)(483,212 records)
#3 (( an?emi* )or( iron near3 deficien* )) or ("Anemia‐" in MJ,MN)(1124 records)
#2 ( an?emi* )or( iron near3 deficien* )(1124 records)
#1 "Anemia‐" in MJ,MN(251 records)
BIOSIS
#1 TS=(an*emi*) OR TS=(iron defien*)
#2 TS=(baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*)
#3 TS=(iron supplement*) OR TS=(iron therap*) OR TS=(iron)
#4 #3 AND #2 AND #1
#5 TS=(random* or trial*)
#6 #5 AND #4
CENTRAL
#1 Anemia, Iron‐Deficiency/
#2 an*emi*
#3 (iron near/3 deficien*).
#4 #1 or #2 or #3
#5 child, preschool/ or infant/
#6 (baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*)
#7 #5 or #6
#8 Dietary Supplements/
#9 (iron near/3 supplement*)
#10 (iron near/3 therap*)
#11 iron
#12 Iron/
#13 #8 or #9 or #10 or #11 or #12
#14 4 and 7 and 13
Appendix 2. Search strategies April 2013
CENTRAL
2011, Issue 1 searched 3 February 2011 2013, Issue 3 searched 23 April 2013 #1 MeSH descriptor: [Anemia, Iron‐Deficiency] explode all trees #2 (anemi* or anaemi*) or iron near/3 deficien* #3 #1 or #2 #4 MeSH descriptor: [Infant] explode all trees #5 child preschool near "MESH check words" #6 (baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*) #7 #4 or #5 or #6 #8 MeSH descriptor: [Dietary Supplements] this term only #9 MeSH descriptor: [Micronutrients] this term only #10 MeSH descriptor: [Trace Elements] this term only #11 MeSH descriptor: [Food, Fortified] this term only #12 MeSH descriptor: [Injections, Intramuscular] this term only #13 (inject* or intramuscular* or intra next muscular*) #14 iron near/3 supplement* #15 iron near/3 therap* #16 iron or "Fe" or ferrous* or ferric* #17 MeSH descriptor: [Iron] this term only #18 MeSH descriptor: [Iron Compounds] this term only #19 MeSH descriptor: [Ferrous Compounds] this term only #20 MeSH descriptor: [Ferrous Compounds] this term only #21 MeSH descriptor: [Iron, Dietary] this term only #22 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 #23 #3 and #7 and #22 Ovid MEDLINE (R)
1948 to January week 3 2011, searched 2 February 2011 1948 to April week 2 2013, searched 22 April 2013 1 Anemia, Iron‐Deficiency/ 2 (anaemi$ or anemi$).tw. 3 (iron adj3 deficien$).tw. 4 or/1‐3 5 infant/ 6 child, preschool/ 7 (baby or babies or infant$ or preschool$ or pre‐school$ or toddler$ or girl$ or boy$ or child$).tw. 8 or/5‐7 9 Food, Fortified/ 10 Dietary Supplements/ 11 Micronutrients/ 12 Trace elements/ 13 Injections, Intramuscular/ 14 (inject$ or intramuscular$ or intra‐muscular$).tw. 15 Iron/ 16 Iron, dietary/ 17 exp iron compounds/ or ferric compounds/ or ferrous compounds/ 18 (iron or Fe or ferrous$ or ferric$).mp. 19 or/9‐18 20 4 and 8 and 19 21 randomized controlled trial.pt. 22 controlled clinical trial.pt. 23 randomi#ed.ab. 24 placebo$.ab. 25 drug therapy.fs. 26 randomly.ab. 27 trial.ab. 28 groups.ab. 29 or/21‐28 30 exp animals/ not humans.sh. 31 29 not 30 32 20 and 31 33 limit 32 to ed=20070501‐20110202 34 32 not 33 EMBASE (Ovid) 1980 to 2011 week 4, searched 2 February 2011 1980 to 2013 week 16, searched 22 April 2013 1 iron deficiency anemia/ 2 (anaemi$ or anemi$).tw. 3 (iron adj3 deficien$).tw. 4 or/1‐3 5 infant/ 6 preschool child/ 7 toddler/ 8 (baby or babies or infant$ or preschool$ or pre‐school$ or toddler$ or girl$ or boy$ or child$).tw. 9 or/5‐8 10 iron/ 11 iron intake/ 12 iron derivative/ or ferric ion/ or ferrous ion/ 13 (iron or Fe or ferrous$ or ferric$).mp. 14 exp trace element/ 15 diet supplementation/ 16 intramuscular drug administration/ 17 (inject$ or intramuscular$ or intra‐muscular$).tw. 18 or/10‐17 19 Clinical trial/ 20 Randomized controlled trial/ 21 Randomization/ 22 Single blind procedure/ 23 Double blind procedure/ 24 Crossover procedure/ 25 Placebo/ 26 Randomi#ed.tw. 27 RCT.tw. 28 (random$ adj3 (allocat$ or assign$)).tw. 29 randomly.ab. 30 groups.ab. 31 trial.ab. 32 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 33 Placebo$.tw. 34 Prospective study/ 35 (crossover or cross‐over).tw. 36 prospective.tw. 37 or/19‐36 38 4 and 9 and 18 and 37 BIOSIS
1969 to 3 February 2011, searched 3 February 2011 BIOSIS was not available to the editorial base or author team in 2013 so the search was not updated
#9 #7 NOT #8 #8 #7 #7 #6 AND #5 AND #4 AND #3 #6 TS=(random* or trial* ) #5 #2 OR #1 #4 TS=(iron or ferric* or ferrous* or Fe) #3 TS=(baby or babies or infant* or preschool* or pre‐school* or toddler* or child*) #2 TS=(anemi* or anaemi*) #1 TS=(iron deficien*) CINAHL (EBSCOhost)
1937 to current, searched 2 February 2011 1937 to current, searched 23 April 2013
S40 S37 NOT S39 S39 S37 and S38 S38 PY 2007‐2011 S37 S4 and S8 and S22 and S36 S36 S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 S35 "cross over*" S34 crossover* S33 (MH "Crossover Design") S32 (tripl* N3 mask*) or (tripl* N3 blind*) S31 (trebl* N3 mask*) or (trebl* N3 blind*) S30 (doubl* N3 mask*) or (doubl* N3 blind*) S29 (singl* N3 mask*) or (singl* N3 blind*) S28 (clinic* N3 trial*) or (control* N3 trial*) Database ‐ CINAHL Plus 113390 Edit S28 S27 (random* N3 allocat* ) or (random* N3 assign*) S26 randomis* or randomiz* S25 (MH "Meta Analysis") S24 (MH "Clinical Trials+") S23 MH random assignment S22 S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 S21 TI (inject* or intramuscular* or intra‐muscular*) S20 (MH "Injections, Intramuscular") S19 (MH "Dietary Supplements") Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost Search Screen ‐ Advanced Search Database ‐ CINAHL Plus 6735 Edit S19 S18 (MH "Food, Fortified") S17 (MH "Dietary Supplementation") S16 "Fe" S15 ferrous* S14 ferric* S13 (MH "Ferrous Compounds") OR (MH "Ferric Compounds") S12 iron* S11 (MH "Micronutrients") S10 (MH "Trace Elements") S9 (MH "Iron") OR (MH "Iron Compounds") S8 S5 or S6 or S7 S7 TI (baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*) or AB (baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*) S6 (MH "Child, Preschool") S5 (MH "Infant") S4 S1 or S2 or S3 S3 TI (iron N3 deficien*) or AB (iron N3 deficien*) S2 AB (anaemi* or anemi*) OR TI (anaemi* or anemi*) S1 (MH "Anemia, Iron Deficiency") PsycINFO (EBSCOhost)
1837 to current, searched 2 February 2011 S33 S30 NOT S31 S32 S30 and S31 S31 PY 2007‐2011 S30 S4 and S8 and S15 and S29 S29 S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 S28 (evaluation N3 stud* or evaluation N3 research*) S27 (effectiveness N3 stud* or effectiveness N3 research*) S26 DE "Placebo" or DE "Evaluation" or DE "Program Evaluation" OR DE "Educational Program Evaluation" OR DE "Mental Health Program Evaluation" S25 (DE "Random Sampling" or DE "Clinical Trials") or (DE "Experiment Controls") S24 "cross over*" S23 crossover* S22 (tripl* N3 mask*) or (tripl* N3 blind*) S21 (trebl* N3 mask*) or (trebl* N3 blind*) S20 (doubl* N3 mask*) or (doubl* N3 blind*) S19 (singl* N3 mask*) or (singl* N3 blind*) S18 (clinic* N3 trial*) or (control* N3 trial*) S17 (random* N3 allocat* ) or (random* N3 assign*) S16 randomis* or randomiz* S15 S9 or S10 or S11 or S12 or S13 or S14 S14 TI (inject* or intramuscul or intra‐muscul*) OR AB(inject* or intramuscul or intra‐muscul*) S13 DE "Injections" OR DE "Intramuscular Injections" S12 TI ( ferrous* or ferric* or Fe) or AB( ferrous* or ferric* or Fe) S11 TI (iron*) or AB (iron*) S10 DE "Dietary Supplements" S9 DE "Iron" S8 S5 or S6 or S7 S7 (ZG "infancy (2‐23 mo)") or (ZG "neonatal (birth‐1 mo)") S6 (ZG "preschool age (2‐5 yrs)") S5 TI(baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*) or AB (baby or babies or infant* or preschool* or pre‐school* or toddler* or girl* or boy* or child*) S4 S1 or S2 or S3 S3 TI (iron N3 deficien*) or AB (iron N3 deficien*) S2 TI (anem* or anaemi*) or AB (anem* or anaemi*) S1 DE "Anemia"
PsycINFO (Ovid)
1806 to April week 3 2013, searched 23 April 2013 1 anemia/ 2 (anem$ or anaemi$).tw. 3 (iron adj3 deficien$).tw. 4 1 or 2 or 3 5 (baby or babies or infant$ or preschool$ or pre‐school$ or toddler$ or boy$ or girl$ or child$).tw. 6 (childhood birth 12 yrs or infancy 2 23 mo or neonatal birth 1 mo or preschool age 2 5 yrs).ag. 7 5 or 6 8 Dietary Supplements/ 9 iron$.tw. 10 (ferrous$ or ferric$ or Fe).tw. 11 Intramuscular Injections/ or Injections/ 12 (inject* or intramuscul$ or intra‐muscul$).tw. 13 iron/ 14 or/8‐13 15 clinical trials/ 16 (randomis$ or randomiz$).tw. 17 (random$ adj3 (allocat$ or assign$)).tw. 18 ((clinic$ or control$) adj trial$).tw. 19 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 20 (crossover$ or "cross over$").tw. 21 random sampling/ 22 Experiment Controls/ 23 Placebo/ 24 placebo$.tw. 25 exp program evaluation/ 26 treatment effectiveness evaluation/ 27 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. 28 or/15‐27 29 4 and 7 and 14 and 28 LILACS (VHL interface)
Searched 3 February 2011 and 23 April 2013 anemi$ or anaemi$ [Words] or "ANEMIA, iron‐deficiency" [Subject descriptor] [Words] AND (iron [Subject descriptor] or iron or ferrous or ferric) [Words] AND child$ or baby or babies or infant$ or pre‐school or preschool [Words] ClinicalTrials.gov
Searched 3 February 2011 and 23 April 2013
anemia AND iron AND children
WHO ICTRP
All years searched 23 April 2013
CONDITION=anemia OR anaemia AND Intervention=iron* AND Trials in Children is selected
Data and analyses
Comparison 1. Iron treatment versus placebo in children with iron deficiency anaemia.
1.1. Analysis.

Comparison 1 Iron treatment versus placebo in children with iron deficiency anaemia, Outcome 1 Tests of psychomotor development performed 5‐30 days after study entry: change in Bayley Scale Psychomotor Development Index (PDI).
1.2. Analysis.

Comparison 1 Iron treatment versus placebo in children with iron deficiency anaemia, Outcome 2 Tests of mental development performed 5‐30 days after study entry: change in Bayley Scale Mental Development Index (MDI).
1.3. Analysis.

Comparison 1 Iron treatment versus placebo in children with iron deficiency anaemia, Outcome 3 Tests of psychomotor development performed 5‐30 days after study entry: post treatment Bayley Scale PDI.
1.4. Analysis.

Comparison 1 Iron treatment versus placebo in children with iron deficiency anaemia, Outcome 4 Tests of mental development performed 5‐30 days after study entry: post treatment Bayley Scale MDI.
1.5. Analysis.

Comparison 1 Iron treatment versus placebo in children with iron deficiency anaemia, Outcome 5 Tests of psychomotor development performed more than 30 days after study entry: change in Bayley Scale PDI.
1.6. Analysis.

Comparison 1 Iron treatment versus placebo in children with iron deficiency anaemia, Outcome 6 Tests of mental development performed more than 30 days after study entry: change in Bayley Scale MDI.
1.7. Analysis.

Comparison 1 Iron treatment versus placebo in children with iron deficiency anaemia, Outcome 7 Tests of psychomotor development and mental development performed more than 30 days after study entry: change in Denver Developmental Screening Test scores.
1.8. Analysis.

Comparison 1 Iron treatment versus placebo in children with iron deficiency anaemia, Outcome 8 Tests of psychomotor development performed more than 30 days after study entry: post treatment Bayley Scale PDI.
1.9. Analysis.

Comparison 1 Iron treatment versus placebo in children with iron deficiency anaemia, Outcome 9 Tests of mental development performed more than 30 days after study entry: post treatment Bayley Scale MDI.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aukett 1986.
| Methods | RCT. Allocation by investigators not connected with study. Investigators, parents and assessors blind to allocation | |
| Participants | 110 children aged 17‐19 months with IDA (Hb 8‐11 g/dL). Community sample | |
| Interventions | Ferrous sulphate 24 mg and vitamin C 10 g or vitamin C 10 mg as identical appearing placebo. Compliance checked twice weekly | |
| Outcomes | Denver Developmental Screening Tests before and 8‐9 weeks after commencement of treatment. Also measured weight change and measures of iron status | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no sufficient information provided |
| Allocation concealment (selection bias) | Low risk | Quote (from report): "Allocation was double blind using consecutively numbered bottles" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors were blind to allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors were blind to allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/54 missing from intervention group; 7/56 missing from control group. Characteristics of these 2 groups were similar to each other and to the group who reattended |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
Driva 1985.
| Methods | RCT. No details of allocation process available. Nursery nurse and assessors blind to allocation | |
| Participants | 40 infants with IDA (Hb < 11.0 g/dL and all suffering from iron deficiency) aged 3‐25 months recruited from an orphanage in Greece | |
| Interventions | IM injection of iron 50 mg in the intervention group | |
| Outcomes | Bayley Scales of infant Development administered pre and 10 days post commencement of intervention | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no sufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no sufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (from the report): "Neither the tester nor the nursery nurse knew the type of therapeutic intervention" Comment: Insufficient information on blinding of investigators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from the report): "Neither the tester nor the nursery nurse knew the type of therapeutic intervention" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures are available for all children. Complete follow‐up in both intervention group and control group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
Honig 1978.
| Methods | RCT. No details of allocation process available. Investigators, parents and assessors blind to allocation | |
| Participants | 24 infants with IDA (Hb < 10.5 g/dL and at least 2 abnormal measures of iron status), aged 9‐26 months, recruited from paediatric clinic in New York | |
| Interventions | Iron dextran complex at a dose calculated to raise Hb to 12 g/dL. Placebo of IM sterile saline | |
| Outcomes | Bayley Scales of infant Development and Bayley Infant Behaviour Record administered pre and 5‐8 days post commencement of intervention. However, only some subscales of the Behaviour Record were reported | |
| Notes | Authors provided unpublished details of allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no sufficient information provided |
| Allocation concealment (selection bias) | Low risk | Comment: Authors provided unpublished details of adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors blind to allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from the report): "Neither the examiner nor the nurse was informed as to which group any infant belonged until Bayley protocols had been scored" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures are available for all children. Complete follow‐up in both intervention group and control group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
Idjradinata 1993.
| Methods | RCT. Allocation sequence derived from table of random numbers, no details provided of who carried this out. Infants grouped by iron status class and randomised separately. Investigators, parents and assessors blind to allocation | |
| Participants | Children aged 12‐18 months: 50 with IDA (Hb ≤ 10.5 g/dL, transferrin saturation < 10% and serum ferritin < 12 mg/dL) recruited from a paediatric clinic in Indonesia. 29 non‐anaemic, iron deficient and 47 iron sufficient infants also randomised to treatment or control | |
| Interventions | Ferrous sulphate 3 mg/kg body weight by mouth for 4 months or placebo syrup of same appearance and flavour. Compliance checked weekly | |
| Outcomes | Bayley Scales of Infant Development administered pre and 4 months post commencement of intervention. Measures of weight, length and Hb status | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from report): "Random assignment of the infants to either the iron treatment or placebo intervention was done with a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no sufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors were blind to allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors were blind to allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/25 missing from intervention group; 2/25 missing from control group. Missing outcome data balanced in numbers across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
Kimmons [pers comm].
| Methods | Allocation sequence generated by random number table prepared by pharmacy and intervention performed by research nurse who was not further involved in study. Assessors blind to allocation | |
| Participants | 42 children aged 6‐24 months with Hb < 10.6 g/dL and MCV < 73 fL were randomised. Children recruited from general paediatric outpatients | |
| Interventions | Iron dextran complex calculated to raise Hb to 12 g/dL or dot of India ink at covered injection site | |
| Outcomes | Bayley Scales of Infant Development and Bayley Infant Behaviour Record administered pre and 1 week post commencement of intervention | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from the report): "Allocation sequence generated by random number table prepared by pharmacy" |
| Allocation concealment (selection bias) | Low risk | Quote (from the report): "Allocation by nurse not connected with study" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no sufficient information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "Assessors were blind to allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/21 missing from either of both groups. Missing outcome data balanced in numbers across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
Lozoff 1982.
| Methods | RCT. Allocation by investigators not involved in other aspects of the study. Investigators, parents and assessors blind to allocation | |
| Participants | 28 children aged 6‐24 months with IDA (Hb < 10.5 g/dL and at least 2 abnormal measures of iron status) and 40 children without IDA (Hb > 12 g/dL) randomised. Volunteers invited to have iron screening from a community in Guatemala | |
| Interventions | Ferrous ascorbate 5 mg/kg body weight orally twice daily for 7 days or identical placebo prepared by pharmacy and administered by researcher blind to allocation | |
| Outcomes | Bayley Scales of Infant Development administered pre and 6‐8 days post commencement of intervention | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no sufficient information provided |
| Allocation concealment (selection bias) | Low risk | Quote (from the report): "Both anaemic and nonanaemic infants were randomly assigned to oral iron or placebo treatment groups by investigators who did not participate in screening , testing" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors were blind to allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors were blind to allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0/15 (MDI) or 3/15 (PDI) missing from intervention group; 0/13 (PDI) or 1/13 (MDI) missing from control group. Missing outcome data almost balanced in numbers across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
Lozoff 1987.
| Methods | RCT. Allocation by investigators not involved in other aspects of the study. Investigators, parents and assessors blind to allocation | |
| Participants | 52 children with IDA (Hb ≤ 10.5 g/dL and at least 2 abnormal measures of iron status) 45 children with Hb > 10.5 to < 12 g/dL and 2 abnormal measures of iron status 21 children with Hb > 12 g/dL and 2 abnormal measures of iron status 38 children with Hb > 12 g/dL and only serum ferritin < 12 μg/dL 35 children with Hb > 12 g/dL and normal measures of iron status Those with Hb < 12 g/dL were randomised to IM iron followed by placebo, IM placebo followed by oral iron or IM placebo followed by oral placebo. The remaining children were randomised to IM placebo followed by either oral iron or oral placebo |
|
| Interventions | IM iron (Imferon) calculated to raise Hb to 12.5 g/dL or IM placebo then ferrous sulphate 5 mg/kg or similar placebo given twice daily by investigators for 7 days | |
| Outcomes | Bayley Scales of Infant Development administered pre and 7 days post commencement of intervention | |
| Notes | Outcome assessments performed on all children at 1 week but data not easily interpretable (see analysis section). The authors report no significant differences between intervention and control groups on MDI or PDI scores | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no sufficient information provided |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "Allocation by investigators not involved in other aspects of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors were blind to allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors were blind to allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up in both intervention group and control group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
Walter 1989.
| Methods | RCT. Method of allocation unclear. Children within each iron‐status group were randomly assigned to intervention and control. Investigators, parents and assessors blind to allocation | |
| Participants | 39 anaemic (Hb < 11.0 g/dL) children aged 12 months allocated to iron (n = 24) or placebo (n = 15). Children recruited from within community‐based RCT of iron supplementation | |
| Interventions | Ferrous sulphate 15 mg 3 times daily for 10 days or placebo | |
| Outcomes | Bayley Scales of Infant Development administered pre and 11 days post commencement of intervention | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no sufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no sufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors were blind to allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "Investigators, parents and assessors were blind to allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome assessments available on all children. Complete follow‐up in both intervention and control group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
Hb: haemoglobin; IDA: iron deficiency anaemia; IM: intramuscular; MCV: mean corpuscular volume; MDI: Mental Development Index; PDI: Psychomotor Development Index; RCT: randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Deinard 1986 | No non‐treated controls |
| Linos 1990 | No non‐treated controls |
| Lozoff 1996 | No non‐treated controls |
| Lozoff 2003 | Children were free of iron‐deficient anaemia |
| Moffatt 1994 | Some children were free of iron‐deficient anaemia |
| Oski 1983 | No non‐treated controls |
| Stoltzfus 2001 | Some children were free of iron‐deficient anaemia |
| Walter 1983 | No non‐treated controls |
Differences between protocol and review
We added 'Risk of bias' tables and a 'Summary of findings' table.
Contributions of authors
All four review authors were involved in the planning and processing of this substantial update. BW and TG selected the trials for inclusion, extracted the data and performed the analyses, while SZ was the referee review author. LL and SZ provided critical input for the statistical analysis and the interpretation of data. All of the review authors took part in the discussion of the results and contributed to the drafting of the final version.
Sources of support
Internal sources
-
Peking University Health Science Center, China.
Allowance
-
Chinese Academy of Medical Sciences, China.
Salary
External sources
-
Ministry of Education, China.
Research fund
Declarations of interest
Bo Wang ‐ none known. Si‐Yan Zhan ‐ none known. Ting Gong ‐ none known. Liming Lee ‐ none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aukett 1986 {published data only}
- Aukett MA, Parks YA, Scott PH, Wharton BA. Treatment with iron increases weight gain and psychomotor development. Archives of Disease in Childhood 1986;61(9):849‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Driva 1985 {published data only}
- Driva A, Kafatos A, Solman M. Iron deficiency and the cognitive and psychomotor development of children: a pilot study with institutionalised children. Early Child Development and Care 1985;22(1):73‐82. [Google Scholar]
Honig 1978 {published data only}
- Honig AS, Oski FA. Developmental scores of iron deficient infants and the effects of therapy. Infant Behavior and Development 1978;1:168‐76. [DOI] [PubMed] [Google Scholar]
- Oski FA, Honig AS. The effects of therapy on the developmental scores of iron‐deficient infants. Journal of Pediatrics 1978;92(1):21‐5. [DOI] [PubMed] [Google Scholar]
Idjradinata 1993 {published data only}
- Idjradinata P, Pollitt E. Reversal of developmental delays in iron‐deficient anaemic infants treated with iron. Lancet 1993;341(8836):1‐4. [DOI] [PubMed] [Google Scholar]
Kimmons [pers comm] {unpublished data only}
- Kimmons G, Moffatt MEK, Lonstaffe S, Whalen‐Besant J (University of Manitoba, Winnipeg, Canada). Short term effects of intramuscular iron on the behaviour of iron‐deficient children: a clinical trial [personal communication]. Letter to: Susana Martins (Federal University of São Paulo, São Paulo, Brazil) 30 November 2000.
Lozoff 1982 {published data only}
- Lozoff B, Brittenham GM, Viteri FE, Abraham WW, Urrutia JJ. The effects of short‐term oral iron therapy on developmental deficits in iron‐deficient anemic infants. Journal of Pediatrics 1982;100(3):351‐7. [DOI] [PubMed] [Google Scholar]
Lozoff 1987 {published data only}
- Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez R, et al. Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatrics 1987;79(6):981‐95. [PubMed] [Google Scholar]
Walter 1989 {published data only}
- Walter T. Infancy: mental and motor development. The American Journal of Clinical Nutrition 1989;50(3):655‐66. [DOI] [PubMed] [Google Scholar]
- Walter T, Andraca I, Chadud P, Perlales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics 1989;84(1):7‐17. [PubMed] [Google Scholar]
References to studies excluded from this review
Deinard 1986 {published data only}
- Deinard AS, List A, Lindgren B, Hunt JV, Chang PN. Cognitive deficits in iron‐deficient and iron deficient anemic children. Journal of Pediatrics 1986;108(5):681‐9. [DOI] [PubMed] [Google Scholar]
Linos 1990 {published data only}
- Gonzalez de Aledo Linos A, Rollán Rollán A, Bonilla Miera C. Prospective study of the prevalence of iron deficiency in breast fed infants in Cantabria, its relation to the introduction of cows milk and psychomotor development [Estudio prospectivo sobre la prevalencia de ferropenia en lactantes de Cantabria, su relacion con la introduccion de la leche de vaca el desarrollo psicomotor]. Anales Españoles de Pediatría 1990;32(1):24‐7. [PubMed] [Google Scholar]
Lozoff 1996 {published data only}
- Lozoff B, Wolf AW, Jimenez E. Iron deficiency anemia and infant development: effects of extended oral iron therapy. Journal of Pediatrics 1996;129(3):382‐9. [DOI] [PubMed] [Google Scholar]
Lozoff 2003 {published data only}
- Lozoff B, Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron‐deficiency anemia in healthy full‐term infants. Pediatrics 2003;112(4):846‐54. [PubMed] [Google Scholar]
Moffatt 1994 {published data only}
- Moffatt MEK, Longstaffe S, Besant J, Dureski C. Prevention of iron deficiency and psychomotor decline in high risk infants through use of iron fortified infant formula: a randomised clinical trial. Journal of Pediatrics 1994;125(4):527–34. [DOI] [PubMed] [Google Scholar]
Oski 1983 {published data only}
- Oski FA, Honig AS, Helu B, Howanitz P. Effects of iron therapy on behaviour performance in nonanemic, iron‐deficient infants. Pediatrics 1983;71(6):877‐80. [PubMed] [Google Scholar]
Stoltzfus 2001 {published data only}
- Stoltzfus RJ, Kvalsvig JD, Chwaya HM, Montresor A, Albonico M, Tielsch JM, et al. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ 2001;323(7326):1389‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walter 1983 {published data only}
- Walter T, Kovalskys J, Stekel A. Effect of mild iron deficiency on infant mental development scores. Journal of Pediatrics 1983;102(4):519‐22. [DOI] [PubMed] [Google Scholar]
Additional references
AHRQ 2006
- Agency for Healthcare Research and Quality. Screening for iron deficiency anemia in childhood and pregnancy. Publication No. AHRQ 06‐0590. [PubMed]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development. 2nd Edition. San Antonio: The Psychological Corporation, 1993. [Google Scholar]
Dallman 1975
- Dallman PR, Simes M, Manies EC. Brain iron: persistent deficiency following short term iron deprivation in the young rat. British Journal of Haematology 1975;31(2):209‐15. [DOI] [PubMed] [Google Scholar]
De Maeyer 1985
- Maeyer E, Adiels‐Tegman M. The prevalence of anaemia in the world. World Health Statistics Quarterly 1985;38(3):302‐16. [PubMed] [Google Scholar]
Felt 1996
- Felt BT, Lozoff B. Brain iron and behaviour of rats are not normalised by treatment of iron deficiency anaemia during early development. Journal of Nutrition 1996;126(3):693‐701. [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Goddard 2011
- Goddard AF, James MW, Mclntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut 2011;60(10):1309‐16. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lansdown 1995
- Lansdown R, Wharton BA. Iron and mental and motor behaviour in children. Iron, Nutrition and Physiological Significance: Report of the British Nutrition Foundation Task Force. London: Chapman and Hall, 1995:65‐78. [Google Scholar]
Larkin 1990
- Larkin EC, Rao GA. Importance of fetal and neonatal iron: adequacy for normal development of the central nervous system. In: Dobbing J editor(s). Brain, Behaviour and Iron in the Infant Diet. London: Springer‐Verlag, 1990:43‐62. [Google Scholar]
Lee 1993
- Lee GR. Wintrobe's Clinical Hematology. 9th Edition. Vol. 1, Philadelphia, PA: Lea & Febiger, 1993. [Google Scholar]
Logan 1997
- Logan S. Commentary on Booth IW, Aukett MA: Iron deficiency anaemia in infancy and early childhood. Archives of Disease in Childhood 1997;76:549‐54 [commentary pp 553‐4]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lozoff 1986
- Lozoff B, Brittenham GM. Behavioural aspects of iron deficiency. Progress in Haematology 1986;XIV:23‐53. [PubMed] [Google Scholar]
Martin 2001
- Martin S, Logan S, Gilbert R. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001444] [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roncagliolo 1998
- Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anaemia at 6 months: delayed maturation of auditory brainstem responses. The American Journal of Clinical Nutrition 1998;68(3):683‐90. [DOI] [PubMed] [Google Scholar]
Sachdev 2004
- Sachdev HPS, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutrition 2004;8(2):117‐32. [DOI] [PubMed] [Google Scholar]
Szajewska 2010
- Szajewska H, Ruszczynski M, Chmielewska A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. The American Journal of Clinical Nutrition 2010;91(6):1684‐90. [DOI] [PubMed] [Google Scholar]
Walter 1994
- Walter T. Effect of iron‐deficiency anaemia on cognitive skills in infancy and childhood. Bailliere's Clinical Haematology 1994;7(4):815‐27. [DOI] [PubMed] [Google Scholar]


