Abstract
Per- and polyfluoroalkyl substances (PFAS) are anthropogenic chemicals of concern that persist in the environment. Environmental monitoring revealed high concentrations of hexafluoropropylene oxide dimer acid (HFPO-DA) and other novel PFAS in the lower Cape Fear River; however, there is limited information on PFAS exposures and effects of this contamination on aquatic biota. Serum concentrations of 23 PFAS in Striped Bass (Morone saxatilis) from the Cape Fear River (n = 58) and a reference population from an aquaculture laboratory on the Pamlico/Tar watershed (n = 29) were quantified using liquid chromatography and high-resolution mass spectrometry, and correlations between PFAS concentrations and health-related serum biomarkers were evaluated. Perfluorooctane sulfonate, the predominant PFAS in Cape Fear River Striped Bass serum, was detectable in every sample with serum concentrations reaching 977 ng/mL. Perfluorononanoic and perfluorodecanoic acid were also detected in all samples, with perfluorohexanesulfonic acid present in > 98% of the samples. HFPO-DA (range < 0.24–5.85 ng/mL) and Nafion byproduct 2 (range < 0.2–1.03 ng/mL) were detected in 48% and 78% of samples, respectively. The mean total PFAS concentration found in domestic Striped Bass raised in well-water under controlled aquaculture conditions was 40 times lower, with HPFO-DA detected in 10% of the samples, and Nafion byproduct 2 was not detected. The elevated PFAS concentrations found in the Cape Fear River Striped Bass were associated with biomarkers of alterations in the liver and immune system.
1. Introduction
Per- and polyfluoroalkyl substances (PFAS) are a class of chemicals used for a variety of industrial applications and in consumer products. Due to their desirable chemical properties, such as stability and heat resistance, PFAS are components of aqueous film forming foams (AFFFs), heat resistant lubricants, non-stick coatings, water and oil repellents, and used as precursors and additives in fluoropolymers and plastics (Swedish Chemical Agency, 2015; Thalheimer et al., 2017). The same physical properties that make these chemicals desirable for industrial applications (longevity and heat-stability) also contribute to their persistence in the environment (Houde et al., 2011).
Since initial production in the 1950s, PFAS have become pervasive worldwide contaminants. The most recent comprehensive analysis from the Organization of Economic Cooperation and Development (OECD) identified 4730 PFAS-related CAS registry numbers, including 947 that were registered in the EPA Toxic Substances Control Act (TSCA) chemical inventory. Of those 947 TSCA registered PFAS, 602 are reported commercially active (OECD, 2018). From this large number of PFAS, there is extensive health and exposure related data for only two compounds (perfluorooctane sulfonate, PFOS and perfluorooctanoic acid, PFOA). There is less toxicity and exposure data available for a handful of additional PFAS, including the PFOA replacement hexafluoropropylene oxide dimer acid (HFPO-DA) and the PFOS replacement perfluorobutane sulfonate (PFBS) (US EPA, 2019). According to the National Health and Nutrition Examination Survey (NHANES), PFOA and/or PFOS were detected in serum of 95% of the United States population (Calafat et al., 2007; Khalil et al., 2016). Evidence from epidemiologic studies has linked increased human exposure to PFOA and PFOS with developmental toxicity, altered immune system function, cancer, metabolic changes and thyroid dysfunction (DeWitt, 2015). Based on the results of a systematic review of animal and epidemiological studies, the United States National Toxicology Program (NTP) has concluded, based on the weight of evidence, that PFOA and PFOS are hazards to the human immune system (DeWitt et al., 2012; Chang et al., 2016; Kielsen et al., 2016). In non-mammalian studies, PFOS was shown to modulate the immune system in zebrafish by altering the structure and function of the liver (Guo et al., 2019). In the Common Carp (Cyprinus carpio), experimental PFOS exposure caused a dose-dependent increase in leakage of liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Those findings were interpreted as PFOS exposure having resulted in hepatic cellular damage and necrosis (Hoff et al., 2003).
Because of their persistence, toxicity and adverse health effects, manufacturers participating in the 2010/2015 PFOA stewardship program report that they no longer produce PFOA and PFOS in the US, although exemptions permitting use and manufacturing still exist (UNEP, 2009). The use and resulting human exposure to PFOA and PFOS has since decreased; however, production and use of replacement (i.e. shorter-chain) fluorinated compounds has increased, based in part on limited data suggesting that some shorter chain PFAS are eliminated more rapidly (Bowman, 2015; Buck et al., 2011).
Surface water sampling data from the Cape Fear River (CFR) in North Carolina revealed that a growing number of novel replacement PFAS and manufacturing byproducts with diverse chemical structures are present in water at least 90 miles downstream from a PFAS production facility (Hopkins et al., 2018; Sun et al., 2016). Novel PFAS detected in the CFR included HFPO-DA (GenX), Nafion byproducts, perfluoro-3,5-dioxahexanoic acid (PFO2HxA), perfluoro-3,5,7-trioxaoctanoic acid (PFO3OA), perfluoro-3,5,7,9-tetraoxadecanoic acid (PFO4DA), and perfluoro-2-methoxyacetic acid (PFMOAA). Exposure to these contaminants of emerging concern has received much public attention because the sum concentration of novel PFAS has exceeded 100,000 ng/L at drinking water treatment plant intakes, these municipal drinking water treatments plants were unable to remove the PFAS, and their toxicity data were lacking (Sun et al., 2016; Zhang et al., 2019). There has also been additional concern about the unknown impacts of PFAS contamination on the health of the aquatic environment, and possible hazards associated with consuming fish and seafood harvested from contaminated waters.
To address the lack of PFAS exposure and health outcome data in wildlife from the CFR, this study examined PFAS serum levels in Striped Bass (Morone saxatilis) using liquid chromatography (LC), high-resolution mass spectrometry (MS), and analyzed associations of PFAS with several blood biomarkers of health. Once plentiful, the CFR Striped Bass population has been in decline for the past 50 years (Rachels and Morgeson, 2018). This economically important fishery has been under a commercial and recreational harvest moratorium since 2009 due to the lack of natural reproduction and poor survival (Hadley, 2015; Patrick and Moser, 2001). As a result, the CFR fishery has been maintained by stocking of hatchery-reared fish, with recent genetic analysis demonstrating that the population is composed almost entirely of these hatchery-reared fish (Rachels and Morgeson, 2018). Unlike more northern populations of Striped Bass that are anadromous and migratory, the CFR Striped Bass are non-migratory and spend their entire lives in the Cape Fear river and estuary system (Anderson et al., 2014; Callihan et al., 2014).
The objectives of this study were to: (1) determine the concentration of 23 legacy and novel PFAS in serum of Striped Bass sampled from both Cape Fear River (n = 58) and (2) determine the association between serum total and individual PFAS concentrations and biomarkers of overall health, and immune, liver, and renal function in both populations of Striped Bass. As a comparator reference population used to define background exposures, PFAS levels were evaluated in hatchery raised Striped Bass reared in water from a deep-well aquifer (Pamlico/Tar River) at the Pamlico Aquaculture Field Laboratory (PAFL).
2. Methods
2.1. Materials
Calibration solutions (n = 23; ranging from 0.1 ng/mL to 100 ng/mL) were prepared from neat standards and combined in calf serum. Standards for perfluoroalkyl carboxylic and sulfonic acids and HFPO-DA were obtained from Wellington Labs (Guelph, ON); the remaining fluoroethers were obtained from the Chemours Company (Wilmington, DE). The internal standard (IS) solution (0.1 M) was prepared by diluting a solution containing 11 mass-labeled PFAS in water and 0.1% formic acid: 13C4-PFBA, 13C2-PFHxA, 18O2-PFBS, 18O2-PFHxS, 13C8-PFOA, 13C4-PFOS, 13C9-PFNA, 13C9-PFDA, 18O2-PFOSA, 13C2-PFUnA, and 13C2-PFDOA (all from Wellington Labs, Guelph, ON).
Acetonitrile (ACN; Optima®, Lot 184819, Fisher Scientific, Hampton NC), methanol (Optima®, Lot 183859, Fisher Scientific), ammonium formate (99%, AC401152500, Fisher Scientific), and formic acid (99.5%, A117-50, Fisher Scientific) was used for extractions and LC eluent, and all laboratory glassware was rinsed with methanol and ACN prior to use. All water used for extractions was purified with a Milli-Q water system, and blanks of purified water were analyzed for contamination prior to use. National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 1957 (Organic contaminants in non-fortified human serum) was analyzed and used as a quality control material for this analysis.
2.2. Striped Bass sample collection
All animal procedures were performed with approval from the North Carolina State University Institutional Animal Care and Use Committee (protocol #19-021-O). Striped Bass serum was sampled in April 2018 from CFR (n = 65 total fish caught; n = 58 serum samples obtained and measured) and in August of 2018 from PAFL (n = 29). CFR wild-caught fish were collected by electroshocking and placed into floating holding tanks with oxygen aeration prior to blood sample collection; fin clips were collected for genetic analysis of parentage, sex was determined, body weight and length were measured. Aquaculture fish were netted and placed into aerated holding tanks. Sex of these juvenile fish was not determined. Prior to blood sample collection, fish weight and length were measured. For blood collection, a 10 mL Luer lock syringe (BD, Franklin Lakes, NJ) with a 20 gauge 1.5 in. (0.9 mm × 40 mm) needle was used to collect 3–5 mL of blood from the caudal vein. Blood was injected into serum blood collection tubes (BD Vacutainer, Franklin Lakes, NJ) and allowed to clot at ambient temperature for at least 30 min, then placed on ice. All sampled fish were released immediately following blood collection. Samples were transported on ice to North Carolina State University (NCSU) where serum was collected following centrifugation (1800g for 10 min at 4 °C). Serum fractions were immediately aliquoted into Teflon-free cryovials and stored at −80 °C until analysis.
2.3. Sample blinding, blanks and controls
Each animal was assigned a randomized numeric code by NCSU researchers prior to measurement and analysis of PFAS and serum biochemistry markers. Samples were decoded for site and length/size only after PFAS measurement/analysis was completed. Samples used for serum biochemistry markers were selected based on the availability of adequate sample volume after PFAS analysis was completed. Identically treated field blanks (n = 5) were made by injecting 5 mL of Milli-Q water into the blank labeled serum tubes at each field collection site. A matrix matched quality control (QC) sample was made by combining randomly selected aliquots of CFR Striped Bass serum (n = 16). That material was used to test method reproducibility and stability (Table S2). Charcoal stripped fetal bovine serum (FBS; Life Technologies, Grand Island, NY; cat #10437, Lot #1754113; total protein 3.7 g/dL) spiked with 5 ng/mL of PFAS standards was used for calibration verification (n = 3); purified water field blanks (n = 5) and FBS blanks (n = 10) were included in the analysis. Reported reference total serum protein values for wild and aquaculture Striped Bass were comparable to levels in FBS used as QC material (Young et al., 1994; Hrubec et al., 2001)
2.4. Sample preparation
All samples were extracted using the following method: 50 μl of sample was added to a 2 mL polypropylene tube with 100 μl of 0.1 M formic acid combined with internal standards (5 ng). After a 3 s vortex, 450 μl of −20 °C ACN was added to the tube and vortexed for 3 s, and the sample was centrifuged at 12,500g for 5 min at room temperature. The supernatant (100 μl) was added to 300 μl of aqueous 0.4 mmol of ammonium acetate, and transferred to polypropylene autosampler vials.
2.5. Detection and quantification of PFAS in serum
Chromatographic separation was performed using a Vanquish UPLC system (Thermo Fisher Scientific, Waltham, MA) and Accucore C18 + column (2.1 mm × 100 mm × 1.5 μ particles) at a flow rate of 300 μl/min, injection volumes of 40 μl, and a binary mobile phase gradient composed of Solvent A (95:5 H2O:ACN, 0.4 mM ammonium acetate) and Solvent B (95:5 ACN:H2O, 0.4 mM ammonium acetate). Mobile phase compositions are detailed in supplemental table 1. PFAS were detected using with a Thermo Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific, Waltham, MA) with a heated electrospray ionization (HESI) source operated in negative mode. Data were collected in data dependent mode for compound validation, with a preferred ion list consisting of the quantitated PFAS standards. Quantitation was based on an eight-point calibration curve (with two injections per concentration) of the internal standard normalized integrated peak area of the extracted ion chromatogram of the [M-H]-ion with a 5-ppm mass tolerance. The r2 values of all calibration curves used for analysis were > 0.98; limits of detection (LOD) were defined as the estimated concentration of blanks plus three standard deviations (Table S-2). Replicate PFAS measurements of SRM 1957 (n = 6) were compared to the values on the Certificate of Analysis for the NIST SRM 1957 standards and were within 5% of expected values.
2.6. Lysozyme assay
A fluorescence based EnzChek® Lysozyme Assay (Thermo Fisher Scientific, Waltham, MA, Cat. E-22013) was used according to manufacturer’s protocols to determine lysozyme activity in serum of a subset of Striped Bass samples (CFR: n = 28, PAFL: n = 29). Samples were diluted 1:5 in sample buffer and analyzed in triplicate, the average relative standard deviation (RSD) for QC and experimental samples were 5% and 7%, respectively.
2.7. Blood chemistry
Blood chemistry values for 14 parameters (listed in supplemental table 3) were obtained for the subset of samples with sufficient volume of serum for analysis (CFR: n = 28, PAFL: n = 7) using a VetScan VS2 Whole Blood chemistry analyzer with Avian/Reptilian Profile Plus and T4/Cholesterol profile rotors (500–0041, 500-1037; Abaxis, Union City, CA). All procedures followed manufacturer’s protocols using 120 μl of serum. Blood chemistry and T4/Cholesterol analysis was previously validated for fish samples (Bowden et al., 2016).
2.8. Data and statistical analysis
Data were analyzed using SPSS (Version 24.0, IBM Corp., Armonk, NY, USA) and JMP Pro (Version 14, SAS, Raleigh, NC, USA). Data visualization utilized Graphpad Prism (version 8, Graphpad Prism Software, La Jolla, CA, USA) and the R statistical programming environment version 3.5.2. A Shapiro-Wilk’s test was used to assess normality of data; for data requiring normalization a natural log or log10 transformation was used. Non-parametric statistics were used for data not meeting analysis model assumptions following data transformations. PFAS concentration values were log10 transformed. Mean values, as well as ranges in concentration, were determined from values above the LOD; when PFAS levels were below the detection limit, the concentration was set to the LOD divided by the square root of two for statistical analysis (Glass and Gray, 2001). Percentages of detection above LOD calculations for each PFAS were determined by dividing the number of samples with detection above LOD by the total number of samples analyzed. Spearman’s rank correlation coefficient or Pearson’s correlation coefficient (PFOS, PFDA, PFNA) between PFAS serum concentrations and serum biomarkers was limited to PFAS detected in > 30% of samples analyzed. Comparisons across serum biomarkers at each site was not done due to controlled rearing conditions at PAFL and known differences in range of juveniles and adults. Sex differences were evaluated using a Student’s t-test with Welch’s correction. Principal component analysis was performed to dimensionally reduce the data using a restricted maximum likelihood estimation (REML) and components with a minimum eigenvalue of 1. Loading scores of individual blood chemistry parameters above ± 0.40 were assessed with further regression analysis (Floyd and Widaman, 1995). A hierarchical linear regression analysis at each site was used to examine the relationship between PFOS levels and serum lysozyme, AST, total protein and albumin, with sex, age, length and weight of fish included as covariates in the statistical model. A minimal level of statistical significance for differences in values among or between groups was considered p < .05.
3. Results
3.1. Striped Bass size and weight distribution
Total length of Striped Bass sampled from the CFR ranged from 37.4 to 80.4 cm (M = 61.2; SD = 8.2; n = 65), and weight ranged from 747 to 5700 g (M = 2894; SD = 1056). Females (M = 3324 g; SD = 1010; n = 36) were heavier than males (M = 2284 g; SD = 943; n = 29), t (64) = 4.32, p < .0001, df = 62.8. Females were also longer than males (female: M = 64.9 cm, SD = 6.62; male: M = 55.7 cm, SD = 10.2), t (64) = 4.51, p < .0001, df = 54. Weight and length were positively correlated, Pearson’s r(65) = 0.971, p < .0001 (Table 1). The total length of Striped Bass sampled from PAFL ranged from 32.6 to 41.9 cm (M = 36.7; SD = 1.97), and total weight ranged from 350 to 930 g (M = 594; SD = 112). Total weight and length were positively correlated, Pearson’s r(29) = 0.906, p < .0001 (Table 1). Study animal characteristics from both populations are detailed in supplemental table 4.
Table 1.
Correlations between serum PFAS concentration, weight and length.
| Cape Fear | |||||||
|---|---|---|---|---|---|---|---|
| Weight | PFNA | PFDA | PFOS | PFHxS | GenX | Nafion BP2 | |
| Length | 0.974 b | 0.272 a | −0.507b | −0.629b | 0.184 | −0.201 | −0.283a |
| Weight | – | 0.262 | −0.502b | −0.628b | 0.165 | −0.158 | −0.285a |
|
| |||||||
| PAFL | |||||||
|
| |||||||
| Weight | PFNA | PFDA | PFOS | PFBS | |||
| Length | 0.847 b | 0.075 | 0.214 | 0.333 | 0.026 | ||
| Weight | – | 0.154 | 0.273 | 0.259 | −0.093 | ||
Shown are correlation coefficients for PFAS with detection frequency > 30%; Cape Fear River n = 55, PAFL n = 29. Significant correlations are indicared in bold,
P < .05,
P ≤ 0.01.
3.2. PFAS detection frequency and serum concentrations
Of the 23 PFAS analyzed, 11 were detected in CFR samples and 8 were detected In PAFL samples (Table 2). The mean total PFAS concentration in Striped Bass serum samples from the CFR was 40 times than the mean concentration observed at PAFL (Fig. 1A). PFOS was the dominant PFAS found in samples from each site, it was detected in 100% of the samples analyzed and accounted for 89% of the total PFAS present in CFR serum samples (M = 490 ng/mL; SD = 186), and 69% of the total PFAS in the PAFL samples (M = 9.4 ng/mL; SD = 2.8), as shown in Fig. 1A–B and Table 2. HFPO-DA (GenX) was detected in 48% of the CFR samples (M = 1.9 ng/mL; SD = 1.6; Fig. 1C), a similar concentration (M = 1.64 ng/mL; SD = 0.95) was present in just 10.3% of samples from PAFL (Table 2). Nafion byproduct 2 was detected in 78% of CFR Striped Bass serum (Fig. 1D), but was not detected in any PAFL sample.
Table 2.
PFAS detection frequency and concentration in Striped Bass serum.
| LOD (ng/mL) | CFR % > LOD (n=58) | PAFL % > LOD (n=29) | Concentration (ng/mL) mean (range) |
||
|---|---|---|---|---|---|
| Cape Fear | PAFL | ||||
|
| |||||
| PFNA | 0.34 | 100 | 96.9 | 4.49 (0.81-11.6) | 0.48 (0.34-0.82) |
| PFDA | 1.68 | 100 | 96.9 | 68.0 (10.2-146) | 2.5 (1.68-4.6) |
| PFOS | 2.47 | 100 | 100 | 490 (122-977) | 9.41 (4.62-16.5) |
| PFHxS | 0.09 | 98.3 | 3.4 | 0.78 (0.15-2.23) | 0.59 |
| Nafion byproduct 2 | 0.25 | 77.6 | 0 | 0.30 (0.25-1.03) | All < LOD |
| GenX | 0.24 | 48.3 | 10.3 | 1.91 (0.31-5.85) | 1.64 (0.24-2.3) |
| PFBS | 0.01 | 24.1 | 44.8 | 0.15 (0.01-1.35) | 0.01 (0.01-0.2) |
| PFO5DoDA | 0.01 | 22.4 | 0 | 0.49 (0.01-1.35) | All < LOD |
| PFOA | 0.16 | 15.1 | 13.8 | 0.57 (0.16-4.29) | 0.16 (0.16-1.14) |
| PMPA | 0.12 | 13.8 | 10.3 | 0.12 (0.12-0.19) | 0.12 (0.12-0.14) |
| PFBA | 0.11 | 13.8 | 0 | 0.11 (0.11-0.18) | All < LOD |
LOD, limit of detection; CFR, Cape Fear River; PAFL, Pamlico aquaculture field laboratory
Fig. 1.
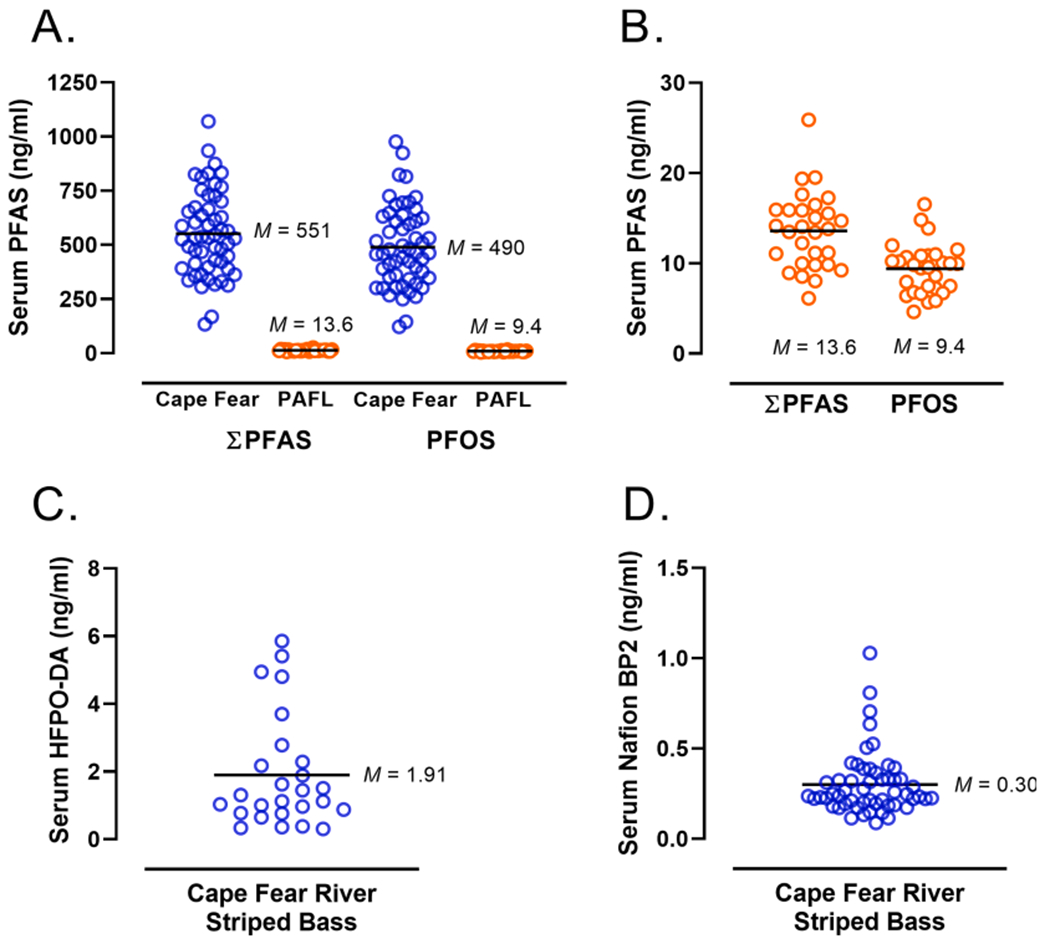
Comparative PFAS concentrations in serum of Striped Bass from the Cape Fear River and Pamlico aquaculture field laboratory. (A) Total serum PFAS (ΣPFAS) concentration (ng/mL or ppb) and PFOS (ng/mL) in Cape Fear River (CFR) and Pamlico aquaculture field laboratory (PAFL) Striped Bass. Mean (M) values are indicated. (B) Comparison of lower levels of total PFAS and PFOS detected in individual Striped Bass at PAFL. (C) Serum levels (ng/mL or ppb) of HFPO-DA (“GenX”) in Cape Fear River Striped Bass (D) Nafion byproduct 2 concentration (ng/mL or ppb) found in CFR Striped Bass; PAFL Striped Bass had no measurable levels of Nafion byproduct 2 in serum.
3.3. Size, weight, and PFAS concentration correlation analysis
In CFR Striped Bass, the PFNA serum concentration was positively correlated with length, (rs(55) = 0.272, p = .047), whereas concentrations of PFDA, PFOS, and Nafion byproduct 2, were negatively correlated with both length and weight (Table 1). For PFAL Striped Bass, no significant correlations between PFAS serum concentrations and length or weight were identified (Table 1).
In CFR fish, serum concentrations of PFDA, PFOS, and Nafion byproduct 2 were positively correlated with each other (Table 3). Serum concentrations of GenX and PFDA (rs(55) = 0.504, p < .0001), and Nafion byproduct 2 and PFHxS, (rs(55) = 0.324, p = .022), were also positively correlated. In PAFL Striped Bass, the serum concentrations of PFDA, PFNA, and PFOS were each positively correlated with one another (Table 3), but not with PFBS.
Table 3.
Correlations between serum concentrations of individual PFAS.
| Cape Fear | ||||||
|---|---|---|---|---|---|---|
| PFNA | PFDA | PFOS | PFHxS | GenX | Nafion BP2 | |
| PFNA | – | 0.229 | 0.191 | 0.707 b | 0.083 | 0.433 b |
| PFDA | – | – | 0.773 b | 0.014 | 0.504 a | 0.693 b |
| PFOS | – | – | – | 0.091 | 0.308 | 0.477 b |
| PFHxS | – | – | – | – | 0.046 | 0.324 a |
| GenX | – | – | – | – | – | 0.175 |
|
| ||||||
| PAFL | ||||||
| PFNA | PFDA | PFOS | PFBS | |||
| PFNA | – | 0.762 b | 0.739 b | 0.077 | ||
| PFDA | – | – | 0.974 b | 0.129 | ||
| PFOS | – | – | – | 0.116 | ||
Shown are correlation coefficients for PFAS with detection frequency > 30%; Cape Fear River n = 55, PAFL n = 29. Bold indicates significant correlations,
P < .05,
P < 0.01.
3.4. Serum biomarker analysis
Principal components analysis indicated that aspartate aminotransferase (AST), creatine kinase (CK), uric acid (UA), total protein (TP), globulin, albumin (ALB), and potassium (K) contributed to 71.1% of the variation in the CFR blood chemistry data (supplemental Fig. 1). Significant correlations between serum concentrations of PFOS, Nafion byproduct 2, PFDA, PFNA and PFHxS and at least one serum biomarker were identified (Table 4).
Table 4.
Correlation between PFAS concentrations and serum biomarkers in Cape Fear River and PAFL Striped Bass.
| Cape Fear | |||||
|---|---|---|---|---|---|
| PFOS | Nafion BP2 | PFDA | PFNA | PFHxS | |
| Lysozyme | 0.440 b | 0.315 | 0.135 | −0.357 | −0.345 |
| AST | 0.373 a | 0.356 a | 0.297 | −0.312 | −0.025 |
| Creatine kinase | 0.311 | 0.432 a | 0.346 | 0.059 | 0.099 |
| Uric acid | −0.055 | −0.326a | −0.054 | 0.322 a | 0.031 |
| Total protein | 0.439 a | 0.283 | 0.432 a | 0.223 | 0.248 |
| Albumin | 0.426 b | 0.462 b | 0.355 b | 0.467 b | 0.488 b |
|
| |||||
| PAFL | |||||
| PFOS | PFDA | ||||
| Lysozyme | 0.287 | 0.071 | |||
| AST | 0.333 | 0.217 | |||
| Creatine kinase | 0.333 | 0.211 | |||
| Uric acid | 0.410 | 0.325 | |||
| Total protein | 0.690 a | 0.564 | |||
| Albumin | 0.633 | 0.604 a | |||
Shown are correlation coefficients for PFAS significantly associated with at least one serum biomarker. Bold indicates significant correlations,
P < .05,
P ≤ 0.01.
Heatmap visualization for the correlation between serum markers and each PFAS in CFR Striped Bass serum are shown in Fig. 2A. No significant correlations between PFAS concentrations and globulin or K were identified. Serum PFOS concentration was positively correlated with AST (r(23) = 0.373, p = .03), TP (r(28) = 0.439, p = .009), and ALB (r(28) = 0.426, p = .01) in CFR Striped Bass. Nafion byproduct 2 was positively correlated with AST (rs (23) = 0.356, p = .047), CK (rs(28) = 0.432, p = .01), and ALB (rs(28) = 0.462, p = .006). PFDA was positively correlated with TP (r(28) = 0.432, p = .019), and ALB (r(28) = 0.388, p = .03). PFNA concentration was positively correlated UA (rs(28) = 0.322, p = .043) and ALB (rs(28) = 0.467, p = .006), and PFHxS and ALB (rs(28) = 0.488, p = .006) were positively correlated. At PAFL, PFOS and PFDA were correlated with serum TP and ALB, respectively (Table 4).
Fig. 2.
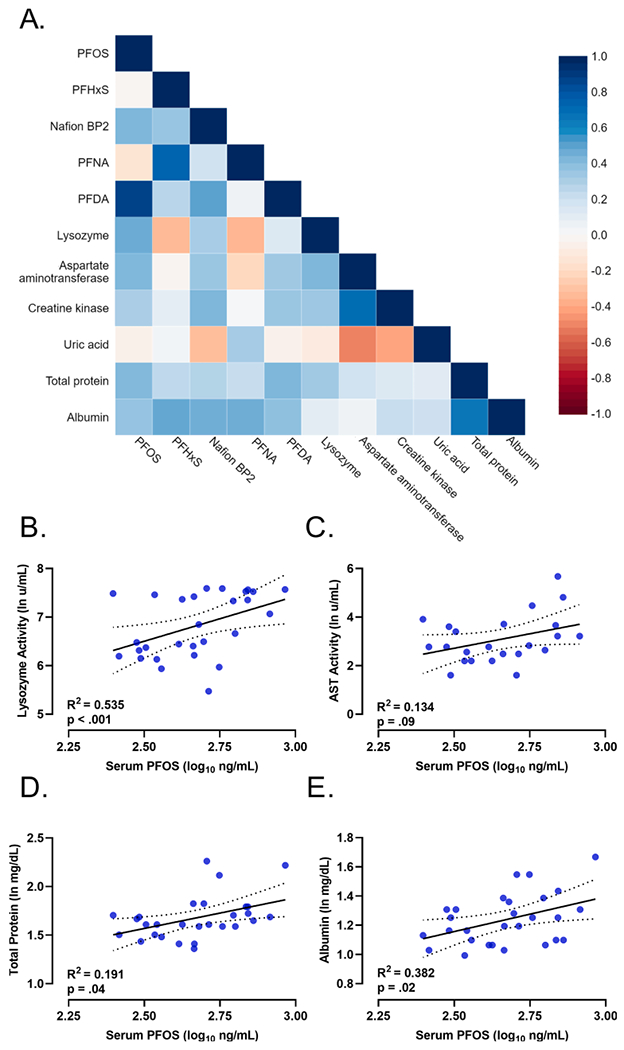
Association of PFAS with serum biomarkers in Cape Fear River Striped Bass. (A) Heatmap of serum PFAS and health markers in Cape Fear River (CFR), n = 28. PFOS, PFDA, Nafion byproduct 2 (BP2), aspartate aminotransferase (AST), creatine kinase (CK), Lysozyme, PFHxS, uric acid (UA), total protein, and albumin were examined with Spearman’s rank correlation coefficient or Pearson’s correlation coefficient (r) and put into a heatmap of increased and decreased r values (blue, r = 1, red, r = −1). (B) Scatterplot of linear regression analysis with 95% confidence intervals for log transformed serum PFOS concentration (ng/mL) and natural log lysozyme activity in CFR fish, (C) natural log transformed aspartate aminotransferase, (D) natural log transformed total protein, and (E) natural log transformed albumin. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Serum PFOS concentration was correlated with serum lysozyme activity (r(28) = 0.440, p < .001) in CFR fish. There was no correlation between serum PFOS concentration and lysozyme activity in PAFL Striped Bass (rs(27) = 0.287, p = .147). Hierarchical linear regression with length, weight, and age included in the statistical model was used to determine if those factors influenced the association between PFOS concentration and lysozyme activity. Model 1 with only PFOS concentration predicted lysozyme concentration [F(1,29) = 6.248p = .004] accounting for 24.7% of the variation in lysozyme concentration with an R2 = 0.247. Model 2 with PFOS, age, length, and weight included as covariants improved the prediction of serum lysozyme by 28.8%, with an R2 = 0.535 [F(4, 26) = 7.48, p < .001] (Fig. 2B). Regression analysis (Fig. 2C–E) in CFR Striped Bass also indicated that PFOS concentration with age, length, and weight considered as covariants had a significant linear relationship with TP, R2 = 0.191 [F(4,26) = 6.217 p = .04], and ALB, R2 = 0.382 [F(4,26) = 5.749, p = .02], and was suggestive for AST, R2 = 0.134 [F(4,24) = 3.396, p = .09].
4. Discussion
Analysis of serum PFAS concentrations in Striped Bass from the Cape Fear River, an important commercial and recreational fishery along the Atlantic Coast of North America, indicated ubiquitous PFAS contamination. Detectable levels of multiple PFAS were present in the serum of every Striped Bass sampled from the Cape Fear River, with much lower background levels of PFAS detectable in every fish reared in an aquaculture facility that used well water. The elevated concentrations of PFOS and several other PFAS found in the CFR Striped Bass were associated with increased lysozyme, and AST activities, suggesting that these exposures were impacting both immune and liver functions. While these data are consistent with effects of PFOS in experimental fish and mammalian models, additional data are needed to determine causal relationships between increasing PFOS concentrations and adverse impacts to the liver and immune system of Striped Bass.
4.1. Unique PFAS profiles and increased PFOS in smaller Striped Bass of Cape Fear River
Striped Bass in the CFR exhibited high levels of PFOS in serum which was correlated with alterations in biomarkers of immune and liver dysfunction. The mean concentration of PFOS detected in CFR Striped Bass (490 ng/mL) is one of the highest levels recorded in serum for any fish species in North America (Reiner and Place, 2015; Giesy and Kannan, 2001). For aquaculture Striped Bass, the mean levels of PFOS (9.4 ng/mL) were comparable to other fish analyzed at relatively remote areas globally (Giesy and Kannan, 2001), and in areas with little or no known manufacturing or sources of aqueous film forming foam (AFFF) contamination (Fair et al., 2019; Bangma et al., 2017). While present, it is concluded that these levels are indicative of a ubiquitous background contamination of PFAS. The levels of PFOS found in CFR Striped Bass suggest upstream source(s) of contamination consistent with historically higher levels of PFOS contamination in the Cape Fear River (Nakayama et al., 2007). The potential use of PFAS-containing AFFFs for fire suppression or training exercises at military bases, airports, and municipalities upstream of the CFR sampling site could contribute as sources of the high levels of exposure observed in CFR Striped Bass (Place and Field, 2012).
In general, PFOS is the PFAS found at highest concentrations in wildlife (Reiner and Place, 2015), and numerous exposure studies have also demonstrated that PFOS is the most frequently detected PFAS in fish tissue and plasma/serum (Fair et al., 2019; Bangma et al., 2018). Although PFOA levels have also been historically high in surface water sampled from the Cape Fear river (Nakayama et al., 2007), PFOA was infrequently detected at relatively low concentrations in CFR Striped Bass. Also, PFOA was infrequently detected at low levels at the PAFL reference site. Surface water monitoring data from 2018 at the CFR site of collection (Lock and Dam #1) are detailed in supplemental table 6; and reveal that PFOA and PFOS was found at a concentration of 6 ng/L and < 3 ng/L, respectively. Striped Bass sampled from the Cape Fear river ranged in age from 2 to 8 years (Patrick and Moser, 2001). Thus, the PFAS levels detected in the Striped Bass serum likely reflect contemporary exposures to PFAS in the Cape Fear River and surrounding estuarine environment.
Long-chain PFOS and PFDA were the main PFAS present in the Striped Bass serum; however, in samples from the CFR a variety of additional PFAS, including recently identified fluoroethers were also frequently detected. Surface water sampling on the CFR has indicated the presence of byproducts of PFAS manufacturing, including PFMOAA, PF02HxA, PF030A, PF04DA, HFPO-DA (“GenX”), and Nafion byproduct 2 (Hopkins et al., 2018). In the Striped Bass samples analyzed here, both GenX and Nafion byproduct 2 were detected frequently with detectable levels of PMPA and PFO5DoDA occurring less frequently. Nafion byproduct 2 and PFO5DoDA were detected exclusively in the CFR fish, with no detections in serum of Striped Bass from the PAFL site. Structurally, Nafion byproduct 2 resembles PFOS (supplemental Fig. 2), and may therefore exhibit similar properties. Further study is needed to determine the half-life of Nafion byproduct 2 in aquatic organisms.
Shorter chain PFAS, such as GenX and PFBS, are used as presumably safer alternatives to PFOA and PFOS, in part because of their shorter biological half-life and lesser potential to bioaccumulate. Both Nafion byproduct 2 and GenX were found at lower serum concentrations than PFOS; although each of these PFAS was detected in over 48% of samples from the CFR. It is notable that mean serum GenX concentrations were more than 136 times higher than concentrations present in contemporary surface water samples collected 1.2 miles downstream from Lock and Dam #1 (supplemental table 6). Nafion byproduct 2 was detected in surface water at the sampling site at 18 ng/L, and average levels were found at 17 times higher concentrations in Striped Bass serum (Zhang et al., 2019). Together, these findings indicate that shorter-chain PFAS are bioaccumulating in Striped Bass, and presumably other fish species. This interpretation is supported by findings of similarly elevated levels of PFAS in pooled liver samples of fish from the Yangtze River (Liu et al., 2018).
Recent experimental studies in Crucian Carp (Carassius carassius) has revealed that the mechanism mediating bioaccumulation and tissue distribution of shorter-chain PFAS involves protein binding (Shi et al., 2018). The observed negative correlation between PFOS, PFDA, and Nafion byproduct 2 serum concentrations and body weight and size of Striped Bass from the CFR fish indicates that PFAS bioaccumulation occurs through a mechanism unrelated to accumulation of lipophilic persistent organic pollutants (POPs) in lipid rich body compartments. Typically, the bioaccumulation of POPs in fat is associated with elevated serum levels in larger sized animals indicative of higher overall body burdens (Guillette et al., 1999). Whereas tissue PFOS concentrations and body size were also inversely correlated in male tilapia, a similar association between PFAS levels and body weight was not found in Striped mullet (Bangma et al., 2018). It is likely that the relative elevation of PFAS in smaller fish sampled in our study results from a combination of factors, including size-related differences in diet and movement within the river, differential tissue distribution, and differences in clearance rate for individual PFAS. Additionally, it is also possible that the larger sexually mature Striped Bass may be traveling further distances into the CFR estuary system, and the smaller fish may remain more localized to sources of contamination in the river system. Further studies are need to define the specific factors influencing exposure, bioaccumulation, and the resulting adverse impacts of PFAS on Striped Bass and other coastal and inland fish.
Due to declining stocks and lack of natural recruitment, there is a capture moratorium in place for Striped Bass on the Cape Fear and surrounding rivers of North Carolina that limits human consumption of these fish. However, several other species within this system (i.e. Flathead catfish, Largemouth Bass) are regularly harvested for consumption. Given the high amount of PFOS found in CFR Striped Bass, it seems likely that other piscivorous fish species share a significant body burden of PFAS. Recreational harvest of CFR fish is likely an important route of exposure for communities living near this site, as fish/seafood consumption is linked to human PFAS exposure (Haug et al., 2010). Currently there are no fish consumption guidelines for PFAS in coastal North Carolina, however a number of states have established fish consumption recommendations for PFAS. For example, the New Jersey Department of Environmental Protection has implemented a health guideline that specify Striped Bass are a ‘do not eat’ species for high-risk populations, including pregnant women and women of child-bearing age, and a once per month guideline for general populations harvesting fish from marine or estuarine waters (NJDEP, 2019). As observed here, examination of fish tissue from 11 waterways across New Jersey found PFOS as the primary PFAS in fish with a maximum concentration of 162.5 ng/g tissue (NJDEP, 2019). Although serum PFAS levels were analyzed here, the high levels of PFOS detected in CFR Striped Bass serum supports the need for additional analysis aimed at characterizing PFAS present in tissues of a variety of different consumed fish from the CFR and other inland waterways. Eliminating this important data gap will be critical in establishing accurate consumption recommendations for fish from inland and coastal waters of North Carolina.
4.2. Association of PFOS with altered immune function
Lysozyme is an important marker of the innate immune system function (Rauta et al., 2012). Lysozyme, along with other innate immunity molecules such as C-reactive protein and interferon are important in maintaining immunologic homeostasis in fish due to their pathogen rich environment (Saurabh and Sahoo, 2008). Lysozyme is secreted by macrophages and circulating enzymatic activity is a biomarker of systemic inflammation (Torsteinsdóttir et al., 1999). Increasing serum PFOS concentrations were positively correlated with lysozyme activity in the CFR Striped Bass, suggesting a relationship between PFOS and increased activity of the innate immune system. This finding is similar to experimental results from a variety of studies in vertebrate species where increased circulating PFOS levels are associated with increased lysozyme activity. For example, lysozyme activity was increased in 14-day old chickens exposed to PFOS from 1 mg/kg egg mass (serum PFOS concentration 154 ng/g) (Peden-Adams et al., 2009), and in female mice, 0.1 mg/kg body weight PFOS (serum PFOS concentration 123 ng/g) (Peden-Adams et al., 2008). Elevated lysozyme activity in both mammalian and non-mammalian species were observed at serum PFOS levels well-below the mean PFOS concentration (490 ng/mL) found in serum of CFR Striped Bass. Additionally, exposure of yellow perch (Perca flavescens) to a suite of PFAS from contaminated upstream aqueous effluent, resulted in altered immune-related gene expression in the liver, and a positive association between PFDA concentration and lysozyme activity was detected (Houde et al., 2014). The consistent finding across diverse taxa of increased PFAS exposure resulting in increased lysozyme activity, suggests that increases in serum lysozyme activity is a fundamental biomarker of PFAS immunotoxicity.
4.3. Associations between PFOS and Nafion byproduct 2 with increased liver enzyme activity
Increased concentrations of PFOS and Nafion byproduct 2 in CFR Striped Bass were also associated with increased AST activity. The positive association between increased PFOA and PFOS exposure and AST activity is well established from results of human epidemiological studies, laboratory toxicology investigations, and wildlife studies (Nian et al., 2019; Xing et al., 2016). In fish, serum concentrations of liver AST of the Common Carp increased with increasing PFOS dose (Hoff et al., 2003). To our knowledge, this is the first study to characterize an association between Nafion byproduct 2 serum levels and altered liver enzyme activity in wildlife species or any experimental model system. These findings suggest that Nafion byproduct 2 exposures may also alter and adversely impact liver function.
Total protein and ALB are also clinical indicators of renal and liver health; however, it is important to note that some PFAS (e.g. PFOS and PFOA) bind to serum albumin (ALB) (Beesoon and Martin, 2015) and can complicate the interpretation of associations between PFAS concentration and changes in these serum protein biomarkers. Associations between ALB and the concentration of PFAS that bind to serum ALB, could result from increases in PFAS causing an increase in hepatic ALB expression. Alternatively, in individuals with higher concentrations of serum ALB, the association may be driven by an increase in PFAS binding sites present in serum, rather than by an adverse impact on hepatic function. The binding properties of most PFAS, with the exception of PFOS and PFOA, for ALB and other serum proteins are uncharacterized (Liu et al., 2019), and careful interpretation of clinical biomarkers data based on associations of PFAS levels and changes in serum protein levels is necessary. Although, interpretation of the observed correlation between PFOS concentration and alterations in serum protein levels is complicated, the alterations in other serum markers of liver and immune dysfunction (i.e. lysozyme and AST enzyme activities) were also observed in Striped Bass from the CFR. The activity of those enzymatic biomarkers is not expected to be influenced by direct binding of PFAS and therefore strengthens the evidence that increasing PFAS concentrations were associated with alterations of both liver and immune function.
It is important to note that several fish species also have species-specific differences in serum protein expression and activities. Certain fish species, including one used for guideline bioaccumulation studies - the Common Carp, express different fatty acid binding proteins, that are not equivalent to those found in human serum albumin; these functions are instead mediated by high density apolipoproteins (De Smet et al., 1998). The affinity of individual PFAS for binding of fatty acid binding proteins (FABP) in the liver or organic anion transporter (OAT) proteins in the kidney may also contribute to species specific differences in PFAS concentrations observed in serum and specific body compartments (Ng and Hungerbühler, 2013; Fang et al., 2014). As a result, for individual PFAS there may be important species-specific differences in patterns of bioaccumulation. Analysis of PFAS levels in a wide range of fish species will be necessary to develop comprehensive understanding of ecosystem health impacts for individual PFAS and for the development of fish consumption guidelines. Future dosing studies in aquatic vertebrates will be needed to determine which novel PFAS and manufacturing byproducts alter the immune system and liver function.
Overall, the Striped Bass population within the Cape Fear River exhibited elevated PFAS serum levels compared to background levels detected in reference aquaculture-reared fish from a different watershed (Pamlico/Tar) within North Carolina. The levels of PFAS detected in CFR Striped Bass represent one of the highest levels of PFOS documented in the serum of any fish species (Delinsky et al., 2010). Measurable levels of PFOS, PFDA, PFNA, and PFBS were detected in the “reference” site supporting the ubiquitous nature of PFAS contamination. No wildlife study to date has shown a site free of PFAS contamination (Giesy and Kannan, 2002). From the Artic, to the remote wilderness of South Africa, PFAS contamination is a global issue (Christie et al., 2016; Young et al., 2007).
Supplementary Material
Acknowledgements
Research reported in this publication was supported in part by NIEHS under award number P30ES025128, R21ES029353, NC SeaGrant Community Collaborative Research Grant, and NC Policy Collaboratory PFAS testing network, Foundation for Food and Agriculture Research (New Innovator in Food and Agriculture Research), and NC State University Agricultural Foundation (William White Endowment). The Striped Bass is a priority species for the USDA National Research Support Project 8 (NRSP-8, National Animal Genome Project), and some fish were provided for this study from the National Program for Genetic Improvement and Selective Breeding for the Hybrid Striped Bass Industry. This is publication number 117 from the NC State University Pamlico Aquaculture Field Laboratory. We are indebted to Thomas Jackson, Dana Hodorovich, and Helen Nguyen who assisted in various aspects of the study and gave critical feed-back on the manuscript.
Although EPA employees contributed to this article, the research presented was not funded by EPA and was conceived, designed, and implemented by NCSU. EPA’s role was limited to advising the PFAS serum analysis and therefore not subject to EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed in the article are solely those of the authors and do not necessarily reflect or represent EPA’s views or policies.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2019.105358.
Footnotes
Declaration of Competing Interest
We declare that we have no conflict of interest.
References
- Swedish Chemical Agency KEMI, 2015. Occurrence and Use of Highly Fluorinated Substances and Alternatives, Report 7/15, vol. 361(164), pp. 112. [Google Scholar]
- Anderson AP, Denson MR, Darden TL, 2014. Genetic Structure of Striped Bass in the Southeastern United States and Effects from Stock Enhancement. North Am. J. Fish. Manag. 34 (3), 653–667. 10.1080/02755947.2014.902409. [DOI] [Google Scholar]
- Bangma JT, Reiner JL, Botha H, Cantu TM, Gouws MA, Guillette MP, Koelmel JP, Luus-Powell WJ, Myburgh J, Rynders O, et al. , 2017. Tissue Distribution of Perfluoroalkyl Acids and Health Status in Wild Mozambique Tilapia (Oreochromis Mossambicus) from Loskop Dam, Mpumalanga, South Africa. J. Environ. Sci. 61, 59–67. 10.1016/j.jes.2017.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangma JT, Reiner JL, Lowers RH, Cantu TM, Scott J, Korte JE, Scheidt DM, McDonough C, Tucker J, Back B, et al. , 2018. Perfluorinated Alkyl Acids and Fecundity Assessment in Striped Mullet (Mugil Cephalus) at Merritt Island National Wildlife Refuge. Sci. Total Environ. 619–620, 740–747. 10.1016/j.scitotenv.2017.11.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesoon S, Martin JW, 2015. Isomer-Specific Binding Affinity of Perfluorooctanesulfonate (PFOS) and Perfluorooctanoate (PFOA) to Serum Proteins. Environ. Sci. Technol 49 (9), 5722–5731. 10.1021/es505399w. [DOI] [PubMed] [Google Scholar]
- Bowden JA, Cantu TM, Chapman RW, Somerville SE, Guillette MP, Botha H, Hoffman A, Luus-Powell WJ, Smit WJ, Lebepe J, et al. , 2016. Predictive Blood Chemistry Parameters for Pansteatitis-Affected Mozambique Tilapia (Oreochromis Mossambicus). PLOS ONE 11 (4), e0153874. 10.1371/journal.pone.0153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JS, 2015. Fluorotechnology Is Critical to Modern Life: The FluoroCouncil Counterpoint to the Madrid Statement. Environ. Health Perspect. 123 (5), A112–A113. 10.1289/ehp.1509910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ, 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag. 7 (4), 513–541. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL, 2007. Serum Concentrations of 11 Polyfluoroalkyl Compounds in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Sci. Technol, vol. 41, 7, pp. 2237–2242. 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- Callihan JL, Godwin CH, Buckel JA, 2014. Effect of Demography on Spatial Distribution: Movement Patterns of the Albemarle Sound-Roanoke River Stock of Striped Bass (Morone Saxatilis) in Relation to Their Recovery. Fish. Bull. 112. 10.7755/fb.112.2-3.3. [DOI] [Google Scholar]
- Chang ET, Adami H-O, Boffetta P, Wedner HJ, Mandel JS, 2016. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit. Rev. Toxicol. 46 (4), 279–331. 10.3109/10408444.2015.1122573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie I, Reiner JL, Bowden JA, Botha H, Cantu TM, Govender D, Guillette MP, Lowers RH, Luus-Powell WJ, Pienaar D, et al. , 2016. Perfluorinated alkyl acids in the plasma of South African Crocodiles (Crocodylus Niloticus). Chemosphere 154, 72–78. 10.1016/j.chemosphere.2016.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet H, Blust R, Moens L, 1998. Absence of Albumin in the Plasma of the Common Carp Cyprinus Carpio: Binding of Fatty Acids to High Density Lipoprotein. Fish Physiol. Biochem. 19 (1), 71–81. 10.1023/A:1007734127146. [DOI] [Google Scholar]
- Delinsky AD, Strynar MJ, McCann PJ, Varns JL, McMillan L, Nakayama SF, Lindstrom AB, 2010. Geographical distribution of perfluorinated compounds in fish from Minnesota Lakes and Rivers. Environ. Sci. Technol. 44 (7), 2549–2554. 10.1021/es903777s. [DOI] [PubMed] [Google Scholar]
- Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances; DeWitt JC, Ed.; Molecular and Integrative Toxicology; Springer International Publishing: Cham, 2015. 10.1007/978-3-319-15518-0. [DOI] [Google Scholar]
- DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR, 2012. Immunotoxicity of perfluorinated compounds: recent developments. Toxicol. Pathol. 40 (2), 300–311. 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- Fair PA, Wolf B, White ND, Arnott SA, Kannan K, Karthikraj R, Vena JE, 2019. Perfluoroalkyl Substances (PFASs) in Edible Fish Species from Charleston Harbor and Tributaries, South Carolina, United States: Exposure and Risk Assessment. Environ. Res. 171, 266–277. 10.1016/j.envres.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Zhao S, Zhang Y, Zhong W, Zhu L, 2014. Distribution of Perfluoroalkyl Substances (PFASs) with Isomer Analysis among the Tissues of Aquatic Organisms in Taihu Lake, China. Environ. Pollut. 193, 224–232. 10.1016/j.envpol.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Floyd FJ, Widaman KF, 1995. Factor analysis in the development and refinement of clinical assessment instruments. Psychol. Assess. 7 (3), 286–299. 10.1037/1040-3590.7.3.286. [DOI] [Google Scholar]
- Giesy JP, Kannan K, 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 35 (7), 1339–1342. 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Kannan K, 2002. Perfluorochemical surfactants in the environment. Environ. Sci. Technol. 36 (7), 146A–152A. 10.1021/es022253t. [DOI] [PubMed] [Google Scholar]
- Guillette L Jr, Brock J, Rooney A, Woodward A, 1999. Serum concentrations of various environmental contaminants and their relationship to sex steroid concentrations and Phallus Size in Juvenile American Alligators. Arch. Environ. Contam. Toxicol. 36 (4), 447–455. 10.1007/PL00006617. [DOI] [PubMed] [Google Scholar]
- Guo J, Wu P, Cao J, Luo Y, Chen J, Wang G, Guo W, Wang T, He X, 2019. The PFOS disturbed immunomodulatory functions via nuclear factor-KB Signaling in Liver of Zebrafish (Danio Rerio). Fish Shellfish Immunol. 91, 87–98. 10.1016/j.fsi.2019.05.018. [DOI] [PubMed] [Google Scholar]
- Hadley J, 2015. . In: An Economic Analysis of Recreational and Commercial Fisheries Occurring in the Middle and Lower Cape Fear River . North Carolina. http://digital.ncdcr.gov/cdm/ref/collection/p16062coll9/id/252966. [Google Scholar]
- Haug LS, Thomsen C, Brantsæter AL, Kvalem HE, Haugen M, Becher G, Alexander J, Meltzer HM, Knutsen HK, 2010. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ. Int. 36 (7), 772–778. 10.1016/j.envint.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Hoff PT, Van Dongen W, Esmans EL, Blust R, De Coen WM, 2003. Evaluation of the toxicological effects of perfluorooctane sulfonic acid in the common Carp (Cyprinus Carpio). Aquat. Toxicol. 62 (4), 349–359. 10.1016/S0166-445X(02)00145-5. [DOI] [PubMed] [Google Scholar]
- Hopkins ZR, Sun M, DeWitt JC, Knappe DRU, 2018. Recently detected drinking water contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids: JOURNAL AWWA. J. - Am. Water Works Assoc. 110 (7), 13–28. 10.1002/awwa.1073. [DOI] [Google Scholar]
- Houde M, De Silva AO, Muir DCG, Letcher RJ, 2011. Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ. Sci. Technol. 45 (19), 7962–7973. 10.1021/es104326w. [DOI] [PubMed] [Google Scholar]
- Houde M, Giraudo M, Douville M, Bougas B, Couture P, De Silva AO, Spencer C, Lair S, Verreault J, Bernatchez L, et al. , 2014. A Multi-Level Biological Approach to Evaluate Impacts of a Major Municipal Effluent in Wild St. Lawrence River Yellow Perch (Perca Flavescens). Sci. Total Environ. 497–498, 307–318. 10.1016/j.scitotenv.2014.07.059. [DOI] [PubMed] [Google Scholar]
- Hrubec TC, Smith SA, Robertson JL, 2001. Age-related changes in hematology and plasma chemistry values of hybrid striped bass (Morone Chrysops × Morone Saxatilis). Vet. Clin. Pathol. 30 (1), 8–15. 10.1111/j.1939-165X.2001.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Glass DC, Gray CN, 2001. Estimating Mean Exposures from Censored Data: Exposure to Benzene in the Australian Petroleum Industry. Ann. Occup. Hyg. 45 (4), 275–282. 10.1093/annhyg/45.4.275. [DOI] [PubMed] [Google Scholar]
- Investigation of Levels of Perfluorinated Compounds in New Jersey Fish, Surface Water, and Sediment. 46. <https://www.nj.gov/dep/dsr/publications/Investigation%20of%20Levels%20of%20Perfluorinated%20Compounds%20in%20New%20Jersey%20Fish,%20Surface%20Water,%20and%20Sediment.pdf> <https://www.nj.gov/dep/dsr/njmainfish.htm>(accessed Jun 6, 2019). [Google Scholar]
- Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC, Kannan K, 2016. Association of Perfluoroalkyl Substances, Bone Mineral Density, and Osteoporosis in the U.S. Population in NHANES 2009–2010. Environ. Health Perspect, vol. 124, 1, pp. 81–87. 10.1289/ehp.1307909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielsen K, Shamim Z, Ryder LP, Nielsen F, Grandjean P, Budtz-Jørgensen E, Heilmann C, 2016. Antibody response to booster vaccination with Tetanus and Diphtheria in Adults Exposed to Perfluorinated Alkylates. J. Immunotoxicol. 13 (2), 270–273. 10.3109/1547691X.2015.1067259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Fang M, Xu F, Chen D, 2019. Characterization of the binding of per- and poly-fluorinated substances to proteins: a methodological review. TrAC Trends Anal. Chem. 116, 177–185. 10.1016/j.trac.2019.05.017. [DOI] [Google Scholar]
- Liu Y, Qian M, Ma X, Zhu L, Martin JW, 2018. Nontarget mass spectrometry reveals new perfluoroalkyl substances in fish from the Yangtze River and Tangxun Lake, China. Environ. Sci. Technol. 52 (10), 5830–5840. 10.1021/acs.est.8b00779. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Strynar MJ, Helfant L, Egeghy P, Ye X, Lindstrom AB, 2007. Perfluorinated compounds in the Cape Fear Drainage Basin in North Carolina. Environ. Sci. Technol. 41 (15), 5271–5276. 10.1021/es070792y. [DOI] [PubMed] [Google Scholar]
- Ng CA, Hungerbühler K, 2013. Bioconcentration of perfluorinated alkyl acids: how important is specific binding? Environ. Sci. Technol. 47 (13), 7214–7223. 10.1021/es400981a. [DOI] [PubMed] [Google Scholar]
- Nian M, Li Q-Q, Bloom M, Qian Z. (Min), Syberg KM, Vaughn MG, Wang S-Q, Wei Q, Zeeshan M, Gurram N, et al. , 2019. Liver Function Biomarkers Disorder Is Associated with Exposure to Perfluoroalkyl Acids in Adults: Isomers of C8 Health Project in China. Environ. Res, vol. 172, pp. 81–88. 10.1016/j.envres.2019.02.013. [DOI] [PubMed] [Google Scholar]
- OECD: New Comprehensive Global Database of PFAS, 2018. (accessed 1 December 2019).
- Patrick WS, Moser ML, 2001. Potential Competition between Hybrid Striped Bass (Morone Saxatilis × M. Americana) and Striped Bass (M. Saxatilis) in the Cape Fear River Estuary, North Carolina. Estuaries 24 (3), 425–429. 10.2307/1353243. [DOI] [Google Scholar]
- Peden-Adams MM, Keller JM, Eudaly JG, Berger J, Gilkeson GS, Keil DE, 2008. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol. Sci. Off. J. Soc. Toxicol. 104 (1), 144–154. 10.1093/toxsci/kfn059. [DOI] [PubMed] [Google Scholar]
- Peden-Adams MM, Stuckey JE, Gaworecki KM, Berger-Ritchie J, Bryant K, Jodice PG, Scott TR, Ferrario JB, Guan B, Vigo C, et al. , 2009. Developmental Toxicity in White Leghorn Chickens Following in Ovo Exposure to Perfluorooctane Sulfonate (PFOS). Reprod. Toxicol. 27 (3), 307–318. 10.1016/j.reprotox.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Place BJ, Field JA, 2012. Identification of novel fluorochemicals in aqueous film-forming foams used by the US Military. Environ. Sci. Technol. 46 (13), 7120–7127. 10.1021/es301465n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachels KT, Morgeson CW, 2018. Cape Fear River Striped Bass Spawning Stock Survey and Proposed Establishment of Put-Grow-Take Fishery. North Carolina Wildlife Resources Commission. Federal Aid in Sport Fish Restoration, Project F-108, Final Report, Raleigh. [Google Scholar]
- Rauta PR, Nayak B, Das S, 2012. Immune system and immune responses in fish and their role in comparative immunity study: a model for higher organisms. Immunol. Lett. 148 (1), 23–33. 10.1016/j.imlet.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Reiner JL, Place BJ, 2015. Perfluorinated alkyl acids in wildlife. Health Eff. Perfluorinated Compd. 10.1007/978-3-319-15518-0_5. [DOI] [Google Scholar]
- Saurabh S, Sahoo PK, 2008. Lysozyme: an important defence molecule of fish innate immune system. Aquac. Res. 39 (3), 223–239. 10.1111/j.1365-2109.2007.01883.x. [DOI] [Google Scholar]
- Shi Y, Vestergren R, Nost TH, Zhou Z, Cai Y, 2018. Probing the Differential Tissue Distribution and Bioaccumulation Behavior of Per- and Polyfluoroalkyl Substances of Varying Chain-Lengths, Isomeric Structures and Functional Groups in Crucian Carp. Environ. Sci. Technol. 52 (8), 4592–4600. 10.1021/acs.est.7b06128. [DOI] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, Pickett A, Smith C, Knappe DRU, 2016. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett. 3 (12), 415–419. 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- Thalheimer AH, McConney LB, Kalinovich IK, Pigott AV, Franz JD, Holbert HT, Mericas D, Puchacz ZJ, 2017. Use and Potential Impacts of AFFF Containing PFASs at Airports. ACRP Res. Rep. No, 173. [Google Scholar]
- Torsteinsdóttir I, Håkansson L, Hällgren R, Gudbjörnsson B, Arvidson N-G, Venge P, 1999. Serum lysozyme: a potential marker of monocyte/macrophage activity in rheumatoid arthritis. Rheumatology 38 (12), 1249–1254. 10.1093/rheumatology/38.12.1249. [DOI] [PubMed] [Google Scholar]
- US EPA, 2019. O. GenX and PFBS Draft Toxicity Assessments <https://www.epa.gov/pfas/genx-and-pfbs-draft-toxicity-assessments>(accessed Jun 17, 2019).
- UNEP, 2009. http://chm.pops.int/TheConvention/ConferenceoftheParties/ReportsandDecisions/tabid/208/Default.aspx.
- Xing J, Wang G, Zhao J, Wang E, Yin B, Fang D, Zhao J, Zhang H, Chen YQ, Chen W, 2016. Toxicity assessment of perfluorooctane sulfonate using acute and subchronic male C57BL/6J Mouse Models. Environ. Pollut. 210, 388–396. 10.1016/j.envpol.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Young G, Brown CL, Nishioka RS, Folmar LC, Andrews M, Cashman JR, Bern HA, 1994. Histopathology, blood chemistry, and physiological status of normal and moribund striped bass (morone saxatilis) involved in summer mortality (‘die-off’) in the Sacramento-San Joaquin Delta of California. J. Fish Biol. 44 (3), 491–512. 10.1111/j.1095-8649.1994.tb01229.x. [DOI] [Google Scholar]
- Young CJ, Furdui VI, Franklin J, Koerner RM, Muir DC, Mabury SA, 2007. Perfluorinated acids in arctic snow: new evidence for atmospheric formation. Environ. Sci. Technol. 41 (10), 3455–3461. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hopkins ZR, McCord J, Strynar MJ, Knappe DRU, 2019. Fate of Perand Polyfluoroalkyl ether acids in the total oxidizable precursor assay and implications for the analysis of impacted water. Environ. Sci. Technol. Lett. 6 (11), 662–668. 10.1021/acs.estlett.9b00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


