Abstract
The consumption of a high-fat diet is linked to the development of obesity and considered a risk factor for cardiovascular diseases. The aim of this study was to evaluate the effect of the methanolic extract of Moringa oleifera leaves (MEML) on the high-fat diet- (HFD-) induced obesity and cardiac damage in rats. MEML, at a dose of 200 mg/kg/bw and 400 mg/kg/bw, was orally administrated to obese rats for 12 weeks. M. oleifera leaves were proved to be rich in nutrients and minerals. Diversity of phenolic compounds in MEML was evidenced via LC-ESI-MS analysis. The chronic administration of HFD in rats led to an increase in the body weight gain, total cholesterol, and triglycerides and reduction in the HDL-C levels. The obtained results indicated a significant increase (p < 0.05) in the cardiac marker enzyme level in obese rats. A significant decrease (p < 0.05) in the levels of cardiac catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) activities was accompanied with an increase of malondialdehyde (MDA) level in the high-fat diet group when compared to those of the control. The treatment with the MEML alleviated abnormalities in the serum biochemical parameters, balanced the antioxidant status, and reestablished the normal histological structure of the heart especially in the case of the higher concentration. Moringa oleifera leaves may be a promising candidate in the management of obesity and its related complications such as heart problems.
1. Introduction
Obesity is a common disease whose prevalence is associated with a combination of genetic, nutritional, and environmental factors. It has now become among the most growing public health issues. A 2016 World Health Organization (WHO) survey on the globally increasing prevalence of obesity reported that 1.9 billion adults were overweight, more than 600 million of which obese [1]. Obesity has been proven to increase various health risks such as cardiovascular disease, diabetes mellitus, fatty liver disease, cancers, and asthma [2]. The strong association between obesity and cardiovascular disease is an area of intensive research. A growing body of evidence suggests that increasing abdominal adiposity is associated with a greater risk of developing heart failure [3].
The existing antiobesity medications have been effectively implemented in regulated weight-loss treatments. However, they pose risk severe adverse effects which outweigh their advantageous properties. Most recent studies on the treatment of obesity and its related comorbidities have focused on the potential role of different plant preparations that can exert a positive effect on the mechanisms involved in this pathology [4]. These potential health benefits may be attributed to macronutrients, micronutrients, and secondary metabolites present in plants.
Moringa oleifera, Lam (Moringaceae), is one of the Brassica aboriginal vegetables, native to the Indian subcontinent and commonly found in tropical and subtropical countries [5]. For centuries, people around the world have used different parts of this plant (roots, leaves, and seeds) for numerous therapeutic applications in the treatment of inflammation and infectious diseases, along with cardiovascular, gastrointestinal, hematological, and respiratory disorders [6, 7]. Recent trials revealed that M. oleifera leaves might contribute to prevent obesity as well as obesity-related complications [8, 9]. The present study evaluates the therapeutic potential of Moringa oleifera leaf extract in the treatment of obesity as well as its protective effect on the cardiac disorders induced by a high-fat diet feeding.
2. Material and Methods
2.1. Chemicals
Gallic acid, Folin-Ciocalteu reagent, catechin, rutin, thiobarbituric acid, catalase (CAT), and malondialdehyde (MDA) were purchased from Sigma–Aldrich Co. (Sigma, St. Louis, USA). Standards (iron (Fe), zinc (Zn), calcium (Ca), potassium (K), magnesium (Mg), manganese (Mn), phosphorus (P), and sodium (Na)) were obtained from Merck (Darmstadt, Germany). Superoxide dismutase (SOD) and glutathione peroxidase (GPx) assay kits were purchased from Randox Laboratory Ltd. Methanol was purchased from PanReac (Barcelona, Spain). All other chemicals and solvents were of analytical grade and were obtained from Sigma–Aldrich (Sigma, St. Louis, USA).
2.2. Plant Collection
Fresh leaves of M. oleifera were collected from the Tataouine region in south Tunisia, in August 2017. The plant was identified by a botanist, Dr. Boulbaba Ltayef (the Faculty of Sciences of Gabes, Tunisia), and a voucher specimen (MO-0317) was deposited in the Herbarium of the Faculty of Sciences, University of Gafsa, Tunisia. The plant leaves were cleaned, shade dried at room temperature, ground into fine powder, and stored at −20°C until use.
2.3. Physicochemical Composition of M. oleifera Leaves
Moisture content of the leaf powder was determined according to the AOAC [10] method by oven drying the sample at 110°C until constant weight was attained. Similarly, ash content was determined according to the AOAC [10] method and the value was obtained by incineration of the sample at 550°C in a muffle furnace until a constant weight. Nitrogen estimation was carried out using the micro-Kjeldahl method with some modifications [11]. Crude proteins were subsequently calculated by multiplying the nitrogen content by 6.25. Ether extracts were determined by means of a Soxhlet extractor, using n-hexane as a solvent. Crude fibers were estimated by acid-base digestion with 1.25% H2SO4 (w/v) and 1.25% NaOH (w/v) solutions [12]. The carbohydrate content was calculated by removing from 100% the amount of moisture, total fat, protein, and ash [10]. The total soluble sugars were measured using phenol-sulfuric according to the method of Al-Harrasi et al. [13]. Pure glucose was used as the standard. The energy value estimation was done by summing values for crude proteins, crude fats, and carbohydrates, respectively, multiplied by the factors (2.44, 8.37, and 3.57), as proposed by Martin and Coolidge [14]. Mineral composition was obtained by applying the method of Rjeibi et al. [15].
2.4. Preparation of M. oleifera Leaf Extract
The fine powder of M. oleifera leaf was extracted with methanol (10 g/200 mL) for 24 h at room temperature and under magnetic stirring (magnetic stirrer, Heidolph, Germany). The extract was centrifuged at 4,500 rpm for 25 min, then filtrated with Whatman Millipore filter paper (0.45 μm) and concentrated on a rotary evaporator (40°C water bath temperature) (EYELA, Tokyo, Japan). Finally, the resulting extract was stored in the dark at 4°C till use.
2.5. Total Phenolic and Flavonoid Contents
The total phenolic content was estimated using the Folin-Ciocalteu method [16]. A calibration curve was obtained using gallic acid as a standard. Total phenolic content was expressed as mg gallic acid equivalent (GAE)/g dry extract. The total flavonoid content was determined by aluminum chloride colorimetric assay [17], and rutin was used as a standard. The results were expressed as mg rutin equivalent (RE)/g dry extract. Tannin content in the M. oleifera extract was determined using the method described by Chupin et al. [18]. A standard curve was prepared with catechin, and the results were expressed as mg catechin equivalent (CE)/g dry extract.
2.6. Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry (LC-ESI-MS) Analysis of Phenolic Compounds
A Shimadzu LC-20ADXR pump with a SIL-20AXR autosampler (40°C) was used for HPLC analysis. The injection volume was 5 μL, and the separation temperature was 75°C in a Discovery BIO Wide Pore C18 (250 mm × 4 mm, 5 μm) column. The flow rate was fixed at 0.4 mL/min, and the mobile phase pooled with eluent A (5% methanol, 95% H2O, and 0.15% acetic acid) and eluent B (50% acetonitrile, 50% water, and 0.15% acetic acid). The eluent flow rates were as follows: 0-14 min from 10 to 20% eluent B, 14-27 min with 20% eluent B, 27-37 min from 20 to 55% eluent B, 37-45 min with 55% eluent B, and 45-52 min from 55 to 100% eluent B. A Shimadzu UFLC XR–2020 single quadrupole mass spectrometer equipped with an electrospray interface (ESI: electrospray ionization) was used for MS analysis. ESI conditions were as follows: an electrospray source (source block temperature, 450°C; desolvation temperature, 280°C; capillary voltage, 2.7 kV; cone voltage, 35 V), a nebulizing gas flow 1.5 L/min, and a drying gas flow 15 L/min. Identification of compound was carried out by comparison with standards.
2.7. Animal Studies
2.7.1. Animals
In this study, three-month-old healthy male Wistar rats (n = 24), with about 120-140 g body weight, obtained from the Central Pharmacy of Tunis, Tunisia, were kept for a two-week adaptation period under controlled conditions of temperature (22 ± 2°C), relative humidity (50% ± 4%), and a constant photoperiod (12 h light/dark cycle). Food and water were provided ad libitum. The rats were treated in accordance with the Tunisian approved code of practice in compliance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Council of Europe No. 123, Strasbourg, 1985).
2.7.2. Induction of Obesity
During the study period, two diets were used: The normal control group was given a normal diet (ND) and the control group was maintained on HFD as illustrated in Table 1 [19]. HFD was prepared by mixing all the ingredients and baking in an oven at 40°C for 24 hours.
Table 1.
Composition of experimental diets.
| Composition (%) | Normal diet (ND) | High-fat diet (HFD)∗ |
|---|---|---|
| Sucrose | 2 | 4 |
| Lard | 2.5 | 34 |
| Soybean oil | 2.5 | 3 |
| α-Corn starch | 10 | 15 |
| Maltodextrin | 29.64 | 6 |
| Casein | 18.5 | 25.5 |
| L-Cystine | 0.26 | 0.36 |
| Cellulose | 6.65 | 6.65 |
| Mineral mix | 4 | 4 |
| Calcium carbonate | 0.2 | 0.24 |
| Vitamin mix | 1.00 | 1.00 |
| Choline bitartrate | 0.25 | 0.25 |
| Total | 100 | 100 |
∗ High-fat diet was modified from the AIN-76 dietary composition [19].
When the Lee index value of rats fed with HFD was above 0.3, the model was considered to be successfully established [20].
2.7.3. Treatment
The rats were randomly divided into four groups of six each. Treatment groups were defined as follows:
Group 1 (C): control rats fed with normal diet
Group 2 (HFD): rats fed with a high-fat diet
Group 3 (HFD+MEML 200): rats fed with a high-fat diet and received MEML at 200 mg/kg.bw by oral gavage
Group 4 (HFD+MEML 400): rats fed with a high-fat diet and treated by gavage with MEML at 400 mg/kg.bw.
Animals did not show any clinical signs of toxicity with the maximum dose of 2,000 mg/kg bw [21]. Rats were dosed by oral gavage at doses of 200 and 400 mg/kg.bw in a volume of 1.5 mL/kg distilled water.
During the experiment, the body weight gained and feed intakes were reported at weekly intervals. The feed efficiency ratio was calculated using the equation: (total weight gain/total feed intake) × 100. At the end of the experiment, all the animals were rapidly sacrificed by decapitation under ether inhalation anesthesia to minimize handling stress. Blood serum was obtained by centrifugation (1,500 rpm, 15 min, 4°C), and the hearts were removed, cleaned of fat, and frozen at -80°C for biochemical and histological studies.
2.8. Biochemical Estimations
The serum levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), creatine kinase-MB (CK-MB), aspartate transaminase (AST), and alanine transaminase (ALT) were evaluated using commercial kits (Spinreact, Girona, Spain) on an automatic biochemical analyser (Vitalab Flexor E, Spankeren, Netherlands) in the biochemical laboratory of the Regional Hospital of Gafsa, Tunisia. Serum low-density lipoprotein cholesterol (LDL-C) values were estimated using the Friedewald equation [22]:
| (1) |
2.9. Assessment of Antioxidant Parameters and Lipid Peroxidation in Heart Tissue
One gram of rat hearts were cut into small pieces and homogenized in 2 mL of tris buffer solution (TBS) (pH 7.4) using an Ultra-Turax homogenizer; then, the mixtures were centrifuged (5,000 rpm, 30 min, 4°C). The supernatants were collected and used to determine (1) the level of lipid peroxidation by measuring the thiobarbituric acid reactive substances (TBARS) according to Yagi's method [23]; (2) the total superoxide dismutase (SOD) activity, following the method of Misra and Fridovich [24]; (3) the glutathione peroxidase (GPx) activity, using the method of Flohe and Gunzler [25]; and (4) the catalase (CAT) activity according to the method of Aebi [26]. The total sulfhydryl groups (TSH) were evaluated at 412 nm after reaction with 5,5′-dithiobis-(2-nitrobenzoic acid) [27].
2.10. Histopathological Analysis
Small pieces of rat heart tissues were immersed in a fixative solution (4% formaldehyde in phosphate buffer, pH 7.6), dehydrated in ethanol, and washed with toluene. The paraffin embedded tissue blocks were made 5 mm thick sections using a rotary microtome and dried overnight in an oven at 37°C. The sections were later stained with hematoxylin and eosin (H&E). Tissue preparations were observed and microphotographed under a light BH2 Olympus microscope.
2.11. Statistical Analysis
All results were presented as mean ± standard deviation (SD). Statistical significance was determined using one-way analysis of variance (ANOVA) followed by a Tukey post hoc test. p < 0.05 was considered statistically significant. Pearson correlation coefficients (r) were used for the estimation of relationships between variables.
3. Results
3.1. Chemical Composition of M. oleifera Leaves
Table 2 presents the proximate composition of M. oleifera leaves on a dry weight basis. The sample was found to contain moisture (6.82%) and ash (10.73%). Crude protein had a value of 25.87%, while ether extract and fiber amounted to 7.28% and 7.98%, respectively. Carbohydrates and sugar content amounted to 41.32% and 9.18%, respectively. The total energy value of 100 g of dry leaves was 350 kcal. The mineral element concentration of the M. oleifera plant leaf powder is also presented in Table 2. Calcium was determined to be the most abundant of all the macroelements analysed herein, followed by K> Na > Mg. Mean contents of the microminerals Fe, Mn, Zn, and Cu were 34.56, 4.72, 2.93, and 0.86, respectively.
Table 2.
Proximate composition and mineral content of Moringa oleifera leaves.
| Components | Amount |
|---|---|
| Moisture (g/100 g DW) | 6.82 ± 0.181 |
| Crude proteins (g/100 g DW) | 25.87 ± 0.340 |
| Crude fats (g/100 g DW) | 7.28 ± 0.173 |
| Ash (g/100 g DW) | 10.73 ± 0.021 |
| Crude fibers (g/100 g DW) | 7.98 ± 0.050 |
| Carbohydrates (g/100 g DW) | 41.32 ± 0.263 |
| Sugar (g/100 g DW) | 9.18 ± 0.012 |
| Energy (kcal/100 g DW) | 350 ± 0.178 |
| Sodium (Na) (mg/100 g) | 660.01 ± 0.151 |
| Calcium (Ca) (mg/100 g) | 1,942.47 ± 11.230 |
| Magnesium (Mg) (mg/100 g) | 464.21 ± 1.214 |
| Potassium(K) (mg/100 g) | 1,134.12 ± 0.463 |
| Phosphorus (P) (mg/100 g) | 103.89 ± 0.521 |
| Iron (Fe) (mg/100 g) | 34.56 ± 0.987 |
| Zinc (Zn) (mg/100 g) | 2.93 ± 0.064 |
| Manganese (Mn) (mg/100 g) | 4.72 ± 0.040 |
Values are means ± SD of three replicate determinations.
3.2. Phenolic, Flavonoid, and Condensed Tannin Contents
The results summarized in Table 3 indicate that M. oleifera leaves possessed a high content of total phenolic (247.53 mg gallic acid equivalents/g extract) and flavonoids (34.13 mg/g mg rutin equivalents/g extract). An appreciable amount of tannins was also detected in the sample extract (10.5 mg catechin equivalents/g extract).
Table 3.
Total phenolic, flavonoid, and tannin contents in Moringa oleifera methanol extract.
| Parameters | Content |
|---|---|
| Total phenols (mg gallic acid equivalents/g extract) | 247.53 ± 3.24 |
| Flavonoids (mg rutin equivalents/g of extract) | 34.13 ± 2.7 |
| Tannins (mg catechin equivalents/g of extract) | 10.5 ± 0.79 |
Values are means ± SD of three replicate determinations.
3.3. HPLC-ESI-MS Analysis
Using 31 authentic standards, we noticed that the LC-ESI-MS analysis of the methanol leaf extract revealed 18 phenolic compounds in which flavonoids were the most concentrated group. Hyperoside (316,822 μg/g extract) and quercetrin (204,685 μg/g extract) were reported as primary compounds (Table 4).
Table 4.
Phenolic compounds identified in methanol extract of Moringa oleifera leaves by LC-ESI-MS.
| No. | Compounds∗ | R t (min) | Molecular formula | Molecular mass | (M-H) (m/z) | Content (μg/g extract) |
|---|---|---|---|---|---|---|
| 1 | Quinic acid | 2,017 | C7H12O6 | 192 | 191 | 16.739 |
| 2 | Protocatchuic acid | 6,485 | C7H6O4 | 154 | 153 | 4.969 |
| 3 | Epicatechin | 16,120 | C15H14O6 | 290 | 289 | 0.091 |
| 4 | p-Coumaric acid | 20,688 | C9H8O3 | 164 | 163 | 0.888 |
| 5 | trans-Ferrelic acid | 22,729 | C10H10O4 | 194 | 193 | 0.958 |
| 6 | Rutin | 23,545 | C27H30O16 | 610 | 609 | 47.401 |
| 7 | Hyperoside | 24,253 | C21H20O12 | 464 | 463 | 316.822 |
| 8 | Naringin | 25,817 | C27H32O14 | 580 | 579 | 9.400 |
| 9 | Quercetrin | 26,138 | C21H20O11 | 448 | 447 | 204.685 |
| 10 | 3,4-di-O-Caffeoylquinic acid | 26,483 | C25H24O12 | 516 | 515 | 1.342 |
| 11 | Salviolonic acid | 27,883 | C36H30O16 | 718 | 717 | 2.330 |
| 12 | Quercetin | 31,570 | C15H10O7 | 302 | 301 | 3.148 |
| 13 | Kaempferol | 31,639 | C15H10O6 | 286 | 285 | 0.489 |
| 14 | Apigenin | 34,193 | C15H10O5 | 270 | 269 | 0.126 |
| 15 | Luteolin | 34,596 | C15H10O6 | 286 | 285 | 1.958 |
| 16 | Cirsiliol | 35,206 | C17H14O7 | 330 | 329 | 17.757 |
| 17 | Cirsilineol | 38,132 | C18H16O7 | 344 | 343 | 0.280 |
| 18 | Acacetin | 39,687 | C16H12O5 | 284 | 283 | 0.483 |
∗Identification was verified using 31 authentic commercial standards.
3.4. Body Weight, Feed Intake, and Feed Efficiency Ratio
Consumption of HFD for 12 weeks caused a significant (p < 0.05) increase in body weight gain in the HFD group when compared to the control group (Table 5). The percentage of body weight gain in the rats, fed the HFD supplemented with both low and high MEML doses, was significantly lower (p < 0.05) than that in the HFD group; in the case of low- and high-dose MEML, the body weight gain percentage reached 145.33% and 126.15%, respectively.
Table 5.
Effect of MEML on body weight gain, feed intake, feed efficiency ratio, serum lipid profiles, and cardiac marker enzymes in obese rats.
| Parameters | C | HFD | MEML 200 | MEML 400 |
|---|---|---|---|---|
| Body weight gain (%) | 98.04 | 162.5∗ | 145.33++ | 126.15++ |
| Feed intake (g/week) | 115.2 ± 3.12 | 122.51±2.7∗∗ | 119.82 ± 1.27++ | 119.09 ± 4.22++ |
| Feed efficiency ratio (%) | 4.8 | 8.19∗∗ | 7.31++ | 6.05+ |
| Total cholesterol(mg/dL) | 89.15 ± 2.16 | 95.76±11.09∗∗ | 93.26 ± 4.32++ | 92.03 ± 1.43++ |
| Triglycerides (mg/dL) | 218.69 ± 4.85 | 265.15±7.12∗∗ | 232.67 ± 2.94++ | 227.50 ± 3.13++ |
| HDL-C (mg/dL) | 63.98 ± 1.5 | 30.22±5.22∗∗ | 39.04 ± 2.45++ | 47.32 ± 5.08++ |
| LDL-C (mg/dL) | 44.65 ± 2.41 | 59.33±0.16∗∗ | 48.15 ± 0.96++ | 44.76 ± 2.20++ |
| CK-MB (UI/L) | 204.30 ± 10.28 | 392.64±8.12∗∗ | 311.55 ± 22.34++ | 226.03 ± 5.23++ |
| AST (UI/L) | 49.5 ± 9.18 | 98.85±7.08∗∗ | 77.56 ± 2.87++ | 60.45 ± 7.22++ |
| ALT (UI/L) | 50.04 ± 12.45 | 102.13±4.56∗∗ | 76.40 ± 13.08++ | 61.88 ± 11.13++ |
Values are expressed as mean ± SD (n = 6). ∗p < 0.05, ∗∗p < 0.01 versus the control group (C). +p < 0.05, ++p < 0.01 versus the high-fat diet group (HFD).
Concerning feed intake, a slight change was detected between the HFD group and the control group or MEML-treated groups. Feed efficiency ratio was significantly increased in the HFD group, as compared to the control group. The oral administration of MEML resulted in a significant decrease (p < 0.05) in feed efficiency ratio levels; the higher dose exhibited a more substantial result than that of the lower dose of the extract (Table 5).
3.5. Assays of Serum Markers
As shown in Table 5, the rats fed with HFD for 12 weeks exhibited a significant increase (p < 0.05) in serum TC (95.76 ± 11.09 mg/dL), TG (265.15 ± 7.12 mg/dL), and LDL-C (59.33 ± 0.16 mg/dL) levels when compared with the control group. However, these rises were significantly (p < 0.01) attenuated by supplementation with different MEML concentrations. Additionally, MEML markedly moderated the serum HDL-C level which had been significantly decreased (p < 0.05) in the HFD rats.
The effects of MEML supplementation on the levels of serum cardiac marker enzyme CK-MB, AST, and ALT are shown in Table 5. In this study, CK-MB, AST, and ALT amounts increased significantly in HDF-treated rats (p < 0.01). Administration with MEML to HDF-treated rats resulted in a significant reduction (p < 0.05) in the levels of CK-MB, AST, and ALT enzymes when compared with the HFD group. The high-dose MEML supplementation (400 mg/kg) appeared to be more effective in enzyme-lowering levels.
3.6. Antioxidative Activities
To determine whether obesity is a primary factor contributing to oxidative stress in the heart tissue, we analysed lipid peroxidation, a marker of oxidative damage. Lipid peroxidation expressed by thiobarbituric acid reactive substances (TBARS) significantly increased (p < 0.05) in the HFD-treated group compared to the control group. However, administration of M. oleifera extract resulted in a significant (p < 0.01) reduction of these TBARS to almost normal values (0.77 ± 0.3 nmol/mg protein). Levels of glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD) in the hearts of both control and experimental rats are illustrated in Table 6. A significant decrease (p < 0.05) in the activities of these antioxidant enzymes were perceived in animals which received a high-fat diet when compared with the control group. As shown in Table 6, antioxidant enzyme levels were significantly, and dose-dependently, enhanced by administration with M. oleifera extract. Moreover, a significant (p < 0.01) decrease in cardiac TSH value was detected in the HFD-treated rats as compared with the control group. In contrast, a considerable improvement in the level of this nonenzymatic antioxidant was observed in the MEML-treated rats when compared with the HFD rats.
Table 6.
Lipid peroxidation levels (TBARS), nonenzymatic antioxidant levels (TSH), and enzymatic antioxidant activities (glutathione peroxidase, catalase, and superoxide dismutase) in the hearts of the different groups.
| Parameters | C | HFD | MEML 200 | MEML 400 |
|---|---|---|---|---|
| TBARS (nmol/mg protein) | 0.61 ± 0.34 | 1.31 ± 0.23∗∗ | 1.05 ± 0.49++ | 0.77 ± 0.3++ |
| SOD (U/mg of protein) | 10.01 ± 0.26 | 6.95 ± 0.08∗ | 7.82 ± 0.21+ | 8.97 ± 1.09++ |
| CAT (μmol/min/mg of protein) | 14.89 ± 2.11 | 9.52 ± 1.2∗∗ | 11.75 ± 0.48++ | 13.01 ± 0.7++ |
| GPx (μmol GSH oxidized/min/mg of protein) | 6.83 ± 0.65 | 4.01 ± 1.1∗∗ | 5.1 ± 1.8++ | 6.05 ± 0.04++ |
| TSH (U/mg protein) | 1.92 ± 0.1 | 0.51 ± 0.02∗∗ | 0.72 ± 0.33++ | 0.89 ± 0.1++ |
Values are expressed as mean ± SD (n = 6). ∗p < 0.05, ∗∗p < 0.01 versus the control group (C). +p < 0.05, ++p < 0.01 versus the high-fat diet group (HFD). C: control rats; HFD: rats fed with high-fat diet; MEML 200: rats fed a high-fat diet and treated with methanolic extract of M. oleifera leaves (200 mg/kg); MEML 400: rats treated with methanolic extract of M. oleifera leaves (400 mg/kg) along with high-fat diet for 12 weeks.
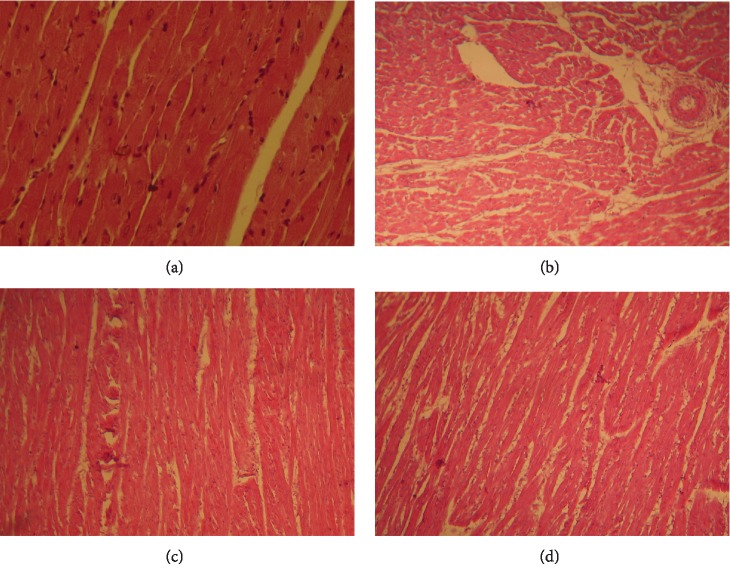
3.7. Histopathological Examination
Microscopic observation of the heart section in control rats showed a normal cardiac histological architecture with regularly arranged cardiac myofibers and muscle bundles (Figure 1(a)). In contrast, the HFD-treated rats revealed damaged heart sections evidenced by disorganized myocardial fibers associated with interstitial edema, inflammatory cell collections, and myocardial fibrosis (Figure 1(b)). However, the histopathological abnormalities were not to the same extent of severity in the MEML-treated animals; the heart revealed soft inflammatory mononuclear collections and absence of necrosis areas (Figure 1(c)). The rats treated with 400 mg/kg MEML exhibited a model of myocardial fiber arrangement similar to that of the control group (Figure 1(d)).
Figure 1.
Representative micrographs from the heart exhibiting the protective effect of methanol M. oleifera leaf extract (MEML) on high-fat diet-induced cardiac injury in rats. (a) Control groups showing normal cardiac architecture. (b) High-fat diet-treated group showing anarchized myocardial fibers associated with interstitial edema and inflammatory cellule collections. (c) High-fat diet group received MEML (200 mg/kg) showing repair in the histological sections. (d) High-fat diet group treated with MEML (400 mg/kg) showing normal structure almost similar to control. Heart sections were stained using hematoxylin-eosin method. Magnifications: ×400.
3.8. Pearson's Correlation Study
Correlation analyses were performed by using Pearson's correlation test to clarify the relation between different parameters. Feed intake, body weight gain, and feed efficiency ratio showed a strong positive correlation with total cholesterol, AST, ALT, and TBARS because the correlation coefficients (r) were all greater than 0.93 (p < 0.001) (Table 7). The body weight gain and feed efficiency ratio were also positively and strongly correlated with CK-MB (r = 0.941 and 0.960, respectively; p < 0.001).
Table 7.
The Pearson correlation coefficients between the parameters.
| Feed intake | Body weight gain | Feed efficiency ratio | |
|---|---|---|---|
| Total cholesterol | 0.993 | 0.991 | 0.984 |
| Triglycerides | 0.895 | 0.881 | 0.886 |
| HDL-C | -0.972 | -0.986 | -0.976 |
| LDL-C | 0.855 | 0.859 | 0.873 |
| CK-MB | 0.898 | 0.941 | 0.960 |
| AST | 0.940 | 0.966 | 0.977 |
| ALT | 0.935 | 0.970 | 0.983 |
| TBARS | 0.935 | 0.971 | 0.984 |
| SOD | -0.963 | -0.994 | -0.999 |
| CAT | -0.978 | -0.985 | -0.985 |
| GPx | -0.958 | -0.978 | -0.984 |
| TSH | -0.963 | -0.949 | -0.924 |
4. Discussion
The study showed that M. oleifera leaves are an interesting source of nutrients. The leaves contained an appreciable amount of crude protein (25.87 g/100 g DW). The high protein value recorded in this study suggests that the leaves can be ranked as a potential source of plant protein and, therefore, could be used as a protein supplement in diet. The obtained results of ether extract analysis (7.28 g/100 g DW) are in agreement with those reported in the literature, ranging from 2.23 to 8% [28, 29]. Available carbohydrate content was moderate, which is consistent with data reported by Aslam et al. [30] (38.21 g/100 g DW). It is worth noting that the levels of fat and carbohydrate of the leaf sample are of particular nutritional significance as they may intervene in the body's energy requirements without causing overweight due to the low caloric value of the leaves. M. oleifera is an interesting source of fiber (7.98 g/100 g DW) that helps to maintain a healthy digestive system and produces a positive adjustment in serum cholesterol levels as well as a measurable reduction in the peak level of serum glucose [31]. Ash content is the reflection of the mineral preserved in the sample. The reported value of ash (10.73 g/100 g DW) indicated that the leaves were a good source of minerals, fairly higher than the range of 5.43 to 5.75 g/100 g DW reported for some edible woody plants [32]. The predominant macroelement in the leaf sample was Ca. Calcium which plays a vital role in the maintenance of bones, thus preventing osteoporosis [33]. High level of potassium was revealed in our sample, accounting for 1,134.12 mg/100 g DW. Potassium is required in the body for maintaining reasonable sodium-potassium ratio assisting the prevention of heart disease and stroke [34]. Iron was the major microelement detected in our sample (34.56 mg/100 g DW). The highly nutritious value of M. oleifera leaves enables its use as a nutraceutical and panacea for many chronic diseases [35]. Moreover, the plant leaves can play a role in monitoring lipid metabolism as well as the prevention of obesity in animal models and even in humans.
Phytochemicals has gained recent interest due to their perceived role in protecting against metabolic diseases. In this study, we found that M. oleifera extract was rich in total phenolic content (247.53 mg gallic acid equivalents/g extract), flavonoids (34.13 mg/g mg rutin equivalents/g extract), and tannins (10.5 mg catechin equivalents/g extract). The obtained result revealed a total phenolic value far greater than that recorded in previous studies [36, 37] reporting the following data for phenolics: 96.30 ± 0.28 mg GAE/g and 48.35 ± 0.05 mg GAE/g of extract, respectively. A considerable body of literature highlights the contribution of oxidative stress and phenolic compounds to, respectively, the pathogenesis and prevention of human diseases [38]. Further, many authors found a high correlation between phenolic compounds and antioxidant activities [39]. Current evidence has proven that phenolics and flavonoids are more efficient antioxidants than vitamins C or E and carotenoids [40]. Hence, plants which contain great amounts of phenolic compounds are believed to exert health-beneficial effects by counteracting oxidative stress, thus reducing the risk of chronic diseases.
In order to identity the different active compounds of our plant leaves, we employed LC-ESI-MS profile analysis. The value of the application of LC-ESI-MS on plant biochemical research stems from its ability to simultaneously monitor retention time of the isolated compound peak and determine accurate molecular weight which is often a characteristic of that particular compound [41]. Results demonstrated that the M. oleifera extract was rich in phenolic acids, especially flavonoids (Table 4).
Using MS analysis, a total of 26 different flavonoids were identified in the methanol extract of M. oleifera [42]. Based on ultra-high-performance liquid chromatography (UHPLC) coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UHPLC-ESI-q TOF-MS), 14 flavonoids were identified in M. oleifera leaves collected in both South Africa and Namibia [43]. It is noteworthy that even in the presence of factors influencing the distribution of flavonoids, such as variations in environmental conditions as well as solvent system or methods of analysis, the content of these phytochemicals in M. oleifera leaves remains highly important. This further confirms the health-promoting effects of the plant leaves.
The use of a rat model of obesity that raised from the administration of HFD consumption allowed us to verify that obesity is associated with an increase in both food efficiency ratio level and body weight gain in rats. The suppression of body weight gain showed in the present study substantiated the antiobesity potential of MEML in HFD-fed rats. These findings are consistent with previous data which reported that M. oleifera leaf extract was efficient in the treatment of weight gain [44]. Several studies confirm an inverse relationship between the intake of dietary fiber, minerals, vitamins, phytochemicals, and antioxidants, and the development of obesity and its comorbidities [45, 46].
In order to determine whether MEML administration had an effect on circulating lipid levels, a comprehensive serum lipid profile assay was carried out. The HFD group revealed a significant increase in the rates of total cholesterol, triglyceride, and LDL-cholesterol in serum, compared to the control group. The supplementation of HFD-fed rats with MEML at 200 and 400 mg/kg doses significantly inhibited the levels of total cholesterol, triglycerides, and LDL-cholesterol and increased HDL-cholesterol level in the serum compared to the HFD-only group. This suggested that MEML consumption-induced alleviation of the hyperlipidemia and harmful cardiovascular effects exerted by HFD feeding.
Serum CK-MB, AST, and ALT are the enzyme biomarkers which allow monitoring of the heart structural integrity and damage and serve as clinical indicators in the diagnosis of heart complications [47]. In the presence of a damage stimulus, additional CK-MB, AST, and ALT are released into the bloodstream and enhance the serum enzyme level reflecting the degree of tissue injury. Our results indicated that CK-MB, AST, and ALT amounts increased significantly in the HDF-treated rats (p < 0.01) compared to the control group. However, it was found that administration of both low and high MEML doses in the HFD-treated group successfully alleviated these abnormalities. The evidence that M. oleifera leaf extract can influence cardiovascular disease risk parameters, when high-fat diet is consumed, could be attributed to the phytochemical content in MEML and its antioxidant potentials.
Oxidative stress supervenes as a result of instability between the levels of antioxidants and oxidants in favor of oxidants [48]. Numerous studies claimed that high-fat diet incites metabolic pattern leading to the generation of reactive oxygen species (ROS) implicated in the etiology of several health complications such as atherosclerosis, diabetes, cancer, cardiovascular disorders, and other chronic diseases [49, 50]. Glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD) are antioxidant enzymes basically included in the antioxidant protective capacity of biological systems against free radical attack [51].
Our study showed that diet-induced obesity markedly impaired the antioxidant status in heart tissues of HDF-treated rats by increasing the levels of TBARS and reducing the SOD, CAT, and GPx activities, compared to controls. Furthermore, the administration of HFD was found to decrease cardiac TSH levels. TSH, like other nonenzymatic antioxidants (including reduced glutathione, vitamins C and E, natural flavonoids, and other compounds), interacts synergistically with the enzymatic antioxidant system and often acts to neutralize or scavenge free radicals by donating electrons [52]. Oral administration of MEML in the diet of HFD-treated rats significantly reduced the lipid peroxidation levels and restored the antioxidant enzyme activities as compared with control rats. These findings strongly suggest that M. oleifera extract is highly effective at preventing heart tissue damage by reducing lipid peroxidation and keeping the ROS levels at acceptable cellular concentrations. These results are consistent with previously reported data [53]. On the other hand, the cardiac histoarchitecture of the HFD-treated rats exhibited myocardial fiber disorganization, inflammatory cell collections, and area of myocardial fibrosis. MEML supplementation also induced a noticeable improvement in the histological alterations in the HFD-fed rats and minimised cardiac injury. Our findings are in agreement with those reported by Zeng et al. [54] and Poudyal et al. [55] who proved degenerative cardiac changes in rats and mice, respectively, in the setting of obesity.
5. Conclusion
In summary, the abovementioned biochemical parameters and histological evidence indicated that the methanolic extract of M. oleifera leaves at 200 and 400 mg/kg bw played an effective role in the treatment of obesity and reduction of cardiometabolic abnormalities. This prominent therapeutic activity is mainly attributed to the considerable amounts of health-promoting nutrients and phenolic compounds in this plant. However, further investigation is necessary to isolate bioactive compounds of Moringa leaf extract to examine their mechanistic therapeutic role and to avoid their potential toxic effects.
Acknowledgments
Authors would like to express special thanks to the Ministry of Higher Education and Scientific Research.
Data Availability
All data generated or analysed during the current study are available from the corresponding author on reasonable request.
Additional Points
Novelty. (i) High nutrients and phenolic compound diversity were assessed in the M. oleifera leaves. (ii) Methanolic extract of M. oleifera leaves exhibited a defensive role against obesity biomarkers and cardiac damages.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.World Health Organization (WHO) Obesity and Overweight. WHO; 2017. World Health Organization Fact sheets. [Google Scholar]
- 2.Mokdad A. H., Ford E. S., Bowman B. A., et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Journal of the American Medical Association. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Hu G., Jousilahti P., Antikainen R., Katzmarzyk P. T., Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121(2):237–244. doi: 10.1161/CIRCULATIONAHA.109.887893. [DOI] [PubMed] [Google Scholar]
- 4.de Freitas Junior L. M., de Almeida E. B., Jr. Medicinal plants for the treatment of obesity: ethnopharmacological approach and chemical and biological studies. American Journal of Translational Research. 2017;9(5):2050–2064. [PMC free article] [PubMed] [Google Scholar]
- 5.Sánchez N. R., Spörndly E., Ledin I. Effect of feeding different levels of foliage of Moringa oleifera to creole dairy cows on intake, digestibility, milk production and composition. Livestock Science. 2006;101(1-3):24–31. doi: 10.1016/j.livprodsci.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Jahn S. A. A. On the introduction of a tropical multipurpose tree to China. Traditional and potential utilization of Moringa oleifera Lamarck. Senckenbergiana Biologica. 1996;75:243–254. [Google Scholar]
- 7.Morimitsu Y., Hayashi K., Nakagawa Y., Horio F., Uchida K., Osawa T. Antiplatelet and anticancer isothiocyanates in Japanese domestic horseradish, wasabi. BioFactors. 2000;13(1-4):271–276. doi: 10.1002/biof.5520130141. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed H. H., Metwally F. M., Rashad H., Zaazaa A. M., Ezzat S. M., Salama M. M. Moringa oleifera offers a multi-mechanistic approach for management of obesity in rats. International Journal of Pharmaceutical Sciences Review and Research. 2014;29:98–106. [Google Scholar]
- 9.Jain P. G., Patil S. D., Haswani N. G., Girase M. V., Surana S. J. Hypolipidemic activity of Moringa oleifera Lam., Moringaceae, on high fat diet induced hyperlipidemia in albino rats. Revista Brasileira de Farmacognosia. 2010;20(6):969–973. doi: 10.1590/S0102-695X2010005000038. [DOI] [Google Scholar]
- 10.AOAC. Official Method of Analysis. Washington: D.C: Association of Official Analytical Chemist; 1990. [Google Scholar]
- 11.Fahey J. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic and prophylactic properties, part 1. Trees for Life. 2005;1:5–10. [Google Scholar]
- 12.DuBois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 13.Al-Harrasi A., Al-Rawahi A., Hussain J., Rehman N., Ali L., Hussain H. Proximate analysis of the resins and leaves of Boswellia sacra. Journal of Medicinal Plants Research. 2012;6(16):3098–3104. doi: 10.5897/jmpr11.1264. [DOI] [Google Scholar]
- 14.Martin E. A., Coolidge A. A. In: Nutrition in Action. 4th. Holt R., Co W., editors. New York, USA: Holt, Rinehart and Winston; 1978. [Google Scholar]
- 15.Rjeibi I., Ncib S., Alimi H., Saad A. B., Saïd I., Souid S. Comparison of phytochemicals, antimicrobial, and antioxidant capacities in different anatomical parts ofFicus microcarpa(Moraceae) Journal of Food Biochemistry. 2017;41(3, article e12354) doi: 10.1111/jfbc.12354. [DOI] [Google Scholar]
- 16.CHEN H., LIN Y., HSIEH C. Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chemistry. 2007;104(4):1418–1424. doi: 10.1016/j.foodchem.2007.02.004. [DOI] [Google Scholar]
- 17.Roussel Y., Wilks M., Harris A., Mein C., Tabaqchali S. Evaluation of DNA extraction methods from mouse stomachs for the quantification of H. pylori by real-time PCR. Journal of Microbiological Methods. 2005;62(1):71–81. doi: 10.1016/j.mimet.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Chupin L., Motillon C., Charrier-El Bouhtoury F., Pizzi A., Charrier B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Industrial Crops and Products. 2013;49:897–903. doi: 10.1016/j.indcrop.2013.06.045. [DOI] [Google Scholar]
- 19.Diet C. B., Diet U., Diet N. P. Report of the American Institute of Nutrition ad hoc committee on standards for nutritional studies. The Journal of Nutrition. 1977;107(7):1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 20.Bernardis L. L., Patterson B. D. Correlation between ‘Lee index’ and carcass fat content in weanling and adult female rats with hypothalamic lesions. Journal of Endocrinology. 1968;40(4):527–528. doi: 10.1677/joe.0.0400527. [DOI] [PubMed] [Google Scholar]
- 21.Adedapo A. A., Mogbojuri O. M., Emikpe B. O. Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. Journal of Medicinal Plants Research. 2009;3:586–591. [Google Scholar]
- 22.Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 23.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochemical Medicine. 1976;15(2):212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 24.Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 25.Flohe L., Gunzler W. A. Assays of glutathione peroxidase. Methods in Enzymology. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 26.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 27.Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical Biochemistry. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 28.Busani M., Patrick J. M., Arnold H., Voster M. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. African Journal of Biotechnology. 2011;10(60):12925–12933. doi: 10.5897/ajb10.1599. [DOI] [Google Scholar]
- 29.Oduro I., Ellis W. O., Owusu D. Nutritional potential of two leafy vegetables: Moringa oleifera and Ipomoea batatas leaves. Scientific Research and Essays. 2008;3:57–60. [Google Scholar]
- 30.Aslam M., Anwar F., Nadeem R., Rashid U., Kazi T. G., Nadeem M. Mineral composition of Moringa oleifera leaves and pods from different regions of Punjab, Pakistan. Asian Journal of Plant Sciences. 2005;4(4):417–421. doi: 10.3923/ajps.2005.417.421. [DOI] [Google Scholar]
- 31.Kaline K., Bornstein S. R., Bergmann A., Hauner H., Schwarz P. E. The importance and effect of dietary fiber in diabetes prevention with particular consideration of whole grain products. Hormone and Metabolic Research. 2007;39(9):687–693. doi: 10.1055/s-2007-985811. [DOI] [PubMed] [Google Scholar]
- 32.Emmanuel T. V., Njoka J. T., Catherine L. W., Lyaruu H. V. M. Nutritive and anti-nutritive qualities of mostly preferred edible woody plants in selected drylands of Iringa District, Tanzania. Pakistan Journal of Nutrition. 2011;10(8):786–791. doi: 10.3923/pjn.2011.786.791. [DOI] [Google Scholar]
- 33.Freyschuss B., Ljunggren O., Saaf M., Mellstrom D., Avenell A. Calcium and vitamin D for prevention of osteoporotic fractures. The Lancet. 2007;370(9605):2098–2099. doi: 10.1016/s0140-6736(07)61896-0. [DOI] [PubMed] [Google Scholar]
- 34.Strazzullo P., D'Elia L., Kandala N. B., Cappuccio F. P. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339, article b4567 doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopalakrishnan L., Doriya K., Kumar D. S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Science and Human Wellness. 2016;5(2):49–56. doi: 10.1016/j.fshw.2016.04.001. [DOI] [Google Scholar]
- 36.El Sohaimy S. A., Hamad G. M., Mohamed S. E., Amar M. H., Al-Hindi R. R. Biochemical and functional properties of Moringa oleifera leaves and their potential as a functional food. Global Advanced Research Journal of Agricultural Science. 2015;4:188–199. [Google Scholar]
- 37.Sultana B., Anwar F., Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14(6):2167–2180. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia E. Q., Deng G. F., Guo Y. J., Li H. B. Biological activities of polyphenols from grapes. International Journal of Molecular Sciences. 2010;11(2):622–646. doi: 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva E. M., Souza J. N. S., Rogez H., Rees J. F., Larondelle Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chemistry. 2007;101(3):1012–1018. doi: 10.1016/j.foodchem.2006.02.055. [DOI] [Google Scholar]
- 40.Ksouri R., Ksouri W. M., Jallali I., et al. Medicinal halophytes: potent source of health promoting biomolecules with medical, nutraceutical and food applications. Critical Reviews in Biotechnology. 2012;32(4):289–326. doi: 10.3109/07388551.2011.630647. [DOI] [PubMed] [Google Scholar]
- 41.Harborne J. B. Phytochemical methods: a guide to modern techniques of plant analysis. London: Chapman & Hall; 1998. [Google Scholar]
- 42.Rodriguez-Perez C., Quirantes-Piné R., Fernández-Gutiérrez A., Segura-Carretero A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Industrial Crops and Products. 2015;66:246–254. doi: 10.1016/j.indcrop.2015.01.002. [DOI] [Google Scholar]
- 43.Makita C., Chimuka L., Steenkamp P., Cukrowska E., Madala E. Comparative analyses of flavonoid content in Moringa oleifera and Moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting. South African Journal of Botany. 2016;105:116–122. doi: 10.1016/j.sajb.2015.12.007. [DOI] [Google Scholar]
- 44.Bais S., Singh G. S., Sharma R. Antiobesity and hypolipidemic activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Advances in Biology. 2014;2014:9. doi: 10.1155/2014/162914.162914 [DOI] [Google Scholar]
- 45.Rodríguez-Pérez C., Segura-Carretero A., Del Mar Contreras M. Phenolic compounds as natural and multifunctional anti-obesity agents: a review. Critical Reviews in Food Science and Nutrition. 2019;59(8):1212–1229. doi: 10.1080/10408398.2017.1399859. [DOI] [PubMed] [Google Scholar]
- 46.Tucker L. A., Thomas K. S. Increasing total fiber intake reduces risk of weight and fat gains in women. Nutrition. 2009;139(3):576–581. doi: 10.3945/jn.108.096685. [DOI] [PubMed] [Google Scholar]
- 47.Hu Z. J., Zhou Y. Q., Zhang H. B., Li L. Clinical value of monitoring serum cardiac biomarkers in pulmonary thromboembolism-induced myocardial injury. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28(10):1853–1855. [PubMed] [Google Scholar]
- 48.Wu J. Q., Kosten T. R., Zhang X. Y. Free radicals, antioxidant defense systems, and schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;46:200–206. doi: 10.1016/j.pnpbp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Bashan N., Kovsan J., Kachko I., Ovadia H., Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiological Reviews. 2009;89(1):27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 50.Tucker P. S., Scanlan A. T., Dalbo V. J. Chronic kidney disease influences multiple systems: describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxidative Medicine and Cellular Longevity. 2015;2015:8. doi: 10.1155/2015/806358.806358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valko M., Morris H., Cronin M. Metals, toxicity and oxidative stress. Current Medicinal Chemistry. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 52.Gümüslü S., Korgun D. K., Bilmen S., Yargiçoglu P., Agar A. Effects of sulfur dioxide Inhalation on plasma vitamin C and ceruloplasmin in ageing rats. Industrial Health. 2000;38(3):319–322. doi: 10.2486/indhealth.38.319. [DOI] [PubMed] [Google Scholar]
- 53.Das N., Sikder K., Ghosh S., Fromenty B., Dey S. Moringa oleifera Lam. leaf extract prevents early liver injury and restores antioxidant status in mice fed with high-fat diet. Indian Journal of Experimental Biology. 2012;50(6):404–412. [PubMed] [Google Scholar]
- 54.Zeng H., Vaka V. R., He X., Booz G. W., Chen J. X. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. Journal of Cellular and Molecular Medicine. 2015;19(8):1847–1856. doi: 10.1111/jcmm.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poudyal H., Campbell F., Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate–, high fat–fed rats. Nutrition. 2010;140(5):946–953. doi: 10.3945/jn.109.117812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during the current study are available from the corresponding author on reasonable request.