Sir: CD30 is a transmembrane receptor in the tumour necrosis factor receptor superfamily, member 8 (TNFRSF8) that was first identified in the malignant Hodgkin and Reed-Sternberg (HRS) cells of classical Hodgkin lymphoma.1 Brentuximab vedotin (SGN-35; BV) is an antibody–drug conjugate used currently in the setting of CD30-positive relapsed or refractory lymphomas. This study demonstrates our experience of relative CD30 expression with tumour response to BV.
Biopsies were collected from all patients receiving BV at our institution. Most recent biopsies from tumour at the time of relapse were reviewed independently by two haematopathologists. Antigen retrieval was performed using a Ventana kit. Rabbit monoclonal antibody to CD30 (clone BerH2, 1:300; Epito-mics, Burlingame, CA, USA) was used.
The percentage of CD30-positive cells of total cellularity, the percentage of CD30-positive cells of the tumour population and the stain strength (mild, moderate, marked) were estimated. The association of each variable with either complete response (CR) or complete and partial response (PR) was evaluated using univariate logistic regression analysis. All calculations used SAS statistical analysis software (SAS Institute Inc., Cary, NC, USA). Two-tailed P-values <0.05 were considered significant.
Patient and tumour characteristics are listed in Table 1. All patients received BV as single agent at 1.8 mg/kg by intravenous infusion once every 3 weeks for a maximum of 16 cycles or until disease progression or toxicity. Clinical response was assessed at average intervals of 3 weeks, 8 weeks and 1 year after starting therapy.
Table 1.
Clinicopathological characteristics
| Case | Diagnosis | Gender | Age (years) | Site of disease | Marrow involvement | Prior therapy | Best response | % CD30 overall | % CD30 of tumour |
|---|---|---|---|---|---|---|---|---|---|
| Non-Hodgkin lymphomas | |||||||||
| 1 | AITL | F | 79 | LN | Positive | CHOP/ICE/Denileukin diftitox/Pralatrexate | CR | 0 | 0 |
| 2 | AITL | M | 62 | LN | Negative | CHOP | CR | 20 | 35 |
| 3 | ALCL, ALK+ | M | 80 | Soft tissue | Positive | Denileukin diftitox/XRT | CR | 8 | 95 |
| 4 | ALCL, ALK− | M | 24 | LN | Positive | EPOCH/AutoSCT | CR | 15 | 100 |
| 5 | cALCL | M | 63 | Tonsil | Negative | CHOP/methotrexate | CR | 18 | 95 |
| 6 | PTCL, NOS | M | 33 | Skin | Negative | Gemcitabine/Romidepsin/Pralatrexate/EPOCH | PR | 0 | 0 |
| 7 | CTCL | F | 55 | LN | Negative | Photopheresis/Targretin | PR | 50 | 70 |
| 8 | PTCL, NOS | M | 63 | LN | Positive | CHOP/AlloSCT/Pralatrexate | CR | 5 | 10 |
| 9 | CTCL | M | 71 | LN | Negative | Total skin irradiation/AlloSCT | CR | 0 | 0 |
| 10 | CTCL | M | 66 | Skin | NA | Methotrexate/Targretin/Photopheresis | SD | 0 | 0 |
| 11 | CTCL | F | 62 | Skin | NA | CHOP/Denileukin diftitox/Methotexate/Targretin | PR | 45 | 45 |
| 12 | CTCL | F | 70 | Tonsil | NA | Photopheresis/IFN-alpha/Targretin/Romidepsin/Pralatrexate/EPOCH | SD | 85 | 97 |
| 13 | DLBCL | F | 20 | LN | Negative | R-CVP/R-EPOCH | PD | 20 | 40 |
| 14 | DLBCL | F | 86 | Bone | Positive | CEOP | SD | 0 | 0 |
| 15 | DLBCL | M | 49 | Soft tissue | Positive | EPOCH-R/RICE/DHAP | PR | 30 | 80 |
| Hodgkin lymphomas | |||||||||
| 16 | CHL | M | 18 | LN | Positive | ABVD/ICE/AutoSCT/COPP/R-DHAP/EPOCH | PR | 18 | 100 |
| 17 | CHL | F | 21 | LN | Negative | ABVD/DHAP/ICE/Auto-SCT/C-MOPP/Bendamustine | PR | 12 | 100 |
| 18 | CHL | M | 84 | LN | NA | ABVD | CR | 5 | 100 |
| 19 | CHL | F | 23 | LN | Negative | ABVD/BEACOPP | CR | 65 | 100 |
| 20 | CHL | F | 65 | liver | Negative | ABVD | PD | 10 | 100 |
| 21 | CHL | M | 38 | LN | NA | ABVD/ICE/AutoSCT/GND/AlloSCT/C-MOPP | CR | 25 | 100 |
| 22 | CHL | F | 28 | LN | NA | ABVD/ICE/AutoSCT | CR | 25 | 100 |
| 23 | CHL | M | 89 | Lung | NA | None | PD | 40 | 100 |
| 24 | CHL | M | 28 | Mediastinal | Positive | ABVD/C-MOPP/AlloSCT | PD | 78 | 100 |
| 25 | CHL | F | 54 | Mediastinal | NA | ABVD/ICE | SD | 3 | 100 |
| 26 | CHL | M | 54 | LN | Negative | ABVD/ICE/AutoSCT | SD | 4 | 100 |
| 27 | CHL | M | 33 | LN | NA | ABVD/ICE/AutoSCT | PR | 5 | 100 |
CR: Complete remission; PR: partial remission; SD: stable disease; PD: progressive disease; LN: lymph node; AITL: angioimmunoblastic T cell lymphoma; ALCL: anaplastic large cell lymphoma; cALCL: cutaneous anaplastic large cell lymphoma; PTCL NOS: peripheral T cell lymphoma, not otherwise specified; CTC:, cutaneous T cell lymphoma; DLBCL: diffuse large B cell lymphoma; CHL: classical Hodgkin lymphoma; R: rituximab; CHOP: cyclophosphamide, hydroxydaunorubicin, oncovine, prednisone; EPOCH: etoposide, prednisone, vincristine, cytoxan, doxorubicin; CEOP: cyclophosphamide, vincristine, epirubicin, prednisone; ICE: ifosfamide, carboplatin, etoposide; DHAP: dexam-ethasone, cytarabine, cisplatin; ABVD: doxorubicin, bleomycin, vinblastine, dacarbazine; COPP: cyclophosphamide, oncovin, procarbazine, prednisone; MOPP: mechlorethamine, vincristine, procarbazine, predisone; BEACOPP: bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; GND: gemcitabine, vinorelbine, doxorubicin; CVP: cyclophosphamide, vincrinstine, prednisone; XRT: radiation therapy; SCT: stem cell transplant.
Reduction in tumour burden was seen in 17 of 25 patients. The overall response rate (ORR) was 72%, with 44% achieving CR. In patients with non-Hodgkin lymphomas (NHL), 78% achieved a response and 50% CR. In classical Hodgkin lymphoma (CHL), the ORR was 64% while CR was seen in 36%.
Positive immunostaining for CD30 assessed as a percentage of total cellularity, or of the percentage of tumour cells showed no correlation with clinical outcome in either NHL or CHL. The strength of CD30 stain on tumour cells showed no association with response in NHL patients. However, in CHL there was a trend of increased response with increased stain strength. Statistical analyses were repeated to assess for correlation between all variables mentioned above at the approximate 1-year interval, which showed no significant correlation. Figure 1 demonstrates the staining patterns of a few of these cases.
Figure 1.
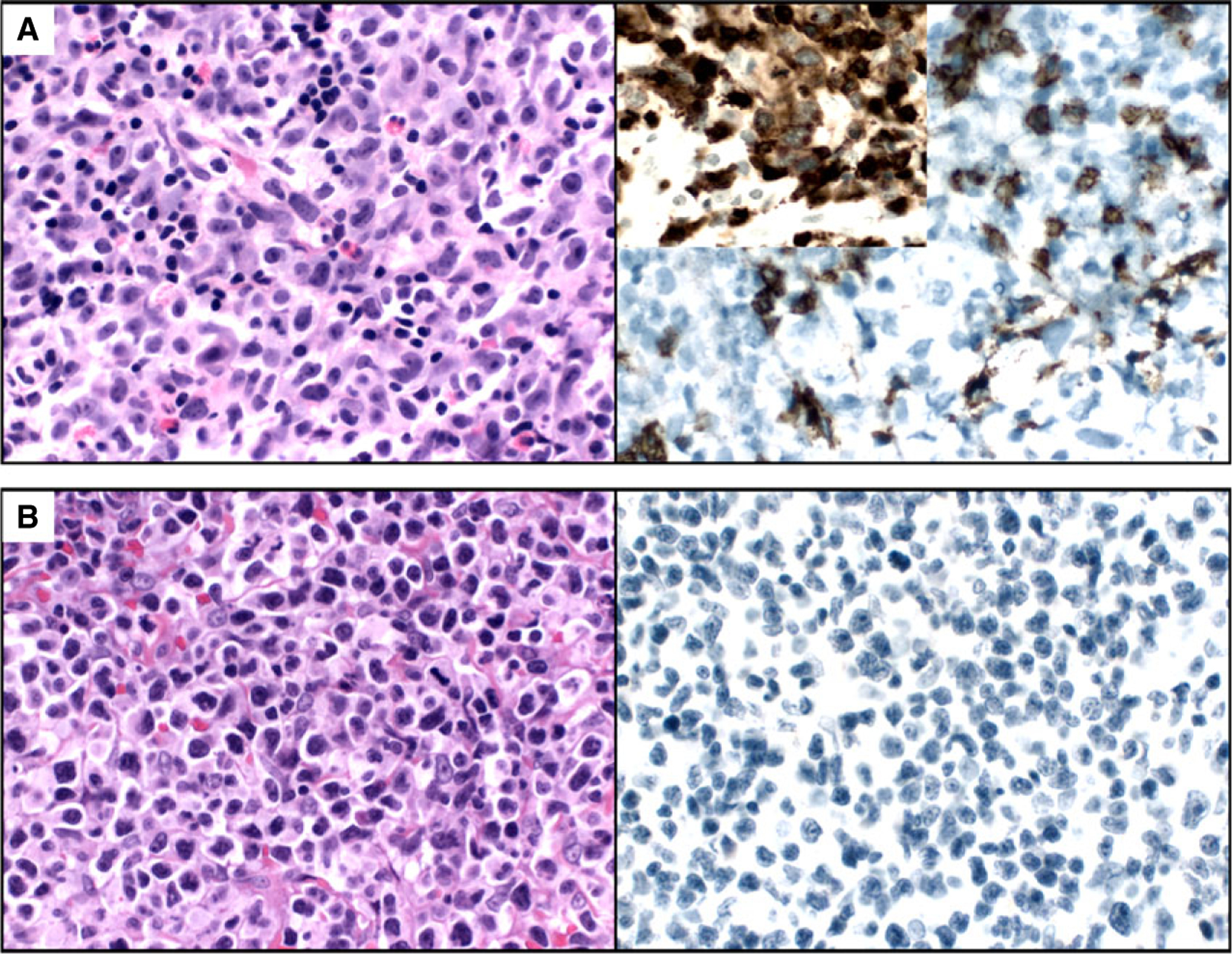
A, Case 7 showing CD30 staining of ~70% of T cell lymphoma cells (inset shows CD3 stain). The patient achieved partial remission. B, Case 1 showing CD30 staining of ~0% of T cell lymphoma cells. The patient achieved complete remission.
Results of logistic regression analysis using clinical response as outcome are represented in Table 2. Odds ratios greater than 1 indicate that with each increase in scoring level of 1 unit (in %CD30 staining or stain strength), there is an increased probability of clinical response.
Table 2.
Logistic regression analysis of CD30 positivity for clinical response to brentuximab vedotin
| CR | CR+PR | |||||
|---|---|---|---|---|---|---|
| Odds ratio | CI | P | Odds ratio | CI | P | |
| All samples (n = 27) | ||||||
| % CD30 of the cellularity | 1.04 | (0.79, 1.35) | 0.774 | 1.29 | (0.65, 2.56) | 0.472 |
| Stain strength | 0.67 | (0.17, 2.67) | 0.574 | 2.49 | (0.58, 10.66) | 0.220 |
| Non-Hodgkin lymphoma (n = 15) | ||||||
| % CD30 of the cellularity | 0.00 | (<0.001, 83.11) | 0.232 | 0.00 | (<0.001, 9.86) | 0.155 |
| % CD30 of all tumour | 0.06 | (0.002, 1.98) | 0.115 | 0.13 | (0.002, 10.39) | 0.357 |
| Stain strength | 0.19 | (0.02, 2.4) | 0.199 | 1.00 | (0.10, 10.073) | 1.000 |
| Classical Hodgkin lymphoma (n = 12) | ||||||
| % CD30 of the cellularity | 1.09 | (0.82, 1.45) | 0.543 | 1.67 | (0.29, 9.54) | 0.563 |
| Stain strength | 1.93 | (0.14–26.79) | 0.625 | 5.90 | (0.46–76.64) | 0.174 |
CR, Complete remission; PR, partial remission; CI, confidence interval.
CD30 is expressed on the cell surface of a wide range of lymphomas, but few non-malignant cells, providing a rational therapeutic target. Although limited by a small sample size, we find that the level of CD30 expression detected by immunohistochemistry is not a good predictor of clinical response to BV. The efficacy demonstrated in our institution is comparable to a large Phase II trial showing an ORR of 75% and CR of 34%.2 A recent study showed a significant correlation between CD30 mRNA levels to CD30 immunohistochemical scores using an identical anti-CD30 antibody clone to the one used here.3
Computer-assisted methods of CD30 detection have been evaluated. A study of 49 diffuse large B cell lymphomas (DLBCLs) in a multi-institutional clinical trial reported no correlation between response and expression of CD30, similar to our findings.4 Interestingly, some tumours negative by visual examination proved to have a quantifiable level of CD30 expression by computer-assisted methods. Unfortunately, computer-assisted methods are not available routinely for clinical use and may not be practical to implement.
Multispectral imaging (MSI) provides another method of possibly increasing sensitivity to low-level CD30 expression. In a Phase II trial of BV in cutaneous T cell lymphoma (CTCL), it was shown that objective clinical response was seen regardless of visual CD30 expression. Some of the positivity visualized later by MSI was found to be co-expressed by elements of the microenvironment, such as macrophages and cytotoxic T cells.5
In conclusion, our study demonstrates a lack of correlation between clinical outcome and CD30 expression on tumour cells as assessed by immunohistochemistry. As such, it may not be rational to use this marker for strict enrolment purposes. Larger, multi-institutional efforts are necessary to validate these findings. The potential that BV activity may be independent of the level of CD30 expression is worth additional investigation in order to elucidate its mechanism and identify eligible patients more effectively.
Footnotes
Ethics approving committee
Yale Human Research Protection Program Human Investigation Committee #1201009533. First approved 1/17/2012; last re-approved: 1/17/2015. This study fulfilled the criteria for waiver of informed, written consent. It was performed according to the Declaration of Helsinki.
Conflict of interests
The authors have no conflicts of interests or sources of funding to disclose.
References
- 1.Schwab U, Stein H, Gerdes J et al. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin’s disease and a subset of normal lymphoid cells. Nature 1982; 299; 65–67. [DOI] [PubMed] [Google Scholar]
- 2.Younes A, Gopal AK, Smith SE et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol 2012; 30; 2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bossard C, Dobay MP, Parrens M et al. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: high correlation with mRNA levels. Blood 2014; 124; 2983–2986. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen ED, Sharman JP, Oki Y et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 2015; 125; 1394–1402. [DOI] [PubMed] [Google Scholar]
- 5.Kim YH, Tavallaee M, Sundram U et al. Phase II investigator-initiated study of brentuximab vedotin in mycosis fungoides and Sezary syndrome with variable cd30 expression level: a multi-institution collaborative project. J. Clin. Oncol 2015; 33; 3750–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]


