Key Points
Question
Is disease progression associated with health-related quality of life among patients with cancer?
Findings
In this cohort study, mixed-model analyses of more than 8000 questionnaires from 2314 patients with metastatic breast, pancreatic, lung, or colorectal cancer showed that disease progression was accompanied by statistically significant and clinically relevant deterioration in many health-related quality-of-life scales.
Meaning
These findings suggest that time to progression and progression-free survival are important additional patient-relevant end points in the early benefit assessment of treatment for advanced cancer.
This cohort study examines the association of disease progression and health-related quality of life among adult patients with breast, lung, pancreatic, or colorectal cancer.
Abstract
Importance
Mortality, morbidity, and health-related quality of life (HRQoL) are patient-relevant end points generally considered in the early benefit assessments of new cancer treatments. Progression-related end points, such as time to progression or progression-free survival, are not included, although patients and physicians testify to the detrimental association of disease progression with HRQoL.
Objective
To examine the association of disease progression and HRQoL in 4 prevalent solid-cancer entities in routine clinical practice.
Design, Setting, and Participants
This cohort study evaluated data from 4 prospective, nonintervention, multicenter registries collected between 2011 and 2018 in 203 centers in Germany. Patients’ HRQoL was assessed regularly for up to 5 years. The change in HRQoL scores after disease progression was examined with linear mixed models, adjusting for demographic and clinical covariates. Patients with metastatic breast, pancreatic, lung, and colorectal cancer were recruited at the start of systemic first-line treatment. Data analysis was performed from February 2019 to April 2019.
Exposures
All patients received systemic, palliative first-line treatment according to their physician’s choice.
Main Outcomes and Measures
The primary outcome was deterioration of HRQoL associated with disease progression, as measured by 4 validated questionnaires: Functional Assessment of Cancer Therapy–General version 4, European Organization for Research and Treatment of Cancer QLQ-C30 version 3.0, European Organization for Research and Treatment of Cancer QLQ-C15-PAL version 1, and Hospital Anxiety and Depression Scale.
Results
More than 8000 questionnaires from 2314 patients with 2562 documented disease progressions were analyzed. In total, 464 patients had breast cancer (464 [100.0%] female; median [range] age, 61.6 [26.4-90.1] years), 807 patients had pancreatic cancer (352 [43.6%] female; median [range] age, 70.0 [39.0-93.0] years), 341 patients had lung cancer (118 [34.6%] female; median [range] age, 65.9 [28.4-88.2] years), and 702 patients had colorectal cancer (248 [35.3%] female; median [range] age, 66.9 [26.9-92.1] years). The first disease progression was associated with a statistically significant worsening of 37 of 45 HRQoL scales; for 17 of these scales, the worsening was clinically meaningful. Scale scores for appetite loss (pancreatic cancer, 10.2 points [95% CI, 6.8-13.5 points]; lung cancer, 10.8 points [95% CI, 5.4-16.2 points]; colorectal cancer, 8.8 points [95% CI, 5.5-12.2]; all P < .001), physical functioning (pancreatic cancer, 6.2 points [95% CI, 3.8-8.5 points]; lung cancer, 8.4 points [95% CI, 5.4-11.5 points]; colorectal cancer, 5.0 points [95% CI, 3.0-7.0 points]; all P < .001), and fatigue (pancreatic cancer, 5.5 points [95% CI, 3.0-7.9 points]; lung cancer, 7.7 points [95% CI, 4.3-11.1]; colorectal cancer, 4.5 points [95% CI, 2.1-6.9 points]; all P < .001) were most affected, irrespective of the type of cancer. The association with global HRQoL was most pronounced in lung cancer (6.7 points [95% CI, 3.5-9.9 points]; P < .001) and pancreatic cancer (5.4 points [95% CI, 3.3-7.5 points]; P < .001) and less in colorectal cancer (3.5 points [95% CI, 1.3-5.7 points]; P = .002) and breast cancer (2.4 points [95% CI, 1.0-3.9 points]; P = .001). The second progression was associated with an even larger decrease in HRQoL.
Conclusions and Relevance
These findings suggest that disease progression is associated with a deterioration in HRQoL among patients with metastatic breast, pancreatic, lung, and colorectal cancer. This evidence highlights the importance of progression-related end points, such as time to progression and progression-free survival, as additional patient-relevant end points when evaluating the benefit of new treatments for patients with metastatic cancer.
Introduction
Approval of novel anticancer medicines is often eagerly awaited by physicians and patients and simultaneously subject to a controversial debate between reimbursement decision-makers and drug companies. In Europe, health technology assessments for reimbursement decisions vary widely between member states, in contrast to the centralized marketing authorization decision.1 Health technology assessments generally encompass both clinical and patient-reported end points, giving an even more comprehensive evaluation of the drug of interest than the dossier for market authorization.2
Time to progression, defined as the time from start of treatment until disease progression, and progression-free survival (PFS), defined as the time from start of treatment until disease progression or death, are established end points in oncology studies. It is a current issue of debate that some health technology assessment authorities, such as in Germany, exclude PFS as a patient-relevant end point, in contrast to other public health technology assessment authorities, such as those in France and England.3,4 In the German early benefit assessments, mortality, morbidity, and health-related quality of life (HRQoL) are considered relevant from a patient perspective, whereas radiologically assessed time to progression and PFS are considered end points not relevant to the patient.3,5
Two systematic reviews6,7 of the association of PFS with HRQoL in clinical trials were inconclusive. Both reviews analyzed the association of PFS with HRQoL on the basis of the assumption that longer PFS results in better HRQoL.6,7 However, greater toxic effects in the treatment group with longer PFS can nullify the presumptive positive association of the longer PFS with HRQoL. Especially in the palliative setting, maintaining HRQoL and minimizing treatment-related adverse effects are vital to most patients.8,9
Is the question of whether longer PFS entails a better HRQoL the right methodological approach in this case? What about the association of disease progression itself with HRQoL? Most of the trials analyzed did not collect data on HRQoL after progression.6,10 Patients and physicians testify to the psychological and physical distress associated with disease progression, caused not only by complications from the progressing cancer or adverse effects of subsequent new treatments, but also by fear of losing control over cancer and fear of death.11
Robust scientific data on the association of disease progression with patient-reported outcomes (PROs) on HRQoL are still scarce. In metastatic breast cancer, results from a small, retrospective German study12 demonstrated no mean differences in QoL scales, whereas a small, retrospective study13 from US community oncology practices reported a detrimental association of disease progression with HRQoL. In metastatic non–small cell lung cancer, an analysis14 from a randomized trial found statistically significant worsening in HRQoL upon disease progression, and a small retrospective study15 of routine care showed substantial and clinically meaningful worsening of symptoms and HRQoL at disease progression. We could not identify any published investigation of the association of disease progression with HRQoL in patients with pancreatic and colorectal cancer.
The objective of the present study was to examine the association of disease progression with various HRQoL domains by analyzing HRQoL before and after progression using 4 large prospective, longitudinal, multicenter registries of patients with metastatic breast, lung, pancreatic, or colorectal cancer. Using these longitudinally collected HRQoL data from real-world databases, HRQoL during the course of metastatic cancer and its association with subsequent disease progressions were investigated.
Methods
Study Design
Patients aged 18 years or older with metastatic cancer of the breast, pancreas, lung, or colon who were undergoing systemic antineoplastic treatment were recruited into the clinical tumor registries for breast cancer, pancreatic cancer, lung cancer, and colorectal cancer. These prospective, multicenter, observational, longitudinal registries in Germany have previously been described in detail.16,17,18,19 They have each been approved by the official responsible ethics committees and are registered at ClinicalTrials.gov (identifiers: Tumour Registry Breast Cancer, NCT01351584; Tumour Registry Pancreatic Cancer, NCT02089269; Tumour Registry Lung Cancer, NCT01192919; and Tumour Registry Colorectal Cancer, NCT00910819). Written informed consent was obtained from all patients. All data were collected during routine clinical care at the participating sites. Institutional review board approval was not sought for the present cohort study because it used deidentified data. All analyses comply with the current laws in Germany, where they were performed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Declaration of Helsinki and its later amendments. This article follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.20
Patient-reported outcome measures used to assess HRQoL and symptom severity included the Functional Assessment of Cancer Therapy–General version 4,21 the Hospital Anxiety and Depression Scale,22 the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 version 3.0,23 and the shortened questionnaire for patients with cancer receiving palliative care, EORTC QLQ-C15-PAL version 1.24 Scoring of the questionnaires was performed according to the respective manuals. The scores of Functional Assessment of Cancer Therapy–General version 4 and Hospital Anxiety and Depression Scale were linearly transformed to a 0 to 100 scale for easier comparison between different scores.
Patients and Procedure
For the present analysis, we included all patients with a completed questionnaire at the start of treatment and at least 1 additional completed questionnaire. Eligible patients were enrolled at 203 sites in Germany. Patients were enrolled at the start of their first-line treatment (hereafter referred to as “start of treatment”). First-line treatment was defined as any systemic palliative treatment (eg, chemotherapy, endocrine therapy, or targeted therapy). Eligible patients with metastatic breast cancer were followed up until death or for 5 years, the database cutoff point was October 10, 2017, and the HRQoL measures were Functional Assessment of Cancer Therapy–General version 4 and Hospital Anxiety and Depression Scale. Patients with metastatic pancreatic cancer were followed up until death or for 2 years, the database cutoff point was June 30, 2018, and the HRQoL measure was the EORTC QLQ-C15-PAL. Patients with metastatic non–small cell lung cancer were followed up until death or for 2 years, the database cutoff point was January 31, 2016, and the HRQoL measure was the EORTC QLQ-C30. Patients with metastatic colorectal cancer were followed up until death or for 3 years, the database cutoff point was March 31, 2018, and the HRQoL measure was the EORTC QLQ-C30. The exact time points and respective questionnaire return rates are shown in the eFigure in the Supplement. Disease progression was determined by the treating physician; because of the observational design, no specifications as to the timing, frequency, or criteria of tumor assessment have been made.
Statistical Analysis
Linear mixed models were applied to examine the change in PRO scores over time. They are an extension of linear regression models, are well established in the analysis of repeated measures, and are recommended by the US Food and Drug Administration.25 Their primary advantage is the high statistical validity resulting from the analysis of HRQoL data of all time points together (taking into account the within-patient correlation of questionnaires). The association of disease progression with HRQoL was assessed by use of the time-dependent multilevel predictor variable progression status, thus enabling the evaluation of multiple tumor progressions in individual patients. In general, methods followed those reported previously.25,26,27 Tumor-specific linear mixed models were calculated using the covariates listed in eTable 1 in the Supplement. Changes of 5% of the instrument range were considered clinically meaningful,28,29,30 and in this longitudinal, within-patient analysis, such changes may be interpreted as moderate-to-large clinically meaningful associations.14,28 There was no imputation of missing data values. Statistical significance was set at 2-sided P < .05, corresponding to 2-sided 95% CIs. All analyses were performed using SAS statistical software version 9.4 (SAS Institute). Data analysis was performed from February 2019 to April 2019.
Results
Patient and Tumor and Characteristics
More than 8000 questionnaires from 2314 patients with 2562 documented disease progressions were analyzed. In total, 464 patients had breast cancer (464 [100.0%] female; median [range] age, 61.6 [26.4-90.1] years), 807 patients had pancreatic cancer (352 [43.6%] female; median [range] age, 70.0 [39.0-93.0] years), 341 patients had lung cancer (118 [34.6%] female; median [range] age, 65.9 [28.4-88.2] years), and 702 patients had colorectal cancer (248 [35.3%] female; median [range] age, 66.9 [26.9-92.1] years). Patient and tumor characteristics and comorbidity status as measured by the Charlson Comorbidity Index31 are shown in the Table by cancer entity. The proportion of patients with Eastern Cooperative Oncology Group performance status of 1 or higher at the start of treatment was 44.4% of patients with breast cancer, 61.2% of patients with pancreatic cancer, 68.0% of patients with lung cancer, and 53.7% of patients with colorectal cancer. The proportion of patients with at least 1 progression was 58.2% of patients with breast cancer, 61.2% of patients with pancreatic cancer, 71.6% of patients with lung cancer, and 60.4% of patients with colorectal cancer (Table). Additional specific characteristics for patients with the different cancers are summarized in eTable 2 in the Supplement.
Table. Patient and Tumor Characteristics.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Breast cancer (n = 464)a | Pancreatic cancer (n = 807)b | Lung cancer (n = 341)c | Colorectal cancer (n = 702)d | |
| Age, median (range), ye | 61.6 (26.4-90.1) | 70.0 (39.0-93.0) | 65.9 (28.4-88.2) | 66.9 (26.9-92.1) |
| Body mass index, mean (SD)e,f | 27.0 (5.5) | 24.7 (4.6) | 25.3 (4.7) | 26.1 (4.8) |
| Sex | ||||
| Female | 464 (100.0) | 352 (43.6) | 118 (34.6) | 248 (35.3) |
| Male | 0 | 455 (56.4) | 223 (65.4) | 454 (64.7) |
| Eastern Cooperative Oncology Group performance statuse | ||||
| 0 | 258 (55.6) | 313 (38.8) | 109 (32.0) | 325 (46.3) |
| ≥1 | 206 (44.4) | 494 (61.2) | 232 (68.0) | 377 (53.7) |
| Comorbidities, Charlson Comorbidity Indexg,h | ||||
| 0 | 389 (83.8) | 593 (73.5) | 189 (55.4) | 542 (77.2) |
| ≥1 | 75 (16.2) | 214 (26.5) | 152 (44.6) | 160 (22.8) |
| Metastasis at diagnosish | ||||
| No (M0, metachronous) | 259 (55.8) | 207 (25.7) | 92 (27.0) | 175 (24.9) |
| Yes (M1, synchronous) | 150 (32.3) | 494 (61.2) | 247 (72.4) | 487 (69.4) |
| Unknown (MX) | 55 (11.9) | 106 (13.1) | 2 (0.6) | 40 (5.7) |
| Progression | ||||
| Patients without progression | 194 (41.8) | 313 (38.8) | 97 (28.4) | 278 (39.6) |
| Patients with ≥1 progression | 270 (58.2) | 494 (61.2) | 244 (71.6) | 424 (60.4) |
Recruited from 2011 to 2016 in 103 centers.
Recruited from 2014 to 2018 in 100 centers.
Recruited from 2011 to 2013 in 71 centers.
Recruited from 2014 to 2017 in 110 centers.
At start of treatment.
Body mass index is calculated as the weight in kilograms divided by height in meters squared.
The Charlson Comorbidity Index is described by Quan et al.31
At primary diagnosis.
As the first line of treatment, 317 of 464 patients with breast cancer (68.3%) received chemotherapy, and 147 (31.6%) received endocrine therapy. Of the 807 patients with pancreatic cancer, most received gemcitabine plus nab-paclitaxel (349 patients [43.2%]), FOLFIRINOX (folinic acid, fluorouacil, irinotecan hydrochloride, and oxaliplatin; 242 patients [29.9%]), or gemcitabine monotherapy (142 patients [17.5%]). Most of the 341 patients with lung cancer received a platinum-based chemotherapy as first-line treatment; the platinum agent was carboplatin for 143 patients (41.9%) and cisplatin for 120 patients (35.2%). The first-line therapy for the 702 patients with colorectal cancer was based on FOLFIRI (folinic acid, fluorouacil, and irinotecan hydrochloride) for 282 patients (40.2%) and on FOLFOX (folinic acid, fluorouacil, and oxaliplatin) for 317 patients (45.2%).
Questionnaire Return Rate and Numbers of Evaluable Questionnaires
The questionnaire return rates for all patients alive who received the respective questionnaire were 86% (3 months after start of treatment) to 50% (30 months) for breast cancer, 91% (3 months) to 31% (24 months) for pancreatic cancer, 96% (2 months) to 76% (10 months) for lung cancer, and 92% (2 months) to 60% (24 months) for colorectal cancer. The intervals between questionnaires were 2 months for pancreatic and lung cancer, 3 to 6 months for breast cancer, and 2 to 4 months for colorectal cancer. The return rate and questionnaire chronology are shown in the eFigure in the Supplement.
The total numbers of individual PRO assessments for the item global quality of life were 1759 for breast cancer, 2927 for pancreatic cancer, 1127 for lung cancer, and 2661 for colorectal cancer. Of these, the following were returned after disease progression: 418 questionnaires (24%) for breast cancer, 862 questionnaires (29%) for pancreatic cancer, 316 questionnaires (28%) for lung cancer, and 708 questionnaires (27%) for colorectal cancer.
Association of Disease Progression With PROs
Controlling for demographic and clinical covariates in entity-specific separate linear mixed models, the first disease progression was accompanied by a statistically significant deterioration in most HRQoL scales in all 4 types of cancer (Figure 1). All covariates are listed in eTable 1 in the Supplement.
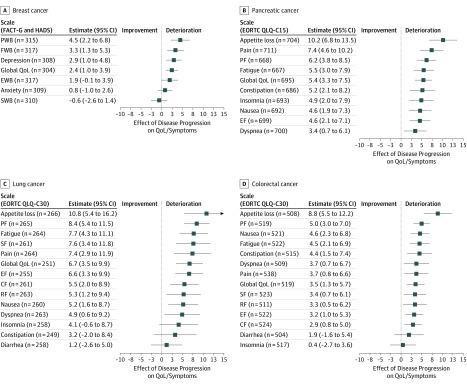
Figure 1. Linear Mixed Model for Association of First Disease Progression With Quality of Life (QoL) and Symptoms.
A, Association of first progression with scales of the Functional Assessment of Cancer Therapy–General version 4 (FACT-G) and Hospital Anxiety and Depression Scale (HADS) questionnaires among patients with breast cancer. B, Association of first progression with scales and symptoms of the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C15 questionnaire among patients with pancreatic cancer. C, Association of first progression with scales and symptoms of the EORTC QLQ-C30 questionnaire among patients with lung cancer. D, Association of first progression with scales and symptoms of the EORTC QLQ-C30 questionnaire among patients with colorectal cancer. Numbers indicate number of questionnaires completed by patients after their first and before the second disease progression. CF indicates cognitive functioning; EF, emotional functioning; EWB, emotional well-being; FWB, functional well-being; PF, physical functioning; PWB, physical well-being; RF, role functioning; SF, social functioning; and SWB, social well-being.
In breast cancer, the instrument scales physical well-being (4.5 points; 95% CI, 2.2 to 6.8 points; P < .001), functional well-being (3.3 points; 95% CI, 1.3 to 5.3 points; P = .001), depression (2.9 points; 95% CI, 1.0 to 4.8 points; P = .002), and global quality of life (2.4 points; 95% CI, 1.0 to 3.9 points; P = .001) showed statistically significant deterioration (Figure 1A). In contrast, social well-being improved slightly (−0.6 point; 95% CI, −2.6 to 1.4 points; P = .54) (Figure 1A). In pancreatic cancer, all scores of the EORTC QLQ-C15 worsened significantly after the first progression, especially for appetite loss (10.2 points; 95% CI, 6.8 to 13.5 points), pain (7.4 points; 95% CI, 4.6 to 10.2 points), physical functioning (6.2 points; 95% CI, 3.8 to 8.5 points), and fatigue (5.5 points; 95% CI, 3.0-7.9 points (all P < .001) (Figure 1B). In lung cancer, all items of the EORTC QLQ-C30 except insomnia, constipation, and diarrhea showed statistically significant worsening after disease progression, especially appetite loss (10.8 points; 95% CI, 5.4 to 16.2 points; P < .001), physical functioning (8.4 points; 95% CI, 5.4 to 11.5 points; P < .001), fatigue (7.7 points; 95% CI, 4.3 to 11.1 points; P < .001), social functioning (7.6 points; 95% CI, 3.4 to 11.8 points; P < .001), and pain (7.4 points; 95% CI, 2.9 points to 11.9 points; P = .001) (Figure 1C). In colorectal cancer, all scores of the EORTC QLQ-C30 except for insomnia and diarrhea statistically significantly deteriorated (Figure 1D), with appetite loss being affected the most (8.8 points; 95% CI, 5.5 to 12.2 points), followed by physical functioning (5.0 points; 95% CI, 3.0 to 7.0 points), nausea (4.6 points; 95% CI, 2.3 to 6.8 points), and fatigue (4.5 points; 95% CI, 2.1 to 6.9 points) (all P < .001). The deterioration in global HRQoL associated with the first progression was 2.4 points (95% CI, 1.0 to 3.9 points; P = .001) in breast cancer, 5.4 points (95% CI, 3.3 to 7.5 points; P < .001) in pancreatic cancer, 6.7 points (95% CI, 3.5 to 9.9; P < .001) in lung cancer, and 3.5 points (95% CI, 1.3 to 5.7 points; P = .002) in colorectal cancer.
Statistically significant worsening after the first progression was seen in the vast majority of HRQoL scales: 37 of 45 scales overall, 4 of 7 in breast cancer, 10 of 10 in pancreatic cancer, 11 of 14 in lung cancer, and 12 of 14 in colorectal cancer. Furthermore, clinically meaningful changes (changes of >5% of the respective scale) after the first progression were seen in 17 of 45 HRQoL scales. These scales were constipation, global quality of life, fatigue, physical well-being, pain, and appetite loss in pancreatic cancer; appetite loss, physical functioning, fatigue, social functioning, pain, global quality of life, emotional functioning, cognitive functioning, role functioning, and nausea in lung cancer; and appetite loss in colorectal cancer. Ten of 14 PRO measures (71%) in lung cancer and 6 of 10 PRO measures (60%) in pancreatic cancer reached clinical relevance. Only the PRO measure appetite loss (1 of 14 [7%]), reached clinical relevance for patients with colorectal cancer. In breast cancer, all scales were below clinical meaningfulness, yet physical well-being came very close with a deterioration of 4.5 points (95% CI, 2.2-6.8 points; P < .001).
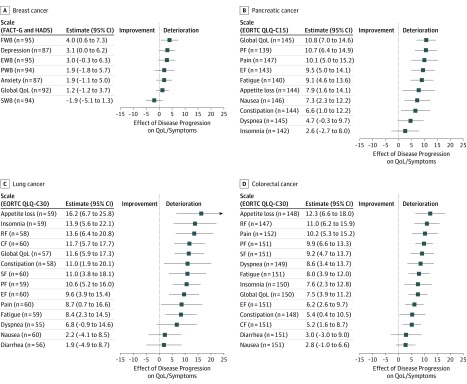
The association of a subsequent second disease progression with the various HRQoL domains is shown in Figure 2. In total, 38 of 45 HRQoL scales showed a greater degree of worsening after the second disease progression than after the first progression. This was seen for the scales depression (3.1 points; 95% CI, 0.0 to 6.2 points; P = .05), anxiety (1.9 points; 95% CI, −1.1 to 5.0 points; P = .21), functional well-being (4.0 points; 95% CI, 0.6 to 7.3 points; P = .02), and emotional well-being (3.0 points; 95% CI, −0.3 to 6.3 points; P = .08) in breast cancer; all scales except for appetite loss and insomnia in pancreatic cancer; and all scales except for nausea in lung cancer and colorectal cancer (Figure 2). Overall, statistically significant worsening after the second progression was seen in 32 of 45 HRQoL scales; likewise, 32 of 45 scales showed clinically meaningful changes. Global HRQoL worsened more after the second disease progression. For patients with breast cancer, the deterioration was moderate (1.2 points; 95% CI, −1.2 to 3.7 points; P = .33), whereas it was severe for patients with pancreatic cancer (10.8 points; 95% CI, 7.0 to 14.6 points; P < .001), lung cancer (11.6 points; 95% CI, 5.9 to 17.3 points; P < .001), and colorectal cancer (7.5 points; 95% CI, 3.9 to 11.2 points; P < .001).
Figure 2. Linear Mixed Model for Association of Second Disease Progression With Quality of Life (QoL) and Symptoms.
A, Association of second progression with scales of the Functional Assessment of Cancer Therapy–General version 4 (FACT-G) and Hospital Anxiety and Depression Scale (HADS) questionnaires among patients with breast cancer. B, Association of second progression with scales and symptoms of the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C15 questionnaire among patients with pancreatic cancer. C, Association of second progression with scales and symptoms of the EORTC QLQ-C30 questionnaire among patients with lung cancer. D, Association of second progression with scales and symptoms of the EORTC QLQ-C30 questionnaire patients with colorectal cancer. Numbers indicate number of questionnaires completed by patients after their second and before the third disease progression. CF indicates cognitive functioning; EF, emotional functioning; EWB, emotional well-being; FWB, functional well-being; PF, physical functioning; PWB, physical well-being; RF, role functioning; SF, social functioning; and SWB, social well-being.
Very few patients had experienced a third or fourth disease progression at the time of analysis. The associations of these progressions with HRQoL are listed in eTable 3 in the Supplement.
Discussion
The objective of this analysis was to determine the association of disease progression with various HRQoL domains in patients with 1 of 4 frequent cancers in clinical routine practice. The findings suggest that disease progression is associated with a deterioration in HRQoL, irrespective of the cancer entity. In general, the scales for appetite loss, physical functioning, and fatigue were most associated with worsening by the first progression. Patients with pancreatic or lung cancer reported much more deterioration in HRQoL than did patients with breast or colorectal cancer. All patients reported further considerable decline in HRQoL after the second progression. To our knowledge, this is the first report analyzing subsequent disease progressions in 4 large, prospectively collected registries with patients from routine care and also is the first report on the association of disease progression with HRQoL in pancreatic and colorectal cancer. These data add important information to the literature, showing that disease progression itself should be considered a relevant criterion to evaluate the benefit of therapies for metastatic cancers.
Regardless of whether the cancer was originating from breast, pancreas, lung, or colon, disease progression was adversely associated with HRQoL in this real-world setting. Looking at the deterioration in global HRQoL upon the first disease progression, with 2.5 points in breast cancer and 3.5 points in colorectal cancer, the deterioration was markedly higher in pancreatic cancer (5.4 points) and lung cancer (6.7 points). This difference may well be associated with the differing prognoses and tumor biological profiles of these entities. The previously published median overall survival times of patients with these metastatic cancers in routine care is 16.8 months (triple-negative tumors) to 38.2 months (hormone receptor–positive and human epidermal growth factor receptor 2–negative tumors) in breast cancer and 16.8 (high-risk group) to 26.5 months (low-risk group) in colorectal cancer, compared with 9.2 months in pancreatic cancer and 11.4 months in lung cancer.16,17,19,32 The different clinical courses entail a quickly deteriorating HRQoL and symptom severity, and they are probably among the reasons for the limited data on HRQoL in pancreatic cancer, which is a severe and rapidly progressive disease. We found that global HRQoL worsened even more after the second disease progression. For patients with breast cancer, the deterioration (1.2 points) was moderate, whereas it was more severe for patients with pancreatic cancer (10.8 points), lung cancer (11.6 points), and colorectal cancer (7.5 points).
Altogether, the first disease progression had a statistically significant detrimental association with 37 of 45 HRQoL scales. Moderate-to-large clinically meaningful changes were seen in 17 of 45 HRQoL scales, highlighting that not only global HRQoL, but also various domains and specific symptom scales, may be affected. Ten of 14 PRO measures (71%) in lung cancer and 6 of 10 PRO measures (60%) in pancreatic cancer reached clinical relevance, further underscoring the high level of aggressiveness of these cancer entities. Interestingly, the first disease progression did not entail a clinically meaningful deterioration of HRQoL scales for patients with breast cancer, and only the PRO measure appetite loss (1 of 14 [7%]), reached clinical relevance for patients with colorectal cancer. A possible explanation might be the questionnaire intervals: these were shorter in pancreatic or lung cancer (2 months) compared with breast cancer (3-6 months) and colorectal cancer (2-4 months), potentially underrepresenting patients with breast or colorectal cancer, who experienced severe deterioration in HRQoL after progression, because those patients most likely could no longer return a subsequent questionnaire.
Few studies12,13,14,15 have published analyses on the association of progression with HRQoL for one of these cancers by examining HRQoL before and after progression. Some other studies33,34,35 have used data from clinical trials to investigate whether longer PFS resulted in better HRQoL or whether HRQoL differed between patients with or without progression. Using these methodological approaches, it must be kept in mind that there is no control for the kind of treatment; thus, the toxic effects of treatment may nullify the positive association of a longer PFS. Conversely, longer PFS may not, per se, be associated with better HRQoL, and it is therefore valid to criticize and challenge a treatment with merely longer PFS if it burdens the patient with greater toxic effects and, thus, has a negative association with the quality of survival.8,9 In the end, the patient’s preference weighing tumor control vs treatment-related toxic effects remains a highly individual decision.
Limitations and Strengths
There are some limitations to this study. Because of the observational design of the study, there are no specifications as to the timing, frequency, or criteria of tumor assessment. Progression assessed in this study reflects the event that the physician considered and communicated as disease progression in routine care and is not necessarily identical to the event progression as determined by radiology according to Response Evaluation Criteria in Solid Tumors reported in clinical trials. A limitation inherent to all PRO assessments is missing data, which are not likely to be missing at random. Patients with deteriorating health and low HRQoL are less likely to return their questionnaires, possibly leading to an underestimation of the association of disease progression with HRQoL.36,37 In the context of this study, this limitation would mean that the association of progression with HRQoL could potentially be even greater than shown. In addition, we examined multiple questionnaires and scales in this study, possibly resulting in random significant findings associated with alpha inflation.
This study also has numerous strengths, including prospective data collection, 4 large multicenter registries leading to robust patient numbers, longitudinal assessment of PROs beyond disease progression, and evaluation of multiple HRQoL domains. In contrast to most other published studies, our analyses were based on a vast number of PROs collected from patients in routine care. The sample was balanced between patients with and without disease progression, further strengthening the results.
Conclusions
Public authorities often disregard disease progression as a patient-relevant end point because radiologically assessed progression might not necessarily be associated with morbidity and because longer PFS is often not statistically significantly associated with longer survival. Here, we show that disease progression is accompanied by a distinct deterioration in HRQoL in 4 common metastatic cancer entities. These data emphasize the importance of disease progression from a patient perspective. We therefore suggest that progression-related end points in metastatic breast, colorectal, lung, or pancreatic cancer should be considered when evaluating the benefit of novel treatments, in addition to survival, morbidity, and HRQoL outcomes.
eTable 1. Covariates Used in Mixed-Model Analyses
eFigure. Questionnaire Return Rate
eTable 2. Additional Patient and Tumor Characteristics
eTable 3. Subsequent Disease Progressions
References
- 1.Kleijnen S, Lipska I, Leonardo Alves T, et al. Relative effectiveness assessments of oncology medicines for pricing and reimbursement decisions in European countries. Ann Oncol. 2016;27(9):-. doi: 10.1093/annonc/mdw233 [DOI] [PubMed] [Google Scholar]
- 2.Köhler M, Haag S, Biester K, et al. Information on new drugs at market entry: retrospective analysis of health technology assessment reports versus regulatory reports, journal publications, and registry reports. BMJ. 2015;350:h796. doi: 10.1136/bmj.h796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giuliani G, Chassagnol F, Traub D, et al. Leveraging EUnetHTA’s conceptual framework to compare HTA decision drivers in France, Italy, and Germany from a manufacturer’s point of view. Health Econ Rev. 2018;8(1):24. doi: 10.1186/s13561-018-0201-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NICE Decision Support Unit, University of Sheffield . Progression-free survival and overall survival (PFS-OS). Accessed May 22, 2019. http://nicedsu.org.uk/methods-development/pfs-os/
- 5.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen . Allgemeine methoden, version 5.0 [in German]. Published July 10, 2017. Accessed May 22, 2019. https://www.iqwig.de/download/Allgemeine-Methoden_Version-5-0.pdf
- 6.Kovic B, Jin X, Kennedy SA, et al. Evaluating progression-free survival as a surrogate outcome for health-related quality of life in oncology: a systematic review and quantitative analysis. JAMA Intern Med. 2018;178(12):1586-1596. doi: 10.1001/jamainternmed.2018.4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutman SI, Piper M, Grant MD, Basch E, Oliansky DM, Aronson N. Progression-Free Survival: What Does It Mean for Psychological Well-being or Quality of Life? Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 8.Jenkins V, Farewell V, May S, et al. Do drugs offering only PFS maintain quality of life sufficiently from a patient’s perspective? results from AVALPROFS (Assessing the ‘VALue’ to patients of PROgression Free Survival) study. Support Care Cancer. 2018;26(11):3941-3949. doi: 10.1007/s00520-018-4273-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fallowfield LJ, Catt SL, May SF, et al. Therapeutic aims of drugs offering only progression-free survival are misunderstood by patients, and oncologists may be overly optimistic about likely benefits. Support Care Cancer. 2017;25(1):237-244. doi: 10.1007/s00520-016-3408-7 [DOI] [PubMed] [Google Scholar]
- 10.Kovic B, Guyatt G, Brundage M, Thabane L, Bhatnagar N, Xie F. Association between progression-free survival and health-related quality of life in oncology: a systematic review protocol. BMJ Open. 2016;6(9):e012909. doi: 10.1136/bmjopen-2016-012909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thong MSY, Mols F, Coebergh J-WW, Roukema JA, van de Poll-Franse LV. The impact of disease progression on perceived health status and quality of life of long-term cancer survivors. J Cancer Surviv. 2009;3(3):164-173. doi: 10.1007/s11764-009-0094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller V, Nabieva N, Häberle L, et al. Impact of disease progression on health-related quality of life in patients with metastatic breast cancer in the PRAEGNANT breast cancer registry. Breast. 2018;37:154-160. doi: 10.1016/j.breast.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 13.Walker MS, Hasan M, Yim YM, Yu E, Stepanski EJ, Schwartzberg LS. Retrospective study of the effect of disease progression on patient reported outcomes in HER-2 negative metastatic breast cancer patients. Health Qual Life Outcomes. 2011;9:46. doi: 10.1186/1477-7525-9-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker MS, Wong W, Ravelo A, Miller PJE, Schwartzberg LS. Effectiveness outcomes and health related quality of life impact of disease progression in patients with advanced nonsquamous NSCLC treated in real-world community oncology settings: results from a prospective medical record registry study. Health Qual Life Outcomes. 2017;15(1):160. doi: 10.1186/s12955-017-0735-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griebsch I, Palmer M, Fayers PM, Ellis S. Is progression-free survival associated with a better health-related quality of life in patients with lung cancer? evidence from two randomised trials with afatinib. BMJ Open. 2014;4(10):e005762. doi: 10.1136/bmjopen-2014-005762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fietz T, Tesch H, Rauh J, et al. ; TMK-Group (Tumour Registry Breast Cancer) . Palliative systemic therapy and overall survival of 1,395 patients with advanced breast cancer: results from the prospective German TMK cohort study. Breast. 2017;34:122-130. doi: 10.1016/j.breast.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 17.Hegewisch-Becker S, Aldaoud A, Wolf T, et al. ; TPK-Group (Tumour Registry Pancreatic Cancer) . Results from the prospective German TPK clinical cohort study: treatment algorithms and survival of 1,174 patients with locally advanced, inoperable, or metastatic pancreatic ductal adenocarcinoma. Int J Cancer. 2019;144(5):981-990. doi: 10.1002/ijc.31751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marschner N, Arnold D, Engel E, et al. Oxaliplatin-based first-line chemotherapy is associated with improved overall survival compared to first-line treatment with irinotecan-based chemotherapy in patients with metastatic colorectal cancer: results from a prospective cohort study. Clin Epidemiol. 2015;7(7):295-303. doi: 10.2147/CLEP.S73857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Verschuer U, Schnell R, Tessen HW, et al. ; TLK-Group (Tumour Registry Lung Cancer) . Treatment, outcome and quality of life of 1239 patients with advanced non-small cell lung cancer: final results from the prospective German TLK cohort study. Lung Cancer. 2017;112:216-224. doi: 10.1016/j.lungcan.2017.07.031 [DOI] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 21.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570-579. doi: 10.1200/JCO.1993.11.3.570 [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 23.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 24.Groenvold M, Petersen MA, Aaronson NK, et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer. 2006;42(1):55-64. doi: 10.1016/j.ejca.2005.06.022 [DOI] [PubMed] [Google Scholar]
- 25.Garcia TP, Marder K. Statistical approaches to longitudinal data analysis in neurodegenerative diseases: Huntington’s disease as a model. Curr Neurol Neurosci Rep. 2017;17(2):14. doi: 10.1007/s11910-017-0723-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locascio JJ, Atri A. An overview of longitudinal data analysis methods for neurological research. Dement Geriatr Cogn Dis Extra. 2011;1(1):330-357. doi: 10.1159/000330228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19(13):1793-1819. doi: [DOI] [PubMed] [Google Scholar]
- 28.Sloan JA, Frost MH, Berzon R, et al. ; Clinical Significance Consensus Meeting Group . The clinical significance of quality of life assessments in oncology: a summary for clinicians. Support Care Cancer. 2006;14(10):988-998. doi: 10.1007/s00520-006-0085-y [DOI] [PubMed] [Google Scholar]
- 29.Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48(11):1713-1721. doi: 10.1016/j.ejca.2012.02.059 [DOI] [PubMed] [Google Scholar]
- 30.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139-144. doi: 10.1200/JCO.1998.16.1.139 [DOI] [PubMed] [Google Scholar]
- 31.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 32.Marschner N, Frank M, Vach W, et al. Development and validation of a novel prognostic score to predict survival in patients with metastatic colorectal cancer: the metastatic colorectal cancer score (mCCS). Colorectal Dis. 2019;21(7):816-826. doi: 10.1111/codi.14600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rugo HS, Diéras V, Gelmon KA, et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol. 2018;29(4):888-894. doi: 10.1093/annonc/mdy012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherrill B, Amonkar MM, Sherif B, Maltzman J, O’Rourke L, Johnston S. Quality of life in hormone receptor-positive HER-2+ metastatic breast cancer patients during treatment with letrozole alone or in combination with lapatinib. Oncologist. 2010;15(9):944-953. doi: 10.1634/theoncologist.2010-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang TJ, Gyawali B. Association between progression-free survival and patients’ quality of life in cancer clinical trials. Int J Cancer. 2019;144(7):1746-1751. doi: 10.1002/ijc.31957 [DOI] [PubMed] [Google Scholar]
- 36.Moinpour CM, Triplett JS, McKnight B, et al. Challenges posed by non-random missing quality of life data in an advanced-stage colorectal cancer clinical trial. Psychooncology. 2000;9(4): 340-354. doi: [DOI] [PubMed] [Google Scholar]
- 37.Stephens R. The analysis, interpretation, and presentation of quality of life data. J Biopharm Stat. 2004;14(1):53-71. doi: 10.1081/BIP-120028506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Covariates Used in Mixed-Model Analyses
eFigure. Questionnaire Return Rate
eTable 2. Additional Patient and Tumor Characteristics
eTable 3. Subsequent Disease Progressions