Abstract
Aim
Laser microdissection (LMD) and liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) enable clinicians to analyse proteins from tissue sections. In nephrology, these methods are used to diagnose diseases of abnormal protein deposition, such as amyloidosis, but they are seldom applied to the diagnosis and pathophysiological understanding of human glomerular diseases.
Methods
Renal biopsy specimens were obtained from five patients with IgA nephropathy (IgAN), five patients with membranous nephropathy (MN) and five kidney transplant donors (as controls). From 10‐μm‐thick sections of formalin‐fixed, paraffin‐embedded specimens, 0.3‐mm2 samples of glomerular tissue were subjected to LMD. The samples were analysed by LC‐MS/MS and investigated clinically and histologically.
Results
From the control glomeruli, we identified more than 300 types of proteins. In patients with IgAN, we detected significant increases not only in IgA1 and in C3, but also in the factors related to oxidative stress and cell proliferation in comparison to the controls. In patients with MN, levels of IgG1, IgG4, C3, C4a and phospholipase‐A2‐receptor were significantly elevated in comparison to the controls, as were the aforementioned factors related to oxidative stress and cell proliferations detected in IgAN.
Conclusion
Application of LMD and LC‐MS/MS to renal biopsy specimens enabled us to identify not only pathognomonic proteins for the diagnosis, but also several factors possibly involved in the pathogenesis of human glomerular diseases.
Keywords: glomerulonephritis, IgA nephropathy, laser microdissection, mass spectrometry, membranous nephropathy
SUMMARY AT A GLANCE
This paper examined the possible application of laser microdissection and liquid chromatography‐tandem mass spectrometry to renal biopsy specimens to clarify the pathogenesis of human glomerular diseases.
1. INTRODUCTION
Identification of proteins in renal biopsy specimens is necessary for diagnosing kidney diseases. Immunofluorescence or immunohistochemistry is generally performed with specific antibodies for the expected protein molecules. Liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) is a powerful tool in proteomics that elucidates unknown protein contents through the measurement of masses of the fragmented peptides. Recently, a novel technique, laser microdissection (LMD) has been developed and introduced into the field of pathology. In LMD, a laser beam is used to dissect any selected fields on histological sections; protein contents can then be analysed by LC‐MS/MS for screening and identification of unknown proteins.
In nephrology, this technique has been used for the diagnosis of protein deposition diseases, such as amyloidosis, which is usually diagnosed by immunohistochemistry. The diagnosis of heavy‐chain amyloidosis or several hereditary forms of amyloidosis, however, is difficult because the corresponding antibodies may be unavailable.1 Sethi et al used this method for diagnosis of amyloidosis and detected novel proteins.2, 3 They reported that the causes of amyloidosis were more diverse than previously recognised. Using this method, other researchers identified a novel and unexpected molecule of DNAJB9 as a marker for fibrillary glomerulonephritis.4, 5
Application of this novel technique to the diagnosis of glomerulonephritis has just begun. To date, IgA nephropathy (IgAN) has been analysed in a few studies, with a focus mainly on deposited immunoglobulin and complements.6, 7 In our study, we analysed the glomerular proteomics of IgAN and membranous nephropathy (MN) by LMD and LC‐MS/MS and we compared our findings with those for normal glomeruli obtained at biopsies from kidney transplant donors. Moreover, we investigated the significance of the detected molecules with regard to the diagnosis and pathogenesis of glomerular diseases.
2. METHODS
2.1. Patients
Five patients with IgAN, five patients with MN and five kidney transplant donors were enrolled in this study. In all 10 patients, disease had been diagnosed with renal biopsy in the Department of Nephrology, Showa University Hospital, Tokyo, from 2014 to 2018. During the study period, IgAN was diagnosed in 69 patients and primary MN in 10 patients. Of these patients, we selected 5 with IgAN and 5 with MN whose specimens contained 10 or more non‐sclerosed glomeruli. All five patients diagnosed with MN in this study showed positive immunostaining for PLA2R in the glomerular capillaries, which could indicate that the MN was primary, but serum anti‐PLA2R was detected by ELISA in three patients out of the five patients with MN. In addition, one autopsied kidney without abnormal renal lesions was used for the studies to clarify the relationship between the number of dissected glomeruli and the number of proteins detected in LC‐MS/MS analysis. Formalin‐fixed paraffin‐embedded (FFPE) tissues from renal biopsy were used to perform LC‐MS/MS analysis. Glomeruli obtained from the autopsied kidney, pre‐transplant kidneys (at the 0‐hour renal biopsy 【0 hour‐RBx】) and post‐transplant kidneys (at the 1‐hour renal biopsy 【1 hour‐RBx】) showed no pathological changes morphologically. The clinical findings of all patients are shown in Table 1.
Table 1.
Clinical features of transplant donors, IgAN and MN patients
| Transplant donor (n = 5) | IgAN (n = 5) | MN (n = 5) | |
|---|---|---|---|
| Age at Bx (years) | 60 ± 11(45‐73) | 37 ± 19(20‐68) | 54 ± 19(24‐73) |
| SEX (M:F) | 1:4 | 2:3 | 1:4 |
| Hematuria (scores) | 0 | 2 | 0 |
| UP‐UCR (g/gCr) | 0 | 0.5 ± 0.5 | 3.1 ± 1.9a |
| Serum creatinine (mg/dL) | 0.75 ± 0.06(0.65‐0.80) | 0.68 ± 0.16(0.48‐0.88) | 0.66 ± 0.19(0.49‐0.90) |
P < .05 compared to the transplant donor.
Abbreviations: Bx: biopsy; IgAN: IgA nephropathy; MN: Membranous nephropathy; UP‐UCR: urinary protein‐creatinine ratio.
2.2. Examination with immunofluorescence staining
The diagnose of IgAN and MN were based on the clinical information, laboratory data and pathological features in this study. Immunofluorescence staining was performed with the use of fluorescein isothiocyanate‐conjugated antibodies against IgG, IgM, IgA and C3.
2.3. Sample collection and protein extraction for LMD and LC‐MS/MS
All FFPE tissues prepared from the human kidney were cut into 10 μm‐thick sections on glass slides (Membrane Slides, N0.11505158, Leica Microsystems, Mannheim, Germany) coated with polyethylene naphthalate. These sections were stained with haematoxylin to clarify the molecular structure and dissected with the use of the Leica dissector (Leica LMD7000; Leica Microsystems). All glomeruli contained in the sections were selected for LMD and subsequent LC‐MS/MS in this study, because in general, the deposition of immunoglobulins and complements are usually seen in almost all glomeruli in the kidneys with IgAN and MN, although the amount of deposition shows some variations among the glomeruli. The dissected glomeruli were collected separately into 40 μL of Tris EDTA buffer and 0.002% Zwittergent 3‐16 detergent. After centrifugation at 10 000g for 1 minute, microdissected glomerular fragments were incubated at 98°C for 90 minutes and sonicated with the ultrasonic cleaner at room temperature for 90 minutes. Solubilised proteins were further digested into peptides by trypsin (Promega, Madison, Wisconsin) overnight. Peptide concentrations of each sample were measured with a fluorometric peptide assay (Thermo Scientific, San Jose, California). The samples were re‐dissolved in formic acid. Then, LC‐MS/MS was performed with a standardised amount of each sample.
2.4. Proteomics by LC‐MS/MS
Samples were subjected to LC‐MS/MS using with the DiNa nano‐LC system (KYA TECH Corporation, Tokyo), coupled online with the Triple TOF 5600+ system (AB Sciex). LC‐MS/MS raw data files were analysed in two different algorithms (Mascot and X! Tandem). The results were recombined and assigned probability scores of proteins and peptides in Scaffold software (Proteome Software, Portland, Oregon) in accordance with the SWISS‐PROT Protein Knowledgebase. The numerical value in the Scaffold software is called spectra value (SV), which shows the number of peptides identified by matching the amino‐acid sequence available in the database. A higher SV indicates a higher confidence in protein identification. A list of the proteins identified by LC‐MS/MS was generated for each sample. Peptide identifications were recognised at 95% probability or more by the Peptide Prophet algorithm,8, 9, 10 and the proteins identified had at least two matching peptides, according to the previous report of Sethi et al2, 3
2.5. Statistical analysis
The data are expressed as means ± standard deviations or as medians with interquartile ranges. Statistical analysis was performed with JMP software, 14.0 (SAS Institute, Inc. Cary, North Carolina). The clinicopathological findings and SV were statistically analysed in an analysis of variance with the non‐parametric Kruskal‐Wallis and Steel‐Dwass tests for multiple comparisons among 1 hour‐RBx, findings in the patients with IgAN and MN. Paired t‐tests were used to compare SV of the 0 hour‐RBx and 1 hour‐RBx findings. Statistical significance was defined as a P value of less than .05.
2.6. Ethical statement
This was a retrospective study; patients had the opportunity to opt out, and those who did not were considered to have given informed consent for their data to be studied. Approval for this study was obtained from the Ethics Committee at Showa University Hospital, Tokyo (approval number 2568).
3. RESULTS
3.1. Relationship between glomerular dissected volume and identified protein numbers
We used three sizes of glomerular volume (3 × 106 μm3, 6 × 106 μm3 and 9 × 106 μm3) were dissected by LMD from the autopsied kidney and analysed for proteins identified by LC‐MS/MS. Each sample was dissected from a 10‐μm‐thick section. The number of detected proteins was 225 ± 13 from the 3 × 106 μm3 glomerular tissue sample, 276 ± 16 from the 6 × 106 μm3 sample and 265 ± 18 from the 9 × 106 μm3 sample; these amounts were not significantly different (Figure 1). According to the result of this preliminary examination, we decided that the dissected glomerular volume should be 3 × 106 μm3 because the minimal numbers of glomeruli were obtained from each specimen.
Figure 1.

The relationship between the dissected glomerular volume and the number of detected proteins. In glomerular tissue volumes, 225 ± 13 types of proteins were detected from the 3 × 106 μm3 sample, 276 ± 16 types were detected from the 6 × 106 μm3 sample and 265 ± 18 types were detected from the 9 × 106 μm3 sample. The difference between these amounts was not significant (N.S.)
3.2. Protein profiles detected in glomeruli from kidney transplant donors and comparison between 0 hour‐RBx and 1 hour‐RBx
To determine whether the presence of a particular protein is pathognomonic for one disease, we must know which proteins exist in normal tissues. Therefore, we first examined the proteins detected from the glomeruli of kidney transplant donors (0 hour‐RBx), which are generally considered normal glomeruli.
From the glomeruli of the 0 hour‐RBx of the five transplant donors, 255 ± 11 types of proteins were detected, whereas from those of 1 hour‐RBx, 202 ± 38 types of proteins were detected. The major proteins detected from 0 hour‐RBx glomeruli are listed in Table 2. The major proteins in the highest numbers were cell structure proteins, such as cytokeratin, alpha‐actinin and tubulin. Podocyte‐related proteins such as synaptopodin, podocin and podocalyxin were also detected but at lower levels of SV. The other detected proteins were nucleus‐related proteins, such as histone and prelamin. Some extracellular matrix‐related proteins, such as type IV collagen alpha‐3 and type IV collagen alpha‐4, were also detected.
Table 2.
Main protein detected from 0 hour RBx
| Spectra value | |
|---|---|
| Name of proteins | Average ± SD |
| 1. Cytoskeleton protein | |
| Vimentin | 423.0 ± 203.0 |
| Keratin, type II cytoskeletal 1 | 171.6 ± 60.0 |
| Keratin, type I cytoskeletal 16 | 125.0 ± 84.0 |
| Alpha‐actinin‐4 | 169.0 ± 43.0 |
| Tubulin alpha‐1A | 109.0 ± 22.0 |
| Actin, cytoplasmic 1 | 155.4 ± 58.0 |
| Myosin‐9 | 130.2 ± 30.0 |
| 2. Nuclear protein | |
| Histone H2A | 74.4 ± 16.0 |
| Histone H2B | 98.8 ± 29.0 |
| Prelamin‐A/C | 20.0 ± 2.0 |
| 3. Extra cellular matrix protein | |
| Collagen alpha‐1(IV) | 1.8 ± 0 |
| Collagen alpha‐2(IV) | 9.4 ± 2.0 |
| Collagen alpha‐3(IV) | 1.6 ± 0 |
| Collagen alpha‐4(IV) | 3.6 ± 1.0 |
| Collagen alpha‐1(VI) | 8.4 ± 1.0 |
| Collagen alpha‐2(VI) | 5.8 ± 1.0 |
| Collagen alpha‐3(VI) | 63.6 ± 1.0 |
| Laminin subunit alpha‐5 | 23.8 ± 4.0 |
| Laminin subunit beta‐2 | 49.0 ± 7.0 |
| Fibronectin | 9.2 ± 2.0 |
| 4. Epithelial cell related protein | |
| Synaptopodin | 10.0 ± 2.0 |
| Podocin | 3.0 ± 0 |
| Podocalyxin | 3.0 ± 0 |
| 5. Endothelial cell related protein | |
| Clathrin | 7.2 ± 1.0 |
| Chloride intracellular channel protein 5 | 3.0 ± 1.0 |
To evaluate the influence of blood components in glomerular samples, we compared the 0 hour‐RBx and 1 hour‐RBx samples of each of the five kidney transplant donors because the blood‐derived proteins were eliminated in the 0 hour‐Rbx samples due to the graft perfusion of preservation solution after removal of the kidneys, whereas the 1 hour‐RBx contained blood‐derived proteins by reperfusion of the blood. Table 3 shows the comparison of SV of the blood concerned proteins identified in the 0 hour‐RBx and 1 hour‐RBx samples. Levels of haemoglobin subunit β (46.2 ± 19.0 vs 133.8 ± 53.0; P = .042), albumin (46.8 ± 27.0 vs 96.6 ± 74.0; P = .048), Igκ light chain (9.4 ± 3.0 vs 19.2 ± 7.0; P = .031), IgG1 (2.0 ± 1.0 vs 6.0 ± 2.0; P = .029) and IgA1 (1.0 ± 0 vs 2.6 ± 1.0; P = .028) were all significantly lower in the 0 hour‐RBx samples than in the 1 hour‐RBx samples. Levels of C3 (3.0 ± 1.0 vs 1.6 ± 1.0; P = .054) and C9 (3.0 ± 1.0 vs 1.8 ± 1.0; P = .054) showed no significant differences. The reason for the increased C3 and C9 in the 0 hour‐RBx samples was uncertain but it may reflect the complement activation due to ischemia after removal of the graft.
Table 3.
Changes of blood‐derived proteins between time‐zero renal biopsy(0 hour‐RBx) and time‐1 hour renal biopsy(1 hour‐RBx) of patients
| Name of proteins | Uniprot accession | Average ± SD | Fold change | P‐Value | |
|---|---|---|---|---|---|
| 0 hour‐RBx | 1 hour‐RBx | ||||
| Blood cell components | |||||
| Hemoglobin subunit alpha | P69905 | 19.8 ± 7.0 | 51.8 ± 19.0 | 2.62 | .052 |
| Hemoglobin subunit beta | P68871 | 46.2 ± 19.0 | 133.8 ± 53.0 | 2.89 | .042a |
| Plasma components | |||||
| Albumin | P02768 | 46.8 ± 12.0 | 96.6 ± 33.0 | 2.06 | .048a |
| Ig kappa chain C region | P01834 | 9.4 ± 3.0 | 19.2 ± 7.0 | 2.04 | .031a |
| Ig gamma‐1 chain C region | P01857 | 2.0 ± 1.0 | 6.0 ± 2.0 | 3.00 | .029a |
| Ig alpha‐1 chain C region | P01876 | 1.0 ± 0 | 2.6 ± 1.0 | 2.60 | .028a |
| Ig gamma‐2 chain C region | P01859 | <1 | <1 | ||
| Complement C3 | P01024 | 3.0 ± 1.0 | 1.6 ± 1.0 | 0.53 | .054 |
| Complement C4‐A | P0C0L4 | 0.8 ± 0 | 1.0 ± 0 | 1.13 | .573 |
| Complement C9 | P02748 | 3.0 ± 1.0 | 1.8 ± 1.0 | 0.60 | .054 |
| Fibrinogen alpha chain | P02671 | <1 | <1 | ||
| Fibrinogen gamma chain | P02679 | <1 | <1 | ||
P < .05 between the patients with 0 hour‐RBx and 1 hour‐RBx.
These results indicate that the blood components present in glomerular capillaries can influence the results of glomerular mass spectrometry in kidney biopsy samples, especially in the interpretation of immunoglobulins and complements as the glomerular depositions in the diagnosis of various forms of glomerulonephritis. For these reasons, 1 hour‐RBx samples obtained from kidney donors were used as the controls in the subsequent studies of IgAN and MN to minimize the effects of blood components present in the glomerular capillaries.
3.3. Glomerular protein profile in IgAN
We detected 483 ± 11 types of proteins from the glomeruli in the five cases of IgAN, and compared them with those detected from the control glomeruli (1 hour‐RBx). The major proteins whose levels were significantly elevated in the glomeruli of IgAN were four types of immunoglobulin proteins, including IgA1 (14.0 ± 2.0 vs 3.0 ± 1.0; P = .003), and four types of complement proteins, including C3 (64.0 ± 25.0 vs 1.6 ± 1.0; P = .037) in comparison with the controls (Table 4). In addition, we found significant elevations in the levels of four types of elongation factors, nine types of heat shock proteins (HSP) and four types of peroxiredoxins, which are associated with cellular proliferation, metabolism and oxidative stress, respectively.
Table 4.
Major proteins that were elevated in IgAN
| Spectra value (average ± SD) | ||||
|---|---|---|---|---|
| IgAN | 1 hour‐RBx | P score | ||
| Immunoglobulin | ||||
| Ig alpha‐1 chain C | IGHA1 | 14.0 ± 2.0 | 3.0 ± 1.0 | .003 |
| Ig gamma‐1 chain C region | IGHG1 | 20.0 ± 2.0 | 6.0 ± 2.0 | .003 |
| Ig gamma‐2 chain C region | IGHG2 | 13.0 ± 1.0 | 0 | .001 |
| Ig mu chain C region | IGHM | 5.0 ± 1.0 | 0 | .001 |
| Complement | ||||
| Complement C3 | C3 | 64.0 ± 25.0 | 1.6 ± 1.0 | .037 |
| Complement C4‐A | C4A | 8.0 ± 2.0 | 1.0 ± 0 | .013 |
| Complement component C9 | C9 | 12.0 ± 4.0 | 1.8 ± 1.0 | .046 |
| Complement receptor type 1 | CR1 | 2.2 ± 0 | 0.2 ± 0 | .003 |
| Peroixiredoxin | ||||
| Peroxiredoxin‐1 | PRDX1 | 10.0 ± 1.0 | 3.0 ± 1.0 | .001 |
| Peroxiredoxin‐2 | PRDX2 | 8.0 ± 1.0 | 2.4 ± 1.0 | .006 |
| Thioredoxin‐dependent peroxide reductase, mitochondrial | PRDX3 | 2.0 ± 0 | 0 | .001 |
| Peroxiredoxin‐5, mitochondrial | PRDX5 | 3.0 ± 0 | 0.4 ± 0 | .001 |
| Heat shock protein | ||||
| Heat shock 60 kDa protein | HSPD1 | 3.0 ± 1.0 | 0.2 ± 0 | .001 |
| Heat shock 70 kDa protein 1A | 30.0 ± 3.0 | 10.6 ± 3.0 | .001 | |
| Heat shock 70 kDa protein 12A | HSPA12A | 3.0 ± 1.0 | 0.2 ± 0 | .006 |
| Stress‐70 protein, mitochondrial | HSPA9 | 1.0 ± 0 | 0 | .020 |
| Heat shock cognate 71 kDa protein | HSPA8 | 28.0 ± 3.0 | 6.4 ± 3.0 | .001 |
| 78 kDa glucose‐regulated protein | HSPA5 | 9.0 ± 1.0 | 2.0 ± 1.0 | .001 |
| Heat shock protein HSP 90‐alpha | HSP90AA1 | 19.0 ± 2.0 | 5.4 ± 2.0 | .001 |
| Endoplasmin | HSP90B1 | 9.0 ± 1.0 | 2.0 ± 1.0 | .001 |
| Elongation factor | ||||
| Elongation factor 1‐alpha 1 | EEF1A1 | 20.0 ± 2.0 | 6.0 ± 3.0 | .003 |
| Elongation factor 1‐delta | EEF1D | 1.0 ± 0 | 0.2 ± 0 | .032 |
| Elongation factor 1‐gamma | EEF1G | 1.0 ± 0 | 0 | .002 |
| Elongation factor 2 | EEF2 | 4.0 ± 1.0 | 0.8 ± 0 | .007 |
3.4. Glomerular protein profile in MN
We detected 426 ± 27 types of proteins from the glomeruli in the five cases of MN and compared them with those detected from the control glomeruli. The major proteins whose levels were significantly elevated in the glomeruli of MN were two types of immunoglobulin proteins, including IgG4 (9.6 ± 3.0 vs 1.6 ± 1.0; P = .030), twelve types of complement proteins, including C3 (102.0 ± 15.0 vs 1.6 ± 1.0; P = .001), C4A (63.0 ± 23.0 vs 1.0 ± 0; P = .028) and phospholipase A2 receptor (PLA2R; 5.3 ± 2.0 vs 0, P = .040; Table 5). In addition, we found elevations in the levels of two types of elongation factors, four types of HSP and three types of peroxiredoxins in MN, as observed in IgAN.
Table 5.
Major proteins that were elevated in MN
| Spectra value (average ± SD) | ||||
|---|---|---|---|---|
| MN | 1 hour‐RBx | P score | ||
| Immunoglobulin | ||||
| Cluster of Ig gamma‐1 chain C region | IGHG1 | 25.2 ± 7.0 | 6.0 ± 2.0 | .030 |
| Ig gamma‐4 chain C region | IGHG4 | 9.6 ± 3.0 | 1.6 ± 1.0 | .030 |
| Secretory phospholipase A2 receptor | PLA2R1 | 5.3 ± 2.0 | 0 | .040 |
| Complement | ||||
| Complement factor H | CFH | 8.0 ± 2.0 | 0 | .001 |
| Complement factor H‐related protein 2 | CFHR2 | 2.0 ± 1.0 | 0 | .041 |
| Complement factor H‐related protein 3 | CFHR3 | 1.0 ± 0 | 0 | .013 |
| Complement factor H‐related protein 5 | CFHR5 | 5.0 ± 2.0 | 0 | .035 |
| Complement C3 | C3 | 102.0 ± 15.0 | 1.6 ± 1.0 | .001 |
| Cluster of complement C4‐A | C4A | 63.0 ± 23.0 | 1.0 ± 0 | .028 |
| Complement C5 | C5 | 13.0 ± 4.0 | 0 | .015 |
| Complement component C6 | C6 | 3.0 ± 1.0 | 0 | .013 |
| Complement component C7 | C7 | 2.0 ± 1.0 | 0 | .038 |
| Complement component C8 beta chain | C8B | 2.0 ± 1.0 | 0 | .027 |
| Complement component C8 gamma chain | C8G | 2.0 ± 1.0 | 0 | .005 |
| Complement component C9 | C9 | 16.0 ± 3.0 | 1.8 ± 1.0 | .002 |
| Peroixiredoxin | ||||
| Peroxiredoxin‐1 | PRDX1 | 7.0 ± 1.0 | 3.0 ± 1.0 | .008 |
| Peroxiredoxin‐2 | PRDX2 | 7.0 ± 1.0 | 2.4 ± 1.0 | .006 |
| Thioredoxin‐dependent peroxide reductase, mitochondrial | PRDX3 | 2.0 ± 0 | 0 | .007 |
| Heat shock protein | ||||
| Stress‐70 protein, mitochondrial | HSPA9 | 1.0 ± 0 | 0 | .002 |
| Heat shock cognate 71 kDa protein | HSPA8 | 19.0 ± 2.0 | 6.4 ± 3.0 | .011 |
| 78 kDa glucose‐regulated protein | HSPA5 | 7.0 ± 1.0 | 2.0 ± 1.0 | .006 |
| Endoplasmin | HSP90B1 | 5.0 ± 0 | 2.0 ± 1.0 | .005 |
| Elongation factor | ||||
| Elongation factor 1‐alpha | EEF1A | 13.0 ± 0 | 6.0 ± 3.0 | .068 |
| Elongation factor 1‐delta | EEF1D | 1.0 ± 0 | 0.2 ± 0 | .012 |
| Elongation factor 1‐gamma | EEF1G | 1.0 ± 0 | 0 | .019 |
3.5. Quantitative property of SV obtained from LC‐MS/MS: a correlation with immunofluorescence intensity
To examine quantitative properties of LC‐MS/MS, the SV of IgA and C3 in IgAN, and those of IgG4, C3 and PLA2R in MN were compared with their immunofluorescence intensities. Significant positive correlations between SV and immunofluorescent intensities were observed in IgA (y = 8.197x + 2.262, r 2 = .940) and C3 (y = 105.38x + 1.189, r 2 = .807) in IgAN, IgG4 (y = 7.021x + 0.237, r 2 = .725) and C3 (y = 73.609x + 11.515, r 2 = .752) in MN (Figures 2 and 3, Figure S1), although no significant correlation was observed with PLA2R. No significant correlations were observed between these SVs of the deposited proteins and the clinical and histological findings in the IgA and MN patients.
Figure 2.
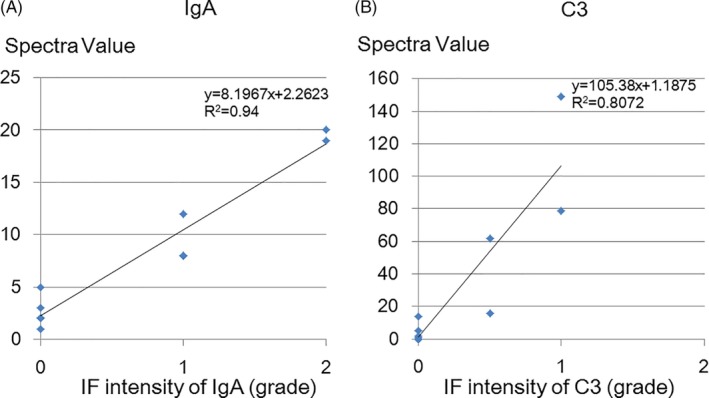
Relationship between SV and IF intensity of IgA and C3 in IgAN and 1 hour‐RBx (control). Significant positive correlations between SV and immunofluorescent intensities of IgA (y = 8.197x + 2.262, r 2 = .940) (A) and C3 (y = 105.38x + 1.189, r 2 = .807) (B) were observed
Figure 3.
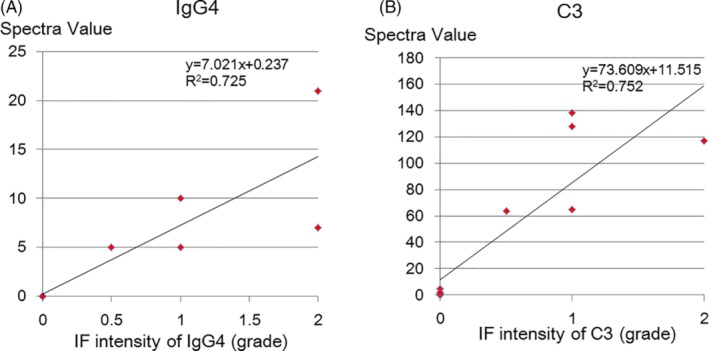
Relationship between SV and IF intensity of IgG4 and C3 in MN and 1 hour‐RBx (control). Significant positive correlations between SV and immunofluorescent intensities of IgG4 (y = 7.021x + 0.237, r 2 = .725) (A) and C3 (y = 73.609x + 11.515, r 2 = .752) (B) were observed
4. DISCUSSION
4.1. Adequate sample type and amount for LMD and LC‐MS/MS analysis
Pathological specimens for subjected to LMD and LC‐MS/MS analysis may be either frozen or from FFPE sections. In renal biopsy, Finne et al11, 12, 13 reported that there was no difference in the protein numbers detected by LMD and LC–MS/MS between the frozen and FFPE sections. Because FFPE blocks usually contained more numbers of glomeruli than frozen specimens, we decided to use FFPE sections for this glomerular proteomics study. Next, we examined adequate amounts of the samples required for LC‐MS/MS. In previous reports,2, 7 a minimum of 4 to 100 glomeruli had to be dissected from 10‐μm‐thick sections. We think that this number depends on the purpose of the study, the amount of the targeted protein, and the sensitivity of mass spectrometer used. Aoki et al14 used a 6 × 106 μm3 glomerular volume for LC‐MS/MS, we used three sizes of glomerular volume: 3 × 106 μm3 (which yielded ~12 glomeruli), 6 × 106 and 9 × 106 μm3, dissected from 10‐μm‐thick sections. There were no significant differences in the number of proteins detected by LC‐MS/MS from the three dissected volumes. The reason why the detected number did not increase as the applied sample volume increased may have been related to a limitation of dissolved peptide numbers in 40 μL of buffer used in this study, or it may have been related to a limitation in the sensitivity of our mass spectrometer. According to the result of this preliminary examination, we decided that the dissected glomerular volume should be 3 × 106 μm3 because the minimal numbers of glomeruli were obtained from each specimen.
4.2. Detection and quantification of glomerular deposits in IgAN and MN by LMD and LC‐MS/MS
Our results confirmed that all proteins detected by IF can be identified by LMD and LC‐MS/MS, with regard to the molecules pathognomonic for certain diseases, such as IgA1 and C3 in IgAN and IgG4, C3, C4A and PLA2R in MN. These results indicated that LMD and LC‐MS/MS can be used for the diagnosis of glomerular disease.
Next, we examined whether LMD and LC‐MS/MS can be utilised for quantitative evaluation. Before we conducted the LC‐MS/MS, we measured the amount of peptides in the sample by the fluorometric peptide assay method, corrected the sample volumes to the standard amount of peptide (300 ng) and then applied them to LC‐MS/MS. Significant positive correlations were observed between SV and semi quantitative grades of IF intensity of IgA and C3 in IgAN and of IgG4 and C3 in MN. Although there are some methodological problems for using LC‐MS/MS in the quantitative analysis, SV obtained by LC‐MS/MS might be helpful as indicators of diagnostic confidence for the diagnosis of renal disease.
An interesting result of this study suggested a possible contribution of the lectin pathway in the pathogenesis of MN, demonstrated by detection of greater amounts of C4A in MN samples than in control and IgAN samples, whereas greater amounts of C1 was not detected. These results are consistent with those of previous reports demonstrating increased activation of lectin pathway in idiopathic MN.15, 16
4.3. Bioactive molecules associated with the pathogenesis of human glomerulonephritis
Because LC‐MS/MS is a method for all‐inclusive proteomics analysis, its results revealed unexpected molecular candidates associated with the pathogenesis of certain diseases. In this study, the levels of several bioactive proteins including peroxiredoxin, elongation factor and HSP were higher in the glomeruli of IgAN and MN samples than in those of the control samples.
Peroxiredoxin is an oxidoreductase present in cytoplasm of various cells, and its function is to protect cells from oxidative stress.17, 18 In an experimental mouse model of renal fibrosis, oxidative stress induced tubular atrophy and interstitial fibrosis were suppressed in the mice that overexpressed peroxiredoxin‐1.19 In humans, expression of peroxiredoxin‐1 and peroxiredoxin‐2 were increased in the podocytes of patients with advanced cases of diabetic nephropathy.20 In various forms of glomerulonephritis, oxidative stress can be enhanced in glomerular cells, and this may induce the expression of peroxiredoxin to protect the cells against oxidative stress.
Our results also revealed a significant elevation in HSP70, 71 and 90 in IgAN and MN samples in comparison with the control samples. HSP is a molecular chaperones that are upregulated when cells are exposed to various stresses such as hyperthermia, cold and anoxia.21 Molecular chaperones function to repair proteins denatured by various stressors, to prevent further denaturation, and to maintain the cellular structure.22 In the patients with chronic glomerulonephritis, increased expression of HSP70 in renal tissue was correlated with an increased degree of proteinuria.23 Therefore, HSP can be expressed in glomeruli of various glomerulonephritis as a protective response to various factors related to glomerular injury.
Elongation factor plays an important role to elongate the peptide during protein synthesis. It is also up‐regulated when cells are damaged by oxidative stress, and it can be used as a marker of oxidative stress.24 In a mouse diabetic model, expression of elongating factor 1α was elevated and induced renal enlargement as a result of increased protein synthesis.25 These results suggested that elongation factor can be associated with mesangial proliferation and increased production of mesangial matrix proteins, such as collagen and fibronectin, in human IgAN.
LC‐MS/MS can detect several unknown proteins associated with cellular function, which can elucidate the cellular responses in human glomerulonephritis. Further investigations are required to elucidate whether peroxiredoxin, HSP, and elongation factor are useful as biomarkers of disease activity and specificity for the diagnosis of IgAN and MN.
Our study has several limitations. First, this is an exploratory study to seek the usefulness of LC‐MS/MS for diagnosis and pathophysiological understanding of glomerular diseases. No novel methodology or new protein which is confirmed by other methods such as immunohistochemistry was reported. Our manuscript demonstrates the practical methods of LC‐MS/MS applying renal biopsy samples to the diagnosis and the detection of some regulatory proteins of glomerular diseases. We also demonstrates the glomerular protein profiles of normal and diseased kidneys detected by LC‐MS/MS, which will be helpful for future studies as a reference. Second, because of the exploratory nature of this study, the numbers of the patients was small to evaluate the significances of the detected proteins or their quantitative correlations to clinical and histological findings; such as Oxford criteria for IgA nephropathy and Ehrenreich‐Churg staging for MN. Third, we have not performed the immunohistochemical study to conform the presence of the candidate proteins which were detected relatively higher in the IgAN and MN than the controls. This should be done in the further studies with increased numbers of the patients and confirmative information as the candidate proteins for the pathogenesis of glomerular diseases. Fourth, the limited protein detection sensitivity of our mass spectrometry apparatus. The improvement of the sensitivity of mass spectrometry will make it possible to perform more precise comprehensive evaluation of proteomics using limited biopsied specimens.
5. CONCLUSION
In this study, we performed proteomics analysis of IgAN and MN by LMD and LC‐MS/MS. Immunoglobulins and complements, which are essential for the diagnosis of both diseases, can be detected by LC‐MS/MS, and certain positive correlations were observed between their immunofluorescence intensities and the SV obtained by LC‐MS/MS. In addition, increases in the levels of several proteins, such as peroxiredoxin, HSP and elongation factor, were detected, which suggest that these molecules might be associated with cellular functions in human glomerulonephritis. Although further study is necessary to elucidate the relevance of LMD and LC‐MS/MS, they can be useful for the diagnosis, pathophysiological understanding and exploration of novel biological markers of human glomerular diseases.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
Supporting information
Appendix S1: Supplementary Material
ACKNOWLEDGEMENTS
We express special thanks to Ms. Tomoko Nagai, Mr. Yosuke Sasaki for the expert technical assistance.
Kawata N, Kang D, Aiuchi T, et al. Proteomics of human glomerulonephritis by laser microdissection and liquid chromatography‐tandem mass spectrometry. Nephrology. 2020;25:351–359. 10.1111/nep.13676
REFERENCES
- 1. Picken MM. Immunoglobulin light and heavy chain amyloidosis AL/AH: renal pathology and differential diagnosis. Contrib Nephrol. 2007;153:135‐155. [DOI] [PubMed] [Google Scholar]
- 2. Sethi S, Vrana JA, Theis JD, et al. Laser microdissection and mass spectrometry‐based proteomics aids the diagnosis and typing of renal amyloidosis. Kidney Int. 2012;82:226‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sethi S, Theis JD, Leung N, et al. Mass spectrometry‐based proteomic diagnosis of renal immunoglobulin heavy chain amyloidosis. Clin J Am Soc Nephrol. 2010;5:2180‐2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dasari S, Alexander MP, Vrana JA, et al. DnaJ heat shock protein family B member 9 is a novel biomarker for fibrillary GN. J Am Soc Nephrol. 2018;29:51‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andeen NK, Yang HY, Dai DF, MacCoss MJ, Smith KD. DnaJ homolog subfamily B member 9 is a putative autoantigen in fibrillary GN. J Am Soc Nephrol. 2018;29:231‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paunas TLF, Finne K, Leh S, et al. Glomerular abundance of complement proteins characterized by proteomic analysis of laser‐captured microdissected glomeruli associates with progressive disease in IgA nephropathy. Clin Proteomics. 2017;30:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ning X, Yin Z, Li Z, et al. Comparative proteomic analysis of urine and laser microdissected glomeruli in IgA nephropathy. Clin Exp Pharmacol Physiol. 2017;44:576‐585. [DOI] [PubMed] [Google Scholar]
- 8. Choi NHNY, Tobe T, Mazda T, et al. Incorporation of SP‐40,40 into the soluble membrane attack complex (SMAC, SC5b‐9) of complement. Int Immunol. 1990;2:413‐417. [DOI] [PubMed] [Google Scholar]
- 9. Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646‐4658. [DOI] [PubMed] [Google Scholar]
- 10. Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383‐5392. [DOI] [PubMed] [Google Scholar]
- 11. Finne K, Vethe H, Skogstrand T, et al. Proteomic analysis of formalin‐fixed paraffin‐embedded glomeruli suggests depletion of glomerular filtration barrier proteins in two‐kidney, one‐clip hypertensive rats. Nephrol Dial Transplant. 2014;29:2217‐2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finne K, Marti HP, Sabine L, et al. Proteomic analysis of minimally damaged renal tubular tissue from two‐kidney‐one‐clip hypertensive rats demonstrates extensive changes compared to tissue from controls. Nephron. 2016;132:70‐80. [DOI] [PubMed] [Google Scholar]
- 13. Vethe H, Finne K, Skogstrand T, et al. Distinct protein signature of hypertension‐induced damage in the renal proteome of the two‐kidney, one‐clip rat model. J Hypertension. 2014;33:126‐135. [DOI] [PubMed] [Google Scholar]
- 14. Aoki M, Kang D, Katayama A, et al. Optimal conditions and the advantages of using laser microdissection and liquid chromatography tandem mass spectrometry for diagnosing renal amyloidosis. Clin Exp Nephrol. 2018;22:871‐880. [DOI] [PubMed] [Google Scholar]
- 15. Yang Y, Wang C, Jin L, et al. IgG4 anti‐phospholipase A2 receptor might activate lectin and alternative complement pathway meanwhile in idiopathic membranous nephropathy: an inspiration from a cross‐sectional study. Immunol Res. 2016;64:919‐930. [DOI] [PubMed] [Google Scholar]
- 16. Zheng W, Lu W, Yanna D, et al. Human anti‐thrombospondin type 1 domain‐containing 7A antibodies induce membranous nephropathy through activation of lectin complement pathway. Biosci Rep. 2018;38:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forshaw TE, Holmila R, Nelson KJ, et al. Peroxiredoxins in cancer and response to radiation therapies. Antioxidants. 2019;11:1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bai B, Lin Y, Hu J, et al. Peroxiredoxin2 downregulation enhances hepatocellular carcinoma proliferation and migration, and is associated with unfavorable prognosis in patients. Oncol Rep. 2019;41:1539‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mei W, Peng Z, Lu M, et al. Peroxiredoxin 1 inhibits the oxidative stress induced apoptosis in renal tubulointerstitial fibrosis. Nephrol Ther. 2015;20:832‐842. [DOI] [PubMed] [Google Scholar]
- 20. Lee E, Lee HS. Peroxidase expression is decreased by palmitate incultured podocytes but increased in podocytes of advanced diabetic nephropathy. J Cell Physiol. 2018;233:9060‐9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krugar K, Reichel T, Zeilinger C. Role of heat shock proteins 70/90 in exercise physiology, exercise immunology and their diagnostic potential in sports. J Appl Physiol. 1985;10:1‐31. [DOI] [PubMed] [Google Scholar]
- 22. Strecher JM. The role of heat shock proteins in regulating receptor signal transduction. Mol Pharmacol. 2019;95:1‐26. [DOI] [PubMed] [Google Scholar]
- 23. Chebotareva N, Bobkova I, Lysenko L, Neprinzeva N, Vinogradov A, Moiseev S. Heat shock protein 70 and anti‐heat shock protein 70 antibodies in patients with chronic glomerulonephritis. Cell Stress Chaperones. 2018;23:1229‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen E, Proestou G, Bourbeau D, Wang E. Rapid up‐regulation of peptide elongation factor EF1‐α protein levels is an immediate early event during oxidative stress‐induced apoptosis. Exp Cell Res. 2000;259:140‐148. [DOI] [PubMed] [Google Scholar]
- 25. Maghrebi MA, Cojocel C, Thompson MS. Regulation of elongation factor‐1 expression by vitamin E in diabetic rat kidneys. Mol Cell Biochem. 2005;273:177‐183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Material


