Abstract
Aim
When selecting treatments for type 2 diabetes (T2D), it is important to consider not only efficacy and safety, but also other treatment attributes that have an impact on patient preference. The objective of this study was to examine preference between injection devices used for two weekly GLP‐1 receptor agonists.
Materials and Methods
The PREFER study was an open‐label, multicentre, randomized, crossover study assessing patient preference for dulaglutide and semaglutide injection devices among injection‐naïve patients receiving oral medication for type 2 diabetes. After being trained to use each device, participants performed all steps of injection preparation and administered mock injections into an injection pad. Time‐to‐train (TTT) for each device was assessed in a subset.
Results
There were 310 evaluable participants (48.4% female; mean age, 60.0 years; 78 participants in the TTT subgroup). More participants preferred the dulaglutide device than the semaglutide device (84.2% vs. 12.3%; P < 0.0001). More participants perceived the dulaglutide device to have greater ease of use (86.8% vs. 6.8%; P < 0.0001). After preparing and using the devices, more participants were willing to use the dulaglutide device (93.5%) than the semaglutide device (45.8%). Training participants to use the dulaglutide device required less time than the semaglutide device (3.38 vs. 8.14 minutes; P < 0.0001).
Conclusions
Participants with type 2 diabetes preferred the dulaglutide injection device to the semaglutide injection device. If patients prefer a device, they may be more willing to use the medication, which could result in better health outcomes. Furthermore, a shorter training time for injection devices may be helpful in busy clinical practice settings.
Keywords: crossover study, dulaglutide, injection devices, preference, semaglutide, type 2 diabetes
1. INTRODUCTION
A recently published consensus report by the American Diabetes Association1 and the European Association for the Study of Diabetes describes patient preference as a “major factor driving the choice of medication” for patients with type 2 diabetes (T2D).2 This consensus report emphasizes that patient preference is influenced by a wide range of treatment attributes including route of administration and injection devices. These attributes can prevent some patients from using a medication even if the medication has proven efficacy. Furthermore, patient preference can have an impact on treatment adherence, which contributes to treatment outcomes.3, 4, 5, 6, 7, 8, 9 Therefore, when making treatment decisions for patients with T2D, it is important to consider not only efficacy and safety, but also other treatment attributes which have an impact on patient preference.
A growing body of literature has examined patient preferences among glucagon‐like peptide‐1 (GLP‐1) receptor agonists,10 which are often recommended for glycaemic control in patients with T2D.2 Medications in this class have been shown to have efficacy for lowering HbA1c with a low risk of hypoglycaemia and a potential benefit of weight loss.11, 12, 13, 14 One of the most commonly prescribed weekly GLP‐1 receptor agonists is dulaglutide, which showed efficacy and safety in the AWARD clinical trial programme15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 and the REWIND trial focusing on cardiovascular outcomes.26 Injectable semaglutide is a newer GLP‐1 receptor agonist that has shown efficacy and safety in the SUSTAIN clinical trial programme20, 27, 28, 29, 30, 31 and a trial focusing on cardiovascular safety.32 In one of the SUSTAIN trials with a head‐to‐head design, semaglutide was superior to dulaglutide in improving glycaemic control.31
Dulaglutide and semaglutide are both administered as weekly subcutaneous injections. However, there are differences between their injection procedures and devices. Dulaglutide is administered with a single‐dose, single‐use, auto‐injector pen that does not require handling the needle.33 Semaglutide is administered via a multi‐use, prefilled injection pen that requires needle attachment/disposal and dose dialing with each use.34 Previous studies have found that patients with T2D consistently prefer injection devices that are easy to use.35, 36, 37, 38 Attributes of the injection device and procedures that have been shown to contribute to patient preference include needle handling requirements, steps for preparing the injection device, dose frequency and overall ease of use.10, 39, 40, 41, 42
The dulaglutide and semaglutide injection devices have been compared in a previous study in which preferences were based on descriptions of the devices with images from the device instructions for use, rather than actual experience of using the devices.43 The primary objective of the current study was to examine preference between the dulaglutide and semaglutide injection devices after participants had been trained to inject with both devices and then used them to administer injections into an injection pad. The gated secondary objective was to compare the two devices with respect to overall ease of use. Exploratory objectives included assessing the training time for each device and willingness to use each device.
2. METHODS
2.1. Study design
This study, called PREFER, was an open‐label, multicentre, randomized, crossover study assessing patient preference for the dulaglutide single‐use pen33 and the semaglutide single‐patient‐use pen34 (which is used multiple times by each patient). Injection‐naïve patients with T2D were recruited at 13 clinical sites in the USA (located in California, Florida, Georgia, Illinois, Kentucky, North Carolina, North Dakota, Pennsylvania, Texas and Virginia), including nine general practice clinics and four endocrinology clinics. The crossover study design was selected to allow participants to directly compare the two injection devices. Patients were randomly assigned in a 1:1 ratio to the two device orders (ie, either dulaglutide first or semaglutide first, followed by the other device). The device order to which each patient was assigned was determined by a computer‐generated random sequence and assigned using a validated interactive web response system (IWRS). After being trained to use each device based on device instructions for use (IFU), participants performed all steps of injection preparation and administered mock injections into an injection pad.
The devices used in this study were the commercialized injection pens as of 2018, containing non‐modified commercial product. The generic and brand names were visible to participants, and questionnaires used the brand names (Trulicity for dulaglutide; Ozempic for semaglutide), which are displayed prominently on the devices. All of the questionnaires asking about preferences or perceptions related to the devices included colour images of the devices along with the medication names to avoid any confusion regarding which device corresponded to each question and response option.
No active drug was administered. The participants were trained with devices that contained the lowest available dose of each medication (dulaglutide, 0.75 mg; semaglutide, 0.25 mg) because these are the recommended starting doses for these two products in the US labels and because of safety concerns (eg, in case of an accidental needle stick). However, the device procedures are virtually identical across all drug dosages. Therefore, the study findings could be applicable to other dulaglutide and semaglutide device doses, as well as other drugs using the same devices.
2.2. Participants
Patients meeting the following inclusion criteria were eligible: aged ≥18 years; diagnosed with T2D; self‐injection‐naïve to any medication; naïve to administering injectable treatments to others; currently receiving treatment with oral medication for T2D; able to read, speak and write in English; able and willing to provide informed consent; and able to complete protocol requirements. Exclusion criteria included a gestational or type 1 diabetes diagnosis; cognitive or physical difficulties that could interfere with their ability to understand study procedures; currently enrolled in another clinical study or any other type of medical research judged not to be scientifically or medically compatible with this study; having participated in a clinical study involving an investigational product within the last 30 days; healthcare practitioner trained in giving injections; investigator, site personnel or immediate family member of site personnel; pregnant; or an employee of Eli Lilly, Novo Nordisk, Evidera or PPD.
Participants were required to provide written informed consent before completing the study procedures, and all of the procedures and materials were approved by an independent institutional review board (Ethical and Independent Review Services, study number 18128–01). This study was conducted in accordance with the Declaration of Helsinki.
2.3. Measures
2.3.1. Global preference item
The primary outcome measure was the global preference item, which evaluated patient preference between the dulaglutide and semaglutide injection devices after being trained and using both devices. The item asked “Overall, which device do you prefer?” Response options were Ozempic, Trulicity or No Preference. After answering this question, participants were also asked an open‐ended question about why they selected their response, and they were permitted to provide multiple reasons.
2.3.2. Diabetes Injection Device Preference Questionnaire
The Diabetes Injection Device Preference Questionnaire (DID‐PQ) was designed to assess patient preferences between two non‐insulin injection devices.44, 45 The 10 items were developed based on qualitative research with patients. Items 1 to 7 focus on specific characteristics of injection delivery systems, and these seven items comprise the device characteristics subscale. Items 8 to 10 are global items assessing preference based on overall satisfaction, ease of use and convenience of the injection devices. Each item is rated on a five‐point scale allowing respondents to indicate whether they prefer or strongly prefer one of the devices over the other. For each item, participants could also respond by selecting the “no preference” response. These five response options are categorical, and mean scores were not calculated. The gated secondary objective of this study was to compare the two injection devices with regard to ease of use as assessed by DID‐PQ item 9 (“Overall ease of use”). Responses to the other nine items were examined in exploratory analyses.
2.3.3. Questions on willingness to use injection devices
Participants completed three questions about willingness to use injection devices, which were adapted from items used in a previous study.46 The first question was administered before the device trainings to assess “willingness to use a diabetes medication that required an injection for each dose”. The other two questions, administered after both trainings and mock injections, asked about willingness to use the dulaglutide and semaglutide injection devices.
2.3.4. Time to train observer recording form
For interviews with time to train (TTT) assessment, an observer timed the device trainings while watching from behind a one‐way mirror. For each device, timing started when the participant finished reading the IFU and the interviewer began training (as described below). The observer recorded the time required for training (including responding to questions from participants) and the time required for mock injection (including device preparation, administering the injection and any additional questions) on the TTT Observer Recording Form. In combination, these two time periods represented the total TTT. The observer also recorded the number of interviewer interventions during each mock injection. Interventions were defined as the interviewer needing to interrupt the injection process to correct an error in device preparation or responding to a question from a participant.
2.4. Interview procedures
Each participant attended one study visit. After signing the informed consent form, the order of exposure to the devices was determined by a computer‐generated random sequence using an IWRS. After answering the question about willingness to use injectable medication, participants were given the IFU (from the Food and Drug Administration‐approved package insert) for the first device and instructed to read it. They were informed that they would be able to refer to the IFU throughout the training and mock injection. When participants indicated that they had finished reading the IFU, the interviewers began training them in how to prepare the injection device and administer the injection following a standardized training guide, which was developed based on the IFU for each device. During the training, the interviewer showed participants each step of preparing and using the injection device. Participants were permitted to ask questions during the training. After the training was complete and participants had no further questions, they were asked to perform the complete process of device preparation and mock injection into the injection pad.
Interviewers were trained to evaluate critical steps of the injection process. Critical steps for semaglutide based on the IFU34 included: remove the pen cap; push the needle straight onto the pen and turn it until it is on tight; remove the outer and inner needle caps; turn the dose selector until the dose counter shows the flow check symbol; press and hold the dose button until the dose counter shows 0 and a drop of medication appears at the needle tip; if no drop appears, repeat the flow check until a drop appears (up to six times); select the dose, and continue turning the dose selector until the dose counter shows 0.25; insert the needle into the pad; press and hold the dose button until the dose counter shows 0; slowly count to six; remove the needle from the pad; and remove the needle from the pen (capped or uncapped). Critical steps for dulaglutide based on the IFU33 included: remove the base cap; place the device on the injection site; unlock the device; press the button and hold the device in place until the grey plunger is visible; and remove it from the injection site.
If participants skipped a critical step or performed a step incorrectly, they were retrained in that particular step so that they could repeat it. Training on each device was considered complete after a participant had successfully performed each critical step and had no further questions. Between training on the first and second devices, participants completed the demographic form. Other questionnaires were administered after completing both device trainings and mock injections.
Four clinical sites were selected (based on availability of an observation room with a one‐way mirror) as a subset for assessment of TTT. All of the participants from these sites were included in the TTT assessment. Interview procedures were identical to those at the other sites, but interviews were conducted in a room with a one‐way mirror so that an observer could watch the interviews from behind the mirror and record the time required for each device.
2.5. Pilot study
A pilot study was conducted to evaluate training procedures for each device and refine the training approach. Pilot study interviews were conducted with 16 participants with T2D. Based on interviewer experience and respondent feedback, the device training procedures were refined and streamlined to maximize clarity. Pilot study data were not included in the main analysis.
2.6. Statistical analysis
Analyses were performed on the evaluable sample, which included participants who had (i) been randomized to a device order, (ii) been exposed to both devices via demonstration regardless of whether they successfully completed the mock injection and (iii) completed the global preference item. No imputations were performed for missing data. All statistical tests were two‐sided with a significance level of 5%. Descriptive statistics (mean, standard deviation, range and frequency) were used to summarize demographic and clinical characteristics, as well as responses to all questionnaires.
For the primary analysis, the Prescott test was used to determine whether there was a statistically significant difference in preference between the semaglutide and dulaglutide devices as indicated by responses to the global preference item, while accounting for neutral responses and the order of the two devices.47, 48 A serial gatekeeping strategy was used to control for type 1 error for the primary and gated secondary objectives.49 The gated secondary hypothesis was planned to be tested only if the primary null hypothesis (ie, that there was no difference in preference between the dulaglutide and semaglutide devices) was rejected.
The analysis of the gated secondary objective used the Prescott test to determine whether there was a statistically significant difference in perceptions of ease of use between the devices, using responses to item 9 of the DID‐PQ. For this analysis, the five response options of the DID‐PQ were collapsed into three response options (responses indicating “prefer” and “strongly prefer” were combined for each device). As exploratory analyses, the Prescott test was also used to examine device preference as indicated by the other nine items of the DID‐PQ.
As a further exploratory analysis, the TTT for each device was compared using a linear mixed model. The model included device (either dulaglutide device or semaglutide device), sequence (either dulaglutide‐semaglutide or semaglutide‐dulaglutide) and period (either trained first or trained second) as fixed effects and patient as a random effect.
3. RESULTS
3.1. Sample characteristics
A total of 312 participants were enrolled, but two were found to be ineligible after randomization because they were not injection‐naïve. Therefore, a total of 310 evaluable participants were included in the sample. Half (n = 155) were randomized to each group (ie, either dulaglutide first or semaglutide first). Mean age was 60.0 years, 48.4% were female and 50% were white (Table 1).
Table 1.
Demographic and clinical characteristics
| Randomization groups | |||
|---|---|---|---|
| Total evaluable sample N = 310 | Dulaglutide device first N = 155 | Semaglutide device first N = 155 | |
| Age, years (mean, SD) | 60.0 (10.86) | 60.5 (11.43) | 59.5 (10.28) |
| Minimum‐maximum | (30–86) | (34–86) | (30–83) |
| Gender, female (n, %) | 150 (48.4%) | 68 (43.9%) | 82 (52.9%) |
| Ethnicity: Hispanic or Latino (n, %)a | 39 (12.6%) | 19 (12.3%) | 20 (12.9%) |
| Racial background (n, %) | |||
| Asian | 10 (3.2%) | 7 (4.5%) | 3 (1.9%) |
| Black or African American | 105 (33.9%) | 52 (33.5%) | 53 (34.2%) |
| White | 155 (50.0%) | 79 (51.0%) | 76 (49.0%) |
| Otherb | 40 (12.9%) | 17 (11.0%) | 23 (14.8%) |
| Employment status (n, %) | |||
| Full‐time work | 106 (34.2%) | 57 (36.8%) | 49 (31.6%) |
| Part‐time work | 43 (13.9%) | 17 (11.0%) | 26 (16.8%) |
| Retired | 98 (31.6%) | 55 (35.5%) | 43 (27.7%) |
| Disabled | 39 (12.6%) | 18 (11.6%) | 21 (13.5%) |
| Otherc | 24 (7.7%) | 8 (5.2%) | 16 (10.3%) |
| Education level (n, %) | |||
| No college degree | 201 (64.8%) | 102 (65.8%) | 99 (63.9%) |
| College degree | 109 (35.2%) | 53 (34.2%) | 56 (36.1%) |
| Type of clinical recruitment site | |||
| General practice | 242 (78.1%) | 120 (77.4%) | 122 (78.7%) |
| Specialist | 68 (21.9%) | 35 (22.6%) | 33 (21.3%) |
| Duration of diabetes (mean years, SD) | 8.06 (6.76) | 8.52 (7.03) | 7.61 (6.47) |
| Current oral medication to treat type 2 diabetes (n, %) | |||
| Sulfonylureas | 74 (23.9%) | 40 (25.8%) | 34 (21.9%) |
| Biguanide | 257 (82.9%) | 130 (83.9%) | 127 (81.9%) |
| DPP‐4 inhibitors | 20 (6.5%) | 11 (7.1%) | 9 (5.8%) |
| SGLT2 inhibitors | 17 (5.5%) | 10 (6.5%) | 7 (4.5%) |
| Thiazolidinediones | 7 (2.3%) | 5 (3.2%) | 2 (1.3%) |
| Combination pills | 35 (11.3%) | 15 (9.7%) | 20 (12.9%) |
| Most recent HbA1c value | |||
| Patients with HbA1c data (n, %) | 304 (98.1%) | 150 (48.4%) | 154 (49.7%) |
| Mean (SD) | 7.29 (1.42) | 7.24 (1.35) | 7.34 (1.48) |
Abbreviations: DPP‐4 inhibitors, dipeptidyl peptidase‐4 inhibitors; SGLT2 inhibitors, sodium‐glucose co‐transporter‐2 inhibitors; SD, standard deviation.
Of the 39 participants with Hispanic or Latino ethnicity, 14 were white and 25 were ‘other’ race.
Race ‘other’ was self‐reported as follows: American Indian or Alaska Native (n = 3); American Indian or Alaska Native + black or African American + white (n = 2); American Indian or Alaska Native + white (n = 3); Asian + black or African American (n = 1); American Indian or Alaska Native + black or African American (n = 1); native Hawaiian or other Pacific Islander (n = 1); Hispanic or Hispanic American (n = 14); Indian (n = 1); Italian (n = 1); Latin (n = 1); Mediterranean (n = 1); Mexican (n = 5); Middle Eastern (n = 1); Puerto Rican (n = 1); mix with Caucasian/Indian (n = 1); not specified (n = 3).
Employment ‘other’ was self‐reported as follows: homemaker/housewife (n = 9); student (n = 1); unemployed (n = 8); stay‐at‐home parent (n = 4); self‐employed (n = 2).
The most common oral medications were metformin (82.9%), combination pills (11.3%) and dipeptidyl peptidase‐4 (DPP‐4) inhibitors (6.5%). The most commonly reported co‐morbid conditions were hypertension (38.1%), arthritis (28.4%), depression (15.8%), heart attack/disease (7.4%) and cancer (7.1%). There were no significant demographic differences between the two randomization groups. The only significant clinical difference between the groups was that the semaglutide‐first group had a slightly higher rate of hypertension than the dulaglutide‐first group (44.5% vs. 31.6%; P = 0.0261).
3.2. Device preference
Based on responses to the global preference item, the dulaglutide injection device was preferred by 84.2% of participants, while 12.3% of participants preferred the semaglutide device and 3.5% reported no preference between the injection devices (Figure 1). The difference in preference between the dulaglutide and semaglutide devices was statistically significant (P < 0.0001), while accounting for neutral responses.
Figure 1.
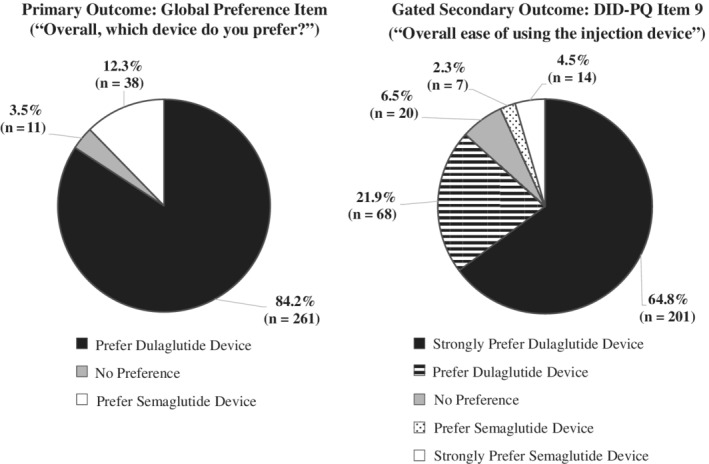
Patient preference between the dulaglutide and semaglutide injection devices (N = 310). Abbreviation: DID‐PQ, Diabetes Injection Device Preference Questionnaire
When asked for an explanation of why they preferred one device over the other, most participants provided multiple reasons. The 261 participants who expressed a preference for the dulaglutide device most commonly reported the device's ease of use (n = 242, 92.7%), reasons related to the needle (eg, the needle is preattached, not visible and does not need to be handled; n = 87, 33.3%) and the ease of learning to use the device (n = 46, 17.6%). Among the 38 participants who expressed a preference for the semaglutide device, the most common reasons were that the device can be used multiple times (n = 15, 39.5%), its ease of use (n = 10, 26.3%) and less generation of plastic waste (ie, because it is a multi‐use device; n = 10, 26.3%).
When asked about “overall ease of using the injection device” (DID‐PQ item 9), 86.8% of participants preferred the dulaglutide device, compared with 6.8% preferring the semaglutide device and 6.5% reporting no preference (Figure 1). This difference between the devices was statistically significant (P < 0.0001). Responses to all other items of the DID‐PQ followed similar patterns with statistically significant results favouring dulaglutide (Table 2; all P < 0.0001).
Table 2.
Responses to each item of the Diabetes Injection Device Preference Questionnaire (DID‐PQ) (N = 310a)
| Response frequency, n (%) | |||||
|---|---|---|---|---|---|
| Item | Strongly prefer dulaglutide device | Prefer dulaglutide device | No preference | Prefer semaglutide device | Strongly prefer semaglutide device |
| 1. Ease of preparing the injection device and medication for use | 180 (58.1%) | 100 (32.3%) | 8 (2.6%) | 10 (3.2%) | 12 (3.9%) |
| 2. Ease of fitting the injection into your routine | 158 (51.0%) | 77 (24.8%) | 50 (16.1%) | 10 (3.2%) | 15 (4.8%) |
| 3. Ease of bringing the injection device with you when it is necessary to inject away from home | 138 (44.5%) | 81 (26.1%) | 50 (16.1%) | 19 (6.1%) | 22 (7.1%) |
| 4. Confidence that the injection device provides the correct dose of medication every time | 148 (47.7%) | 86 (27.7%) | 40 (12.9%) | 17 (5.5%) | 19 (6.1%) |
| 5. Confidence that you are using the injection device correctly | 160 (51.6%) | 85 (27.4%) | 40 (12.9%) | 9 (2.9%) | 15 (4.8%) |
| 6. The size of the needle | 106 (34.2%) | 53 (17.1%) | 121 (39.0%) | 13 (4.2%) | 16 (5.2%) |
| 7. The time it takes to prepare and inject each dose of medication | 187 (60.3%) | 83 (26.8%) | 24 (7.7%) | 5 (1.6%) | 10 (3.2%) |
| 8. Overall satisfaction with the injection device | 178 (57.4%) | 77 (24.8%) | 25 (8.1%) | 11 (3.5%) | 19 (6.1%) |
| 9. Overall ease of using the injection device (gated secondary outcome) | 201 (64.8%) | 68 (21.9%) | 20 (6.5%) | 7 (2.3%) | 14 (4.5%) |
| 10. Overall convenience of using the injection device | 197 (63.5%) | 70 (22.6%) | 17 (5.5%) | 8 (2.6%) | 18 (5.8%) |
All 310 participants responded to items 1 to 4 and 8 to 10. There was one missing response for items 5, 6 and 7.
3.3. Willingness to use injection devices
Willingness to use injection devices is presented in Figure 2. Prior to receiving training on the two injection devices, a total of 65.2% of participants reported that they would be willing (ie, very willing or somewhat willing) to use a diabetes medication that required a self‐injection for each dose, while 18.7% were neutral and 16.1% were not willing (ie, not willing or somewhat not willing). Following the device trainings and mock injections, 93.5% of participants were willing to use the dulaglutide device while 3.5% were unwilling. In contrast, 45.8% were willing to use the semaglutide device and 34.8% were not willing.
Figure 2.
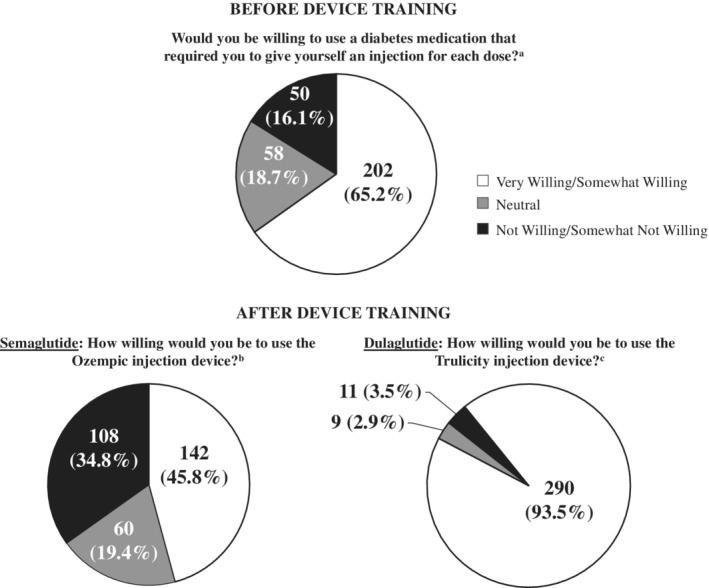
Willingness to use injection devices (N = 310) a Willing includes “somewhat willing” (34.5%) and “very willing” (30.6%); Not willing includes “somewhat not willing” (6.5%) and “not willing” (9.7%). b Willing includes “somewhat willing” (32.6%) and “very willing” (13.2%); Not willing includes “somewhat not willing” (18.7%) and “not willing” (16.1%). c Willing includes “somewhat willing” (19.7%) and “very willing” (73.9%); Not willing includes “somewhat not willing” (2.3%) and “not willing” (1.3%)
3.4. TTT
The TTT subgroup included 78 participants (dulaglutide first, 37; semaglutide first, 41). The mean total time for training and mock injections with the semaglutide device was approximately 2.4 times longer than the dulaglutide device (8.14 vs. 3.38 minutes; P < 0.0001). Figure 3 presents the total TTT and separate times for the training and mock injection.
Figure 3.
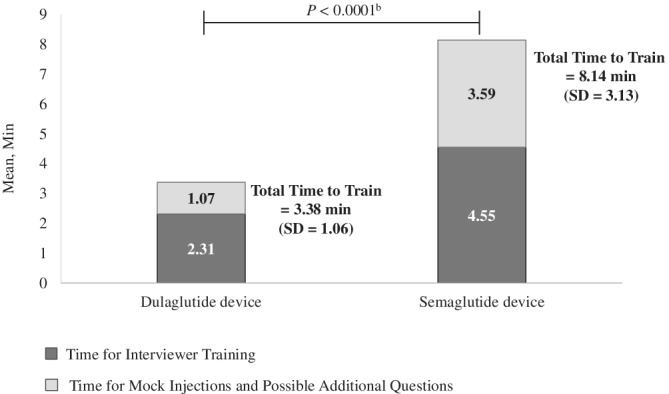
Time to traina on the dulaglutide and semaglutide injection devices (N = 78).Abbreviation: SD, standard deviation. a The training procedures were based on the instructions for use included as a package insert with each device. b The P‐value is based on a linear mixed model comparing time to train for dulaglutide and semaglutide. The model included device, sequence and period as fixed effects, and patient as a random effect. Sequence effect (P‐value = 0.93) and period effect (P‐value = 0.61) were not significant
The semaglutide device required more interviewer interventions than the dulaglutide device during the mock injections (means: 3.10 vs. 0.69). Over half of the patients in the TTT subgroup (n = 43, 55.1%) required no interviewer intervention during the mock injection with the dulaglutide device, while 11.5% (n = 9) required no intervention with the semaglutide device.
4. DISCUSSION
In this controlled study with a randomized crossover design, significantly more participants with T2D preferred the dulaglutide device than the semaglutide device (84.2% vs. 12.3%). When asked which device had greater ease of use, significantly more chose the dulaglutide device than the semaglutide device (86.8% vs. 6.8%).
These findings are consistent with those from four previous studies comparing the dulaglutide device to either the semaglutide device or the very similar liraglutide device, despite differences in study methods.36, 37, 43, 50 The dulaglutide device was preferred over the comparator device by 83.1% to 94.5% of participants in three studies, in which patients learned about the devices from written descriptions accompanied by illustrations or videos.36, 37, 43 In another study with a group of 58 patients who had used the dulaglutide and liraglutide devices, patients were asked to compare their current device to their previous treatment, which was discontinued over a year earlier on average. The dulaglutide device was perceived to have greater ease of use by 70.7% of patients.50 In contrast, the current study had a rigorous randomized crossover design in which each patient was trained based on the approved IFUs to prepare and use both injection devices immediately prior to performing injections with the actual devices. This study design randomized device order, avoided recall bias and ensured that all participants had a good understanding of both devices when stating their preferences.
Only one published study, a discrete choice experiment (DCE), contradicts the current results by reporting no significant difference in preference between the dulaglutide and semaglutide devices.51 Participants in this DCE were given brief device descriptions without any further information (“single‐use, disposable prefilled pen, with no dose adjustment possible” and “multi‐dose prefilled pen, used with disposable injection needles, with dose adjustment possible”). With these minimal descriptions, respondents may not have understood the differences in procedures for preparing the injection devices and administering injections. Therefore, the non‐significant result in this DCE may not be an accurate representation of preference between these two devices.
Exploratory outcomes revealed other advantages of the dulaglutide device. After both mock injections, more participants were willing to use the dulaglutide device (93.5%) than the semaglutide device (45.8%), while more were unwilling to use the semaglutide device (34.8%) than the dulaglutide device (3.5%). This injection‐naïve sample represents the type of patient who could benefit from injectable medication if they fail to maintain glycaemic control with oral treatment. With patients like these, reluctance to initiate injectable medication is a potential barrier to effective treatment.52, 53, 54, 55 If patients are more willing to use a device, they may be more likely to initiate injectable therapy and remain adherent to treatment. Therefore, a device that patients are more willing to use could help them achieve and maintain glycaemic control, which is associated with improved health outcomes and a reduced risk of diabetes‐related complications.1, 2
The shorter training time for participants to learn to use the dulaglutide device, which was significantly less than the training time for the semaglutide device (3.38 vs. 8.14 minutes; P < 0.0001), is highly relevant for busy clinical practices where care must be delivered quickly. A shorter training time could improve office flow by improving efficiency as medical professionals help their patients make the transition to injectable treatment.
When interpreting this study's findings, it is important to remember that all of the results relate only to the injection devices and injection processes. These findings may inform clinical decisions when choosing among medications delivered via different injection devices. Because preferences were related to the devices rather than the medications, the current results could be relevant to other medications delivered via these injection devices. However, when selecting a treatment for individual patients, device preference must be considered in the context of other factors such as efficacy, safety, cost‐effectiveness and any potential risks of the device or medication. In the current study, participants were not exposed to treatment or provided information about these other treatment attributes that might influence patient preference in real‐world clinical settings. Therefore, treatment decisions should not be made solely on the basis of the current results. Instead, clinicians should consider injection device preference in combination with efficacy, safety and other potentially important treatment attributes.
Some study limitations should be considered. First, it should be noted that participants used each device only once, and the degree to which preferences would remain stable after long‐term repeated use is unknown. It is possible that the strength of preference for one device over another could decrease over time as patients become more familiar with the devices. Second, participants injected medication into an injection pad, and it is not known whether preferences would be different if they had injected themselves instead. For example, there could be aspects of injection comfort related to factors such as the size of the needle or volume of liquid that are not apparent while performing a mock injection into an injection pad. Third, the injection devices were not blinded. Therefore, it is possible that responses could have been biased by the labelling, package design or prior knowledge about either medication. Fourth, although the sample was geographically and racially diverse within the USA, generalizability to other countries is unknown. It is possible that patients from other locations or cultures could have different preferences.
Overall, the PREFER study was a controlled randomized crossover study that highlights advantages of the dulaglutide device with regard to patient preference, ease of use, willingness to inject and time required for injection device training. This is the first study to examine these device preferences in a large sample using rigorous controlled clinical trial methods, and the results were consistent with previous research using different methodology. Although the study outcomes focused on the device rather than the clinical effects of the medication, the results may have important clinical implications. If patients prefer a device, perceive it as easy to use, and are more willing to use it, they may adopt injectable medication more easily. Further research is needed to examine the links between preference, adherence and outcomes in patients with T2D.
CONFLICT OF INTEREST
L. S. M., K. D. S., K. S. C., K. N. C., J. J., B. C., K. G. M. and K. J. I. are employees of Evidera, a company that received funding from Eli Lilly for the time spent conducting this research study. K. S. B., P. K. W., Q. W., M. Y., R. T. H. and L.‐E. G.‐P. are employees of and own stock in Eli Lilly and Company.
AUTHOR CONTRIBUTIONS
L. S. M., K. S. B. and L.‐E. G.‐P. co‐directed this study. L. S. M., K. S. B., K. D. S., K. S. C. and L.‐E. G.‐P. were the primary contributors to the study design, while all the other authors provided input on aspects of the study design. L. S. M., K. S. B., J. J. and R. T. H. directed protocol development. K. N. C., K. D. S. and P. K. W. directed data collection. M. Y., Q. W., K. J. I. and K. G. M. were responsible for the statistical considerations in the analysis and study design. L. S. M., K. S. B. and K. D. S. drafted the manuscript and all of the other authors provided input and approval.
5.
ACKNOWLEDGMENTS
The authors would like to thank Adebimpe Atanda, Gordon Parola, Haylee Andrews, Melissa Garcia, Natalie Taylor, Peter Chongpinitchai and Timothy Howell for assistance with data collection; Robyn Cyr for statistical programming; Sandra Macker for data management; and Amara Tiebout, Dawn Ri'chard, Fritz Hamme and Kawthar Nakayima for editorial assistance. Funding for this study was provided by Eli Lilly and Company.
Matza LS, Boye KS, Stewart KD, et al. Assessing patient PREFERence between the dulaglutide pen and the semaglutide pen: A crossover study (PREFER). Diabetes Obes Metab. 2020;22:355–364. 10.1111/dom.13902
Funding information Eli Lilly and Company
DATA AVAILABILITY
Data are available upon request.
REFERENCES
- 1. American Diabetes Association . Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41. [Google Scholar]
- 2. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461‐2498. [DOI] [PubMed] [Google Scholar]
- 3. Hixson‐Wallace JA, Dotson JB, Blakey SA. Effect of regimen complexity on patient satisfaction and compliance with warfarin therapy. Clin Appl Thromb Hemost. 2001;7:33‐37. [DOI] [PubMed] [Google Scholar]
- 4. Morris LS, Schulz RM. Medication compliance: the patient's perspective. Clin Ther. 1993;15:593‐606. [PubMed] [Google Scholar]
- 5. Raue PJ, Schulberg HC, Heo M, Klimstra S, Bruce ML. Patients' depression treatment preferences and initiation, adherence, and outcome: a randomized primary care study. Psychiatr Serv. 2009;60:337‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schaller M, Sigurgeirsson B, Sarkany M. Patient‐reported outcomes from two randomised studies comparing once‐weekly application of amorolfine 5% nail lacquer to other methods of topical treatment in distal and lateral subungual onychomycosis. Mycoses. 2017;60:800‐807. [DOI] [PubMed] [Google Scholar]
- 7. Shikiar R, Rentz A, Barone J, Duncanson F, Katz E. Patient satisfaction with ofloxacin (F) and polymyxin B/Neomycin/Hydrocortisone© in the treatment of otitis externa: results from two randomized clinical trials. J Manag Care Med. 2002;6:24‐27. [Google Scholar]
- 8. Shikiar R, Rentz AM. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004;7:204‐215. [DOI] [PubMed] [Google Scholar]
- 9. Shingler SL, Bennett BM, Cramer JA, Towse A, Twelves C, Lloyd AJ. Treatment preference, adherence and outcomes in patients with cancer: literature review and development of a theoretical model. Curr Med Res Opin. 2014;30:2329‐2341. [DOI] [PubMed] [Google Scholar]
- 10. Thieu VT, Robinson S, Kennedy‐Martin T, Boye KS, Garcia‐Perez LE. Patient preferences for glucagon‐like peptide 1 receptor‐agonist treatment attributes. Patient Prefer Adherence. 2019;13:561‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aroda VR, Henry RR, Han J, et al. Efficacy of GLP‐1 receptor agonists and DPP‐4 inhibitors: meta‐analysis and systematic review. Clin Ther. 2012;34:1247‐1258. [DOI] [PubMed] [Google Scholar]
- 12. Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon‐like peptide‐1 receptor agonists in type 2 diabetes: a systematic review and mixed‐treatment comparison analysis. Diabetes Obes Metab. 2017;19:524‐536. [DOI] [PubMed] [Google Scholar]
- 13. Tofe S, Arguelles I, Mena E, et al. Real‐world GLP‐1 RA therapy in type 2 diabetes: a long‐term effectiveness observational study. Endocrinol Diabetes Metab. 2019;2:e00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trujillo JM, Nuffer W, Ellis SL. GLP‐1 receptor agonists: a review of head‐to‐head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blonde L, Jendle J, Gross J, et al. Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet. 2015;385:2057‐2066. [DOI] [PubMed] [Google Scholar]
- 16. Dungan KM, Povedano ST, Forst T, et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet. 2014;384:1349‐1357. [DOI] [PubMed] [Google Scholar]
- 17. Dungan KM, Weitgasser R, Perez Manghi F, et al. A 24‐week study to evaluate the efficacy and safety of once‐weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD‐8). Diabetes Obes Metab. 2016;18:475‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care. 2015;38:2241‐2249. [DOI] [PubMed] [Google Scholar]
- 19. Ludvik B, Frias JP, Tinahones FJ, et al. Dulaglutide as add‐on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD‐10): a 24‐week, randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2018;6:370‐381. [DOI] [PubMed] [Google Scholar]
- 20. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care. 2014;37:2149‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pozzilli P, Norwood P, Jodar E, et al. Placebo‐controlled, randomized trial of the addition of once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD‐9). Diabetes Obes Metab. 2017;19:1024‐1031. [DOI] [PubMed] [Google Scholar]
- 22. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate‐to‐severe chronic kidney disease (AWARD‐7): a multicentre, open‐label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605‐617. [DOI] [PubMed] [Google Scholar]
- 23. Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care. 2014;37:2168‐2176. [DOI] [PubMed] [Google Scholar]
- 24. Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once‐weekly dulaglutide versus sitagliptin after 2 years in metformin‐treated patients with type 2 diabetes (AWARD‐5): a randomized, phase III study. Diabetes Obes Metab. 2015;17:849‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care. 2014;37:2159‐2167. [DOI] [PubMed] [Google Scholar]
- 26. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121‐130. [DOI] [PubMed] [Google Scholar]
- 27. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2018;41:258‐266. [DOI] [PubMed] [Google Scholar]
- 28. Lingvay I, Desouza CV, Lalic KS, et al. A 26‐week randomized controlled trial of semaglutide once daily versus liraglutide and placebo in patients with type 2 diabetes suboptimally controlled on diet and exercise with or without metformin. Diabetes Care. 2018;41:1926‐1937. [DOI] [PubMed] [Google Scholar]
- 29. Nauck MA, Petrie JR, Sesti G, et al. A phase 2, randomized, dose‐finding study of the novel once‐weekly human GLP‐1 analog, semaglutide, compared with placebo and open‐label liraglutide in patients with type 2 diabetes. Diabetes Care. 2016;39:231‐241. [DOI] [PubMed] [Google Scholar]
- 30. O'Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double‐blind, placebo and active controlled, dose‐ranging, phase 2 trial. Lancet. 2018;392:637‐649. [DOI] [PubMed] [Google Scholar]
- 31. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275‐286. [DOI] [PubMed] [Google Scholar]
- 32. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 33. Eli Lilly and Company . Instructions for Use: TRULICITY® (Trū‐li‐si‐tee) (dulaglutide) injection, for subcutaneous use – 0.75 mg/0.5 mL Single‐Dose Pen once weekly. 2017; http://pi.lilly.com/us/trulicity-lowdose-ai-ifu.pdf
- 34.Novo Nordisk. Instructions for Use: OZEMPIC® (semaglutide) injection, for subcutaneous use – 0.5 mg/1 mg. 2017; https://www.novo-pi.com/ozempic.pdf
- 35. Bailey T, Thurman J, Niemeyer M, Schmeisl G. Usability and preference evaluation of a prefilled insulin pen with a novel injection mechanism by people with diabetes and healthcare professionals. Curr Med Res Opin. 2011;27:2043‐2052. [DOI] [PubMed] [Google Scholar]
- 36. Gelhorn HL, Bacci ED, Poon JL, Boye KS, Suzuki S, Babineaux SM. Evaluating preferences for profiles of glucagon‐like peptide‐1 receptor agonists among injection‐naive type 2 diabetes patients in Japan. Patient Prefer Adherence. 2016;10:1337‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gelhorn HL, Poon JL, Davies EW, Paczkowski R, Curtis SE, Boye KS. Evaluating preferences for profiles of GLP‐1 receptor agonists among injection‐naive type 2 diabetes patients in the UK. Patient Prefer Adherence. 2015;9:1611‐1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nadeau DA, Campos C, Niemeyer M, Bailey T. Healthcare professional and patient assessment of a new prefilled insulin pen versus two widely available prefilled insulin pens for ease of use, teaching and learning. Curr Med Res Opin. 2012;28:3‐13. [DOI] [PubMed] [Google Scholar]
- 39. Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12:219‐230. [DOI] [PubMed] [Google Scholar]
- 40. Matza LS, Boye KS, Jordan JB, et al. Patient preferences in Italy: health state utilities associated with attributes of weekly injection devices for treatment of type 2 diabetes. Patient Prefer Adherence. 2018;12:971‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matza LS, Boye KS, Stewart KD, Davies EW, Paczkowski R. Health state utilities associated with attributes of weekly injection devices for treatment of type 2 diabetes. BMC Health Serv Res. 2017;17:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP‐1 products–liraglutide and exenatide–for the treatment of type 2 diabetes. J Med Econ. 2010;13:655‐661. [DOI] [PubMed] [Google Scholar]
- 43. Boye KS, Matza LS, Stewart KD, et al. Patient preferences and health state utilities associated with dulaglutide and semaglutide injection devices among patients with type 2 diabetes in Italy. J Med Econ. 2019;22(8):806‐813. [DOI] [PubMed] [Google Scholar]
- 44. Matza LS, Boye KS, Stewart KD, Paczkowski R, Jordan J, Murray LT. Development of the Diabetes Injection Device Experience Questionnaire (DID‐EQ) and Diabetes Injection Device Preference Questionnaire (DID‐PQ). J Patient Rep Outcomes. 2018;2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matza LS, Stewart KD, Paczkowski R, Coyne KS, Currie B, Boye KS. Psychometric evaluation of the Diabetes Injection Device Experience Questionnaire (DID‐EQ) and Diabetes Injection Device Preference Questionnaire (DID‐PQ). J Patient Rep Outcomes. 2018;2:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poon JL, Boye KS, Thieu VT, Norrbacka K, Hassan SW, Gelhorn HL. Preferences for attributes of medications among patients with type 2 diabetes: a cross‐medication class comparison of injection therapies. Curr Res Diabetes Obes J. 2018;6. [Google Scholar]
- 47. Pictor A. Analysing Binary Outcome Data from a Crossover Design Study using the SAS® System. 2003; https://www.lexjansen.com/views/2003/statpharm/st02.pdf
- 48. Prescott R. The comparison of success rates in cross‐over trials in the presence of an order effect. J R Stat Soc C Appl. 1981;30:9‐15. [Google Scholar]
- 49. Westfall PH, Krishen A. Optimally weighted, fixed sequence and gatekeeper multiple testing procedures. J Stat Plan Infer. 2001;99:25‐40. [Google Scholar]
- 50. Matza LS, Boye KS, Currie BM, et al. Patient perceptions of injection devices used with dulaglutide and liraglutide for treatment of type 2 diabetes. Curr Med Res Opin. 2018;34:1457‐1464. [DOI] [PubMed] [Google Scholar]
- 51. Brooks A, Langer J, Tervonen T, Hemmingsen MP, Eguchi K, Bacci ED. Patient preferences for GLP‐1 receptor agonist treatment of type 2 diabetes mellitus in Japan: a discrete choice experiment. Diabetes Ther. 2019;10:735‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abu Hassan H, Tohid H, Amin RM, Badrulnizam Long Bidin M, Muthupalaniappen L, Omar K. Factors influencing insulin acceptance among type 2 diabetes mellitus patients in a primary care clinic: a qualitative exploration. BMC Fam Pract. 2013;14:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matza LS, Curtis SE, Jordan JB, Adetunji O, Martin SA, Boye KS. Physician perceptions of GLP‐1 receptor agonists in the UK. Curr Med Res Opin. 2016;32:857‐864. [DOI] [PubMed] [Google Scholar]
- 54. Polonsky WH, Fisher L, Guzman S, Villa‐Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28:2543‐2545. [DOI] [PubMed] [Google Scholar]
- 55. Tan AM, Muthusamy L, Ng CC, Phoon KY, Ow JH, Tan NC. Initiation of insulin for type 2 diabetes mellitus patients: what are the issues? A qualitative study. Singapore Med J. 2011;52:801‐809. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.


