Abstract
Objective
To explore herpes zoster (HZ) rates and live zoster vaccine (LZV) safety in a subset of patients with rheumatoid arthritis who received LZV before tofacitinib ± methotrexate (MTX), or adalimumab (ADA) plus MTX in the ORAL Strategy.
Methods
ORAL Strategy was a 1‐year, phase IIIb/IV, randomized, triple‐dummy, active‐comparator–controlled study. MTX‐inadequate responder patients received tofacitinib 5 mg twice daily (BID), tofacitinib 5 mg BID plus MTX, or ADA 40 mg every other week plus MTX (1:1:1 randomization). Eligible patients age ≥50 years could opt to receive LZV 28 days before initiating study treatment. HZ incidence rates (IRs; patients with events per 100 patient‐years) were calculated. Opportunistic HZ infections (multidermatomal/disseminated), serious HZ events, and LZV‐related adverse events were monitored.
Results
In ORAL Strategy, 216 of 1,146 patients (18.8%) received LZV. Overall, 18 patients (1.6%) developed HZ (vaccinated: n = 3; nonvaccinated: n = 15). HZ IRs were 1.1 (95% confidence interval [95% CI] 0.3–2.9), 2.3 (95% CI 1.0–4.6), and 1.7 (95% CI 0.6–3.7) for tofacitinib monotherapy, tofacitinib plus MTX, and ADA plus MTX, respectively, and were generally similar between vaccinated and nonvaccinated patients. Three multidermatomal, 1 disseminated, and 2 serious HZ events occurred. No vaccinated patients had zoster‐like lesions within 42 days of vaccination; 1 patient had vaccination‐site erythema.
Conclusion
LZV was well tolerated, and HZ IRs were generally similar between treatment groups and vaccinated versus nonvaccinated patients. However, ORAL Strategy was not powered for comparisons between vaccinated and nonvaccinated patients because <20% of all patients were vaccinated. Furthermore, LZV has been shown to be effective only in ~50% of individuals.
INTRODUCTION
Herpes zoster (HZ) is a common and sometimes debilitating infection that most frequently affects elderly and/or immunocompromised individuals 1. Patients with rheumatoid arthritis (RA) have a 1.5–2‐fold higher risk of developing HZ versus the general population 2. This risk may be further increased by RA therapies such as biologic disease‐modifying antirheumatic drugs (bDMARDs) 3 and targeted synthetic DMARDs, such as Janus kinase inhibitors 4, 5. The mechanism for this increase in risk is considered to be multifactorial and is currently not well understood 4.
SIGNIFICANCE & INNOVATIONS.
Patients with rheumatoid arthritis (RA) are at greater risk of developing herpes zoster (HZ) than the general population, and this risk can be increased by some RA therapies, including tofacitinib.
The American College of Rheumatology and European League Against Rheumatism recommend vaccination with live zoster vaccine (LZV) in patients with RA.
However, there are limited prospective data to clearly define the effects of HZ vaccination and the effects of RA therapies on HZ vaccination efficacy.
In the Oral Rheumatoid Arthritis Trial Strategy, LZV was generally well tolerated in patients who were vaccinated, and no patients developed zoster‐like lesions within 42 days of vaccination.
As HZ can be prevented in many patients, the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) recommend using live zoster vaccine (LZV) in patients with RA where appropriate, unless there are contraindications 6, 7. However, LZV efficacy has been shown to be limited and to reduce with increasing age, with efficacy (assessed for up to 4.9 years) of only 51.3% reported in immunocompetent subjects age ≥60 years, versus 37.6% in those subjects age ≥70 years 8.
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. Tofacitinib has been reported to increase HZ risk in patients with RA, and the risk is higher still in older patients, those receiving corticosteroids 4, 9, and in certain Asian populations, particularly Japanese and Korean patients 9. In an analysis of data from phase II, phase III, and long‐term extension (LTE) studies in patients with RA who received tofacitinib 5 or 10 mg twice daily (BID) without prior LZV (per protocol, some patients may have received LZV), the HZ incidence rate (IR) was 4.4 per 100 patient‐years (95% confidence interval [95% CI] 3.8–4.9) globally and 9.2 per 100 patient‐years (95% CI 7.5–11.4) in Japan/Korea 4. Analysis of phase I, phase II, phase III, and LTE study data showed that most HZ events in tofacitinib‐treated patients with RA were not serious (per the investigator's assessment) and resolved with standard antiviral treatment 9. Increased HZ risk versus placebo has also been observed with another Janus kinase inhibitor, baricitinib, in an analysis of data pooled from phase I, phase II, phase III, and LTE studies. HZ rates with baricitinib were higher in Asia versus other geographic regions, and higher in Japan versus the rest of Asia 5.
Given that an increased HZ risk has been reported in patients with RA, considering whether this risk can be mitigated by vaccination is important. The ORAL Strategy was a 1‐year, global phase IIIb/IV study evaluating the efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate (MTX), and adalimumab (ADA) with MTX 10. Although not protocol‐mandated, eligible patients could receive LZV at the investigators’ discretion before starting study treatment. In this post hoc analysis, we explore the rate of HZ events by treatment arm and LZV safety (in terms of vaccine‐related adverse reactions, injection‐site reactions, and development of zoster‐like lesions), which were secondary objectives in ORAL Strategy.
PATIENTS AND METHODS
Study design
The full design of ORAL Strategy has been reported previously 10. Briefly, ORAL Strategy was a 1‐year, phase IIIb/IV, double‐blind, head‐to‐head, randomized, triple‐dummy, active‐comparator–controlled study 10. The study was conducted at 194 centers in 25 countries. All procedures were in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice and were approved by the institutional review board/ethics committee at each study center. All patients provided written informed consent. For data sharing information, see Supplementary Appendix A, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.24010/abstract.
Patients
Eligible patients were age ≥18 years with active RA based on the ACR/EULAR criteria, despite receiving continuous MTX for ≥4 months and at 15–25 mg/week for ≥6 weeks before baseline. Concomitant oral corticosteroids (≤10 mg/day of prednisone or equivalent) were permitted. Patients were excluded if they currently had, or had a history of, recurrent (>1 episode) or disseminated (a single episode) HZ or disseminated herpes simplex.
Randomization and treatment
Between September 11, 2014 and December 28, 2015, patients were blindly randomized 1:1:1 to receive oral tofacitinib 5 mg BID monotherapy (tofacitinib monotherapy), oral tofacitinib 5 mg BID with MTX (tofacitinib plus MTX), or subcutaneous ADA 40 mg every other week with MTX (ADA plus MTX).
Live zoster vaccination procedure
In countries where LZV was available and allowed by local regulations, eligible consenting patients age ≥50 years could receive LZV at the investigators’ discretion, 28 days (± 1 week) before the initiation of study treatment. Full exclusion criteria are shown in Supplementary Appendix B, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.24010/abstract.
Post hoc analysis of vaccination and HZ events
For all patients treated in ORAL Strategy and for those with HZ events, the proportion who were vaccinated was reported, together with demographics and baseline characteristics by treatment group and stratified by vaccination status. Baseline varicella zoster virus serology checks were not protocol‐mandated. All HZ events were monitored, including potential opportunistic infection HZ events, which were evaluated by an external adjudication committee and defined in this study as events that were disseminated or multidermatomal (occurring in nonadjacent or >2 adjacent dermatomes). Serious HZ events were defined as those that were life‐threatening, required parenteral antiviral treatment or hospitalization, or resulted in death, birth defect, or persistent/significant disability. LZV safety was assessed: zoster vaccine–related adverse events were reported, including injection‐site reactions and development of zoster‐like lesions.
Statistical analysis
Because self‐reported vaccination status may not be reliable and was not verifiable, patients self‐reporting previous vaccination were categorized as nonvaccinated. HZ events were summarized descriptively. HZ IRs (patients with events per 100 patient‐years) and 95% CIs were calculated for each treatment group and for vaccinated versus nonvaccinated patients. Crude HZ IRs were also calculated for vaccinated and nonvaccinated patients stratified by age ≥50 years.
RESULTS
Live zoster vaccination
In ORAL Strategy, 1,146 patients received the study treatment (tofacitinib monotherapy, n = 384; tofacitinib plus MTX, n = 376; or ADA plus MTX, n = 386). Of these patients, 549 (47.9%) were eligible by age (≥50 years) to receive LZV, excluding those in Russia (n = 57) due to regulatory restrictions. Of all 1,146 patients, 216 (18.8%) received LZV prior to study treatment and 930 (81.2%) did not. Of the 549 patients eligible by age, 333 (60.7%) did not receive LZV due to institutional review board/investigator discretion and/or patient decision, other protocol exclusions, or lack of availability of frozen LZV in Canada, Israel, and Thailand at the beginning of ORAL Strategy. Of the 216 patients who received LZV, 7 (3.2%) were age <50 years and were considered to be protocol deviations. Overall, 30 patients (2.6%) self‐reported vaccination prior to the study and were categorized as nonvaccinated in this analysis since the vaccination was not verifiable.
Supplementary Table 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.24010/abstract, shows LZV use stratified by treatment group and country. Overall, the proportions of patients who received LZV were similar among patients receiving tofacitinib monotherapy (n = 69 of 384 [18.0%]), tofacitinib plus MTX (n = 75 of 376 [19.9%]), and ADA plus MTX (n = 72 of 386 [18.7%]). Demographics and baseline characteristics of patients in ORAL Strategy who received study treatment stratified by vaccination status are shown in Table 1 (all patients) and in Supplementary Table 2 (patients age ≥50 years), available at http://onlinelibrary.wiley.com/doi/10.1002/acr.24010/abstract.
Table 1.
Demographics and baseline characteristics of patients in ORAL Strategy by treatment group, stratified by LZV vaccination statusa
| Tofacitinib 5 mg BID monotherapy (n = 384) | Tofacitinib 5 mg BID + MTX (n = 376) | ADA 40 mg Q2W + MTX (n = 386) | ||||
|---|---|---|---|---|---|---|
| Vaccinated (n = 69) | Nonvaccinated (n = 315) | Vaccinated (n = 75) | Nonvaccinated (n = 301) | Vaccinated (n = 72) | Nonvaccinated (n = 314) | |
| Age, years | 58.7 ± 7.0 | 47.7 ± 12.3 | 58.2 ± 7.3 | 47.9 ± 13.7 | 60.5 ± 7.5 | 48.4 ± 13.4 |
| Sex, no. (%) | ||||||
| Male | 11 (15.9) | 54 (17.1) | 16 (21.3) | 49 (16.3) | 15 (20.8) | 51 (16.2) |
| Female | 58 (84.1) | 261 (82.9) | 59 (78.7) | 252 (83.7) | 57 (79.2) | 263 (83.8) |
| Geographic region, no. (%) | ||||||
| North Americab | 15 (21.7) | 47 (14.9) | 25 (33.3) | 46 (15.3) | 28 (38.9) | 45 (14.3) |
| Latin America | 18 (26.1) | 75 (23.8) | 21 (28.0) | 70 (23.3) | 13 (18.1) | 79 (25.2) |
| Europe | 13 (18.8) | 146 (46.3) | 9 (12.0) | 141 (46.8) | 16 (22.2) | 138 (43.9) |
| Asia | 20 (29.0) | 23 (7.3) | 18 (24.0) | 20 (6.6) | 14 (19.4) | 28 (8.9) |
| Australia/New Zealand | 2 (2.9) | 5 (1.6) | 1 (1.3) | 5 (1.7) | 0 (0.0) | 5 (1.6) |
| Rest of the world | 1 (1.4) | 19 (6.0) | 1 (1.3) | 19 (6.3) | 1 (1.4) | 19 (6.1) |
| TJC28 score | 14.7 ± 7.1 | 15.5 ± 6.4 | 14.7 ± 7.0 | 15.8 ± 6.3 | 14.3 ± 7.4 | 15.3 ± 6.5 |
| SJC28 score | 11.1 ± 5.8 | 11.2 ± 5.5 | 10.3 ± 5.3 | 12.2 ± 5.8 | 10.8 ± 6.0 | 11.0 ± 5.2 |
| CRP, mg/liter | 16.1 ± 18.2 | 16.7 ± 19.5 | 17.4 ± 19.3 | 19.0 ± 22.5 | 16.4 ± 16.7 | 16.6 ± 22.2 |
| ESR, mm/hour | 48.2 ± 27.2 | 48.0 ± 26.1 | 51.0 ± 31.4 | 49.1 ± 26.8 | 43.9 ± 25.3 | 48.1 ± 25.6 |
| CDAI score | 36.8 ± 13.1 | 39.0 ± 12.4 | 36.3 ± 12.4 | 40.6 ± 12.7 | 36.5 ± 13.8 | 38.6 ± 12.7 |
| DAS28 4 (ESR) score | 6.4 ± 0.9 | 6.5 ± 0.9 | 6.3 ± 0.9 | 6.6 ± 0.9 | 6.2 ± 1.0 | 6.5 ± 1.0 |
| Baseline corticosteroid use, | ||||||
| no. (%) | 41 (59.4) | 187 (59.4) | 44 (58.7) | 171 (56.8) | 45 (62.5) | 178 (56.7) |
| Daily dose, mg | 5.2 ± 2.8 | 7.6 ± 14.7 | 5.6 ± 2.6 | 6.3 ± 4.1 | 9.9 ± 32.1 | 6.5 ± 6.8 |
| Weekly MTX dose, mg | 16.2 ± 3.6 | 16.7 ± 3.4 | 16.0 ± 3.8 | 16.9 ± 3.6 | 17.1 ± 3.6 | 16.3 ± 3.7 |
| Diabetes mellitus, no. (%) | ||||||
| Yes | 14 (20.3) | 25 (7.9) | 9 (12.0) | 25 (8.3) | 11 (15.3) | 22 (7.0) |
| no | 55 (79.7) | 290 (92.1) | 66 (88.0) | 276 (91.7) | 61 (84.7) | 292 (93.0) |
| ALC, 103 cells/mm3 | 1.7 ± 0.6 | 1.8 ± 0.6 | 1.9 ± 0.9 | 1.8 ± 0.7 | 1.8 ± 0.7 | 1.8 ± 0.6 |
Values are the mean ± SD unless indicated otherwise. Data for patients age <50 and ≥50 years. LZV = live zoster vaccine; BID = twice daily; MTX = methotrexate; ADA = adalimumab; Q2W = every other week; TJC28 = tender joint count (28 joints); SJC28 = swollen joint count (28 joints); CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; CDAI = Clinical Disease Activity Index; DAS28 4 = Disease Activity Score in 28 joints using 4 variables; ALC = absolute lymphocyte count.
US and Canada.
HZ events
Overall, 18 of the 1,146 patients (1.6%) who received the study treatment developed HZ. The demographics and baseline characteristics of these patients, stratified by vaccination status, are shown in Table 2. HZ events occurred in 3 of 216 vaccinated patients (1.4%) and 15 of 930 nonvaccinated patients (1.6%) (1 event per patient) and are summarized descriptively in Supplementary Table 3, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.24010/abstract. Notably, all patients receiving tofacitinib monotherapy who developed HZ received corticosteroids at baseline.
Table 2.
Demographics and baseline characteristics of patients with HZ in ORAL Strategy by treatment group, stratified by vaccination statusa
| Tofacitinib 5 mg BID monotherapy (n = 384; patients with HZ 4 [1.0%]) | Tofacitinib 5 mg BID + MTX (n = 376; patients with HZ 8 [2.1%]) | ADA 40 mg Q2W + MTX (n = 386; patients with HZ 6 [1.6%]) | ||||
|---|---|---|---|---|---|---|
| Vaccinated (n = 1) | Nonvaccinated (n = 3) | Vaccinated (n = 2) | Nonvaccinated (n = 6) | Vaccinated (n = 0) | Nonvaccinated (n = 6) | |
| Age, years | 75.0 | 40.7 | 58.0 | 58.8 | – | 52.2 |
| Male/female, no. | 0/1 | 0/3 | 1/1 | 0/6 | – | 2/4 |
| Geographic region, no.b | ||||||
| North America | 0 | 1 | 1 | 3 | – | 2 |
| Latin America | 0 | 1 | 0 | 1 | – | 0 |
| Eastern Europe | 0 | 1 | 0 | 2 | – | 4 |
| Western Europe | 0 | 0 | 1 | 0 | – | 0 |
| Asia | 1 | 0 | 0 | 0 | – | 0 |
| TJC28 score | 17.0 | 17.0 | 8.0 | 12.0 | – | 17.3 |
| SJC28 score | 7.0 | 13.0 | 10.0 | 10.8 | – | 10.5 |
| CRP, mg/liter | 5.2 | 35.7 | 24.1 | 14.6 | – | 14.5 |
| ESR, mm/hour | 19.0 | 41.0 | 27.0 | 52.8 | – | 37.0 |
| CDAI score | 36.5 | 40.3 | 32.1 | 34.9 | – | 40.5 |
| DAS28 4 (ESR) score | 5.9 | 6.7 | 5.7 | 6.3 | – | 6.4 |
| Baseline corticosteroid use, no. | 1 | 3 | 1 | 4 | – | 3 |
| Daily dose, mg | 2.5 | 6.3 | 2.5 | 3.5 | – | 3.7 |
| Weekly MTX dose, mg | 0.0 | 0.0 | 22.5 | 17.1 | – | 16.3 |
| Diabetes mellitus, no. | ||||||
| Yes | 1 | 0 | 0 | 0 | – | 2 |
| No | 0 | 3 | 2 | 6 | – | 4 |
| Baseline ALC, 103 cells/mm3 | 1.3 | 1.9 | 1.0 | 1.8 | – | 1.7 |
Values are the mean unless indicated otherwise. HZ = herpes zoster; BID = twice daily; MTX = methotrexate; ADA = adalimumab; Q2W = every other week; TJC28 = tender joint count (28 joints); SJC28 = swollen joint count (28 joints); CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; CDAI = Clinical Disease Activity Index; DAS28 4 = Disease Activity Score in 28 joints using 4 variables; ALC = absolute lymphocyte count.
USA (n = 7), Mexico (n = 2), Latvia (n = 1), Bulgaria (n = 1), Poland (n = 3), Russia (n = 1), Bosnia and Herzegovina (n = 1), UK (n = 1), and Taiwan (n = 1).
In vaccinated patients, 1 HZ event was adjudicated as an opportunistic infection (multidermatomal, tofacitinib monotherapy), and none were classified as serious (per the definition in Patients and Methods, above). No vaccinated patients had zoster‐like lesions within 42 days of vaccination; 1 patient, treated with tofacitinib monotherapy, had vaccination‐site erythema. In nonvaccinated patients, 3 HZ events were adjudicated as opportunistic infections (multidermatomal, tofacitinib monotherapy, n = 1; disseminated, ADA plus MTX, n = 1; multidermatomal, ADA plus MTX, n = 1), and 2 were classified as serious (tofacitinib plus MTX, n = 1; ADA plus MTX, n = 1). Of all HZ events: most (17 of 18 [94.4%]) were described by the investigator as mild or moderate in severity; all events resolved (72.2% treated with antiviral therapy); 1 case of varicella adjudicated as an opportunistic infection was included as an HZ event (nonvaccinated, ADA plus MTX). Although strain testing was not performed systematically, in the 2 patients who were tested (vaccinated, tofacitinib monotherapy, n = 1; nonvaccinated, tofacitinib plus MTX, n = 1), wild type strains were reported.
Incidence of HZ
HZ IRs in ORAL Strategy were 1.1 (95% CI 0.3–2.9) for tofacitinib monotherapy, 2.3 (95% CI 1.0–4.6) for tofacitinib plus MTX, and 1.7 (95% CI 0.6–3.7) for ADA plus MTX. In vaccinated patients, HZ IRs were 1.5 (95% CI 0.0–8.3) for tofacitinib monotherapy, 3.0 (95% CI 0.4–10.8) for tofacitinib plus MTX, and 0 (95% CI 0.0–5.8) for ADA plus MTX. In nonvaccinated patients, HZ IRs were 1.0 (95% CI 0.2–3.0) for tofacitinib monotherapy, 2.2 (95% CI 0.8–4.7) for tofacitinib plus MTX, and 2.1 (95% CI 0.8–4.5) for ADA plus MTX (Figure 1A).
Figure 1.
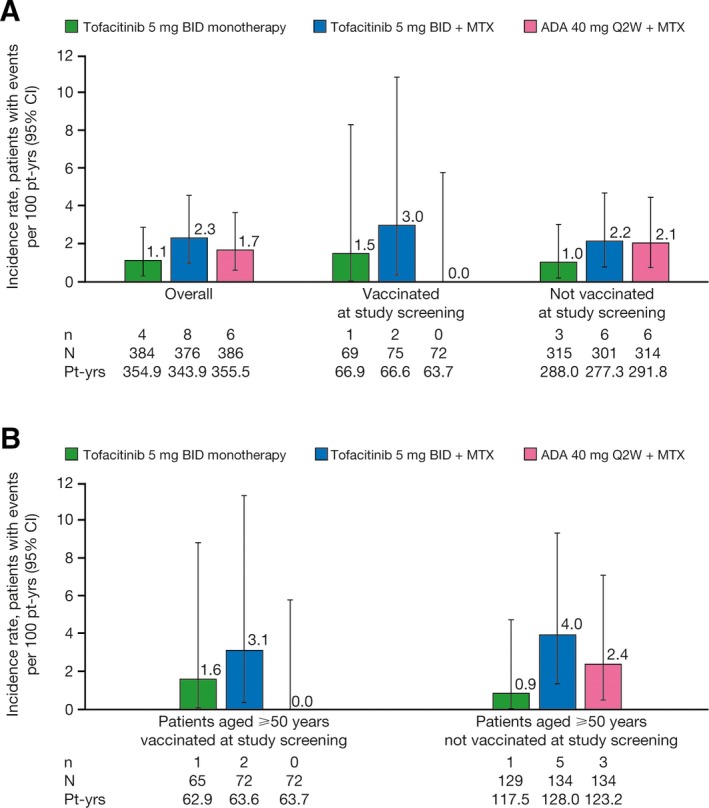
Incidence rates of herpes zoster (serious and nonserious) in the ORAL Strategy by treatment group, stratified by vaccination status, in A, all patients and B, patients age ≥50 years. BID = twice daily; MTX = methotrexate; ADA = adalimumab; Q2W = every other week; pt‐yrs = patient‐years; 95% CI = 95% confidence interval.
In patients age ≥50 years who were vaccinated, HZ IRs were 1.6 (95% CI 0.0–8.9) for tofacitinib monotherapy, 3.1 (95% CI 0.4–11.4) for tofacitinib plus MTX, and 0 (95% CI 0.0–5.8) for ADA plus MTX. In nonvaccinated patients, HZ IRs were 0.9 (95% CI 0.0–4.7) for tofacitinib monotherapy, 4.0 (95% CI 1.3–9.3) for tofacitinib plus MTX, and 2.4 (95% CI 0.5–7.1) for ADA plus MTX (Figure 1B).
No HZ events were reported in the 7 patients age <50 years who were vaccinated (protocol deviations). HZ events occurred in 6 of 930 patients (0.6%) age <50 years who were nonvaccinated (per protocol): tofacitinib monotherapy, n = 2; tofacitinib plus MTX, n = 1; ADA plus MTX, n = 3.
DISCUSSION
In this post hoc exploratory analysis of data from the ORAL Strategy, we evaluated the effect of LZV in a subset of patients with RA who received the vaccine prior to treatment with tofacitinib monotherapy, tofacitinib plus MTX, or ADA plus MTX. HZ IRs were generally similar across treatment groups and between vaccinated and nonvaccinated patients, with wide and overlapping 95% CIs. ORAL Strategy was not designed/powered, however, for comparisons between vaccinated and nonvaccinated patients; vaccination was not protocol‐mandated and <20% of patients received LZV. Moreover, a proportion of patients who self‐reported HZ vaccination history were analyzed as nonvaccinated since the date/year of vaccination occurrence was not verifiable. Additionally, LZV administration was not randomized, in contrast with the randomization of study drugs in ORAL Strategy; confounding is therefore possible. ORAL Strategy was not conducted in Japan, where HZ risk in tofacitinib‐treated patients with RA is increased versus other countries 4, 9; therefore, this analysis may not give a full representation of LZV in tofacitinib‐treated patients. The findings should also be interpreted in the context of the limited efficacy (~50%) that has been observed for LZV in immunocompetent subjects with up to 4.9 years of follow‐up 8; in contrast, ORAL Strategy was limited to just 1 year of follow‐up.
All patients receiving tofacitinib monotherapy who developed HZ were receiving corticosteroids at baseline. This result appears consistent with data from phase III studies in patients with RA receiving tofacitinib without prior LZV, in which HZ IRs were lowest with tofacitinib 5 mg BID without corticosteroids or conventional synthetic DMARDs (csDMARDs; 0.56 per 100 patient‐years [95% CI 0.07–2.01]) and highest with tofacitinib 10 mg BID with corticosteroids and csDMARDs (5.44 per 100 patient‐years [95% CI 3.72–7.68]) 9.
HZ IRs for the 3 treatment groups were generally similar in patients age ≥50 years, stratified by vaccination status. None of the 7 patients age <50 years who received LZV (considered protocol deviations) developed HZ, whereas 6 of 15 patients (40.0%) who did not receive LZV and developed HZ were age <50 years. Although this analysis was not designed/powered to detect the effect of age on LZV efficacy, in the general population (which, in 1 study, included immunosuppressed individuals and those with disorders previously associated with HZ, such as RA), the efficacy of LZV is reduced with increasing age 8, 11. Meanwhile, HZ risk increases with age both in the general population 1 and in patients with RA 2, including those treated with tofacitinib 4, 9.
Overall, evidence suggests that patients with RA should be vaccinated against HZ once eligible. Notably, EULAR guidelines recommend that vaccination against HZ should be considered only in patients who are less severely immunosuppressed 6; ACR recommends considering LZV before initiating RA therapies and while receiving csDMARD monotherapy or csDMARD combination therapy but not when receiving bDMARDs 7. A US National Institutes of Health trial (VERVE; NCT02538341) is assessing LZV use in patients with RA treated with biologic tumor necrosis factor inhibitor therapies; preliminary results from the pilot trial at 6 weeks showed no safety issues 12.
Given the limitations of LZV, interest has focused on newer vaccines that appear to offer improved efficacy. For example, a new adjuvanted HZ subunit vaccine (HZ/su) is indicated for use in adults age ≥50 years 13 and differs significantly from LZV. Since HZ/su is not a live vaccine, it is not contraindicated in those who are immunosuppressed due to disease or therapy. HZ/su efficacy has been reported to be 97.2% in immunocompetent subjects age ≥50 years and does not appear to decrease with advancing age: efficacy was 96.6%, 97.4%, and 97.9% in patients ages 50–59, 60–69, and ≥70 years, respectively 14. The reactogenicity of HZ/su should be noted: 81.5% and 66.1% of subjects were reported to experience injection‐site reactions and systemic reactions, respectively 14, which may be attributable to the mode of action and/or strength of the adjuvant. The effect of HZ/su has not yet been evaluated in patients with RA.
In addition to evaluating the effect of LZV on HZ IRs in this post hoc analysis of ORAL Strategy, LZV safety was monitored: no vaccinated patients had zoster‐like lesions in the 42 days following vaccination, and 1 patient had vaccination‐site erythema. This result supports findings of a previous phase II trial in which patients age ≥50 years received LZV 2–3 weeks prior to initiating tofacitinib 5 mg BID or placebo with background MTX 15. LZV was well tolerated, but 1 patient who lacked pre‐existing varicella zoster virus immunity developed cutaneous vaccine dissemination, which resolved with antiviral treatment and discontinuation of tofacitinib 15.
In this post hoc analysis of data from ORAL Strategy, HZ IRs were generally similar between treatment groups and between vaccinated and nonvaccinated patients, and safety findings were consistent with previous reports. These data suggest that LZV is well tolerated in patients with RA treated with tofacitinib with or without background MTX. However, due to the limitations of the analysis, definitive conclusions on vaccine efficacy cannot be drawn from these data. Further studies are necessary to fully compare LZV efficacy in patients with RA versus the general population and to identify potential modifiable factors, such as concomitant medications, to achieve maximal HZ prevention. Additionally, the efficacy and safety of the new HZ/su vaccine should be investigated in patients with RA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr Takiya had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Tatulych, Soma, Luo, Fleischmann.
Acquisition of data
Abud‐Mendoza, Lee, Tatulych, Soma, Fleischmann.
Analysis and interpretation of data
Calabrese, Abud‐Mendoza, Lindsey, Takiya, Iikuni.
ROLE OF THE STUDY SPONSOR
The study was sponsored by Pfizer. Pfizer was involved in the study design and in data collection. All authors, including those employed by Pfizer, had a role in data analysis, data interpretation, and writing the manuscript. Medical writing support, under the guidance of the authors, was provided by Sarah Piggott, MChem, of CMC Connect, a division of McCann Health Medical Communications, Glasgow, UK, and was funded by Pfizer in accordance with Good Publication Practice guidelines. Publication of this article was not contingent upon approval by Pfizer.
Supporting information
Appendix A
ACKNOWLEDGMENTS
The authors thank the study participants.
ClinicalTrials.gov identifier: NCT02187055.
Supported by Pfizer.
Dr. Calabrese has received consulting fees from AbbVie, Amgen, Bristol‐Myers Squibb, Crescendo, Eli Lilly and Company, Genentech, Gilead, GlaxoSmithKline, Horizon, Janssen, Pfizer, Sanofi‐Genzyme, and UCB (less than $10,000 each) and speaking fees from Genentech, Horizon, Novartis, and Sanofi‐Regeneron (more than $10,000 each). Dr. Abud‐Mendoza has received consulting fees from Bristol‐Myers Squibb, Pfizer, and Roche (less than $10,000 each) and speaking fees from Bristol‐Myers Squibb, Merck‐Serono, Pfizer, Roche, and UCB (more than $10,000 each). Dr. Lindsey has received consulting fees and speaking fees from Pfizer (more than $10,000). Drs. Tatulych, Takiya, Iikuni, Soma, and Luo own stock or stock options in Pfizer. Dr. Fleischmann has received consulting fees from Acea, Akros, Amgen, Bristol‐Myers Squibb, Janssen, Novartis, Samsung, Sandoz, Tahio (less than $10,000 each), AbbVie, Eli Lilly and Company, EMDSerano, GlaxoSmithKline, and Pfizer (more than $10,000 each) and research support from AbbVie, Acea, Amgen, AstraZeneca, Bristol‐Myers Squibb, Celgene, Centrexion, Eli Lilly and Company, EMDSerano, Genentech, GlaxoSmithKline, Novartis, Pfizer, Resolve, Roche, Samumed, Sandoz, Sanofi‐Aventis, UCB, and Unity. No other disclosures relevant to this article were reported.
References
- 1. Harpaz R, Ortega‐Sanchez IR, Seward JF. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008;57:1–30. [PubMed] [Google Scholar]
- 2. Yun H, Yang S, Chen L, Xie F, Winthrop K, Baddley JW, et al. Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol 2016;68:2328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yun H, Xie F, Delzell E, Chen L, Levitan EB, Lewis JD, et al. Risks of herpes zoster in patients with rheumatoid arthritis according to biologic disease‐modifying therapy. Arthritis Care Res (Hoboken) 2015;67:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winthrop KL, Yamanaka H, Valdez H, Mortensen E, Chew R, Krishnaswami S, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol 2014;66:2675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winthrop KL, Lindsey S, Weinblatt M, Takeuchi T, Hyslop D, Issa M, et al. Herpes zoster in patients with moderate to severe rheumatoid arthritis treated with baricitinib [abstract]. Arthritis Rheumatol 2016;68 Suppl 10:3027. [Google Scholar]
- 6. Van Assen S, Agmon‐Levin N, Elkayam O, Cervera R, Doran MF, Dougados M, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2011;70:414–22. [DOI] [PubMed] [Google Scholar]
- 7. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 8. Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005;352:2271–84. [DOI] [PubMed] [Google Scholar]
- 9. Winthrop KL, Curtis JR, Lindsey S, Tanaka Y, Yamaoka K, Valdez H, et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol 2017;69:1960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double‐blind, head‐to‐head, randomised controlled trial. Lancet 2017;390:457–68. [DOI] [PubMed] [Google Scholar]
- 11. Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post‐herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013;10:e1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curtis JR, Turner A, Thomas C, Cofield S, Parks R, Ku JH, et al. Safety, clinical and immunologic effectiveness of the live zoster vaccine administered to patients receiving anti‐TNF biologics [abstract]. Arthritis Rheumatol 2015;67 Suppl 10:1520. [Google Scholar]
- 13. US Food and Drug Administration . SHINGRIX (zoster vaccine recombinant, adjuvanted) suspension for intramuscular injection, highlights of prescribing information. 2017. URL: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM581605.pdf.
- 14. Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez‐Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087–96. [DOI] [PubMed] [Google Scholar]
- 15. Winthrop KL, Wouters AG, Choy EH, Soma K, Hodge JA, Nduaka CI, et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized Phase II trial. Arthritis Rheumatol 2017;69:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A


