Summary
Background
Tildrakizumab is a specific anti‐interleukin‐23p19 monoclonal antibody approved for the treatment of plaque psoriasis.
Objectives
To evaluate the long‐term efficacy and safety of tildrakizumab treatment for patients with moderate‐to‐severe psoriasis for up to 148 weeks.
Methods
Pooled analysis from two double‐blind, randomized controlled trials: reSURFACE 1 and reSURFACE 2. Efficacy was assessed for responders (≥ 75% improvement in Psoriasis Area and Severity Index; PASI 75) and partial responders (PASI 50–75) to tildrakizumab 100 mg and 200 mg at week 28 who were maintained on the same dose (administered every 12 weeks), and for partial responders or nonresponders (PASI < 50) to etanercept 50 mg at week 28 who, after a 4‐week washout, were switched to tildrakizumab 200 mg (administered at weeks 32 and 36, and every 12 weeks thereafter). Safety was assessed in the all‐patients‐as‐treated population. Three different methods of imputing missing data were used: nonresponder imputation (NRI), multiple imputation and observed cases. The Clinicaltrials.gov numbers are NCT01722331 (reSURFACE 1) and NCT01729754 (reSURFACE 2).
Results
At week 148 (NRI), 72·6%, 53·8% and 28·9% of tildrakizumab 100‐mg responders and 80·2%, 59·9% and 32·6% of tildrakizumab 200‐mg responders had PASI 75, 90 and 100 responses, respectively. For partial responders to tildrakizumab 100 mg and 200 mg, the proportions of patients achieving PASI 75, 90 and 100 responses were 32·5%, 25·0% and 10·0%; and 47·1%, 27·5% and 12·8%, respectively. For patients who were partial responders or nonresponders to etanercept, the proportions of patients achieving PASI 75, 90 and 100 responses were 66·9%, 43·8% and 14·9% at week 148. Rates of discontinuations due to adverse events [tildrakizumab 100 mg: 1·7 per 100 patient‐years (PYs); tildrakizumab 200 mg: 1·2 per 100 PYs] and exposure‐adjusted rates of serious adverse events (5·9 per 100 PYs; 5·5 per 100 PYs), severe infections (1·1 per 100 PYs; 1·1 per 100 PYs), malignancies (0·6 per 100 PYs; 0·4 per 100 PYs) and major adverse cardiovascular events (0·4 per 100 PYs; 0·5 per 100 PYs) were low.
Conclusions
Tildrakizumab was well tolerated and efficacy was well maintained in week 28 responders who continued tildrakizumab treatment through 3 years, or improved among etanercept partial responders or nonresponders who switched to tildrakizumab.
What's already known about this topic?
Tildrakizumab 100 mg and 200 mg are efficacious and well tolerated with short‐term use in the treatment of patients with moderate‐to‐severe plaque psoriasis.
What does this study add?
High levels of efficacy are maintained for up to 3 years of psoriasis treatment with tildrakizumab.
There is a favourable long‐term safety profile with both tildrakizumab 100 mg and 200 mg, with a low incidence of adverse events of special interest through 3 years.
Short abstract
https://doi.org/10.1111/bjd.18831 available online
Interleukin (IL)‐23 is a key regulatory cytokine that is essential in differentiation and survival of T helper 17 cells, which are cells fundamental in the pathogenesis of psoriasis. Thus, specific and selective targeting of IL‐23 has become an effective therapeutic strategy,1, 2, 3 without targeting IL‐12. Tildrakizumab is a monoclonal antibody that specifically targets the p19 subunit of IL‐23, and that is approved for the treatment of plaque psoriasis in the U.S.A. and European Union.4, 5
The objective of this manuscript is to report the 148‐week pooled efficacy and safety results in patients from two phase III trials, reSURFACE 1 and reSURFACE 2,5 who were responders [≥ 75% improvement in Psoriasis Area and Severity Index (PASI 75)] or partial responders (≥ 50 to < 75% improvement in PASI) to tildrakizumab 100 mg or 200 mg at week 28 and who continued treatment with the same dose of tildrakizumab. The evolution of patients who were partial responders or nonresponders to etanercept 50 mg at week 28 and who were then treated with tildrakizumab 200 mg is also presented. The results of these trials to week 28 have already been published.5, 6
Patients and methods
Patients and study design
Both reSURFACE 1 (64 weeks) and reSURFACE 2 (52 weeks) were three‐part, parallel‐group, double‐blinded, randomized, placebo‐controlled phase III trials. The detailed methodology and patient characteristics have been published previously.5
In part 1 (week 0 to week 12), patients were randomized 2 : 2 : 1 to tildrakizumab 200 mg, tildrakizumab 100 mg or placebo (reSURFACE 1), or 2 : 2 : 1 : 2 to tildrakizumab 200 mg, tildrakizumab 100 mg, placebo or etanercept 50 mg (reSURFACE 2) (Fig. 1). In part 2 (week 12 to week 28), placebo patients were rerandomized 1 : 1 to tildrakizumab 200 mg or tildrakizumab 100 mg. In part 3 (week 28 to week 52 or 64), PASI 75 responders in reSURFACE 1 were rerandomized to continue the same tildrakizumab dose or to receive placebo (with retreatment with the same tildrakizumab dose upon relapse); in reSURFACE 2, PASI 75 responders to tildrakizumab 200 mg were rerandomized to tildrakizumab 200 mg or tildrakizumab 100 mg, while PASI 75 responders to tildrakizumab 100 mg maintained the same dose.
Figure 1.
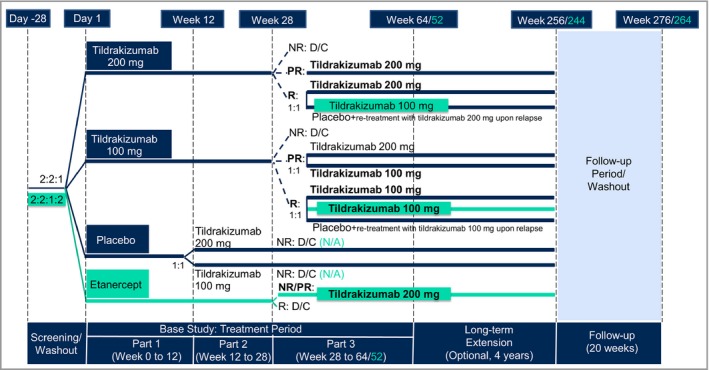
Integrated design of the reSURFACE 1 and reSURFACE 2 trials. Differences in design for reSURFACE 2 vs. reSURFACE 1 are shown in turquoise colour. Groups of interest for efficacy analyses are marked in bold. D/C, discontinued; N/A, not applicable; NR, nonresponders [< 50% improvement in Psoriasis Area and Severity Index (PASI 50)]; PR, partial responders (PASI 50–75); R, responders (PASI ≥ 75).
In both studies, partial responders to tildrakizumab 200 mg continued the same dose, while partial responders to tildrakizumab 100 mg were randomized 1 : 1 to tildrakizumab 200 mg or tildrakizumab 100 mg. Tildrakizumab 100‐mg and 200‐mg doses were given at week 0, week 4 and every 12 weeks thereafter. In reSURFACE 2, etanercept 50 mg was given twice weekly in part 1 and once a week in part 2. At week 28, partial responders and nonresponders to etanercept 50 mg were crossed over to tildrakizumab 200 mg after a 4‐week washout period. Tildrakizumab 200 mg was administered at week 32, week 36 and every 12 weeks thereafter. At week 52 or 64, patients who completed part 3 with at least a PASI 50 response entered an optional and still ongoing extension period of up to extension week 256 (reSURFACE 1) or up to extension week 244 (reSURFACE 2).
Both studies were conducted in a double‐blinded manner for the entire duration of the base study (parts 1, 2 and 3). The first 28 weeks of reSURFACE 2 employed double‐dummy blinding, administering matching placebo doses of tildrakizumab and etanercept with identical prefilled syringes. Once the base study was completed and locked at week 52 (reSURFACE 2) or week 64 (reSURFACE 1), patients in the extension were provided open‐label study medication for the rest of the study.
The main inclusion and exclusion criteria at baseline were similar for reSURFACE 1 and reSURFACE 2. Patients fulfilled the following inclusion criteria: age 18 years or older, diagnosis of plaque psoriasis ≥ 6 months previously, ≥ 10% of body surface area affected, Physician's Global Assessment score ≥ 3, PASI score ≥ 12, and a candidate for phototherapy or systemic therapy. In reSURFACE 2, patients who had previously used etanercept or were sensitive or allergic to latex were excluded. The study protocols were approved by local institutional review boards or ethics committees, and all patients provided written informed consent.
Assessments
Efficacy was assessed based on PASI 75, 90 or 100 response rates (proportions of patients achieving ≥ 75%, ≥ 90% or 100% improvement) and the proportions of patients with absolute PASI scores < 1, < 3 or < 5. In part 3 and the extension period up to week 148, efficacy measurements were done at weeks 28, 32, 36, 40, 52, 60 and 64, and then every 12 weeks until week 148.
Safety assessments consisted of overall adverse events (AEs) and AEs of special interest. Severe infections were defined as any infection meeting the regulatory definition of a serious AE (i.e. resulted in death, was life‐threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, or required intervention to prevent one of the other outcomes listed), or any infection requiring intravenous antibiotics. Extended major adverse cardiovascular events (MACEs) included nonfatal myocardial infarction, nonfatal stroke, unstable angina, coronary revascularization, resuscitated cardiac arrest, and cardiovascular deaths that were confirmed as ‘cardiovascular’ or ‘sudden’. Deaths and serious cardiovascular events were adjudicated by an external clinical adjudication committee. Safety measurements were done at all study visits, which coincided between the two studies, except at weeks 44, 48 and 56 (reSURFACE 1 only) and week 46 (reSURFACE 2 only).
Statistical analyses
Responders were defined as patients with ≥ 75% improvement in PASI; partial responders were defined as patients with ≥ 50 to < 75% improvement in PASI; and nonresponders were defined as patients with < 50% improvement in PASI.
The current analyses focus on 148‐week efficacy data in patients who were responders or partial responders to tildrakizumab 100 mg or 200 mg at week 28 and who continued treatment with the same tildrakizumab dose, and in patients who were partial responders or nonresponders to etanercept 50 mg at week 28 and who were crossed over to tildrakizumab 200 mg (Fig. 1). Safety data for the 148‐week period, pooled across reSURFACE 1 and reSURFACE 2, were also included.
The patient populations were different for the safety and efficacy analyses. Efficacy analyses were performed on the full analysis set of part 3, which consisted of all patients who entered part 3 (at week 28) and received at least one dose of study treatment in part 3, based on the treatment assigned. Responders to tildrakizumab 100 mg, responders to tildrakizumab 200 mg, partial responders to tildrakizumab 100 mg, partial responders to tildrakizumab 200 mg, and partial responders or nonresponders to etanercept 50 mg were analysed as separate subgroups.
The presented data are based on nonresponder imputation (NRI), where patients with missing data were treated as nonresponders. Observed cases (OC) and multiple imputation (MI) data are presented as sensitivity analyses. A monotone missing data pattern and regression were used to generate multiple imputed datasets according to the different treatment schemes. The imputation model used for analysis of dichotomized PASI response based on the multiple imputed PASI score included demographic variables and baseline disease characteristics such as age, sex, body mass index, presence of psoriatic arthritis, prior exposure to biologics, baseline PASI, baseline Physician's Global Assessment or baseline body surface area, as covariates. After imputation, dichotomized responses (PASI 75, 90 or 100, PASI < 1, < 3 or < 5) were computed for each dataset and then reported as n (%) computing the average from the 10 imputed datasets.
The 95% confidence interval (CI) for the proportion with NRI was computed as
where = proportion with NRI; N NRI = sample size per treatment; CI NRI = 95% CI for and z α/2 is the 1 − α/2 quantile of the standard normal distribution (i.e. z 0·05/2 = 1·96).
The 95% CI for the proportion with OC was computed as
where = proportion with OC; N OC = sample size per week and treatment; CI OC = 95% CI for and z α/2 = 1·96.
For MI data, the proportion used for the computation of the 95% CI was obtained using the average of the 10 proportions (i.e. 10 imputed datasets) from MI analysis. The 95% CI was then computed as
where = proportion with MI; N MI = sample size per treatment; CI MI = 95% CI for and z α/2 = 1·96.
The number of patients used (sample size) for NRI and MI (N NRI and N MI) were 329, 227, 40, 102 and 121 for responders to tildrakizumab 100 mg, responders to tildrakizumab 200 mg, partial responders to tildrakizumab 100 mg, partial responders to tildrakizumab 200 mg and partial responders or nonresponders to etanercept 50 mg, respectively.
Safety analyses were conducted on all of the tildrakizumab‐treated patients and were based on the all‐patients‐as‐treated population, defined as all randomized patients who received at least one dose of study treatment based on the treatment received. Data were pooled from patients treated with either tildrakizumab 100 mg (patients who received tildrakizumab 100 mg in at least one part of the study), tildrakizumab 200 mg (patients who received tildrakizumab 200 mg in at least one part of the study) or placebo (patients who received placebo in at least one part of the study). Medical Dictionary for Regulatory Activities preferred terms were assigned to tildrakizumab 100 mg, tildrakizumab 200 mg or placebo if the AE occurred when the patient was actively being treated with tildrakizumab 100 mg, tildrakizumab 200 mg or placebo, respectively. The etanercept 50‐mg group is presented alongside the pooled data groups. Preferred terms were assigned to etanercept 50 mg if the AE occurred when the patient was actively being treated with etanercept 50 mg. Patients were counted in each assigned treatment group after starting a different treatment.
Exposure‐adjusted incidence rates (EAIRs) [i.e. events per 100 patient‐years (PYs) of exposure] for overall AEs and AEs of special interest, are reported. EAIRs were computed using the number of events for a particular event, adjusting by the total PYs of follow‐up for each treatment, where total PYs of follow‐up was the sum of the individual exposures in years for all patients by treatment. Different crossovers due to the study design were taken into account (e.g. patients who were taking tildrakizumab 100 mg or 200 mg during part 1 and then crossed to placebo in part 3). Computation of EAIRs by treatment was as follows:
Using the previous formula for the computation of EAIRs, the 95% CI for an EAIR was computed from the standard error (SE) for an incidence rate, estimated as
The 95% CI for the (estimated) incidence rate (IR) was then computed as:
where zα/2 = 1·96.
Results
Efficacy population
At week 28, 329 patients who were responders to tildrakizumab 100 mg, 227 patients who were responders to tildrakizumab 200 mg, 40 patients who were partial responders to tildrakizumab 100 mg and 102 patients who were partial responders to tildrakizumab 200 mg were maintained on the same dose. In addition, 121 patients who were partial responders (70%) or nonresponders (30%) to etanercept 50 mg were switched to tildrakizumab 200 mg (Fig. 2). Few patients discontinued due to lack of efficacy or AEs during part 3 or the extension period (Fig. 2). For example, in the tildrakizumab 100‐mg (n = 302) and 200‐mg (n = 213) analysis groups, there were three (1·0%) and one (0·5%), respectively, who discontinued due to lack of efficacy, and five (1·7%) and five (2·3%), respectively, due to an AE during the extension period. The demographics and disease characteristics at baseline were similar across treatment groups (Table 1), and were also similar to those of the general trial population.
Figure 2.
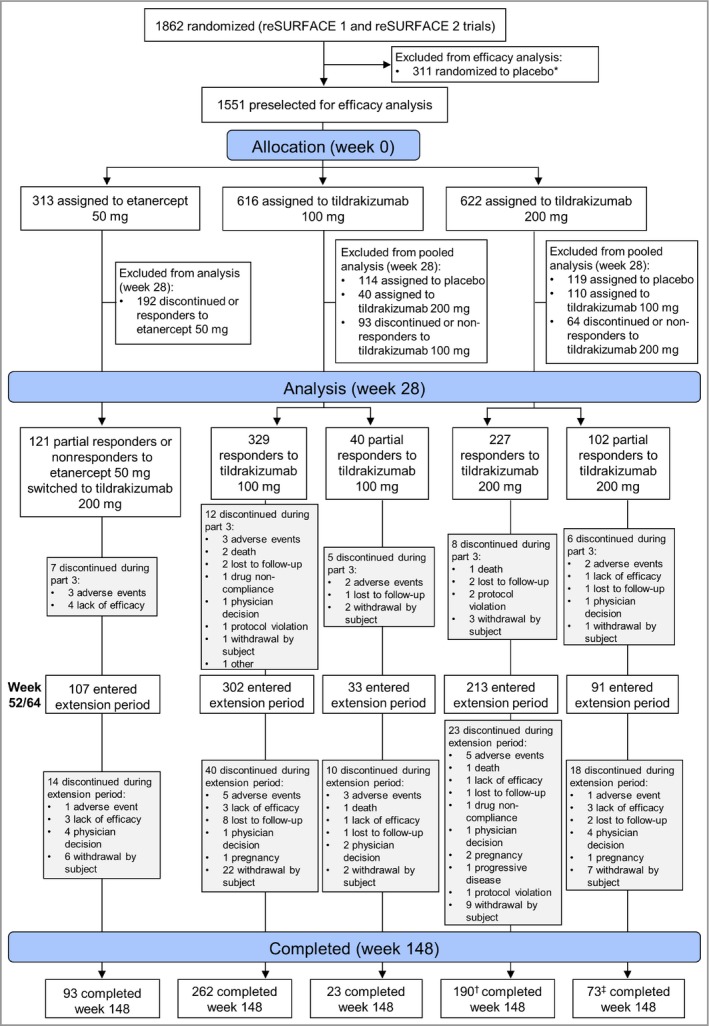
Patient disposition. *One patient did not receive study medication (placebo) and was excluded from the all‐patients‐as‐treated population. †Seven patients with observed Psoriasis Area and Severity Index score at week 148 discontinued thereafter. ‡Four patients with observed Psoriasis Area and Severity Index score at week 148 discontinued thereafter.
Table 1.
Baseline demographics and disease characteristics of the efficacy population
| Tildrakizumab 100 mg (n = 616) | Tildrakizumab 200 mg (n = 622) | Placebo (n = 310) | Etanercept 50 mg (n = 313) | |
|---|---|---|---|---|
| Male, n (%) | 427 (69·3) | 451 (72·5) | 212 (68·4) | 222 (70·9) |
| Age (years), mean ± SD | 45·5 ± 13·4 | 45·8 ± 13·4 | 47·1 ± 12·9 | 45·8 ± 14·0 |
| Weight (kg), mean ± SD | 88·9 ± 23·0 | 88·6 ± 22·7 | 88·1 ± 24·4 | 88·0 ± 21·5 |
| BMI (kg m−2), mean ± SD | 30·0 ± 7·1 | 29·8 ± 7·4 | 30·1 ± 7·7 | 28·9 ± 6·4 |
| BSA (%), mean ± SD | 32·0 ± 18·1 | 31·4 ± 17·5 | 30·4 ± 16·1 | 31·6 ± 16·6 |
| PASI, mean ± SD | 20·2 ± 7·7 | 20·2 ± 8·0 | 19·6 ± 7·3 | 20·2 ± 7·4 |
| DLQI, mean ± SD | 14·4 ± 7·0 | 13·3 ± 7·0 | 13·6 ± 7·1 | 14·5 ± 7·2 |
| PGA category, n (%) | ||||
| ≤ 3 | 414 (67·2) | 409 (66·1) | 209 (67·6) | 200 (63·9) |
| ≥ 4 | 202 (32·8) | 210 (33·9) | 100 (32·4) | 110 (35·1) |
| Psoriatic arthritis (yes), n (%) | 102 (16·6) | 102 (16·4) | 42 (13·6) | 41 (13·1) |
| Previously treated with biologics, n (%) | 110 (17·9) | 109 (17·5) | 54 (17·4) | 37 (11·8) |
| Previously treated with nonbiologic systemic therapy (excluding phototherapy), n (%) | 196 (31·8) | 213 (34·2) | 99 (31·9) | 117 (37·4) |
BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; PGA, Physician's Global Assessment.
Efficacy results
Responders to tildrakizumab 100 mg or 200 mg
The proportions of responders to tildrakizumab 100 mg (Fig. 3a) or 200 mg (Fig. 3b) achieving PASI 75, PASI 90 and PASI 100 responses at weeks 28, 52 and 148 are shown in Table S1 (see Supporting Information). Responses were maintained at week 148 (2‐year extension period). The proportions of responders to tildrakizumab 100 mg (Fig. 4a) or 200 mg (Fig. 4b) achieving absolute PASI < 5, < 3 and < 1 at weeks 28, 52 and 148 are shown in Table S2 (see Supporting Information).
Figure 3.
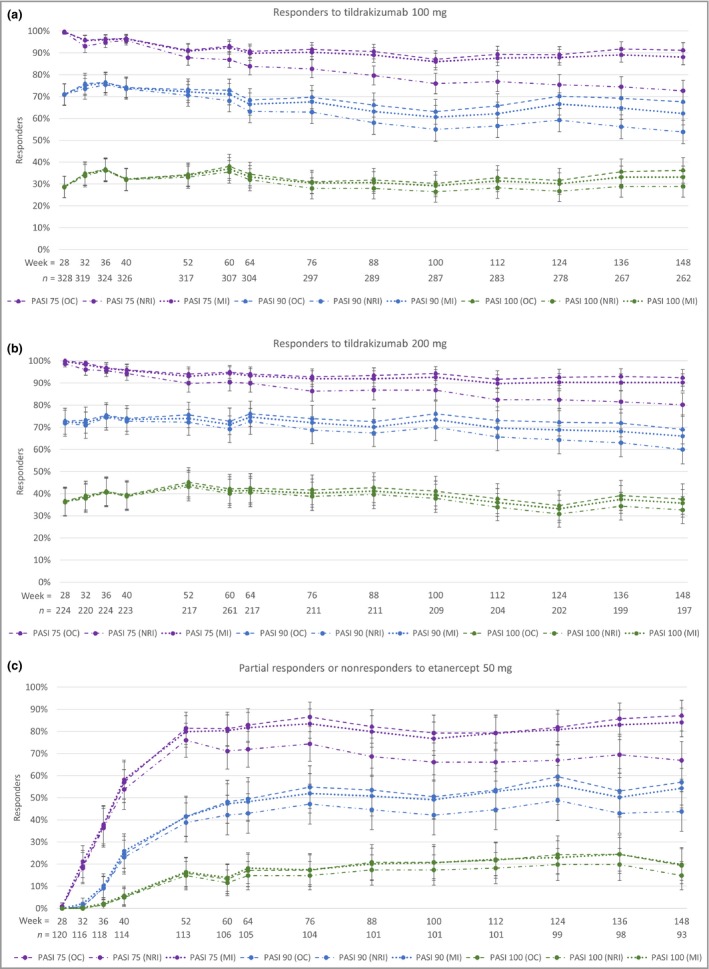
Proportions of patients achieving ≥ 75% improvement in Psoriasis Area and Severity Index (PASI 75), PASI 90 and PASI 100 responses through week 148. (a) Responders to tildrakizumab 100 mg. (b) Responders to tildrakizumab 200 mg. (c) Partial responders or nonresponders to etanercept 50 mg who switched to tildrakizumab 200 mg. OC, observed cases; MI, multiple imputation; NRI, nonresponder imputation. n = number of cases in the OC analyses. Error bars represent 95% confidence intervals.
Figure 4.
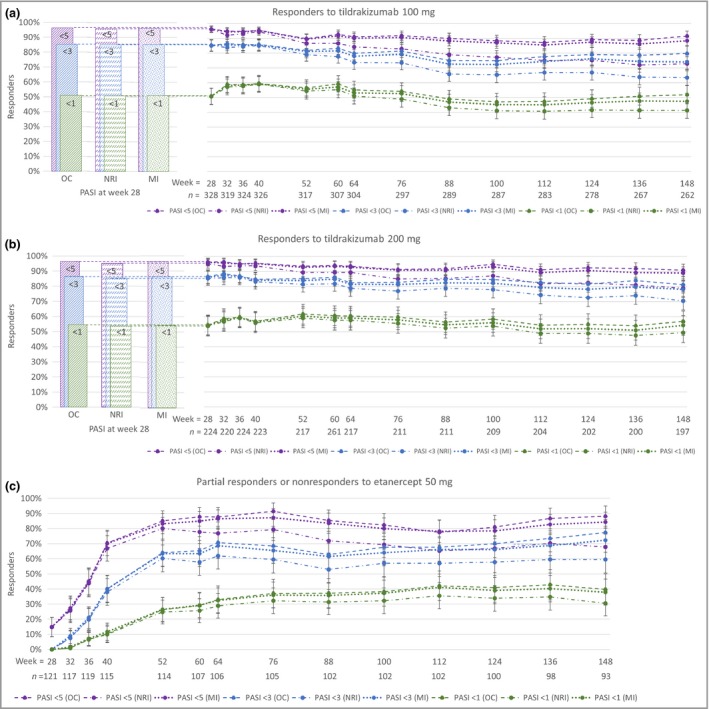
Proportions of patients achieving absolute Psoriasis Area and Severity Index (PASI) of < 5, < 3 and < 1 through week 148. (a) Responders to tildrakizumab 100 mg. (b) Responders to tildrakizumab 200 mg. (c) Partial responders or nonresponders to etanercept 50 mg who switched to tildrakizumab 200 mg. OC, observed cases; MI, multiple imputation; NRI, nonresponder imputation. n = number of cases in the OC analyses. Error bars represent 95% confidence intervals.
Partial responders to tildrakizumab 100 mg or 200 mg
The proportions of partial responders to tildrakizumab 100 mg or 200 mg achieving PASI 75, PASI 90 and PASI 100 at weeks 52 and 148 are shown in Table S3 (see Supporting Information). Again, responses were maintained during the 2‐year extension period at week 148. The proportions of partial responders to tildrakizumab 100 mg or 200 mg achieving absolute PASI < 5, < 3 and < 1 are shown in Table S4 (see Supporting Information).
Partial responders or nonresponders to etanercept 50 mg who were treated with tildrakizumab 200 mg
Table S5 (see Supporting Information) and Figure 3(c) show the proportions of patients who were partial responders or nonresponders to etanercept 50 mg who had PASI 75, PASI 90 and PASI 100 responses with tildrakizumab 200 mg at weeks 52 and 148. The responses were maintained during the 2‐year extension period at week 148. The proportions with absolute PASI < 5, < 3 and < 1 are shown in Figure 4(c) and Table S6 (see Supporting Information).
Safety results
In the 148‐week period (base study plus 2‐year extension period; total exposure to tildrakizumab 100 mg and 200 mg of 4061·2 PYs), EAIRs for treatment‐emergent AEs for tildrakizumab 100 mg, tildrakizumab 200 mg, placebo and etanercept were 35·2, 37·2, 148·6 and 148·6 events per 100 PYs, respectively (Table 2). The most common treatment‐emergent AE in all treatment groups was nasopharyngitis (10·2, 9·8, 22·4 and 41·1 events per 100 PYs for tildrakizumab 100 mg, tildrakizumab 200 mg, placebo and etanercept, respectively). Other frequent AEs (occurring at a frequency ≥ 5% in one or more treatment groups) were infections such as upper respiratory tract infection, influenza, bronchitis and sinusitis (Table 3). Candida infections were infrequent (0·10 events per 100 PYs in the tildrakizumab 100‐mg group and 0·39 events per 100 PYs in the tildrakizumab 200‐mg group).
Table 2.
148‐Week cumulative exposure‐adjusted incidence rates of adverse events (AEs) (events per 100 patient‐years of exposure)
| Tildrakizumab 100 mg | Tildrakizumab 200 mg | Placebo | Etanercept 50 mg | |
|---|---|---|---|---|
| Total patient‐years of follow‐up | 2014·49 | 2046·71 | 205·30 | 153·42 |
| TEAE | 709; 35·20 (32·55–37·84) | 761; 37·18 (34·49–39·88) | 305; 148·6 (131·6–165·6) | 228; 148·6 (128·9–168·3) |
| SAE | 118; 5·86 (4·78–6·94) | 112; 5·47 (4·44–6·51) | 13; 6·33 (2·82–9·84) | 20; 13·04 (7·21–18·87) |
| Deaths | 6; 0·30 (0·05–0·54) | 3; 0·15 (0·00–0·32) | 0; 0·00 | 0; 0·00 |
| Drug‐related AE | 229; 11·37 (9·87–12·87) | 263; 12·85 (11·27–14·43) | 73; 35·56 (27·23–43·88) | 112; 73·00 (59·21–86·80) |
| Drug‐related SAE | 16; 0·79 (0·40–1·19) | 11; 0·54 (0·21–0·86) | 2; 0·97 (0·00–2·35) | 5; 3·26 (0·34–6·17) |
| Discontinued due to AE | 34; 1·69 (1·11–2·27) | 25; 1·22 (0·73–1·71) | 4; 1·95 (0·00–3·90) | 9; 5·87 (1·96–9·78) |
| Discontinued due to SAE | 19; 0·94 (0·51–1·38) | 16; 0·78 (0·39–1·17) | 1; 0·49 (0·00–1·46) | 5; 3·26 (0·34–6·17) |
| Discontinued due to drug‐related AE | 15; 0·74 (0·36–1·13) | 7; 0·34 (0·08–0·60) | 1; 0·49 (0·00–1·46) | 4; 2·61 (0·00–5·21) |
| Discontinued due to drug‐related SAE | 7; 0·35 (0·08–0·61) | 4; 0·20 (0·00–0·39) | 0; 0·00 | 1; 0·65 (0·00–1·96) |
The data are represented as n; events per 100 patient‐years of exposure (95% confidence interval). TEAE, treatment‐emergent AE; SAE, serious AE.
Table 3.
148‐Week cumulative exposure‐adjusted incidence rates of the most common adverse events (AEs)a
| Tildrakizumab 100 mg | Tildrakizumab 200 mg | Placebo | Etanercept 50 mg | |
|---|---|---|---|---|
| Nasopharyngitis | 205; 10·18 (8·75–11·60) | 201; 9·82 (8·44–11·21) | 46; 22·41 (15·80–29·01) | 63; 41·06 (30·72–51·41) |
| Injection‐site erythema | 9; 0·45 (0·15–0·74) | 10; 0·49 (0·18–0·80) | 2; 0·97 (0·00–2·35) | 28; 18·25 (11·35–25·15) |
| Upper respiratory tract infection | 76; 3·77 (2·91–4·64) | 114; 5·57 (4·53–6·61) | 32; 15·59 (10·08–21·10) | 11; 7·17 (2·85–11·49) |
| Headache | 54; 2·68 (1·95–3·41) | 63; 3·08 (2·30–3·85) | 12; 5·85 (2·47–9·22) | 19; 12·38 (6·70–18·07) |
| Injection‐site reaction | 6; 0·30 (0·05–0·54) | 10; 0·49 (0·18–0·80) | 2; 0·97 (0·00–2·35) | 17; 11·08 (5·71–16·46) |
| Influenza | 49; 2·43 (1·74–3·13) | 65; 3·18 (2·39–3·96) | 18; 8·77 (4·63–12·90) | 5; 3·26 (0·34–6·17) |
| Urinary tract infection | 40; 1·99 (1·36–2·61) | 29; 1·42 (0·89–1·94) | 9; 4·38 (1·46–7·31) | 12; 7·82 (3·31–12·34) |
| Cough | 48; 2·38 (1·69–3·07) | 60; 2·93 (2·17–3·69) | 15; 7·31 (3·53–11·08) | 8; 5·21 (1·53–8·90) |
| Pruritus | 31; 1·54 (0·99–2·09) | 29; 1·42 (0·89–1·94) | 11; 5·36 (2·13–8·59) | 11; 7·17 (2·85–11·49) |
| Viral upper respiratory tract infection | 139; 6·90 (5·73–8·07) | 138; 6·74 (5·59–7·89) | 3; 1·46 (0·00–3·15) | 2; 1·30 (0·00–3·15) |
| Arthralgia | 68; 3·38 (2·56–4·19) | 57; 2·78 (2·05–3·52) | 9; 4·38 (1·46–7·31) | 10; 6·52 (2·40–10·64) |
| Back pain | 55; 2·73 (1·99–3·47) | 58; 2·83 (2·09–3·58) | 8; 3·90 (1·14–6·65) | 9; 5·87 (1·96–9·78) |
| Hypertension | 53; 2·63 (1·91–3·35) | 53; 2·59 (1·88–3·30) | 8; 3·90 (1·14–6·65) | 9; 5·87 (1·96–9·78) |
| Gastroenteritis | 37; 1·84 (1·23–2·44) | 42; 2·05 (1·42–2·69) | 6; 2·92 (0·54–5·31) | 8; 5·21 (1·53–8·90) |
| Diarrhoea | 51; 2·53 (1·82–3·24) | 43; 2·10 (1·46–2·74) | 4; 1·95 (0·00–3·90) | 8; 5·21 (1·53–8·90) |
| Oropharyngeal pain | 27; 1·34 (0·82–1·86) | 35; 1·71 (1·13–2·29) | 5; 2·44 (0·26–4·61) | 8; 5·21 (1·53–8·90) |
| Sinusitis | 48; 2·38 (1·69–3·07) | 37; 1·81 (1·21–2·40) | 10; 4·87 (1·79–7·95) | 4; 2·61 (0·00–5·21) |
| Bronchitis | 40; 1·99 (1·36–2·61) | 53; 2·59 (1·88–3·30) | 5; 2·44 (0·26–4·61) | 5; 3·26 (0·34–6·17) |
| Nausea | 26; 1·29 (0·78–1·80) | 28; 1·37 (0·85–1·89) | 3; 1·46 (0·00–3·15) | 3; 1·96 (0·00–4·21) |
The data are represented as n; events per 100 patient‐years of exposure (95% confidence interval). aDefined as adverse events occurring at a frequency of ≥ 5% in one or more treatment groups. Ordered by the highest exposure‐adjusted incidence rate in any of the treatment groups.
Few patients discontinued due to AEs (1·69 and 1·22 events per 100 PYs of exposure to tildrakizumab 100 mg or 200 mg), and the rates of drug‐related serious AEs per 100 PYs of exposure to either tildrakizumab 100 mg or 200 mg were 0·79 and 0·54 events per 100 PYs, respectively (Table 2). Nine deaths (six patients in the tildrakizumab 100‐mg group and three patients in the tildrakizumab 200‐mg group) occurred during the trials. Six deaths (already reported by Blauvelt et al.)7 occurred during the base study, and were due to steatohepatitis and alcoholic cardiomyopathy, acute myeloid leukaemia, respiratory arrest, myocardial infarction, aneurysm and sepsis. During the extension period of reSURFACE 2, three additional deaths were reported: (i) an intoxication by the combined effects of fluoxetine and cyclobenzaprine; (ii) an unknown reason of death; and (iii) a case of asphyxiation due to a tractor accident. All deaths were assessed by the investigators to be not related to the study medication. One case of suicide attempt was reported in the tildrakizumab 200‐mg group, which resulted in a suicide attempt rate of 0·05 events per 100 PYs.
Incidences of severe infections, malignancies, confirmed extended MACEs and hypersensitivity reactions were low and comparable across treatment groups, although rates of severe infections tended to be higher for etanercept 50 mg (Table 4). The most common severe infections were cellulitis – three patients in the tildrakizumab 100‐mg group (0·15 events per 100 PYs), four patients in the tildrakizumab 200‐mg group (0·20 events per 100 PYs), two patients in the placebo group (0·97 events per 100 PYs) and one patient in the etanercept 50‐mg group (0·65 events per 100 PYs); herpes zoster – one patient in the tildrakizumab 200‐mg group (0·05 events per 100 PYs) and one patient in the etanercept 50‐mg group (0·65 events per 100 PYs) and urosepsis – one patient in the etanercept 50‐mg group (0·65 events per 100 PYs).
Table 4.
148‐Week cumulative exposure‐adjusted incidence rates of adverse events (AEs) of special interest
| Tildrakizumab 100 mg | Tildrakizumab 200 mg | Placebo | Etanercept 50 mg | |
|---|---|---|---|---|
| Severe infectiona | 23; 1·14 (0·67–1·62) | 23; 1·12 (0·66–1·59) | 2; 0·97 (0·00–2·35) | 3; 1·96 (0·00–4·21) |
| Malignancy (excluding NMSC) | 11; 0·55 (0·22–0·88) | 8; 0·39 (0·11–0·67) | 0; 0·00 | 2; 1·30 (0·00–3·15) |
| NMSC | 10; 0·50 (0·18–0·81) | 10; 0·49 (0·18–0·80) | 2; 0·97 (0·00–2·35) | 2; 1·30 (0·00–3·15) |
| Melanoma | 1; 0·05 (0·00–0·15) | 0; 0·00 | 0; 0·00 | 0; 0·00 |
| Confirmed extended MACEb | 8; 0·40 (0·12–0·68) | 11; 0·54 (0·21–0·86) | 1; 0·49 (0·00–1·46) | 1; 0·65 (0·00–1·96) |
| Injection‐site reactionc | 39; 1·94 (1·32–2·56) | 47; 2·30 (1·63–2·97) | 11; 5·36 (2·13–8·59) | 62; 40·41 (30·15–50·68) |
| Drug‐related hypersensitivity reactiond | 6; 0·30 (0·05–0·54) | 3; 0·15 (0·00–0·32) | 1; 0·49 (0·00–1·46) | 0; 0·00 |
The data are represented as n; events per 100 patient‐years of exposure (95% confidence interval). MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; SAE, serious AE. aDefined as any infection meeting the regulatory definition of an SAE, or any infection requiring intravenous antibiotics whether reported as an SAE or not. bDefined as nonfatal myocardial infarction, nonfatal stroke, unstable angina, coronary revascularization, resuscitated cardiac arrest, and cardiovascular deaths that were confirmed as ‘cardiovascular’ or ‘sudden’. cThe following preferred terms were included: injection‐site bruising, injection‐site discomfort, injection‐site dryness, injection‐site erythema, injection‐site haematoma, injection‐site haemorrhage, injection‐site hypoaesthesia, injection‐site induration, injection‐site inflammation, injection‐site oedema, injection‐site pain, injection‐site papule, injection‐site paraesthesia, injection‐site pruritus, injection‐site reaction, injection‐site swelling and injection‐site urticaria. This information was only collected for AEs in the base study. dThe following preferred terms were included: angio‐oedema, hypersensitivity, injection‐site urticaria, lip swelling, swelling face, swollen tongue and urticaria.
The most common malignancies (excluding nonmelanoma skin cancer) were breast cancer – one patient in the tildrakizumab 100‐mg group (0·05 events per 100 PYs), one patient in the tildrakizumab 200‐mg group (0·05 events per 100 PYs) and one patient in the etanercept 50‐mg group (0·65 events per 100 PYs) and lung adenocarcinoma – one patient in the etanercept 50‐mg group (0·65 events per 100 PYs). The most common nonmelanoma skin cancer was basal cell carcinoma – six patients in the tildrakizumab 100‐mg group (0·30 events per 100 PYs), seven patients in the tildrakizumab 200‐mg group (0·34 events per 100 PYs), one patient in the placebo group (0·49 events per 100 PYs) and two patients in the etanercept 50‐mg group (1·30 events per 100 PYs). Malignant melanoma occurred in one patient in the tildrakizumab 100‐mg group (0·05 events per 100 PYs).
The most common MACEs were coronary artery disease – four patients in the tildrakizumab 200‐mg group (0·20 events per 100 PYs); acute myocardial infarction – three patients in the tildrakizumab 200‐mg group (0·15 events per 100 PYs) and one patient in the tildrakizumab 100‐mg group (0·05 events per 100 PYs) and cerebellar infarction – one patient in the placebo group (0·49 events per 100 PYs). One case of suspected new‐onset Crohn disease occurred in the tildrakizumab 100‐mg group, which resulted in a rate of Crohn disease of 0·05 events per 100 PYs.
Discussion
The treatment of moderate‐to‐severe plaque psoriasis has rapidly progressed in the last 20 years, and there are now many effective therapies. Numerous factors, including frequency of injection and presence of comorbidities, affect selection of therapies.8, 9 For many patients, IL‐23p19 inhibitors have demonstrated high levels of efficacy and safety, effectively confirming a key regulatory role of IL‐23 in disease pathogenesis.10, 11 As a chronic disease, psoriasis requires long‐term treatment. Thus, data on long‐term maintenance of clinical responses and long‐term safety are needed for all treatment options, especially new biologic agents. The pooled analyses from the reSURFACE 1 and reSURFACE 2 trials presented here demonstrate long‐term efficacy and safety data for tildrakizumab 100 mg and 200 mg from over 1500 patients with moderate‐to‐severe psoriasis treated for up to 3 years, with approximately 4000 PYs of follow‐up. While tildrakizumab 100 mg is the recommended dose in major regions of the world, the European label accepts the use of 200 mg in certain conditions (e.g. high disease burden, bodyweight ≥ 90 kg).
Overall, these analyses indicate that most patients who initially respond to tildrakizumab (achieving PASI 75 after 28 weeks) and who continue treatment with tildrakizumab maintain this efficacy over time. PASI 75 responses were well maintained with continued tildrakizumab 100 mg or 200 mg in 8 out of every 10 patients through 148 weeks of treatment (NRI). PASI 90 and PASI 100 responses were also stable throughout the analysis period, and approximately 60% of patients had PASI 90 responses at week 148. Looking at absolute PASI scores, about 70% and 50% of patients, respectively, maintained PASI scores of < 3 and < 1 at week 148. In this regard, looking at absolute PASI may provide additional clinical value to PASI response in clinical practice.
In addition, these data show that tildrakizumab has increasing efficacy over time in certain subgroups of patients. For example, a substantial proportion of patients with initial partial responses achieved good levels of response if treatment was maintained. Continuing treatment with tildrakizumab in partial responders beyond week 28 led to PASI 75 responses in approximately 60% of patients at week 52. Also, PASI 90 responses were achieved in approximately one‐third of patients who were partial responders at week 28. Notably, clinical responses were maintained through 148 weeks. Therefore, continuing tildrakizumab should be considered in patients who have shown partial responses after the first 28 weeks of therapy.
In partial responders or nonresponders to etanercept 50 mg at week 28, switching to tildrakizumab 200 mg resulted in PASI 75 responses in approximately 8 out of every 10 patients and PASI 90 responses in 4 out of every 10 patients after only two doses. Additionally, absolute PASI scores < 3 and < 1 were achieved by approximately 60% and 25% of these patients at week 52. These responses were maintained at week 148. Thus, tildrakizumab may be a good alternative for those patients with moderate‐to‐severe plaque psoriasis who have not responded well to etanercept.
These results are in line with published data on other IL‐23p19 inhibitors such as guselkumab, which is also approved for the treatment of moderate‐to‐severe plaque psoriasis. Results from the phase III VOYAGE 1 trial showed that clinical responses in guselkumab‐treated patients were maintained through 2 years.12 Other IL‐23p19 subunit‐specific antibodies approved or under development are risankizumab and mirikizumab; however, no data beyond week 52 or week 16, respectively, have been published for these drugs to date.13, 14
The combination of durable efficacy and favourable safety, together with infrequent dosing (once every 8–12 weeks), characterize IL‐23p19 inhibitors. Regarding safety, no new or unexpected AEs have been described with these drugs over time.7, 13, 14, 15 Here, we have specifically demonstrated low rates of severe infections, malignancies and MACEs for treatment with tildrakizumab 100 mg and 200 mg over a 148‐week period. EAIRs of severe infections, malignancies, nonmelanoma skin cancer, melanoma skin cancer and extended MACEs with tildrakizumab were low and comparable with placebo, indicating no increased risk of these events with tildrakizumab treatment. Also, the frequency of Candida infections was very low. Only one event of inflammatory bowel disease was reported (0·05 events of Crohn disease per 100 PYs), even though an increased risk of incident Crohn disease among patients with psoriasis has been reported (relative risk of 4).16 Aside from these prespecified AEs, no new or unexpected AEs were reported with tildrakizumab, and rates of discontinuations due to AEs were very low. Although these data are very promising, patients with moderate‐to‐severe psoriasis may need treatment for years or even decades. Thus, longer‐term safety data are needed for tildrakizumab and the other IL‐23p19 inhibitors. Of note, the reSURFACE 1 and reSURFACE 2 trials are both 5‐year trials, with long‐term results to be published upon their completion.
Our analyses had several limitations. Nonresponders to tildrakizumab 100 mg or 200 mg discontinued at week 28 and were not included in this report. However, more conservative NRI data from week 28 onwards are presented as a primary analysis. Etanercept was the standard of care when the trials were designed and was chosen as a comparator in the reSURFACE 2 clinical trial. Patients responding to etanercept were withdrawn from the study at week 28 as per the study design; therefore, no active comparator is available beyond that time point. Finally, patients in the extension period were provided open‐label study medication.
In conclusion, in patients who initially responded to either tildrakizumab 100 mg or 200 mg, efficacy with continued treatment was maintained over time up to 148 weeks. Among partial responders or nonresponders to etanercept, tildrakizumab demonstrated high levels of efficacy after a few doses, and efficacy was also maintained over time. Lastly, the safety profile of tildrakizumab was favourable over 3 years, with low rates of severe infections, malignancies and MACEs.
Supporting information
Table S1 Responders to tildrakizumab 100 mg or 200 mg: changes in Psoriasis Area and Severity Index.
Table S2 Responders to tildrakizumab 100 mg or 200 mg: absolute Psoriasis Area and Severity Index.
Table S3 Partial responders to tildrakizumab 100 mg or 200 mg: changes in Psoriasis Area and Severity Index.
Table S4 Partial responders to tildrakizumab 100 mg or 200 mg: absolute Psoriasis Area and Severity Index.
Table S5 Partial responders or nonresponders to etanercept 50 mg who were treated with tildrakizumab 200 mg: changes in Psoriasis Area and Severity Index.
Table S6 Partial responders or nonresponders to etanercept 50 mg who were treated with tildrakizumab 200 mg: absolute Psoriasis Area and Severity Index.
Video S1 Author video.
Powerpoint S1 Journal Club Slide Set.
Acknowledgments
The authors would like to acknowledge Eva Mateu, PhD of TFS S.L. for editorial assistance and writing support, and Andreu Schoenenberger of Almirall S.A. for statistical support.
Conflicts of interest: K.R. has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Forward Pharma, Fresenius Medical Care, GlaxoSmithKline, Janssen‐Cilag, Kyowa Hakko Kirin, LEO Pharma, Lilly, Medac, Merck Sharp & Dohme, Miltenyi Biotec, Novartis, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Takeda, UCB, Valeant and Xenoport. R.B.W. has received grants and/or honoraria from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Janssen, Lilly, LEO Pharma, Novartis, Pfizer, Sanofi, UCB Pharma and Xenoport. L.I. has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Janssen‐Cilag, Kyowa, LEO Pharma, MSD, Novartis, Pfizer and UCB. L.P. has received grants or research support from or participated in clinical trials (paid to the institution) for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, Roche, Sanofi and UCB; has received honoraria or consultation fees from AbbVie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Gebro, Janssen, LEO Pharma, Lilly, Merck‐Serono, Merck Sharp & Dohme, Mylan, Novartis, Pfizer, Regeneron, Roche, Samsung Bioepis, Sandoz, Sanofi and UCB; and has participated in company‐sponsored speakers’ bureaus for Celgene, Janssen, Lilly, Merck Sharp & Dohme, Novartis and Pfizer. A.I. has received honoraria for serving on advisory boards from Maruho, Janssen, AbbVie, Novartis, Eli Lilly Japan, Celgene and Sun Pharma Japan. M.O. has received honoraria for serving on advisory boards from Maruho, Janssen, AbbVie, Novartis, Eli Lilly Japan, Celgene, Eisai, Kyowa Hakko Kirin, LEO Pharma, Pfizer Japan, Mitsubishi Tanabe Pharma, Boehringer Ingelheim, Bristol‐Myers Squibb Company and Sun Pharma Japan. I.P.‐C. and M.F. are employees of Almirall. M.H. has served as a statistical consultant for Almirall. S.R. is an employee of Sun Pharmaceuticals. M.G.L. has received research funds (paid to the institution) from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Incyte, Janssen/Johnson & Johnson, Kadmon, LEO Pharma, Medimmune/AstraZeneca, Novartis, Ortho‐dermatologics, Pfizer, Sciderm, UCB5 and Vidac; and is a consultant for Allergan, Aqua, Arcutis, Boehringer Ingelheim, Bristol‐Myers Squibb, LEO Pharma, Menlo, Mitsubishi Pharma/Neuroderm LTD, Promius/Dr Reddy, Regeneron, Theravance Biopharma and Verrica. W.C. has received honoraria or fees for serving on advisory boards or as a speaker from Sun Pharmaceuticals, Lilly, Novartis and Janssen. A.B. has served as a scientific adviser and/or clinical study investigator for AbbVie, Aclaris, Akros, Allergan, Almirall, Amgen, Arena, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermavant, Dermira, Inc., Eli Lilly and Company, FLX Bio, Galderma, Genentech/Roche, GlaxoSmithKline, Janssen, LEO, Meiji, Merck Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Revance, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB Pharma, Valeant and Vidac; and as a paid speaker for Janssen, Regeneron and Sanofi Genzyme. D.T. has received honoraria or fees for serving on advisory boards, or as a speaker or consultant, from AbbVie, Almirall, Bioskin, Boehringer Ingelheim, Celgene, Dignity, Galapagos, GlaxoSmithKline, Janssen, LEO Pharma, Lilly, Medac, Merck Sharp & Dohme, Morphosys, Novartis, Pfizer, Regeneron, Samsung, Sandoz‐Hexal, Sanofi, Sun Pharmaceuticals and UCB; and has received grants from Celgene and Novartis.
Funding sources This publication was funded by Almirall R&D, Barcelona, Spain.
Conflicts of interest Conflicts of interest statements can be found in the Appendix.
https://doi.org/10.1111/bjd.18831 available online
References
- 1. Reich K, Armstrong AW, Foley P et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76:418–31. [DOI] [PubMed] [Google Scholar]
- 2. Blauvelt A, Papp KA, Griffiths CEM et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Am Acad Dermatol 2017; 76:405–17. [DOI] [PubMed] [Google Scholar]
- 3. Papp KA, Blauvelt A, Bukhalo M et al Risankizumab versus ustekinumab for moderate‐to‐severe plaque psoriasis. N Engl J Med 2017; 376:1551–60. [DOI] [PubMed] [Google Scholar]
- 4. Papp K, Thaçi D, Reich K et al Tildrakizumab (MK‐3222), an anti‐interleukin‐23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo‐controlled trial. Br J Dermatol 2015; 173:930–9. [DOI] [PubMed] [Google Scholar]
- 5. Reich K, Papp KA, Blauvelt A et al Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017; 390:276–88. [DOI] [PubMed] [Google Scholar]
- 6. Papp KA, Reich K, Blauvelt A et al Efficacy of tildrakizumab for moderate‐to‐severe plaque psoriasis: pooled analysis of three randomized controlled trials at weeks 12 and 28. J Eur Acad Dermatol Venereol 2019; 33:1098–106. [DOI] [PubMed] [Google Scholar]
- 7. Blauvelt A, Reich K, Papp KA et al Safety of tildrakizumab for moderate‐to‐severe plaque psoriasis: pooled analysis of three randomized controlled trials. Br J Dermatol 2018; 179:615–22. [DOI] [PubMed] [Google Scholar]
- 8. Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient. Focus on special populations and chronic infections. J Am Acad Dermatol 2019; 80:43–53. [DOI] [PubMed] [Google Scholar]
- 9. Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient. Psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol 2019; 80:27–40. [DOI] [PubMed] [Google Scholar]
- 10. Ibler E, Gordon KB. IL‐23 inhibitors for moderate‐to‐severe psoriasis. Semin Cutan Med Surg 2018; 37:158–62. [DOI] [PubMed] [Google Scholar]
- 11. Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol 2018; 45:264–72. [DOI] [PubMed] [Google Scholar]
- 12. Griffiths CEM, Papp KA, Kimball AB et al Long‐term efficacy of guselkumab for the treatment of moderate‐to‐severe psoriasis: results from the phase 3 VOYAGE 1 trial through two years. J Drugs Dermatol 2018; 17:826–32. [PubMed] [Google Scholar]
- 13. Gordon KB, Strober B, Lebwohl M et al Efficacy and safety of risankizumab in moderate‐to‐severe plaque psoriasis (UltIMMa‐1 and UltIMMa‐2): results from two double‐blind, randomised, placebo‐controlled and ustekinumab‐controlled phase 3 trials. Lancet 2018; 392:650–61. [DOI] [PubMed] [Google Scholar]
- 14. Reich K, Rich P, Maari C et al Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate‐to‐severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol 2019; 181:88–95. [DOI] [PubMed] [Google Scholar]
- 15. Galluzzo M, D'Adamio S, Campione E et al A safety evaluation of guselkumab for the treatment of psoriasis. Expert Opin Drug Saf 2018; 17:741–51. [DOI] [PubMed] [Google Scholar]
- 16. Li W‐Q, Han J‐L, Chan AT, Qureshi AA. Psoriasis, psoriatic arthritis and increased risk of incident Crohn's disease in US women. Ann Rheum Dis 2013; 72:1200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Responders to tildrakizumab 100 mg or 200 mg: changes in Psoriasis Area and Severity Index.
Table S2 Responders to tildrakizumab 100 mg or 200 mg: absolute Psoriasis Area and Severity Index.
Table S3 Partial responders to tildrakizumab 100 mg or 200 mg: changes in Psoriasis Area and Severity Index.
Table S4 Partial responders to tildrakizumab 100 mg or 200 mg: absolute Psoriasis Area and Severity Index.
Table S5 Partial responders or nonresponders to etanercept 50 mg who were treated with tildrakizumab 200 mg: changes in Psoriasis Area and Severity Index.
Table S6 Partial responders or nonresponders to etanercept 50 mg who were treated with tildrakizumab 200 mg: absolute Psoriasis Area and Severity Index.
Video S1 Author video.
Powerpoint S1 Journal Club Slide Set.


