Abstract
Background
Assessment of treatment effects in clinical trials requires valid information on treatment adherence, adverse events and symptoms. Paper‐based diaries are often inconvenient and have limited reliability, particularly for outpatient trials.
Objectives
To investigate the utility of an electronic diary (e‐diary) application for patients with skin diseases in outpatient clinical trials.
Methods
An e‐diary application was developed and technically validated. Treatment adherence was defined as topical administration by the patient, and patient‐reported outcomes, i.e. pain and itch, were evaluated by the e‐diary in six clinical trials on newly tested topical drugs. Additionally, the proportion of patients capturing the applied topical drug by camera and filling in the pain and itch scores was defined as e‐diary adherence, and patients’ perception of usefulness and acceptability of the e‐diary were evaluated.
Results
Treatment adherence rates of the included 256 patients were high (median 98%, range 97–99%). E‐diary adherence was also high with a median of 93% (range 87–97%) for capturing the applied drug by camera, and 89% (range 87–96%) and 94% (range 87–96%) for entering respectively the itch and pain score. Daily symptom scores provided good insights into the disease burden, and patients rated the e‐diary as good to excellent with respect to user acceptability.
Conclusions
The results suggest that the e‐diary is an excellent way to ensure proper treatment administration, indicated by both the high user acceptability scores and high treatment adherence. Moreover, the e‐diary may also be valuable for frequent and reliable monitoring of patient‐reported outcomes in daily clinical practice.
Introduction
Treatment adherence is the degree to which patients take their medications as prescribed or as instructed by their treating physician1 and is defined as taking ≥80% of the prescribed medicines.2, 3, 4, 5, 6, 7 It is known that adherence to long‐term therapy for chronic illnesses in developed countries is only approximately 50%5 and adherence to topical treatments is even poorer than oral treatments.8 To estimate the clinical efficacy of drugs and to examine new drugs in clinical trials, treatment adherence is of main importance. Safety, pharmacodynamics and efficacy can only be adequately assessed and interpreted if patient data on treatment adherence are available. The impact of poor adherence varies across numerous chronic skin disorders.9, 10 For instance, non‐adherence to topical regimens leads to increased scores on the six area, six sign atopic dermatitis (SASSAD) severity scale, indicating the disease severity in patients with atopic dermatitis.11 For this reason, increasing adherence may even have a larger impact on patient‐reported outcomes than the improvement of the treatment itself.5
Whereas good insight in the treatment adherence and symptoms of the patient is essential, patient‐reported outcome measures are often recorded during visits and by use of paper diaries. This requires a good memory of the patient and depends on translation by the doctor/researcher, which can both lead to erroneous interpretation and over‐ or under‐reporting of medication use or symptoms. Paper diaries have a high recall bias,12 a low‐to‐moderate adherence rate and a limited reliability and are therefore considered as inappropriate to reliably measure treatment adherence.13, 14, 15, 16 Advancements in technology have enabled the widespread use of electronic diaries (e‐diaries) for both the monitoring of patient outcomes and the improvement of treatment adherence in clinical trials.13, 17 In 2018, Svendsen et al. performed a randomized, controlled trial with a smartphone application for currently used topical treatment in patients with psoriasis and showed an improved short‐term treatment adherence of 27% more adherence than the non‐intervention group.3
The purpose of this study was to investigate the utility of an e‐diary in 256 patients with various skin diseases participating in six clinical trials. In this study, treatment adherence and patient‐reported outcomes were measured by an e‐diary in six clinical trials on newly investigated topical drugs. Additionally, patient perception of usefulness and acceptability of the e‐diary were evaluated.
Materials and methods
Subjects and design
From December 2014 to March 2018, six randomized, double‐blind, placebo‐controlled clinical trials were performed including various skin diseases. Two different topical formulations were examined in cutaneous warts (CW), atopic dermatitis (AD), genital warts (GW) and vulvar high‐grade squamous intraepithelial lesions (HSILs). The Declaration of Helsinki was the guiding principle for trial execution, and all subjects gave informed consent before any procedure. The studies were approved by the Dutch Medical Ethics Committee (‘Stichting Beoordeling Ethiek Biomedisch Onderzoek’, Assen, the Netherlands). The clinical efficacy and safety results of these studies have been or will be reported elsewhere.18, 19, 20, 21
E‐diary application
An iOS application was developed using Xcode 7 and Objective‐C according to predefined User Requirement Specifications and subsequently technically validated using pertaining guidelines (see Figure S1). The application was installed on an iPod Touch or iPhone. The patients received oral, paper and digital (in the e‐diary) instructions regarding the use of the e‐diary. The subjects were instructed to take pictures of the amount of the topical drug applied using the integrated camera. A maximum of four scheduled e‐diary notifications were repeated every 30 minutes until the picture was taken. Subjects were instructed to apply the drug daily and to directly answer questions about patient‐reported outcomes. Data were saved and securely transferred to the on‐site server using encryption the following day.
Treatment adherence
Treatment adherence (i.e. actual administrations divided by expected administrations) was measured by evaluating whether a patient had applied the topical drug, based on the presence of a picture in the e‐diary or if absent (i.e. when, for instance, a technical issue occurred) after consultation of the patient. Expected entries were based on the number of patients and treatment days and calculated with the formula: number of patients times the amount of entries per day times treatment period in days.
E‐diary adherence
E‐diary adherence was positive if the e‐diary was used as intended, i.e. a picture and symptom scores were entered in the e‐diary for one specific day. E‐diary adherence was expressed as a percentage and was measured by dividing the total number of actual entries (present pictures and/or NRS scores) by expected entries in the entire treatment period as defined per protocol.
Patient‐reported outcomes
Severity ratings of the disease or treatment‐related symptoms pain and itch were assessed daily by a numeric rating scale (NRS) in the e‐diary. The NRS was selected to assess pain and itch intensity once daily on a scale from 0 to 100 (0: no pain/itch and 100: worst pain/itch possible), if applicable, see Table 1. The symptom assessments were used to visualize the course of symptoms during the diseases, and only patients who received placebo treatment were included in these analyses.
Table 1.
Clinical characteristics of patients participating in the six clinical trials
| Trial number | 1 | 2 | 3 | 4 | 5 | 6: |
|---|---|---|---|---|---|---|
| Trial ID | NCT02333643 | NCT02456480 | NCT03091426 | NCT02849262 | NCT03334240 | NCT02596074 |
| Disease | Cutaneous warts | Atopic dermatitis | Atopic dermatitis | Genital warts | Genital warts | Vulvar HSIL |
| N | 80 | 36 | 80 | 24 | 24 | 12 |
| Age (SD) | 25.8 (10.6) | 24.9 (7.8) | 24.4 (6.5) | 34.4 (11.6) | 30.8 (10.6) | 49.8 (11.0) |
| Female | 49 (61%) | 27 (75%) | 44 (55%) | 9 (38%) | 5 (20.8%) | 12 (100%) |
| Male | 31 (39%) | 9 (25%) | 36 (45%) | 15 (63%) | 19 (79.2%) | 0 (0%) |
| Treatment | ICVT | Omiganan | Omiganan | Omiganan | ICVT | Omiganan |
| Dose strength | Digoxin + furosemide, digoxin, furosemide | 1%, 2.5% | 1%, 1.75%, 2.5% | 2.5% | Digoxin + furosemide | 2.5% |
| Active: placebo | 1:1:1:1 | 1:1:1 | 1:1:1:1 | 2:1 | 3:1 | 2:1 |
| Treatment period (weeks) | 6 | 4 | 4 | 12 | 6 | 12 |
| Regimen treatment | Once daily | Once daily | Twice daily | Once daily | Once daily | Once daily |
| NRS pain | – | – | – | Once daily | Once daily | Once daily |
| NRS itch | – | Twice daily | Twice daily | Once daily | Once daily | Once daily |
Age is shown as mean in years. Sex is described as number of patients. Treatment period is described in weeks. The e‐diary was filled in during the entire treatment period.
HSIL, high‐grade squamous intraepithelial lesion; ICVT, ionic contra‐viral therapy; NRS, numeric rating scale.
User acceptability of the e‐diary
At the end of the treatment period, all patients were asked to fill out a 14‐item questionnaire (in Dutch) regarding their experience using the e‐diary (Supporting Information, questionnaire translated to English). The questionnaire consisted of multiple‐choice questions and Likert‐type scales regarding general user experience, technical aspects of the e‐diary and adherence. Two open‐ended questions allowed patients to report the strengths and weaknesses of the e‐diary and to fill in any comments or suggestions.
Data analysis
Descriptive analyses and visualization were performed using IBM SPSS (version 23, IBM Corporation, Armonk, New York, USA) and GraphPad Prism (version 6.05 for Windows, GraphPad Software, La Jolla, California, USA). Adherence was described in percentage and as the median percentage for all studies together.
Results
Patient characteristics
The use of the e‐diary was evaluated in 256 patients in all treatment arms, including placebo (Table 1). The patient population in this study was the sum of patients enrolled and analysed in the six trials, as there were no patients lost to follow‐up. Patients included in the trials received financial incentives.
Treatment and e‐diary adherence
The overall median treatment adherence, i.e. the proportion of patients applying the topical drug, was 98% (Table 2). This was very consistent in the different trials indicated by a narrow range of mean adherence of 97–99%. The median e‐diary adherence, i.e. the proportion of patients capturing the applied topical drug by camera, was 93% (range 87–97%), see Table 3. The main reasons for not filling in the e‐diary were either technical (empty device battery, no possibility of data entry after midnight) or patients forgot to take the photograph before application of the topical drug. The mean overall adherence of filling in the NRS for itch and pain was 90% for all trials together, see Table 4.
Table 2.
Treatment adherence
| Trial | Expected adminsa | Actual adminsb | Overall treatment adherencec | Number of subjects with ≥80% treatment adherence |
|---|---|---|---|---|
| 1 (CW) | 3280 | 3187 | 97% | 79/80 (99%) |
| 2 (AD) | 1013 | 993 | 98% | 35/36 (97%) |
| 3 (AD) | 4318 | 4233 | 98% | 79/80 (99%) |
| 4 (GW) | 1960 | 1942 | 99% | 24/24 (100%) |
| 5 (GW) | 1008 | 998 | 99% | 24/24 (100%) |
| 6 (vulvar HSIL) | 1020 | 1009 | 99% | 12/12 (100%) |
| Overall mean | 12599 | 12360 | 98% | 253/256 (99%) |
| Median (range) | 98% (97–99%) | 100% (97–100%) |
Expected administrations of study drugs based on number of patients and treatment days (number of patients x treatment period in days).
Actual administrations based on photographs imported via the e‐diary and recall of administration asked via mail or phone.
Treatment adherence is the percentage of actual admins divided by the expected admins.
AD, atopic dermatitis; CW, cutaneous warts; GW, genital warts; HSIL, high‐grade squamous intraepithelial lesion.
Table 3.
E‐diary adherence
| Trial | Expected entriesa | Actual entriesb | e‐diary adherencec | Number of subjects with ≥80% e‐diary adherence |
|---|---|---|---|---|
| 1 (CW) | 3280 | 3187 | 97% | 79/80 (99%) |
| 2 (AD) | 1013 | 963 | 95% | 35/36 (97%) |
| 3 (AD) | 4318 | 3958 | 92% | 72/80 (90%) |
| 4 (GW) | 1960 | 1710 | 87% | 17/24 (71%) |
| 5 (GW) | 1008 | 963 | 96% | 23/24 (96%) |
| 6 (vulvar HSIL) | 1020 | 907 | 89% | 11/12 (92%) |
| Overall mean | 12599 | 11695 | 93% | 237/256 (93%) |
| Median (range) | 93% (87–97%) | 94% (71–98%) |
Expected entries of images in e‐diary based on number of patients and treatment days (number of patients x treatment period in days).
Actual entries are the imported images of topical drug amount.
e‐diary treatment adherence is the percentage of actual entries divided by the expected entries.
AD, atopic dermatitis; CW, cutaneous warts; GW, genital warts; HSIL, high‐grade squamous intraepithelial lesion.
Table 4.
Adherence of NRS of itch and pain
| Trial | Itch | Pain | ||||
|---|---|---|---|---|---|---|
| Expected entriesa | Actual entriesb | NRS adherencec | Expected entriesa | Actual entriesb | NRS adherencec | |
| 2 (AD) | 3192 | 2845 | 89% | N.A. | N.A. | N.A. |
| 3 (AD) | 4480 | 3909 | 87% | N.A. | N.A. | N.A. |
| 4 (GW) | 2016 | 1759 | 87% | 2016 | 1760 | 87% |
| 5 (GW) | 999 | 962 | 96% | 999 | 962 | 96% |
| 6 (vulvar HSIL) | 1020 | 957 | 94% | 1020 | 957 | 94% |
| All studies | 11707 | 10432 | 89% | 4035 | 3679 | 91% |
| Median (range) | 2016 | 1759 | 89% (87–96%) | 1020 | 962 | 94% (87–96%) |
Expected entries pain/itch scores based on patients and treatment days (number of patients x treatment period in days).
Actual pain/itch scores entered in the e‐diary.
NRS pain/itch adherence is the percentage of actual entries divided by the expected entries.
AD, atopic dermatitis; CW, cutaneous warts; GW, genital warts; HSIL, high‐grade squamous intraepithelial lesion; N.A. , not applicable.
In patients with atopic dermatitis, itch was assessed twice daily.
Patient‐reported outcomes
Patients with AD experienced more severe itch with a higher inter‐patient variability compared to patients with GW and vulvar HSIL (Fig. 1a). The inter‐patient variability of pain in the GW and vulvar HSIL trials was also minimal, and most patients (10/14 and 2/4, respectively) experienced no pain (Fig. 1b). When examining the intra‐patient variability of itch in the AD patients, there was an extensive variability in itch scores in course of disease during the 4 weeks but also between the morning and evening scores (data not shown). There was a minimal intra‐patient variability of pain and itch in the GW and vulvar HSIL trials (data not shown).
Figure 1.
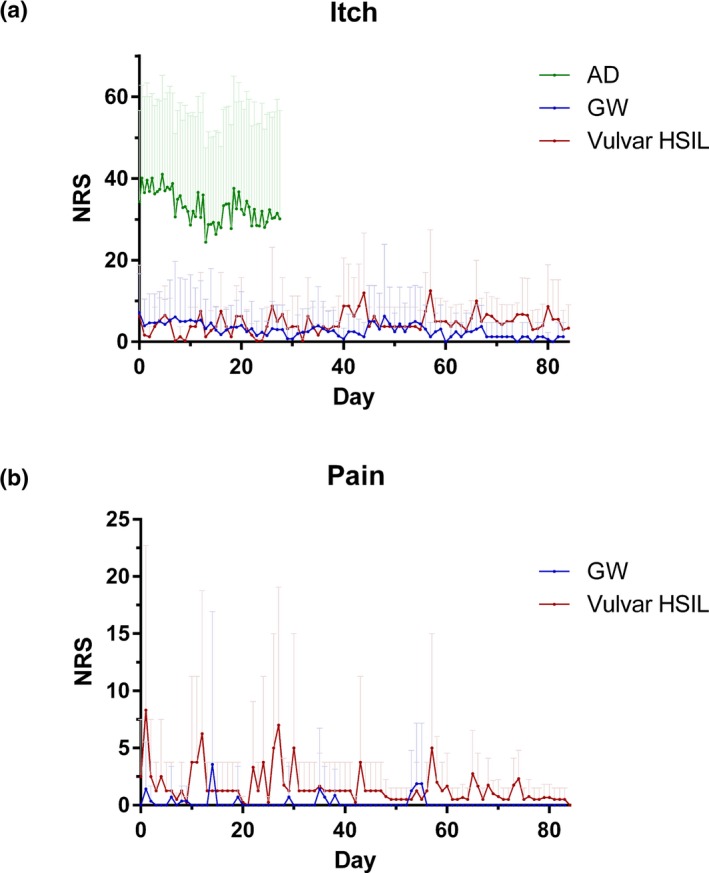
Symptoms itch (a) and pain (b) over time as monitored with the e‐diary of patients in the placebo group. The symptoms itch and pain are monitored by using a numerical rating scale (NRS) from 0 to 100 (0 no pain/itch and 100 worst pain/itch). Per study day, the mean itch of all subjects is shown +SD. AD, atopic dermatitis (N = 32), GW, genital warts (N = 14), HSIL, high‐grade squamous intraepithelial lesion (N = 4).
User acceptability of the e‐diary
A total of 249 (97%) patients completed the evaluation form (Table 5). In general, the e‐diary was rated good to excellent by 89% of the patients and the user‐friendliness was experienced as being good to excellent by 94% of the patients. Most patients (84%) reported that it took less than 5 minutes per day to use the e‐diary. Of all patients, 67% never experienced any error and 23% of the patients reported a technical problem once or twice, i.e. empty device battery. In the open‐ended questions regarding the strengths and weaknesses of the e‐diary, most patients commented that they found the e‐diary user‐friendly mainly because of its simplicity. Some patients experienced problems with filling in the e‐diary before midnight and also suggested to consider developing the e‐diary also for android‐based operating systems.
Table 5.
Evaluation of e‐diary
| General user experience | N | % | |
|---|---|---|---|
| How user‐friendly was the app? | Excellent | 108 | 43% |
| Good | 126 | 51% | |
| Average | 11 | 4% | |
| Fair | 2 | 1% | |
| Poor | 2 | 1% | |
| In general, how would you rate the app? | Excellent | 63 | 25% |
| Good | 159 | 64% | |
| Average | 20 | 8% | |
| Fair | 5 | 2% | |
| Poor | 1 | 0% | |
| How much time did it take to use the app each day? | 1–5 min | 209 | 84% |
| 5–10 min | 37 | 15% | |
| 10–15 min | 2 | 1% | |
| 15–20 min | 0 | 0% | |
| >20 min | 1 | 0% | |
| How were the instructions given? | Excellent | 130 | 52% |
| Good | 110 | 44% | |
| Average | 9 | 4% | |
| Fair | 0 | 0% | |
| Poor | 0 | 0% | |
| Technical aspects | N | % | |
|---|---|---|---|
| How often did technical problems occur (iPod, App or Camera)? | Never | 165 | 67% |
| 1–2 times | 57 | 23% | |
| 3–4 times | 12 | 5% | |
| 5–10 times | 9 | 4% | |
| >10 times | 5 | 1% | |
| How would you rate the photo function of the app? | Excellent | 67 | 27% |
| Good | 117 | 47% | |
| Average | 53 | 21% | |
| Fair | 8 | 3% | |
| Poor | 2 | 1% | |
| How would you rate the reminder function on the app? | Excellent | 46 | 19% |
| Good | 80 | 33% | |
| Average | 79 | 32% | |
| Fair | 39 | 16% | |
| Poor | 2 | 1% | |
| Did the reminder function support you to apply the gel on time? | Definitely | 105 | 43% |
| Maybe | 52 | 21% | |
| No | 90 | 36% | |
| Adherence | N | % | |
|---|---|---|---|
| If you would have used a paper diary, what would your compliance have been? With a paper diary I would have forgotten to apply the gel | More often | 74 | 30% |
| Occasionally | 59 | 24% | |
| Similarly | 74 | 30% | |
| Less often | 1 | 0% | |
| Never | 5 | 2% | |
| I do not know | 33 | 13% | |
| How do you estimate the burden of using the app compared to a paper diary? The app is | Much less work | 146 | 59% |
| Less work | 56 | 23% | |
| Similar work | 15 | 6% | |
| More work | 8 | 3% | |
| Much more work | 8 | 3% | |
| I do not know | 13 | 5% | |
| What do you prefer to use for subsequent studies? | E‐diary | 229 | 93% |
| Paper diary | 4 | 2% | |
| I do not know | 13 | 5% | |
N, sum of all patients of all studies.
Discussion
This study is the first to show that a mobile e‐diary application enhances the monitoring of patient‐reported outcomes and is associated with a high treatment adherence in patients with skin disorders in an outpatient clinical trial setting. Overall, patients appreciated the e‐diary and reported that the application was easy to use.
The observed treatment adherence in the current study was high compared to previously reported low adherence rates for topical treatment; i.e., up to 80% of psoriasis patients are classified as non‐adherent, and also, adherence in atopic dermatitis patients is very poor.5, 8, 22 However, before we draw convincing conclusions, there are a number of considerations that should be taken into account. At first, patients might have felt more responsible or obliged to be adherent due to a combination of our reminder strategy (i.e. patients received a second reminder when they did not correctly fill in the e‐diary) and the financial incentive received. Second, we did not take the efficacy or tolerability of the drug into account, which could have influenced the adherence rate.
An additional limitation of our study is the lack of a head‐to‐head comparison with a paper diary. However, previous studies have already shown that paper diaries yield a much lower adherence; for instance, Stone et al. found that the actual adherence of filling in pain scores with a paper diary was only 11%, while adherence with an e‐diary was as high as 94%.13
When interpreting the treatment adherence rates, it is important to additionally consider the trial protocol guidelines and their relation with real‐world clinical practice. The e‐diary adherence in trial 4 (GW) was lowest with 87%, as patients experienced problems when applying the topical drug on a specific calendar day. As demanded by the study protocol of a well‐controlled trial, the time window for application was set at midnight, which was unfeasible for some patients. Therefore, the time window in the study protocol in trial 5 (GW) was extended, which resulted in an improvement of e‐diary adherence from 87 to 96%. The e‐diary adherence in the trial involving patients with vulvar HSIL was marginally lower (89%) than in other trials, mainly caused by one subject who showed a very low treatment adherence of 30% due to not understanding the e‐diary and device. It should be noted that the higher age of this population and lack of experience with mobile applications might have been a limiting factor. This is a clear indication that mobile apps do not provide a one‐fits‐all solution but that the use of an application needs to be carefully considered per specific age group and additional training may be required.
Altogether, we believe that our results indicate that this mobile e‐diary platform can be used for the assessment of safety, efficacy and patient‐reported outcomes in clinical trials in the future. We hypothesize that the reminder function of the e‐diary does improve treatment adherence of patients in the six trials and can be applied to prevent under‐ and overdosing of topical treatments, as previously published results indicate that 67–95% of the patients using topical treatments underdose their medication.23, 24 The e‐diary will also enable the monitoring of disease‐specific patient‐reported outcomes and adverse events, and this will support the clinician in daily clinical practice. In research settings, remote visits and monitoring could enhance recruitment and lower the burden for participants.25 Despite the promising features of the e‐diary platform, mobile apps generally do not provide a one‐fits‐all solution. We should take notion of the age of future user groups, as our results also demonstrated that older patients experienced difficulties while using the application. Additional training may be required.
In conclusion, this study shows that a mobile e‐diary application can be used to remotely monitor patient outcomes and treatment adherence in clinical trials with various skin disorders. Therefore, its use for personalized monitoring in the outpatient setting should be further explored. Further development of e‐diaries may improve the collection of real‐life patient‐reported outcomes and treatment adherence, which may also lead to the improvement of disease outcomes in clinical practice.
Supporting information
Figure S1. Screenshots of the e‐diary (English translation from Dutch original).
Acknowledgements
The authors would like to thank all patients for their participation in the clinical trials and Karen Broekhuizen, PhD, for her careful revision of the manuscript.
Conflict of interest
The authors have declared that no conflict of interest exists.
Funding source
The six clinical trials were funded by Cutanea Life Science, Wayne, Pennsylvania, USA.
References
- 1. Vrijens B, De Geest S, Hughes DA et al A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012; 73: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]
- 3. Svendsen MT, Andersen F, Andersen KH et al A smartphone application supporting patients with psoriasis improves adherence to topical treatment: a randomized controlled trial. Br J Dermatol 2018; 179: 1062–1071. [DOI] [PubMed] [Google Scholar]
- 4. Nieuwlaat R, Wilczynski N, Navarro T et al Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014; 11: CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabaté E (Ed.). Adherence to long‐term therapies: Evidence for action. World Health Organization, Geneva, Switzerland, 2003. [Google Scholar]
- 6. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc 2011; 86: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sackett DL, Haynes RB, Gibson ES et al Randomised clinical trial of strategies for improving medication compliance in primary hypertension. Lancet 1975; 1: 1205–1207. [DOI] [PubMed] [Google Scholar]
- 8. Furue M, Onozuka D, Takeuchi S et al Poor adherence to oral and topical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale‐8. Br J Dermatol 2015; 172: 272–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murage MJ, Tongbram V, Feldman SR et al Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence 2018; 12: 1483–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Devaux S, Castela A, Archier E et al Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol 2012; 26(Suppl 3): 61–67. [DOI] [PubMed] [Google Scholar]
- 11. Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. Am J Clin Dermatol 2006; 7: 231–236. [DOI] [PubMed] [Google Scholar]
- 12. Okupa AY, Sorkness CA, Mauger DT, Jackson DJ, Lemanske RF Jr. Daily diaries vs retrospective questionnaires to assess asthma control and therapeutic responses in asthma clinical trials: is participant burden worth the effort? Chest 2013; 143: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials 2003; 24: 182–199. [DOI] [PubMed] [Google Scholar]
- 14. Krogh AB, Larsson B, Salvesen O, Linde M. A comparison between prospective Internet‐based and paper diary recordings of headache among adolescents in the general population. Cephalalgia 2016; 36: 335–345. [DOI] [PubMed] [Google Scholar]
- 15. Carter MC, Burley VJ, Nykjaer C, Cade JE. Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. J Med Internet Res 2013; 15: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ireland AM, Wiklund I, Hsieh R, Dale P, O'Rourke E. An electronic diary is shown to be more reliable than a paper diary: results from a randomized crossover study in patients with persistent asthma. J Asthma 2012; 49: 952–960. [DOI] [PubMed] [Google Scholar]
- 17. Burton C, Weller D, Sharpe M. Are electronic diaries useful for symptoms research? A systematic review J Psychosom Res 2007; 62: 553–561. [DOI] [PubMed] [Google Scholar]
- 18. Rijsbergen M, van der Kolk TN, Hogendoorn G et al A randomized controlled proof‐of‐concept trial of digoxin and furosemide in adults with cutaneous warts. Br J Dermatol 2018; 180: 1058–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rijsbergen MRR, van der Kolk T, Klaassen ES et al A randomized controlled proof‐of‐pharmacology trial of omiganan in patients with external genital warts. NVED 2019;29(1): 53. [Google Scholar]
- 20. Buters T.P.N‐vdKT, Krouwels L., Boltjes J. et al Omiganan, a topical antimicrobial peptide, normalizes dysbiosis but does not improve atopic dermatitis clinically in a phase II randomized controlled trial. NVED 2019; 29: 63. [DOI] [PubMed] [Google Scholar]
- 21. Buters TPFG, van Doorn MBA, Burggraaf J, Rissmann R. Omiganan demonstrates pharmacodynamic and clinical activity in patients with mild to moderate atopic dermatitis in a phase 2 proof‐of‐concept trial. NVED 2018; 28: 60–61. [Google Scholar]
- 22. Krejci‐Manwaring J, Tusa MG, Carroll C et al Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol 2007; 56: 211–216. [DOI] [PubMed] [Google Scholar]
- 23. Storm A, Benfeldt E, Andersen SE, Serup J. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol 2008; 59: 975–980. [DOI] [PubMed] [Google Scholar]
- 24. Yang MY, Jin H, Shim WH et al High rates of secondary non‐adherence causes decreased efficacy of 0.1% topical tacrolimus in adult eczema patients: results from a multicenter clinical trial. J Dermatolog Treat 2018; 29: 129–134. [DOI] [PubMed] [Google Scholar]
- 25. Sommer C, Zuccolin D, Arnera V et al Building clinical trials around patients: evaluation and comparison of decentralized and conventional site models in patients with low back pain. Contemp Clin Trials Commun 2018; 11: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Screenshots of the e‐diary (English translation from Dutch original).


