Abstract
Aim
To investigate the effects of semaglutide versus comparators on major adverse cardiovascular events (MACE: cardiovascular [CV] death, nonfatal myocardial infarction [MI] and nonfatal stroke) and hospitalization for heart failure (HF) in the SUSTAIN (subcutaneous semaglutide) and PIONEER (oral semaglutide) trials across subgroups of varying CV risk.
Methods
Post hoc analyses of individual patient‐level data combined from SUSTAIN 6 and PIONEER 6 were performed to assess MACE and HF. MACE were analysed in subjects with and without: established CV disease and/or chronic kidney disease; prior MI or stroke; and prior HF. MACE in the SUSTAIN and PIONEER glycaemic efficacy trials were also assessed.
Results
In SUSTAIN 6 and PIONEER 6 combined, the hazard ratio (HR) for effect of semaglutide versus placebo on overall MACE was 0.76 (95% CI 0.62, 0.92), which was mainly driven by the effect on nonfatal stroke (HR 0.65 [95% CI 0.43, 0.97]). The HR for hospitalization for HF was 1.03 (95% CI 0.75, 1.40). The HRs for MACE were <1.0 in all subgroups, except for those with prior HF (HR 1.06 [95% CI 0.72, 1.57]); P‐values for interaction of subgroup on treatment effect were >0.05, except for HF (0.046). In the combined glycaemic efficacy trials, the HR for effect of semaglutide versus comparators on MACE was 0.85 (95% CI 0.55, 1.33).
Conclusions
In SUSTAIN and PIONEER combined, glucagon‐like peptide‐1 analogue semaglutide showed consistent effects on MACE versus comparators across varying CV risk. No effect of semaglutide on MACE was observed in subjects with prior HF.
Keywords: cardiovascular disease, clinical trial, glucagon‐like peptide‐1 analogue, phase III study, type 2 diabetes
1. INTRODUCTION
Cardiovascular (CV) disease (CVD) is the leading cause of morbidity and mortality in patients with type 2 diabetes (T2D).1 CV outcomes trials (CVOTs) have demonstrated the CV safety of several glucose‐lowering drugs. Moreover, some drugs, such as the glucagon‐like peptide‐1 (GLP‐1) receptor agonists (GLP‐1RAs) liraglutide,2 albiglutide3 and dulaglutide,4 and sodium‐glucose co‐transporter‐2 inhibitors (SGLT‐2is) empagliflozin5 and canagliflozin,6 have shown a reduction in major adverse cardiovascular events (MACE) versus placebo. SGLT‐2is have also been shown to reduce the risk of hospitalization for heart failure (HF),5, 6, 7 an endpoint for which liraglutide and dulaglutide demonstrated a neutral effect in CVOTs.2, 4 The majority of CVOTs include patients with established CVD. The strength of these data is now reflected in treatment guidelines, which recommend a GLP‐1RA or an SGLT‐2i in those with CVD.8, 9
There are several mechanisms through which GLP‐1RAs may mediate their CV effects, including improving cardiac function and attenuating atherothrombosis,10 with the strongest evidence supporting an effect on atherosclerosis.11 CV benefits with GLP‐1RAs could, therefore, be expected in those with atherosclerosis without established CVD, although they may take longer to manifest. Indeed, there is evidence to support CV benefits of GLP‐1RAs in such patients, including data from a CVOT in which a majority of patients did not have established CVD.4 As the incidence of MACE in patients without established CVD is lower than in those with, analyses of large datasets are required to verify whether CV benefits apply to these patients.
Semaglutide (Novo Nordisk, Denmark) is a long‐acting human GLP‐1 analogue, which can be administered once weekly subcutaneously (s.c.), or once daily orally when co‐formulated with the absorption enhancer sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate (commonly known as ‘SNAC’).12, 13 The half‐life (~7 days) and clinical effects of semaglutide are similar irrespective of mode of administration.14, 15 Both formulations lowered HbA1c and body weight versus comparators across the continuum of care in subjects with T2D in the SUSTAIN (s.c.)16, 17, 18, 19, 20, 21, 22 and PIONEER (oral)23, 24, 25, 26, 27, 28, 29, 30, 31 phase 3a clinical trial programmes.
SUSTAIN 6 and PIONEER 6 were preapproval CVOTs of s.c. and oral semaglutide, respectively, with similar designs and populations.32, 33 Key differences included planned duration and treatment arms. SUSTAIN 6 was time‐ and event‐driven (≥104 weeks’ exposure and ≥122 primary outcome events),32 whereas PIONEER 6 was solely event‐driven (≥122 primary outcome events).33 SUSTAIN 6 was a four‐armed trial (semaglutide 0.5 mg and 1.0 mg once weekly, and volume‐matched placebo), with the primary analysis performed on pooled semaglutide and placebo groups,32 whereas PIONEER 6 was a two‐armed trial (oral semaglutide target dose 14 mg once daily and placebo).33 The primary outcome in both trials was the time to first occurrence of a three‐component MACE (CV death, nonfatal myocardial infarction [MI], or nonfatal stroke).32 There were fewer MACE with semaglutide versus placebo in both trials: the hazard ratios (HRs) for SUSTAIN 6 and PIONEER 6 were 0.74 (95% confidence interval [CI] 0.58, 0.95) and 0.79 (0.57, 1.11), respectively.32, 33 In SUSTAIN 6, the results were significant for noninferiority and superiority, although the latter was not prespecified.32
SUSTAIN 6 and PIONEER 6 aimed to rule out an excess risk of CV events with semaglutide versus placebo, defined by the US Food and Drug Administration as having an upper bound of the 95% CI <1.8 in preapproval trials. Both trials, therefore, enrolled fewer subjects and were shorter in duration (median follow‐up: 2.1 years in SUSTAIN 632 and 1.3 years in PIONEER 633) than CVOTs, with the aim of showing safety in a postapproval setting or powered to show superiority.
The aim of this analysis was to assess the effects of semaglutide (s.c. and oral) versus placebo on MACE and hospitalization for HF in subjects with T2D in a combined population from SUSTAIN 6 and PIONEER 6, and to evaluate the effect across clinically relevant CV risk subgroups. To support these analyses, MACE in the combined phase 3a glycaemic efficacy trials were also analysed. Our expectation was that analyses of all available datasets would enable a more robust assessment of the effect of semaglutide on MACE in subjects with T2D across the diverse spectrum of CV risk encountered in clinical practice.
2. MATERIALS AND METHODS
2.1. Trial design and participants
The previously reported32, 33 trial designs for SUSTAIN 6 and PIONEER 6 are summarized in Table S1. In both, randomization was stratified based on the same criteria for evidence of CVD at baseline: established CVD and/or chronic kidney disease (CKD), or CV risk factors only.
Trial designs and endpoints for the SUSTAIN and PIONEER phase 3a glycaemic efficacy trials (SUSTAIN 1–5 and two SUSTAIN JAPAN trials, and PIONEER 1–5, 7–8 and the two PIONEER Japanese trials [9 and 10]) are summarized in Tables S2 and S3, and have also been reported previously.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31
All studies were approved by Independent Ethics Committees and Institutional Review Boards at each participating centre and conducted in compliance with the International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. All subjects provided written informed consent before any trial‐related activities. All authors had full access to all study data and take responsibility for their integrity and analyses.
2.2. Outcomes and subgroups
In our post hoc analysis, the incidences of the adjudicated three‐component MACE and its individual components were analysed using individual patient‐level data from the combined population of SUSTAIN 6 and PIONEER 6. In addition to the fatal MI and stroke components of MACE, the incidences of fatal plus nonfatal MI and fatal plus nonfatal stroke events, as well as hospitalization for HF, were assessed in the combined population.
The incidences of adjudicated MACE in subgroups from the combined population of SUSTAIN 6 and PIONEER 6 were also analysed. Subgroups were defined according to the same criteria for evidence of CVD at screening and CV risk strata in the trials’ inclusion criteria (Table S1). In two other subgroup analyses, subjects were defined according to occurrence of prior MI or stroke, or prior HF (New York Heart Association [NYHA] Class II–III) as assessed by medical history (yes/no).
The incidences of MACE were also analysed across the combined population from the phase 3a glycaemic efficacy trials (SUSTAIN 1–5 and two SUSTAIN JAPAN trials, and PIONEER 1–5, 7–8 and the two PIONEER Japanese trials [9 and 10]). In these trials, MACE were recorded as adjudicated adverse events (AEs) using the same terms as the CVOTs (MI, stroke and CV death). The individual components of MACE are not reported for this population because of the low numbers of events.
2.3. Statistical analyses
Post hoc analyses of SUSTAIN 6 and PIONEER 6 data combined on a patient level were conducted to increase the number of events available for analysis. The incidences of three‐component MACE and other CV outcomes were analysed using a stratified Cox proportional hazards model with treatment as a categorical fixed factor stratified by trial (SUSTAIN 6 or PIONEER 6) and CV risk at screening (established CVD and/or CKD, or CV risk factors only).
For the subgroup analyses, the subgroup was added as a categorical fixed factor and interaction term. Consistency across the subgroups of the treatment effect on time to first occurrence of MACE was assessed using interaction P‐values.
For the post hoc analyses of data from the SUSTAIN and PIONEER glycaemic efficacy trials combined, times from randomization to first event adjudication committee‐confirmed MACE were analysed using a Cox proportional hazards model with treatment as a categorical fixed factor and stratified by trial.
All analyses were performed using data from randomization to follow‐up (“in‐trial” observation period).
3. RESULTS
3.1. Subject disposition and baseline characteristics
SUSTAIN 6 and PIONEER 6 included 6480 subjects, of whom 3239 were randomized to semaglutide (s.c. or oral) and 3241 to placebo. Key baseline characteristics are provided for this combined population overall and by treatment group (Table 1), and for each subgroup (Table S4). Individual trial data for each subgroup are shown in Tables S5 and S6. The treatment groups in SUSTAIN 6 and PIONEER 6 trials, individually and as a combined population, were well balanced in terms of baseline characteristics, CVD, and CV risk factors.32, 33
Table 1.
Baseline characteristics and demographics of the combined SUSTAIN 6 and PIONEER 6 population
| Semaglutide | Placebo | Total | |
|---|---|---|---|
| n = 3239 | n = 3241 | N = 6480 | |
| Baseline characteristics, mean (SD) (except where n [%]) | |||
| Age, years | 65.3 (7.2) | 65.5 (7.4) | 65.4 (7.3) |
| Female, n (%) | 1142 (35.3) | 1160 (35.8) | 2302 (35.5) |
| Diabetes duration, years | 14.4 (8.4) | 14.3 (8.3) | 14.4 (8.3) |
| BMI, kg/m2 | 32.5 (6.4) | 32.5 (6.3) | 32.5 (6.4) |
| HbA1c, % | 8.4 (1.5) | 8.4 (1.6) | 8.4 (1.6) |
| SBP, mmHg | 135.7 (17.5) | 135.5 (17.2) | 135.6 (17.4) |
| DBP, mmHg | 76.6 (10.1) | 76.5 (10.0) | 76.6 (10.0) |
| Total cholesterol, mmol/L (geometric mean [CoV]) | 4.1 (26.9) | 4.1 (26.5) | 4.1 (26.7) |
| Current smoker, n (%) | 388 (12.0) | 367 (11.3) | 755 (11.7) |
| CVD, n (%) | |||
| Prior MI | 1090 (33.7) | 1131 (34.9) | 2221 (34.3) |
| Prior stroke or TIA | 499 (15.4) | 522 (16.1) | 1021 (15.8) |
| Prior arterial revascularization | 1427 (44.1) | 1489 (45.9) | 2916 (45.0) |
| ≥50% stenosis on angiography | 991 (30.6) | 1050 (32.4) | 2041 (31.5) |
| History of symptomatic coronary heart disease | 606 (18.7) | 611 (18.9) | 1217 (18.8) |
| Asymptomatic cardiac ischaemia | 172 (5.3) | 163 (5.0) | 335 (5.2) |
| HF (NYHA Class II–III) | 473 (14.6) | 488 (15.1) | 961 (14.8) |
| CKD (eGFR <60 mL/min/1.73 m2) | 849 (26.2) | 844 (26.0) | 1693 (26.1) |
| CV risk factors, n (%) | |||
| Microalbuminuria or proteinuria | 309 (9.5) | 314 (9.7) | 623 (9.6) |
| Hypertension and left ventricular hypertrophy | 204 (6.3) | 185 (5.7) | 389 (6.0) |
| Left ventricular systolic or diastolic dysfunction† | 134 (4.1) | 127 (3.9) | 261 (4.0) |
| Ankle/brachial index <0.9 | 59 (1.8) | 56 (1.7) | 115 (1.8) |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; CoV, coefficient of variation; CV, cardiovascular; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; MI, myocardial infarction; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation; TIA, transient ischaemic attack.
Dysfunction was determined from imaging by the investigators according to local procedures. Data were combined for semaglutide and placebo treatment groups in SUSTAIN 6 and PIONEER 6.
Key baseline characteristics of subjects in the SUSTAIN and PIONEER glycaemic efficacy trials and their combined population are provided in Table S7. These trials included subjects from across the continuum of T2D care who were at a lower risk of CV events compared with subjects enrolled in the CVOTs.
3.2. Time to first occurrence of CV outcomes in the CVOTs
Cumulative incidence plots of time to first occurrence of MACE in SUSTAIN 6 and PIONEER 6 have been reported for each trial individually,32, 33 and are shown in Figure 1 (data from the combined population are not presented owing to differences in trial durations).
Figure 1.
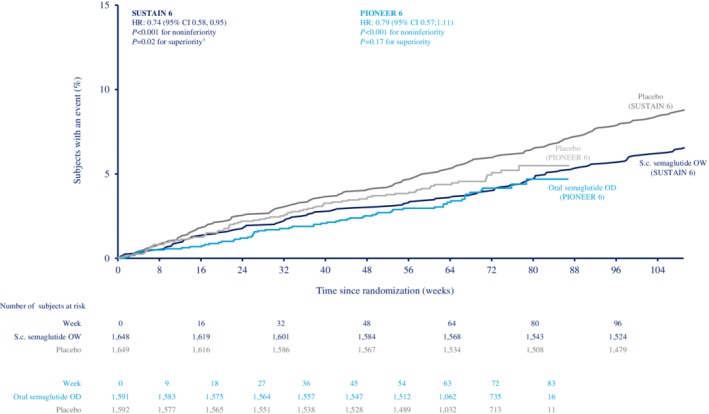
Cumulative incidence plot of time to first occurrence of event adjudication committee‐confirmed three‐component major adverse cardiovascular events (MACE) (cardiovascular [CV] death, nonfatal myocardial infarction [MI] and nonfatal stroke) with semaglutide versus placebo in SUSTAIN 6 and PIONEER 6. Combined population omitted owing to different trial durations; SUSTAIN 6 and PIONEER 6 have already been individually reported.32, 33 Data are “in‐trial”, analysed using Cox proportional hazards models with treatment as categorical fixed factor, stratified by CV risk at screening (established cardiovascular disease [CVD] and/or chronic kidney disease [CKD], or CV risk factors only). †Not prespecified. CI, confidence interval; HR, hazard ratio; OD, once daily; OW, once weekly; s.c., subcutaneous
In SUSTAIN 6 and PIONEER 6 combined, the incidence rates of MACE were 3.1 and 4.2 events per 100 subject‐years with semaglutide and placebo, respectively (HR 0.76 [95% CI 0.62, 0.92]; Figure 2). The HRs for each individual component of MACE were <1.0, although the upper limit of the 95% CI was <1.0 for nonfatal stroke only (Figure 2).
Figure 2.
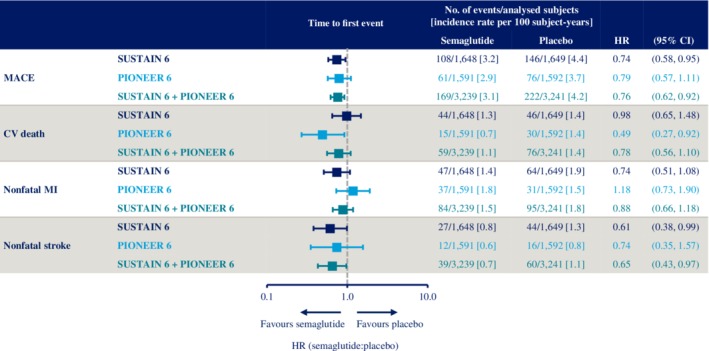
Time to first occurrence of event adjudication committee‐confirmed three‐component major adverse cardiovascular events (MACE) (cardiovascular [CV] death, nonfatal myocardial infarction [MI] and nonfatal stroke) and its components with semaglutide versus placebo in SUSTAIN 6 and PIONEER 6. SUSTAIN 6 and PIONEER 6 have already been individually reported.32, 33 Data are “in‐trial”, analysed using Cox proportional hazards models with treatment as a categorical fixed factor, stratified by trial (SUSTAIN 6 or PIONEER 6) and CV risk at screening (established cardiovascular disease [CVD] and/or chronic kidney disease [CKD], or CV risk factors only). CI, confidence interval; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular events; MI, myocardial infarction
When fatal and nonfatal MI events were analysed together in SUSTAIN 6 and PIONEER 6 combined, the incidence rates were 1.7 and 1.9 events per 100 subject‐years with semaglutide and placebo, respectively (HR 0.89 [95% CI 0.67, 1.18]; Figure S1). For fatal and nonfatal stroke events, these were 0.8 and 1.2 events per 100 subject‐years (HR 0.68 [95% CI 0.46, 1.00]; Figure S1).
The incidence rates for hospitalization for HF in the combined population from the CVOTs with semaglutide and placebo, respectively, were 1.5 and 1.4 events per 100 subject‐years (HR 1.03 [95% CI 0.75, 1.40]; Figure S1).
3.3. Time to first occurrence of MACE in subgroups of CV risk in the CVOTs
The incidence rates and HRs (95% CIs) for the effects of semaglutide versus placebo on MACE in each CV risk subgroup in SUSTAIN 6 and PIONEER 6 individually and combined are shown in Figure 3.
Figure 3.
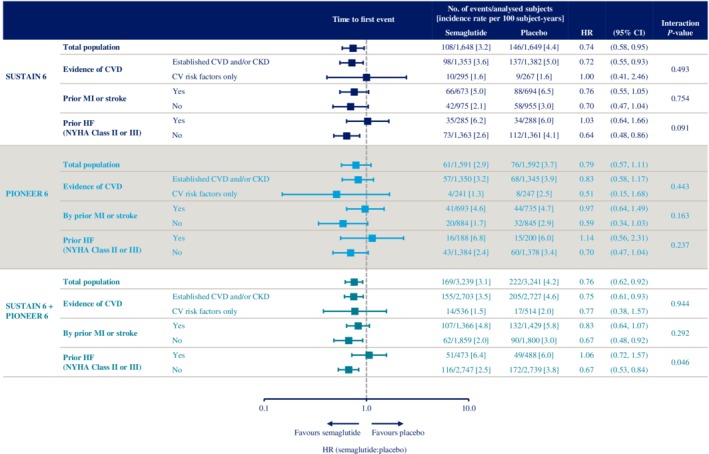
Time to first occurrence of event adjudication committee‐confirmed three‐component major adverse cardiovascular events (MACE) (cardiovascular [CV] death, nonfatal myocardial infarction [MI] and nonfatal stroke) with semaglutide versus placebo in SUSTAIN 6 and PIONEER 6 CV risk subgroups. SUSTAIN 6 and PIONEER 6 have already been individually reported.32, 33 Data are “in‐trial”, analysed using Cox proportional hazards models with treatment and subgroup as categorical fixed factors, with interaction term between treatment and subgroup, stratified by trial and CV risk at screening. P‐value: unadjusted interaction test between treatment and subgroups. CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; NYHA, New York Heart Association
In the combined population, the HRs for MACE were <1.0 in each CV risk subgroup, with the exception of those with prior HF (HR 1.06 [95% CI 0.72, 1.57]; Figure 3). The 95% CIs spanned 1.0 for the following subgroups: CV risk factors only, prior MI or stroke, and prior HF (Figure 3).
P‐values for the interaction of the presence of CVD and/or CKD at baseline, and prior MI or stroke on MACE were not significant (P > 0.05), suggesting no heterogeneity in the treatment effects across these subgroups. The P‐value for the interaction of prior HF was nominally significant (P = 0.046); however, this was not controlled for multiple comparisons.
3.4. Time to first occurrence of MACE in the phase 3a glycaemic efficacy trials
In the combined SUSTAIN and PIONEER glycaemic efficacy trials, the incidence rates for MACE were 0.7 and 0.9 events per 100 subject‐years with semaglutide and comparator, respectively (HR 0.85 [95% CI 0.55, 1.33]; Figure 4). A sensitivity analysis was performed, in which all subjects who were receiving an SGLT‐2i or a GLP‐1RA at baseline or initiated such therapies during the trial were excluded. In this analysis, the HR for MACE was 0.77 (95% CI 0.47, 1.27) with semaglutide versus comparators.
Figure 4.
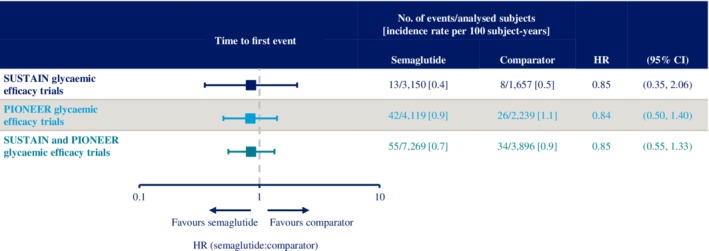
Time to first occurrence of major adverse cardiovascular events (MACE) (cardiovascular [CV] death, nonfatal myocardial infarction [MI] and nonfatal stroke) across the SUSTAIN and PIONEER phase 3a glycaemic efficacy trials. Data are “in‐trial” from SUSTAIN 1–5 and two SUSTAIN JAPAN trials, and PIONEER 1–5, 7–8 and the two PIONEER Japanese trials (9 and 10). Times from randomization to first event adjudication committee‐confirmed MACE analysed using a stratified Cox proportional hazards model with treatment as a categorical fixed factor and stratified by trial. See Tables S2 and S3 for details of comparators. CI, confidence interval; HR, hazard ratio
4. DISCUSSION
In the combined SUSTAIN 6 and PIONEER 6 population, semaglutide reduced the risk of MACE versus placebo in subjects with T2D at high risk of CV events. While the HRs for each component of MACE favoured semaglutide versus placebo, the HR for overall MACE was mainly driven by the effect of semaglutide on nonfatal stroke and the HRs were not statistically significant for CV death or nonfatal MI. When fatal and nonfatal MI, and fatal and nonfatal stroke events were analysed in the combined CVOT population, there was no significant effect of semaglutide versus placebo, consistent with the effect of semaglutide on the CV death component of the primary endpoint. Furthermore, no significant effect of semaglutide versus placebo on hospitalization for HF was observed in the combined analysis of the CVOTs. The effect of semaglutide on overall MACE in the combined population of SUSTAIN 6 and PIONEER 6 was consistent irrespective of baseline CV risk, with the exception of subjects with prior HF.
The subgroup analysis by subjects’ history of CVD (established CVD and/or CKD vs. CV risk factors only) was based on the inclusion criteria of the CVOTs, which can differ across trials. Although these inclusion strata are suitable for identifying subjects at high risk of MACE, they are not ideal for clinical decision‐making. In practice, decisions are typically based on the presence or absence of a prior CV event, making subgroup analyses based on prior MI or stroke, or history of HF, more relevant. It should be noted that the subgroup analyses were not mutually exclusive.
Findings from the SUSTAIN and PIONEER glycaemic efficacy trials also suggest a potentially beneficial effect of semaglutide on MACE in subjects with T2D at low risk of MACE. These data were included to investigate the effects of semaglutide in a population of patients with T2D considered to be at a lower CV risk than those in the CVOTs. However, the number of events was small; accordingly, the 95% CIs were broad and not statistically significant. Furthermore, the pooling of placebo and active comparators (including liraglutide and empagliflozin, for which CV benefits have been demonstrated)2, 5 may confound the ability of this analysis to detect a benefit of semaglutide. Results were similar, however, when subjects receiving a GLP‐1RA or an SGLT‐2i were excluded from the analysis.
Once absorbed, s.c. and oral semaglutide share similar pharmacokinetic profiles and effects.14, 15 It has been shown that median exposure of s.c. semaglutide 1.0 mg is higher than oral semaglutide 14 mg. However, the exposure range following orally administered semaglutide is wider than for s.c. semaglutide, with a considerable overlap between oral semaglutide 14 mg and s.c. semaglutide 1.0 mg. This indicates similar exposure levels across the two formulations. Furthermore, similar exposure−response relationships were observed for the efficacy (HbA1c and body weight reduction) and tolerability of semaglutide, regardless of the route of administration.34 The HRs for MACE in SUSTAIN 6 and PIONEER 6 were similar,32, 33 despite the different administration routes. These factors support a similar potential of oral and s.c. semaglutide in conferring clinical benefits and mediating CV effects at the doses used in the CVOTs, implying that either could be used in clinical practice. Additionally, the similarity in trial designs, study populations and outcomes in SUSTAIN 6 and PIONEER 6 adds to the comparability of the trials, and supports the scientific validity of a combined analysis.
Although comparison across CVOTs is not straightforward, owing to subjects in each trial having different risk profiles, our combined SUSTAIN 6 and PIONEER 6 findings are consistent with other trials. The superiority of a GLP‐1RA versus placebo has been demonstrated in LEADER,2 HARMONY3 and REWIND,4 and noninferiority demonstrated in EXSCEL35 and ELIXA.36 Subjects’ CV risk in these trials varied from all having established CVD (HARMONY) to only one third having established CVD (REWIND). There are, however, conflicting data for the effects of GLP‐1RAs on MACE in a population at high CV risk. In ELIXA, the HR for the effect of lixisenatide versus placebo on MACE was 1.02 but nonsignificant in a population who had experienced a recent acute coronary event,36 arguably one of the highest risk patient populations encountered in clinical practice.
A prespecified analysis of REWIND showed similar estimated effects of dulaglutide on MACE in subjects with and without established CVD4; however, it should be noted that the definition of “established CVD” can differ across CVOTs. In analyses of GLP‐1RA CVOTs in which MACE in subjects with and without prior HF were assessed (LEADER, EXSCEL and HARMONY), the HRs were always <1.0 irrespective of subgroup, and the tests for interaction nonsignificant (P > 0.05), suggesting consistent effects irrespective of subjects’ CV risk at baseline.2, 3, 4, 35
Post hoc analyses of CVOTs also support the CV effects of GLP‐1RAs in subjects with T2D across the spectrum of CV risk. MACE in LEADER have been assessed in three different subgroup analyses: by prior MI or stroke status37; by baseline vascular disease38; and by prior HF (NYHA Class I–III, yes/no).39 The HRs favoured liraglutide versus placebo in all subgroups, apart from the subgroups at lowest CV risk in the former two analyses.37, 38, 39 The interaction of CV risk status at baseline on the treatment effect of liraglutide was not significant in any analysis, suggesting consistent CV effects across clinically relevant subgroups.37, 38 These findings may seem inconsistent with a prespecified subgroup analysis of LEADER, in which a significant interaction of treatment effect on MACE was observed in those aged ≥50 years with established CVD or CKD versus those aged ≥60 years with risk factors for CVD.2 However, considering the broad 95% CI in the CV risk factor only subgroup (0.86, 1.67), further study is warranted. In a post hoc analysis of subjects with and without prior MI or stroke in REWIND, the HR for MACE was <1.0 in each subgroup and the interaction nonsignificant, supporting a consistent benefit of dulaglutide.4 Although the effect of exenatide extended release on MACE was not superior to placebo in the full population of EXSCEL, a post hoc analysis of subjects with prior CVD showed an HR of 0.90 (95% CI 0.816, 0.999) for the effect on MACE versus placebo.40
A recent meta‐analysis of GLP‐1RAs suggests that their CV benefits should be regarded as a class effect, and that timing of exposure is the most prominent factor in trial heterogeneity, with increased exposure associated with improved outcomes.41 The benefits of GLP‐1RAs on CV outcomes in subjects with T2D have also recently been confirmed in an analysis of the seven GLP‐1RA CVOTs described above, and are consistent irrespective of CVD at baseline.42
In our analyses, a significant interaction of prior HF status on the effect of semaglutide versus placebo on MACE was detected. However, the numbers of events were low (51 and 49 [with prior HF], and 116 and 172 [without prior HF], with semaglutide and placebo, respectively), resulting in a broad 95% CI and a nominally significant P‐value uncontrolled for multiplicity. The proportion of subjects with prior HF in this analysis (~15%) is similar to the proportions in the prespecified subgroup analyses of LEADER2 and EXSCEL,35 in which the CIs also spanned 1.0. In the post hoc analysis of MACE by prior HF in LEADER, there were 142 and 170 (with prior HF), and 466 and 524 (without prior HF) events with liraglutide and placebo, respectively.39 The interaction P‐value suggested no effect of prior HF status on the treatment effect on MACE (P = 0.528).39
When considering HF as an endpoint in its own right, no effect of liraglutide, semaglutide (s.c. or oral), exenatide extended release or dulaglutide was observed compared with placebo in their respective CVOTs.2, 4, 32, 33, 35 However, a benefit was observed with albiglutide in HARMONY3 and, furthermore, a recent meta‐analysis that included data from seven CVOTs showed that treatment with a GLP‐1RA results in a small but significant reduction in the risk of hospitalization for HF versus placebo.42 Conversely, in two small studies of liraglutide in subjects with HF with reduced ejection fraction (with or without T2D), there was no difference in the incidence of death or rehospitalization for HF, but a higher incidence of cardiac serious AEs compared with placebo, although the types of events were heterogeneous (ventricular tachycardia, atrial fibrillation, acute coronary syndrome and worsening of chronic HF) and the overall incidence was low.43, 44 It has been suggested that the benefits of GLP‐1RAs on HF are secondary to the reduced risk of MI, which would account for the results in the HARMONY trial, in which the risk reduction for hospitalization for HF and MI was greater than in other GLP‐1RA CVOTs.42 Consistent with this, in our analysis of SUSTAIN 6 and PIONEER 6 combined, semaglutide had neutral effects on both MI and hospitalization for HF compared with placebo. Taken together, these data suggest that GLP‐1RAs have a consistent effect on MACE in subjects with T2D and CVD, there are no overall safety concerns regarding their effects on HF, and they should be considered in those with T2D and HF, as suggested in recent guideline updates.11
Reporting safety data is beyond the scope of the current analysis; however, the safety profile of semaglutide in each SUSTAIN and PIONEER trial individually has previously been reported.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33
The limitations of our analysis include the low number of CV events and the short trial durations of SUSTAIN 6 (planned exposure 104 weeks and median follow‐up 2.1 years) and PIONEER 6 (median follow‐up 1.3 years) compared with other CVOTs. This resulted in small sample sizes and event numbers. Although findings from the SUSTAIN and PIONEER glycaemic efficacy trials support those of the CVOTs, the small number of events mean they should be interpreted with caution. Indeed, interpretation of subgroup analyses also requires caution, as our analyses (as with others discussed here) have not been controlled for multiplicity. There are additional limitations inherent to post hoc (versus prospective) analyses of randomized clinical trials.
Limitations aside, the data reported here add to the growing body of evidence on the CV benefits of GLP‐1RAs, specifically semaglutide, in patients with T2D across the spectrum of CV risk. It will be important to analyse the results of other randomized clinical trials in subjects with T2D by key demographics and baseline disease measures to confirm these findings and further inform optimal T2D care and CV risk management.
In conclusion, this post hoc analysis of a combined population from the SUSTAIN 6 and PIONEER 6 CVOTs showed that semaglutide reduced the risk of MACE versus placebo in subjects with T2D by 24%. This effect appeared to be consistent across the s.c. and oral formulations of semaglutide, and across several clinically relevant subgroups, namely those with and without established CVD and/or CKD, and with and without prior MI or stroke. In subjects with prior HF (NYHA Class II–III), no effect of semaglutide versus placebo on MACE was observed, although the overall incidence in the subgroup with prior HF was low. These findings are further supported by an analysis of combined data from the SUSTAIN and PIONEER glycaemic efficacy trials, which compared semaglutide with placebo and active comparators in subjects with T2D at a relatively low risk of CV events. Collectively, these analyses suggest a CV benefit of semaglutide not only in those with a history of CVD, but also in lower CV risk subgroups, and that the effect was similar despite different routes of treatment administration.
CONFLICT OF INTEREST
M.H. discloses personal fees for advisory panel consultancy and speaker honoraria from Boehringer Ingelheim and Janssen Inc.; grants for investigator‐initiated research studies and personal fees for advisory panel consultancy from AstraZeneca and Merck & Co; personal fees for advisory panel consultancy from Roche; and grants for investigator‐initiated preclinical research and personal fees for advisory panel consultancy and speaker honoraria from Novo Nordisk. S.C.B. discloses grants, personal fees and other potential conflicts of interest from Novo Nordisk, and personal fees and other potential conflicts of interest from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme and Sanofi‐Aventis, all for honoraria, teaching and research sponsorship or grants; also discloses other potential conflicts of interest from Elsevier and Medscape for development of educational programmes, and nonfinancial potential conflicts of interest from the All‐Wales Medicines Strategy Group, and the National Institute for Health and Care Excellence UK for providing expert advice; and discloses a potential conflict of interest for owning shares in Glycosmedia. O.K.J., R.S. and M.B.T. are full‐time employees of Novo Nordisk; O.K.J. and M.B.T. also report potential conflicts of interest for owning shares in Novo Nordisk. I.L. discloses institutional grant payments from Novo Nordisk, Novartis, Pfizer, Merck, Mylan and GI Dynamics; and personal fees from AstraZeneca, Eli Lilly, TARGETPharma, Novo Nordisk, Boehringer Ingelheim, Jenssen, Mannkind, Valeritas, Intarcia and Sanofi for consultancy. T.V. discloses personal fees from Amgen, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Bristol‐Myers Squibb, Merck Sharp & Dohme, Novo Nordisk and Sun Pharma for advisory panels and consultancy.
AUTHOR CONTRIBUTIONS
All authors were involved in data interpretation and analysis, and manuscript preparation and approval. M.H., S.C.B., I.L., M.B.T., and T.V. were involved in the study design and/or conduct of at least one of the trials reported in this manuscript.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
We thank all the patients, investigators and trial‐site staff members who were involved in the conduct of these trials; Thomas Hansen and Signe Harring (Novo Nordisk) for review and suggestions for revising the manuscript; and Ellen Robertshaw, PhD, at AXON Communications (funded by Novo Nordisk) for medical writing and editorial assistance.
These studies were funded by Novo Nordisk A/S, Denmark.
Husain M, Bain SC, Jeppesen OK, et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. 2020;22:442–451. 10.1111/dom.13955
Funding information These studies were funded by Novo Nordisk A/S, Denmark.
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13955.
† http://clinicaltrials.gov: NCT02054897, NCT01930188, NCT01885208, NCT02128932, NCT02305381, NCT01720446, NCT02906930, NCT02863328, NCT02607865, NCT02863419, NCT02827708, NCT02692716, NCT02849080, NCT03021187, NCT03018028, NCT03015220.
‡EudraCT: 2013‐000632‐94, 2012‐004827‐19, 2012‐004826‐92, 2013‐004392‐12, 2013‐004502‐26, 2012‐002839‐28, 2015‐005622‐19, 2015‐005209‐36, 2015‐001351‐71, 2015‐005210‐30, 2015‐005326‐19, 2015‐003563‐10, 2015‐005593‐38, 2016‐000988‐16.
REFERENCES
- 1. Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013:653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519‐1529. [DOI] [PubMed] [Google Scholar]
- 4. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121‐130. [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 7. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347‐357. [DOI] [PubMed] [Google Scholar]
- 8. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation. 2019;140:e596‐e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255‐323. [DOI] [PubMed] [Google Scholar]
- 10. Khat DZ, Husain M. Molecular mechanisms underlying the cardiovascular benefits of SGLT2i and GLP‐1RA. Curr Diab Rep. 2018;18:45. [DOI] [PubMed] [Google Scholar]
- 11. Rakipovski G, Rolin B, Nøhr J, et al. The GLP‐1 analogs liraglutide and semaglutide reduce atherosclerosis in apoE‐/‐ and LDLr‐/‐ mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3:844‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lau J, Bloch P, Schäffer L, et al. Discovery of the once‐weekly glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide. J Med Chem. 2015;58:7370‐7380. [DOI] [PubMed] [Google Scholar]
- 13. Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon‐like peptide‐1 receptor agonist. Sci Transl Med. 2018;10:eaar7047. [DOI] [PubMed] [Google Scholar]
- 14. Granhall C, Donsmark M, Blicher TM, et al. Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP‐1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes. Clin Pharmacokinet. 2019;58:781‐791. [DOI] [PubMed] [Google Scholar]
- 15. Davies M, Pieber TR, Hartoft‐Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318:1460‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251‐260. [DOI] [PubMed] [Google Scholar]
- 17. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341‐354. [DOI] [PubMed] [Google Scholar]
- 18. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2018;41:258‐266. [DOI] [PubMed] [Google Scholar]
- 19. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:355‐366. [DOI] [PubMed] [Google Scholar]
- 20. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103:2291‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seino Y, Terauchi Y, Osonoi T, et al. Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab. 2018;20:378‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaku K, Yamada Y, Watada H, et al. Safety and efficacy of once‐weekly semaglutide versus additional oral antidiabetic drugs, in Japanese subjects with inadequately controlled T2D: a randomised trial. Diabetes Obes Metab. 2018;20:1202‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial comparing the efficacy and safety of oral semaglutide monotherapy with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724‐1732. [DOI] [PubMed] [Google Scholar]
- 24. Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide vs empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 Trial. Diabetes Care. 2019;42:2272‐2281. [DOI] [PubMed] [Google Scholar]
- 25. Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double‐blind, phase 3a trial. Lancet. 2019;394:39‐50. [DOI] [PubMed] [Google Scholar]
- 27. Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo‐controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515‐527. [DOI] [PubMed] [Google Scholar]
- 28. Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open‐label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:528‐539. [DOI] [PubMed] [Google Scholar]
- 29. Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety and tolerability of oral semaglutide versus placebo added to insulin ± metformin in patients with type 2 diabetes: the PIONEER 8 Trial. Diabetes Care. 2019;42:2262‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamada Y, Katagiri H, Hamamoto Y, et al. Efficacy and safety of oral semaglutide monotherapy vs placebo or liraglutide in Japanese T2D patients: PIONEER 9 trial. Paper presented at: Asian Association for the Study of Diabetes, February 7–9, 2019; Osaka, Japan.
- 31. Yabe D, Nakamura J, Kaneto H, et al. Safety and efficacy of oral semaglutide vs dulaglutide in Japanese T2D patient: the PIONEER 10 trial. Paper presented at: Asian Association for the Study of Diabetes, February 7–9, 2019; Osaka, Japan.
- 32. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 33. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841‐851. [DOI] [PubMed] [Google Scholar]
- 34. Overgaard RV, Navarria A, Hertz CL, Ingwersen SH. Similar efficacy and gastrointestinal tolerability versus exposure for oral and subcutaneous semaglutide. Presentation at the 55th Annual Meeting of the European Association for the Study of Diabetes, Barcelona, Spain, September 16–20, 2019.
- 35. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247‐2257. [DOI] [PubMed] [Google Scholar]
- 37. Verma S, Poulter NR, Bhatt DL, et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without history of myocardial infarction or stroke. Circulation. 2018;138:2884‐2894. [DOI] [PubMed] [Google Scholar]
- 38. Verma S, Bhatt DL, Bain SC, et al. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER trial. Circulation. 2018;137:2179‐2183. [DOI] [PubMed] [Google Scholar]
- 39. Husain M, Bain SC, Mann JFE, et al. No increased risk of heart failure hospitalization of major cardiovascular events observed with liraglutide in patients with or without a history of heart failure – results from the LEADER trial. Paper presented at: American College of Cardiology, March 10–12, 2018; Orlando, FL.
- 40. Mentz RJ, Thompson VP, Aguilar D, et al. Effects of once‐weekly exenatide on clinical outcomes in patients with preexisting cardiovascular disease. Circulation. 2018;138:2576‐2578. [DOI] [PubMed] [Google Scholar]
- 41. Caruso I, Cignarelli A, Giorgino F. Heterogeneity and similarities in GLP‐1 receptor agonist cardiovascular outcomes trials. Trends Endocrinol Metab. 2019;30:578‐589. [DOI] [PubMed] [Google Scholar]
- 42. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;10:776‐785. [DOI] [PubMed] [Google Scholar]
- 43. Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon‐like peptide‐1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)‐a multicentre, double‐blind, randomised, placebo‐controlled trial. Eur J Heart Fail. 2017;19:69‐77. [DOI] [PubMed] [Google Scholar]
- 44. Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


