Abstract
Objective
This post hoc analysis evaluated the efficacy and safety of adjunctive perampanel 4 mg/d received as modal dose, which may have differed from randomized dose, for treatment of focal seizures.
Methods
Data were pooled from four randomized, double‐blind, placebo‐controlled, phase III studies of adjunctive perampanel in patients (aged ≥12 years) with focal seizures, with/without focal to bilateral tonic‐clonic (FBTC) seizures: studies 304 (NCT00699972), 305 (NCT00699582), 306 (NCT00700310), and 335 (NCT01618695). Efficacy assessments included median percentage reductions in seizure frequency per 28 days and seizure‐freedom rates for patients receiving placebo and perampanel 4 mg/d (modal dose). Treatment‐emergent adverse events (TEAEs) were assessed in patients receiving perampanel 4 mg/d at their TEAE onset. Outcomes were also assessed with/without enzyme‐inducing antiseizure medications (EIASMs).
Results
The full analysis set included 979 patients with focal seizures (placebo: n = 616 [235 with FBTC seizures]; perampanel 4 mg/d: n = 363 [134 with FBTC seizures]). Compared with placebo, perampanel 4 mg/d conferred significantly greater median percentage reductions in seizure frequency per 28 days for focal (12.6% vs 21.1%; P = .0004) and FBTC seizures (17.4% vs 49.8%; P < .0001), and seizure‐freedom rates for focal (0.8% vs 3.6%; P = .0018) and FBTC seizures (11.1% vs 18.7%; P = .0424). Seizure improvements with perampanel 4 mg/d were greater without EIASMs than with EIASMs. For assessment of TEAEs, overall 1376 patients with focal seizures received perampanel 4 mg/d at any time (FBTC seizures, n = 499). TEAEs with perampanel 4 mg/d occurred in 419 of 1376 (30.5%) and 148 of 499 (29.7%) patients with focal and FBTC seizures, respectively; most common was dizziness. The proportion of TEAEs was similar with or without EIASMs.
Significance
This post hoc analysis showed adjunctive perampanel 4 mg/d was efficacious and well tolerated in patients with focal seizures, with or without FBTC seizures. This dose may be a valuable treatment option in patients unable to tolerate higher perampanel doses up to 12 mg/d.
Keywords: AMPA receptor antagonist, antiseizure medications, epilepsy, focal to bilateral tonic‐clonic
Key Points.
This post hoc analysis reports efficacy and safety outcomes with adjunctive perampanel 4 mg/d received as modal dose for focal seizures
Compared with placebo, adjunctive perampanel 4 mg/d conferred significantly greater reductions in focal seizure frequency per 28 days
Responder and seizure‐freedom rates were also significantly greater with perampanel 4 mg/d compared with placebo
The proportion of patients experiencing treatment‐emergent adverse events with perampanel 4 mg/d was relatively low (30.5%)
Perampanel 4 mg/d may be an appropriate treatment option for patients who are unable to tolerate higher perampanel doses
1. INTRODUCTION
Perampanel is a once‐daily oral antiseizure medication (ASM) for focal seizures (previously referred to as partial‐onset seizures), with or without focal to bilateral tonic‐clonic (FBTC) seizures (previously secondarily generalized seizures), and generalized tonic‐clonic seizures (previously primary generalized tonic‐clonic seizures).1, 2 The approval of adjunctive perampanel for the treatment of focal seizures was based on the results of three international, randomized, double‐blind, placebo‐controlled, phase III studies in patients aged ≥12 years with uncontrolled focal seizures, with or without FBTC seizures: studies 304 (ClinicalTrials.gov identifier: NCT00699972), 305 (NCT00699582), and 306 (NCT00700310).3, 4, 5 A subsequent randomized, double‐blind, placebo‐controlled, phase III study 335 (NCT01618695) also demonstrated the efficacy and safety of adjunctive perampanel for the treatment of uncontrolled focal seizures, with or without FBTC seizures, in patients aged ≥12 years from the Asia‐Pacific region.6
For focal seizures, the recommended maintenance dose range for perampanel is 8‐12 mg/d; however, some patients may respond to the lower 4‐mg/d dose.1 A pooled analysis of studies 304, 305, and 306 supported the efficacy of perampanel doses of 4, 8, and 12 mg/d, as they were associated with significantly greater reductions in focal seizure frequency per 28 days and 50% responder rates compared with placebo, based on randomized dose.7 However, the 4‐mg/d dose did not achieve significant reductions in seizure frequency in study 335.6 This may have been attributable to the more refractory population in study 335 compared with studies 304, 305, and 306, and/or the high proportion of patients receiving treatment with concomitant enzyme‐inducing ASMs (EIASMs), which can reduce the systemic exposure of perampanel.6, 8 Notably, patients receiving perampanel 4 mg/d without concomitant EIASMs experienced greater reductions in seizure frequency in study 335 compared with placebo that were more aligned with those observed in studies 304, 305, and 306 (data on file, Eisai Co Ltd). Furthermore, perampanel 4 mg/d may have tolerability benefits, because the randomized perampanel 4‐mg/d dose was associated with a lower overall frequency of treatment‐emergent adverse events (TEAEs) compared with perampanel 8 and 12 mg/d in a pooled analysis of studies 304, 305, and 306, and in study 335.6, 7 Therefore, perampanel 4 mg/d may be an appropriate dose for patients who cannot tolerate higher perampanel doses due to TEAEs. Overall, there is a clear need to better characterize the efficacy and safety profiles of the 4‐mg/d dose.
At the time that the randomized, double‐blind, phase III studies were conducted, the International League Against Epilepsy (ILAE) 1981 seizure classification was still in use; however, in accordance with the new ILAE 2017 seizure classification, “partial‐onset seizures” and “secondarily generalized seizures” have been updated to focal seizures and FBTC seizures, respectively, throughout this article.9
This post hoc pooled analysis of studies 304, 305, 306, and 335 was performed to further evaluate the efficacy and safety of adjunctive perampanel 4 mg/d in patients with focal seizures, with or without FBTC seizures. To ensure that all patients who received perampanel 4 mg/d during the four studies were included, these analyses were based on the modal (actual) dose of perampanel received, which may have differed from the perampanel dose to which the patient was originally randomized. Because EIASMs have previously been shown to lower perampanel exposure,8 efficacy outcomes were also assessed by EIASM use.
2. MATERIALS AND METHODS
2.1. Study designs
The designs of studies 304, 305, 306, and 335 have been previously reported in detail.3, 4, 5, 6 Briefly, patients aged ≥12 years with uncontrolled focal seizures (≥5 at baseline), with or without FBTC seizures, despite receiving treatment with 1‐3 concomitant ASMs, were randomized to receive once‐daily placebo or adjunctive perampanel 8 or 12 mg/d in studies 304 and 305; 2, 4, or 8 mg/d in study 306; and 4, 8, or 12 mg/d in study 335. Perampanel was administered across a 19‐week double‐blind treatment phase (6‐week titration and 13‐week maintenance periods). During the titration period, perampanel was uptitrated from 2 mg/d in weekly 2‐mg increments to the target randomized dose. Patients experiencing intolerable adverse events could have their perampanel dose reduced at the investigator's discretion. During the maintenance period, patients continued to receive perampanel at the dose achieved during titration.
In the study protocols, only one EIASM (defined as carbamazepine, phenytoin, phenobarbital, or primidone) was permitted.3, 4, 5, 6 Although eslicarbazepine acetate and oxcarbazepine were not defined as EIASMs in the original study protocols, they have since been shown to decrease plasma levels of perampanel, and as such, they have been included as EIASMs in the current analyses; neither phenobarbital nor primidone was defined as an EIASM in the current analyses, because they have not been shown to significantly affect plasma levels of perampanel.1, 2 Therefore, in this post hoc analysis, EIASMs were defined as carbamazepine, eslicarbazepine acetate, oxcarbazepine, and phenytoin.
Although patients were randomized to a particular perampanel dose at the start of these studies, the study designs allowed for dose adjustments to be made based on the clinical judgment of the investigators to account for tolerability issues.3, 4, 5, 6 Patients who experienced TEAEs could have their dose reduced. If tolerability improved with the decreased dose, patients could have their dose increased.3, 4, 5, 6 As such, patients may have received a different (lower) perampanel dose from that to which they were originally randomized.
All studies were performed in accordance with the Declaration of Helsinki, Good Clinical Practice ICH‐E6 Guideline CPMP/ICH/135/95, European Clinical Trial Directive 2001/83/EC, and the US Code of Federal Regulations Part 21.3, 4, 5, 6 Trial protocols, amendments, and informed consent were reviewed by national regulatory authorities in each country and independent ethics committees or institutional review boards for each site. All patients gave written informed consent before participation.3, 4, 5, 6
2.2. Pooled post hoc analysis
This post hoc analysis included pooled data from studies 304, 305, 306, and 335. Efficacy analyses and assessments of TEAEs were performed for all patients with focal seizures, with a subanalysis of patients who experienced FBTC seizures during the prerandomization (baseline) period. Patient demographics and clinical characteristics were recorded during baseline for the safety analysis set, which consisted of patients who received ≥1 dose of study drug (placebo or perampanel at a modal dose of 4 mg/d) and had ≥1 postdose safety assessment. The modal dose represents the actual daily perampanel dose that a patient received most frequently and for the longest duration during the treatment period of studies 304, 305, 306, and 335. The modal dose may or may not have been the perampanel dose to which the patient was initially randomized.
2.3. Assessment of efficacy
Efficacy assessments were based on patients in the full analysis set, which consisted of patients who received ≥1 dose of study drug (placebo or perampanel 4 mg/d [modal dose]) and had any seizure frequency data during the double‐blind treatment phase (6‐week titration period as well as 13‐week maintenance periods). These analyses included all patients who received a modal dose of perampanel 4 mg/d, meaning that these patients received perampanel 4 mg/d for a longer period compared with all other perampanel doses. This could have occurred at any time during the study and was not necessarily on consecutive days of dosing. Efficacy assessments included median percentage reductions in seizure frequency per 28 days from baseline to the double‐blind treatment phase, 50% and 75% responder rates (defined as the proportions of patients with a ≥50% or ≥75% reduction in seizure frequency per 28 days from baseline to the maintenance period; last observation carried forward), and seizure‐freedom rates (defined as the proportion of patients who completed the studies and had no seizures during the maintenance period). All seizure endpoints were assessed for all focal seizures in all patients, and also for FBTC seizures in patients who had experienced FBTC seizures during baseline. Efficacy outcomes were also assessed by EIASM use (with or without).
2.4. Assessment of TEAEs
A TEAE was defined as an adverse event that (1) emerged during treatment, having been absent at pretreatment; or (2) re‐emerged during treatment, having been present at pretreatment but having stopped prior to treatment. TEAEs, using Medical Dictionary for Regulatory Activities (MedDRA) search terms, were assessed in patients who were receiving the perampanel 4‐mg/d dose at the onset of their TEAE(s) at any time during the double‐blind treatment phase (titration and maintenance periods). Serious TEAEs, TEAEs leading to discontinuation, and TEAEs related to hostility and/or aggression were also assessed in the same patient population. These patients may or may not have been receiving a modal dose of perampanel 4 mg/d; as such, this population is different from the safety analysis set defined above, in which all patients received a modal dose of perampanel 4 mg/d. Due to the differences in the lengths of time that patients received treatment with placebo versus perampanel 4 mg/d in the assessment of TEAEs, safety data are not reported here for placebo‐treated patients, as this would not be an appropriate comparison. TEAEs were also assessed by concomitant EIASM use.
2.5. Statistical analysis
Changes in seizure frequency were analyzed using rank analysis of covariance, with treatment as a factor and baseline seizure frequency as a covariate. The median difference from placebo and 95% confidence intervals (CIs) were based on the Hodges‐Lehmann method. Responder and seizure‐freedom rates were analyzed using the Cochran‐Mantel‐Haenszel test, stratified by country.
3. RESULTS
3.1. Patients
The safety analysis set comprised 983 patients with focal seizures (placebo, n = 618; perampanel 4 mg/d modal dose, n = 365), and the full analysis set comprised 979 patients with focal seizures (placebo, n = 616; perampanel 4 mg/d modal dose, n = 363). Both populations included 369 patients who had experienced FBTC seizures during baseline (placebo, n = 235; perampanel 4 mg/d modal dose, n = 134).
In the safety analysis set, there were no clinically meaningful differences in patient clinical characteristics between the placebo and perampanel groups (Table 1). The most common seizure type during baseline in both the placebo and perampanel 4 mg/d groups was focal impaired awareness seizures (80.9% and 76.4% of patients, respectively). The majority of patients in the placebo and perampanel 4 mg/d groups were receiving two (n = 286 [46.3%] and n = 168 [46.0%], respectively) or three ASMs during baseline (n = 261 [42.4%] and n = 164 [44.9%], respectively). Overall, 376 (60.8%) patients in the placebo group and 214 (58.6%) patients in the perampanel 4 mg/d group were receiving EIASMs during baseline; the most common were carbamazepine (n = 222 [35.9%] and n = 130 [35.6%], respectively) and oxcarbazepine (n = 112 [18.1%] and n = 53 [14.5%], respectively).
Table 1.
Patient demographics and clinical characteristics during prerandomization (baseline; safety analysis set)
| All patients | Patients with FBTC seizures during baseline | |||
|---|---|---|---|---|
| Placebo, n = 618 | Perampanel 4 mg/d, n = 365 | Placebo, n = 235 | Perampanel 4 mg/d, n = 134 | |
| Mean age, y (SD) | 34.4 (13.4) | 34.1 (13.3) | 32.6 (13.5) | 31.8 (12.4) |
| Female, n (%) | 312 (50.5) | 192 (52.6) | 105 (44.7) | 71 (53.0) |
| Race, n (%) | ||||
| Caucasian | 344 (55.7) | 134 (36.7) | 126 (53.6) | 62 (46.3) |
| Asiana | 246 (39.8) | 229 (62.7) | 90 (38.3) | 71 (53.0) |
| Black/African American | 14 (2.3) | 0 (0.0) | 10 (4.3) | 0 (0.0) |
| Otherb | 13 (2.1) | 2 (0.5) | 8 (3.4) | 1 (0.7) |
| Seizure types, n (%) | ||||
| Focal aware without motor signs | 132 (21.4) | 73 (20.0) | 47 (20.0) | 28 (20.9) |
| Focal aware with motor signs | 156 (25.2) | 106 (29.0) | 58 (24.7) | 39 (29.1) |
| Focal impaired awareness | 500 (80.9) | 279 (76.4) | 171 (72.8) | 85 (63.4) |
| Focal with FBTC | 237 (38.3) | 135 (37.0) | 235 (100.0) | 134 (100.0) |
Abbreviations: FBTC, focal to bilateral tonic‐clonic, SD, standard deviation.
Includes Chinese, Japanese, and other Asian.
Includes American Indian or Alaskan Native, Native Hawaiian or other Pacific Islander, and other.
Of the 365 patients in the safety analysis set who received a modal perampanel dose of 4 mg/d, 321 patients were randomized to perampanel 4 mg/d and 44 patients were initially randomized to perampanel 8 or 12 mg/d (Figure S1). Of the 44 patients who were randomized to perampanel 8 or 12 mg/d, 14 patients completed the study and 30 discontinued. Reasons for discontinuation are shown in Figure S1 and included adverse events (n = 18), lack of efficacy (n = 1), and other reasons (n = 11).
For assessment of TEAEs, a total of 1376 patients received perampanel 4 mg/d at any time during the double‐blind treatment phases of the four studies. Of these, 499 patients had experienced FBTC seizures during baseline. Mean (standard deviation [SD]) duration of exposure to perampanel 4 mg/d was 5.1 (7.1) weeks for all 1376 patients who received perampanel 4 mg/d at any time during the studies (with EIASMs: 4.9 [7.0] weeks [n = 859]; without EIASMs: 5.3 [7.2] weeks [n = 517]). In comparison, mean (SD) duration of exposure to placebo was 17.9 (3.9) weeks in all 618 patients who were randomized to placebo (with EIASMs: 18.0 [3.8] weeks [n = 375]; without EIASMs: 17.8 [4.1] weeks [n = 243]). Given the differences in the lengths of time that patients received treatment with placebo (randomized) versus perampanel 4 mg/d (modal dose), placebo data are not included in the assessment of TEAEs.
3.2. Efficacy outcomes
During baseline, median seizure frequency per 28 days was 10.7 in the placebo group and 9.6 in the perampanel 4 mg/d group. Median percentage reductions in focal seizure frequency per 28 days were 12.6% with placebo and 21.1% with perampanel 4 mg/d (P = .0004; Figure 1); median (95% CI) difference from placebo was −10.4% (−16.1 to −4.7). For patients who had FBTC seizures during baseline, median percentage reductions in FBTC seizure frequency per 28 days were 17.4% and 49.8% for placebo and perampanel 4 mg/d, respectively (P < .0001; Figure 1); median (95% CI) difference from placebo was −27.2% (−40.6 to −13.9).
Figure 1.
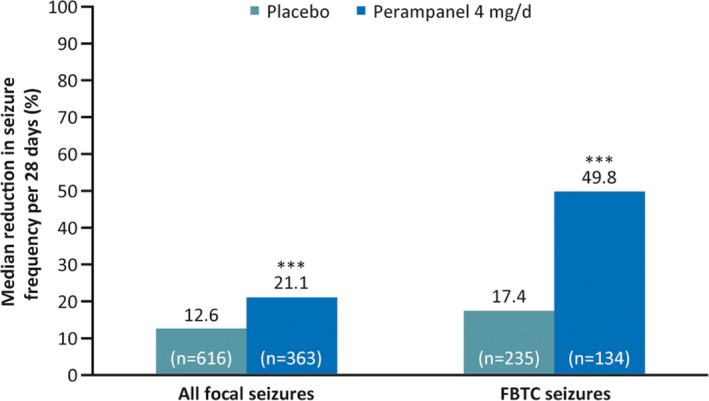
Median percentage reductions in seizure frequency per 28 days for all focal seizures (all patients) and focal to bilateral tonic‐clonic (FBTC) seizures (patients who had FBTC seizures during baseline; full analysis set). ***P < .001 vs placebo
Compared with placebo, perampanel 4 mg/d was also associated with significantly greater 50% and 75% responder rates for focal seizures (P = .0021 and P = .0026, respectively) and FBTC seizures (P = .0053 and P = .0070, respectively; Figure 2A,B), and significantly greater seizure‐freedom rates (focal seizures: 0.8% vs 3.6%, respectively; P = .0018; FBTC seizures: 11.1% vs 18.7%, respectively; P = .0424; Figure 2C).
Figure 2.
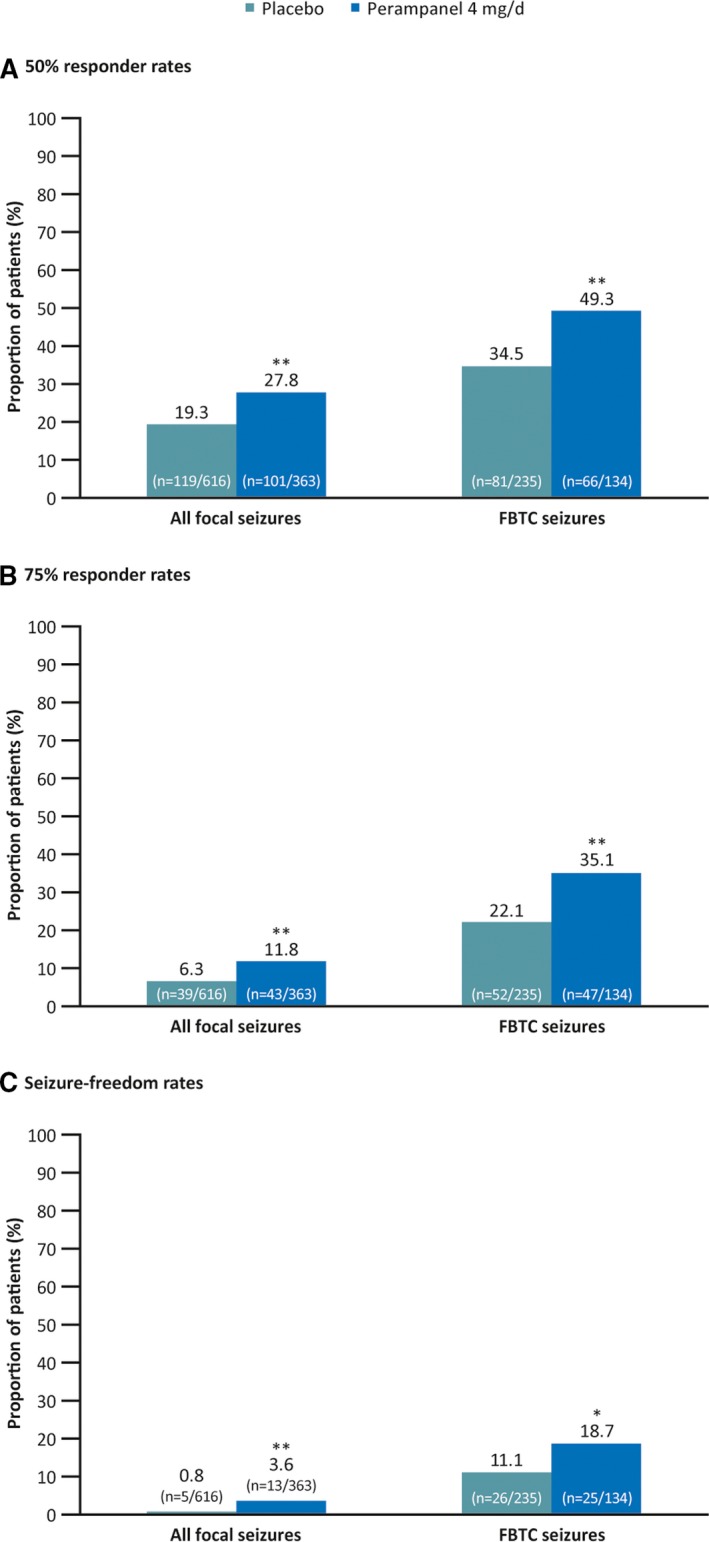
Fifty percent responder rates (A), 75% responder rates (B), and seizure‐freedom rates (C) during maintenance for all focal seizures (all patients) and focal to bilateral tonic‐clonic (FBTC) seizures (patients who had FBTC seizures during baseline; full analysis set). *P < .05 and **P < .01 vs placebo
In patients with focal seizures who were not receiving EIASMs, and compared with placebo, perampanel 4 mg/d was associated with significantly greater median percentage reductions in seizure frequency per 28 days (P = .0019; Figure 3), 50% responder rates (P < .0001; Figure 4A), 75% responder rates (P = .0108; Figure 4B), and seizure‐freedom rates (P = .0027; Figure 4C). In patients who were receiving EIASMs, no statistically significant differences were observed for these efficacy outcomes (Figures 3 and 4A‐C).
Figure 3.

Median percentage reductions in seizure frequency per 28 days for all focal seizures (all patients) and focal to bilateral tonic‐clonic (FBTC) seizures (patients who had FBTC seizures during baseline) in patients with and without enzyme‐inducing antiseizure medications (EIASMs; full analysis set). **P < .01 vs placebo
Figure 4.

Fifty percent responder rates (A), 75% responder rates (B), and seizure‐freedom rates (C) during maintenance for all focal seizures (all patients) and focal to bilateral tonic‐clonic (FBTC) seizures (patients who had FBTC seizures during baseline) in patients with and without enzyme‐inducing antiseizure medications (EIASMs; full analysis set). *P < .05, **P < .01, ***P < .001 vs placebo
In patients with FBTC seizures who were not receiving EIASMs, and compared with placebo, perampanel 4 mg/d was associated with significantly greater median percentage reductions in seizure frequency per 28 days (P = .0049; Figure 3) and 50% responder rates (P = .0118, Figure 4A). Seventy‐five percent responder and seizure‐freedom rates were also greater with perampanel 4 mg/d compared with placebo; however, statistical significance was not reached (P = .0648 and P = .0767, respectively; Figure 4B,C). Similarly to all focal seizures, in patients with FBTC seizures who were receiving EIASMs, there were no statistically significant differences reported for these efficacy outcomes (Figures 3 and 4A‐C).
For all focal seizures, median percentage reductions in seizure frequency per 28 days tended to be lower during the first weeks of treatment compared with the later weeks of treatment for both placebo (weeks 1‐2, 8.9%; weeks 15‐19, 18.7%) and perampanel 4 mg/d (weeks 1‐2, 16.3%; weeks 15‐19, 29.7%; Figure S2A). A similar pattern was seen in patients with focal seizures who were receiving concomitant EIASMs (Figure S2B), whereas in patients who were not receiving concomitant EIASMs, the early weeks of treatment with perampanel 4 mg/d showed similar efficacy to the later weeks (Figure S2C). In patients who had FBTC seizures during baseline, median percentage reductions in seizure frequency per 28 days were also lower during the first weeks of treatment compared with later weeks for placebo; however, the highest levels of efficacy appeared to be during the first 1‐4 weeks of treatment with perampanel 4 mg/d for all patients (Figure S2A), those receiving concomitant EIASMs (Figure S2B), and those not receiving concomitant EIASMs (Figure S2C). For both focal seizures and FBTC seizures, median percentage reductions in seizure frequency were consistently higher during each treatment week without concomitant EIASMs versus with concomitant EIASMs (Figures S2B,C). Further analysis may be required to determine whether any of these differences are statistically significant.
3.3. Safety outcomes
TEAEs were experienced by 419 of 1376 (30.5%) patients with focal seizures while receiving perampanel 4 mg/d at the onset of their TEAE(s) (Table 2). In the subgroup of patients who had FBTC seizures during baseline, 148 of 499 (29.7%) patients experienced TEAEs while receiving perampanel 4 mg/d at the onset of their TEAE(s) (Table 2). With perampanel 4 mg/d, the most common TEAEs in all patients, and in those who had FBTC seizures during baseline, were dizziness, somnolence, headache, and nasopharyngitis (Table 2). TEAEs were experienced by 248 of 859 (28.9%) patients with focal seizures receiving perampanel 4 mg/d with EIASMs and 171 of 517 (33.1%) patients without EIASMs.
Table 2.
Overall incidence of TEAEs and most frequent TEAEs (occurring in ≥1% of patients) in patients who were receiving perampanel 4 mg/d at the onset of their TEAE(s)
| All patients, n = 1376 | Patients with FBTC seizures during baseline, n = 499 | |
|---|---|---|
| TEAEs, n (%) | 419 (30.5) | 148 (29.7) |
| Dizziness | 100 (7.3) | 26 (5.2) |
| Somnolence | 49 (3.6) | 15 (3.0) |
| Headache | 43 (3.1) | 16 (3.2) |
| Nasopharyngitis | 38 (2.8) | 19 (3.8) |
| Fatigue | 20 (1.5) | 5 (1.0) |
| Vertigo | 19 (1.4) | 5 (1.0) |
| Irritability | 17 (1.2) | 4 (0.8) |
| Upper respiratory tract infection | 17 (1.2) | 5 (1.0) |
| Nausea | 15 (1.1) | 3 (0.6) |
| Weight increased | 14 (1.0) | 3 (0.6) |
| Rash | 9 (0.7) | 5 (1.0) |
Patients with ≥2 adverse events with the same preferred term are counted only once for that preferred term.
Abbreviations: FBTC, focal to bilateral tonic‐clonic; TEAE, treatment‐emergent adverse event.
Serious TEAEs were reported in 17 of 1376 (1.2%) patients, including six of 499 (1.2%) patients with FBTC seizures during baseline (Table S1). Convulsion was the only serious TEAE that occurred in >1 patient with focal seizures (n = 2). There was also one patient from study 335 who died while receiving perampanel 4 mg/d; however, the cause of death was unknown and was not deemed to be related to the study drug by the investigator.
TEAEs led to the discontinuation of 27 of 1376 (2.0%) patients, including seven of 499 (1.4%) patients with FBTC seizures during baseline (Table S2). Those that led to the discontinuation of more than one patient with focal seizures included convulsion (n = 5), vertigo (n = 4), dizziness (n = 3), and fatigue (n = 2). There were no TEAEs that led to the discontinuation of more than one patient in the subgroup of patients who had FBTC seizures during baseline.
Based on narrow and broad Standardized MedDRA Query terms, TEAEs related to hostility and/or aggression were reported in 23 of 1376 (1.7%) patients, including nine of 499 (1.8%) patients with FBTC seizures during baseline (Table S3). In patients with focal seizures, the most common TEAEs related to hostility and/or aggression were irritability (n = 17) and aggression (n = 5); in the subgroup of patients who had FBTC seizures during baseline, irritability and aggression were reported in four patients each. Of the 17 patients with focal seizures who experienced irritability while receiving perampanel 4 mg/d, four patients had a prior history of psychiatric disorders (depression [n = 2], abnormal behavior [n = 1], and insomnia [n = 1]); one patient who had a prior history of depression also had a history of irritability. Of the five patients with focal seizures who experienced aggression while receiving perampanel 4 mg/d, one patient had a prior history of psychiatric disorders (abnormal behavior, bruxism, insomnia, and poverty of speech) and four patients had no prior history of psychiatric disorders; none of these patients had a prior history of aggression or irritability.
4. DISCUSSION
This post hoc analysis of pooled phase III data supports the efficacy and tolerability of adjunctive perampanel 4 mg/d in patients (aged ≥12 years) with focal seizures, with or without FBTC seizures. Compared with placebo, perampanel 4 mg/d was associated with significantly greater reductions in seizure frequency and increased responder rates.
Findings were consistent with efficacy outcomes from a previous pooled analysis of data from studies 304, 305, and 306,7 although, as described in the introduction, the original analysis of study 335 data did not demonstrate significant reductions in seizure frequency with the 4‐mg/d dose (possibly due to the highly refractory population and frequent use of concomitant EIASMs).6 An important distinction is that these previous analyses were based on the randomized dose, whereas the present analysis was based on the modal dose. The modal dose may have differed from the randomized dose, because patients were permitted to have their dose reduced during the double‐blind treatment phase due to tolerability concerns. Therefore, although analyses based on randomized dose may be a more conservative approach, analyses based on modal dose may provide a more accurate reflection and ensure that all patients who received the relevant dose are included. For example, across studies 304, 305, 306, and 335, a total of 346 patients were included in the randomized 4‐mg/d dose group for efficacy assessments,6, 7 whereas in our analyses we identified a total of 363 patients who received a modal dose of 4 mg/d during these studies.
Previous analyses have shown that EIASMs can stimulate the metabolism of various coadministered ASMs, including valproic acid, tiagabine, ethosuximide, lamotrigine, topiramate, oxcarbazepine, zonisamide, felbamate, and to some extent, levetiracetam, which may cause a reduction in serum concentrations of these ASMs.10 Consistent with the finding that the randomized perampanel 4‐mg/d dose did not confer significant efficacy versus placebo in study 335, possibly due to the frequent use of EIASMs,6 results from our analysis showed that patients who received a modal dose of perampanel 4 mg/d in combination with EIASMs did not show improvements in seizure control versus placebo. In contrast, patients who received a modal dose of perampanel 4 mg/d without concomitant EIASMs showed significantly greater median percentage reductions in seizure frequency and responder rates compared with placebo. In addition, our data showed that during each treatment week, median percentage reductions in seizure frequency for all focal seizures were greater in patients receiving perampanel 4 mg/d without concomitant use of EIASMs versus with concomitant EIASMs, and that a higher level of efficacy was achieved during the early stages of perampanel treatment in those patients not receiving EIASMs. Similar to other common ASMs,10 it has previously been shown that EIASMs lower perampanel exposure8; therefore, patients receiving concomitant EIASMs may require a higher dose of perampanel to achieve similar efficacy to patients not receiving concomitant EIASMs.
In the present post hoc analysis, TEAE incidence was assessed in patients who were receiving perampanel 4 mg/d at the onset of their TEAE(s). This aimed to capture all TEAEs at the 4‐mg/d dose, not just in patients who were randomized to perampanel 4 mg/d, but also in those who received perampanel 4 mg/d during uptitration in the titration period even if this was only for a brief period, or who had their dose reduced to 4 mg/d due to tolerability concerns. Based on this approach, the proportion of patients who experienced a TEAE with perampanel 4 mg/d was relatively low (n = 419/1376 [30.5%]) compared with the incidences reported in analyses based on randomized dose (pooled TEAE incidences at randomized perampanel doses of 4 mg/d for studies 304, 305, and 306: n = 111/172 [64.5%]; study 335, n = 121/176 [68.8%]).6, 7 Similarly, the incidence of serious TEAEs in our analyses was also lower than previous analyses based on randomized dose (1.2% vs 3.4%–3.5%, respectively), and the incidence of TEAEs leading to discontinuation was similar to previous analyses (2.0% vs 2.9%‐4.5%, respectively).6, 7 In this analysis, the proportion of patients experiencing TEAEs was similar in patients with and without EIASMs. When interpreting the results of our study, it is important to consider the smaller patient populations of the analyses based on randomized dose and that a large number of patients in the current analyses received perampanel 4 mg/d for only a brief period prior to being uptitrated to higher perampanel doses.
Potential limitations of this analysis include those inherent to post hoc analyses and that this analysis was conducted across four different studies with different patient populations. In addition, given that this analysis was based on the actual dose of perampanel received, the duration for which each patient received the 4‐mg/d dose of perampanel will have differed. Due to the differences in the lengths of time that patients received treatment with placebo (randomized) versus perampanel 4 mg/d (modal dose) in this analysis (mean duration of exposure = 17.9 vs 5.1 weeks in the overall population with focal seizures), the incidence of TEAEs with perampanel 4 mg/d could not be compared with that reported in placebo‐treated patients, because this would not be an appropriate comparison.
5. CONCLUSION
These findings support the use of perampanel 4 mg/d as an efficacious treatment option for patients aged ≥12 years with focal seizures who are unable to tolerate higher perampanel doses (up to 12 mg/d). These data also support existing recommendations to start patients at a low dose of perampanel and increase the dose slowly until a clinical effect is achieved, thereby minimizing the potential for adverse events.1, 2 However, consideration should be given to those patients who are receiving concomitant EIASMs to determine whether perampanel 4 mg/d is an appropriate treatment option in these patients.
CONFLICT OF INTEREST
B.J.S. has received speaker's honoraria from Desitin Pharma, Eisai, GW Pharmaceuticals, Hikma, Novartis, Sandoz, and UCB Pharma; and has served as a paid consultant for B. Braun, Desitin Pharma, Eisai, GW Pharmaceuticals, and UCB Pharma. A.P. is an employee of Eisai Ltd. B.W. is a former employee of Eisai Inc, and has carried out consultancy work for Eisai Inc. M.M. is an employee of Eisai Inc. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
All authors provided substantial contributions to the conception and design of the post hoc analyses, acquisition of data, or the data analysis. All authors were involved in the interpretation of the results, in the reviewing and approval of the manuscript, and in the decision to submit the article for publication. All authors also confirm accountability for the accuracy and integrity of the work.
Supporting information
ACKNOWLEDGMENTS
Medical writing support, under the direction of the authors, was provided by Daniela DiBiase, MS, MPH, of CMC Affinity, McCann Health Medical Communications, funded by Eisai Inc, in accordance with Good Publication Practice (GPP3) guidelines. Studies 304, 305, and 306, as well as the present analysis, were funded by Eisai Inc. Study 335 was funded by Eisai Co Ltd. Some of the data reported in this paper have been previously presented at the 72nd Annual Meeting of the American Epilepsy Society, New Orleans, Louisiana, November 30 to December 4, 2018, the Annual Meeting of the Association of British Neurologists, Edinburgh, UK, May 21‐23, 2019, and the Annual Meeting of the Canadian League Against Epilepsy, Winnipeg, Manitoba, Canada, September 20‐22, 2019.
Steinhoff BJ, Patten A, Williams B, Malhotra M. Efficacy and safety of adjunctive perampanel 4 mg/d for the treatment of focal seizures: A pooled post hoc analysis of four randomized, double‐blind, phase III studies. Epilepsia. 2020;61:278–286. 10.1111/epi.16428
REFERENCES
- 1. Food and Drug Administration . Highlights of prescribing information. May 2019. Available at: https://www.fycompa.com/-/media/Files/Fycompa/Fycompa_Prescribing_Information.pdf. Accessed January 9, 2020.
- 2. European Medicines Agency . Annex I: summary of product characteristics. April 2017. Available at: https://www.ema.europa.eu/en/documents/product-information/fycompa-epar-product-information_en.pdf. Accessed January 9, 2020.
- 3. French JA, Krauss GL, Biton V, et al. Adjunctive perampanel for refractory partial‐onset seizures: randomized phase III study 304. Neurology. 2012;79:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. French JA, Krauss GL, Steinhoff BJ, et al. Evaluation of adjunctive perampanel in patients with refractory partial‐onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54:117–25. [DOI] [PubMed] [Google Scholar]
- 5. Krauss GL, Serratosa JM, Villanueva V, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial‐onset seizures. Neurology. 2012;78:1408–15. [DOI] [PubMed] [Google Scholar]
- 6. Nishida T, Lee SK, Inoue Y, Saeki K, Ishikawa K, Kaneko S. Adjunctive perampanel in partial‐onset seizures: Asia‐Pacific, randomized phase III study. Acta Neurol Scand. 2018;137:392–9. [DOI] [PubMed] [Google Scholar]
- 7. Steinhoff BJ, Ben‐Menachem E, Ryvlin P, et al. Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: a pooled analysis of three phase III studies. Epilepsia. 2013;54:1481–9. [DOI] [PubMed] [Google Scholar]
- 8. Gidal BE, Laurenza A, Hussein Z, et al. Perampanel efficacy and tolerability with enzyme‐inducing AEDs in patients with epilepsy. Neurology. 2015;84:1972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–30. [DOI] [PubMed] [Google Scholar]
- 10. Johannessen SI, Landmark CJ. Antiepileptic drug interactions—principles and clinical implications. Curr Neuropharmacol. 2010;8:254–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


