Abstract
The epidermal cells of flowers come in different shapes and have different functions, but how they evolved remains largely unknown. Floral micro‐texture can provide tactile cues to insects, and increases in surface roughness by means of conical (papillose) epidermal cells may facilitate flower handling by landing insect pollinators. Whether flower microstructure correlates with pollination system remains unknown.
Here, we investigate the floral epidermal microstructure in 29 (congeneric) species pairs with contrasting pollination system. We test whether flowers pollinated by bees and/or flies feature more structured, rougher surfaces than flowers pollinated by non‐landing moths or birds and flowers that self‐pollinate.
In contrast with earlier studies, we find no correlation between epidermal microstructure and pollination system. The shape, cell height and roughness of floral epidermal cells varies among species, but is not correlated with pollinators at large. Intriguingly, however, we find that the upper (adaxial) flower surface that surrounds the reproductive organs and often constitutes the floral display is markedly more structured than the lower (abaxial) surface.
We thus conclude that conical epidermal cells probably play a role in plant reproduction other than providing grip or tactile cues, such as increasing hydrophobicity or enhancing the visual signal.
Keywords: Colour, epidermal cones, grip, micro‐papillae, microstructure, pollination
The shape and size of floral epidermal cells differs between different flower sides, but is not associated with pollinator guild across different genera.
Introduction
The variety in shape and structure of flower surfaces and their function in terms of interactions between plants and insects has intrigued scientists for decades (e.g. Kay et al. 1981; Lee 2007; Whitney et al. 2011; Papiorek et al. 2014; van der Kooi et al. 2014; Ojeda et al. 2016). There are three types of epidermal cell shapes in flowers, distinguished by their shape. Flat cells are the least common type, but are characteristic of flowers in the buttercup genera Ranunculus and Ficaria (Ranunculaceae), contributing to their glossy appearance (Kay et al. 1981; Vignolini et al. 2012; van der Kooi et al. 2017). More common types of shapes are convex and cone‐shaped (Fig. 1). The epidermal cells of leaves and stems are generally flat, why then do flowers of so many species have cone‐shaped epidermal cells?
Figure 1.
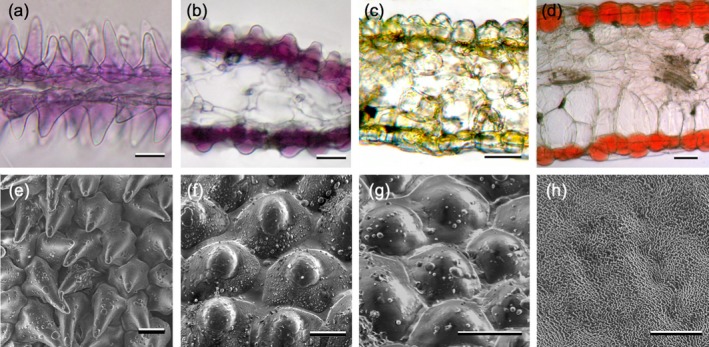
Variation in epidermal surface shape and structure of flowers. (a) Solanum citrullifolium, (b) Aquilegia vulgaris, (c) Impatiens scabrida, (d) Phaseolus coccineus, (e) Solanum citrullifolium, (f) Impatiens sodenii, (g) Nicotiana bonariensis, (h) Clarkia breweri. Scale bars: a, b, e–h: 20 μm, c, d: 50 μm.
At least three non‐mutually exclusive hypotheses as to the functional significance of cone‐shaped epidermal cells have been proposed. First, the cone shape may contribute to the flower’s visual signal by focusing or scattering incident light. Modelling studies suggested that under perpendicular illumination, conical epidermal cells could act as micro‐lenses that focus incident light on the floral pigments (Gorton & Vogelmann 1996; Wilts et al. 2018), resulting in reflected light being more strongly filtered by pigments and thus generating stronger flower coloration. However, experimental studies with bees showed that presence or absence of cones does not change the flower’s salience as perceived by bees (Dyer et al. 2007). Further, given the often strongly varying illumination in nature and varying epidermal cell shape and spacing – even between neighbouring cells (Fig. 1; van der Kooi et al. 2014; Fritz et al. 2017), it remains unclear whether focusing effects play a biologically meaningful role in natural conditions (reviewed by van der Kooi et al. 2019a).
Second, the roughness of a flower surface may play a role in the flower’s hydrophobicity and self‐cleaning. Surface roughness, by means of epidermal cones or cuticle striations, reduces the contact area – and thus adhesion – of water droplets and the flower, causing water to roll of the flower (Neinhuis & Barthlott 1997; Barthlott & Neinhuis 1997; Taneda et al. 2015). Indeed, both modelling and experimental approaches showed that epidermal cell shape (in combination with a structured cuticle) determines the wettability of flowers (Taneda et al. 2015).
Third, the flower surface may provide a tactile cue and/or mediate the amount of grip an insect has upon landing on the flower. A tactile role of flower surfaces was first shown by Kevan & Lane (1985), who demonstrated that bees can discriminate flowers based on surface microstructure. Further, the minute claws and hairs at the tarsi of flower‐visiting insects indeed adhere better to rough flower‐like surfaces (Voigt et al. 2012; Bräuer et al. 2017). Studies using isogenic Antirrhinum majus lines and flower surface replicas that differed solely in epidermal cell shape, confirmed that bees can discriminate flowers based on touch alone and that epidermal cones may provide grip to bees that visit the flowers (Whitney et al. 2009). The importance of microstructures in providing grip to visiting insects is further likely to increase with verticality of the floral display.
If conical epidermal cells provide grip to landing insects then, conversely, their absence could be an ‘anti‐bee’ adaptation (sensu Castellanos et al. 2004). For example, (nearly) flat and smooth epidermal cells may hamper flower handling by floral antagonists such as nectar‐robbing insects (Papiorek et al. 2014; Ojeda et al. 2017). The pitchers of some species of carnivorous plants (e.g. Nepenthes) feature flat epidermal cells that are indeed slippery, causing prey to slide into the pitcher and be digested (Gaume et al. 2002). By the same reasoning, there may be differences in surface structure between flower areas that are frequently touched by pollinating insects (e.g. the landing area or surfaces surrounding the nectaries and anthers) versus areas that will rarely be touched by pollinators, such as the lower (abaxial) side of the floral display.
Following the hypothesis that flower surfaces may provide grip and/or tactile cues to specific insect pollinators and having an unstructured surface can deter floral antagonists, we may expect epidermal surfaces to correlate with pollinator guild. Whereas bees and flies land on the flower to forage nectar and pollen, birds and hawkmoths do not land but hover in front of the flower while feeding. Some birds sit on a nearby branch while drinking nectar from the flower. Similarly, flowers that reproduce via uniparental reproduction (e.g. self‐pollination or asexual reproduction) will also not need to provide grip or tactile cues to landing insects, so surface structure may be one of the multiple (pollinator‐attracting) traits that degenerate in flowers of self‐pollinating plants (e.g. Goodwillie et al. 2010; Sicard & Lenhard 2011; Dart et al. 2012).
In this study, we investigate the evolution of flower surfaces by virtue of comparing closely related species with contrasting pollination or mating systems. We include 13 genera across nine angiosperm families, and in total compare the floral epidermal cell shape for 29 congeneric sister species pairs with vertically presented flowers that differ in pollination or mating system. We test whether (i) flower surfaces that are frequently touched and/or seen by insect pollinators are more structured (i.e. have higher and/or more pointy, conical epidermal cells) than areas that are invisible/inaccessible to pollinators, and whether (ii) surfaces of bee‐ and fly‐pollinated flowers will be more structured than species that are pollinated by hawkmoths, birds or via self‐fertilisation. We found that there is no large‐scale correlation of flower surface and pollinator guild or mating system, but that the adaxial surfaces of flowers are markedly more structured than abaxial surfaces, hinting at abiotic and/or visual effects as main drivers for flower surface evolution.
Material and Methods
Species used
Plant species pairs were chosen via a thorough literature research. We included species that were specialised in their pollinator guild inasmuch as that they are exclusively bee‐/fly‐, moth‐ or bird‐pollinated, not mixed. In choosing our study species, we followed the most effective pollinator principle and not the ‘pollination syndrome’ concept, because this has been proven to not hold for numerous plant groups (Ollerton et al. 2009; Funamoto & Ohashi 2017). For the same reason, we included only plant species for which actual pollinating species were reported, thus excluding cases for which only flower visitors were known. Although we realise that within each category a plant may be serviced by a wide array of different species (Waser et al. 1996), these pollinators have similar behaviour with respect to flower handling, that is, the plants are functionally specialised (sensu Ollerton et al. 2007). Species were classified as (obligate) selfer only when there was clear evidence to suggest that selfing was the main reproductive mode, because – as for the pollination syndrome concept – presence of a phenotypical trait that typically occurs in selfers need not be good evidence that the species is actually largely selfing (e.g. Lozada‐Gobilard et al. 2019). Phylogenetic relatedness was determined using recently published phylogenies (see Supplementary Information for information on pairs and references). We thus included 46 species of 13 genera in nine plant families that covered 29 species pairs pollinated by guilds with contrasting flower handling behaviour (Table 1). Flowers were taken from plants grown from seed in the University greenhouse (LD: 14 h:10 h, day:night temperature 22:20 °C) or in Botanical Gardens (see Supplementary Information for details on sources).
Table 1.
Summary of the taxa and pollinator mode included. Details regarding species pairs, pollinator guild/mating system and references are provided in the supplementary data file.
| family | genus | comparison groups (number of pairs) |
|---|---|---|
| Acanthaceae | Ruellia | Bee‐bird (1) |
| Balsaminaceae | Impatiens | Bee‐bird (4), bee‐moth (2) |
| Campanulaceae | Lobelia | Bee‐bird (1) |
| Hippobroma a | Bee‐moth (1) | |
| Caryophyllaceae | Silene | Bee‐bird (1), bee‐moth (1), outcrossing‐selfing (1) |
| Lamiaceae | Salvia | Bee‐bird (2) |
| Onagraceae | Clarkia | Bee‐moth (1) |
| Oenothera | Outcrossing‐selfing/asexual (1) | |
| Phrymaceae | Mimulus | Bee‐bird (1), bee‐moth (3), outcrossing‐selfing (1) |
| Polemoniaceae | Leptosiphon | Outcrossing‐selfing (1) |
| Solanaceae | Nicotiana | Bee‐bird (2), bee‐moth (1) |
| Petunia | Bee‐bird (1), bee‐moth (1) | |
| Solanum | Outcrossing‐selfing (2) |
All species pair comparisons are within one genus, except moth‐pollinated Hippobroma longiflora, which was compared with the closely related bee‐pollinated Lobelia siphilitica.
Investigation of flower surfaces
We first compared the adaxial and abaxial surfaces of 12 bee‐/fly‐pollinated species. For the comparison between pollinator guilds and mating systems, we compared the adaxial sides, for in the studied species the adaxial surface will be seen and touched by pollinators. The surface of the floral organ that constitutes the visual display (generally the petal) was examined regardless of its colour (pattern) using dental impression material (Provil Novo Light, Heraeus Kulzer, Hanau, Germany) as per van der Kooi et al. (2014). Immediately after picking the flower, it was pressed into the freshly prepared dental impression material that solidifies within minutes. The mould was subsequently filled with transparent nail polish (base coat, Hema, the Netherlands), creating a positive surface replica, i.e. a cast. The casts were examined under a microscope (Nikon Diaphot 300) with a 40× objective. The surface structure of the whole floral organ was examined and the most dominant structure (generally that on the central/distal area) was photographed with a Nikon D70 camera. Epidermal cell shape dimensions (below) were measured in Fiji (ImageJ, NIH). To avoid observer bias, the flower surfaces were observed and measured by an observer (MK) who had no prior knowledge of the pollinator, i.e. the observer was ‘blind’ (Holman et al. 2015; Kardish et al. 2015). Transverse sections of fresh flowers shown in Fig. 1 were obtained by photographing a transverse section of a piece of flower embedded in 6% agarose (for details, see van der Kooi & Stavenga 2019).
Analyses of surface parameters
For cells with a round or (quasi‐)hexagonal base, we measured the diameter of the cell’s base, and for cells with a rectangular base, we measured cell length and width (Fig. 2a, b). The protruding height was measured similarly for both types of epidermal cell (Fig. 2c, d). Preferably, we measured the floral surfaces of three individual plants per species, for which we succeeded in 34 out of 46 species (74%), but for nine species we could sample only two individuals and for three species only one. The approximate roughness of a surface was calculated via a ‘roughness index’, which is the ratio of the lateral, 3D surface area to the projected, geometric surface area of the cell’s base (following Aideo & Mohanta 2018). For cone‐shaped cells, the lateral surface area of the cone is described by:
and the cell base size by:
with L being the cone’s long side and d b the cell’s base diameter (see Fig. 2e). For rectangular cells, the lateral surface area was calculated as:
and the cell base size as:
with l, L and w being the base length, the long side length and width of the cells, respectively (see Fig. 2f). The roughness index is then the ratio of the lateral surface to the ground plane:
Figure 2.
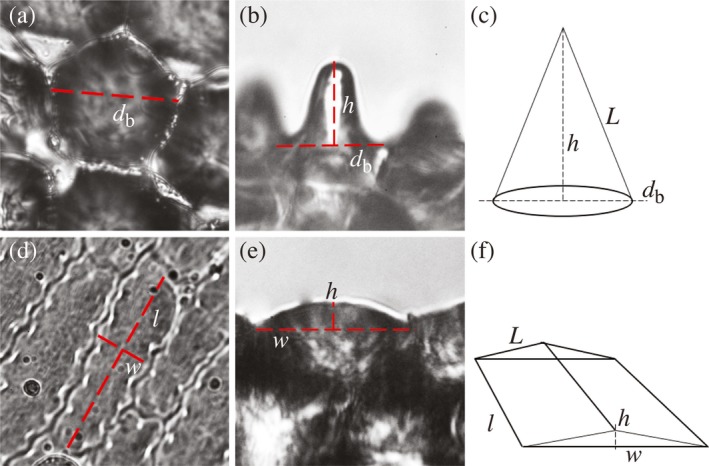
Measuring different types of epidermal cell shapes. Convex (or dome‐shaped) cells generally have a rectangular/elongated base cell shape, whereas conical epidermal cells have a circular/hexagonal base cell shape. Left column: shape and measurement of conical epidermal cells; right column: shape and measurement of convex cells. (a,d) top view, (b,e) side view, (c,f): measurements and calculations of different cells shapes. The roughness index was calculated as the ratio of the 3D lateral surface of the cell to the ground plane of the cell’s base (see Methods) db: diameter of cell base, l: cell base length, w: cell base width, h: protruding height, L: long side length.
For the exemplary cases shown in Fig. 1a–d, mean cell widths are 27, 22, 32 and 44 μm, respectively, and mean cell heights (measured as in Fig. 2c) are 34, 13, 10, 8 μm, respectively. The calculated roughness indices for these species then are 2.7, 1.6, 1.2 and 1.1, respectively, which is in line with the differences in roughness seen in Fig. 1a–d.
Statistical significance was tested via a parametric bootstrap test, using the pbkrtest package in R (R Foundation for Statistical Computing, Vienna, Austria). In the linear models, species was nested within genus as a random effect when testing the effect of flower side, and pair was nested within genus as random effect when testing the effect of pollination system. A likelihood ratio test (LRT) was used to see whether a model with the response variable (pollination system or flower side) fits better to the data than the same model without the response variable (1000 simulations). R script and data files are provided as Supporting Information.
Results
Our analysis of cell anatomy revealed significant differences between the adaxial and abaxial side of flowers (Fig. 3). The cell surface area is similar on both sides (LRT = 1.93, P = 0.18), but the adaxial cell height is on average twice that of the abaxial side (LRT = 48.98, P < 0.001). As cell height does not adequately capture overall surface curvature, we also calculated a roughness index, which is the ratio of the cell’s outer surface to that of the flat, projected cell surface area (Methods, Fig. 2). Analogous to the aspect ratio, the roughness index thus summarises the cell’s curvature relative to its ground plane. Indeed, the adaxial roughness index values are significantly larger than those of the abaxial surface (Fig. 3c; LRT = 44.3, P < 0.001). In ten out of the 12 studied species, the adaxial epidermal cells are higher than the abaxial cells; in one species it is approximately the same and in one there is a small decrease in height; the roughness shows a similar effect (Fig. 3b, c). In the vast majority, cone‐shaped epidermal cells are thus particularly prominent at the adaxial side, and species with cone‐shaped cells on both sides (e.g. Solanum citrullifolium; Fig. 1a) are relatively rare.
Figure 3.
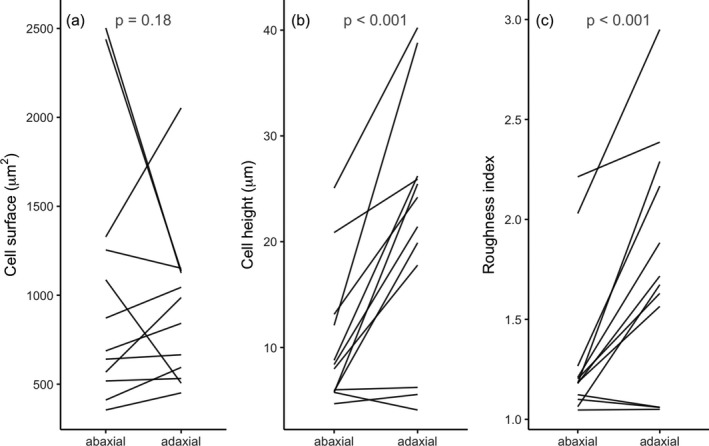
Floral epidermal cell shape for different flower sides. (a) Cell surface, (b) cell height, (c) roughness index. Each line connects the mean values of different sides of the flower. [Correction added on 10 January 2020 after first publication: the figure caption has been updated in this version.]
In contrast to observations between different sides of the flower, adaxial epidermal cell shape does not consistently differ between species with contrasting pollinators (Fig. 4; n = 29 species pairs, for all comparisons). Neither cell surface area (LRT = 2.57, P = 0.11), cell height (LRT = 0.21, P = 0.2) nor roughness index (LRT = 0.41, P = 0.52) differs significantly between sister species. Also, when compared per pollinator guild, none are significantly different in epidermal cell height or roughness (Table S1).
Figure 4.
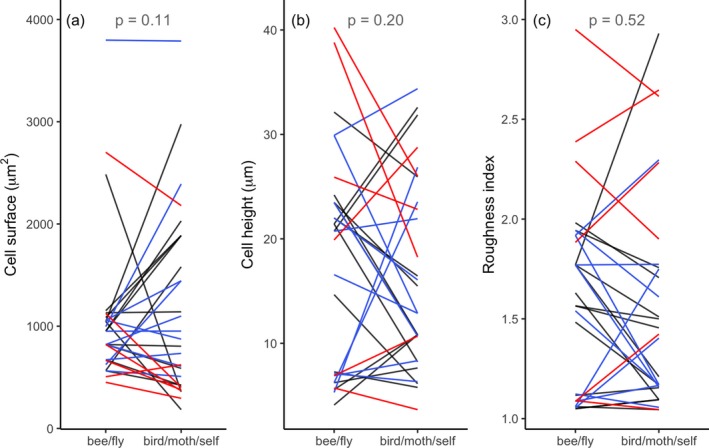
Floral epidermal cell shape in species pairs with different pollination systems. (a) Cell surface, (b) cell height, (c) roughness index. Each line connects the mean values of a species‐pair. Different colours represent different pollinator guilds or mating systems, bee/fly versus bird (black), moth (blue) and self‐pollination (red).
In line with the reduction in floral display size commonly found in selfers (Goodwillie et al. 2010; Sicard & Lenhard 2011; Dart et al. 2012) is our observation that epidermal cell size is smaller in five out of six outcrossing–selfing species pairs (see the red curves in Fig. 4a). Floral epidermal cells of selfers are approximately 35% smaller in surface area than epidermal cells in related outcrossers, a difference that is marginally significant (P = 0.036, n = 6 species pairs; Table S1). A similar trend is visible in epidermal cells of moth‐pollinated flowers, although this is not significant (P = 0.072, n = 10 species pairs; Table S1).
Discussion
Flower surface does not correlate with pollination system
In our investigation of the microstructure of flowers with contrasting pollinators, we found no indication of large‐scale correlation of epidermal cell shape and pollination system (Fig. 4). The fact that the adaxial surface, which generally faces pollinators and is close to the reproductive organs, is markedly more structured than the abaxial surface (Fig. 3) suggests, however, that surface roughness fulfils a biologically meaningful function. Further, there is an effect of mating system inasmuch as that selfing plants have smaller epidermal cells than their outcrossing relatives (Fig. 4a; Table S1). This indicates that the reduction in floral display size commonly found in selfers (Goodwillie et al. 2010; Sicard & Lenhard 2011; Dart et al. 2012) is due, at least partly, to a reduction in cell size. Whether the number of epidermal cells, as well as the size and number of interior cells, also decrease in selfers requires additional investigation.
The fact that we find no correlation of flower epidermal cell shape and pollinator is not because sister species have not (yet) diverged sufficiently to develop phenotypic differences or because our method is inadequate to detect a biological signal. Indeed, we found up to three‐fold differences in epidermal cell surface, height and roughness index between sister species, albeit in opposite directions (Fig. 4); hence the absence of an overall pattern. In addition, (genetic) studies corroborate that shape of floral epidermal cells is evolutionarily labile (Glover et al. 1998; van Houwelingen et al. 1998; Di Stilio et al. 2009; Ojeda et al. 2017). Even within species, floral epidermal cell shape can vary; for example, a recent study on conical epidermal cells of 32 (cultivated) Vicia faba lines showed that 13% of the lines featured deviant epidermal cell shapes (Bailes & Glover 2018).
Our study does not invalidate previous experiments on tactile or grip effects of flower surfaces (Kevan & Lane 1985; Whitney et al. 2009), yet it does suggest that providing grip and/or tactile cues are not the main function of epidermal cones, at least not across angiosperms broadly. Thus, other functions are expected to shape flower surface microstructure. Our findings deviate from some earlier studies. Papiorek et al (2014) reported differences in epidermal cell shape of 58 species with bird or bee pollination; however, as they compared largely unrelated species, the observed phenotypic differences may be due to, for example, phylogenetic effects rather than pollinator guild. Ojeda et al. (2016) compared insect‐ and bird‐pollinated flowers and found that a transition to bird pollination confers a loss in epidermal cones. The discrepancy with our results may be explained by the fact that their sampling included relatively few species pairs (five comparison groups) and/or was based on categorical classification of cell shape. Our results dovetail those recently reported in two species of Nymphaeaceae with contrasting pollinators but no difference in flower surface (Coiro & Barone Lumaga 2018), and with those in a study on surfaces of 11 (unrelated) plant species with different pollinators (Costa et al. 2017).
It remains unknown how striations in the flower’s cuticle contribute to the mechanical interaction with pollinators. In the studied species, the frequent lack of cuticle structure precluded analysis of whether cuticle differences, if any, are linked to specific pollinators. Deeper species sampling would help to elucidate the importance of phylogeny on flower surface evolution. Additionally, in the systems so far considered, bird pollination was derived from bee/fly pollination and not vice versa, reflecting the asymmetry in the direction of transitions between insect and bird pollination found globally (Thomson & Wilson 2008; Barrett 2013). It remains to be investigated whether transitions from bird to bee pollination yield a systematic difference in epidermal cell shape, although we consider that unlikely.
Adaxial flower side markedly more structured than abaxial side
The marked differences between the adaxial and abaxial surfaces (Fig. 3), combined with our observation that epidermal cell shape does not correlate with pollinator guild, suggest that cone‐shaped epidermal cells occur for reasons other than providing grip. Adaxial surfaces generally surround the reproductive organs, at least in the studied species, and having cone‐shaped cells may increase water repellence and even self‐cleaning of the flower (Neinhuis & Barthlott 1997; Barthlott & Neinhuis 1997; Watanabe‐Taneda & Taneda 2019), which could benefit flower visibility and longevity, and ultimately the plant’s reproductive success. Cone‐shaped epidermal cells could also modify the reflection of light by reducing the surface gloss and thus create a uniform visual signal that is well visible to pollinators approaching from different angles (van der Kooi et al. 2019a). Similar cone‐like structures were found to reduce surface gloss of some animal organs, such as moth eyes and snake skin (Stavenga et al. 2006; Spinner et al. 2013). Further, a higher surface roughness means an increase in surface area, which may lead to changes in transpiration rates (Buschhaus et al. 2015), flower temperature (van der Kooi et al. 2019b) and scent emission (Effmert et al. 2005).
The asymmetry in epidermal cell shape between adaxial and abaxial flower sides may be linked with the pigmentary aspects of the flower’s visual signal. Many species that have flowers with conical epidermal cells have pigments that occur in the epidermal cells only (e.g. flavonoids and anthocyanins; Kay et al. 1981; Grotewold 2006; Lee 2007; van der Kooi et al. 2016). Anthocyanin‐pigmented flowers often feature clear differences in pigmentation between abaxial and adaxial flower sides, resulting in only the pigmented side being strongly coloured, creating marked differences in coloration between the different sides (Stavenga & van der Kooi 2016; van der Kooi et al. 2016). Asymmetry in flower side coloration can be an efficient way of filtering reflected light with relatively little pigment (van der Kooi et al. 2016), and having only one coloured side can reduce conspicuousness to floral antagonists when the flower is closed (Kemp & Ellis 2019). Whether (asymmetry in) epidermal cell shape and pigmentation are indeed coupled requires further anatomical and optical investigation. Of particular interest would be to study the surfaces of species with hanging, pendant flowers where the abaxial flower side constitutes the visual signal.
Author contribution
CJvdK designed the study, both authors collected the data, MK made the photographs and performed measurements, both authors performed the analyses and CJvdK wrote the manuscript. Both authors approved the final version of the manuscript.
Supporting information
Table S1. P‐values for different comparisons. The p‐values obtained for the different sublevels were Bonferroni corrected for multiple testing.
Data S1. Information on species‐pairs, data file and R script.
Acknowledgements
The authors thank the Botanical Garden of Utrecht and in particular Hans Persoon for generously granting access to their collection and on‐site support. We further thank Carol Goodwillie, Dena Grossenbacher, Kathleen Kay, Francesca Quattrocchio and Mario Vallejo‐Marin for generously providing seeds. Doekele Stavenga, Marten Staal and Hein Leertouwer are acknowledged for their assistance throughout the study, and three reviewers for providing comments. This research was funded by a NWO Veni grant (number 016.Veni.181.025) and AFOSR/EOARD (grant FA9550‐15‐1‐0068). Data for this study were obtained during MK’s internship for her studies at Hanze University of Applied Sciences.
References
- Aideo S.N., Mohanta D. (2018) Surface‐wettability and structural colouration property of certain Rosaceae cultivars with off‐to‐dark pink appearances. Journal of Bionic Engineering, 15, 1012–1024. [Google Scholar]
- Bailes E.J., Glover B.J. (2018) Intraspecific variation in the petal epidermal cell morphology of Vicia faba L. (Fabaceae). Flora, 244–245, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett S.C.H. (2013) The evolution of plant reproductive systems: how often are transitions irreversible? Proceedings of the Royal Society B: Biological Sciences, 280, 20130913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthlott W., Neinhuis C. (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta, 202, 1–8. [Google Scholar]
- Bräuer P., Neinhuis C., Voigt D. (2017) Attachment of honeybees and greenbottle flies to petal surfaces. Arthropod‐Plant Interactions, 11, 171–192. [Google Scholar]
- Buschhaus C., Hager D., Jetter R., Feldmann K.A. (2015) Wax layers on Cosmos bipinnatus petals contribute unequally to total petal water resistance. Plant Physiology, 167, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos M.C., Wilson P., Thomson J.D. (2004) “Anti‐bee” and “pro‐bird” changes during the evolution of hummingbird pollination in Penstemon flowers. Journal of Evolutionary Biology, 17, 876–885. [DOI] [PubMed] [Google Scholar]
- Coiro M., Barone Lumaga M.R. (2018) Disentangling historical signal and pollinator selection on the micromorphology of flowers: an example from the floral epidermis of the Nymphaeaceae. Plant Biology, 20, 902–915. [DOI] [PubMed] [Google Scholar]
- Costa V.B.S., Pimentel R.M.M., Chagas M.G.S., Alves G.D., Castro C.C. (2017) Petal micromorphology and its relationship to pollination. Plant Biology, 19, 115–122. [DOI] [PubMed] [Google Scholar]
- Dart S.R., Samis K.E., Austen E., Eckert C.G. (2012) Broad geographic covariation between floral traits and the mating system in Camissoniopsis cheiranthifolia (Onagraceae): multiple stable mixed mating systems across the species’ range? Annals of Botany, 109, 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stilio V.S., Martin C., Schulfer A.F., Connelly C.F. (2009) An ortholog of MIXTA‐like2 controls epidermal cell shape in flowers of Thalictrum . New Phytologist, 183, 718–728. [DOI] [PubMed] [Google Scholar]
- Dyer A.G., Whitney H.M., Arnold S.E.J., Glover B.J., Chittka L. (2007) Mutations perturbing petal cell shape and anthocyanin synthesis influence bumblebee perception of Antirrhinum majus flower colour. Arthropod‐Plant Interactions, 1, 45–55. [Google Scholar]
- Effmert U., Große J., Röse U.S.R., Ehrig F., Kägi R., Piechulla B. (2005) Volatile composition, emission pattern, and localization of floral scent emission in Mirabilis jalapa (Nyctaginaceae). American Journal of Botany, 92, 2–12. [DOI] [PubMed] [Google Scholar]
- Fritz B., Hünig R., Schmager R., Hetterich M., Lemmer U., Gomard G. (2017) Assessing the influence of structural disorder on the plant epidermal cells’ optical properties: a numerical analysis. Bioinspiration & Biomimetics, 12, 036011. [DOI] [PubMed] [Google Scholar]
- Funamoto D., Ohashi K. (2017) Hidden floral adaptation to nocturnal moths in an apparently bee‐pollinated flower, Adenophora triphylla var. japonica (Campanulaceae). Plant Biology, 19, 767–774. [DOI] [PubMed] [Google Scholar]
- Gaume L., Gorb S., Rowe N. (2002) Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytologist, 156, 479–489. [DOI] [PubMed] [Google Scholar]
- Glover B.J., Perez‐Rodriguez M., Martin C. (1998) Development of several epidermal cell types can be specified by the same MYB‐related plant transcription factor. Development, 125, 3497–3508. [DOI] [PubMed] [Google Scholar]
- Goodwillie C., Sargent R.D., Eckert C.G., Elle E., Geber M.A., Johnston M.O., Kalisz S., Moeller D.A., Ree R.H., Vallejo‐Marin M. (2010) Correlated evolution of mating system and floral display traits in flowering plants and its implications for the distribution of mating system variation. New Phytologist, 185, 311–321. [DOI] [PubMed] [Google Scholar]
- Gorton H.L., Vogelmann T.C. (1996) Effects of epidermal cell shape and pigmentation on optical properties of Antirrhinum petals at visible and ultraviolet wavelengths. Plant Physiology, 112, 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E. (2006) The genetics and biochemistry of floral pigments. Annual Review of Plant Biology, 57, 761–780. [DOI] [PubMed] [Google Scholar]
- Holman L., Head M.L., Lanfear R., Jennions M.D. (2015) Evidence of experimental bias in the life sciences: Why we need blind data recording. PLoS Biology, 13, e1002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houwelingen A., Souer E., Spelt K., Kloos D., Mol J., Koes R. (1998) Analysis of flower pigmentation mutants generated by random transposon mutagenesis in Petunia hybrida . The Plant Journal, 13, 39–50. [DOI] [PubMed] [Google Scholar]
- Kardish M.R., Mueller U.G., Amador‐Vargas S., Dietrich E.I., Ma R., Barrett B., Fang C.C. (2015) Blind trust in unblinded observation in ecology, evolution, and behavior. Frontiers in Ecology and Evolution, 3, 51. [Google Scholar]
- Kay Q.O.N., Daoud H.S., Stirton C.H. (1981) Pigment distribution, light reflection and cell structure in petals. Botanical Journal of the Linnean Society, 83, 57–83. [Google Scholar]
- Kemp J.E., Ellis A.G. (2019) Cryptic petal coloration decreases floral apparency and herbivory in nocturnally closing daisies. Functional Ecology, 33, 2130–2141. [Google Scholar]
- Kevan P.G., Lane M.A. (1985) Flower petal microtexture is a tactile cue for bees. Proceedings of the National Academy of Sciences of the United States of America, 82, 4750–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi C.J., Stavenga D.G. (2019) Vividly coloured poppy flowers due to dense pigmentation and strong scattering in thin petals. Journal of Comparative Physiology. A, 205, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi C.J., Wilts B.D., Leertouwer H.L., Staal M., Elzenga J.T.M., Stavenga D.G. (2014) Iridescent flowers? Contribution of surface structures to optical signaling. New Phytologist, 203, 667–673. [DOI] [PubMed] [Google Scholar]
- van der Kooi C.J., Elzenga J.T.M., Staal M., Stavenga D.G. (2016) How to colour a flower: on the optical principles of flower coloration. Proceedings of the Royal Society B: Biological Sciences, 283, 20160429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi C.J., Elzenga J.T.M., Dijksterhuis J., Stavenga D.G. (2017) Functional optics of glossy buttercup flowers. Journal of the Royal Society Interface, 17, 20160933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi C.J., Dyer A.G., Kevan P.G., Lunau K. (2019a) Functional significance of the optical properties of flowers for visual signalling. Annals of Botany, 123, 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi C.J., Kevan P.G., Koski M.H. (2019b) The thermal ecology of flowers. Annals of Botany, 124, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.W. (2007) Nature’s palette. The science of plant color. University of Chicago Press, Chicago, IL, USA. [Google Scholar]
- Lozada‐Gobilard S., Weigend M., Fischer E., Janssens S.B., Ackermann M., Abrahamczyk S. (2019) Breeding systems in Balsaminaceae in relation to pollen/ovule ratio, pollination syndromes, life history and climate zone. Plant Biology, 21, 157–166. [DOI] [PubMed] [Google Scholar]
- Neinhuis C., Barthlott W. (1997) Characterization and distribution of water‐repellent, self‐cleaning plant surfaces. Annals of Botany, 79, 667–677. [Google Scholar]
- Ojeda D.I., Valido A., Fernández De Castro A.G., Ortega‐Olivencia A., Fuertes‐Aguilar J., Carvalho J.A., Santos‐Guerra A. (2016) Pollinator shifts drive petal epidermal evolution on the Macaronesian Islands bird‐flowered species. Biology Letters, 12, 20160022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda D.I., Jaén‐Molina R., Santos‐Guerra A., Caujape‐Castells J., Cronk Q. (2017) Temporal, but not spatial, changes in expression patterns of petal identity genes are associated with loss of papillate conical cells and the shift to bird pollination in Macaronesian Lotus (Leguminosae). Plant Biology, 19, 420–427. [DOI] [PubMed] [Google Scholar]
- Ollerton J., Killick A., Lamborn E., Watts S., Whiston M. (2007) Multiple meanings and modes: on the many ways to be a generalist flower. Taxon, 56, 717–728. [Google Scholar]
- Ollerton J., Alarcon R., Waser N.M., Price M.V., Watts S., Cranmer L., Hingston A., Peter C.I., Rotenberry J. (2009) A global test of the pollination syndrome hypothesis. Annals of Botany, 103, 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiorek S., Junker R.R., Lunau K. (2014) Gloss, colour and grip: multifunctional epidermal cell shapes in bee‐and bird‐pollinated flowers. PLoS ONE, 9, e112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A., Lenhard M. (2011) The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Annals of Botany, 107, 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner M., Kovalev A., Gorb S.N., Westhoff G. (2013) Snake velvet black: Hierarchical micro‐ and nanostructure enhances dark colouration in Bitis rhinoceros . Scientific Reports, 3, 1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga D.G., van der Kooi C.J. (2016) Coloration of the Chilean Bellflower, Nolana paradoxa, interpreted with a scattering and absorbing layer stack model. Planta, 243, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga D., Foletti S., Palasantzas G., Arikawa K. (2006) Light on the moth‐eye corneal nipple array of butterflies. Proceedings of the Royal Society B: Biological Sciences, 273, 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneda H., Watanabe‐Taneda A., Chhetry R., Ikeda H. (2015) A theoretical approach to the relationship between wettability and surface microstructures of epidermal cells and structured cuticles of flower petals. Annals of Botany, 115, 923–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J.D., Wilson P. (2008) Explaining evolutionary shifts between bee and hummingbird pollination: convergence, divergence, and directionality. International Journal of Plant Sciences, 169, 23–38. [Google Scholar]
- Vignolini S., Thomas M.M., Kolle M., Wenzel T., Rowland A., Rudall P.J., Baumberg J.J., Glover B.J., Steiner U. (2012) Directional scattering from the glossy flower of Ranunculus: how the buttercup lights up your chin. Journal of the Royal Society Interface, 9, 1295–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt D., Schweikart A., Fery A., Gorb S. (2012) Leaf beetle attachment on wrinkles: isotropic friction on anisotropic surfaces. Journal of Experimental Biology, 215, 1975–1982. [DOI] [PubMed] [Google Scholar]
- Waser N.M., Chittka L., Price M.V., Williams N.M., Ollerton J. (1996) Generalization in pollination systems, and why it matters. Ecology, 77, 1043–1060. [Google Scholar]
- Watanabe‐Taneda A., Taneda H. (2019) Interspecific variations in the surface wettability and morphological traits of petals across 125 plant species. Flora, 257, 151417. [Google Scholar]
- Whitney H.M., Chittka L., Bruce T.J.A., Glover B.J. (2009) Conical epidermal cells allow bees to grip flowers and increase foraging efficiency. Current Biology, 19, 948–953. [DOI] [PubMed] [Google Scholar]
- Whitney H.M., Bennett K.M.V., Dorling M., Sandbach L., Prince D., Chittka L., Glover B.J. (2011) Why do so many petals have conical epidermal cells? Annals of Botany, 108, 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilts B.D., Rudall P.J., Moyroud E., Gregory T., Ogawa Y., Vignolini S., Steiner U., Glover B.J. (2018) Ultrastructure and optics of the prism‐like petal epidermal cells of Eschscholzia californica (California poppy). New Phytologist, 219, 1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. P‐values for different comparisons. The p‐values obtained for the different sublevels were Bonferroni corrected for multiple testing.
Data S1. Information on species‐pairs, data file and R script.


