Abstract
Nasopharyngeal carcinoma (NPC) is originated from the epithelial cells of nasopharynx, Epstein–Barr virus (EBV)‐associated and has the highest incidence and mortality rates in Southeast Asia. Late presentation is a common issue and early detection could be the key to reduce the disease burden. Sensitivity of plasma EBV DNA, an established NPC biomarker, for Stage I NPC is controversial. Most newly reported NPC biomarkers have neither been externally validated nor compared to the established ones. This causes difficulty in planning for cost‐effective early detection strategies. Our study systematically evaluated six established and four new biomarkers in NPC cases, population controls and hospital controls. We showed that BamHI‐W 76 bp remains the most sensitive plasma biomarker, with 96.7% (29/30), 96.7% (58/60) and 97.4% (226/232) sensitivity to detect Stage I, early stage and all NPC, respectively. Its specificity was 94.2% (113/120) against population controls and 90.4% (113/125) against hospital controls. Diagnostic accuracy of BamHI‐W 121 bp and ebv‐miR‐BART7‐3p were validated. Hsa‐miR‐29a‐3p and hsa‐miR‐103a‐3p were not, possibly due to lower number of advanced stage NPC cases included in this subset. Decision tree modeling suggested that combination of BamHI‐W 76 bp and VCA IgA or EA IgG may increase the specificity or sensitivity to detect NPC. EBNA1 99 bp could identify NPC patients with poor prognosis in early and advanced stage NPC. Our findings provided evidence for improvement in NPC screening strategies, covering considerations of opportunistic screening, combining biomarkers to increase sensitivity or specificity and testing biomarkers from single sampled specimen to avoid logistic problems of resampling.
Keywords: systematic comparison, biomarkers, early detection, prognosis, Epstein–Barr virus, nasopharyngeal carcinoma
Short abstract
What's new?
Plasma Epstein–Barr virus (EBV) DNA is an established nasopharyngeal carcinoma (NPC) biomarker, but not all cases are associated with EBV and its sensitivity for stage I NPC remains controversial. Meanwhile, most newly‐reported NPC biomarkers have neither been externally validated nor compared to established biomarkers. This study systematically evaluates six established and four new biomarkers in NPC cases, population controls, and hospital controls. The findings provide evidence to policymakers for improvement in NPC screening and monitoring strategies, covering considerations of opportunistic screening, combining biomarkers to increase sensitivity/specificity, and testing multiple biomarkers on single specimens to avoid the logistic problems of resampling.
Abbreviations
- AJCC
American Joint Committee on Cancer
- ASR
age standardized rate
- AUC
area under curve
- EA
early antigen
- EBNA‐1
EBV nuclear antigen 1
- EBV
Epstein–Barr virus
- ICC
intraclass correlation coefficient
- LMICs
low‐ and middle‐income countries
- NPC
nasopharyngeal carcinoma
- qPCR
quantitative polymerase chain reaction
- ROC
receiver operating characteristic
- RT
reverse transcription
- VCA
viral capsid antigen
Background
Nasopharyngeal carcinoma (NPC) is an epithelial malignancy originating from the fossa of Rosenmüller of the nasopharynx. Its distribution is geographically distinct, with natives of Borneo Island, people in Southeast Asia and the Southern part of China having high age standardized rate (ASR) but is uncommon in most part of the world.1, 2 Among the top 20 countries with highest incidence and mortality rates of NPC,3 17 are low‐ and middle‐income countries (LMICs), 10 of which are located in Southeast Asia. It is known that the family members of NPC patients have two to nine folds higher risk in developing NPC.4, 5, 6, 7 The lowest social class group had 4.1 odds ratio in developing NPC.8 NPC is radiosensitive when treated early, with 5‐year overall survival rate ranging from 78% to 100% (early stage) to as low as 26% (late stage and recurrent cases).9, 10, 11 Recently, a study revealed that over 75% of cancer patients in Southeast Asia experienced death or financial catastrophe within 1 year of cancer diagnosis, mainly due to the lack of early detection and affordable cancer care.12 As the majority of NPC patients present at late stage,13 early detection could be the key to reduce the disease burden caused by NPC in LMICs.
Interaction among genes, environmental exposure and the Epstein–Barr virus (EBV) are the key events leading to NPC pathogenesis. Majority of NPC cases (>95%, except for the WHO keratinizing NPC subtype) are associated with EBV.14 EBV is commonly detected in the tumor cells, blood and urine of NPC patients.15 Over decades of research, EBV serology and plasma EBV DNA tests have become the established circulating biomarkers known to have high diagnostic performance in distinguishing NPC from controls.15 Recent evidences showed that combination of serum viral capsid antigen (VCA) IgA and EBV nuclear antigen 1 (EBNA‐1) IgA tests by ELISA could outperform single serology marker test in a case–control study16 as well as in a cluster randomized screening trial17 among the southern Chinese populations. The percentage of early stage NPC cases (Stages I and II) detected by the combination of these two serology markers during screening were higher (68.3%) as compared to unscreened populations in the screening towns (36.0%) and control towns (25.7%).17 However, the seropositive rate of about 3% in a screening setting may still lead to a considerable burden on the resource low health care system in LMICs to conduct close follow‐up for individuals with positive screening results. Meanwhile, plasma EBV DNA test is long known to have high sensitivity and specificity to distinguish NPC from controls when optimal experimental protocols were carried out, but there were concerns about its utility in detecting early stage NPC and recurrent NPC.15, 18 Of note, these EBV DNA case–control studies analyzed small sample size of Stage I NPC cases.15, 19 Recently, a large NPC screening study conducted in Hong Kong demonstrated that plasma EBV DNA test (BamHI‐W 76 bp) could identify a significantly higher proportion of participants with early stage NPC as compared to the unscreened historical cohort (70.6% vs. 19.2%).20 The same study group subsequently reported that EBV DNA fragment size profiles of NPC patients are different from the small subset of general population who was transiently positive for plasma EBV DNA.21
According to the US National Cancer Institute's Early Detection Research Network, there are five phases for developing and validating biomarkers.22 Despite the established EBV DNA tests and EBV serology tests which had already reach Phase 5 (Cancer Control), the pursuit of new NPC biomarkers continues for two main reasons: (i) keratinizing NPC subtype and recurrent NPC have reduced or absence of biomarkers originating from EBV,15 and (ii) EBV is also associated with many other diseases23 and biomarkers of non‐EBV origin may help to reduce the false positive rate. Among the newly reported circulating biomarkers for NPC, serum ebv‐BART2‐5p, plasma ebv‐miR‐BART7‐3p, ebv‐miR‐BART13‐3p, hsa‐miR‐29a‐3p, hsa‐miR‐103a‐3p, hsa‐miR‐483‐5p and hsa‐let‐7c had moderately good diagnostic accuracy (area under curve [AUC] > 0.7) in detecting NPC against controls.24, 25, 26 Meanwhile, other newly reported circulating biomarkers had AUC < 0.7,27, 28, 29 were identified from studies with normalization methods which are suboptimal for circulating biomarkers30, 31 and/or required additional processing or enrichment steps.19, 32 The reliability and diagnostic accuracy of these new biomarkers for early detection of NPC await validation by external independent studies (Phase 2, Clinical Assay and Validation) and should be evaluated together with the established EBV DNA and serology tests.
Malaysia is a country inhabited by multiethnic groups with different ASRs of NPC. Highest ASR of NPC (30 per 100,000) was observed in Bidayuh males, followed by Bidayuh females, Chinese males, Iban males and Kadazan males (10–20 per 100,000). Malay males, Chinese females, Iban females and Kadazan females have intermediate ASR of NPC (3.3–5.9 per 100,000), while lowest ASR of NPC (0.6–1.3 per 100,000) was observed in Malay females, Indian males and females.33, 34, 35 According to the Malaysian National Cancer Registry Report 2007–2011, NPC was the cancer with the highest ASR among Malaysian men between 26 and 45 years old.35 Despite the progress of NPC screening studies in southern China, NPC screening is yet to be adopted in Malaysia, due to less characterized population baseline values and uncertainty in the application of single or combination of biomarkers for screening. In Malaysia, histological examination of nasoendoscopic biopsy samples remains the gold standard to diagnose NPC. Computerized tomography is limited to major centers while magnetic resonance imaging and positron emission tomography are not routinely available to most NPC patients. Due to the confusing and nonspecific nature of early stage NPC symptoms,13 as well as the invasive and difficult accessibility of nasoendoscopic biopsy tests mandatory to confirm the presence of tumor (nasoendoscopy is only performed by trained otorhinolaryngologists in major centers), late presentation is a common issue.13
Our study aimed to evaluate the diagnostic performance of six established NPC biomarkers, consisting of two EBV DNA (BamHI‐W 76 bp and EBNA1 99 bp) and four anti‐EBV antibodies (early antigen [EA] IgA, EA IgG, EBNA‐1 IgA and VCA IgA), in local NPC cases, population controls and hospital controls. In addition, the performance of four newly reported NPC biomarkers, including one EBV DNA (BamHI‐W 121 bp) and three miRNAs (ebv‐miR‐BART7‐3p, hsa‐miR‐29a‐3p and hsa‐miR‐103a‐3p) were evaluated in a subset of our study. It is hoped that single or combination of tests optimal for early detection and prognosis of NPC can be identified to improve strategies for NPC screening and monitoring.
Materials and Methods
Participants and blood samples collection
Participants were recruited from hospitals and National Blood Bank from year 2008 to 2017. Ethics approval was obtained from the Medical Research and Ethics Committee, Ministry of Health Malaysia. Signed informed consent was obtained from histologically confirmed NPC patients, population controls (apparently healthy asymptomatic individuals) and hospital controls (patients without any cancer, EBV related diseases or ear‐nose‐throat diseases). Blood samples were collected in EDTA tubes and processed within 4 hr. Blood tubes were centrifuged at room temperature for 10 min at 2,500 RPM, and plasma aliquoted into separate cryogenic tubes and stored at −80°C. The numbers of samples analyzed for each test are stated in Table 1. Staging for NPC was based on the American Joint Committee on Cancer (AJCC) 7th edition and completion of radical treatment was defined as receiving a minimum of 66 Gy of radiotherapy. Survival information was retrieved from National Registration Department, Ministry of Home Affairs.
Table 1.
Diagnostic performance of 10 plasma biomarkers for detection of NPC
| Comparison | BamHI‐W 76 bp | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | TP | FN | TN | FP | Accuracy | Sensitivity | Specificity | AUC | |
| Stage I NPC vs. PC | >0 copy/ml | 29 | 1 | 113 | 7 | 94.7% | 96.7% | 94.2% | 0.9726 |
| Stages I and II NPC vs. PC | >0 copy/ml | 58 | 2 | 113 | 7 | 95.0% | 96.7% | 94.2% | 0.9756 |
| All NPC vs. PC | >0 copy/ml | 226 | 6 | 113 | 7 | 96.3% | 97.4% | 94.2% | 0.9832 |
| Stage I NPC vs. HC | >0 copy/ml | 29 | 1 | 113 | 12 | 91.6% | 96.7% | 90.4% | 0.9615 |
| Stages I and II NPC vs. HC | >0 copy/ml | 58 | 2 | 113 | 12 | 92.4% | 96.7% | 90.4% | 0.9679 |
| All NPC vs. HC | >0 copy/ml | 226 | 6 | 113 | 12 | 95.0% | 97.4% | 90.4% | 0.9796 |
| Comparison | EBNA1 99 bp | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | TP | FN | TN | FP | Accuracy | Sensitivity | Specificity | AUC | |
| Stage I NPC vs. PC | >0 copy/ml | 22 | 8 | 119 | 1 | 94.0% | 73.3% | 99.2% | 0.8650 |
| Stages I and II NPC vs. PC | >0 copy/ml | 48 | 12 | 119 | 1 | 92.8% | 80.0% | 99.2% | 0.8988 |
| All NPC vs. PC | >0 copy/ml | 200 | 32 | 119 | 1 | 90.6% | 86.2% | 99.2% | 0.9303 |
| Stage I NPC vs. HC | >0 copy/ml | 22 | 8 | 124 | 1 | 94.2% | 73.3% | 99.2% | 0.8608 |
| Stages I and II NPC vs. HC | >0 copy/ml | 48 | 12 | 124 | 1 | 93.0% | 80.0% | 99.2% | 0.8941 |
| All NPC vs. HC | >0 copy/ml | 200 | 32 | 124 | 1 | 90.8% | 86.2% | 99.2% | 0.9281 |
| Comparison | EA IgA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | TP | FN | TN | FP | Accuracy | Sensitivity | Specificity | AUC | |
| Stage I NPC vs. PC | <1,006 U/ml1 | 4 | 4 | 83 | 29 | 72.5% | 50.0% | 74.1% | 0.5368 |
| Stages I and II NPC vs. PC | >1,852 U/ml | 17 | 13 | 81 | 31 | 69.0% | 56.7% | 72.3% | 0.6226 |
| All NPC vs. PC | >1,510 U/ml | 136 | 53 | 71 | 41 | 68.8% | 72.0% | 63.4% | 0.6835 |
| Stage I NPC vs. HC | >823.0 U/ml | 7 | 1 | 44 | 9 | 83.6% | 87.5% | 83.0% | 0.8514 |
| Stages I and II NPC vs. HC | > 815.0 U/ml | 27 | 3 | 44 | 9 | 85.5% | 90.0% | 83.0% | 0.9094 |
| All NPC vs. HC | >1,002 U/ml | 172 | 17 | 48 | 5 | 90.9% | 91.0% | 90.6% | 0.9567 |
| Comparison | EBNA‐1 IgA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | TP | FN | TN | FP | Accuracy | Sensitivity | Specificity | AUC | |
| Stage I NPC vs. PC | >6,147 U/ml | 4 | 4 | 107 | 6 | 91.7% | 50.0% | 94.7% | 0.6565 |
| Stages I and II NPC vs. PC | >4,409 U/ml | 10 | 19 | 100 | 13 | 77.5% | 34.5% | 88.5% | 0.6196 |
| All NPC vs. PC | >5,217 U/ml | 58 | 130 | 104 | 9 | 53.8% | 30.9% | 92.0% | 0.6476 |
| Stage I NPC vs. HC | >5,080 U/ml | 4 | 4 | 50 | 3 | 88.5% | 50.0% | 94.3% | 0.7476 |
| Stages I and II NPC vs. HC | >2,988 U/ml | 15 | 14 | 48 | 5 | 76.8% | 51.7% | 90.6% | 0.7586 |
| All NPC vs. HC | >1,791 U/ml | 117 | 71 | 43 | 10 | 66.4% | 62.2% | 81.1% | 0.7886 |
| Comparison | EA IgG | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | TP | FN | TN | FP | Accuracy | Sensitivity | Specificity | AUC | |
| Stage I NPC vs. PC | >1,605 U/ml | 8 | 0 | 60 | 52 | 56.7% | 100.0% | 53.6% | 0.7031 |
| Stages I and II NPC vs. PC | >1,575 U/ml | 28 | 2 | 60 | 52 | 62.0% | 93.3% | 53.6% | 0.7991 |
| All NPC vs. PC | >5,322 U/ml | 156 | 34 | 84 | 28 | 79.5% | 82.1% | 75.0% | 0.8612 |
| Stage I NPC vs. HC | >1,642 U/ml | 8 | 0 | 50 | 3 | 95.1% | 100.0% | 94.3% | 0.9670 |
| Stages I and II NPC vs. HC | >1,481 U/ml | 28 | 2 | 49 | 4 | 92.8% | 93.3% | 92.5% | 0.9415 |
| All NPC vs. HC | >1,642 U/ml | 185 | 5 | 50 | 3 | 96.7% | 97.4% | 94.3% | 0.9765 |
| Comparison | VCA IgA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | TP | FN | TN | FP | Accuracy | Sensitivity | Specificity | AUC | |
| Stage I NPC vs. PC | >731.5 U/ml | 8 | 0 | 71 | 42 | 65.3% | 100.0% | 62.8% | 0.7378 |
| Staged I and II NPC vs. PC | >731.5 U/ml | 27 | 2 | 71 | 42 | 69.0% | 93.1% | 62.8% | 0.7667 |
| All NPC vs. PC | >731.5 U/ml | 180 | 8 | 71 | 42 | 83.4% | 95.7% | 62.8% | 0.7979 |
| Stage I NPC vs. HC | >1,055 U/ml | 6 | 2 | 51 | 2 | 93.4% | 75.0% | 96.2% | 0.9033 |
| Stages I and II NPC vs. HC | >964.5 U/ml | 25 | 4 | 50 | 3 | 91.5% | 86.2% | 94.3% | 0.9115 |
| All NPC vs. HC | >1,022 U/ml | 170 | 18 | 51 | 2 | 91.7% | 90.4% | 96.2% | 0.9498 |
| Comparison | BamHI‐W 121 bp | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | TP | FN | TN | FP | Accuracy | Sensitivity | Specificity | AUC | |
| Stage I NPC vs. PC | >0 copy/ml | 14 | 6 | 48 | 1 | 89.9% | 70.0% | 98.0% | 0.8459 |
| Stages I and II NPC vs. PC | >0 copy/ml | 28 | 8 | 48 | 1 | 89.4% | 77.8% | 98.0% | 0.8861 |
| All NPC vs. PC | >0 copy/ml | 48 | 14 | 48 | 1 | 86.5% | 77.4% | 98.0% | 0.8845 |
| Stage I NPC vs. HC | >0 copy/ml | 14 | 6 | 8 | 4 | 68.8% | 70.0% | 66.7% | 0.6333 |
| Stages I and II NPC vs. HC | >0 copy/ml | 28 | 8 | 8 | 4 | 75.0% | 77.8% | 66.7% | 0.6736 |
| All NPC vs. HC | >0 copy/ml | 48 | 14 | 8 | 4 | 75.7% | 77.4% | 66.7% | 0.7218 |
| Comparison | ebv‐miR‐BART7‐3p | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | TP | FN | TN | FP | Accuracy | Sensitivity | Specificity | AUC | |
| Stage I NPC vs. PC | >5.565 FCOD | 15 | 4 | 38 | 5 | 85.5% | 78.9% | 88.4% | 0.8550 |
| Stages I and II NPC vs. PC | >4.145 FCOD | 30 | 5 | 31 | 12 | 78.2% | 85.7% | 72.1% | 0.8399 |
| All NPC vs. PC | >4.085 FCOD | 52 | 19 | 31 | 12 | 72.8% | 73.2% | 72.1% | 0.7737 |
| Stage I NPC vs. HC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Stages I and II NPC vs. HC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| All NPC vs. HC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Comparison | hsa‐miR‐29a‐3p | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | TP | FN | TN | FP | Accuracy | Sensitivity | Specificity | AUC | |
| Stage I NPC vs. PC | >9.760 FCOD1 | 6 | 12 | 23 | 0 | 70.7% | 33.3% | 100.0% | 0.6763 |
| Stages I and II NPC vs. PC | >9.760 FCOD1 | 9 | 23 | 23 | 0 | 58.2% | 28.1% | 100.0% | 0.5639 |
| All NPC vs. PC | <8.200 FCOD | 25 | 21 | 15 | 8 | 58.0% | 54.3% | 65.2% | 0.5071 |
| Stage I NPC vs. HC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Stages I and II NPC vs. HC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| All NPC vs. HC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Stages II to IVC NPC vs. PC | <8.300 FCOD | 22 | 6 | 14 | 9 | 70.6% | 78.6% | 60.9% | 0.6250 |
| Comparison | hsa‐miR‐103a‐3p | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | TP | FN | TN | FP | Accuracy | Sensitivity | Specificity | AUC | |
| Stage I NPC vs. PC | >10.89 FCOD1 | 5 | 13 | 20 | 3 | 61.0% | 27.8% | 87.0% | 0.5060 |
| Stages I and II NPC vs. PC | <9.270 FCOD | 19 | 13 | 15 | 8 | 61.8% | 59.4% | 65.2% | 0.5618 |
| All NPC vs. PC | <9.390 FCOD | 31 | 15 | 15 | 8 | 66.7% | 67.4% | 65.2% | 0.6144 |
| Stage I NPC vs. HC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Stages I and II NPC vs. HC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| All NPC vs. HC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Stages II to IVC NPC vs. PC | <9.390 FCOD | 22 | 6 | 15 | 8 | 72.5% | 78.6% | 65.2% | 0.6918 |
All cutoff values were calculated based on Youden index from ROC analysis except BamHI‐W 76 bp, EBNA‐1 99 bp and BamHI‐W 121 bp which had cutoff set as >0 copy/ml.
Cutoff is not practical due to biomarker not suitable for detection of early stage NPC.
Abbreviations: FCOD, fold change over detection limit; HC, hospital controls; PC, population controls; ND, not determined.
Measurement of plasma anti‐EBV antibodies using ELISA
Plasma VCA IgA, EBNA‐1 IgA, EA IgA and EA IgG were measured according to manufacturer's instructions (IBL International, Hamburg, Germany). The microtiter strips of VCA IgA (RE57341), EBNA‐1 IgA (RE57321), EA IgA (RE56211) and EA IgG (RE57311) ELISA kits were precoated with VCA gp 125 affinity purified from P3HR1 cells, recombinant EBNA‐1 p72 antigen expressed in Sf9‐cells, an immunodominant region of EA‐D which was affinity purified from RAJI cells, and recombinant EA p54 expressed in Escherichia coli, respectively. First, plasma samples were diluted in diluent buffer (1:401). Standard, control or diluted samples were aliquoted (100 μl each) into duplicate wells of microtiter plates, followed by 60 min incubation at 25°C or 37°C and three times washing (each time with 350 μl wash buffer per well). Then, 30 min or 1 hr incubation with 100 μl of enzyme conjugate was carried out at 25°C or 37°C, followed by another washing step as described previously. Twenty or 30 min incubation with 100 μl of 3,3′,5,5′‐tetramethylbenzidine substrate solution was subsequently carried out in the dark and the reaction was stopped by addition of 100 μl stop solution. Optical density was measured at 450 nm and average results from duplicate wells were calculated. Levels of anti‐EBV antibodies in Unit/ml were interpolated from standard curve.
Plasma DNA and RNA extractions
Frozen plasma samples were thawed and centrifuged at room temperature for 10 min at 3,000 RPM to remove any cell debris prior to DNA or RNA extractions. DNA extraction from 200 to 400 μl plasma per sample was performed using QIAamp DNA Mini kit, while automated extraction of RNA from 400 μl plasma per sample was carried out using miRNeasy Micro Kit with QIAcube according to manufacturer's protocols (Qiagen, Hilden, Germany). In order to account for possible plasma RNA extraction bias, 500 attomole of synthetic miRNA cel‐miR‐39 (Integrated DNA Technologies, Coralville, IA) was spiked into all plasma samples after mixing with QIAzol from the miRNeasy Micro Kit (Qiagen). All DNA and RNA samples were eluted in 50 and 25 μl of nuclease free water (Qiagen), respectively.
Quantification of plasma EBV DNA level
Three EBV DNA tests with different primers and hydrolysis probes were conducted in our study (Supporting Information Table S1). Quantitative polymerase chain reaction (qPCR) was carried out using TaqMan Fast Advanced Master Mix in the ABI7500 Fast Real‐Time PCR system (Applied Biosystems, Foster City, CA) according to manufacturer's instructions. A total of 5 μl eluted DNA was used in 20 μl total reaction volume in each qPCR well, and each sample was analyzed in triplicate wells. Each qPCR plate contained no‐template‐control and serially diluted Namalwa cell DNA samples as standard points for the construction of EBV DNA copy number standard curve. Namalwa cells are known to have two integrated EBV genomes per cell.36 Accurate dilution of Namalwa cell DNA standard points and quantification of EBV copy numbers by EBNA1 99 bp test were validated by calibrating these Namalwa cell DNA standard points to the 1st WHO International Standard for EBV for Nucleic Acid Amplification Techniques37 (NIBSC code: 09/260, Supporting Information Table S2). Thermal cycling conditions include 50°C for 2 min, 95°C for 20 sec, and 40 cycles of 95°C for 3 sec and 56°C for 30 sec. EBV DNA copy number was interpolated from the Namalwa cell DNA standard curve and plasma EBV DNA level was calculated using the following formula:
where c = intercept, m = slope of the standard curve, V e = DNA elution volume, V f = final DNA volume used per qPCR well, a = ml of plasma used for DNA extraction.
RT‐qPCR validation of differential miRNA expression
Pooled reverse transcription (RT) of cel‐miR‐39, hsa‐miR‐29a‐3p and hsa‐miR‐103a‐3p was carried out using commercially available assays (Applied Biosystems) according to optimized protocol which showed high reliability and consistency.38, 39 RT protocol, primers and probe sequences of ebv‐miR‐BART7‐3p were according to Zhang et al.24 RT products of each sample, negative and positive controls were analyzed in duplicate wells using TaqMan 2X Universal PCR Master Mix, No AmpErase UNG in ABI7500 Fast Real‐Time PCR system (Applied Biosystems) according to manufacturer's instructions. Data were normalized to cel‐miR‐39 (spike‐in control) and fold change over detection limit was calculated.38, 39
Statistical analysis
In GraphPad Prism software, Mann–Whitney test was used to compare the mean rank differences between NPC and controls. AUC values were generated from receiver operating characteristic (ROC) curve analysis. In SPSS software, intraclass correlation coefficient (ICC) was obtained from average‐measurement, absolute‐agreement, two‐way mixed‐effects model. Decision tree models for NPC detection and prediction of overall survival were built with sample size, decision tree growing methods, criteria and validation parameters stated in Table 2.
Table 2.
Comparison of decision tree models and single tests for the detection and prognosis of NPC
| Biomarker test | Diagnostic performance | Cutoff | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Decision tree algorithm | Growing method | BamHI‐W as first testing criteria | Validation | BamHI‐W 76 bp | EBNA1 99 bp | EA IgG | EA IgA | VCA IgA | EBNA‐1 IgA | Name | True negative | False negative | False positive | True positive | Accuracy | Sensitivity | Specificity | AUC | BamHI‐W 76 bp (copy/ml) | EBNA1 99 bp (copy/ml) | EA IgG (U/ml) | VCA IgA (U/ml) | |
| Detection of NPC1 | Classification and Regression Trees with Gini impurity measure | PN ≥ 50, CN ≥ 2, TD ≤ 5 | No | 20‐fold CV | + | − | − | − | + | − | Model 1 | 106 | 7 | 0 | 180 | 97.6% | 96.3% | 100.0% | ND | >0.2459 | NA | NA | >512.6 |
| Yes | 50% train | + | − | − | − | − | − | Model 2 | 45 | 2 | 0 | 102 | 98.7% | 98.1% | 100.0% | ND | >0.1810 | NA | NA | NA | |||
| 50% test | 58 | 2 | 3 | 81 | 96.5% | 97.6% | 95.1% | NA | NA | NA | |||||||||||||
| Classification and Regression Trees with Twoing impurity measure | No | 20‐fold CV | + | − | − | − | + | − | Model 3 | 106 | 7 | 0 | 180 | 97.6% | 96.3% | 100.0% | ND | >0.2459 | NA | NA | >512.6 | ||
| Yes | 50% train | + | − | − | − | + | − | Model 4 | 60 | 0 | 0 | 88 | 100.0% | 100.0% | 100.0% | ND | >0.6786 | NA | NA | >541.8 | |||
| 50% test | 46 | 10 | 0 | 89 | 93.1% | 89.9% | 100.0% | ||||||||||||||||
| CHAID with likelihood ratio Chi‐square statistics | PN ≥ 50, CN ≥ 2, TD ≤ 3 | No | 20‐fold CV | + | − | − | − | − | − | Model 5 | 102 | 4 | 4 | 183 | 97.3% | 97.9% | 96.2% | ND | >0 | NA | NA | NA | |
| + | − | + | − | − | − | Model 6 | 94 | 1 | 12 | 186 | 95.6% | 99.5% | 88.7% | ND | >0 | NA | >97,619 | NA | |||||
| Yes | 50% train | + | − | − | − | − | − | Model 7 | 44 | 1 | 1 | 91 | 98.5% | 98.9% | 97.8% | ND | >0 | NA | NA | NA | |||
| 50% test | 58 | 3 | 3 | 92 | 96.2% | 96.8% | 95.1% | ||||||||||||||||
| BamHI‐W 76 bp | 102 | 4 | 4 | 183 | 97.3% | 97.9% | 96.2% | 0.987 | >0 | NA | NA | NA | |||||||||||
| Prognosis of NPC2 | Classification and Regression Trees with Gini impurity measure | PN ≥ 50, CN ≥ 2, TD ≤ 5 | No | 20‐fold CV | − | + | − | − | − | − | Model 8 | 31 | 8 | 16 | 25 | 70.0% | 75.8% | 66.0% | ND | NA | >14.0598 | NA | NA |
| Yes | 50% train | − | + | − | − | − | − | Model 9 | 15 | 5 | 10 | 16 | 67.4% | 76.2% | 60.0% | ND | NA | >14.0451 | NA | NA | |||
| 50% test | 16 | 3 | 6 | 9 | 73.5% | 75.0% | 72.7% | ||||||||||||||||
| Classification and Regression Trees with Twoing impurity measure | No | 20‐fold CV | − | + | − | − | − | − | Model 10 | 31 | 8 | 16 | 25 | 70.0% | 75.8% | 66.0% | ND | NA | >14.0598 | NA | NA | ||
| Yes | 50% train | − | + | − | − | − | − | Model 11 | 19 | 3 | 7 | 14 | 76.7% | 82.4% | 73.1% | ND | NA | >14.0598 | NA | NA | |||
| 50% test | 12 | 5 | 9 | 11 | 62.2% | 68.8% | 57.1% | ||||||||||||||||
| CHAID with likelihood ratio Chi‐square statistics | PN ≥ 50, CN ≥ 2, TD ≤ 3 | No | 20‐fold CV | − | + | − | − | − | − | Model 12 | 31 | 9 | 16 | 24 | 68.8% | 72.7% | 66.0% | ND | NA | >14.4270 | NA | NA | |
| Yes | 50% train | − | + | − | − | − | − | Model 13 | 24 | 13 | 3 | 6 | 65.2% | 31.6% | 88.9% | ND | NA | >137.998 | NA | NA | |||
| 50% test | 16 | 7 | 4 | 7 | 67.6% | 50.0% | 80.0% | ||||||||||||||||
| EBNA1 99 bp | 31 | 8 | 16 | 25 | 70.0% | 75.8% | 66.0% | 0.709 | NA | >14.0600 | NA | NA | |||||||||||
| BamHI‐W 76 bp | ND | ND | ND | ND | ND | ND | ND | 0.680 | ND | NA | NA | NA | |||||||||||
| EA IgA | ND | ND | ND | ND | ND | ND | ND | 0.518 | ND | NA | NA | NA | |||||||||||
| EA IgG | ND | ND | ND | ND | ND | ND | ND | 0.545 | ND | NA | NA | NA | |||||||||||
| EBNA‐1 IgA | ND | ND | ND | ND | ND | ND | ND | 0.525 | ND | NA | NA | NA | |||||||||||
| VCA IgA | ND | ND | ND | ND | ND | ND | ND | 0.516 | ND | NA | NA | NA | |||||||||||
Data set included 187 NPC patients and 106 population controls who had test results of six established biomarkers. Positive = NPC; Negative = Population control.
Data set included 80 NPC patients who completed radical treatment, had overall survival information and test results of six established biomarkers. Positive = Dead; Negative = Alive.
Abbreviations: +, included in decision tree; −, not included in decision tree; AUC, area under curve; CHAID, Chi‐square Automatic Interaction Detector; CN, child node; CV, cross validation; NA, not applicable ND, not determined; PN, parental node; TD, tree depth.
Results
Plasma EBV DNA
Demographic and clinicopathological characteristics of NPC patients and controls are shown in Supporting Information Table S3. All 10 plasma biomarkers analyzed in our study did not correlate with age and were not significantly different between different sex and ethnic groups (Supporting Information Table S4). In our hands, results of EBV DNA test from DNA extraction replicates had excellent test–retest reliability (ICC > 0.95, Supporting Information Fig. S1 a). We also found that prior to plasma processing, plasma EBV DNA load was fairly stable up to 6 hr in EDTA blood tube kept on bench at room temperature (Supporting Information Fig. S1 b).
Comparison of plasma EBV DNA load as measured by two established EBV DNA tests (BamHI‐W 76 bp and EBNA1 99 bp, Figs. 1 a and 1 b) were carried out between NPC patients and controls. In general, plasma EBV DNA loads were significantly higher in NPC patients compared to controls, and only low levels of plasma EBV DNA load was observed in a small subset of controls (Figs. 1 a and 1 b). The level of plasma EBV DNA increases with more advanced stages (Figs. 1 a and 1 b). Similar to the large cohort NPC screening study in Hong Kong,20 plasma EBV DNA load of >0 copy/ml was set as positive for both plasma EBV DNA tests (Table 1). This resulted in 94.2% and 99.2% specificity, respectively for BamHI‐W 76 bp and EBNA1 99 bp to identify NPC against population controls. Specificity for BamHI‐W 76 bp and EBNA1 99 bp to identify NPC against hospital controls were 90.4% and 99.2%, respectively (Table 1). BamHI‐W 76 bp being the EBV DNA test with highest sensitivity to detect NPC had 96.7% (29/30) sensitivity to detect Stage I NPC, 96.7% (58/60) sensitivity to detect early stage (Stages I and II) NPC and 97.4% (226/232) sensitivity to detect all NPC (Table 1). Based on recent findings that NPC patients had significantly longer fragment lengths of plasma EBV DNA compared to non‐NPCs,21 the new BamHI‐W 121 bp test was evaluated in a subset of our study samples with more early stage NPC cases as well as cases with false positive results as determined by the two common EBV DNA tests (Fig. 2 a). When testing NPC against controls, improved specificity but decreased sensitivity was found with BamHI‐W 121 bp as compared to BamHI‐W 76 bp (Supporting Information Fig. S2).
Figure 1.
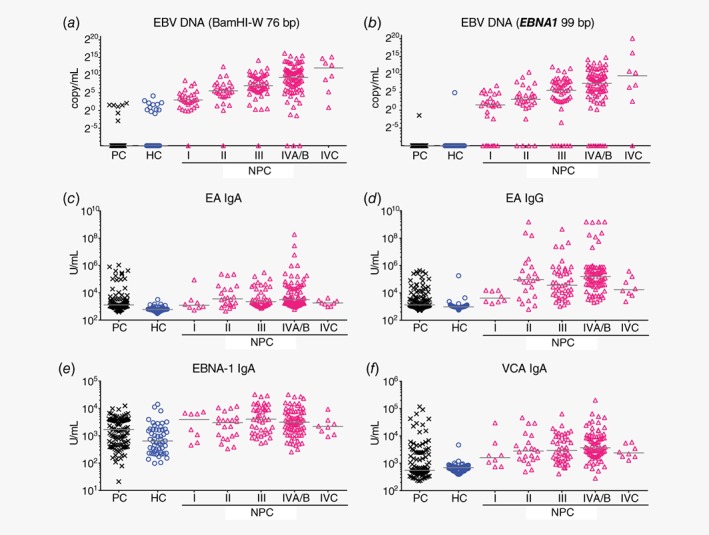
Evaluation of established plasma biomarkers to detect NPC against controls in our study. (a, b) Only low levels of plasma EBV DNA was observed in small subset of population controls and hospital controls. The levels of plasma EBV DNA increased with the stages of NPC. (c–f) NPC patients generally had higher plasma levels of anti‐EBV antibodies as compared to controls but no obvious trend within NPC subgroups was observed. Samples with undetectable plasma BamHI‐W 76 bp and plasma EBNA1 99 bp were arbitrarily set as 0.001 copy/ml. Abbreviations: HC, hospital control; PC, population control. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
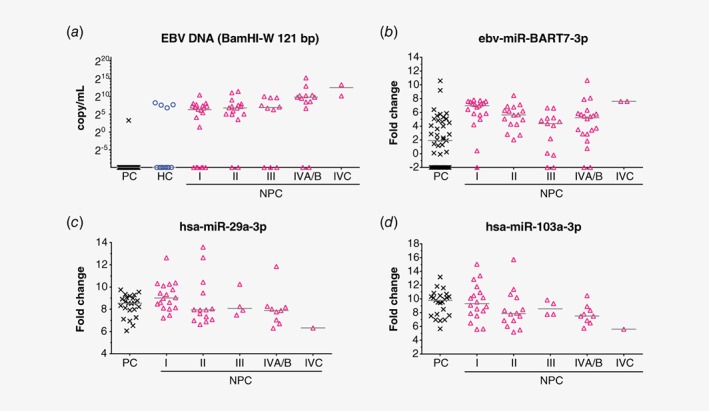
Evaluation of newly reported plasma biomarkers to detect NPC against controls in our study subset. (a) Plasma EBV DNA trend as measured by BamHI‐W 121 bp is similar to the other two EBV DNA tests in Figure 1. (b) NPC patients generally had higher plasma levels of ebv‐miR‐BART7‐3p. A portion of healthy donors also had detectable level of plasma ebv‐miR‐BART7‐3p. (c, d) Decreasing plasma levels of hsa‐miR‐29a‐3p and hsa‐miR‐103a‐3p were observed from early stage NPC to advanced stage NPC. Plasma levels of these two human miRNAs were not significantly different between population controls and Stage I NPC (p > 0.05). Samples with undetectable plasma BamHI‐W 121 bp were arbitrarily set as 0.001 copy/ml and samples with undetectable plasma ebv‐miR‐BART7‐3p were arbitrarily set as −2 fold change over detection limit. Abbreviations: HC, hospital control; PC, population control. [Color figure can be viewed at http://wileyonlinelibrary.com]
Plasma anti‐EBV antibodies
Moderately good to excellent test–retest reliability (ICC of 0.837–0.998) was achieved by commercially available ELISA tests measuring plasma VCA IgA, EBNA‐1 IgA, EA IgA and EA IgG (Supporting Information Fig. S1 c).
Comparison of ELISA results between NPC patients and controls showed that plasma level of anti‐EBV antibodies was generally higher in NPC patients as compared to controls. No obvious trend was observed across different NPC stages and high levels of plasma anti‐EBV antibodies were observed in some controls (Figs. 1 c–1 f). Among these four anti‐EBV antibody tests evaluated in our study, VCA IgA and EA IgG consistently had higher AUC values to detect early stage NPC against all controls, while EBNA‐1 IgA consistently showed the lowest AUC values among the established biomarkers (Fig. 3 and Table 1).
Figure 3.
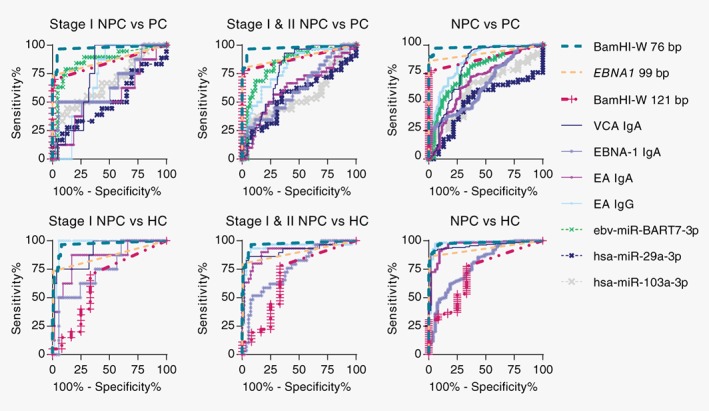
ROC analysis of 10 plasma biomarkers. BamHI‐W 76 bp test (dark green dash line) consistently appeared to be the test with highest AUC values while EBNA‐1 IgA (purple line) consistently appeared to be the test with lowest AUC values among the six established biomarkers. AUC values and numbers of test subjects can be viewed in Table 1. [Color figure can be viewed at http://wileyonlinelibrary.com]
Plasma miRNAs
Plasma ebv‐miR‐BART7‐3p, hsa‐miR‐29a‐3p and hsa‐miR‐103a‐3p were shortlisted for validation in a subset of our study samples enriched with more early stage NPC cases (Table 1). In general, plasma ebv‐miR‐BART7‐3p levels were higher in NPC compared to population controls and a portion of population controls also had detectable plasma ebv‐miR‐BART7‐3p (Fig. 2 b and Table 1). Similar median levels of plasma hsa‐miR‐29a‐3p and hsa‐miR‐103a‐3p were observed between population controls and Stage I NPC (Figs. 2 c and 2 d). It appeared that there was a decreasing trend in plasma hsa‐miR‐29a‐3p and hsa‐miR‐103a‐3p with the advancement of NPC stage (Figs. 2 c and 2 d).
Combination of plasma biomarkers for the detection of NPC
In order to evaluate if combination of plasma biomarkers may improve NPC detection, decision tree modeling was carried out on our data set comprising of 187 NPC cases and 106 population controls with available results of six plasma biomarkers (Table 2 and Supporting Information Table S3). BamHI‐W 76 bp test alone appeared to be sufficient for the detection of NPC, and appeared to be essential in all seven decision tree models (Table 2). Models 2, 5 and 7 suggested that BamHI‐W 76 bp test alone is sufficient (Table 2). Models 1, 3, and 4 suggested that combining VCA IgA with BamHI‐W 76 bp test can improve specificity at the expense of reduced sensitivity while Model 6 suggested that combining EA IgG with BamHI‐W 76 bp test can further increase sensitivity at the expense of decreased specificity (Table 2 and Supporting Information Fig. S3).
Plasma EBV DNA load as a prognosis marker for NPC overall survival
Survival information and test results of six plasma biomarkers were available for a subset of our NPC cases who had completed radical treatment (n = 80, Supporting Information Table S3). ROC analysis and decision tree modeling were carried out to evaluate if any of these six plasma biomarkers had prognostic value for the survival of these NPC patients (Table 2). According to ROC analysis, EBNA1 99 bp was the only biomarker with AUC > 0.7 (Table 2). With a cutoff at 14.06 copy/ml, EBNA1 99 bp could identify NPC patients with poor overall survival (Fig. 4 a) as well as poor progression‐free survival (Fig. 4 b) in both early stage and late stage NPC (Fig. 4). Decision tree modeling supported findings from this ROC analysis, revealing that EBNA1 99 bp with cutoff at about 14 copy/ml (Models 8–12) is sufficient for prognosis of survival while increasing EBNA1 99 bp cutoff to 138 copy/ml (Model 13) led to higher specificity but lower sensitivity in prognosis of survival (Table 2). Notably, EBNA1 99 bp with cutoff at about 14 copy/ml was still the only biomarker chosen by decision tree modeling even though additional information including age, sex, ethnicity, WHO type and AJCC staging were added into the analysis (data not shown).
Figure 4.
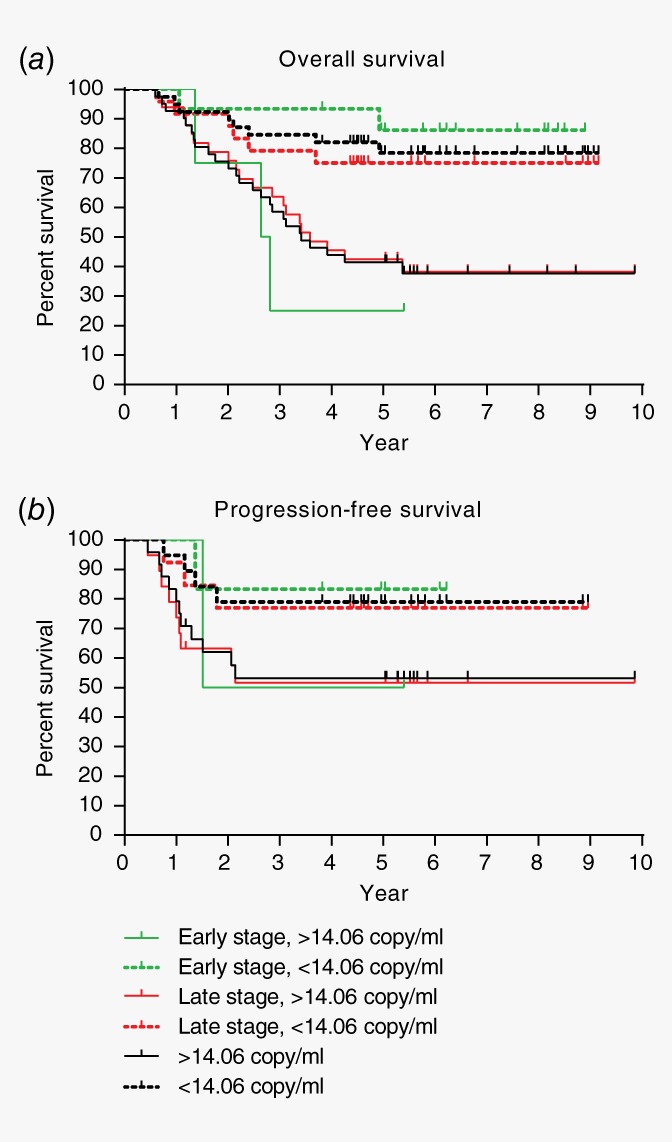
Prognostic value of EBNA1 99 bp test. NPC patients with plasma EBNA1 99 bp >14.06 copy/ml had poorer (a) overall survival and (b) progression‐free survival as compared to those with less plasma EBNA1 99 bp level. EBNA1 99 bp is a good prognostic marker regardless of early or late stages. [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
In our study, 10 plasma biomarkers (BamHI‐W 76 bp, BamHI‐W 121 bp, EBNA1 99 bp, EA IgA, EA IgG, EBNA‐1 IgA, VCA IgA, ebv‐miR‐BART7‐3p, hsa‐miR‐29a‐3p and hsa‐miR‐103a‐3p) were systematically analyzed for early detection and prognosis of NPC. These included established and newly reported NPC biomarkers of EBV and human origin.
To our knowledge, published case–control studies which reported 50–86% sensitivity of plasma EBV DNA test for Stage I NPC had only analyzed two to 22 cases.15, 19 Our study which include larger sample size of Stage I NPC (n = 30) for plasma EBV DNA test revealed 96.7% (29/30) sensitivity to detect Stage I NPC. Besides larger sample size, our improved sensitivity findings may be due to lower qPCR platform detection limit (25 copy/ml) achieved with usage of more advanced qPCR master mix in our study as compared to other studies.15, 19 Our findings from comparison of BamHI‐W 121 bp test and BamHI‐W 76 bp test (Supporting Information Fig. S2) support the notion that the larger the qPCR amplicon size, the more specific but less sensitive is the EBV DNA qPCR test. This is consistent with the findings reported earlier which compared the performance of EBNA1 213 bp test and EBNA1 99 bp test.40 It is estimated that increase in input volume by eight times may compensate the sensitivity issue of BamHI‐W 121 bp test as compared to BamHI‐W 76 bp test, hypothetically from qPCR Cq 40 (undetected) to Cq 37, but will incur higher cost and larger effort in sample processing and DNA extraction. Interestingly, four hospital controls were positive in both plasma BamHI‐W 76 bp and BamHI‐W 121 bp tests (Supporting Information Fig. S2). It is possible that these tests were sensitive enough to detect NPC in cases which were too early to be detected clinically. A follow‐up on these individuals to check on event of NPC will be interesting.
In the EBV genome, there is only one copy of EBNA1 gene while BamHI‐W region may be reiterated by 7 to 11 repeats.41 Prevalent EBV in different populations may differ in the numbers of BamHI‐W region repeats, making prognostic cutoff value of pretreatment plasma BamHI‐W 76 bp level deduced from one cohort not optimal for another cohort.42, 43, 44 If plasma BamHI‐W 76 bp test results are intended to be used for prognosis, EBV DNA clearance rate calculated from pretreatment and posttreatment plasma EBV DNA load may be analyzed to rule out interindividual variability. Indeed, in a systematic review and meta‐analysis on the prognosis of NPC by plasma BamHI‐W 76 bp test, Zhang et al. showed that cutoff for EBV DNA clearance rate was comparable among studies cohort.42 In our study, pretreatment plasma EBNA1 99 bp and BamHI‐W 76 bp tests had similar prognostic values (AUC 0.709 and 0.680, respectively). Unlike BamHI‐W 76 bp test, pretreatment plasma EBNA1 99 bp test is not affected by interindividual variability and do not require multiple sampling to calculate EBV DNA clearance rate. It would be interesting to investigate if the cutoff value of pretreatment plasma EBNA1 99 bp level deduced in our study is applicable to future follow‐up studies.
Our study had served as an external independent study and validated the diagnostic performance of two newly reported biomarkers (BamHI‐W 121 bp and ebv‐miR‐BART7‐3p). Specificity of ebv‐miR‐BART7‐3p appeared to be less optimal as it was detected in about 28% (12/43) of population controls (Table 1), which is in line with recent findings from Ramayanti et al.32 but not Gao et al.29 The discrepancy may be due to the differences in PCR primers. Meanwhile the diagnostic performance of hsa‐miR‐29a‐3p and hsa‐miR103a‐3p reported elsewhere25 could not be reproduced in our study, possibly due to inclusion of more early stage NPC and less advanced stage NPC in our analysis. Consistent with findings from a previous report,25 differences in plasma hsa‐miR‐29a‐3p levels seemed to be more apparent only when comparing controls to advanced stage NPC (Fig. 2 c). It is possible that four other miRNAs (ebv‐BART2‐5p, ebv‐miR‐BART13‐3p, hsa‐miR‐483‐5p and hsa‐let‐7c) that are not included for validation in our study may perform well as early diagnosis markers for NPC. EBV DNA markers are already well established for NPC screening. From a clinical utility viewpoint, the additional value of including non‐EBV markers may be higher than the additional value of including another EBV marker in the NPC detection panel. Our study indicates that much effort is still needed to identify a combination panel of EBV markers and non‐EBV markers that will benefit the detection of not only the majority of NPC cases which are EBV positive but also the small subset of NPC cases which are EBV negative.
Conclusions
Our study provides important information to policy makers in LMICs who have limited health care resources to plan a more cost‐effective NPC screening and monitoring strategy for the apparently healthy asymptomatic controls. We showed that the diagnostic performance of established biomarkers to detect NPC in local general population were comparable to findings of studies from another NPC endemic area15 and plasma BamHI‐W 76 bp test is superior for early detection of NPC. Comparison of plasma biomarkers in NPC patients and local hospital controls suggests that plasma EBV DNA test could identify NPC cases among individuals who visit the hospital for other conditions in local setting, thus allowing for opportunistic screening. Combined biomarker tests from single sampled specimens can improve NPC detection specificity (with slight decrease in sensitivity) and avoid logistic problems of resampling. Plasma EBNA1 99 bp test may have important prognostic value and could be used to stratify NPC patients for different clinical management.
Supporting information
Supporting Information Fig. S1 Consistency and reproducibility of plasma EBV DNA and anti‐EBV antibody test results in this study. (A) Plasma EBV DNA test results had high test–retest reliability, with intraclass correlation coefficient (ICC) > 0.95. Each dot represents one individual and DNA extraction duplicates from 6 individuals were analyzed. (B) Minimal change in plasma EBV DNA load was observed within six hours of blood sampling to plasma processing time in room temperature. Each line represents one individual and each data point represents an EBV DNA test result from a plasma sample processed at the specified duration. (C) Anti‐EBV antibodies test results had ICC ranging from 0.837 to 0.998. Each data point represents one individual and duplicates of plasma aliquots from 43 to 44 individuals were analyzed. Samples with undetectable plasma BamHI‐W 76 bp and plasma EBNA1 99 bp were arbitrarily set as 0.001 copy/mL. CI, confidence interval.
Supporting information Fig. S2. Comparison of BamHI‐W tests of different amplicon sizes. BamHI‐W 121 bp test had increased specificity (less false positive from population control) but decreased sensitivity (less true positive for NPC) as compared to BamHI‐W 76 bp test. Each dot represents qPCR results from one individual. Samples with undetectable plasma EBV DNA were arbitrarily set as 0.001 copy/mL.
Supporting information Fig. S3. Representative decision tree models. Combination of (A) VCA IgA or (B) EA IgG with BamHI‐W 76 bp for the classification of NPC and population control. Samples with undetectable plasma BamHI‐W 76 bp were arbitrarily set as 0.001 copy/mL.
Supporting Information Table S1 Primer/Probe sequences and final working concentrations for 3 EBV DNA tests.
Supporting information Table S2. Validation of serially diluted standard points for EBV DNA tests.
Supporting information Table S3. Demographic and clinicopathological characteristic of NPC patients and controls.
Supporting information Table S4. The levels of ten plasma biomarker did not seem to be significantly affected by known demographic factors.
Acknowledgements
The authors thank the Director General of Health Malaysia for his approval of the publication of this article. The authors also thank the Director of Institute for Medical Research Malaysia for her support in this study. The authors are grateful to all staff from the Molecular Pathology unit, Biospecimen Bank at the Institute for Medical Research and hospitals involved in this study for their assistance in sample and data collection.
Conflict of interest: The authors declare no conflict of interest.
Author contributions: L.P.T., G.W.T., S.V.M., S.L.G., C.S.L., T.B.B.M.M., C.C.N. conducted experiments and acquired experimental data. W.R.K., X.J.N., N.S.M.D., C.S.H.O., C.Y.W., C.A.O., Y.Y.Y., F.H., K.C.P., C.E.K. and the M.N.C.S.G. recruited patients, acquired and interpreted clinical data. L.P.T. and G.W.T. analyzed and interpreted data. L.P.T. wrote the manuscript. A.S.B.K. and G.W.T. critically reviewed and improved the manuscript.
Data availability
The data that support the findings of our study are available from the corresponding author upon reasonable request.
References
- 1. Devi BC, Pisani P, Tang TS, et al. High incidence of nasopharyngeal carcinoma in native people of Sarawak, Borneo Island. Cancer Epidemiol Biomarkers Prev 2004;13:482–6. [PubMed] [Google Scholar]
- 2. Yu MC. Nasopharyngeal carcinoma: epidemiology and dietary factors. IARC Sci Publ 1991;105:39–47. [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4. Liu Z, Chang ET, Liu Q, et al. Quantification of familial risk of nasopharyngeal carcinoma in a high‐incidence area. Cancer 2017;123:2716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jia WH, Feng BJ, Xu ZL, et al. Familial risk and clustering of nasopharyngeal carcinoma in Guangdong. China Cancer 2004;101:363–9. [DOI] [PubMed] [Google Scholar]
- 6. Friborg J, Wohlfahrt J, Koch A, et al. Cancer susceptibility in nasopharyngeal carcinoma families–a population‐based cohort study. Cancer Res 2005;65:8567–72. [DOI] [PubMed] [Google Scholar]
- 7. Liu Z, Fang F, Chang ET, et al. Cancer risk in the relatives of patients with nasopharyngeal carcinoma‐a register‐based cohort study in Sweden. Br J Cancer 2015;112:1827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armstrong RW, Imrey PB, Lye MS, et al. Nasopharyngeal carcinoma in Malaysian Chinese: salted fish and other dietary exposures. Int J Cancer 1998;77:228–35. [DOI] [PubMed] [Google Scholar]
- 9. Chee Ee Phua V, Loo WH, Yusof MM, et al. Treatment outcome for nasopharyngeal carcinoma in University Malaya Medical Centre from 2004‐2008. Asian Pac J Cancer Prev 2013;14:4567–70. [DOI] [PubMed] [Google Scholar]
- 10. El‐Sherbieny E, Rashwan H, Lubis SH, et al. Prognostic factors in patients with nasopharyngeal carcinoma treated in Hospital Kuala Lumpur. Asian Pac J Cancer Prev 2011;12:1739–43. [PubMed] [Google Scholar]
- 11. Cheah SK, Lau FN, Yusof MM, et al. Treatment outcome with brachytherapy for recurrent nasopharyngeal carcinoma. Asian Pac J Cancer Prev 2014;14:6513–8. [DOI] [PubMed] [Google Scholar]
- 12. ACTION Study Group , Kimman M, Jan S, et al. Catastrophic health expenditure and 12‐month mortality associated with cancer in Southeast Asia: results from a longitudinal study in eight countries. BMC Med 2015;13:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pua KC, Khoo AS, Yap YY, et al. Nasopharyngeal carcinoma database. Med J Malaysia 2008;63:59–62. [PubMed] [Google Scholar]
- 14. Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol 2017;11:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yip TT, Ngan RK, Fong AH, et al. Application of circulating plasma/serum EBV DNA in the clinical management of nasopharyngeal carcinoma. Oral Oncol 2014;50:527–38. [DOI] [PubMed] [Google Scholar]
- 16. Liu Y, Huang Q, Liu W, et al. Establishment of VCA and EBNA1 IgA‐based combination by enzyme‐linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: a two‐stage design with a preliminary performance study and a mass screening in southern China. Int J Cancer 2012;131:406–16. [DOI] [PubMed] [Google Scholar]
- 17. Liu Z, Ji MF, Huang QH, et al. Two Epstein‐Barr virus‐related serologic antibody tests in nasopharyngeal carcinoma screening: results from the initial phase of a cluster randomized controlled trial in Southern China. Am J Epidemiol 2013;177:242–50. [DOI] [PubMed] [Google Scholar]
- 18. Leung SF, Lo YM, Chan AT, et al. Disparity of sensitivities in detection of radiation‐naive and postirradiation recurrent nasopharyngeal carcinoma of the undifferentiated type by quantitative analysis of circulating Epstein‐Barr virus DNA1,2. Clin Cancer Res 2003;9:3431–4. [PubMed] [Google Scholar]
- 19. Yang X, Dai W, Kwong DL, et al. Epigenetic markers for noninvasive early detection of nasopharyngeal carcinoma by methylation‐sensitive high resolution melting. Int J Cancer 2015;136:E127–35. [DOI] [PubMed] [Google Scholar]
- 20. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein‐Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med 2017;377:513–22. [DOI] [PubMed] [Google Scholar]
- 21. Lam WKJ, Jiang P, Chan KCA, et al. Sequencing‐based counting and size profiling of plasma Epstein‐Barr virus DNA enhance population screening of nasopharyngeal carcinoma. Proc Natl Acad Sci U S A 2018;115:E5115–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wagner PD, Srivastava S. New paradigms in translational science research in cancer biomarkers. Trans Res 2012;159:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan G, Hashim MJ. Global burden of deaths from Epstein‐Barr virus attributable malignancies 1990‐2010. Infec Agents Cancer 2014;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang G, Zong J, Lin S, et al. Circulating Epstein‐Barr virus microRNAs miR‐BART7 and miR‐BART13 as biomarkers for nasopharyngeal carcinoma diagnosis and treatment. Int J Cancer 2015;136:E301–12. [DOI] [PubMed] [Google Scholar]
- 25. Wang HY, Yan LX, Shao Q, et al. Profiling plasma microRNA in nasopharyngeal carcinoma with deep sequencing. Clin Chem 2014;60:773–82. [DOI] [PubMed] [Google Scholar]
- 26. Jiang C, Chen J, Xie S, et al. Evaluation of circulating EBV microRNA BART2‐5p in facilitating early detection and screening of nasopharyngeal carcinoma. Int J Cancer 2018;143:3209–17. [DOI] [PubMed] [Google Scholar]
- 27. He B, Zeng J, Chao W, et al. Serum long non‐coding RNAs MALAT1, AFAP1‐AS1 and AL359062 as diagnostic and prognostic biomarkers for nasopharyngeal carcinoma. Oncotarget 2017;8:41166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng XH, Cui C, Ruan HL, et al. Plasma microRNA profiling in nasopharyngeal carcinoma patients reveals miR‐548q and miR‐483‐5p as potential biomarkers. Chin J Cancer 2014;33:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao W, Wong TS, Lv KX, et al. Detection of Epstein‐Barr virus (EBV)‐encoded microRNAs in plasma of patients with nasopharyngeal carcinoma. Head Neck 2019;41:780–92. [DOI] [PubMed] [Google Scholar]
- 30. Liu X, Luo HN, Tian WD, et al. Diagnostic and prognostic value of plasma microRNA deregulation in nasopharyngeal carcinoma. Cancer Biol Ther 2013;14:1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gourzones C, Ferrand FR, Amiel C, et al. Consistent high concentration of the viral microRNA BART17 in plasma samples from nasopharyngeal carcinoma patients–evidence of non‐exosomal transport. Virol J 2013;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramayanti O, Verkuijlen S, Novianti P, et al. Vesicle‐bound EBV‐BART13‐3p miRNA in circulation distinguishes nasopharyngeal from other head and neck cancer and asymptomatic EBV‐infections. Int J Cancer 2019;144:2555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ooi CH, Mastulu W. Epidemiology of cancer in Sarawak 2001–2005. Sarawak: Sarawak Cancer Registry, Sarawak Health Department, 2009. [Google Scholar]
- 34. Maria S, Nirmal K, Nafisah A, et al. Sabah cancer registry report 2006–2010. Sabah: Sabah Cancer Registry, Sabah State Health Department, 2015. [Google Scholar]
- 35. Azizah AM, Nor Saleha IT, Noor Hashimah A, et al. In: Manan AA, ed. Malaysian National Cancer Registry Report 2007‐2011. Malaysia: Ministry of Health Malaysia, 2016. [Google Scholar]
- 36. Lawrence JB, Villnave CA, Singer RH. Sensitive, high‐resolution chromatin and chromosome mapping in situ: presence and orientation of two closely integrated copies of EBV in a lymphoma line. Cell 1988;52:51–61. [DOI] [PubMed] [Google Scholar]
- 37. Fryer JF, Heath AB, Wilkinson DE, et al. A collaborative study to establish the 1st WHO International Standard for Epstein‐Barr virus for nucleic acid amplification techniques. Biologicals 2016;44:423–33. [DOI] [PubMed] [Google Scholar]
- 38. Tan GW, Khoo AS, Tan LP. Evaluation of extraction kits and RT‐qPCR systems adapted to high‐throughput platform for circulating miRNAs. Sci Rep 2015;5:9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tan GW, Tan LP. High‐throughput RT‐qPCR for the analysis of circulating MicroRNAs. Methods Mol Biol 2017;1580:7–19. [DOI] [PubMed] [Google Scholar]
- 40. Stevens SJ, Verkuijlen SA, Hariwiyanto B, et al. Diagnostic value of measuring Epstein‐Barr virus (EBV) DNA load and carcinoma‐specific viral mRNA in relation to anti‐EBV immunoglobulin A (IgA) and IgG antibody levels in blood of nasopharyngeal carcinoma patients from Indonesia. J Clin Microbiol 2005;43:3066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ryan JL, Fan H, Glaser SL, et al. Epstein‐Barr virus quantitation by real‐time PCR targeting multiple gene segments: a novel approach to screen for the virus in paraffin‐embedded tissue and plasma. J Mol Diagn 2004;6:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang W, Chen Y, Chen L, et al. The clinical utility of plasma Epstein‐Barr virus DNA assays in nasopharyngeal carcinoma: the dawn of a new era? A systematic review and meta‐analysis of 7836 cases. Medicine 2015;94:e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang J, Shu C, Song Y, et al. Epstein‐Barr virus DNA level as a novel prognostic factor in nasopharyngeal carcinoma: a meta‐analysis. Medicine 2016;95:e5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee VH, Kwong DL, Leung TW, et al. The addition of pretreatment plasma Epstein‐Barr virus DNA into the 8th edition of nasopharyngeal cancer TNM stage classification. Int J Cancer 2018;144:1713–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Fig. S1 Consistency and reproducibility of plasma EBV DNA and anti‐EBV antibody test results in this study. (A) Plasma EBV DNA test results had high test–retest reliability, with intraclass correlation coefficient (ICC) > 0.95. Each dot represents one individual and DNA extraction duplicates from 6 individuals were analyzed. (B) Minimal change in plasma EBV DNA load was observed within six hours of blood sampling to plasma processing time in room temperature. Each line represents one individual and each data point represents an EBV DNA test result from a plasma sample processed at the specified duration. (C) Anti‐EBV antibodies test results had ICC ranging from 0.837 to 0.998. Each data point represents one individual and duplicates of plasma aliquots from 43 to 44 individuals were analyzed. Samples with undetectable plasma BamHI‐W 76 bp and plasma EBNA1 99 bp were arbitrarily set as 0.001 copy/mL. CI, confidence interval.
Supporting information Fig. S2. Comparison of BamHI‐W tests of different amplicon sizes. BamHI‐W 121 bp test had increased specificity (less false positive from population control) but decreased sensitivity (less true positive for NPC) as compared to BamHI‐W 76 bp test. Each dot represents qPCR results from one individual. Samples with undetectable plasma EBV DNA were arbitrarily set as 0.001 copy/mL.
Supporting information Fig. S3. Representative decision tree models. Combination of (A) VCA IgA or (B) EA IgG with BamHI‐W 76 bp for the classification of NPC and population control. Samples with undetectable plasma BamHI‐W 76 bp were arbitrarily set as 0.001 copy/mL.
Supporting Information Table S1 Primer/Probe sequences and final working concentrations for 3 EBV DNA tests.
Supporting information Table S2. Validation of serially diluted standard points for EBV DNA tests.
Supporting information Table S3. Demographic and clinicopathological characteristic of NPC patients and controls.
Supporting information Table S4. The levels of ten plasma biomarker did not seem to be significantly affected by known demographic factors.
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request.


