Abstract
Background
The epidemic phenomenon leading to a progressive increase in benzodiazepine prescriptions represents a challenge for healthcare systems. In the hospital setting, indicators of prescription variation and potential of overuse are lacking and are rarely monitored. Inter‐hospital monitoring/benchmarking, via peer‐pressure, can foster the motivation to change. The aim of this investigation was to analyse whether, the reduction in new benzodiazepine prescriptions obtained thanks to a Choosing Wisely campaign, also contributed to reducing inter‐hospital variation.
Methods
Secondary analysis of a multicentre longitudinal intervention in a network of five teaching hospitals in Switzerland. We set out to explore the effect, on inter‐hospital benzodiazepine prescription variation, of a continuous monitoring/benchmarking strategy, which was proven effective in reducing the intra‐hospital prescription rate. The variance was used to assess inter‐hospital variation. To investigate the impact of the intervention a segmented regression analysis of interrupted time series was performed.
Results
A total of 36 299 admissions over 42 months were analysed (1 July 2014 to 31 December 2017). Before the intervention a significant constant upward trend in inter‐hospital variability was found (+0.901; SE 0.441; P < .05). After the intervention, the variance trend line significantly changed, decreasing by −0.257 (SE 0.005: P < .001) and producing by December 2017, a 27% absolute reduction.
Conclusions
Thanks to a multimodal approach based on monitoring‐benchmarking, a significant reduction in inter‐hospital benzodiazepine prescription variation was obtained. Aligning to peer strategy is a spontaneous consequence of open benchmarking that can be used to convert a variation‐based suspicion of overuse, into an occasion to actively review prescription habits.
Keywords: benchmarking, benzodiazepine, choosing wisely, intervention study, monitoring, overuse, peer‐pressure, variation
What’s known
The increasing trend in benzodiazepine consumption has epidemic characteristics leading potentially to harmful consequences for patients.
Variation in diagnostic and therapeutic prescriptions is a well‐known marker of under/over‐use.
Healthcare systems increase their efforts to reduce variation; however, most interventions have been targeted at reducing under‐ rather than over‐use.
What’s new
With a multi‐level strategy based on peer‐pressure and educational interventions, we obtained a significant reduction in both, benzodiazepine prescriptions and inter‐institutional prescription variation in a network of teaching hospitals.
This study suggests and motivates the necessity, as an adequacy parameter, of monitoring both, prescription trends and variations.
1. BACKGROUND
With the aim of improving the quality of care, healthcare systems increase their efforts in reducing variations in diagnostic and therapeutic prescriptions.1, 2 Large variations in healthcare delivery could in fact lead to worse patient outcomes and to unfavourable economic consequences.3, 4
Traditionally, unwarranted variations were analysed to explore the magnitude of underuse,5, 6 however, in recent years, mainly in western countries, a widespread, pervasive and latent phenomenon represented by overuse of medical treatments has come to light.7, 8
Unnecessary care is not only an economic waste; it could lead to significant harmful consequences for patients.9
Nevertheless, most interventions have been targeted at reducing underuse rather than overuse.10
In the hospital setting, however, indicators of variation in overuse are often lacking and rarely monitored in a sustained way by healthcare providers.
In our hospital network, called “Ente Ospedaliero Cantonale” (EOC), composed of five teaching hospitals in the south of Switzerland, we successfully experimented an integrated, multi‐level approach aimed at curbing the in‐hospital prescription of drugs known to be over‐used [(ie benzodiazepines (BZD),11 and proton pump inhibitors (PPI)]12 and to reduce inappropriate laboratory blood testing during the hospital stay.13
A fundamental underlying issue was discussed during the implementation of our experimental strategy: would the intervention reduce drug and blood test prescriptions in the hospitals of the network in an independent way, or rather produce a levelling in the prescription rate, expressed mainly by a reduction in variation? The first hypothesis suggests the absence of an ideal prescription rate of the drug under study and/or an important overuse, whereas the second one could suggest the contrary, or the presence of a peer effect generated by the psychological pressure to be observed and judged by colleagues (called “peer‐pressure”).
Reducing the inter‐providers variation in drug prescriptions could help in improving, patients’ care experience and clinical outcomes, and in reducing healthcare costs.14, 15
Unwarranted variations in prescriptions could be the result of different individual strategies but also of acquired habits. To inform individuals, thanks to benchmarking, about their prescription attitude is essential to generate awareness and promote change.16
A better understanding of the underlying components determining variation is useful also for the healthcare institutions and for health systems in order to promote initiatives aimed at improving the quality and appropriateness of care at the network level. Causes of variation can be recognised at different levels and include: individual peculiarities in providers’ attitudes, inhomogeneous consistency and knowledge of best practices, individual culture, patient preferences.17, 18 Individual differences in prescription strategies will then translate into between‐service and between‐hospital differences. This means that in the end patients will experience different clinical outcomes depending on the hospital mix of providers’ attitudes.
No previous studies investigated the impact, on between‐hospital variations, of an intervention targeted at curbing the over‐prescription of individual drugs. Hence, in the present analysis we primarily aimed to explore the effect on inter‐hospital variation of BZD prescriptions, of a strategy based on peer‐pressure, carried out with an open monitoring‐benchmarking web‐based computer tool. Secondly, we strove to elucidate the hospitals’ interdependency, analysing the impact on the network of changes registered at the hospital level.
2. METHODS
2.1. Design, setting, intervention
Secondary analysis of a multicentre longitudinal, before and after study conducted in a network of five teaching hospitals in Switzerland,11 aimed at exploring the effect of an intervention based on education and peer‐pressure, on between hospital variations of benzodiazepine prescription rate.
Five teaching hospitals (H1‐H5), belonging to a public network, sharing the same internal guidelines and IT platform were involved. Data of admissions in the internal medicine departments, over three and a half years (from July 1, 2014 to December 31, 2017) were analysed. Demographic data and information on diagnoses and case mix (CM) were collected from the electronic patient records. The CM, as relative value assigned to the patients' Swiss Diagnosis‐Related Group at hospital discharge, is an indicator of illness severity used to calculate hospitals' reimbursements. Data collected were part of the standard hospital monitoring and did not contain patient information. The study was reviewed by the Swiss Ethics Committee, which confirmed that, involving anonymous secondary data only, it was exempt from institutional board approval.19
The original intervention, demonstrating a significant reduction in in‐hospital benzodiazepine, PPI and blood test prescriptions, started in January 2016 and was widely described elsewhere.11, 12, 13
Briefly, the strategy proposed consisted in educational interventions (targeted meetings, audit and feedback) combined with an open monitoring and benchmarking, called “Reporting Wisely”, of new BZD prescriptions at hospital discharge. The web‐based system used for the monitoring, was able to provide the physicians’ and nurses’ staff, a continuously updated benchmarking (with trend lines and histograms) up to the network level, on BZD prescription rates. The web interface appeared to be user‐friendly and was exploited during ad hoc educational meetings, to promote discussions on individual prescription attitudes, to define critical points and to target following interventions.
The present analysis was aimed at exploring the specific effect of the applied strategy on inter‐hospital benzodiazepine prescription variation.
2.2. Statistical analysis
In primary analysis, descriptive statistics were used to evaluate characteristics of the hospitals during the study period, using the median and interquartile range (IQR) or frequencies and percentages (%), as appropriate.
In order to investigate the inter‐hospital variation in new BZD prescriptions, we preliminarily generated funnel plots. They allow to produce control charts in which the outcome of interest is plotted against a measure of its precision. Each funnel plot provides a graphical representation of variations in the outcome (new BZD prescription rate, y‐axis) as a function of the number of patients, admitted quarterly in the hospitals network (x‐axis) during the study years. On the x‐axis, only patients admitted without an on‐going BZD prescription were considered. We then examined the variation across the hospitals network in the preintervention (years 2014‐2015) and intervention (2016‐2017) periods, separately. Confidence intervals for the funnel plots were calculated on the basis of a Wilson binomial formula. Limits of 95% and 80% were chosen to represent the expected variation in the data.
To explore the effect of the intervention in reducing the inter‐hospital prescription rate variation, a segmented regression analysis of interrupted time series was performed. The variation in the prescription rate per hospital was assessed by calculating the inter‐hospital variance (σ2‐BZD) for each of the quarters considered. To determine whether specific adjustments were required, partial autocorrelation functions were analysed. The serial autocorrelation in the regression models was tested using the R’s GLS function from the NLME package in the fitted segmented regression. For the time‐series regression model, we calculated: (a) the β0 coefficient, which estimates the inter‐hospital prescription rate variation at the beginning of the observation; (b) the β1 coefficient, which estimates the baseline inter‐hospital trend; (c) the β2 coefficient, which estimates the change in inter‐hospital variation during the intervention, and (d) the β3 coefficient, which represents the change in the slope of the trend of inter‐hospital BZD prescription variability after the start of the intervention. In all models, the 95% standard errors and associated P‐values were also shown.
In order to investigate the interdependence in the between hospital prescription rate, we used a vector autoregressive (VAR) modelling.
VAR modelling allows for inspecting the interaction between hospitals as a function of the time in a multivariate system, in which each variable is regressed on all variables at the previous time point. This analysis detects the temporal dynamics of a system of related variables as a function of time and can help in investigating causal relationships between mutual prescription changes. VAR modelling assumes a specified lag structure, and the Schwarz Bayesian information criterion (SBIC), the Hannan‐Quinn information criterion (HQIC) the Akaike information criterion, and the forecast prediction error (FPE) statistics were used to determine the optimal number of lags to include. After building the VAR models, we used the SBIC, HQIC and FPE, to determine the choice between competing models. In order to inspect the model's dynamic behaviour of interdependencies across hospitals over time, orthogonalised impulse response functions were used.
All analyses were performed using r Statistical Software (http://www.r-project.org, version 3.2.0). Statistical significance for all outcomes was set at P ≤ .05.
3. RESULTS
3.1. Characteristics of the study population and variation in benzodiazepine prescriptions across hospitals
Demographic and clinical characteristics of the study population in the periods before (years 2014‐2015) and after (2016‐2017) the intervention are shown in Table 1. A total of 36 299 hospital admissions were analysed. The hospitals of the network showed similar characteristics for respectively, gender and age of the patients, and CM. New BZD prescription rates and inter‐hospital variances (σ2‐BZD), before and after the intervention were 7.1% vs 5.7%. and 0.005 vs. 0.002.
Table 1.
Characteristics of the study population by study period (years 2014‐2017; 36 299 admissions)
| Before intervention | After intervention | |
|---|---|---|
| Age (y) | 75 (63‐83) | 76 (63‐84) |
| Gender (females), (%) | 49.36 | 50.40 |
| Case mix | 0.70 (0.52‐0.923) | 0.71 (0.51‐0.96) |
| BZD at admission, n (%) | 31.07 | 31.07 |
| BZD at discharge, n (%) | 31.62 | 31.04 |
| New BZD‐prescriptions at discharge (%) | 7.09 | 5.69 |
| Inter‐hospital variance of BZD prescriptions (σ2‐BZD) | 0.005 | 0.002 |
Data are expressed as median, interquartile range (Q1‐Q3) or as absolute (n) and relative (%) frequencies.
Abbreviation: BZD, benzodiazepines.
Variation in New BZD prescriptions across the hospital network was preliminarily investigated considering, the non‐intervention (years 2014‐2015) and the intervention (years 2016‐2017) periods separately. A graphical representation of new BZD prescription variation as a function of the number of patients quarterly admitted in the hospitals of the network was provided using funnel plots (Figure 1A,B). The number of patients admitted in the hospital network, without an on‐going BZD prescription, ranged from 1067 to 2391 in the non‐intervention period and from 1397 to 3116 in the intervention period.
Figure 1.
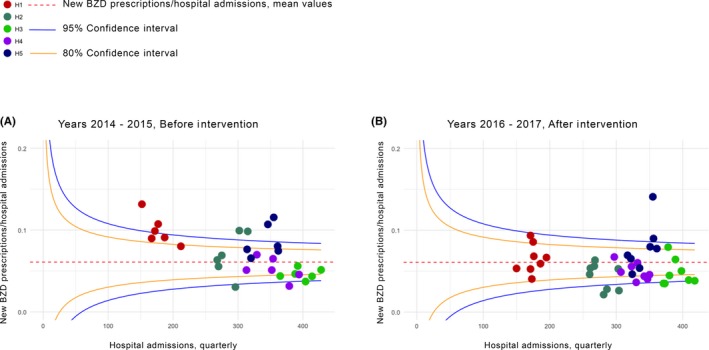
Between hospital variability in benzodiazepine prescriptions by study period. In these funnel plots, variability in benzodiazepine prescriptions/hospital admissions (y‐axes) is plotted against total hospital admissions (x‐axes). Each spot represents an individual hospital of the network (H1, H2, H3, H4, H5). Plots inspect the across hospital variability before (A, years 2014‐2015) and after the intervention (B, years 2016‐2017). New benzodiazepine prescriptions/hospital admissions (horizontal dashed red lines) with the 80 (solid yellow lines) and 95% confidence intervals (blue solid lines) are shown
Overall mean new BZD prescriptions/admissions was 6.1% (range 5%‐9% in the non intervention period, and 3%‐8% in the intervention period).
Before the intervention most of the new BZD prescriptions of three of the five hospitals of the network were over the mean, with 30% new BZDs presciption over the 95% confidence limit.
After the intervention only one hospital presents more then 50% of new BZD prescriptions up the mean and overall 10% of new BZD prescritions were in the 95% confidence limit.
In total, before the intervention 70% of new BZDs prescriptions fell in the the 95% and 80% control limits vs 90% after the intervention. Overall the mean inter‐hospitals reduction in BZDs prescriptions were significantly reduced after the intervention: −2% (0.2%‐3%; P < .03).
3.2. Time‐trend analysis of the inter‐hospital variability of the new BZD prescription rate
Across the hospital network, before the intervention, a significant constant upward trend in inter‐hospital variation of new BZD prescriptions, with a slope of 0.901 (SE 0.441; P < .05), was found. After the implementation of the intervention the inter‐hospital variation trend line significantly changed, decreasing by −0.257 (SE 0.005: P < .001) (Figure 2). By December 2017, 2 years after the start of the intervention, we estimated that the trimestral rate of inter‐hospital variation was 96% less than would have been expected if the intervention had not taken place. A tabular version of the Interrupted Time Series Regression Analysis of inter‐hospital variation is shown in Table 2.
Figure 2.
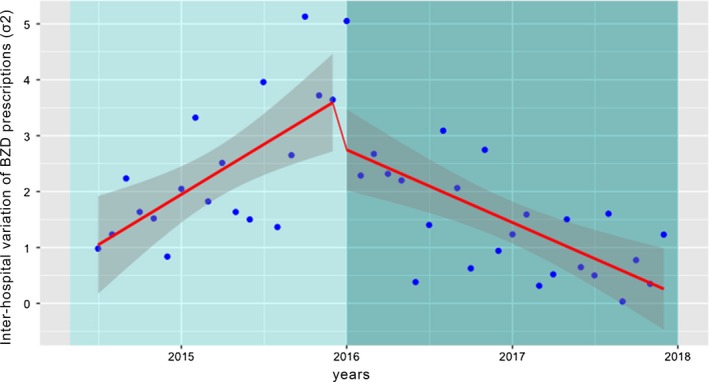
Interrupted time‐series regression analysis of the inter‐hospital BZD prescription variation. Monthly inter‐hospital variation in new BZD prescriptions across hospitals (blue dots: BZD inter‐hospital variance) during the study period. Light green screen represents the preintervention period; Dark green screen the post. Solid red line indicates the variation trend
Table 2.
Interrupted time‐series regression analysis of the inter‐hospital BZD prescription variation
| Inter‐hospital BZD prescription variation (σ2‐BZD) | β coefficient | SE | P‐value |
|---|---|---|---|
| Base level (β0) | 0.901 | 0.441 | <.05 |
| Base trend of variation in BZD prescriptions (β1) | 0.149 | 0.040 | <.001 |
| Change in level (β2) | 3.90 | 0.397 | <.001 |
| Trend change of variability in BZD prescriptions after the intervention (β3) | −0.257 | 0.005 | <.001 |
3.3. Inter‐hospital mutual influence on prescription variation
The inter‐hospital mutual influence on prescription variation was assessed using VAR models. A graphical representation of the impact of a change in new BZD prescription rates in one of the hospitals on the others, over a period of 10 trimesters, is provided (Figure 3). In order to show the results in the easiest comprehensible way, we present the effect of a 1% change in the prescription rate (Figure 3 and Table S1). Trends suggestive of mutual influence can be easily recognised; significant results however, were seen only between hospitals H2 and H4 (0.7%; 95% CI 0.0 to −1.1%), and H5 and H1 (0.8%; 95% CI 0.0‐1.2%).
Figure 3.

Impact of the multimodal strategy in one of the hospitals on the change of new BZD prescriptions on the other ones. Orthogonalised impulse response functions based on vector‐autoregressive models. The graphs show the percentage change in new BZD prescriptions by hospital (y‐axes, continuous blue lines), over time (quarterly, x‐axes) with 95% bootstrapped error bands (dashed blue lines). Impacts of isolated changes in new BZD prescriptions are depicted following the order, from top left to bottom right: H1 (A), H2 (B); H3 (C); H4 (D), H5 (E)
4. DISCUSSION
Between physicians/healthcare‐institutions, practice variations are widely recognised as a marker of potential overuse/overtreatment.20
Even if numerous studies targeted to waste in healthcare have been published in recent years,11, 12, 13 interventions aiming at reducing variation in treatments and diagnostic procedures, are lacking.21
One of the reasons is that an intervention can be promoted at the service or hospital level following the temporal trend of the targeted prescription behaviour; while investigating the impact on variation implies to act at the network level analysing and benchmarking before and after data. Interventions at the network level can be performed collecting data manually or, if the network has a common IT platform like ours, directly extracting data.
We implemented a software able not only to continuously collect data, but also to benchmark and report results of drug and laboratory test prescriptions to clinicians.
A similar strategy offers the possibility to expose healthcare providers of the network to an open benchmarking and to the psychological pressure related to being observed and eventually judged by peers (called “peer‐pressure”).
In a network an intervention, if successful, can theoretically produce an independent change in the chosen indicator in each one of the institutions under study and/or an alignment towards a common prescription level. The second possibility suggests a drift towards a common compromise between over‐ and under‐use and/or the effect of the open benchmarking associated with the related peer‐pressure. Regardless of the root cause, a reduction in variation has to be seen as a potential indicator of prescription adequacy.
We decided therefore to perform a secondary analysis of a previous before and after study performed at the network level and targeted to reduce new in‐hospital benzodiazepine prescriptions, aimed at evaluating the impact of the intervention on inter‐hospital variation.11
Over a period of three and half years, we succeeded in a significant absolute 27% reduction of between‐hospital variability in BZD prescriptions. We can obviously not calculate in the context of a multifaceted intervention the impact of each individual measure; we believe however that the use of a “comparative variation data tool”, did significantly contribute in changing the prescription habits of clinicians and in minimising overuse.
These results are even more of interest because benzodiazepine overuse represents an epidemic phenomenon worldwide. A survey published by the Organisation for Economic Co‐operation and Development (OECD) in 2015, showed that in OECD countries, excluding Switzerland, on average 2.5% of the adults are chronic BZD users, while 6.4% of older adults have received at least one BZD prescription within 1 year. In the same survey, moreover, a large variability among countries in the rate of patients older than 65 years treated with BZD was found, ranging from 1% to 20%.22
Recent data estimate that among the general Swiss population older than 15 years, 7.5% are using BZDs, with the highest consumption rate in women older than 70 years.23 A report of a Swiss health insurance company published in 2017, documented that psycholeptics represent, leading drugs in Switzerland, accounting, along with painkillers, anti‐inflammatory and anti‐rheumatic drugs, for one fifth of the totality of drugs prescribed, with higher rates in elderly homes.24 Furthermore, a national pharmacy survey showed in 2007 a 1‐year prevalence in BZD prescriptions of 14.5%, with 56% of patients receiving a long‐term therapy.25 This meaning that, data on BZD prescriptions in Switzerland shows rates in the upper distribution compared with other countries.26, 27, 28, 29
As far as prescription trends over years, alarming data about BZD consumption and mortality related to BZD overdose in the United States were recently published. To note BZD and opioids and other sedative co‐prescriptions, quadrupled and respectively doubled between 2003 and 2015.29
The large variation in BZD consumption among regions and countries could be explained by specific treatment guidelines as well as prescribing and reimbursement policies, by differences in the prevalence of diseases and by cultural phenomena. In Switzerland BZDs can be dispensed under medical order only and prescribing is regulated at the legislative level, with a specific federal law, which indicates the terms of narcotic and psychotropic substance prescription.30 Prescriptions of drugs can be repeatable or not, and for drugs for which repeatability apply, the maximum duration is 6 months. Furthermore, it is a specific task of the pharmacist to verify that the dosage ordered by the physician matches the number of boxes of the drug collected by the patient.
Compared with existing work on prescribing variation, this study provides a unique example for several aspects. Previous studies investigated variation mainly in a static way or outside the context of a clinical study, and in ours, we examined the impact of an intervention at the network level.
Even examining two previous studies about drug prescription adequacy with variation analysis, the first one focused on opioids and the second one on antibiotics, the impact on variability shown in our data stands out.31, 32, 33
We can hypothesise that the greater reduction in variation in our study could be the consequence of several intrinsic aspects of our intervention.
First, the medication to which the intervention was directed; benzodiazepines, is a class of drugs for which not only the misuse of prescription guidelines is implicated, but also a vicious circle in which an unsuccessful shared decision process and health advocacy intervenes (renew of prescriptions on the basis of a postulated patient dependence, lack of offer of alternative therapies for the treatment of insomnia, failure to educate patients about the risks of the medication).34
In accordance with the principles of the “Choosing Wisely” campaign, our goal was also the improvement of the shared‐decision‐making process, empowering patients with informed conversations about their treatment options.
Moreover, our study highlighted the important effect of the peer‐to‐peer comparison on prescription habits.
Other studies, previously, investigated the role of a constant comparison between peers on prescriptions, as an efficacy nonfinancial strategy to improve mainly guideline compliance and underuse.35
It is however, the first time that this aspect is evaluated during a medication prescription overuse targeted intervention in a network of hospitals. Peer‐to‐peer discussions on one's prescription behaviour, in a formally non‐judgmental setting, can help in recognising the harmful consequences of one's attitudes and habits, and the need to promote a cultural change between providers and patients.
The setting of the study with hospitals sharing the same internal guidelines, allowed us to explore, along with the evolution of the variance, using a dynamic interdependency model, the influence on the other hospitals of changes in the prescription pattern that took place in one of them.
Although marginally significant, interdependency among the hospitals of the network was seen. In particular, a fluctuation in BZD prescriptions in the hospital H2 and H5 did translate in similar trends in the hospitals H4 and H1, respectively. In which way and whether the mutual influence can positively impact on the prescription habits can however not be stated on the basis of the present data. We have however to acknowledge some limitations of our study; the fact that the observation results from a secondary analysis and the impossibility of evaluating individual healthcare provider factors. Furthermore, the study was a non‐randomised interventional one, without a control group. Last but not least, even if a general feeling of appreciation of the intervention emerged from healthcare providers involved, we have to acknowledge that the present study is limited by the lack of a systematic process of collection of qualitative information on how clinicians perceived the pro‐active involvement in the education, monitoring and benchmarking processes.
In the context, a longitudinal study aiming at directly establishing the relationship between peer‐pressure and reduction of BZD variability is needed.
In conclusion, through a multi‐level strategy based on peer‐pressure and educational interventions, we obtained a reduction in variation in new benzodiazepine prescriptions in a network of teaching hospitals. We believe that a similar strategy could contribute to reducing variation and overuse and to promoting best care practices, also in other areas.
DECLARATION OF INTEREST
The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in, or financial conflict, with the subject matter or materials discussed in the manuscript.
ETHICS APPROVAL
The study was reviewed by the Swiss Ethics Committee, which confirmed that, involving anonymous secondary data only, it was exempt from institutional board approval.
CONSENT FOR PUBLICATION
All authors have provided consent to publish.
DATA SHARING STATEMENT
The datasets from the current study are not publicly available but are available from the corresponding author on request.
AUTHOR CONTRIBUTIONS
RDG, LG, AO: Conceptualisation; RDG, KS: Formal analysis; RDG, AO, KS, AG, AC, FK: Data Curation; RDG, AO: Investigation; RDG, AO, AG, AC, FK: Methodology; LG: Supervision; RDG., AO, LG: Writing original draft; RDG, AO, LG, AG, AC, FK: Writing review and editing.
Supporting information
Del Giorno R, Ottini A, Greco A, et al. Peer‐pressure and overuse: The effect of a multimodal approach on variation in benzodiazepine prescriptions in a network of public hospitals. Int J Clin Pract. 2020;74:e13448 10.1111/ijcp.13448
Rosaria Del Giorno and Andrea Ottini share joint first authorship.
Contributor Information
Rosaria Del Giorno, Email: rosaria.delgiorno@eoc.ch, Email: rosaria.delgiorno@eoc.ch.
Luca Gabutti, Email: luca.gabutti@eoc.ch.
REFERENCES
- 1. Stammen LA, Stalmeijer RE, Paternotte E, et al. Training physicians to provide high‐value, cost‐conscious care: a systematic review. JAMA. 2015;314(22):2384‐2400. [DOI] [PubMed] [Google Scholar]
- 2. Baicker K, Chandra A. Medicare spending, the physician workforce, and beneficiaries’ quality of care. Health Aff. 2004;23:w184‐w197. [DOI] [PubMed] [Google Scholar]
- 3. Pozo‐Rodríguez F, Castro‐Acosta A, Alvarez CJ, et al. Determinants of between‐hospital variations in outcomes for patients admitted with COPD exacerbations: findings from a nationwide clinical audit (AUDIPOC) in Spain. Int J Clin Pract. 2015;69(9):938‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mbuagbaw L, Medley N, Darzi AJ, et al. Health system and community level interventions for improving antenatal care coverage and health outcomes. Cochrane Database Syst Rev. 2015;12:1‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelley AS, Bollens‐Lund E, Covinsky KE, Skinner JS, Morrison RS. Prospective identification of patients at risk for unwarranted variation in treatment. J Palliat Med. 2018;21(1):44‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schang L, Morton A, DaSilva P, Bevan G. From data to decisions? Exploring how healthcare payers respond to the NHS atlas of variation in healthcare in england. Health Policy. 2014;114(1):79‐87. [DOI] [PubMed] [Google Scholar]
- 7. Moon S, Choi M. The effect of usual source of care on the association of annual healthcare expenditure with patients' age and chronic disease duration. Int J Environ Res Public Health. 2018;15;pii:E1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60‐65. [DOI] [PubMed] [Google Scholar]
- 9. Korenstein D, Chimonas S, Barrow B, Keyhani S, Troy A, Lipitz‐Snyderman A. Development of a conceptual map of negative consequences for patients of overuse of medical tests and treatments. JAMA Intern Med. 2018;178(10):1401‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sadana D, Pratzer A, Scher LJ, et al. Promoting high‐value practice by reducing unnecessary transfusions with a patient blood management program. JAMA Intern Med. 2018;178(1):116‐122. [DOI] [PubMed] [Google Scholar]
- 11. Del Giorno R, Greco A, Zasa A, et al. Combining prescription monitoring, benchmarking, and educational interventions to reduce benzodiazepine prescriptions among internal medicine inpatients; a multicenter before and after study in a network of Swiss Public Hospitals. Postgrad Med. 2018;130(7):627‐636. [DOI] [PubMed] [Google Scholar]
- 12. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in‐hospital over‐prescription of proton pump inhibitors: a longitudinal multicenter quasi‐experimental before‐and‐after study. Eur J Intern Med. 2018;50:52‐59. [DOI] [PubMed] [Google Scholar]
- 13. Erard Y, Del Giorno R, Zasa A, et al. A multi‐level strategy for a long lasting reduction in unnecessary laboratory testing: a multicenter before and after study in a teaching hospital network. Int J Clin Pract. 2018;73:e13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emanuel E, Tanden N, Altman S, et al. A systemic approach to containing health care spending. N Engl J Med. 2012;367:949‐954. [DOI] [PubMed] [Google Scholar]
- 15. National Priorities Partnership . National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, DC: National Quality Forum; 2008. [Google Scholar]
- 16. Watkins C, Harvey I, Carthy P, Moore L, Robinson E, Brawn R. Attitudes and behaviour of general practitioners and their prescribing costs: a national cross sectional survey. Qual Saf Health Care. 2003;12(1):29‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schpero WL. Limiting low‐value care by “Choosing Wisely”. Virtual Mentor. 2014;16(2):131‐134. [DOI] [PubMed] [Google Scholar]
- 18. Brownlee S, Chalkidou K, Doust J, et al. Evidence for overuse of medical services around the world. Lancet. 2017;390(10090):156‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. http://www.kofam.ch/it/domanda-e-procedura/progetti-non-soggetti-allobbligodiautorizzazione/ Accessed 11 November 2017.
- 20. Morgan DJ, Leppin AL, Smith CD, Korenstein D. A practical framework for understanding and reducing medical overuse: conceptualizing overuse through the patient‐clinician interaction. J Hosp Med. 2017;12(5):346‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parchman ML, Henrikson NB, Blasi PR, et al. Taking action on overuse: creating the culture for change. Healthc (Amst). 2017;5(4):199‐203. [DOI] [PubMed] [Google Scholar]
- 22. OECD . Health at a Glance 2015: OECD Indicators. Paris, France: OECD Publishing, 2015. 10.1787/health_glance-2015-en [DOI] [Google Scholar]
- 23. Corolar (Continuous Rolling Survey of Addictive Behaviours and Related Risks) . Häufigkeit und Dauer der Einnahme von Schlaf‐ und Beruhigungsmitteln, nach Geschlecht und Alter, 2016. http://www.suchtmonitoring.ch (accessed 30 October 2019).
- 24. Schneider R, Schur N, Reinau D, Schwenkglenks M, Meier C Helsana‐Arzneimittelreport für die Schweiz 2017. Auswertungsergebnisse der Helsana Arzneimitteldaten aus den Jahren 2013 bis 2016. https://www.helsana.ch/docs/arzneimittelreport-2017.pdf
- 25. Petitjean S, Ladewig D, Meier CR, Amrein R, Wiesbeck GA. Benzodiazepine prescribing to the Swiss adult population: results from a national survey of community pharmacies. Int Clin Psychopharmacol. 2007;22(5):292‐298. [DOI] [PubMed] [Google Scholar]
- 26. Lagnaoui R, Depont F, Fourrier A, et al. Patterns and correlates of benzodiazepine use in the French general population. Eur J Clin Pharmacol. 2004;60:523‐529. [DOI] [PubMed] [Google Scholar]
- 27. Windle A, Elliot E, Duszynski K, Moore V. Benzodiazepine prescribing in elderly Australian general practice patients. Aust N Z J Public Health. 2007;31:379‐381. [DOI] [PubMed] [Google Scholar]
- 28. Spanemberg L, Nogueira EL, Belem da Silva CT, Dargél AA, Menezes FS, Neto AC. High prevalence and prescription of benzodiazepines for elderly: data from psychiatric consultation to patients from an emergency room of a general hospital. Gen Hosp Psychiatry. 2011;33:45‐50. [DOI] [PubMed] [Google Scholar]
- 29. Agarwal D, Bruce E. Patterns in Outpatient Benzodiazepine Prescribing in the United States Sumit D. Landon JAMA Netw Open. 2019;2:e187399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Federal Act of 3 October 1951 on Narcotics and Psychotropic Substances (Narcotics Act, NarcA). https://www.admin.ch/opc/it/classifiedcompilation/19981989/index.html (accessed 30 October 2019).
- 31. Smieszek T, Pouwels KB, Dolk FCK, et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother. 2018;73(suppl_2):ii36‐ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sedrak A, Anpalahan M, Luetsch K. Enablers and barriers to the use of antibiotic guidelines in the assessment and treatment of community‐acquired pneumonia–a qualitative study of clinicians' perspectives. Int J Clin Pract. 2017;71(6):e12959. [DOI] [PubMed] [Google Scholar]
- 33. Burton JH, Hoppe JA, Echternach JM, Rodgers JM, Donato M. Quality improvement initiative to decrease variability of emergency physician opioid analgesic prescribing. West J Emerg Med. 2016;17(3):258‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gerlach LB, Maust DT, Leong SH, Mavandadi S, Oslin DW. Factors associated with long‐term benzodiazepine use among older adults. JAMA Intern Med. 2018;178(11):1560‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Navathe AS, Emanuel EJ. Physician peer comparisons as a nonfinancial strategy to improve the value of care. JAMA. 2016;316(17):1759‐1760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


