Abstract
Objectives
A treatment regimen consisting of bendamustine and brentuximab vedotin (BV) has been described as a highly potent salvage therapy and as an effective induction therapy leading to high response rates before autologous stem cell transplantation (ASCT) in patients with classical Hodgkin lymphoma (cHL). In this retrospective analysis, we aimed to assess this therapy's efficacy in unselected patients with cHL and CD30+ peripheral T‐cell lymphoma (PTCL).
Patients and methods
Data of 28 patients with cHL and five patients with PTCL treated with a combination of bendamustine and BV at three Austrian tertiary cancer centers were analyzed.
Results
In patients with cHL, the ORR was 100% (78.6% CR, 21.4% PR). After 17 months median follow‐up, median survival times were not reached; 1‐year PFS was 81.9%, and 1‐year OS was 95.7%. Thirteen eligible patients (46.4%) successfully underwent planned ASCT after salvage therapy with bendamustine and BV and subsequent high‐dose chemotherapy. Three of the five PTCL patients achieved CR, while two did not respond and died during or shortly after therapy.
Conclusion
A combination of bendamustine and BV is an effective salvage and induction therapy before ASCT in patients with relapsed/refractory cHL. Further research is warranted to evaluate the use in patients with PTCL.
Keywords: bendamustine, brentuximab vedotin, Hodgkin lymphoma, induction therapy, peripheral T‐cell lymphoma, PTCL, salvage therapy
1. INTRODUCTION
Classical Hodgkin lymphoma (cHL) and some subtypes of peripheral T‐cell lymphoma (PTCL) are lymphoid malignancies characterized by a strong expression of CD30 on tumor cells.
Most patients with cHL can be cured with conventional first‐line chemotherapy (with or without radiation therapy). However, a fraction of patients is primary refractory to treatment or relapses after first‐line treatment. While immunotherapy has been shown to be effective in patients with chemotherapy‐resistant cHL and might be incorporated in future therapy regimens,1 the current standard of treatment for those patients is autologous stem cell transplantation (ASCT) after intensive salvage chemotherapy. Ideally, salvage chemotherapy should achieve complete metabolic remission (CR) since this is favorable when aiming for long‐term control of disease.2 Commonly used salvage chemotherapy regimens like ICE (ifosfamide, carboplatin, and etoposide), DHAP (cisplatin, cytarabine, and dexamethasone), and ESHAP (etoposide, steroids, ara‐C, and cisplatin) yield CR rates of between 20% and 50% and are associated with significant toxicities.3, 4, 5 Recently, a phase 1/2 trial by Garcia‐Sanz et al investigated the addition of brentuximab vedotin (BV) to ESHAP in patients with relapsed or refractory cHL as induction therapy before planned ASCT. The combination showed improved efficacy when indirectly compared to ESHAP alone while still being tolerable.6 BV in combination with other chemotherapy agents (doxorubicin, vinblastine, and dacarbazine) also has already been shown to be superior to standard chemotherapy with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) in the frontline treatment of advanced HL in the ECHELON‐1 trial.7 Two‐phase 1/2 studies by LaCasce et al8 and O'Connor et al9 in 2018 showed that therapy with BV could achieve high CR rates as salvage therapy and was still effective in heavily pretreated patients, while having manageable side effects when indirectly compared to platinum‐based therapies. A phase 2 study by Friedberg et al10, comparing BV and bendamustine to BV in combination with dacarbazine as frontline therapy in patients over the age of 60, also showed a very high efficacy of bendamustine and BV (100% ORR, 88% CR), albeit associated with a significantly higher toxicity in these patients.
While the encouraging efficacy of bendamustine and BV has been shown in Hodgkin lymphoma, and BV in combination with other chemotherapy agents has already been proven effective and safe in the frontline treatment of CD30 positive PTCL,11 only very limited data exist for the treatment of PTCL with bendamustine and BV. Dumont et al12 recently reported of nine patients with advanced PTCL that were treated with bendamustine and BV outside of prospective clinical trials, two of which achieved a CR. Although the study of O'Connor et al also included patients with PTCL, only one patient with anaplastic large T‐cell lymphoma was included in it and subsequently treated with bendamustine and BV.
Due to the paucity of data, we therefore wanted to assess the efficacy and safety of a bendamustine and BV regimen and its suitability as induction therapy before high‐dose chemotherapy and subsequent ASCT in clinical practice, in unselected patients with Hodgkin lymphoma and PTCL, who were in part heavily pretreated.
2. PATIENTS AND METHODS
2.1. Patients
First, we identified patients with cHL and PTCL treated with bendamustine and BV from the Austrian Brentuximab Vedotin registry of the Austrian Study Group of Medical Tumor Therapy (AGMT) (415‐E/1942). Then, we added patients from two additional tertiary Austrian cancer centers to achieve sufficient patient numbers. Overall, we identified 28 patients with histologically confirmed cHL and five patients with PTCL, which were treated with a combination of bendamustine (70 or 90 mg/m2 on day 1 and 2 of 3‐week cycles) and BV (1.8 mg/kg on day 1 of 3‐week cycles) between 2015 and 2019. One patient received prophylactic G‐CSF. Treatments were chosen at the discretion of the treating institutions, and all patients signed an informed consent form agreeing to being treated with an off‐label regimen. Clinical characteristics and outcome were retrospectively analyzed by chart‐based review. Assessment of response was done according to the Lugano FDG‐PET/CT criteria.13
2.2. Statistical analysis
Clinical characteristics, progression‐free survival (PFS), and overall survival (OS) after therapy with bendamustine and BV were retrospectively analyzed by chart‐based review. PFS was defined as the time from start of treatment until disease progression or death from any cause. OS was defined as the time from treatment start until death from any cause. All statistical analyses were performed using IBM® SPSS® statistics software, version 24. Kaplan‐Meier curve analysis was used for estimation of survival.
3. RESULTS
3.1. Patients with classical Hodgkin lymphoma
3.1.1. Patient characteristics and outcome
The detailed patient characteristics can be found in Table 1. As expected, patients with cHL were younger than the patients with PTCL (median age 43.5 vs 70 years, P = .01) and most of them had a good performance status, with only two patients (7.1%) having an ECOG score higher than one. Eighteen patients (64.3%) were male, and ten patients (35.7%) were female. Notably, one patient developed Hodgkin lymphoma through Richter transformation of a pre‐existing chronic lymphocytic leukemia. The patients received a median of one treatment regimen before treatment with bendamustine and BV, ranging from zero to four therapy lines beforehand. Five patients underwent ASCT before therapy with bendamustine and BV, and one patient underwent both autologous and allogeneic stem cell transplantation beforehand. Only two patients were treated with an immune checkpoint inhibitor before therapy with bendamustine and BV, and one patient received immune checkpoint inhibitor therapy in a subsequent therapy line.
Table 1.
Detailed patient characteristics of all patients with classical Hodgkin lymphoma and peripheral T‐cell lymphoma
| cHL | PTCL | |
|---|---|---|
| n = 28 | n = 5 | |
| Sex | ||
| Male | 64.3% (18) | 60.0% (3) |
| Female | 35.7% (10) | 40.0% (2) |
| Age at primary diagnosis (y) | ||
| Median | 35.5 | 74 |
| Mean ± SD | 40.45 ± 20.80 | 67.80 ± 10.11 |
| Range | 9‐84 | 54‐76 |
| Age at start bendamustine + BV (y) | ||
| Median | 43.5 | 75 |
| Mean ± SD | 46.21 ± 19.36 | 69.40 ± 9.86 |
| Range | 21‐84 | 54‐77 |
| Bendamustine + BV treatment line | ||
| First‐line | 14.3% (4) | 20.0% (1) |
| Second‐line | 42.9% (12) | 60.0% (3) |
| Third‐line | 32.1% (9) | 20.0% (1) |
| Fourth‐line | 3.6% (1) | – |
| Fifth‐line | 7.1% (2) | – |
| ECOG before bendamustine + BV | ||
| 0 | 46.4% (13) | 80.0% (4) |
| 1 | 42.9% (12) | 20.0% (1) |
| 2 | 7.1% (2) | – |
| Missing | 3.6% (1) | – |
| Response to bendamustine + BV | ||
| Overall response rate | 100% (28) | 60.0% (3) |
| Complete remission | 78.6% (22) | 40.0% (2) |
| Partial remission | 21.4% (6) | 20.0% (1) |
| No response | – | 50.0% (2) |
| Bendamustine + BV as induction before ASCT | ||
| Yes | 46.4% (13) | – |
| No | 53.6% (15) | 100.0% (5) |
| Stem cell collection during therapy | ||
| Planned | 9 (69.2% of ASCT) | – |
| Successful | 9 (100%) | – |
| BV maintenance given | ||
| No | 67.9% (19) | 80.0% (4) |
| Yes | 32.1% (9) | 20.0% (1) |
| Documented adverse events | ||
| Infections (any grade) | 35.7% (10) | 40.0% (2) |
| Neutropenia (±fever) necessitating G‐CSF | 10.7% (3) | 40.0% (2) |
| Skin‐related adverse events | 39.3% (11) | – |
| Peripheral polyneuropathy | 10.7% (3) | – |
| Infusion‐related reactions | 35.7% (10) | – |
| Therapy delay | 21.4% (6) | – |
| Dose reduction | 28.6% (8) | 20.0% (1) |
| Death during therapy | 3.6% (1) | 20.0% (1) |
Abbreviations: ASCT, autologous stem cell transplantation; BV, brentuximab vedotin; cHL, classical Hodgkin lymphoma; G‐CSF, granulocyte‐colony‐stimulating factor; PTCL, peripheral T‐cell lymphoma; SD, standard deviation.
The difference between the median age at primary diagnosis (35.5 years) and the age at which patients received therapy with bendamustine and BV (43.5 years) was 8 years, which can be explained by the inclusion of some patients with late relapses of disease and patients treated with up to four previous lines of therapy. The median number of treatment cycles with bendamustine and BV received was three (range 1‐6). Nine patients (32.1%) received further BV maintenance therapy after completion of this combination therapy. The median number of BV doses (including in combination with bendamustine) given was 5 (range 1‐17). After a median follow‐up time of 17 months, the median PFS and OS after therapy with bendamustine and BV were not reached yet. The 1‐year PFS was 82.7%, and the 1‐year OS was 95.8%. The Kaplan‐Meier survival curves are shown in Figure 1. No significant difference in PFS between patients receiving maintenance therapy and those who did not could be observed; however, the 1‐year PFS of those receiving maintenance was higher than that of the rest (100% vs 77.4%, P = .243).
Figure 1.
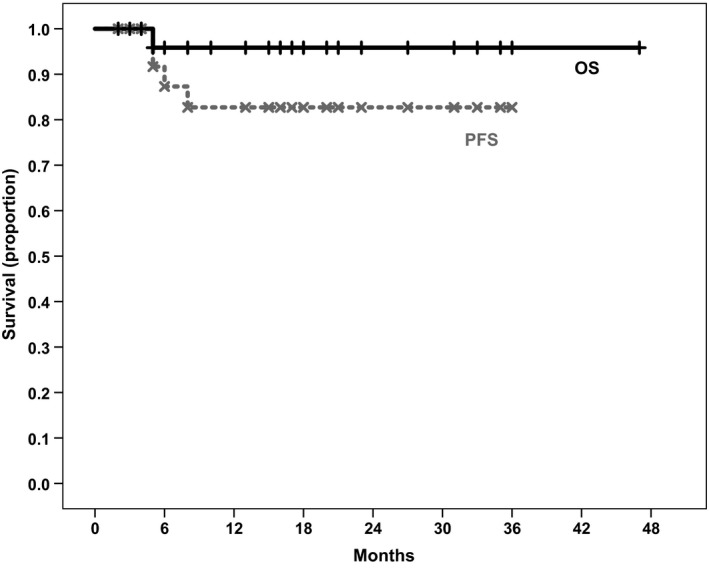
Overall survival and progression‐free survival curves of all patients with classical Hodgkin lymphoma. The 1‐y overall and progression‐free survival were 95.8% and 82.7%, respectively. Median survival was not reached yet
Figure 2 compares the PFS of different lines of therapy with bendamustine and BV. As expected, patients receiving bendamustine and BV in earlier lines of therapy (first‐line median PFS not reached, 1‐year PFS 100%; second‐ and third‐line median PFS not reached, 1‐year PFS 87.8%) had a significantly longer PFS than patients receiving therapy later (fourth‐line or later median PFS 8.0 months [95% CI: 3.2‐12.8 months], 1‐year PFS 33.3%; overall P = .039). When patients receiving bendamustine plus BV as first‐line therapy are excluded from the analysis, the difference between PFS of patients receiving treatment as second‐ or third‐line therapy and those receiving it later remains statistically significant (median PFS not reached vs 8.0 months [95% CI: 3.2‐12.8 months], P = .033). However, the percentage of patients achieving CR after therapy did not significantly differ between earlier and later lines of therapy (P = .493). The OS of patients did not differ between the therapy lines, with no median survival calculable due to all except one patient still being alive at time of data collection (P = .80).
Figure 2.
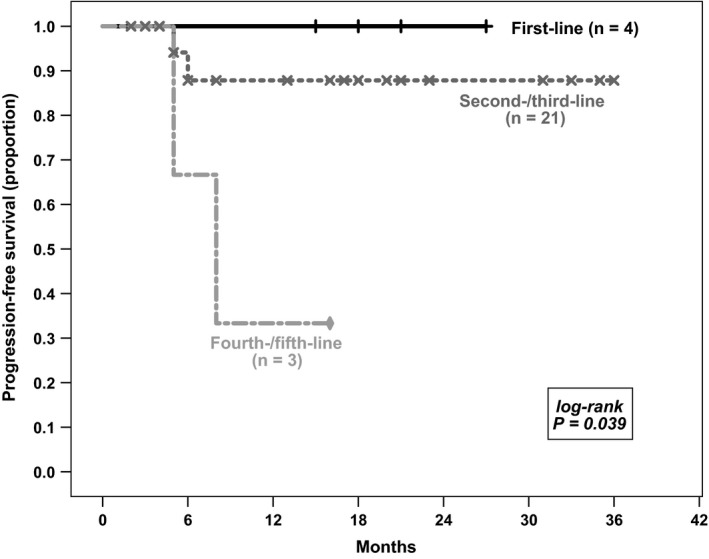
Comparison of patients with classical Hodgkin lymphoma receiving bendamustine and brentuximab vedotin in different therapy lines. Progression‐free survival (PFS) of patients treated in earlier lines was significantly higher than of those treated later (first‐line median PFS not reached, 1‐y PFS 100%; second‐ and third‐line median PFS not reached, 1‐y PFS 87.1%; fourth‐ and fifth‐line median PFS 8.0 mo (95% CI: 3.2‐12.8 mo), 1‐y PFS 33.3%; overall P = .048)
The overall response rate (ORR) in all patients was 100%. For 22 patients (78.6%), achievement of a CR after completion of chemotherapy was documented and six patients (21.4%) achieved a partial remission. The rate of CR and PR in only those 24 patients with relapsed or refractory cHL was 75.0% and 25.0%, respectively.
3.1.2. Use as an induction therapy before autologous stem cell transplantation
In thirteen patients (46.4%), bendamustine and BV were used as planned induction therapy before high‐dose chemotherapy (HDT) and subsequent ASCT. The other patients either were too old had already undergone ASCT or were treated with bendamustine and BV as first‐line therapy. In nine of the patients undergoing ASCT, stem cell collection was successfully done during or after chemotherapy with bendamustine and BV, and a median of 4.0 M/kg stem cells, ranging from 0.7 M/kg (collection was not continued as there were already 2.5 M/kg cells stored from collection during earlier therapy lines) to 6.8 M//kg cells, was collected. Stem cell harvest was done after the first or second cycle of bendamustine and BV in seven patients, while in the other two patients, collection was done after finishing treatment with bendamustine and BV with G‐CSF mobilization. Stem cell collection had been done during earlier treatment lines in the remaining four patients. Of all thirteen patients that underwent ASCT, nine did so as second‐line therapy, three patients underwent ASCT as third‐line therapy and one patient as fifth‐line therapy. The median number of treatment cycles with bendamustine and BV before ASCT was three (range: 2‐5). The ORR to bendamustine and BV as planned induction was 100%, resulting in eleven cases with CR and two cases with PR, one of which with a singular lesion with a Deauville score of 3 in FDG‐PET/CT. One of the patients in PR achieved CR after ASCT and had no relapse of disease 8 months after ASCT. Restaging of the second patient in PR was not done yet at the time of data collection, which was 1 month after ASCT. After a median follow‐up of 13 months, the 1‐year PFS and OS of patients undergoing ASCT after receiving bendamustine and BV induction therapy were 80.8% and 100%, respectively, and the median PFS and OS were not reached yet (Figure 3). Five patients (38.5%) received further BV maintenance therapy after successful ASCT. Two patients had a relapse of disease after ASCT; the first patient underwent ASCT as first salvage therapy after being refractory to BEACOPP, and the second patient underwent ASCT as fourth salvage therapy after relapsing from ABVD as frontline and second‐line therapy and being primary refractory to BRECADD and Nivolumab as third‐ and fourth‐line therapy, respectively.
Figure 3.
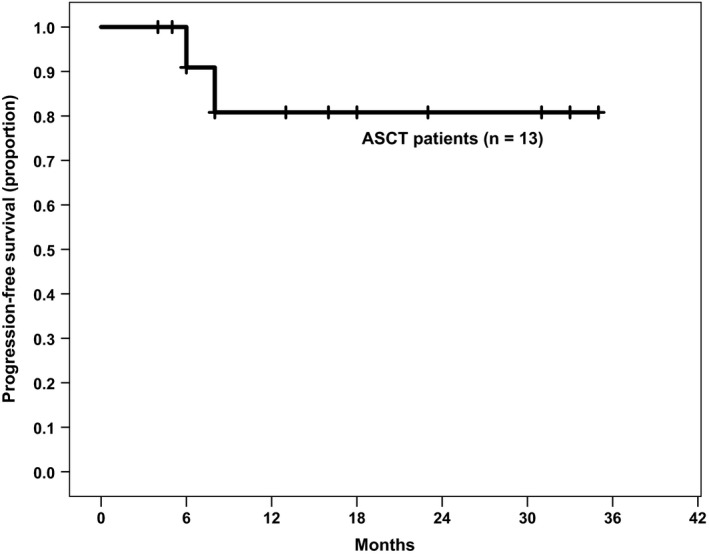
Progression‐free survival of patients with classical Hodgkin lymphoma who underwent autologous stem cell transplantation (n = 13). The progression‐free survival at 1 y was 80.8%, the median progression‐free survival was not reached yet
3.1.3. Adverse events
The most frequently documented adverse events were skin events (in 39.03% of patients), infusion‐related reactions (in 35.7% of patients), and infections requiring intervention or hospitalization (in 35.7% of patients). Notably, only one patient was administered prophylactic G‐CSF from the start of therapy and further three patients were administered G‐CSF in consecutive cycles after developing severe neutropenia. Therapy discontinuation due to the severity of adverse events affecting the skin was not necessary in any patient, as only mild and moderate events occurred. Three patients (10.7%) developed peripheral polyneuropathy during treatment, one of which had to discontinue therapy because of it. Administration of the next treatment cycle had to be delayed due to side effects at least once in 21.4% of patients, and in 28.6% of patients, the bendamustine dose had to be lowered due to toxicities. One patient died in partial remission 9 weeks after last chemotherapy due to unknown reasons, which we counted as therapy related. No other deaths occurred during therapy.
3.2. Patients with peripheral T‐cell lymphoma
We collected data of five patients with PTCL—two PTCL NOS, one ALCL, one lymphoepithelioid (=“Lennert's”) lymphoma and one with a composite lymphoma of PTCL and HL. The median age was 75 years, three patients were male, and two patients were female. All patients either had an ECOG score of zero or one. The detailed characteristics can be found in Table 1. With a median follow‐up of 20 months, the median PFS was 20.0 months (95% CI: 0.0‐45.0 months) and the median OS was not reached yet. Three of five patients responded to the treatment and achieved a CR; of the responding patients, one patient was treated with bendamustine and BV as first‐line therapy, one as second‐line and the last as third‐line therapy. One patient had a relapse of disease after 20 months, and the other two had no sign of relapse at time of their last follow‐up visits, with a current PFS of 21 and 2 months, respectively. Two patients received the therapy in second‐line after not responding to first‐line therapy; one of them died after the first cycle of treatment, and the other patient died after discontinuing treatment after three cycles without response. None of the patients with PTCL underwent ASCT after therapy with bendamustine and BV. The detailed course of disease in the patients with PTCL can be found in Table 2.
Table 2.
Important characteristics and course of disease in the five patients with peripheral T‐cell lymphoma
| Histology | Sex | Age | ECOG | Previous therapy lines | Response | PFS (months) | OS (months) | Relapse | |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | PTCL, NOS | F | 75 | 0 | 1 | Death after one cycle | 0 | 0 | – |
| Patient 2 | ALCL | M | 65 | 0 | 2 | CR | 21 | 21 | No |
| Patient 3 | PTCL, NOS | F | 77 | 0 | 0 | CR | 20 | 20 | Yes |
| Patient 4 | composite (PTCL, NOS + HL) | M | 54 | 1 | 1 | Death after 3 cycles | 4 | 4 | – |
| Patient 5 | Lennert's lymphoma | M | 76 | 0 | 1 | CR | 5 | 5 | No |
Abbreviations: ALCL, anaplastic large cell lymphoma; HL, Hodgkin lymphoma; NOS, not otherwise specified; PTCL, peripheral T‐cell lymphoma.
4. DISCUSSION
With an high overall response rate of 100%, a CR rate of 77.8% in all patients and a CR rate of 73.9% in patients receiving bendamustine and BV only as first or later salvage therapy, our data could further substantiate the results of previous clinical trials, showing that a combination of bendamustine and BV is a highly effective choice of salvage treatment in patients with relapsed or refractory classical Hodgkin lymphoma. Two prior prospective clinical trials investigated the use of bendamustine and BV in a relapsed or refractory setting. In the phase 2 part of the clinical trial conducted by O'Connor et al9, the ORR of the 37 included patients was 78%, with 43% complete responses. Patients in the trial had a median of three previous therapies before receiving bendamustine and BV, possibly explaining the lower response rate. The phase 1‐2 trial by LaCasce et al8, in which all 53 enrolled patients received bendamustine and BV as first salvage therapy, showed an ORR of 93%, with 74% of patients achieving a CR, which is very similar to our results. The 1‐year PFS of 80% was also similar to the 81.9% 1‐year PFS we calculated for our patients.
The toxicities reported in our analysis were also in line with other studies, consisting mostly of infusion‐related reactions, adverse events affecting the skin and infections.8, 9 As always in retrospective studies, assessment of adverse events is hampered by incomplete and not standardized documentation. Therefore, we used extensions of the planned intervals between treatment cycles and reductions of drug doses during treatment as surrogate markers for toxicities, leaving us with a rate of around 20% events leading to dose reduction, delay of therapy administration or discontinuation of therapy, which is a realistic assumption when compared with other published data. Friedberg et al10 reported a markedly more pronounced toxicity of bendamustine and BV in patients over the age of 60. We can also confirm the higher mortality during treatment of older patient, as the patient dying due to treatment toxicity was over the age of 70.
In contrast to most other studies, bendamustine and BV were also given as first‐line therapy in four patients; one of them changing to bendamustine and BV due to significant toxicities of treatment with BEACOPPesc. Friedman et al10 also analyzed bendamustine and BV or dacarbazine and BV as frontline treatment in elderly patients over the age of 60 with cHL. In that study, the combination of bendamustine and BV resulted in a superior ORR of 100% with 88% of patients achieving CR, albeit with a significantly higher toxicity. Since the number of patients we treated in a first‐line setting was low and neither the rate of response, nor the 1‐year PFS or OS did significantly differ when compared to the other patients, the inclusion of this data in our analysis should not significantly affect the overall results.
Nine patients received additional BV maintenance therapy after completion of chemotherapy. While we could see a non‐significantly higher 1‐year PFS in the patients with maintenance therapy, the median follow‐up was short (only 13 months in this patient group, compared to 20 months in the other patients) and many patients still had not completed maintenance at time of data collection. Therefore, additional data and longer follow‐up would be needed to evaluate the effect of maintenance therapy after treatment with bendamustine and BV.
Current data support the use of a bendamustine and BV combination as salvage therapy in second‐line, followed by autologous stem cell transplantation. All thirteen of our patients which were intended to undergo ASCT could do so after relatively well tolerable salvage therapy with bendamustine and BV. Only two of them did not achieve a complete remission, but a partial remission, before high‐dose chemotherapy. However, three patients in our analysis underwent ASCT only later during therapy: Two patients underwent ASCT as third‐line therapy, after radiotherapy only and non‐response to DHAP as first salvage therapy, respectively. One patient received bendamustine and BV followed by ASCT as fifth‐line therapy, following a re‐induction therapy with ABVD after 8 years in CR as second‐line therapy and not responding to salvage therapy with BRECADD and Nivolumab after the second relapse of disease. Of the 12 patients treated with bendamustine and BV as first salvage therapy, three patients did not undergo ASCT afterward as they were all over the age of 70 and therefore not suitable for stem cell transplantation. Generally, the 13 patients reported that underwent ASCT with this new form of induction treatment compared favorably to historical data using conventional induction chemotherapy before autologous transplantation at first relapse of HL that had resulted in a 3‐year PFS of 72% and a significant number of patients, who could not proceed to ASCT. In this large multicenter trial, 13% of patients who did not complete two cycles of conventional induction chemotherapy mainly due to progression or toxicity and further 6% did not proceed to ASCT due to various reasons.14 In another trial, single‐agent BV was used in patients with relapsed and refractory Hodgkin lymphoma. While the treatment was well tolerated and 89% of patients were able to proceed to ASCT, the ORR to BV was 68%, with only 35% of patients achieving a CR.15 In conclusion, salvage therapy with bendamustine and BV before ASCT seems to be better tolerable than traditional therapy with DHAP, while resulting in an excellent response rate, which is reflected in 100% of our patients intended to undergo ASCT actually proceeding to ASCT after therapy.
Concerning the feasibility of stem cell collection during or after receiving bendamustine‐containing chemotherapy, we did not run into relevant problems in our patients. Of the nine patients from which no stem cells were collected during previous therapy lines, we could mobilize and collect stem cells easily after few cycles of treatment. Stem cell collection was successfully done after treatment with bendamustine and BV in the other two patients. La'Casce et al also successfully collected stem cells during treatment with bendamustine and BV in 39 of their 41 patients. While there is no more data about stem cell collection with this regimen, promising data about bendamustine‐containing regimens in myeloma and aggressive lymphoma exist, with successful stem cell mobilization and harvest in most of the treated patients.16, 17 We therefore conclude that stem cell mobilization is feasible during the early cycles of therapy with bendamustine and brentuximab or after therapy in most patients.
The two relapses of cHL after ASCT in our 13 patients were observed in one patient refractory to BEACOPP and the other one underwent ASCT late as fourth salvage therapy. Apart from these two relapses of disease, two other relevant clinical events occurred so far: one patient who also received bendamustine and BV as fifth‐line therapy was primary refractory to the combination treatment, and one patient died due to treatment toxicity. We therefore suspect that high age being refractory to previous therapies and later treatment lines seem to be associated with a worse prognosis.
Only one patient with PTCL has been included in prospective clinical studies investigating a combination of bendamustine and BV so far,9 but the effectivity of monotherapy with either substance has been demonstrated in previous studies. Patients with relapsed or refractory PTCL treated with single‐agent bendamustine showed an ORR of 50%, with 28% of patients in CR.18 Single‐agent BV therapy in patients with relapsed or refractory CD30+ PTCL resulted in an ORR of 41%, with a CR rate of 24%.19 However, the duration of response to single‐agent bendamustine or BV is short, and therefore, new treatment combinations are needed. In the ECHELON‐2 trial, patients with CD30+ PTCL were treated with either BV and cyclophosphamide, doxorubicin, and prednisone (A+CHP) or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), showing that the BV including regimen was more effective and well tolerable.11 Dumont et al treated nine patients with PTCL with bendamustine and BV, observing a CR in two patients and a PR in further five patients. We therefore decided for off‐label use in the five patients described in our analysis and observed a response in 60% of patients, with all three patients responding to therapy achieving a CR, lasting for 5, 20, and 21 months, respectively. Two patients were still in CR at time of data collection, one patient had a relapse of disease. Notably, only two patients with PTCL did not respond to treatment with bendamustine and BV. Further clinical data are still needed to assess the efficacy of bendamustine and BV in patients with PTCL, but our results are promising when compared to historical data.
Limitations of our study include the retrospective nature of the study design, the not standardized and possibly incomplete documentation of adverse events in some patients and the relatively small number of patients. One major limitation of our study is the lack of long‐term follow‐up. The heterogeneity of our patient population regarding number and type of previous therapy and other patient characteristics complicates the comparison to other data, but resembles clinical practice.
In conclusion, a combination of bendamustine and BV is a highly effective salvage therapy for patients with relapsed or refractory cHL and can be efficiently used as induction therapy before HDT and subsequent ASCT, due to the high number of complete responses. The side effects are usually well manageable and a high rate of patients intended to undergo ASCT can do so. However, special caution must be taken when treating older patients with this combination. Further studies with a higher number of patients are needed to directly compare the efficacy of this treatment to other options for salvage therapy.
Further studies are also needed to evaluate the possible use and efficiency of bendamustine and BV in patients with PTCL compared to other treatment regimens.
CONFLICT OF INTEREST
SW, TMa, and MP received honoraria from Takeda; TMe and RG received honoraria from Mundipharma and Takeda; AE, PS, IS, BL CS, and CS have no conflict of interest; FK received honoraria and scientific grant from Mundipharma and Takeda; UJ received honoraria and research support from Takeda Millennium.
AUTHOR CONTRIBUTIONS
SW and CS did the statistical analysis and the primary assessment of the clinical data. All authors were involved in the management of the patients, wrote, and critically revised the manuscript.
Wagner SM, Melchardt T, Egle A, et al. Treatment with brentuximab vedotin plus bendamustine in unselected patients with CD30‐positive aggressive lymphomas. Eur J Haematol. 2020;104:251–258. 10.1111/ejh.13368
REFERENCES
- 1. Greil R, Pleyer L, Jansko B, et al. Sequential immunotherapy in a patient with primary refractory Hodgkin lymphoma and novel mutations. Oncotarget. 2018;9(29):20928‐20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116(23):4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2‐step comprehensive high‐dose chemoradiotherapy second‐line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3):616. [DOI] [PubMed] [Google Scholar]
- 4. Ribrag V, Nasr F, Bouhris JH, et al. VIP (etoposide, ifosfamide and cisplatinum) as a salvage intensification program in relapsed or refractory Hodgkin's disease. Bone Marrow Transplant. 1998;21:969. [DOI] [PubMed] [Google Scholar]
- 5. Labrador J, Cabrero‐Calvo M, Pérez‐López E, et al. ESHAP as salvage therapy for relapsed or refractory Hodgkin's lymphoma. Ann Hematol. 2014;93(10):1745‐1753. [DOI] [PubMed] [Google Scholar]
- 6. Garcia‐Sanz R, Sureda A, de la Cruz F, et al. Brentuximab vedotin and ESHAP is highly effective as second‐line therapy for Hodgkin lymphoma patients (long‐term results of a trial by the Spanish GELTAMO Group). Ann Oncol. 2019;30(4):612‐620. [DOI] [PubMed] [Google Scholar]
- 7. Connors JM, Jurczak W, Straus DJ, et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin's Lymphoma. N Engl J Med. 2017;378(4):331‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LaCasce AS, Bociek RG, Sawas A, et al. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018;132(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Connor OA, Lue JK, Sawas A, et al. Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin's lymphoma: an international, multicentre, single‐arm, phase 1–2 trial. Lancet Oncol. 2018;19(2):257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedberg JW, Forero‐Torres A, Bordoni RE, et al. Frontline brentuximab vedotin in combination with dacarbazine or bendamustine in patients aged ≥60 years with HL. Blood. 2017;130(26):2829. [DOI] [PubMed] [Google Scholar]
- 11. Horwitz S, O'Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30‐positive peripheral T‐cell lymphoma (ECHELON‐2): a global, double‐blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dumont M, Ram‐Wolff C, Roelens M, et al. Efficacy and safety of brentuximab vedotin plus bendamustine in advanced‐stage primary cutaneous T‐cell lymphomas. Br J Dermatol. 2019;181(6):1315‐1317. [DOI] [PubMed] [Google Scholar]
- 13. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol: Off J Am Soc Clin Oncol. 2014;32(27):3059‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Josting A, Müller H, Borchmann P, et al. Dose intensity of chemotherapy in patients with Relapsed Hodgkin's lymphoma. J Clin Oncol. 2010;28(34):5074‐5080. [DOI] [PubMed] [Google Scholar]
- 15. Chen R, Palmer JM, Martin P, et al. Results of a multicenter phase II Trial of brentuximab vedotin as second‐line therapy before autologous transplantation in relapsed/refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2015;21(12):2136‐2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poenisch W, Plötze M, Holzvogt B, et al. Stem cell mobilization and autologous stem cell transplantation after pretreatment with bendamustine, prednisone and bortezomib (BPV) in newly diagnosed multiple myeloma. J Cancer Res Clin Oncol. 2015;141(11):2013‐2022. [DOI] [PubMed] [Google Scholar]
- 17. Budde LE, Wu D, Martin DB, et al. Bendamustine with rituximab, etoposide and carboplatin (T(R)EC) in relapsed or refractory aggressive lymphoma: a prospective multicentre phase 1/2 clinical trial. Br J Haematol. 2018;183(4):601‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Damaj G, Gressin R, Bouabdallah K, et al. Results from a prospective, open‐label, phase II trial of bendamustine in refractory or relapsed T‐cell lymphomas: the BENTLY trial. J Clin Oncol. 2012;31(1):104‐110. [DOI] [PubMed] [Google Scholar]
- 19. Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T‐cell lymphomas with single‐agent brentuximab vedotin. Blood. 2014;123(20):3095‐3100. [DOI] [PMC free article] [PubMed] [Google Scholar]


