Abstract
Background:
Safety concerns associated with long-acting β2-agonists (LABAs) have led to many US Food and Drug Administration (FDA) regulatory activities for this class of drugs. Little is known about the effect of these regulatory activities on use of LABA-containing agents or other asthma medications.
Methods:
We created rolling cohorts of pediatric and adult asthmatic patients in the Mini-Sentinel Distributed Database between January 2005 and June 2011. The proportions of asthmatic patients using LABA-containing products, inhaled corticosteroids (ICSs), leukotriene modifiers, short-acting β2-agonists, oral corticosteroids, other bronchodilators, and no medications were measured on a monthly basis, and the changes were evaluated by using interrupted time series with segmented regression analysis.
Results:
When the 2005 regulatory activity was announced, there were statistically significant decreases in the use of fixed-dose ICS-LABA agents in children (−0.98 percentage points) and adults (−1.24 percentage points). Increased use of ICSs and leukotriene modifiers was observed just after the regulatory activities were announced in both children and adults. Although of smaller magnitude, continued favorable changes in the use of LABA agents were observed after the 2010 FDA regulatory activity.
Conclusion:
The 2005 and 2010 FDA regulatory activities might have contributed to reduced use of LABA agents, as intended; however, their effect, independent of other factors, cannot be determined. Use of other classes of asthma medications was similarly affected.
Keywords: Long-acting β2-agonist, asthma, US Food and Drug Administration, medication use, drug safety
Asthma is one of the most common health conditions in the United States.1,2 Pharmacologic therapy is important for treating and maintaining control of asthma symptoms and preventing exacerbations. Several classes of medications are used to treat asthma either as monotherapy or in combination with other asthma medications.3 Long-acting β2-agonists (LABAs) were first marketed in the United States for asthma management in 1994. Asthma clinical guidelines issued subsequent to market entry recommended use of LABA agents in combination with inhaled corticosteroids (ICSs) in patients with moderate persistent to severe persistent asthma.3
Safety concerns associated with LABAs surfaced soon after market entry, both within and outside the United States.4–7 Clinical trials, such as the Salmeterol Multicenter Asthma Research Trial, provided evidence that LABA monotherapy was associated with a small increase in the risk of severe asthma exacerbations, including asthma-related death.8 In response to the Salmeterol Multicenter Asthma Research Trial findings, the US Food and Drug Administration (FDA) held an advisory committee meeting in 2005. As a result of that meeting, a public health advisory on the safety of LABA-containing products was issued, and requests for label changes for LABA-containing products were made to manufacturers of such products to reflect the risk of severe asthma exercerbations.9 In 2007, the asthma clinical guidelines were also updated to reflect the safety issues associated with LABA agents.10 Clinicians were advised to avoid prescribing LABA monotherapy for long-term control of asthma and to assess the risk/benefit ratio of adding a LABA to an established dose of ICS versus increasing the ICS dose. The FDA held additional advisory committee meetings in 2008 and 2010. The 2008 meeting led to additional labeling recommendations (which were rolled out in 2010), whereas the 2010 meeting discussed the design of postmarketing safety trials.11–14 The 2010 labeling changes strengthened the message that LABAs should not be used alone and provided guidance on initiating LABA use.13 In addition, the FDA recommended that LABA agents be used for the shortest duration possible and that fixed-dose ICS-LABA combination products be prescribed for pediatric patients instead of single-agent LABA products.
Despite all of the warnings and guideline recommendations, trends in asthma medication use after the 2005 and 2010 LABA FDA regulatory activities are not well established. The goal of this study was to examine changes in trends of LABA and other asthma medication use as a result of both the 2005 and 2010 FDA regulatory activities and recommendations for LABA-containing products. We expected that adherence to the regulatory activities would result in less LABA dispensing.
METHODS
Data source
This study used the Mini-Sentinel program, which was created to help the FDA develop a national system for monitoring the safety of medical products and to assess the effect of FDA regulatory activity.15,16 The Mini-Sentinel Distributed Database (MSDD) was created as part of the Mini-Sentinel program’s analytic and surveillance capabilities. Data partners, consisting of regional and national health plans and state Medicaid programs, maintain local databases (behind firewalls) that include demographic, enrollment, diagnostic, procedure, laboratory, and prescription medication data from electronic medical records and administrative data. Health plans have joined the Mini-Sentinel program as data partners at various stages since the program’s inception in 2009. As the number of data partners has grown, so too has the number of members represented in the MSDD. Currently, there are more than 178 million members represented. For this study, a rolling cohort of asthmatic patients was created from 9 data partners contributing data to the MSDD from January 2004 through June 2011.17
Study population
Eligibility for the rolling cohort was assessed on the first of each month. Patients were included if they had continuous enrollment with pharmacy benefits, were between the ages of 1 and 65 years, and had a diagnosis of asthma all within 365 days of the first of each month. Asthma was defined by International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 493 for at least 2 office visits, 1 emergency department visit, or 1 hospitalization (primary diagnosis). We excluded patients with any of the following conditions: chronic obstructive pulmonary disease (ICD-9-CM codes 491, 492, and 496), cystic fibrosis (ICD-9-CM code 277.0x), bronchiectasis (ICD-9-CM code 494), pulmonary hypertension or embolism (ICD-9-CM codes 416.0 and 415.1), bronchopulmonary dysplasia (ICD-9-CM code 770.7), or congestive heart failure (ICD-9-CM code 428).18,19
Outcomes
To examine overall trends of prevalent asthma medication use within the rolling cohort of asthmatic patients, we measured the proportion of asthmatic patients using single-agent LABAs, fixed-dose ICS-LABA combinations, and other medication regimens for asthma, including ICSs, leukotriene modifiers (LMs), other asthma controller medications that are less commonly used (theophylline, cromolyn, nedocromil, and omalizumab), and other medications, such as oral corticosteroids, short-acting β2-agonists, and other bronchodilators (tiotropium, ipratropium, and ipratropium/albuterol), on a monthly basis. Medication days’ supply, the number of days of medication a patient is dispensed based on the prescription quantity and the frequency of dosing, was used to measure medication use. In addition to assessing asthma medication use, we measured the use of no asthma medications, which we defined as the proportion of asthmatic patients who did not have a day’s supply of any asthma medication during the month.
Statistical analysis
We used interrupted time series (ITS) with segmented regression analyses to model changes in the levels and trends of asthma medication use associated with the LABA-related 2005 and 2010 FDA regulatory activities. The interrupted times series design controls for preregulatory activity (eg, policy) levels and trends in outcomes, therefore producing more valid evidence of a regulatory effect than pre/post-research designs.20,21 Because 2 major regulatory activities occurred during our study period (November 2005 and February 2010), the ITS model included 3 periods: January 2005 to November 2005, December 2005 to January 2010, and February 2010 to June 2011. As a result, the ITS models could have up to 5 parameter estimates (ie, intercept, baseline trend, level change in 2005, change in trend after 2005, level change in 2010, and change in trend after 2010). The intercept estimated the baseline level of drug use in January 2004 for this study. The baseline trend estimated the change in use of medications that occurred within each month before November 2005. A level change, the change in the trend in medication use after the regulatory activity compared with the monthly trend before the regulatory activity, represented the immediate effect of the regulatory activity when it was announced. For example, the sum of the baseline trend and the change in trend after 2005 is the slope between December 2005 and January 2010. We used stepwise elimination of nonsignificant terms (P ≥.2); the final most parsimonious model contained only the intercept and retained only parameter estimates with P values of less than .2. Changes in the proportion of prevalent asthma medication use are described in both absolute and relative terms.22 We adjusted for seasonal variation on a monthly basis using autoregressive integrated moving average models23 and controlled for significant autocorrelation terms by using backstep elimination.20 We considered estimates to be statistically significant when the P value was less than .0167 (.05/3) because separate models were run in children (age <18 years), adults, and overall. We used PROC X11 to transform the original data series into seasonally adjusted monthly series, and PROC AUTOREG was carried out on the seasonally adjusted monthly series (SAS Institute, Cary, NC).
A power calculation was performed by using 10,000 simulations based on modifications of existing algorithms in SAS software, and the first 200 sample points were dropped to maintain the stability of the generation. We used the first 2 segments (January 2005 to November 2005 and December 2005 to January 2010) to calculate power because they have fewer time points (less power) than the last 2 segments. Assuming no trend in values at baseline (before conducting the study) and 11 months before and 50 months after FDA regulatory activity, a usual autocorrelation at 0.3 and α level at .05, and an estimated effect size of 1.5 (level change/SD), we estimated that the power is 89% using simulations.24
RESULTS
More than 1.5 million children and adults with asthma were enrolled over the study period. Table I shows age, sex, and medication use among the first members eligible for the rolling cohort in January 2005. The mean ages of the pediatric and adult populations were 8 and 42 years, respectively. Although almost two thirds of the pediatric population was male, two thirds of the adult population was female. In January 2005, ICSs (21.9%) and LMs (17.5%) were the most commonly used controller medications in children (Table I). The fixed-dose ICS-LABA agents were used by 7.1% of the pediatric cohort, and less than 1.0% of children with asthma filled a single-agent LABA prescription. Short-acting β2-agonists were the most commonly used noncontroller medications (20.8%). More than half of the children with asthma were not using any asthma medications at the start of the study (55.9%). In adults ICS agents were also the most commonly used controller medications (25.0%), followed by fixed-dose ICS-LABA combination (16.0%) and LM (15.0%) agents (Table I). Single-agent LABA use was 4.6%. In January 2005, fewer adults were not using any asthma medication than children (47.1% adults vs 55.9% children).
TABLE I.
Characteristics and medication use among asthmatic patients in January 2005
| Pediatric patients (no.) | 93,392 |
| Male sex | 59.9% |
| Mean age (y) | 8.0 |
| Single-agent LABA | 0.8% |
| Fixed-dose ICS-LABA | 7.1% |
| ICS | 21.9% |
| LM | 17.5% |
| OCM | 0.4% |
| OB | 0.5% |
| OCS | 5.5% |
| SABA | 20.8% |
| No medication | 55.9% |
| Adult patients (no.) | 123,868 |
| Male sex | 30.4% |
| Mean age (y) | 42.0 |
| Single-agent LABA | 4.6% |
| Fixed-dose ICS-LABA | 16.0% |
| ICS | 25.0% |
| LM | 15.0% |
| OCM | 2.2% |
| OB | 4.0% |
| OCS | 7.3% |
| SABA | 22.8% |
| No medication | 47.1% |
OB, Other bronchodilators (ipratropium, ipratropium/albuterol, and tiotropium); OCM, other controller medications (theophylline, nedocromil, cromolyn, and omalizumab); OCS, oral corticosteroids; SABA, short-acting β2-agonists.
Asthma medication use trends changed over the course of the study period (Figs 1 and 2 and Table II). Among pediatric patients, there was a 13.55% decrease in use of fixed-dose ICS-LABA agents immediately after the 2005 regulatory activity was announced compared with the January 2005 intercept (0.98 percentage point absolute decrease, P < .001; Table I). The decrease in fixed-dose ICS-LABA use was largely offset by a nonsignificant increase in ICS use immediately after the regulatory activity was announced (0.54 percentage points, P = .046), an increasing trend after regulatory activity (0.06 percentage points per month, P < .001), and also a nonstatistically significant increase in LM use after the announcement (0.44 percentage points, P =.068). There were no level or trend changes in fixed-dose ICS-LABA use associated with the 2010 regulatory activity in children.
FIG 1.

Percentage of asthma medication use before, between, and after the 2005 and 2010 FDA regulatory activities for LABA-containing agents in asthmatic children.
FIG 2.
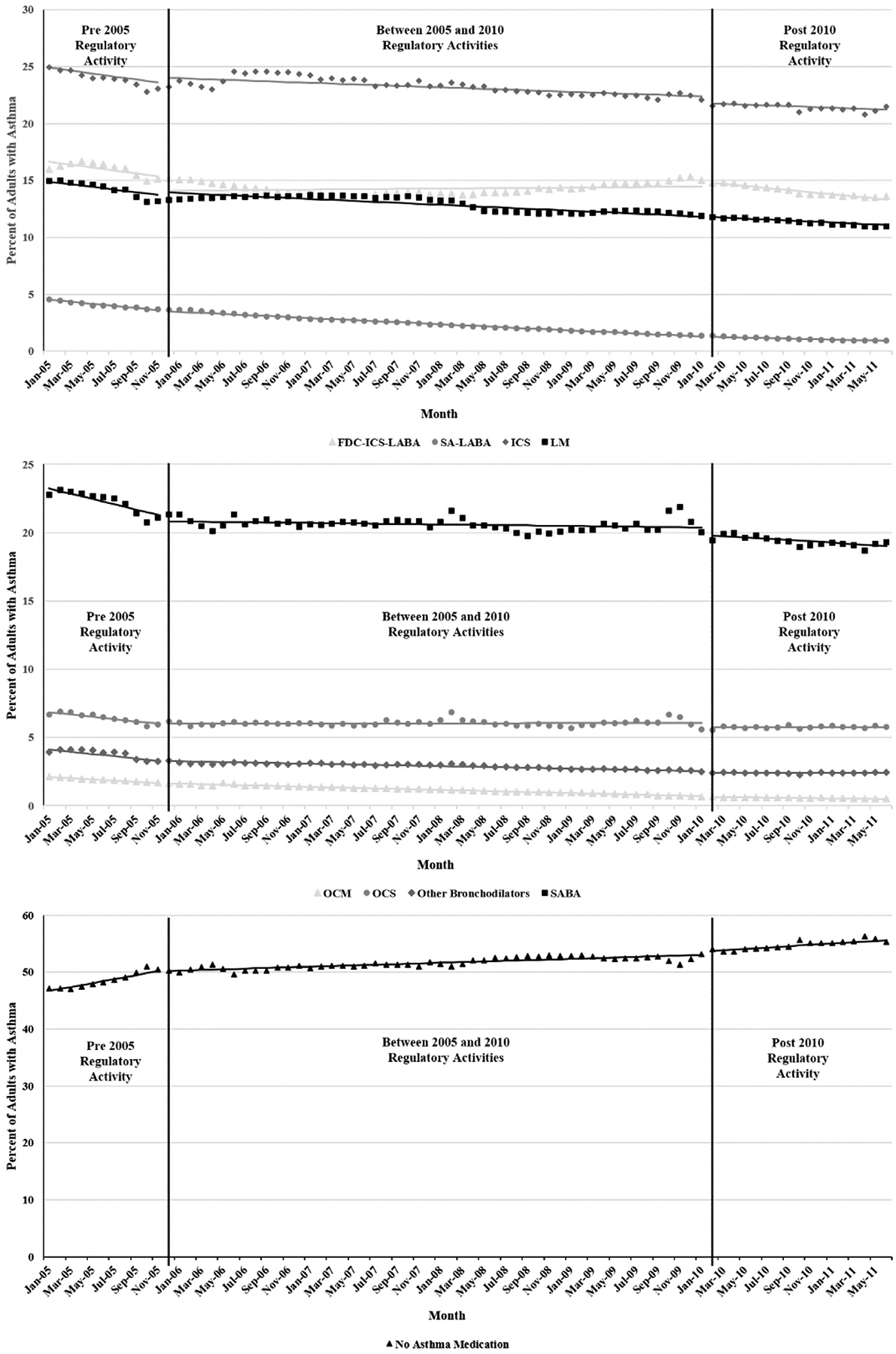
Percentage of asthma medication use before, between, and after the 2005 and 2010 FDA regulatory activities for LABA-containing agents in asthmatic adults.
TABLE II.
Parameter estimates, 95% CIs, and P values from the most parsimonious segmented regression models predicting monthly rates of asthma medication use
| Parameter | Estimate | 95% CI | P value | ||
|---|---|---|---|---|---|
| Children | |||||
| Single-agent LABA | Intercept | 0.840 | 0.804 | 0.876 | <.0001 |
| Baseline trend | −0.024 | −0.028 | −0.020 | <.0001 | |
| 2005 trend change | 0.014 | 0.010 | 0.018 | <.0001 | |
| 2010 trend change | 0.008 | 0.005 | 0.010 | <.0001 | |
| Fixed-dose ICS-LABA | Intercept | 7.219 | 7.041 | 7.397 | <.0001 |
| Baseline trend | −0.028 | −0.031 | −0.024 | <.0001 | |
| 2005 level change | −0.978 | −1.217 | −0.740 | <.0001 | |
| ICS | Intercept | 21.946 | 21.538 | 22.353 | <.0001 |
| 2005 level change | 0.539 | 0.010 | 1.069 | .0462 | |
| 2005 trend change | 0.058 | 0.047 | 0.069 | <.0001 | |
| 2010 trend change | −0.102 | −0.156 | −0.048 | .0003 | |
| LM | Intercept | 17.881 | 16.852 | 18.91 | <.0001 |
| 2005 level change | 0.438 | −0.033 | 0.910 | .0680 | |
| 2005 trend change | −0.050 | −0.078 | −0.022 | .0006 | |
| 2010 level change | −0.357 | − 0.828 | 0.115 | .1359 | |
| OCM | Intercept | 0.371 | 0.356 | 0.387 | <.0001 |
| Baseline trend | −0.005 | − 0.005 | −0.004 | <.0001 | |
| Other bronchodilators | Intercept | 0.589 | 0.541 | 0.636 | <.0001 |
| Baseline trend | −0.011 | −0.016 | −0.006 | .0001 | |
| 2005 trend change | 0.009 | 0.003 | 0.014 | .0018 | |
| OCS | Intercept | 5.212 | 4.769 | 5.654 | <.0001 |
| Baseline trend | −0.035 | −0.082 | 0.013 | .1534 | |
| 2005 trend change | 0.045 | −0.007 | 0.096 | .0869 | |
| 2010 trend change | −0.043 | −0.071 | −0.016 | .0025 | |
| SABA | Intercept | 21.838 | 20.82 | 22.852 | <.0001 |
| Baseline trend | −0.148 | −0.256 | −0.039 | .0084 | |
| 2005 trend change | 0.173 | 0.058 | 0.289 | .0039 | |
| 2010 trend change | −0.108 | −0.167 | −0.050 | .0004 | |
| No medication use | Intercept | 55.374 | 53.965 | 56.784 | <.0001 |
| Baseline trend | 0.120 | −0.063 | 0.303 | .1946 | |
| 2005 level change | −0.653 | − 1.654 | 0.349 | .1980 | |
| 2005 trend change | −0.123 | −0.312 | 0.065 | .1970 | |
| 2010 trend change | 0.150 | 0.063 | 0.236 | .0009 | |
| Adults | |||||
| Single-agent LABA | Intercept | 4.621 | 4.487 | 4.755 | <.0001 |
| Baseline trend | −0.091 | −0.107 | −0.076 | <.0001 | |
| 2005 level change | −0.073 | −0.132 | −0.014 | .0167 | |
| 2005 trend change | 0.046 | 0.030 | 0.063 | <.0001 | |
| 2010 trend change | 0.023 | 0.014 | 0.033 | <.0001 | |
| Fixed-dose ICS-LABA | Intercept | 16.775 | 16.228 | 17.323 | <.0001 |
| Baseline trend | −0.130 | −0.211 | −0.049 | .0020 | |
| 2005 level change | −1.244 | −1.780 | −0.708 | <.0001 | |
| 2005 trend change | 0.137 | 0.056 | 0.218 | .0012 | |
| 2010 level change | 0.428 | −0.062 | 0.918 | .0860 | |
| 2010 trend change | −0.099 | −0.141 | −0.056 | <.0001 | |
| ICS | Intercept | 25.040 | 24.213 | 25.868 | <.0001 |
| Baseline trend | −0.131 | −0.229 | −0.033 | .0095 | |
| 2005 level change | 0.437 | −0.085 | 0.959 | .0994 | |
| 2005 trend change | 0.098 | −0.005 | 0.201 | .0630 | |
| 2010 level change | −0.591 | −1.085 | −0.096 | .0200 | |
| LM | Intercept | 15.014 | 14.370 | 15.658 | <.0001 |
| Baseline trend | −0.117 | −0.188 | −0.047 | .0014 | |
| 2005 level change | 0.262 | 0.041 | 0.483 | .0209 | |
| 2005 trend change | 0.074 | −0.000 | 0.149 | .0512 | |
| OCM | Intercept | 2.166 | 2.128 | 2.204 | <.0001 |
| Baseline trend | −0.045 | −0.050 | −0.039 | <.0001 | |
| 2005 level change | − 0.026 | −0.062 | 0.011 | .1613 | |
| 2005 trend change | 0.026 | 0.020 | 0.031 | <.0001 | |
| 2010 level change | −0.049 | −0.081 | −0.016 | .0042 | |
| 2010 trend change | 0.010 | 0.007 | 0.013 | <.0001 | |
| Other bronchodilators | Intercept | 4.207 | 3.999 | 4.413 | <.0001 |
| Baseline trend | −0.082 | −0.105 | −0.060 | <.0001 | |
| 2005 trend change | 0.067 | 0.042 | 0.092 | <.0001 | |
| 2010 level change | −0.111 | −0.255 | 0.032 | .126 | |
| 2010 trend change | 0.013 | −0.004 | 0.030 | .136 | |
| OCS | Intercept | 6.933 | 6.710 | 7.156 | <.0001 |
| Baseline trend | −0.084 | −0.108 | −0.060 | <.0001 | |
| 2005 trend change | 0.085 | 0.060 | 0.110 | <.0001 | |
| 2010 level change | −0.303 | −0.430 | −0.177 | <.0001 | |
| SABA | Intercept | 23.427 | 22.884 | 23.969 | <.0001 |
| Baseline trend | −0.193 | −0.271 | −0.116 | <.0001 | |
| 2005 level change | −0.462 | −0.961 | 0.037 | .0691 | |
| 2005 trend change | 0.184 | 0.106 | 0.263 | <.0001 | |
| 2010 level change | −0.538 | −1.034 | −0.041 | .0343 | |
| 2010 trend change | −0.039 | −0.085 | 0.007 | .0972 | |
| No medication use | Intercept | 46.356 | 45.584 | 47.129 | <.0001 |
| Baseline trend | 0.347 | 0.263 | 0.431 | <.0001 | |
| 2005 trend change | −0.289 | −0.379 | −0.199 | <.0001 | |
| 2010 level change | 0.602 | −0.095 | 1.300 | .0895 | |
| 2010 trend change | 0.059 | −0.009 | 0.127 | .0892 | |
OCM, Other controller medications (theophylline, cromolyn, nedocromil, and omalizumab); OCS, oral corticosteroids; SABA, short-acting β2-agonists.
Although neither of the regulatory activities had an immediate effect on single-agent LABA use, there were changes in slope after each activity in the pediatric population. Single-agent LABA use decreased by a monthly slope of −0.024 from the beginning of the study up to the 2005 regulatory activity. After the 2005 regulatory activity, the slope flattened to −0.010, and after 2010, the slope was close to a flat line (slope −0.002). Similar to the LABA-containing products, the 2010 regulatory activity had no immediate effect on use of any of the other asthma medication classes. Despite the increases in use around the 2005 regulatory activity, LM use started to decrease soon afterward, whereas ICS use began to decrease after the 2010 regulatory activity. The proportion of children not using asthma medication began to increase after the 2010 regulatory activity (Fig 1 and Table II).
In adults there was a 7.42% relative decrease in the use of fixed-dose ICS-LABA use after the 2005 regulatory activity was announced compared with the January 2005 intercept (1.24 percentage point absolute decrease, P < .001). Fixed-dose ICS-LABA use decreased gradually from the beginning of the study with a monthly slope of −0.130. After a decreasing baseline slope of −0.091, single-agent LABA use had a small immediate decrease after the 2005 regulatory activity (−0.073 percentage points, P = .017). Between December 2005 and January 2010, use continued to decrease; the rate of decrease slowed by 51% (−0.046/−0.091).
In the adult population the 2010 regulatory activity had no immediate effect on single-agent LABA, fixed-dose ICS-LABA, LM, or ICS use. After the 2010 regulatory activity, the slope for fixed-dose ICS-LABA use decreased once again, whereas the slope for single-agent LABA use flattened. Lastly, there was a trend leading to an increase in the proportion of adults not using asthma medication when the 2010 regulatory activities were announced and thereafter (Fig 2 and Table II).
DISCUSSION
After FDA regulatory activities for LABA-containing products, there were small shifts in the use of LABA agents, other asthma medications, and no medication use. Although the effect was fairly small, the most important trend was the effectiveness of the FDA in reducing LABA dispensing. The 2005 regulatory activity had a larger immediate effect on fixed-dose ICS-LABA use than the 2010 regulatory activity in both children and adults; however, among adults, the relative magnitude of change in trend after the 2010 regulatory activity was larger than after the 2005 regulatory activity for fixed-dose ICS-LABA use. The effect of the 2005 regulatory activity on single-agent LABA users was only larger than the effect of the 2010 regulatory activity among adults.
Initial increases ICS and LM use after the 2005 regulatory activity raise questions about whether these medications were being substituted for LABA-containing agents. After the 2010 regulatory activity, use of all asthma medications decreased, and there was an increase in the number of patients no longer taking asthma medication. The latter effect could be the result of asthma conditions changing over time, leading to changes in medication requirements or patients being more concerned about asthma medications in general. Additionally, safety concerns with LMs surfaced during the study and could have led to a reduction in use.25
Few studies have examined asthma medication use across time in children and adults separately or without pooling the data over several years. In the literature describing prevalent use of asthma medication before the 2005 regulatory activities and between the 2005 and 2010 regulatory activities, ICSs were the most commonly prescribed asthma controller medications in children in several studies.19,26,27 However, LM use differed by country. One US study found that LMs were the next most commonly prescribed controller medication, which is consistent with our findings26; however, LMs were infrequently used in the United Kingdom.26,27 LABA use was very low in pediatric patients,19,26,27 and many pediatric patients were not prescribed asthma medication.26,27
Before our study period, changes in asthma medication use were observed among pediatric patients. ICS use had decreased in the United Kingdom from 1998 to 2004.27 In addition, the number of children not prescribed asthma medication increased between 1998 and 2004.27 During our study period, prevalent LABA use in children and adults decreased from 2006 to 2008 in a Michigan Medicaid asthmatic population.28 Another study measuring counts of patients prescribed select LABA-containing and ICS products from 2005 to 2011 also showed decreases in LABA-containing product use among children and adults.29 Our results differed with respect to trends in ICS use in the pediatric population. ICS use consistently increased throughout their study period, whereas use started to decrease in 2010 in our analysis. The relative decrease in LABA use was greater in children than in adults, which is consistent with our findings.28,29
This study has several strengths. The MSDD is comprised of a geographically and demographically diverse population. We used a rolling cohort design, and therefore subjects who were not eligible at one point because of a lack of continuous enrollment could be included at a later time if enrollment criteria were met. We did not restrict our study population based on age of approved asthma medication use. Without the restriction, we are able to assess how asthma medications, including LABA-containing products, are actually being used in practice. We were able to assess the effect of 2 regulatory activities during the study period. Doing so allowed us to have a better perspective of the true effect of the 2010 regulatory activity.
Despite the strengths of this study, several limitations merit discussion. FDA regulatory activities are applicable across the whole nation, and therefore it was not feasible to have a concurrent comparator to measure the effects of these regulatory activities. As a result, it is possible that contemporaneous factors other than the FDA’s regulatory activities influenced the observed changes. Furthermore, medication use in the pediatric population might be underestimated because some clinicians do not document a diagnosis of asthma until a child is 2 to 4 years old and our study required a diagnosis.19 Korelitz et al26 found that pediatric patients were often prescribed asthma medications with no asthma diagnosis. We could not assess whether the decreasing use of asthma medication was due to decreasing asthma severity or some other cause because administrative claims data do not contain information on symptomatology. We were not able to assess whether the effect of these regulatory activities differed by prescriber specialty. Lastly, it is possible that more follow-up time is needed to assess the long-term effect of the 2010 regulatory activity.
In summary, our findings suggest decreasing use of LABA-containing products and increasing use of other asthma controller medications during a period of FDA LABA-related regulatory activities (2005–2011). The 2005 FDA regulatory activity might have contributed to less use of LABA agents, as intended; however, its effect independent of other factors cannot be determined. Use of other classes of asthma medications was similarly affected. Although of smaller magnitude, continued favorable changes in the use of LABA agents were observed after the 2010 FDA regulatory activity. Although these changes appear small in magnitude, they represent thousands of children and adults with asthma using less LABA agents.
Key messages.
The 2005 FDA regulatory activity for LABA-containing products had a larger effect on use of LABA agents than the 2010 FDA regulatory activity.
Use of asthma medications, such as ICSs and LMs, increased as LABA use decreased.
Over time, all asthma medication use decreased.
Acknowledgments
Mini-Sentinel is funded by the US Food and Drug Administration (FDA) through the Department of Health and Human Resources (contract HHSF223200910006I). Mini-Sentinel is a pilot project sponsored by the FDA to inform and facilitate development of a fully operational active surveillance system, the Sentinel System, for monitoring the safety of FDA-regulated medical products. Mini-Sentinel is one piece of the Sentinel Initiative, a multifaceted effort by the FDA to develop a national electronic system that will complement existing methods of safety surveillance. Mini-Sentinel Collaborators include Data and Academic Partners that provide access to health care data and ongoing scientific, technical, methodological, and organizational expertise.
We thank Marsha Reichman, PhD, and Monika Houstoun, PharmD, from the Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, FDA, Silver Spring, Maryland, for their scientific and administrative support.
Abbreviations used
- FDA
US Food and Drug Administration
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- ICS
Inhaled corticosteroid
- ITS
Interrupted time series
- LABA
Long-acting β2-agonist
- LM
Leukotriene modifier
- MSDD
Mini-Sentinel Distributed Database
Footnotes
Disclosure of potential conflict of interest: M. G. Butler has received research support from the US Food and Drug Administration (FDA) and the Agency for Healthcare Research & Quality (AHRQ) and has received travel support from the FDA. A. C. Wu has received research support from the FDA and the National Institutes of Health (NIH) and is employed by Boston Children’s Hospital. P. Wu has received research support from the NIH and AHRQ. S. Toh has received research support from the FDA. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Bloom B, Jones LI, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10 2013;(258):1–81. [PubMed] [Google Scholar]
- 2.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10 2014;(260):1–161. [PubMed] [Google Scholar]
- 3.National Heart, Lung, and Blood Institute. Expert panel report 2: guidelines for the diagnosis and management of asthma. 1997. Available at: http://www.nhlbi.nih.gov/guidelines/archives/epr-2/asthgdln_archive.pdf. Accessed January 15, 2014.
- 4.Castle W, Fuller R, Hall J, Palmer J. Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ 1993;306:1034–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarus SC, Boushey HA, Fahy JV, Lazarus SC, Boushey HA, Fahy JV, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285:2583–93. [DOI] [PubMed] [Google Scholar]
- 6.Lemanske RF Jr, Sorkness CA, Mauger EA, Lemanske RF Jr, Sorkness CA, Mauger EA, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA 2001;285: 2594–603. [DOI] [PubMed] [Google Scholar]
- 7.Mann M, Chowdhury B, Sullivan E, Mann M, Chowdhury B, Sullivan E, et al. Serious asthma exacerbations in asthmatics treated with high-dose formoterol. Serious asthma exacerbations in asthmatics treated with high-dose formoterol. Chest 2003;124:70–4. [DOI] [PubMed] [Google Scholar]
- 8.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 129:15–26. [DOI] [PubMed] [Google Scholar]
- 9.FDA. Information for healthcare professionals—salmeterol xinafoate (marketed as Serevent Diskus). 2005. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm162673.htm. Accessed January 15, 2012.
- 10.National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnosis and management of asthma—full report 2007. 2007. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed January 15, 2012.
- 11.Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting beta agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med 2006;144:904–12. [DOI] [PubMed] [Google Scholar]
- 12.FDA. Advair Diskus, Advair HFA, Brovana, Foradil, Perforomist, Serevent Diskus, and Symbicort Information (Long Acting Beta Agonists). 2008. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm108111.htm. Accessed January 15, 2012.
- 13.FDA. FDA drug safety communication: new safety requirements for long-acting inhaled asthma medications called long-acting beta-agonists (LABAs). 2010. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed January 15, 2012.
- 14.McMahon AW, Levenson MS, McEvoy BW, Mosholder AD, Murphy D. Age and risks of FDA approved long-acting beta-adrenergic receptor agonists. Pediatrics 2011;128:e1147–54. [DOI] [PubMed] [Google Scholar]
- 15.Behrman RE, Benner JS, Brown JS, McClellan M, Woodcock J, Platt R. Developing the Sentinel System—a national resource for evidence development. N Engl J Med 2011;364:498–9. [DOI] [PubMed] [Google Scholar]
- 16.Platt R, Carnahan RM, Brown JS, Chrischilles E, Curtis LH, Hennessy S, et al. The U.S. Food and Drug Administration’s Mini-Sentinel program: status and direction. Pharmacoepidemiol Drug Saf 2012;21(suppl 1):1–8. [DOI] [PubMed] [Google Scholar]
- 17.Curtis LH, Weiner MG, Boudreau DM, Cooper WO, Daniel GW, Nair VP, et al. Design considerations, architecture, and use of the Mini-Sentinel distributed data system. Pharmacoepidemiol Drug Saf 2012;21(suppl 1):23–31. [DOI] [PubMed] [Google Scholar]
- 18.Blanchette CM, Culler SD, Ershoff D, Gutierrez B. Association between previous health care use and initiation of inhaled corticosteroid and long-acting beta2-adrenergic agonist combination therapy among US patients with asthma. Clin Ther 2009;31:2574–83. [DOI] [PubMed] [Google Scholar]
- 19.Thomas M, Murray-Thomas T, Fan T, Williams T, Taylor S. Prescribing patterns of asthma controller therapy for children in UK primary care: a cross-sectional observational study. BMC Pulm Med 2010;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- 21.Shadish WR. Experimental and quasi-experimental designs for generalized causal inference. Boston: Houghton Mifflin; 2002. [Google Scholar]
- 22.Zhang F, Wagner AK, Soumerai SB, Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol 2009;62:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bobbit LG, Otto MC. “Effects of forecasts on the revisions of seasonally adjusted data using the X-11 adjustment procedure,” Proceedings of the Business and Economic Statistics Section of the American Statistical Association. 1990; 449–53. [Google Scholar]
- 24.Zhang F, Wagner AK, Ross-Degnan D. Simulation-based power calculation for designing interrupted time series analyses of health policy interventions. J Clin Epidemiol 2011;64:1252–61. [DOI] [PubMed] [Google Scholar]
- 25.FDA. Early Communication about an Ongoing Safety Review of Montelukast (Singu-lair). 2008. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm070618.htm. Accessed May 30, 2014.
- 26.Korelitz JJ, Zito JM, Gavin NI, Masters MN, McNally D, Irwin DE, et al. Asthma-related medication use among children in the United States. Ann Allergy Asthma Immunol 2008;100:222–9. [DOI] [PubMed] [Google Scholar]
- 27.Turner S, Thomas M, von Ziegenweidt J, Price D. Prescribing trends in asthma: a longitudinal observational study. Arch Dis Child 2009;94:16–22. [DOI] [PubMed] [Google Scholar]
- 28.Wasilevich EA, Clark SJ, Cohn LM, Dombkowski KJ. Long-acting β-agonist monotherapy among children and adults with asthma. Am J Manag Care 2011; 17:e91–5. [PubMed] [Google Scholar]
- 29.DiSantostefano RL, Yeakey AM, Raphiou I, Stempel DA. An evaluation of asthma medication utilization for risk evaluation and mitigation strategies (REMS) in the United States: 2005–2011. J Asthma 2013;50:776–82. [DOI] [PubMed] [Google Scholar]


