Abstract
Nearly one third of the encoded proteome is comprised of secretory proteins that enable communication between cells and organ systems, playing a ubiquitous role in human health and disease. High-throughput detection of secreted proteins would enhance efforts to identify therapies for secretion-related diseases. Using the Z mutant of alpha-1 antitrypsin as a human secretory model, we have developed 1536-well high-throughput screening assays that utilize acoustic droplet ejection to transfer nanoliter volumes of sample for protein quantification. Among them, the acoustic reverse phase protein array (acoustic RPPA) is a multiplexable, low-cost immunodetection technology for native, endogenously secreted proteins from physiologically relevant model systems like stem cells that is compatible with plate-based instrumentation. Parallel assay profiling with the LOPAC1280 chemical library validated performance and orthogonality between a secreted bioluminescent reporter and acoustic RPPA method by consistently identifying secretory modulators with comparable concentration response relationships. Here, we introduce a robust, multiplexed drug discovery platform coupling extracellular protein quantification by acoustic RPPA with intracellular and cytotoxicity analyses from single wells, demonstrating proof-of-principle applications for human induced pluripotent stem cell-derived hepatocytes.
Secretory proteins constitute a substantial fraction of the encoded proteome, acting across a broad range of biological processes1, 2. Numerous diseases arise from abnormally secreted proteins or the secretion of aberrant proteins modified by genetic or environmental factors2, 3. Thus, methodologies for high-throughput cell-based secretion detection represent an attractive approach towards identifying novel therapies for secretory disorders that could have a significant impact on drug discovery.
In high-throughput screening (HTS), reporter-based cellular assays represent a popular approach for protein detection4-7. However, reporter-specific chemical assay artifacts can complicate screening by enriching for compounds with uncertain relevance6-10. More direct antibody-based HTS assays measure unlabeled, endogenously secreted proteins, although not without challenges, especially in 1536-well format. For instance, the “sandwich” enzyme-linked immunosorbent assay (ELISA), a mainstay of proteomic research, finds limited use in 1536-well HTS11, 12 because two compatible primary antibodies are required, high costs, variability, and extensive processing. AlphaLISA was developed as a 1536-well compatible bead-based derivative of the sandwich ELISA, however similar antibody and cost concerns remain, and the homogenous format (i.e. no wash) can exacerbate test compound interference13-15.
The reverse phase protein array (RPPA), in which nanoliter spots of cellular lysates are applied to a protein-binding substrate, enables endogenous protein quantification using standard immunochemical protocols16-18. Printed by pin- or tip-based arrayers onto nitrocellulose-coated glass slides, RPPA is comparably sensitive to ELISA and AlphaLISA, multiplexable, and requires minimal sample input using a single antibody per antigen19, with each spot representing a complete collection of analytes17. Despite these advantages, arrayer incompatibility and nitrocellulose-coated slide costs can make RPPA prohibitively expensive for large HTS campaigns.
After considering existing technologies, we designed HTS assays to evaluate large chemical libraries for modulators of protein secretion using Z mutant alpha-1 antitrypsin (ATZ), a model misfolded secretory protein the majority of which is retained within hepatocytes causing liver and lung disease through alpha-1 antitrypsin deficiency20-22. Fusing ATZ to secretable NanoLuc (secNLuc-ATZ) enabled development of 1536-well secretion assays detectable through both reporter bioluminescence and immunochemistry after transferring nanoliter volumes of media by acoustic droplet ejection from live cell source plates to recipient assay plates. After constructing a 3D-printed, inexpensive nitrocellulose plate alternative to coated slides for RPPA, both assays were evaluated in parallel quantitative HTS (qHTS) experiments using a chemical library (LOPAC1280) to orthogonally identify secretion enhancers and inhibitors of secNLuc-ATZ. The acoustically arrayed immunoassay, which we have termed acoustic RPPA, demonstrated picogram sensitivity for native, endogenously secreted protein. Lastly, multiplexing acoustic RPPA with high content imaging in human iPSC-derived hepatocytes enabled intra- and extracellular quantification of endogenously secreted proteins to generate comprehensive biological profiles from individual wells. This study introduces widely applicable acoustic HTS methodologies to evaluate secretory protein biology in stem cells and other physiologically relevant model systems for drug discovery.
RESULTS
Bioluminescent Protein Secretion Assay by Acoustic Dispensing.
To develop protein secretion assays for 1536-well qHTS, we generated a model reporter cell line in U2-OS cells from which secreted protein was detectable in cell culture media. In alpha-1 antitrypsin deficiency, the majority of destabilized, polymerogenic ATZ accumulates within the early secretory pathway, eventually becoming degraded or forming toxic polymers and insoluble aggregates20-22 (Fig. 1A). Compounds that modulate ATZ secretion could therefore have therapeutic significance in preventing polymer formation. A bioluminescent fusion protein reporter was constructed encoding a secretable NanoLuc fused to ATZ (secNLuc-ATZ; Fig. S1A). A corresponding wild-type reporter (AAT) was also constructed for comparison (secNLuc-AAT).
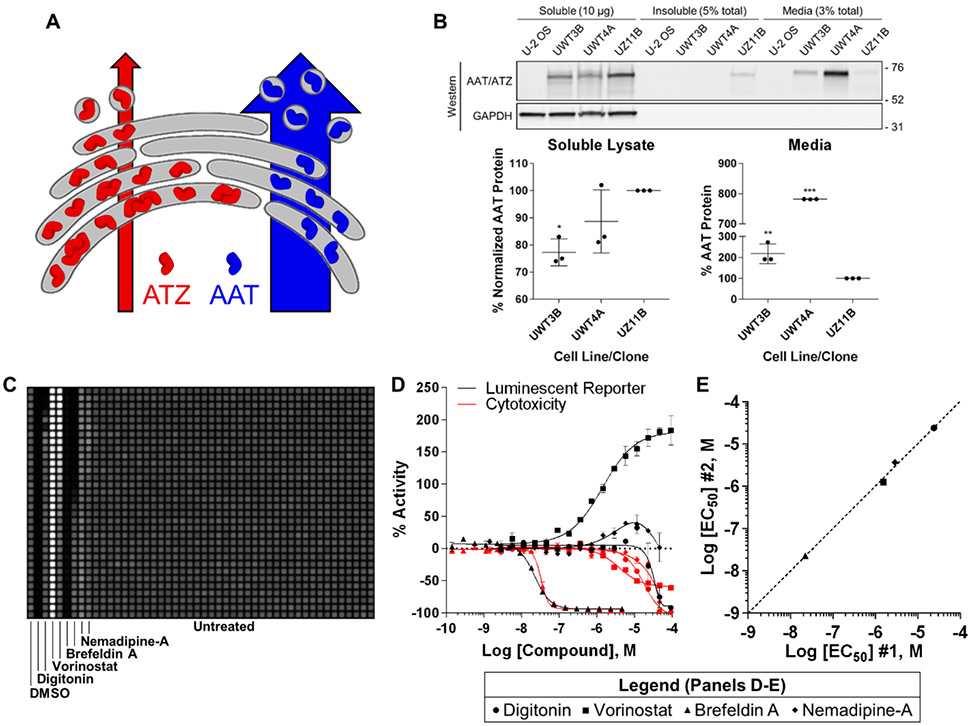
Figure 1 ∣.
Development of a secretion assay for qHTS using acoustic droplet ejection. (A) Conformational instability causes the majority of ATZ to be retained in the endoplasmic reticulum and degraded, while a fraction is capable of forming insoluble toxic polymers. (B) Representative western blot (see also Fig. S1B-E) confirming secNLuc-AAT and secNLuc-ATZ protein levels across cellular fractions from monoclones expressing similar levels of reporter. Data represent the mean of three replicate experiments. (C) Representative luminescent receiving plate image for one-to-one 50 nL acoustic transfer from 1536-well plate containing compound-treated UZ11B cells incubated for 24 h. (D) Compound CRCs for the UZ11B bioluminescent secretion (black) and cytotoxicity (red) assays. Data plotted are derived from the mean ± S.E.M. of two replicate experiments, each containing duplicate compound treatments. (E) Log [EC50] correlation plot for compound CRCs from D describing potency reproducibility during modulation of secNLuc-ATZ media levels, determined by CRCs generated in the two replicate experiments; R2 = 0.996. Bars in B are mean ± SD with statistical analysis by one-way ANOVA with Tukey’s posthoc test; (*) p < 0.05; (**) p < 0.005; (***) p < 0.0001. AAT, alpha-1 antitrypsin protein; ATZ, Z mutant alpha-1 antitrypsin protein; LU, luminescence units; CRC, concentration response curve; EC50, half-maximal effective concentration.
Clones from secNLuc-AAT and secNLuc-ATZ reporter cell lines were characterized for reporter expression and biological comparison, with clone UZ11B recapitulating Z mutant pathology by producing an insoluble fraction of secNLuc-ATZ and significantly less reporter secretion than two secNLuc-AAT clones with similar reporter expression (Fig. 1B, bottom panel and Fig. S1B-E). These data portrayed UZ11B recapitulation of ATZ biology and rationalized further assay development.
Cell-free testing of various media transfer methods between 1536-well source and recipient plates demonstrated acoustic droplet ejection as the most precise (3.7% CV; Fig. S2A-B). Subsequently UZ11B cells were plated into 1536-well source plates and incubated 24 h before acoustic media transfer into receiving plates. Importantly, no disturbance to the UZ11B monolayer was observed from the transfer (Fig. S2C), and transfers were precise and accurate in the presence of cells (Fig. S3A-B). The bioluminescent assay response to known pharmacological modulators of protein secretion and an NLuc-specific reporter-interfering compound yielded excellent assay performance statistics by Z’-factor (≥ 0.5)23 for secretory modulators and a variety of potential secretory concentration response outcomes (Fig. 1C-D and Fig. S3C). Concentration response curves (CRCs) were well-defined and reproducible according to minimum significant ratios (MsRs) ≤ 3, a measure of potency reproducibility24, 25 (Fig. S3D). A correlation plot of log half-maximal effective concentrations (EC50) between replicate experiments further supported CRC reproducibility (Fig. 1E). As a counter-screen for media loss-of-signal caused by cytotoxicity, CellTiter-Glo analysis was performed in a separate parallel assay (Fig. 1D and Fig. S3E-F).
Digitonin, a cytotoxicity control causing acute necrosis by disrupting plasma membrane integrity26, effected a strong reduction in media luminescence corresponding with increasing cell death. The HDAC inhibitor vorinostat, reported to correct ATZ secretion in other cellular models27, increased secNLuc-ATZ secretion and became a control for secretion enhancement. The well-studied secretion inhibitor brefeldin A performed consistently, although its cytotoxic effects overlapped with the half-maximal inhibitory concentration (IC50) for extracellular secNLuc-ATZ. The dihydropyridine and known NLuc-inhibiting artifact nemadipine-A was included as a reporter control to characterize signal outputs derived from its stabilizing effect on NLuc half-life28, 29, which was reproducible as observed through the biphasic “bell-shaped” CRC.
To biochemically confirm these observations, UZ11B cells were treated in 6-well plates with increasing concentrations of vorinostat and brefeldin A and soluble, insoluble, and media fractions were analyzed for secNLuc-ATZ levels. Western blotting confirmed secretion assay observations (Fig. S4). Collectively, these data suggested the generation of a highly reproducible 1536-well qHTS assay.
Secretory Protein Detection by Acoustic RPPA.
We next sought to develop an acoustically dispensed 1536-well secretory immunoassay based on the RPPA methodology by delivering media onto a protein-binding substrate with the dimensions of a microtiter plate. As commercially available options were limited to nitrocellulose-coated glass slides incompatible with plate-based instrumentation, we constructed a 3D-printed prototype nitrocellulose membrane recipient plate (Fig. S5) capable of adsorbing transferred protein and referred to this new methodology as acoustic RPPA. Secreted secNLuc-ATZ (~74 kDa) was detected in media acoustically delivered onto the nitrocellulose recipient plate from a 1536-well plate containing UZ11B cells (Fig. S6A) using a primary antibody targeting AAT/ATZ (~54 kDa) validated for specificity by western blotting (Fig. S6B).
Highly reproducible immunofluorescent acoustic arrays of media-containing secNLuc-ATZ were generated, as spot size and signal intensity increased with volume transferred (Fig. S6C-D). Identical transfers from the same source plate were evaluated by bioluminescent secretion assay, demonstrating that acoustic RPPA performed comparably (Fig. S6E). However, unlike the well-based bioluminescent secretion assay, acoustic RPPA signal output displayed a nonlinear relationship with increasing transfer volumes since samples were converted from 3D liquid volume to 2D surface spot, with spot size dependent on the absorbent and adsorbent properties of the substrate (e.g. nitrocellulose membrane) (Fig. S6C).
Similar precision was observed between 50 nL and 200 nL transfer volumes across the nitrocellulose recipient plate (Fig. S7A-B), with the bioluminescent secretion assay yielding slightly lower intraplate variability (Fig. S7C-D). As an additional assay technique and to demonstrate that acoustic RPPA is non-denaturing, media delivered onto nitrocellulose receiving plates was submerged in bioluminescent reagent and imaged. Luminescent arrays were generated by enzymatically active secNLuc-ATZ, verifying the functional state of the transferred reporter (Fig. S7E-F).
For acoustic RPPA performance comparison between our prototype nitrocellulose membrane recipient plates and commercially available nitrocellulose-coated glass slides, we generated a 3D-printed glass slide holder in plate format to immobilize four slides across its top surface with acoustic dispenser compatibility. Strikingly, nitrocellulose plates exhibited less variability than coated slides for identically dispensed 10 nL and 50 nL arrays (Fig. S8A-D). Larger 50 nL spots resulted in greater overall signal on coated slides compared to membrane, implying that differences in the absorptive and adsorptive properties of substrates can cause variations in signal linearity and output. Cost analysis also suggested that a more than 20-fold reduction in price per data point could be attained by using the nitrocellulose membrane recipient plate versus coated slides (Fig. S8E-F). Together, these data illustrate three techniques for monitoring secreted protein reporter using acoustic dispensing and a prototype nitrocellulose recipient plate. Notably, NLuc transferred by acoustic RPPA retained enzymatic activity.
Multiplexing Secretion and Cytotoxicity Assays.
Secretion assays were pharmacologically compared using a diverse panel of compounds (Table S1) to determine if cellular stress or death could inadvertently increase reporter levels in media. UZ11B cells were treated for 24-48 h before media was transferred from the same source plate and evaluated using bioluminescent and acoustic RPPA methodologies. Since acoustic dispensing does not disrupt the cell monolayer, a multiplexed cytotoxicity counter-screen was performed within the source plate after media transfers, increasing workflow efficiency and reducing variability.
Most compounds that produced well-defined, measurable secretory responses displayed comparable CRCs and EC50s between methodologies (Fig. 2A-C), with most MSRs ≤ 3.0 (Table S2A) and Z’-factors typically higher at 48 h (Table S2B-C). As illustrated by vorinostat (Fig. 2D-E) and bortezomib (Fig. S9A), longer treatment produced bell-shaped CRCs likely caused by cytotoxicity. As a secretion enhancement control, vorinostat scored excellent Z’-factors for the bioluminescent assay (> 0.5) and acoustic RPPA (≥ 0.48), suggesting an ideal orthogonal qHTS assay pair.
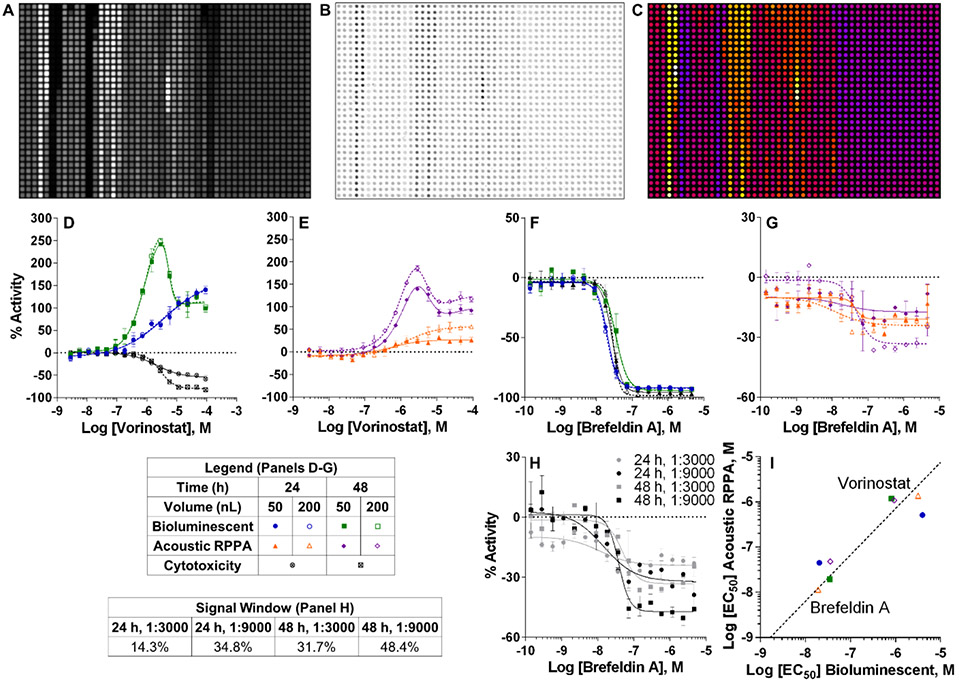
Figure 2 ∣.
Comparison between bioluminescent reporter and acoustic RPPA secretion assays after titrated compound treatment. UZ11B cells were plated and treated with compound within 1 h, then incubated for 24-48 h prior to evaluation. Multiplexed cytotoxicity was conducted within the source plate from which media was dispensed. Representative images of (A) bioluminescent reporter and (B) acoustic RPPA immunofluorescence assays after compound treatment, each with 200 nL total media transferred in 10 nL droplets. (C) Heat map representation of the acoustic RPPA assay in B. Graphs of vorinostat gain-of-signal (D-E) and brefeldin A loss-of-signal (F-G) secretory responses display % activity normalized to DMSO of secNLuc-ATZ luminescence or fluorescence in media transferred to respective recipient plates after 24-48 h. Cytotoxicity was determined by CellTiter-Glo luminescent viability assay at 24-48 h. CRC data plotted are mean ± SD of duplicate compound treatments at each concentration. (H) Graph of CRCs for brefeldin A after 24-48 h treatments comparing changes to signal window between AAT antibody concentrations. (I) Log [EC50] correlation plot for compound CRCs from D-G describing potency reproducibility between bioluminescent reporter and acoustic RPPA assays. EC50 values for each assay were determined by CRCs generated from duplicate compound treatments at each concentration tested. 24 h, 50 nL R2 = 0.96; 48 h, 50 nL R2 = 0.98; 24 h, 200 nL R2 = 0.98; 48 h, 200 nL R2 = 0.99. LU, luminescence units; FU, fluorescence units; CRC, concentration response curve; EC50, half-maximal effective concentration.
Although the bioluminescent assay produced a superior loss-of-signal window and Z’-factor for secretion inhibition by brefeldin A, IC50 measurements were consistent between assays (Fig. 2F-G and Table S2A-C). The compressed acoustic RPPA loss-of-signal windows for brefeldin A contributed to low Z’-factors (Table S2B-C) but MSRs < 3.0 indicated potency reproducibility (Table S2A). Notably, acoustic RPPA signal windows improved considerably after reducing antibody concentration (Fig. 2H). This, in addition to IC50 consistency between assays, suggested the bioluminescent and acoustic RPPA methodologies could also function as orthogonal loss-of-signal qHTS assays. To support assay orthogonality for gain- and loss-of-signal, a plot of CRC potencies for control compounds vorinostat and brefeldin A correlated strongly between assays (Fig. 2I). Multiplexed cytotoxicity within the source plate performed consistently, providing a useful toxicity filter against which to interpret a loss-of-signal response (Fig. 2F and Table S2D).
CRCs for additional evaluated compounds offered noteworthy observations. The effects of stress-inducing and cytotoxic compounds on secNLuc-ATZ media levels were compound-dependent and unpredictable based upon mechanisms of action, with some demonstrating increased media signal with concurrent increases in cytotoxicity (Fig. S9A-F and Table S2A-D). The multiplexed cytotoxicity assay was therefore useful in segregating genuine secretion-modifying compounds from potential cytotoxicity-induced phenomena. Additionally, while the dihydropyridine NLuc artifact nemadipine-A caused bell-shaped CRCs in the bioluminescent assay as previously observed in Fig. 1D, this response was seemingly undetectable by acoustic RPPA (Fig. S9G).
Acoustic qHTS Identifies secNLuc-ATZ Secretion Modulators.
We performed a seven-concentration qHTS study of the bioluminescent and acoustic RPPA secretion assays using the LOPAC1280 collection with a 24 h compound exposure (Table S3A). The screen was multiplexed with cytotoxicity, and a parallel enzymatic counter-screen for NLuc activity was performed to identify potential compound artifacts mediated by direct reporter inhibition or light attenuation. All CRCs were initially evaluated using four-parameter logistic curve fitting. To ensure bell-shaped actives were not inadvertently missed by poor fitting, secretion data were also sorted by maximum signal and fit with five-parameter logistic curves where applicable.
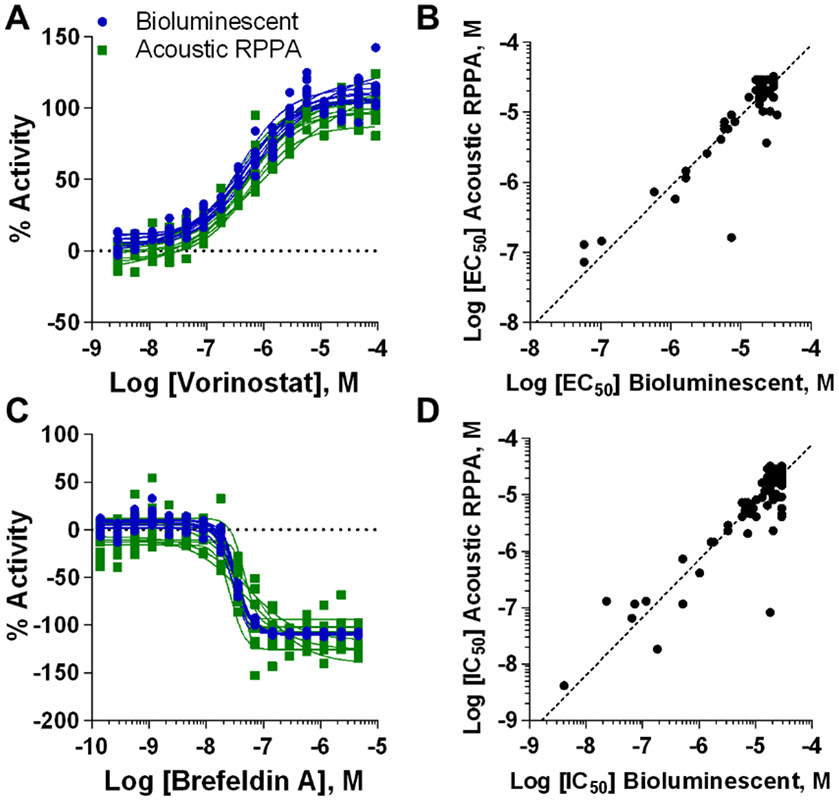
Gain-of-signal vorinostat control CRCs and EC50s displayed highly reproducible responses between screens, with MSRs ≤ 2.6 (Fig. 3A and Table 1). Vorinostat produced an excellent 0.52 bioluminescent screen Z’-factor (Table S3B). While the acoustic RPPA Z’-factor was lower (0.38, Table S3B), we share the view that MSR is more appropriate for evaluating qHTS since it characterizes potency reproducibility between titrated compound responses24, while single concentration Z’-factor assessments disregard potency reproducibility. Both assays were highly selective for identifying compounds that enhanced secNLuc-ATZ levels in media after normalization against vorinostat. High quality sigmoidal actives30 equaling 0.5% in the bioluminescent and 0.3% in the acoustic RPPA screens varied in known mechanisms of action (Table S3C). Between assays, most active CRCs reproduced strongly with comparable EC50s (Fig. S10A-D and Table S3D), in addition to lower quality sigmoid, bell-shaped or single-point activity CRCs30 (Fig. S10E-H). Interestingly, CRC artifacts by dihydropyridine and other NLuc inhibitors were detectable in the bioluminescent screen at concentrations mirroring NLuc inhibition but not for acoustic RPPA (Fig. S10I-L), consistent with earlier observations (Fig. S9). A potency correlation plot for gain-of-signal CRCs underscored the compatibility observed between screening methodologies (Fig. 3B). Table S2E-F contains complete LOPAC1280 results for secretion enhancement, multiplexed cytotoxicity, and NLuc enzymatic inhibition.
Figure 3 ∣.
Comparison of secretion-modulating activity observed in bioluminescent and acoustic RPPA LOPAC1280 qHTS. (A) CRCs displaying % activity with secretion normalized to vorinostat (secretion enhancement). (B) Log [EC50] correlation plot between bioluminescent and acoustic RPPA screens for all secretion-enhancing CRCs after normalization to vorinostat (R2 = 0.84). (C) CRCs displaying % activity with secretion normalized to brefeldin A (secretion inhibition). (D) Log [EC50] correlation plot between screens for all secretion-inhibiting CRCs after normalization to brefeldin A (R2 = 0.80). CRC, concentration response curve; EC50, half-maximal effective concentration.
Table 1 ∣.
Performance of bioluminescent and acoustic RPPA assays for LOPAC1280 qHTS. Potency estimates and MSRs for vorinostat (secretion enhancement, Fig. 3A) and brefeldin A (secretion inhibition, Fig. 3C) controls during LOPAC1280 qHTS. Log [EC50]s were calculated as the mean of 9 independent 16-point, 1:2 dilution CRCs (one from each assay plate of the screen) fit as four-parameter sigmoidal responses. MSRs were calculated based on the SD of log [EC50] calculations from the 9 independent CRCs as indicated in Supplementary Methods. EC50, half-maximal effective concentration; MSR, minimum significant ratio; CRC, concentration response curve.
| Control | Vorinostat – Secretion Enhancer | Brefeldin A – Secretion Inhibitor | ||||
|---|---|---|---|---|---|---|
| Assay | Bioluminescent | Acoustic RPPA | Both Assays | Bioluminescent | Acoustic RPPA | Both Assays |
| Log [EC50] | −6.27 | −6.26 | −6.26 | −7.48 | −7.43 | −7.46 |
| Log [EC50] Parameter Fit | 4 | 4 | 4 | 4 | 4 | 4 |
| MSR | 2.4 | 2.6 | 2.5 | 1.2 | 2.4 | 1.9 |
Loss-of-signal brefeldin A control CRCs and IC50s also showed strong consistency (MSRs ≤ 2.4) (Fig. 3C and Table 1). Brefeldin A Z’-factors of 0.57 and 0.04 for the bioluminescent and acoustic RPPA screens, respectively (Table S2G), reiterated that Z’-factor cannot account for high CRC and potency reproducibility and underscored a dissonance between MSR and Z’-factor in qHTS. To support MSR effectiveness as the more appropriate performance standard, both screens identified brefeldin A as a potent inhibitor of secNLuc-ATZ secretion with high quality, overlapping CRCs (Fig. S11A). High quality sigmoidal actives totaled 0.9% and 1.3% for the bioluminescent and acoustic RPPA screens, respectively, after filtering out compounds with overlapping cytotoxicity (Table S2H). Nearly all loss-of-signal responses regardless of cytotoxicity produced highly comparable CRCs and IC50s and also represented a range of known mechanisms of action (Fig. S11B-D and Table S2I). This was also observed for lower quality and single-point inhibitory CRCs likely caused by cytotoxicity at maximum concentration (Fig. S11E-H). Some NLuc enzymatic inhibitors identified as single-point inhibitory compounds, likely due to cytotoxicity (Fig. S11I-K). A loss-of-signal potency correlation plot again supported assay orthogonality (Fig. 3D). Table S2J contains all LOPAC1280 results for secretion inhibition, multiplexed cytotoxicity, and NLuc enzymatic inhibition.
Several bell-shaped secretory CRCs without associated NLuc enzymatic inhibition were likely caused by cytotoxicity at higher concentrations, confounding secretion increases at lower concentrations (Fig. S12A-G). Because sigmoidal curve fitting mischaracterized these curves (28), they were manually sorted and identified based on maximum CRC signal. Also identified was the PKC activator phorbol 12-myristate 13-acetate (Fig. S12H), where an incomplete CRC likely represents the upper asymptote of a highly potent response with a nanomolar maximum efficacy plateau before acute cytotoxicity. These LOPAC1280 screening results indicate strong gain-of and loss-of-signal cross-validation between bioluminescent and acoustic RPPA assays as confirmed through mutual identification of secretory enhancers and inhibitors with activity in the UZ11B cell line.
Detecting and Quantifying Endogenous Protein Secretion with Acoustic RPPA.
We next evaluated endogenous AAT secretion after titrated compound treatment (Table 1) in HepG2 cells. After primary antibody validation by western blotting (Fig. S13A), cells in 1536-well source plates were treated 24-48 h before media was acoustically transferred onto our prototype nitrocellulose recipient plate for AAT detection (Fig. S13B-C). Endogenous AAT media levels were reduced or unaffected by compound treatment as efficacy and signal window increased with time. Importantly, most measurable CRCs were highly reproducible (MSR ≤ 3) (Table S4A). Several compounds presented overlapping media and cytotoxicity CRCs similar to brefeldin A that could not be distinguished from cytotoxicity (Fig. S13D-F), while others decreased secretion without overlapping cytotoxicity (Fig. S13G-I). Performance metrics for single concentration treatments were satisfactory (Table S4B-C). These data illustrate the functionality of acoustic RPPA to effectively monitor endogenous, native secretory protein in titration-based and single concentration treatment formats.
To demonstrate antigen quantification with acoustic RPPA, 1 mg/mL purified AAT was serially diluted in media (10% FBS) and acoustically transferred well-to-spot in 50 nL and 200 nL volumes onto a nitrocellulose recipient plate. Consistent titrations were generated across receiving plates yielding protein standard curves with R2 ≥ 0.97 (Fig. S14A-B). Further analysis approximated a 3-log dynamic range with 2-log linear signal and a 45.5 pg limit of detection (LOD) for 50 nL transfer (Fig. S14C). It is important to note that signal output is a function of spot intensity and spot size, so greater signal was generated as transfer volume increased for equal concentrations of protein.
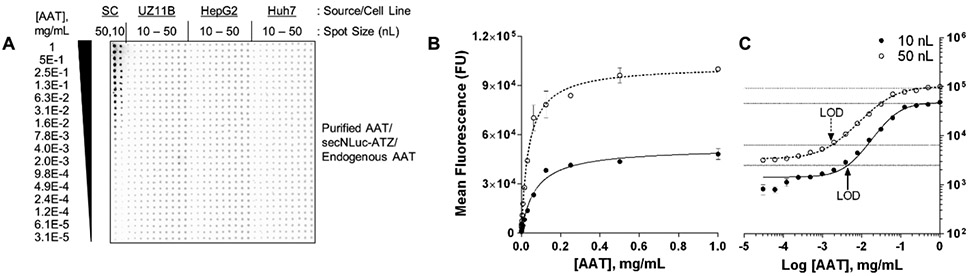
To quantify media protein concentration using live cells, UZ11B, HepG2, and Huh7 cells were incubated in a 1536-well source plate for 24 h, with protein standards included alongside cultured cells prior to acoustic transfer (Fig. 4A). Curves yielded R2 ≥ 0.99 (Fig. 4B) and exhibited 2- to 3-log dynamic ranges with a 44.1 pg LOD for 10 nL transfer (Fig. 4C). Based on calculated media protein concentrations and transfer volumes, as low as 50 pg endogenously secreted AAT was detectable from 10 nL Huh7 media (Fig. S14D-F). Notably, AAT concentration in Huh7 media was lower compared to secNLuc-ATZ concentration in UZ11B media, corresponding with secNLuc-ATZ/AAT gene expression (Fig. S1E). These data illustrate the incorporation of protein standards in acoustic RPPA for quantifying media protein concentration with implications for biological assays without available controls and the monitoring of unmodified, naturally secreted proteins.
Figure 4 ∣.
Quantification of secreted protein using acoustic RPPA. (A) Acoustic RPPA of untreated UZ11B, HepG2, and Huh7 cells cultured in a 1536-well source plate using 10-50 nL media transfers. Standard curves (SC) were generated with 50 nL and 10 nL volumes in duplicate 16-point, 1:2 titrations of purified AAT. Concentrations of titrated AAT in each row are indicated to the left. (B-C) Protein standard curve generated by AAT serial dilutions from A in 50 nL and 10 nL volumes. LOD (3SEM from bottom) in C are 44.1 pg (4.41 pg/nL) and 85.3 pg (1.71 pg/nL), respectively. For standard curves, while signal intensity is consistent at a given concentration, differences in signal are a function of transfer volume and the resulting spot size. Horizontal lines in graphs represent 3SEM from curve maximum and minimum. AAT, alpha-1 antitrypsin; FU, fluorescence units; LOD, limit of detection; SC, standard curve.
A Multiplexed Platform for Human iPSC-Derived Hepatocytes Amenable with Drug Discovery.
Human induced pluripotent stem cells (iPSCs) provide unprecedented access to patient- and disease-specific cell types for disease modeling and drug discovery. The non-destructive nature of media sampling by acoustic RPPA makes it an ideal technique for multiplexing with orthogonal methodologies to monitor endogenous protein expression over time in stem cell cultures. To demonstrate this, we monitored iPSC hepatocyte differentiation by analyzing the temporal expression of cell type-specific markers AAT and albumin (ALB), which increase over time with maturation31. Media from iPSC-derived hepatocytes cultured in 384- and 1536-well formats were acoustically transferred onto a nitrocellulose recipient plate before fixing cells for immunofluorescence imaging. Purified AAT and ALB standards were also transferred adjacent to media samples, with multiplexed immunodetection of both proteins on the same recipient plate.
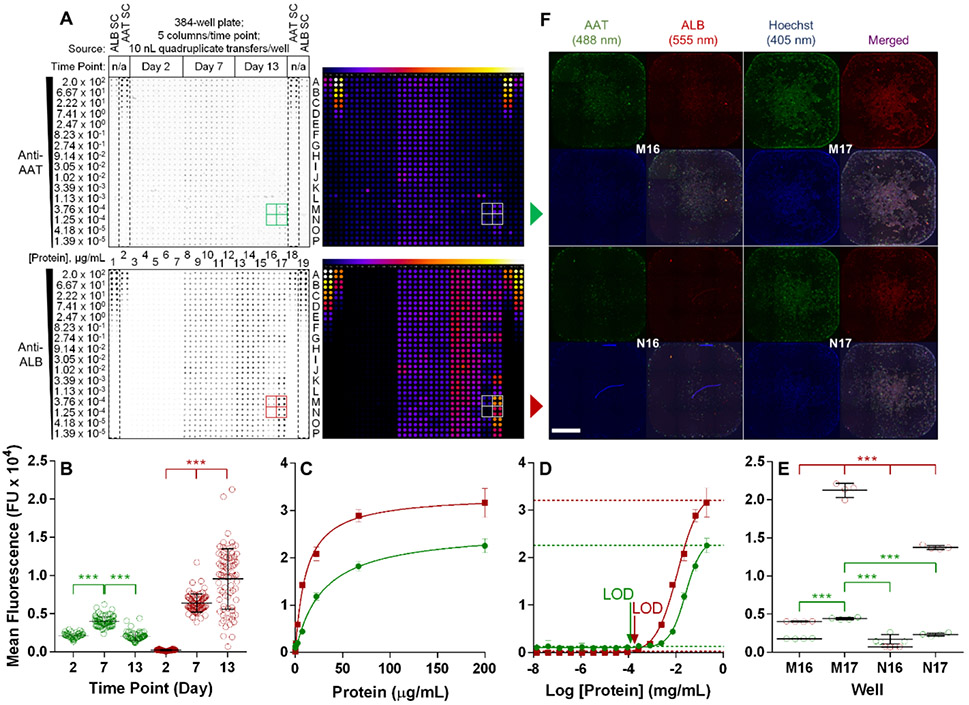
For 384-well format, 10 nL quadruplicate transfers detected significant fluctuations for both AAT and ALB expression between time points (Fig. 5A-B), with both protein standards (dashed boxes) generating consistent curves (R2 ≥ 0.97; Fig. 5C-D). What appeared to be reciprocal cross-reactivity between antibodies and standards for AAT and ALB, two of the most abundant serum proteins32, was actually standard contamination from the vendor and not antibody promiscuity as confirmed by mass spectrometry and western blotting (Fig. S15A-C and Table S5). Importantly, target antigen signal was significantly greater within the signal range of the assay, with the option to normalize by subtracting background signal (Fig. S15D). The assay detected as low as 21.7 pg (23.7 pg normalized) AAT at Day 2 and 18.2 pg (9 pg normalized) ALB at Day 7 (Fig. 5D and Fig. S16A-B). Significant changes to protein expression were also detectable in 1536-well format between Days 2 and 7 (Fig. S16C-D). While the observed increases to ALB protein over time in 384- and 1536-well formats were consistent with hepatocyte maturation, concurrent fluctuations to AAT protein were less interpretable.
Figure 5 ∣.
Temporal and orthogonal evaluation of iPSC-derived hepatocyte maturation by multiplexed secretion and imaging analyses. In 384-well plates, untreated iPSC-derived hepatocytes were incubated for the indicated durations in a hepatocyte maturation protocol. At each time point, media was collected and cells were fixed for imaging. (A) Media was acoustically transferred onto an acoustic RPPA recipient plate alongside 16-point, 1:3 serial dilutions of purified AAT and albumin (ALB) (dashed boxes). Transfers were 10 nL performed in quadruplicate. Protein standard concentrations in each quadruplicate row are indicated left of the receiving plate. Multiplexed AAT (top panels) and ALB (bottom panels) detection was imaged simultaneously. Right panels indicate heat map representations of the left recipient membranes. Partitioned boxes for AAT (green) and ALB (red) indicate adjacent sample wells at Day 13 used for further analyses. (B) Signal intensity of all wells and time points for AAT and ALB. Each symbol represents the mean of the quadruplicate well transfers. (C, D) Generation of protein standard curves by averaging quadruplicate transfers at each concentration on the receiving plate. For C: AAT R2 = 0.97, ALB R2 = 0.99. For D: AAT and ALB R2 = 0.99. Horizontal lines in graphs represent 3SEM from curve maximum and minimum. LOD in D at 10 nL are 7.3 pg AAT, 5.0 pg ALB. (E) Signal intensity of indicated wells (partitioned in A) at Day 13 for AAT and ALB. Each symbol represents one of the quadruplicate well transfers. (F) Whole well immunofluorescence of indicated wells by montage of 10X magnification images from the media source plate used in A as an orthogonal analysis to acoustic RPPA. After media collection, cells were fixed and stained for AAT (green) and ALB (red) with Hoechst nuclear counterstain to confirm cell density. Scale bar = 1000 μm. Bars in B and E are mean ± SD with statistical analysis by two-way ANOVA with Tukey’s posthoc test; (***) p < 0.0001. AAT, alpha-1 antitrypsin; ALB, serum albumin; FU, fluorescence units; LOD, limit of detection; SC, standard curve.
The utility of a multiplexed imaging and acoustic RPPA platform for protein expression and identifying sources of variation was illustrated when some 384-well Day 13 wells displayed high media protein heterogeneity, especially adjacent wells M16, M17, N16, and N17 (Fig. 5A, E and Fig. S16E-F). Whole well (Fig. 5F) and higher resolution (Fig. S17A) microscopy confirmed acoustic RPPA protein levels, but also revealed cell number variation. Laser cytometry not only verified cell density discrepancies that arose over time, but also indicated that wells with greater cell numbers (M17, N17) exhibited higher AAT and ALB protein intensity per object (Fig. S17B), consistent with previous reporting that hepatocyte differentiation is cell density-dependent33. In summary, acoustic RPPA, independently or multiplexed with orthogonal detection methodologies, can quantify minute differences in endogenous protein levels from individual wells and is compatible with iPSC-derived cellular models and drug discovery.
DISCUSSION
Acoustic droplet ejection has emerged as a preferred method for nanoliter liquid transfer. Applications continue to grow, and while some liquids present challenges due to acoustic droplet dependence on surface tension34, 35, this study demonstrates that acoustic media transfer enables endogenous secretory protein quantification directly from cells in culture using nanoliter sample volumes for 1536-well acoustic qHTS. Non-destructive sampling also allows further multiplexing with imaging or cytotoxicity assays, maximizing data collection and efficiency while decreasing costs and variability.
Monitoring secretory proteins by acoustic transfer has far-reaching implications for understanding secretory protein biology and therapeutic discovery. Alpha-1 antitrypsin deficiency (AATD) is one of many secretory proteins associated with protein conformational diseases36-39. While this study exhibits acoustic RPPA applications for monitoring secreted protein levels, alternative assays characterizing protein conformation may be developed. Using transthyretin-related amyloidosis as an example, anti-amyloid antibodies and high-throughput quantification by acoustic RPPA could have aided in identifying Tafamidis and diflunisal36 as clinical treatments for reducing transthyretin dissociation into amyloid fibrils.
Here, we also demonstrate acoustic RPPA for developmental biology applications such as examining the temporal expression of pluripotency and tissue-specific markers like those from iPSC-derived hepatocytes (Fig. 5). Furthermore, the observation that secreted NLuc retains enzymatic activity after adsorption onto nitrocellulose membrane (Fig. S7E-F) presents evidence that secretory proteins may remain functional after acoustic transfer. This could have implications for further surface-level enzymatic or affinity assay development requiring native protein structure such as antibody, lectin, or receptor screening in which target variants are spotted then blotted with antigen, analyte or ligand.
In its simplest form, acoustic RPPA could significantly impact HTS by merging high-tech liquid handling with low-tech antigen detection. Minimal sample volumes generate protein arrays detectable by immunoblotting protocols and spotted arrays are relatively stable, with fluorescent secondary antibodies equally durable when protected from light. Multiplexing capabilities can expand using near-IR fluorescence to enable multi-antigen detection. While the main limitation is antibody specificity, this study describes a viable 1536-well single-antibody methodology that provides orthogonality to reporter assay alternatives to the sandwich ELISA and AlphaLISA on a reusable frame. For quantitative secretory screening in iPSC-derived disease models (Fig. 5), we envision acoustic RPPA HTS will complement technologies aimed at enhancing our understanding of pathophysiology to accelerate therapeutic discovery for a broad range of disorders.
METHODS
Generation of bioluminescent secNLuc-ATZ and secNLuc-ATM fusion protein reporter constructs.
The pNLF1-secN [CMV/Hygro] vector (Promega) was used as a backbone for generating the secNLuc-ATz and secNLuc-ATM fusion protein reporters. First, wild-type ATM cDNA lacking a signal sequence was PCR amplified with primers 5’-CTTCCGAATTCAGAGCTGAGGATCCCCAGGGAGAT-3’ (forward) and 5’-AAATCTAGATATCCGCGTTATTTTTGGGTGGGATTCACC-3’ (reverse) (Thermo Fisher) from a plasmid encoding full-length ATM (a gift from Richard Sifers). These primers contained overhangs complementary to the pNLF1-secN multiple cloning site for Gibson assembly (New England Biolabs), with EcoRI and XbaI restriction sites flush with the 5’ and 3’ ends of ATM, respectively. The pNLF1-secN backbone was linearized by PCR amplification with primers 5’-AGCTCTGAATTCGGAAGC-3’ (forward) and 5’-CGCGGATATCTAGATTTGG-3’ (reverse). Gibson assembly was performed according to manufacturer’s protocol after PCR to generate a circularized pNLF1-secN plasmid encoding secNLuc-ATM with the incorporated flexible peptide linker (Fig. S1A). The secNLuc-ATZ reporter was generated subsequently using the Quikchange II site-directed mutagenesis kit (Agilent) by inserting the Z mutation (E342K) with the primers 5’-GCTTCAGTCCCTTTCTTGTCGATGGTCAGCACA-3’ (forward) and 5’-TGTGCTGACCATCGACAAGAAAGGGACTGAAGC-3’ (reverse) (Integrated DNA Technologies). All plasmid construction and mutagenesis were confirmed by DNA sequencing.
Cell line maintenance and construction.
All cells were cultured at 37°C, 5% CO2, and 95% humidity, and were tested for mycoplasma contamination every 2 to 4 weeks with MycoAlert PLUS (Lonza #LT07-710). The U2-OS parental cell line was obtained from ATCC (#HTB-96). Cells were maintained in McCoy’s 5A modified medium at 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. To generate secNLuc-ATZ – U2-OS and secNLuc-ATM – U2-OS fusion reporter cell lines, 3 x 105 cells/well were plated into 6-well plates and transfected using 9.9 μL FuGENE 6 reagent (Promega #E2691) and 3.3 μg DNA for a 3:1 ratio of reagent to DNA, following manufacturer’s protocol. Two days later, media was exchanged for hygromycin selection at 200 μg/mL for 2 weeks during which hygromycin-resistant cells were expanded until near confluence. After confirming media luminescence with the Nano-Glo luciferase assay system per manufacturer’s protocol (Promega #N1120) on a ViewLux uHTS Microplate Imager (PerkinElmer), single cell clonal isolation was performed by serial dilution into 96-well plates. During clonal expansion, media luminescence was continually monitored after media changes to confirm stable integration and expression of the secreted luminescent reporters by transferring 10-20 μL cell culture media into a 96-well plate, adding and mixing an equal volume of Nano-Glo luciferase assay reagent, incubating 10 min, then imaging on a ViewLux. HepG2 cells were obtained from ATCC (#HB-8065). Huh7 cells were a gift from David Gerhold (NCATS). Both cell lines were maintained in DMEM medium at 10% FBS and 1% penicillin/streptomycin.
Characterization of secretion reporter monoclones.
Once secNLuc-ATZ – U2-OS and secNLuc-ATM – U2-OS monoclones were expanded into frozen stocks, reporter protein levels in soluble and insoluble lysate and media fractions were evaluated by western blot using a primary antibody targeting the human AAT region of the reporter (Sigma Aldrich #A0409). Monoclones were plated at 3 x 105 cells/well into 6-well plates in 2 mL complete media. After incubation for 24 h, cells were washed 3x in 1X PBS and incubated for 3 h in 1 mL serum-free media to accumulate secreted reporter, then put on ice. Media was removed and centrifuged at 4°C, 200 x g for 5 min, then transferred to a new tube. Media samples were mixed with 4X Laemmli sample buffer + 10% β-mercaptoethanol, then heated at 100°C for 10 min. After media was removed, cells were washed 3x in ice-cold 1X PBS, then lysed on ice in 200 μL freshly prepared lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 1X Xpert protease inhibitor cocktail) for 30 min. Lysates were homogenized by pipetting, collected and spun at 4°C, 16,000 x g for 30 min, after which the supernatant was collected as the soluble lysate fraction. Soluble fractions were quantified for total protein concentration by BCA assay prior to mixing with 4X Laemmli sample buffer + 10% β-mercaptoethanol, then heated at 100°C for 10 min. The insoluble pellet was washed by heavy vortexing in 1 mL ice-cold 1X PBS, then centrifuged at 4°C, 16,000 x g for 5 min. After removing PBS, pellets were dissolved in 200 μL 2X Laemmli sample buffer + 10% β-mercaptoethanol with heavy vortexing, then heated at 100°C for 10 min followed by heavy vortexing for 30 sec. All samples were allowed to return to room temperature after heating before centrifugation at 25°C, 16,000 x g for 5 min prior to either 10% or 4-20% SDS-PAGE as indicated. Soluble fractions were normalized to GAPDH using an anti-GAPDH antibody (Proteintech #60004-1-Ig). Western blotting occurred using the primary antibodies indicated. Secondary antibodies (Thermo Fisher) goat anti-rabbit Alexa Fluor 647 (#A21244) and goat anti-mouse Alexa Fluor 488 (#A11001) were used for primary antibody detection. Fluorescent western blot imaging occurred on a Typhoon FLA 9500 Biomolecular Imager (GE Healthcare).
For RT-qPCR analysis of monoclones, each cell line was plated at 1 x 104 cells/well into 96-well plates in 100 μL complete media. Huh7 cells were included as an experimental reference for endogenous SERPINA1 gene expression which encodes AAT protein. After incubation for 24 h, cells were washed with ice-cold 1X PBS and lysed inwell for RNA purification using the RNeasy Mini Kit (Qiagen #74104) per manufacturer’s protocol including on-column DNase digestion. After RNA quantification, 140 ng total RNA from each cell line was reverse transcribed with the High-Capacity RNA-to-cDNA Kit (Thermo Fisher #4387406) per manufacturer’s protocol. The resulting single-stranded cDNA was quantified and approximately 280 ng (1 μL) cDNA from each cell line was used for qPCR reactions with TaqMan Gene Expression Master Mix (Thermo Fisher #4369016) in triplicate 20 μL reactions per manufacturer’s protocol using the probes SERPINA1 FAM-MGB (Thermo Fisher #4331182) for AAT transcript detection and GAPDH VICMGB_PL (Thermo Fisher #4448484) for GAPDH transcript detection and normalization. Two independent experiments were performed in a 384-well plate on a ViiA 7 Real-Time PCR System (Thermo Fisher). To evaluate SERPINA1 gene expression, Ct values were normalized to GAPDH, with relative expression calculated by comparative ΔCt40.
Comparison of liquid transfer methodologies.
Three liquid handling instruments were compared to evaluate performance for transferring cell culture media from secNLuc-ATZ – U2-OS Clone UZ11B cells between 1536-well plates. Cell culture media was obtained by culturing UZ11B cells for approximately 48 h in a T75 flask with complete media until expansion to 80-90% confluency. Media was removed from the flask and centrifuged at 25°C, 200 x g for 5 min to remove cells and debris before dispensing into a black, clear-bottom 1536-well source plate (Aurora Microplates #ABI121000A) using a Multidrop Combi (Thermo Fisher), then centrifuged at 800 x g for 5 min to remove any bubbles in wells. This source plate was used by each method throughout the evaluation to transfer approximately 100 nL of media into a white, solid-bottom 1536-well recipient plate (Greiner Bio One #789175-F) containing 3 μL/well of 1X PBS. All transfer methods were evaluated in triplicate. After media transfers, all recipient plates received 3 μL/well Nano-Glo luciferase assay reagent by BioRAPTR FRD (Beckman Coulter) and were incubated for 10 min prior to imaging with a ViewLux. The Wako Pintool (23 nL capillary pins, 1536-pin head) was dipped into the source plate then transferred to the recipient plate 4x without washing to approximate a 100 nL transfer of media. Plate-at-once imaging conditions for the Pintool transfers were: clear filter with 10 sec integration, slow speed, high sensitivity, and 2X binning. The TTP Labtech Mosquito HTS with 16-tip head aspirated 100 nL media from each source plate well then transferred to the recipient plate; this was performed without changing tips between well transfers, which did not represent the intended format for screening but prevented excessive use of tips during optimization. Because the 10 sec integration caused overexposure of the recipient plate, plate-at-once imaging conditions for the Mosquito HTS were: clear filter with 1 sec integration, slow speed, high sensitivity, and 2X binning. The EDC Biosystems ATS-100 acoustic dispenser used a calibration file specific to McCoy’s 5A complete medium (10% FBS, 1% penicillin/streptomycin) to transfer 100 nL media in 10 nL droplets one-to-one from the source plate to the recipient plate. Plate-at-once imaging conditions for the ATS-100 were: clear filter with 10 sec integration, slow speed, high sensitivity, and 2X binning. Data represent the mean ± SD of three replicate transfers.
Cell-based bioluminescent reporter secretion assay by acoustic dispensing.
For initial optimization without compound, untreated UZ11B cells were plated at 1200 cells/well in 5 μL of McCoy’s 5A complete medium into 1536-well black, clear-bottom source plates and incubated 24 h. Prior to dispensing, source plates were centrifuged at 200 x g for 10 min to remove bubbles in wells. For the full plate transfer, the ATS-100 acoustic dispenser used a calibration file specific to McCoy’s 5A complete medium to transfer 100 nL cell culture medium in 10 nL droplets one-to-one from the source plate to a white, solid-bottom 1536-well recipient plate containing 3 μL/well of 1X PBS. After media transfers, all receiving plates received 3 μL/well Nano-Glo luciferase assay reagent by BioRAPTR FRD and were incubated for 10 min prior to imaging with a ViewLux. For the checkerboard transfer, the same source plate, transfer, and detection protocols were used, only alternating wells were acoustically transferred from source to receiving plate. Data represent the mean ± SD of three replicate transfers. Imaging to confirm maintenance of the cell monolayer before and after acoustic dispensing was performed with an IN Cell Analyzer 2000 (Ge Healthcare) at 10X magnification.
For testing the bioluminescent reporter assay response to pharmacological modulation, UZ11B cells were plated at 1200 cells/well in 5 μL of McCoy’s 5A complete medium into 1536-well black, clear-bottom source plates. Within 1 h, cells were treated with a single concentration or 16-point, 1:2 duplicate titration of the indicated compounds using a Wako Pintool (23 nL capillary pins, 1536-pin head, 0.46% DMSO final concentration), then returned to the incubator for 24 h incubation. Prior to dispensing, source plates were centrifuged at 200 x g for 10 min to remove bubbles in wells. Full plate transfers were performed by the ATS-100 acoustic dispenser using a calibration file specific to McCoy’s 5A complete medium to transfer 50 nL cell culture medium in 10 nL droplets one-to-one from the source plate to a white, solid-bottom 1536-well recipient plate containing 3 μL/well of 1X PBS. After media transfers, recipient plates received 2 μL/well Nano-Glo luciferase assay reagent by BioRAPTR FRD and were incubated for 10 min prior to imaging with a ViewLux. Plate-at-once imaging conditions with the bioluminescent reporter assay for compound-treated cells were: clear filter with 5 sec integration, slow speed, high sensitivity, and 2X binning. Data represent the mean ± SD of two replicate transfers.
Biochemical confirmation of compound-mediated secretory reporter modulation.
UZ11B cells were plated at 3.5 x 105 cells/well into 6-well plates in 2 mL complete media. After 1 h, cells were treated with compound at the indicated concentrations and incubated for 24 h. Cells were then washed 3x in 1X PBS and incubated for 5 h in 1 mL serum-free media to accumulate secreted reporter, then put on ice. Media was removed and centrifuged at 4°C, 200 x g for 5 min, then transferred to a new tube. Media samples were mixed with 4X Laemmli sample buffer + 10% β-mercaptoethanol, then heated at 100°C for 10 min. After media was removed, cells were washed 3x in ice-cold 1X PBS, then lysed on ice in 200 μL freshly prepared lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 1X Xpert protease inhibitor cocktail) for 30 min. Lysates were homogenized by pipetting, collected and spun at 4°C, 16,000 x g for 30 min, after which the supernatant was collected as the soluble lysate fraction. Soluble fractions were quantified for total protein concentration by BCA assay prior to mixing with 4X Laemmli sample buffer + 10% β-mercaptoethanol, then heated at 100°C for 10 min. The insoluble pellet was washed by heavy vortexing in 1 mL ice-cold 1X PBS, then centrifuged at 4°C, 16,000 x g for 5 min. After removing PBS, pellets were dissolved in 200 μL 2X Laemmli sample buffer + 10% β-mercaptoethanol with heavy vortexing, then heated at 100°C for 10 min followed by heavy vortexing for 30 sec. All samples were allowed to return to room temperature after heating before centrifuging at 25°C, 16,000 x g for 5 min prior to either 10% or 4-20% SDS-PAGE as indicated. Western blotting occurred as previously described.
Acoustic reverse phase protein array (RPPA) and comparison with other secretion assays.
The prototype nitrocellulose recipient plates for acoustic RPPA were created to match the exterior dimensions of the 1536-well white, solid-bottom, high-base plates used in the bioluminescent reporter assay. Using an Ultimaker 3 3D printer and polylactic acid, a microplate base frame was printed with interior edge supports to hold a removable 1/16-inch thick sheet of clear acrylic cut to fit using a laser cutter. The acrylic functioned as a support for a nitrocellulose membrane cut to fit and held in place by a 3D-printed retainer frame that snapped into the top of the microplate base frame.
For initial optimization untreated UZ11B cells were plated at 1200 cells/well into 1536-well black, clear-bottom source plates and incubated for 24 h. Using the same dispensing protocol as the bioluminescent reporter assay, media was acoustically dispensed well-to-spot onto the top surface of the nitrocellulose receiving plate using the ATS-100 acoustic dispenser at the volumes indicated and allowed to dry at room temperature. Once dried, membranes were removed and immunoblotted by blocking with 5% nonfat milk/1X TBS-T (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% Tween 20) for 1 h at room temperature, followed by incubation with a primary antibody against human AAT (1:3000 dilution in 1% nonfat milk/1X TBS-T, as indicated) for 3 h at room temperature. After washing 3x in 1X TBS-T for 10 min each, incubation with goat anti-rabbit Alexa Fluor 647 secondary antibody (1:2000) occurred for 1 h at room temperature, followed by washing 3x in 1X TBS-T for 10 min each, then 1X TBS rinse and storage. Image acquisition was performed with a Typhoon FLA 9500. After imaging the acoustic RPPA receiving plate, the image was imported into ImageJ, processed and analyzed using the Protein Array Analyzer macro toolset plugin freely available to the public41. During this process, the 16-bit image was converted to 8-bit followed by a linear background subtraction with a 2D rolling ball radius of 25. Once information corresponding to the array was entered (e.g. number of rows and columns, analysis circle radius, etc.), the upper left, upper right, and bottom left corners were selected to align the grid for densitometric analysis. Data was then generated corresponding to each spot’s size and intensity, which could be visualized by the software’s heat mapping feature. ImageJ was also used to determine spot diameter based on volume dispensed using built-in scaling and measuring features.
For the untreated comparison with the bioluminescent reporter assay, well-to-well transfers were performed from the same source plate at the volumes indicated into a 1536-well white, solid-bottom target plate and processed as previously described. To evaluate whether the secNLuc-ATZ reporter transferred during acoustic RPPA remained enzymatically active, the same transfers were performed well-to-spot from the same source plate at the volumes indicated onto the top surface of the nitrocellulose receiving plate (bioluminescent surface assay). Spots were allowed to dry at room temperature for 24 h, then plates were submerged in Nano-Glo luciferase assay reagent at room temperature for 10 min prior to imaging and luminescent quantification with a ViewLux. Plate-at-once imaging conditions for the NLuc reporter surface assay were: clear filter with 60 sec integration, slow speed, high sensitivity, and 2X binning. Data represent the mean ± SD of all well transfers at volumes indicated from a single 1536-well plate.
To compare the nitrocellulose membrane recipient plates with commercially available nitrocellulose-coated glass slides (Grace Bio-Labs #705278), a 3D-printed glass slide holder was generated to match the exterior frame of the prototype acoustic RPPA plates with a flat, solid bottom support printed within the frame instead of using removable acrylic. The slide holder immobilized four adjacent slides across its top surface using a 3D-printed spring mechanism that applied pressure to the side of the end slide. Cell-free conditioned UZ11B media was used for the evaluation as previously described, with membrane and slides processed identically using the acoustic RPPA immunochemical protocol.
For comparing bioluminescent and acoustic RPPA assay responses to pharmacological modulation, well-to-well and well-to-spot transfers were performed from the same compound-treated source plate at the volumes indicated as previously described for the bioluminescent assay. For each source plate, media was first dispensed into a white, solid-bottom 1536-well receiving plate containing 3 μL/well of 1X PBS for the bioluminescent assay, then the acoustic RPPA nitrocellulose recipient plate. During media transfers all plates were covered with lids and kept at room temperature. After media transfers, bioluminescent recipient plates were processed and indicated as previously described. Acoustic RPPA receiving plates were allowed to dry overnight at room temperature prior to the immunoblotting protocol described previously with a primary antibody against human AAT (1:3000 or 1:9000) and goat anti-rabbit Alexa Fluor 647 secondary antibody (1:2000). Acoustic RPPA to measure endogenous AAT secretion in HepG2 cells was performed using the previously described protocol at 3000 cells/well. Data represent the mean ± SD of two duplicate compound treatments and were analyzed as outlined in subsequent sections.
Protein standard curves were generated during acoustic RPPA with purified AAT (Athens Research and Technology #16-16-011609) diluted in McCoy’s 5A complete media to the indicated concentrations. Whole-plate standard curves were generated by averaging spots for each concentration in the 16-point, 1:2 duplicate titration starting at 1 mg/mL (n= 96 for each concentration) in Prism software. For the acoustic RPPA assay quantifying secreted secNLuc-ATZ and endogenous AAT levels in untreated cells, standard curves were generated by the single 16-point, 1:2 duplicate titrations starting at 1 mg mL−1 (n=2 for each concentration). AAT protein concentrations in media were calculated from the standard curve from the formula:
where Kd = half-maximal concentration, Smax = signal maximum, x = protein concentration, and y = signal output. UZ11B (1200 cells/well), HepG2 (1600 cells/well), and Huh7 (1600 cells/well) untreated cells were plated and incubated for 24 h before following acoustic RPPA protocol as previously described and dispensed at the indicated volumes.
Cytotoxicity analysis.
Cytotoxicity was determined using the CellTiter-Glo luminescent cell viability assay (Promega #G7572). For the initial separate cytotoxicity assay, UZ11B cells were plated and treated identically to the bioluminescent assay protocol. After 24 h incubation, 3 μL/well CellTiter-Glo reagent was added by BioRAPTR FRD, incubated for 10 min, and then imaged on a ViewLux. Plate-at-once imaging conditions for the separate cytotoxicity assay were: clear filter with 10 sec integration, fastest speed, high sensitivity, and 2X binning. For the multiplexed cytotoxicity assay, after acoustic media transfer 3 μL/well CellTiter-Glo reagent was added by BioRAPTR FRD to the black, clear-bottom source plates containing treated cells, incubated for 10 min, and then imaged with a ViewLux. During the 10 min incubation, plates were centrifuged at 800 x g for 5 min to remove bubbles. Plate-at-once imaging conditions for the multiplexed cytotoxicity assay were: clear filter with 10 sec integration, slow speed, high sensitivity, and 2X binning.
LOPAC1280 qHTS.
Screening was performed in a semi-automated manner in 7-point interplate qHTS with DMSO plates at the beginning and end of pinning. Clone UZ11B cells used for screening were consistently passaged 4x from thawing before screening and confirmed negative for mycoplasma. Cells were grown in McCoy’s 5A complete medium (10% FBS, 1% penicillin/streptomycin) before and during screening.
For qHTS screening, UZ11B cells were plated at 1200 cells/well in 5 μL medium into 1536-well black, clear-bottom source plates using a Multidrop Combi. Within 1-2 h of plating, cells were treated with compounds using a Wako Pintool (23 nL capillary pins, 1536-pin head, 0.46% DMSO final concentration), then returned to the incubator for 24 h incubation. Controls were plated at a single concentration (n=16) and in a 16-point, 1:2 titration. In addition to controls, plates contained 0.46% DMSO as a neutral control (n=32). Prior to acoustic dispensing, source plates were centrifuged at 200 x g for 10 min to remove bubbles in wells. Full plate transfers were performed by the ATS-100 acoustic dispenser using a calibration file specific to McCoy’s 5A complete medium to dispense 200 nL cell culture medium in 10 nL droplets one-to-one from the source plate. For each source plate, media was first dispensed into white, solid-bottom 1536-well receiving plates containing 3 μL/well of 1X PBS for the bioluminescent assay, then the acoustic RPPA nitrocellulose recipient plate. During media transfers all plates were covered with lids and kept at room temperature. After media transfers, bioluminescent recipient plates received 2 μL/well Nano-Glo luciferase assay reagent by BioRAPTR FRD and were incubated for 10 min prior to imaging with a ViewLux. Plate-at-once imaging conditions with the bioluminescent assay for compound-treated cells were: clear filter with 5 sec integration, slow speed, high sensitivity, and 2X binning. Acoustic RPPA receiving plates were allowed to dry overnight at room temperature prior to the immunoblotting protocol described previously with a primary antibody against human AAT (1:9000) and goat anti-rabbit Alexa Fluor 647 secondary antibody (1:2000). For the multiplexed cytotoxicity assay, after acoustic media transfer 3 μL/well CellTiter-Glo reagent was added by BioRAPTR FRD to the black, clear-bottom source plates containing treated cells, incubated for 10 min, and then imaged with a ViewLux. During the 10 min incubation, plates were centrifuged at 800 x g for 5 min to remove bubbles. Plate-at-once imaging conditions for the multiplexed cytotoxicity assay were: clear filter with 10 sec integration, slow speed, high sensitivity, and 2X binning. Data were analyzed as outlined in subsequent sections.
NLuc enzymatic inhibition assay.
Biochemical NLuc enzymatic inhibition assays were derived from a previously described assay 29, with all reagent dispenses performed by a BioRAPTR FRD. Medium was collected from secNLuc-expressing S16 cells, centrifuged for 5 min at 200 x g, and filtered through a 0.22 μm filter. Based on luminescent output during optimization, secNLuc media was diluted 1/20 in 1X PBS and dispensed into a 1536-well white, solid-bottom plate at 2 μL/well followed by short centrifugation at 200 x g. Compounds were transferred using a Wako Pintool as in the cell-based assays, then incubated at room temperature for 20 min. Nano-Glo luciferase assay reagent was added at 2 μL/well and incubated for 10 min at room temperature prior to imaging on a ViewLux. Plate-at-once imaging conditions were: clear filter with 5 sec integration, slow speed, high sensitivity, and 2X binning.
Multiplexed analysis of iPSC-derived hepatocytes.
Human iPSC-derived hepatocytes (Cellular Dynamics International #R1027) were handled and maintained per manufacturer’s protocol. On Day 0, cells were plated into either 1536- or 384-well black, clear-bottom plates using a Multidrop Combi. Plating medium was manually changed by multichannel pipet daily until the Day 5 switch to maintenance medium with media renewal every two days. For 1536-well cell-containing source plates, at the indicated time points protein standards were added and plates were analyzed by acoustic RPPA in 10 nL volumes as previously described. However, image processing could not proceed because of substantial cell loss after manual washing and fixing steps.
For 384-well cell-containing source plates, at the indicated time points protein standards were added, media from each well was manually transferred in quadruplicate to a 1536-well acoustic source plate, and analysis by acoustic RPPA was performed in 10 nL volumes as previously described. After media removal, cells were processed as follows at room temperature: 2x 1X PBS wash; 20 min 4% formaldehyde/PBS fixation; 2x 1X PBS wash; 20 min 0.4% Triton X-100/PBS permeabilization; 1 h 10% goat serum block; 3 h primary antibody/1% goat serum incubation; 2x 1X PBS wash; 1 h secondary antibody/Hoechst/1% goat serum incubation; 2x 1X PBS wash. Primary antibodies were against human AAT (1:1000) and human albumin (Cedarlane #CL2513A, 1:250). Secondary antibodies were goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 555 (1:1000) incubated with Hoechst (1:5000). Multiplexed acoustic RPPA was imaged with a CLx (LICOR). Microscopy imaging was performed with an IN Cell Analyzer 2200. Laser cytometry was performed with an Acumen Cellista (TTP Labtech).
Data analysis for assay performance and characterization.
Assays were evaluated based on performance after compound treatment at single concentrations or a 16-point, 1:2 duplicate titration of compound to generate concentration response curves (CRCs). For single concentration treatments, Z’-factors23 were calculated for each compound according to indicated transfer volumes and time points using the intraplate DMSO column as a neutral control. Compound CRCs were generated and fit using Prism software (Graphpad) to determine CRC potency and evaluate by minimum significant ratio (MSR)24. Each CRC was fit with Prism’s 3- or 4-parameter (Hill Equation) or 5- or 6-parameter (bell-shaped) models, or a customized 7-parameter (bell-shaped) model formula where applicable. Models were selected based on an extra-sum-of-squares F test for each CRC. The 7-parameter model consisted of:
where S0 is the half-maximal signal, S1 is signal maximum, S2 is signal minimum, a is the log base 10 of the midpoint compound concentration, b is the log base 10 compound concentration at the signal midpoint, n1 is the ascending hill slope, n2 is the descending hill slope, x is the log10 of compound concentration, and y = response in units. For the 7-parameter or 5- and 6-parameter model CRCs in which log [EC50] or log [IC50] were not appropriately determined by Prism, a table of xy coordinates for the CRC fit was generated in Prism and imported into Microsoft Excel, where the inflection points of the ascending (i.e. EC50) and descending (i.e. IC50) segments of the curve were calculated as the half maximal effective and half maximal inhibitory concentrations, respectively.
MSR, a measure of potency reproducibility, calculates the smallest ratio between two potency determinations that is significant. An MSR ≤3 is generally satisfactory for an assay to enter primary screening, and an increasing MSR corresponds with decreasing reproducibility24, 25. For bioluminescent and acoustic RPPA assay performance and characterization, intraplate potencies were determined from de-interlaced 16-point, 1:2 duplicate titrations for each compound as described elsewhere (27), and MSR values were calculated using the intrarun formula:
where σ is the standard deviation of log [EC50] or log [IC50] differences between duplicates.
Concentration response data fitting and compound activity classification in qHTS.
Data from the LOPAC1280 qHTS screens were normalized plate-wise to corresponding intraplate controls as previously described30. These controls were also used to determine Z’-factors23 (single concentration) and MSR values24, 25 (16-point, 1:2 titration) for each plate that contributed to overall scores for both screens. For LOPAC1280 qHTS, interplate MSR values across the nine screening plates (seven test concentrations, two DMSO at the beginning and end) were calculated using the interrun formula:
where σ is the standard deviation of log [EC50] or log [IC50] differences of the indicated controls across the nine plates from each respective screen.
For the bioluminescent and acoustic RPPA screens, secretion signal output was normalized to the median readings of the indicated single concentration controls (23.0 μM vorinostat or 92.2 nM brefeldin A, n=16) and DMSO (n=32). For the NLuc enzymatic counter-screen, luminescent signal output was normalized to the median readings of the single concentration control (114.9 μM cilnidipine, n=32) and DMSO (n=32). For the multiplexed CellTiter-Glo cytotoxicity counter-screen, luminescent signal output was normalized to the median readings of DMSO (n=32). Normalized data from each screening plate was corrected using the DMSO screening plates at the beginning and end of the screens. Interplate titration data for each compound was fit using automated in-house software to the standard hill equation and classified into curve class as previously described42. CRCs were created by Prism software.
Most compounds that generated bell-shaped CRCs were misclassified by the standard hill equation fit. To identify these, post-normalized screening results were manually sorted by maximum signal generated across the CRC and selected based on visual inspection of curve shape and corresponding NLuc enzymatic inhibition or cytotoxicity. These CRCs were then re-fit using 5- or 6-parameter models with potency determinations as previously described.
Protein digestion and HPLC-MS analysis.
Approximately 50 μg of protein from each sample was used for in-solution digestion using RapidGest (Waters Corporation, Milford, MA) to enhance enzymatic digestion of protein, followed by denature, reduction, alkylation and incubated with Trypsin/Lys-C Mix (1:50, trypsin/Lys-C: protein) at 37°C for approximately 12 hours. Acidified tryptic peptides were desalted using an HPLC C18 column on an Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA), lyophilized and then re-suspended in 3% acetonitrile (ACN), 97.9% water and 0.1% formic acid (FA) buffer for LC-MS/MS analysis. Approximately 1 μg of the digested samples were loaded into an Agilent LC-MS system comprised of a 1200 LC system coupled to a 6550 QTOF via an HPLC Chip Cube interface. Agilent Polaris-HR-Chip-3C18 chip (360 nL, 180 A C18 trap with a 75 μm i.d., 150 mm length, 180 Å C18 analytical column) was used for the peptide capture and separation prior to MS analysis. Elution of peptides from the analytical column was performed using a gradient starting at 97% A (A: 99.9% water, 0.1% FA) at 300 nL/min. The mobile phase was 3–10% B for 2 min, 10–35% B for 16 min, 35–99% for 1 min, and maintained at 99% B (B: 90% ACN, 9.9% water, 0.1% FA) for 4 min, followed by re-equilibration of column with 3% B for 6 min. Data dependent (autoMS2) MS acquisition was performed by an Agilent 6550 QTOF at 2 GHz. Precursor MS spectra were acquired from m/z 315 to 1700 and the top 6 peaks were selected for MS/MS analysis. Product scans were acquired from m/z 50 to 1700 at a scan rate of 3 spectra per second. A medium isolation width (~4 amu) was used, and a collision energy of slope 3.6 V/100 Da with a 2.9 V offset was applied for fragmentation. A dynamic exclusion list was applied with precursors excluded for 0.50 min after two MS/MS spectrum was acquired.
Database searching.
All LC-MS/MS raw data were converted to Mascot generic format (.mgf) by ProteoWizard MS Convert release: 3.0.9134 (2015-11-11). Mascot version 2.5 was used to search against either the Swiss-Prot human database (2018.07) which consists of 20,386 sequences for peptide sequence assignments using the following parameters: precursor ion mass tolerance of 50 ppm and a fragment ion mass tolerance of 0.2 daltons. Peptides were searched using fully tryptic cleavage constraints, and up to two internal cleavage sites were allowed for tryptic digestion. Fixed modifications consisted of carbamidomethylation of cysteine. Variable modifications that were considered were oxidation of methionine residues.
Supplementary Material
Acknowledgments
We thank P. Dranchak for laboratory assistance, E. Wallgren for diagrams of the acoustic RPPA prototype plate, A. Simeonov for critical review of the manuscript, and the Alpha-1 Foundation scientific advisory board (B. Gooptu, D. Kotton, R. Sifers, J. Teckman, A. Wanner). This work was supported by the intramural program of NCATS, National Institutes of Health, project 1ZIATR000048-03 (J.I.) and the Alpha-1 Foundation (J.I. and M.J.I.).
Footnotes
Supporting Information Available: This material is available free of charge via the internet.
References
- 1.Uhlen M; Fagerberg L; Hallstrom BM; Lindskog C; Oksvold P; Mardinoglu A; Sivertsson A; Kampf C; Sjostedt E; Asplund A; Olsson I; Edlund K; Lundberg E; Navani S; Szigyarto CA; Odeberg J; Djureinovic D; Takanen JO; Hober S; Alm T; Edqvist PH; Berling H; Tegel H; Mulder J; Rockberg J; Nilsson P; Schwenk JM; Hamsten M; von Feilitzen K; Forsberg M; Persson L; Johansson F; Zwahlen M; von Heijne G; Nielsen J; Ponten F, Proteomics. Tissue-based map of the human proteome. Science 2015, 347 (6220), 1260419. [DOI] [PubMed] [Google Scholar]
- 2.Brown KJ; Seol H; Pillai DK; Sankoorikal BJ; Formolo CA; Mac J; Edwards NJ; Rose MC; Hathout Y, The human secretome atlas initiative: implications in health and disease conditions. Biochim Biophys Acta 2013, 1834 (11), 2454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M; Kaufman RJ, Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529 (7586), 326–35. [DOI] [PubMed] [Google Scholar]
- 4.Milligan G, Applications of bioluminescence- and fluorescence resonance energy transfer to drug discovery at G protein-coupled receptors. Eur J Pharm Sci 2004, 21 (4), 397–405. [DOI] [PubMed] [Google Scholar]
- 5.Milligan G; Feng GJ; Ward RJ; Sartania N; Ramsay D; McLean AJ; Carrillo JJ, G protein-coupled receptor fusion proteins in drug discovery. Curr Pharm Des 2004, 10 (17), 1989–2001. [DOI] [PubMed] [Google Scholar]
- 6.Inglese J; Johnson RL; Simeonov A; Xia M; Zheng W; Austin CP; Auld DS, High-throughput screening assays for the identification of chemical probes. Nat Chem Biol 2007, 3 (8), 466–79. [DOI] [PubMed] [Google Scholar]
- 7.Thorne N; Inglese J; Auld DS, Illuminating insights into firefly luciferase and other bioluminescent reporters used in chemical biology. Chem Biol 2010, 17 (6), 646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auld DS; Inglese J, Interferences with Luciferase Reporter Enzymes. In Assay Guidance Manual, Sittampalam GS; Coussens NP; Brimacombe K; Grossman A; Arkin M; Auld D; Austin C; Baell J; Bejcek B; Caaveiro JMM; Chung TDY; Dahlin JL; Devanaryan V; Foley TL; Glicksman M; Hall MD; Haas JV; Inglese J; Iversen PW; Kahl SD; Kales SC; Lal-Nag M; Li Z; McGee J; McManus O; Riss T; Trask OJ Jr.; Weidner JR; Wildey MJ; Xia M; Xu X, Eds. Bethesda (MD), 2004. [PubMed] [Google Scholar]
- 9.Thorne N; Auld DS; Inglese J, Apparent activity in high-throughput screening: origins of compound-dependent assay interference. Curr Opin Chem Biol 2010, 14 (3), 315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho PI; Yue K; Pandey P; Breault L; Harbinski F; McBride AJ; Webb B; Narahari J; Karassina N; Wood KV; Hill A; Auld DS, Reporter enzyme inhibitor study to aid assembly of orthogonal reporter gene assays. ACS Chem Biol 2013, 8 (5), 1009–17. [DOI] [PubMed] [Google Scholar]
- 11.Cassaday J; Shah T; Murray J; O'Donnell GT; Kornienko O; Strulovici B; Ferrer M; Zuck P, Miniaturization and automation of an ubiquitin ligase cascade enzyme-linked immunosorbent assay in 1,536-well format. Assay Drug Dev Technol 2007, 5 (4), 493–500. [DOI] [PubMed] [Google Scholar]
- 12.Gul S; Hadian K, Protein-protein interaction modulator drug discovery: past efforts and future opportunities using a rich source of low- and high-throughput screening assays. Expert Opin Drug Discov 2014, 9 (12), 1393–404. [DOI] [PubMed] [Google Scholar]
- 13.Bielefeld-Sevigny M, AlphaLISA immunoassay platform- the "no-wash" high-throughput alternative to ELISA. Assay Drug Dev Technol 2009, 7 (1), 90–2. [DOI] [PubMed] [Google Scholar]
- 14.Schorpp K; Rothenaigner I; Salmina E; Reinshagen J; Low T; Brenke JK; Gopalakrishnan J; Tetko IV; Gul S; Hadian K, Identification of Small-Molecule Frequent Hitters from AlphaScreen High-Throughput Screens. J Biomol Screen 2014, 19 (5), 715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasgar A; Jadhav A; Simeonov A; Coussens NP, AlphaScreen-Based Assays: Ultra-High-Throughput Screening for Small-Molecule Inhibitors of Challenging Enzymes and Protein-Protein Interactions. Methods Mol Biol 2016, 1439, 77–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishizuka S; Charboneau L; Young L; Major S; Reinhold WC; Waltham M; Kouros-Mehr H; Bussey KJ; Lee JK; Espina V; Munson PJ; Petricoin E 3rd; Liotta LA; Weinstein JN, Proteomic profiling of the NCI-60 cancer cell lines using new high-density reverse-phase lysate microarrays. Proc Natl Acad Sci U S A 2003, 100 (24), 14229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan SM; Ermann J; Su L; Fathman CG; Utz PJ, Protein microarrays for multiplex analysis of signal transduction pathways. Nat Med 2004, 10 (12), 1390–6. [DOI] [PubMed] [Google Scholar]
- 18.Spurrier B; Ramalingam S; Nishizuka S, Reverse-phase protein lysate microarrays for cell signaling analysis. Nat Protoc 2008, 3 (11), 1796–808. [DOI] [PubMed] [Google Scholar]
- 19.Zhu H; Qian J, Applications of functional protein microarrays in basic and clinical research. Adv Genet 2012, 79, 123–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teckman JH; Burrows J; Hidvegi T; Schmidt B; Hale PD; Perlmutter DH, The proteasome participates in degradation of mutant alpha 1-antitrypsin Z in the endoplasmic reticulum of hepatoma-derived hepatocytes. J Biol Chem 2001, 276 (48), 44865–72. [DOI] [PubMed] [Google Scholar]
- 21.Graham KS; Le A; Sifers RN, Accumulation of the insoluble PiZ variant of human alpha 1-antitrypsin within the hepatic endoplasmic reticulum does not elevate the steady-state level of grp78/BiP. J Biol Chem 1990, 265 (33), 20463–8. [PubMed] [Google Scholar]
- 22.Gooptu B; Dickens JA; Lomas DA, The molecular and cellular pathology of alpha(1)-antitrypsin deficiency. Trends Mol Med 2014, 20 (2), 116–27. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JH; Chung TD; Oldenburg KR, A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen 1999, 4 (2), 67–73. [DOI] [PubMed] [Google Scholar]
- 24.Eastwood BJ; Farmen MW; Iversen PW; Craft TJ; Smallwood JK; Garbison KE; Delapp NW; Smith GF, The minimum significant ratio: a statistical parameter to characterize the reproducibility of potency estimates from concentration-response assays and estimation by replicate-experiment studies. J Biomol Screen 2006, 11 (3), 253–61. [DOI] [PubMed] [Google Scholar]
- 25.MacArthur R; Leister W; Veith H; Shinn P; Southall N; Austin CP; Inglese J; Auld DS, Monitoring compound integrity with cytochrome P450 assays and qHTS. J Biomol Screen 2009, 14 (5), 538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz I, Permeabilizing cells: some methods and applications for the study of intracellular processes. Methods Enzymol 1990, 192, 280–300. [DOI] [PubMed] [Google Scholar]
- 27.Bouchecareilh M; Hutt DM; Szajner P; Flotte TR; Balch WE, Histone deacetylase inhibitor (HDACi) suberoylanilide hydroxamic acid (SAHA)-mediated correction of alpha1-antitrypsin deficiency. J Biol Chem 2012, 287 (45), 38265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasson SA; Fogel AI; Wang C; MacArthur R; Guha R; Heman-Ackah S; Martin S; Youle RJ; Inglese J, Chemogenomic profiling of endogenous PARK2 expression using a genome-edited coincidence reporter. ACS Chem Biol 2015, 10 (5), 1188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inglese J; Dranchak P; Moran JJ; Jang SW; Srinivasan R; Santiago Y; Zhang L; Guha R; Martinez N; MacArthur R; Cost GJ; Svaren J, Genome editing-enabled HTS assays expand drug target pathways for Charcot-Marie-tooth disease. ACS Chem Biol 2014, 9 (11), 2594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inglese J; Auld DS; Jadhav A; Johnson RL; Simeonov A; Yasgar A; Zheng W; Austin CP, Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A 2006, 103 (31), 11473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ang LT; Tan AKY; Autio MI; Goh SH; Choo SH; Lee KL; Tan J; Pan B; Lee JJH; Lum JJ; Lim CYY; Yeo IKX; Wong CJY; Liu M; Oh JLL; Chia CPL; Loh CH; Chen A; Chen Q; Weissman IL; Loh KM; Lim B, A Roadmap for Human Liver Differentiation from Pluripotent Stem Cells. Cell Rep 2018, 22 (8), 2190–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hortin GL; Sviridov D; Anderson NL, High-abundance polypeptides of the human plasma proteome comprising the top 4 logs of polypeptide abundance. Clin Chem 2008, 54 (10), 1608–16. [DOI] [PubMed] [Google Scholar]
- 33.Mallanna SK; Duncan SA, Differentiation of hepatocytes from pluripotent stem cells. Curr Protoc Stem Cell Biol 2013, 26, Unit 1G 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulesskiy E; Saarela J; Turunen L; Wennerberg K, Precision Cancer Medicine in the Acoustic Dispensing Era: Ex Vivo Primary Cell Drug Sensitivity Testing. J Lab Autom 2016, 21 (1), 27–36. [DOI] [PubMed] [Google Scholar]
- 35.Wong EY; Diamond SL, Advancing microarray assembly with acoustic dispensing technology. Anal Chem 2009, 81 (1), 509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ankarcrona M; Winblad B; Monteiro C; Fearns C; Powers ET; Johansson J; Westermark GT; Presto J; Ericzon BG; Kelly JW, Current and future treatment of amyloid diseases. J Intern Med 2016, 280 (2), 177–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiti F; Dobson CM, Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu Rev Biochem 2017, 86, 27–68. [DOI] [PubMed] [Google Scholar]
- 38.Ekeowa UI; Freeke J; Miranda E; Gooptu B; Bush MF; Perez J; Teckman J; Robinson CV; Lomas DA, Defining the mechanism of polymerization in the serpinopathies. Proc Natl Acad Sci U S A 2010, 107 (40), 17146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gooptu B; Lomas DA, Polymers and inflammation: disease mechanisms of the serpinopathies. J Exp Med 2008, 205 (7), 1529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmittgen TD; Livak KJ, Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008, 3 (6), 1101–8. [DOI] [PubMed] [Google Scholar]
- 41.Carpentier G Protein Array Analyzer for ImageJ. http://image.bio.methods.free.fr/ImageJ/?Protein-Array-Analyzer-for-ImageJ&lang=en.
- 42.Southall N; Jadhav A; Huang R; Nguyen T; Wang Y, Enabling the Large-Scale Analysis of Quantitative High-Throughput Screening Data In Handbook of Drug Screening, 2nd ed.; Seethala R; Zhang L, Eds. Informa Healthcare: 2009; Vol. 196, pp 442–464. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.