Abstract
Objectives
The aim of this study was to examine the effect of three different doses of aerobic exercise training (corresponding to approximately 50%, 100% and 150% of the National Institutes of Health consensus guidelines) on endothelial function in sedentary obese postmenopausal women with elevated blood pressure. Aerobic exercise training improves endothelial function in individuals with cardiovascular risk; however, it is unknown whether these adaptations occur in a dose-dependent manner.
Methods
Obese postmenopausal women (n=155) with elevated blood pressure (systolic blood pressure between 120 and 159.0 mm Hg) were randomly assigned to one of four groups: 4, 8 or 12 kilocalories per kilogram of energy expenditure per week (kcal/kg/week) or a non-exercise control group for 6 months. Endothelial function was assessed via flow-mediated dilation (FMD) at baseline and post-intervention.
Results
After exercise training, there was a similar improvement (1.02–1.5%) in FMD in all three exercise groups (p<0.05) compared with control (–0.5%). Change in FMD after exercise training was significantly correlated with FMD at baseline (r= −0.35, p<0.001). Post hoc analyses found a significant improvement in FMD in exercisers (all exercise groups combined) with endothelial dysfunction (FMD < 5.5%) at baseline (1.8%, 95% CI: 1.17 to 2.38; p<0.001) compared with exercisers with normal endothelial function (FMD ≥ 5.5%) (–1.2%; 95% CI: −1.17 to 0.69; p=0.60).
Conclusions
Aerobic exercise training was associated with improved FMD in postmenopausal women with elevated blood pressure. In addition, exercise training may be more efficacious in improving endothelial function in postmenopausal women with endothelial dysfunction than individuals with normal endothelial function at baseline.
INTRODUCTION
Menopause is associated with atherogenic changes, including a worsening of cardiovascular disease (CVD) risk factors1–3 and decreased aerobic fitness4 compared with premenopausal women. Similarly, vascular endothelial function measured via flow-mediated dilation (FMD) is impaired after menopause.5,6 This initially may be due to a decrease in endogenous oestrogen levels,7 and likely further exacerbated by increased adiposity8 and reductions in exercise habits or physical activity levels.8 Because endothelial dysfunction may represent one of the early subclinical events in atherogenesis,9 and has been shown to predict future risk of hypertension10 and cardiovascular events11 in postmenopausal women, effective intervention strategies capable of improving endothelial function are needed.
Aerobic exercise training as an intervention has been shown to improve endothelial function consistently in individuals with CVD risk12–15 and hypertension.14,16,17 However, because previous research has typically compared one standardised exercise intervention with a control group, it is unknown whether improvements in FMD with exercise training occur in a dose-dependent manner. Aerobic exercise is believed to improve endothelial function by increasing antegrade vascular shear stress, which leads to upregulation of nitric oxide synthase, and greater nitric oxide release.13 Therefore, it may be possible that longer exposure to antegrade shear stress via increased aerobic exercise dose (greater amount of kilocalories expended) may improve FMD to a greater extent than lower-dose training. The purpose of the present study is to investigate the effect of three different doses of aerobic exercise training on endothelial function in overweight and obese postmenopausal women with elevated blood pressure from the Dose Response to Exercise in Women (DREW) trial.
METHODS
Study design and participants
The full design and methodology for DREW has been published previously.18 In brief, DREW was a randomised controlled trial evaluating the dose response of aerobic exercise training with increasingly higher doses of energy expenditure in 464 sedentary postmenopausal women with elevated blood pressure. A subset of 200 participants underwent FMD measurements at baseline and follow-up. The research protocol was reviewed and approved annually by the Cooper Institute institutional review board and subsequently approved by Pennington Biomedical Research Center for continued analysis. Written informed consent was obtained from all participants before enrolment. Women recruited for this study were healthy, postmenopausal, overweight or obese (body mass index (BMI) 25.0–43.0 kg/m2), sedentary (no participation in greater than 20 min of exercise 3 times a week), had elevated systolic blood pressure (SBP) (120–159.0 mm Hg), and were physically capable of participating in an exercise program. Notable exclusion criteria included the presence of significant medical conditions, elevated low-density lipoprotein (≥ 3.36 mmol/l) or weight loss of ≥9.1 kg in the previous year.18
Fitness testing
Fitness testing was performed using a Lode Excalibur Sport cycle ergometer (Groningen, The Netherlands), an electronic, rate-independent ergometer. Participants cycled at 30 W for 2 min, 50 W for 4 min, followed by increases of 20 W every 2 min until they could no longer maintain a pedal cadence of 50 revolutions per minute. Respiratory gases were measured using a Truemax 2400 Metabolic Measurement Cart (Parvomedics, Sandy, Utah, USA). Volume and gas calibrations were conducted before each test. Gas-exchange variables (VO2, CO2 production, ventilation and respiratory exchange ratio) were collected breath by breath and averaged into 15-s intervals. Two fitness tests were performed on separate days at baseline and at follow-up, and the mean of the VO2 peak obtained from these two tests was defined as cardiorespiratory fitness.
FMD measurements
Endothelial function was evaluated in the morning, in the fasted state without the influence of vasoactive substances (ie, medications, caffeine, alcohol). FMD measurements were obtained using a high-resolution ultrasound (Acuson Aspen, Mountain View, California, USA) with a 5–11 MHz linear array probe (L-10) in accordance with published guidelines.19 Ultrasound images were observed in longitudinal view approximately 4 cm proximal to the olecranon process, in the arterial/medial plane by a well-trained sonographer. All images were performed on the left arm with the forearm extended and slightly supinated, while the participant rested supine in a hospital bed. A rapid inflation/deflation pneumatic cuff (E 20 Rapid Cuff Inflator; E.C. Hokanson, Bellevue, Washington, USA) was placed around the forearm approximately 1 cm distal to the olecranon process. Once the baseline images were obtained, the pneumatic cuff was rapidly inflated to 200 mm Hg (or >50 mm Hg if participant’s resting SBP was >150 mm Hg) for 5 min. B-mode images of the brachial artery were recorded during a 30 s time period at rest and during 2 min after cuff deflation.
Images were captured during each cardiac cycle at end diastole, as defined by the onset of the QRS complex, and were analysed in a blinded fashion using vascular imaging software (Brachial Tools Analysis Workstation; Medical Imaging Applications, Iowa City, Iowa, USA). Vessel diameter was measured along a 4–10 mm segment of the brachial artery and was measured from the media-adventitial border on the near wall to the media-adventitial border on the far wall. The resting diameter was quantified as a mean value from 5 to 10 frames recorded before cuff occlusion. The peak diameter was determined from five consecutive frames with the largest diameters obtained during the 2 min post-occlusion period. FMD (%) was defined as the peak brachial diameter after cuff release minus the mean diameter at rest divided by mean diameter at rest. Blood flow velocity was measured via pulsed Doppler at baseline during 7–10 cardiac cycles and immediately after the cuff release during the first 3–5 cardiac cycles to obtain peak velocity. FMD measures were obtained at baseline and approximately 3 days after the last exercise session at follow-up.
FMD reproducibility
We examined the reproducibility of FMD measurements on two occasions (7–10 days apart) in 10 participants aged 22–35 years. The mean brachial diameter of 3.67±0.38 mm at rest and 3.95±0.38 mm during reactive hyperaemia yielded a mean FMD of 7.74±2.47% during the first measurement. Mean brachial diameter during the second measurements at rest was 3.67±0.41 mm and during reactive hyperaemia was 3.94±0.44 mm. The mean FMD calculated from the second measurements was 7.59±2.48%. The mean difference in FMD between studies was 0.14±0.71% (r=0.96).
Randomisation
A consort diagram for the present study is provided in figure 1. In the final analysis, there were 23 participants randomised to the control group, 68 randomised to the 4 kcal/kg/week group, 32 randomised to the 8 kcal/kg/week and 32 randomised to the 12 kcal/kg/week group. The unequal distribution of participants between groups was due to the fact that FMD measures were added to the study protocol after the DREW trial had already begun, and the over-recruitment of the 4 kcal/kg/week group in DREW was decided a priori to increase statistical power because of the smaller anticipated improvements in the 4 kcal/kg/week group.18
Figure 1.
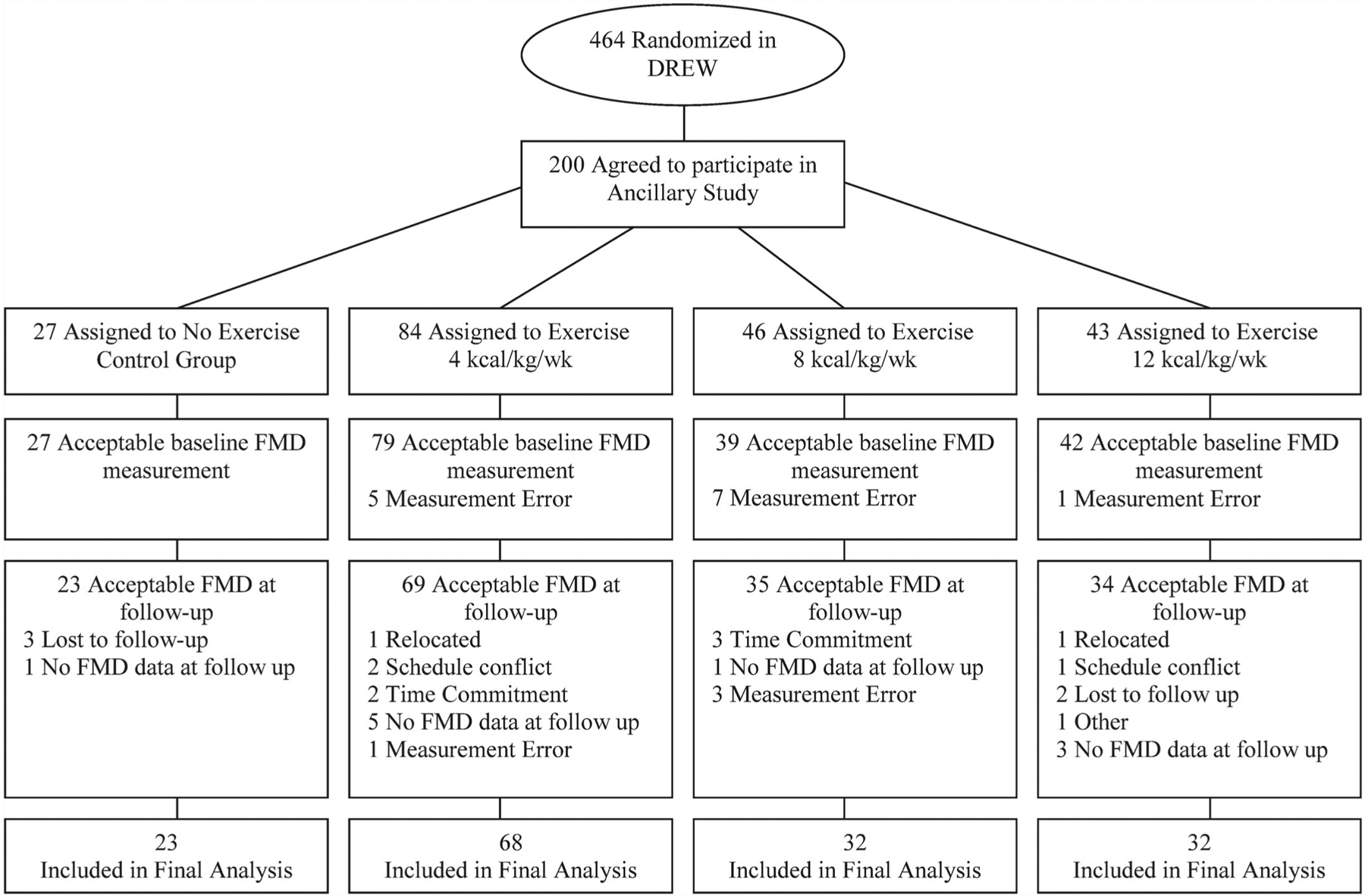
Consort diagram.
Exercise training
We calculated the exercise energy expenditure for women in the DREW age range associated with meeting the consensus public health recommendation from the National Institutes of Health (NIH) and other organisations.20,21 Details of these calculations are presented in the DREW design and methods report.18 A major objective of DREW was to evaluate exercise levels 50% below and 50% above public health recommendations to test whether the lower dose provides any benefit and whether the higher dose provides proportionally more benefit than the standard exercise levels of 8 kcal/kg/week.
Participants attended three or four training sessions each week for 6 months with training intensity at the heart rate associated with 50% of each woman’s VO2 peak. During the first week, each group expended 4 kcal/kg. Those assigned to that level continued to expend 4 kcal/kg/week for 6 months. All other groups increased their energy expenditure by 1 kcal/kg a week until they reached the level required for their group. All exercise sessions were supervised in an exercise laboratory with strict monitoring of the amount of exercise completed during each session. Participants were weighed each week, and their weight was multiplied by their exercise dosage to determine the number of calories to be expended for the week. Women in the exercise groups alternated training sessions on semirecumbent cycle ergometers and treadmills. Adherence to exercise training was calculated by dividing the kilocalories expended during exercise training by the kilocalories prescribed for the training period multiplied by 100%. Women in the non-exercise control group were asked to maintain their level of activity during the 6-month study period. There were separate intervention and assessment teams, and assessment staff members were blinded to the randomisation of study participants.
Statistical procedure
Statistical analyses were performed using SAS version 9.1. Descriptive data were tabulated as means (SD). One-way analysis of variance (ANOVA) analyses were performed to evaluate group differences between randomisation groups for continuous variables. χ2 analyses were performed to evaluate group differences in categorical variables. For all analyses, a p value less than 0.05 was used to reject the null hypothesis. Differences in FMD, body composition and fitness as a result of exercise training were analysed using ANOVA. Results are presented as least-square means with 95% CIs. Spearman correlations were performed between baseline parameters and endothelial function. In addition, correlations were analysed between the change of FMD after exercise training, and change in body composition, fitness and biomedical variables after exercise training.
Because it has been suggested that endothelial dysfunction at baseline may be an important factor affecting the efficacy of exercise training on endothelial function,13 we divided the participants who randomised to exercise (n=132) into two groups: normal endothelial function at baseline (n=39, FMD ≥ 5.5%) and endothelial dysfunction at baseline (n=93, FMD <5.5%). This criterion was chosen based on previous studies that have classified individuals with endothelial dysfunction.22–26 An ANOVA was performed to evaluate whether the change in FMD after completion of exercise training was different between the normal and endothelial dysfunction groups.
RESULTS
Baseline demographic data are presented in table 1. The study population had a mean (SD) age of 56.7 (6.0) years; had a weight of 84.4 (12.5) kg; had a BMI of 31.7 (3.7) kg/m2; and approximately 46.4% were Caucasian. There were approximately 34% of participants taking antihypertensive medications, of whom 11% were taking ACE inhibitors, 23% were taking diuretics, 11% were taking calcium channel blockers and 0.6% were taking β-blockers. In addition, 33% of participants were taking oestrogen replacement and 32% were taking medication for hyperlipidemia. There were no significant differences in baseline characteristics or medication usage between groups.
Table 1.
Descriptive subject characteristics
| All | Control | 4 kcal/kg/week | 8 kcal/kg/week | 12 kcal/kg/week | |
|---|---|---|---|---|---|
| N | 155 | 23 | 68 | 32 | 32 |
| Age (year) | 56.7 (6.0) | 56.8 (5.4) | 57.4 (5.8) | 55.9 (6.0) | 56.3 (6.8) |
| Caucasian (%) | 46.4 | 26.1 | 45.6 | 50.0 | 59.4 |
| Weight | 84.4 (12.5) | 85.2 (10.9) | 83.4 (12.6) | 86.3 (13.0) | 83.7 (12.9) |
| BMI (kg/m2) | 31.7 (3.7) | 32.2 (3.0) | 31.5 (3.1) | 32.7 (4.3) | 30.7 (3.1) |
| VO2 peak (l/min) | 1.3 (0.25) | 1.3 (0.3) | 1.3 (0.2) | 1.3 (0.2) | 1.3 (0.3) |
| Systolic blood pressure (mm Hg) | 137.2 (13.6) | 141.2 (15.4) | 136.9 (13.6) | 137.6 (15.5) | 134.8 (9.9) |
| Diastolic blood pressure (mm Hg) | 80.4 (8.8) | 81.2 (8.8) | 80.9 (9.4) | 79.7 (7.3) | 79.6 (9.1) |
| Exercise training adherence (%) | 97.9 (9.3) | – | 97.7 (9.9) | 98.1 (4.8) | 96.6 (13.7) |
| Waist circumference (cm) | 96.7 (11.0) | 96.6 (11.7) | 96.8 (11.2) | 97.5 (11.0) | 95.8 (10.3) |
| Triglycerides (mg/dl) | 116.1 (53.7) | 105.1 (45.4) | 116.3 (53.8) | 134.6 (60.1) | 105.4 (49.2) |
| HDL cholesterol (mg/dl) | 58.7 (15.9) | 59.43 (16.8) | 60.96 (17.5) | 54.22 (13.9) | 58.06 (12.9) |
| LDL cholesterol (mg/dl) | 117.7 (25.1) | 124.7 (20.1) | 114.1 (26.8) | 119.1 (23.1) | 119.1 (26.2) |
| Fasting glucose (mg/dl) | 93.0 (9.3) | 93.7 (12.8) | 91.8 (8.7) | 93.0 (8.0) | 95.0 (8.5) |
| HRT (%) | 32.9 | 26.1 | 32.4 | 37.5 | 34.4 |
| Ace-inhibitors (%) | 11.1 | 8.7 | 8.8 | 15.6 | 12.5 |
| Diuretics (%) | 23.2 | 8.7 | 22.1 | 31.3 | 28.1 |
| Calcium channel blockers (%) | 11.0 | 4.4 | 16.2 | 12.5 | 3.1 |
| Hyperlipidaemia medication (%) | 20.7 | 21.7 | 25.0 | 18.8 | 12.5 |
| Brachial artery diameter (mm) | 3.76 (0.5) | 3.76 (0.6) | 3.97 (0.5) | 3.89 (0.4) | 3.79 (0.5) |
| FMD (%) | 4.13 (2.5) | 4.7 (2.4) | 4.0 (2.6) | 4.4 (2.4) | 3.7 (2.6) |
Baseline characteristics of 155 obese postmenopausal women enrolled in the DREW trial who underwent FMD measurements. Continuous variables are presented as mean (SD). Categorical variables are expressed as (%).
BMI, body mass index; FMD, flow-mediated dilation, HDL, high density lipoprotein, HRT, hormone replacement therapy; LDL, low-density lipoprotein, VO2 peak, peak oxygen consumption.
Fitness and body composition
Participants were sedentary with a VO2 peak of 1.3 (0.25) l/min. As shown in table 2, there was a significant improvement in VO2 peak with exercise training in the 8 kcal/kg/week (p=0.006) and 12 kcal/kg/week (p<0.001) exercise groups, but not in the 4 kcal/kg/week (p=0.191) group compared with control. In addition, there was no significant change in waist circumference, blood pressure, BMI or weight after exercise training in any of the exercise groups compared with control (all p values >0.05).
Table 2.
The effect of exercise training on fitness, anthropometric, brachial artery diameter and blood pressure
| Control | Exercise groups | |||
|---|---|---|---|---|
| 4 kcal/kg/week | 8 kcal/kg/week | 12 kcal/kg/week | ||
| VO2 peak (l/min) | −0.02 (−0.08 to 0.05) | 0.04 (0.00 to 0.07) | 0.11 (0.05 to 0.16)* | 0.17 (0.12 to 0.23)* |
| Systolic blood pressure (mm Hg) | 2.1 (−3.51 to 7.74) | 3.5 (0.26 to 6.71) | 0.5 (−4.23 to 5.19) | −0.4 (−5.12 to 4.37) |
| Diastolic blood pressure (mm Hg) | 2.6 (−0.72 to 5.83) | 2.7 (0.78 to 4.57) | 1.6 (−1.20 to 4.33) | 1.3 (−1.46 to 4.10) |
| Waist circumference (cm) | −2.7 (−5.41 to 0.00) | −3.4 (−4.94 to −1.76) | −2.8 (−5.14 to −0.48) | −3.9 (−6.15 to −1.56) |
| Weight (kg) | −0.9 (−2.08 to 0.25) | −0.5 (−1.13 to 0.20) | −1.3 (−2.28 to −0.35) | −1.4 (−2.40 to −0.47) |
| Brachial artery diameter (mm) | 0.02 (−0.12 to 0.16) | −0.10 (−0.18 to −0.02) | −0.03 (−0.15 to 0.08) | 0.01 (−0.11 to 0.12) |
Changes in fitness, anthropometric, brachial artery diameter and blood pressure following exercise training in the non-exercise control and 4, 8 and 12 kcal/kg/week groups. Values are expressed as the absolute mean change for each group from pre-intervention to postintervention, and are presented as least square means with 95% CIs.
p value < 0.05 compared with control.
BMI, body mass index, VO2 peak, maximal oxygen consumption.
Brachial artery diameter
The mean brachial artery diameter of all participants before the exercise training was 3.16 mm (0.5). There was no significant difference in brachial artery at baseline between groups (p>0.05). In addition, there was no significant change in brachial artery diameter after exercise training in either the exercise groups or the control (p>0.05).
Flow-mediated dilation
The mean FMD (%) (SD) of all participants before the exercise training was 4.13% (2.5). Baseline medication use did not significantly affect baseline FMD (all p values >0.05). FMD at baseline was significantly correlated with SBP (r=–0.24, p=0.003) and VO2 peak (r=0.18, p=0.02) but was not significantly associated with age, weight, BMI, triglycerides, diastolic blood pressure or waist circumference (all p values >0.05).
There was a significant absolute improvement in FMD in the 4 kcal/kg/week (1.0%, 95% CI: 0.29 to 1.76), 8 kcal/kg/week (1.5%, 95% CI: 0.48 to 2.62) and 12 kcal/kg/week (1.2%, 95% CI: 0.10 to 2.24) exercise groups compared with control (−0.5%, 95% CI: −1.79 to 0.74) as shown in figure 2. However, there were no significant differences in change in FMD between the three exercise groups (p>0.05). The correlation between change in FMD with exercise training and change in anthropometric, fitness and biomedical variables are summarised in table 3. Change in FMD after exercise training was significantly, but weakly, associated with change in waist circumference (r=–0.17, p=0.05). In addition, the correlation between change in diastolic blood pressure and change in FMD approached significance (r=–0.16, p=0.06).
Figure 2.
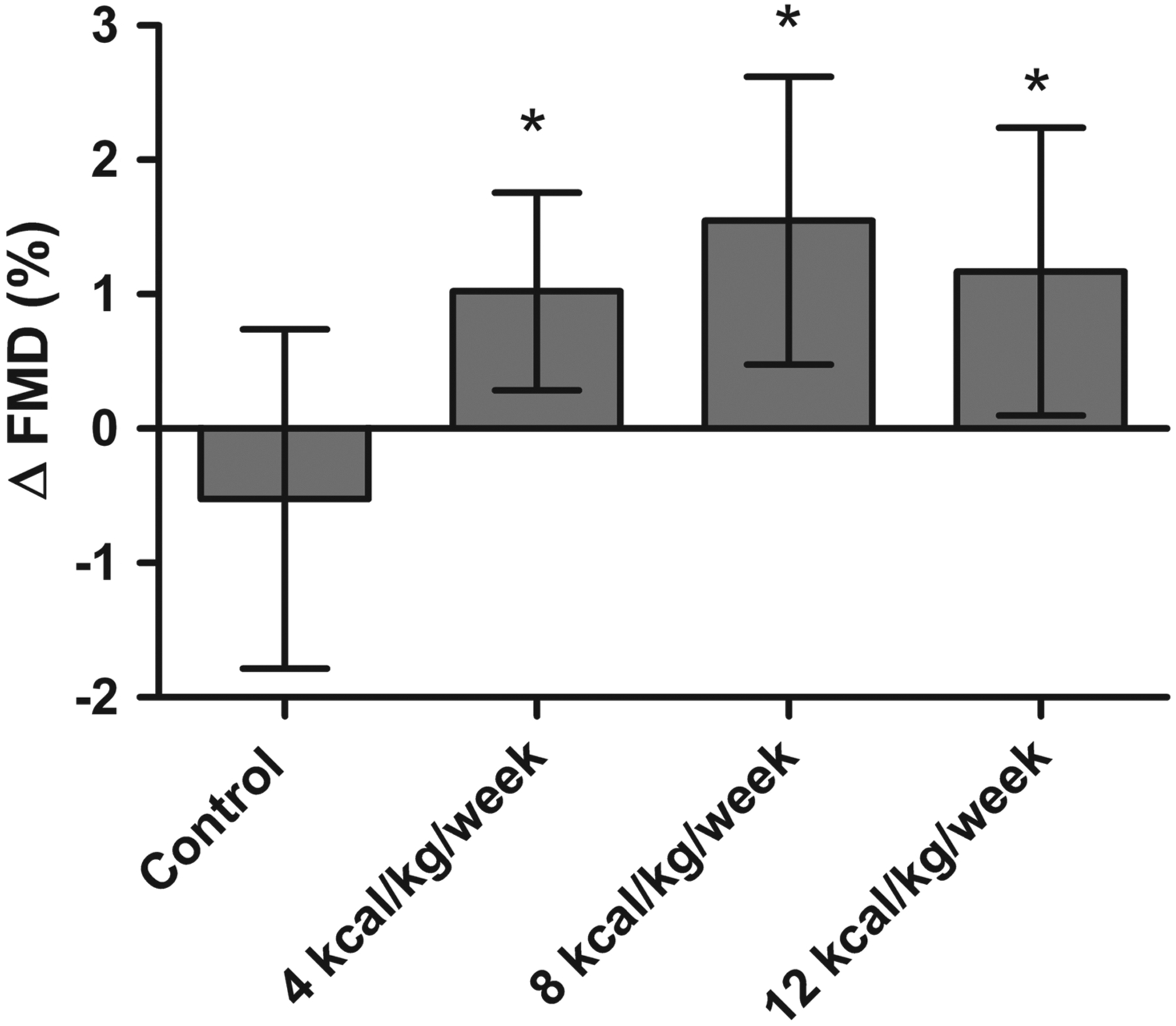
The effect of exercise training on endothelial function among different doses of exercise training. Shown are the absolute change in FMD in the non-exercise control group and the 4, 8 and 12 kcal/kg/week exercise groups. Change in FMD is expressed as the mean difference of the post-training FMD from the pretraining value in each group. A p value <0.05 was designated as the criterion for statistical significance. FMD, flow-mediated dilation.
Table 3.
The correlation of anthropometric, fitness and biomedical variables with change in FMD after exercise training
| Variable | r | p-value |
|---|---|---|
| Baseline FMD (%) | −0.35 | <0.001* |
| Age | −0.05 | 0.56 |
| Δ VO2 peak (l/min) | 0.02 | 0.80 |
| Δ Systolic blood pressure (mm Hg) | 0.04 | 0.61 |
| Δ Diastolic blood pressure (mm Hg) | −0.16 | 0.06 |
| Δ Waist circumference (cm) | −0.17 | 0.05* |
| Δ Weight (kg) | 0.03 | 0.76 |
| Δ Triglycerides (mg/dl) | −0.10 | 0.21 |
| Δ Low-density lipoprotein (mg/dl) | 0.02 | 0.81 |
| Δ High density lipoprotein (mg/dl) | −0.08 | 0.38 |
Spearman correlations for the change in anthropometric, fitness, and biomedical variables with change in FMD following exercise training in exercising subjects (n=132).
Indicates a significant correlation (p <0.05).
FMD, flow-mediated dilation.
In addition, we observed a significant absolute improvement in FMD (1.8%, 95% CI: 1.17 to 2.38; p<0.001) in subjects with endothelial dysfunction at baseline after exercise training, which was significantly greater (p<0.001) than the change in FMD in participants without endothelial dysfunction at baseline (−1.2%; 95% CI: −1.17 to 0.69; p=0.61) (figure 3).
Figure 3.
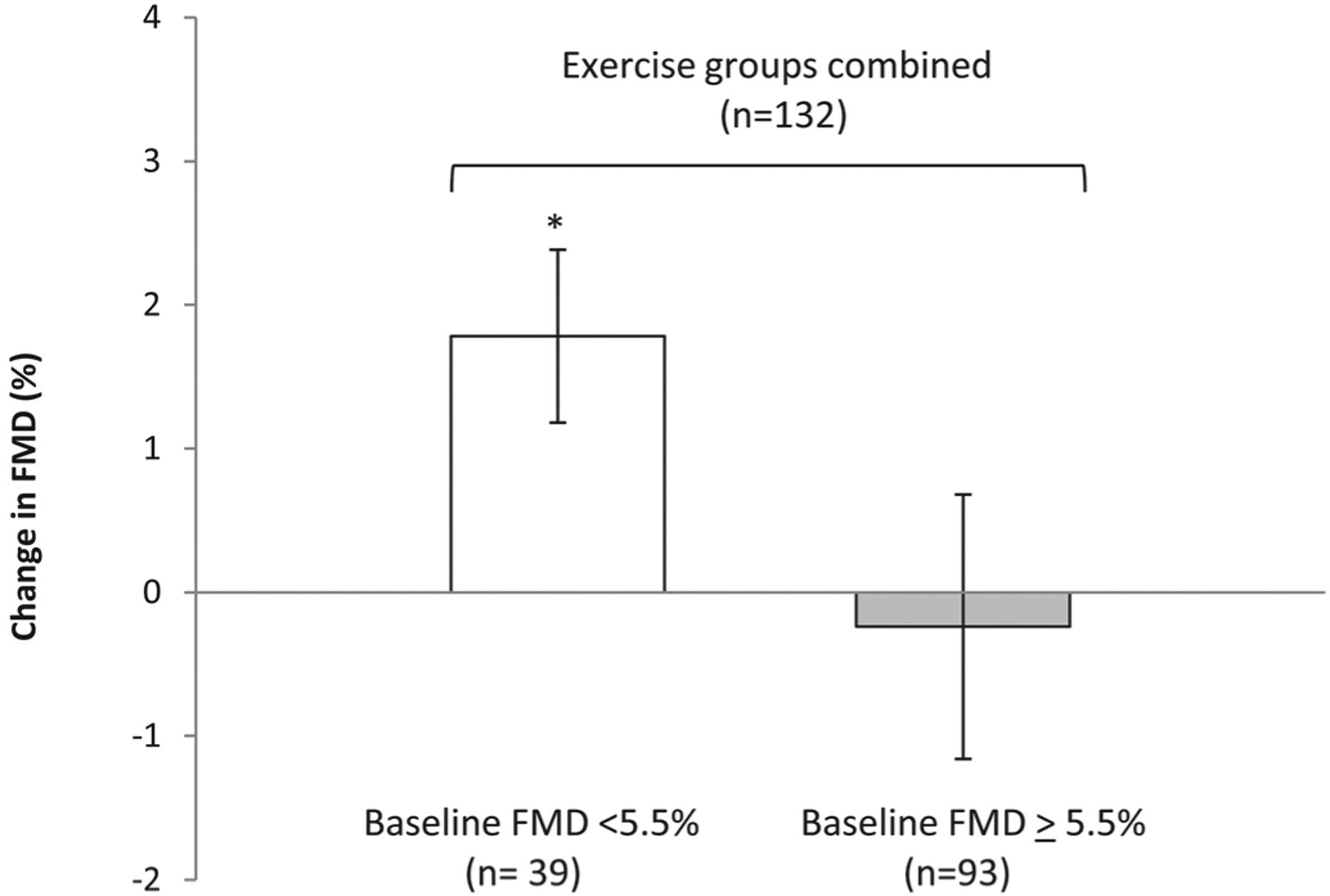
The effect of exercise training on exercising subjects (all exercise groups combined, n=132) with endothelial dysfunction (FMD <5.5%, n=93) and normal endothelial function (FMD ≥ 5.5%, n=39). Shown are the mean absolute change in FMD after exercise training in subjects with impaired FMD at baseline, normal FMD. Change in FMD is expressed as the mean difference between the post-training FMD and the pretraining value in each group. *Indicates significant difference (p<0.05) compared with exercisers with endothelial dysfunction. FMD, flow-mediated dilation.
DISCUSSION
The primary finding of this ancillary study is aerobic exercise training exerts a beneficial dose-independent effect on endothelial function in obese postmenopausal women with elevated blood pressure, as a higher dose of exercise training did not improve FMD to a greater extent than the lowest dose. The results of the present study suggest that moderate intensity aerobic exercise training may have cardioprotective benefits because improvements in endothelial function are associated with reduced risk of cardiovascular events in postmenopausal women.11 The DREW trial was designed to encompass a wide range of exercise doses because our participants were exercising at approximately 50%, 100% and 150% of the NIH consensus Panel physical activity recommendations; thus, it is likely that we captured doses of exercise that would be reasonable for postmenopausal women to perform. Because the present study found that increased exercise dose did not result in greater improvements in FMD, this may suggest that there is a relatively low threshold for increasing endothelial function for sedentary postmenopausal women with elevated blood pressure. In addition, the improvements in FMD after exercise training occurred independent of changes in SBP but may be related to reductions in waist circumference and possibly diastolic blood pressure. It is important to note that because FMD was evaluated at baseline and follow-up, we are unable to evaluate whether the rate of change in FMD was different between exercise groups, as a more rapid improvement in endothelial function may represent a more favourable response.
There are three published studies to our knowledge which have found no improvement in endothelial function in healthy postmenopausal women after aerobic exercise training.27–29 Casey et al27 found no significant change in FMD after 18 weeks of treadmill training in healthy subjects (n=10). Black et al28 found no significant change in FMD after 12 weeks, and approached significance (p=0.07) for improvement in FMD in sedentary postmenopausal women (n=6) after 24 weeks of combined treadmill and cycling exercise training (but found improvements in FMD/nitroglycerine-mediated dilation ratio). Recently, Pierce et al29 found no improvement in FMD in postmenopausal women with no CVD, but they found improvement in older men. Our results are in line with previous studies, because differences in improvements in endothelial function after exercise training are likely explained by the fact that our subjects had elevated blood pressure (which is associated with endothelial dysfunction),30 and presented with lower FMD values at baseline (4.1%) than the previous training studies in postmenopausal women already mentioned (Casey et al: 9.9%, Black et al: 5.3%, Pierce et al: 5.1%).27–29 In addition, subject populations with elevated blood pressure have shown improvement in FMD with aerobic exercise training.14,16,17
The lack of a dose response in FMD with exercise dose may be due to a ‘ceiling effect’ or a change in the rate of increase in FMD after exercise training by 6 months. It is also possible that vascular remodelling (structural adaptations to the conduit artery) with exercise training may also play a role, because it may normalise shear stress and reduce endothelium-dependent vasodilation. Tinken et al31 observed evidence of vascular remodelling during cycle ergometer exercise training in young healthy subjects because FMD increased after 2 and 4 weeks but was not significantly different from baseline at 6 or 8 weeks. In addition, the authors found that vasodilatory capacity (a surrogate measure of vascular remodelling) increased after each week of exercise. However, to our knowledge, a similar study has not been conducted in subjects with endothelial dysfunction. If vascular remodelling normalises the antegrade shear stress during training sessions, this may limit the improvement of FMD with increasing exercise dose.
The other major finding of the present study is that we observed a significant improvement in FMD in subjects with endothelial dysfunction, but no significant change in subjects with normal FMD before exercise training. Approximately, 70% of exercisers in the present study presented with endothelial dysfunction, which was expected because our participants had elevated blood pressure30 and were post-menopausal.5 Our results demonstrate that aerobic exercise will benefit individuals with greatest need for improving endothelial function. In addition, it suggests that some of the variation in the change in FMD with exercise training may be due to the presence of endothelial dysfunction at baseline. For sedentary individuals who present with normal FMD, exercise training may have a role in the maintenance of normal endothelial function. This may explain why there is generally a consistent improvement in endothelial function in individuals with CVD risk, and inconsistent results in apparently healthy subjects in response to exercise training.13
The main strengths of the current study are that DREW was a randomised controlled study with a large and diverse subject population as only 46% of our total population was Caucasian. However, there are several limitations. Our participants exercised at 50% of VO2 peak, so it is possible that other exercise intensities may produce different results. In addition, we did not measure endothelial independent dilation, so we cannot evaluate whether exercise dose affected smooth muscle function. Finally, because the data were collected before the regular use of duplex mode ultrasounds, FMD values were not corrected for shear area under the curve (AUC). However, there is evidence that shear stress normalisation may only be appropriate for young adults, as Thijssen et al32 observed no significant correlation between FMD and various methods of shear stress correction in older adults. In addition, FMD normalisation via shear AUC may violate several statistical assumptions33 and may even introduce errors into the measurement.32 Lastly, previous studies have observed significant relationships between FMD uncorrected for shear and CVD risk.11,34,35
In conclusion, we found that aerobic exercise training significantly improved FMD in previously sedentary postmenopausal women independent of exercise dose. However, we observed that only participants with endothelial dysfunction at baseline showed an improvement in FMD after the intervention, which may suggest that aerobic exercise maintains normal endothelial function and improves FMD in participants with endothelial dysfunction. Future research should investigate the rate of improvement of FMD with different doses of exercise, and the effect of different intensities of aerobic exercise training on endothelial function.
Acknowledgements
The authors thank The Cooper Institute Scientific Advisory Board and the DREW participants. In addition, they acknowledge the NIH T-32 postdoctoral fellowship (Obesity from Genes to man) awarded to Pennington Biomedical Research Center.
Funding This work was supported by National Institute of Health (grant number HL66262) and unrestricted research support from Coca-Cola.
Footnotes
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Schroeder S, Enderle MD, Ossen R, et al. Noninvasive determination of endothelium-mediated vasodilation as a screening test for coronary artery disease: pilot study to assess the predictive value in comparison with angina pectoris, exercise electrocardiography, and myocardial perfusion imaging. Am Heart J 1999;138:731–9. [DOI] [PubMed] [Google Scholar]
- 2.Gokce N, Keaney JF Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 2003;41:1769–75. [DOI] [PubMed] [Google Scholar]
- 3.Shechter M, Issachar A, Marai I, et al. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 2009;134:52–8. [DOI] [PubMed] [Google Scholar]
- 4.Lynch NA, Ryan AS, Berman DM, et al. Comparison of VO2max and disease risk factors between perimenopausal and postmenopausal women. Menopause 2002;9:456–62. [DOI] [PubMed] [Google Scholar]
- 5.Taddei S, Virdis A, Ghiadoni L, et al. Menopause is associated with endothelial dysfunction in women. Hypertension 1996;28:576–82. [DOI] [PubMed] [Google Scholar]
- 6.Walters JF, Hampton SM, Deanfield JE, et al. Circadian variation in endothelial function is attenuated in postmenopausal women. Maturitas 2006;54:294–303. [DOI] [PubMed] [Google Scholar]
- 7.Harvey PJ, Morris BL, Kubo T, et al. Hemodynamic after-effects of acute dynamic exercise in sedentary normotensive postmenopausal women. J Hypertens 2005;23:285–92. [DOI] [PubMed] [Google Scholar]
- 8.Poehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at menopause: a controlled longitudinal study. Ann Intern Med 1995;123:673–5. [DOI] [PubMed] [Google Scholar]
- 9.Cybulsky MI, Lichtman AH, Hajra L, et al. Leukocyte adhesion molecules in atherogenesis. Clin Chim Acta 1999;286:207–18. [DOI] [PubMed] [Google Scholar]
- 10.Rossi R, Chiurlia E, Nuzzo A, et al. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol 2004;44:1636–40. [DOI] [PubMed] [Google Scholar]
- 11.Rossi R, Nuzzo A, Origliani G, et al. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 2008;51:997–1002. [DOI] [PubMed] [Google Scholar]
- 12.Maiorana A, O’Driscoll G, Cheetham C, et al. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 2001;38:860–6. [DOI] [PubMed] [Google Scholar]
- 13.Green DJ, Maiorana A, O’Driscoll G, et al. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol (Lond) 2004;561:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavrencic A, Salobir BG, Keber I. Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Arterioscler Thromb Vasc Biol 2000;20:551–5. [DOI] [PubMed] [Google Scholar]
- 15.Vona M, Rossi A, Capodaglio P, et al. Impact of physical training and detraining on endothelium-dependent vasodilation in patients with recent acute myocardial infarction. Am Heart J 2004;147:1039–46. [DOI] [PubMed] [Google Scholar]
- 16.Westhoff TH, Franke N, Schmidt S, et al. Beta-blockers do not impair the cardiovascular benefits of endurance training in hypertensives. J Hum Hypertens 2007;21:486–93. [DOI] [PubMed] [Google Scholar]
- 17.Moriguchi J, Itoh H, Harada S, et al. Low frequency regular exercise improves flow-mediated dilatation of subjects with mild hypertension. Hypertens Res 2005;28:315–21. [DOI] [PubMed] [Google Scholar]
- 18.Morss GM, Jordan AN, Skinner JS, et al. Dose Response to Exercise in Women aged 45–75 yr (DREW): design and rationale. Med Sci Sports Exerc 2004;36:336–44. [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–65. [DOI] [PubMed] [Google Scholar]
- 20.National Institute of Health. Physical activity and cardiovascular health: NIH consensus development panel on physical activity and cardiovascular health. JAMA 1996;276;3:241–6. [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. Physical Activity and Health. A Report of the Surgeon General. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, 1996. [Google Scholar]
- 22.Kitta Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol 2009;53:323–30. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care 2009;32:810–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brevetti G, Martone VD, de Cristofaro T, et al. High levels of adhesion molecules are associated with impaired endothelium-dependent vasodilation in patients with peripheral arterial disease. Thromb Haemost 2001;85:63–6. [PubMed] [Google Scholar]
- 25.Sfikakis PP, Papamichael C, Stamatelopoulos KS, et al. Improvement of vascular endothelial function using the oral endothelin receptor antagonist bosentan in patients with systemic sclerosis. Arthritis Rheum 2007;56:1985–93. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Guo F, Li G, et al. Prognostic role of brachial reactivity in patients with ST myocardial infarction after percutaneous coronary intervention. Coron Artery Dis 2009;20:467–72. [DOI] [PubMed] [Google Scholar]
- 27.Casey DP, Pierce GL, Howe KS, et al. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol 2007;100:403–8. [DOI] [PubMed] [Google Scholar]
- 28.Black MA, Cable NT, Thijssen DH, et al. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 2009;297:H1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierce GL, Eskurza I, Walker AE, et al. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci 2010;120:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JB, Charbonneau F, Schiffrin EL. Correlation of endothelial function in large and small arteries in human essential hypertension. J Hypertens 2001;19:415–20. [DOI] [PubMed] [Google Scholar]
- 31.Tinken TM, Thijssen DH, Black MA, et al. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol (Lond) 2008;586:5003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thijssen DH, Bullens LM, van Bemmel MM, et al. Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. Am J Physiol Heart Circ Physiol 2009;296:H57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris RA, Nishiyama SK, Wray DW, et al. Ultrasound assessment of flow-mediated dilation. Hypertension 2010;55:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation 2004;109:613–19. [DOI] [PubMed] [Google Scholar]
- 35.Yeboah J, Crouse JR, Hsu FC, et al. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 2007;115:2390–7. [DOI] [PubMed] [Google Scholar]


