Abstract
Pathogenic variants in pre-messenger RNA (pre-mRNA) splicing factor 31, PRPF31, are the second most common genetic cause of autosomal dominant retinitis pigmentosa (adRP) in most populations. This remains a completely untreatable and incurable form of blindness, and it can be difficult to predict the clinical course of disease. In order to design appropriate targeted therapies, a thorough understanding of the genetics and molecular mechanism of this disease is required. Here, we present the structure of the PRPF31 gene and PRPF31 protein, current understanding of PRPF31 protein function and the full spectrum of all reported clinically relevant variants in PRPF31. We delineate the correlation between specific PRPF31 genotype and RP phenotype, suggesting that, except in cases of complete gene deletion or large-scale deletions, dominant negative effects contribute to phenotype as well as haploinsufficiency. This has important impacts on design of targeted therapies, particularly the feasibility of gene augmentation as a broad approach for treatment of PRPF31-associated RP. We discuss other opportunities for therapy, including antisense oligonucleotide therapy and gene-independent approaches and offer future perspectives on treatment of this form of RP.
Highlights
-
•
PRPF31 is the second most common cause of autosomal dominant retinitis pigmentosa and a potential target for gene therapy.
-
•
We present all reported pathogenic variants in PRPF31 as a resource for clinicians, diagnostic genetics labs, and researchers.
-
•
Genotype-phenotype correlations suggest that, dominant negative effects contribute to disease in addition to haploinsufficiency.
-
•
This finding has important impacts on the suitability of gene augmentation approaches across all mutation types.
-
•
This finding may aid prognosis of disease in PRPF31-associated RP patients.
1. Introduction
1.1. Pre-mRNA splicing
Human pre-mRNA splicing factor 31 (PRPF31) is a component of the spliceosome, the huge macromolecular ribonucleoprotein (RNP) complex which catalyses the splicing of pre-messenger RNAs (pre-mRNAs) to remove introns and produce mature mRNAs(Will and Luhrmann, 2011).
Pre-mRNA splicing is a core function in all eukaryotic cells. The vast majority of genes have multiple exons and introns, and around 95% of these multiexon genes undergo alternative splicing(Pan et al., 2008). Alternative splicing allows increased organism complexity without increasing genome size, and helps to explain the c-value paradox; the observation that phenotypic complexity in the eukaryotic domain is not proportional to genome size.
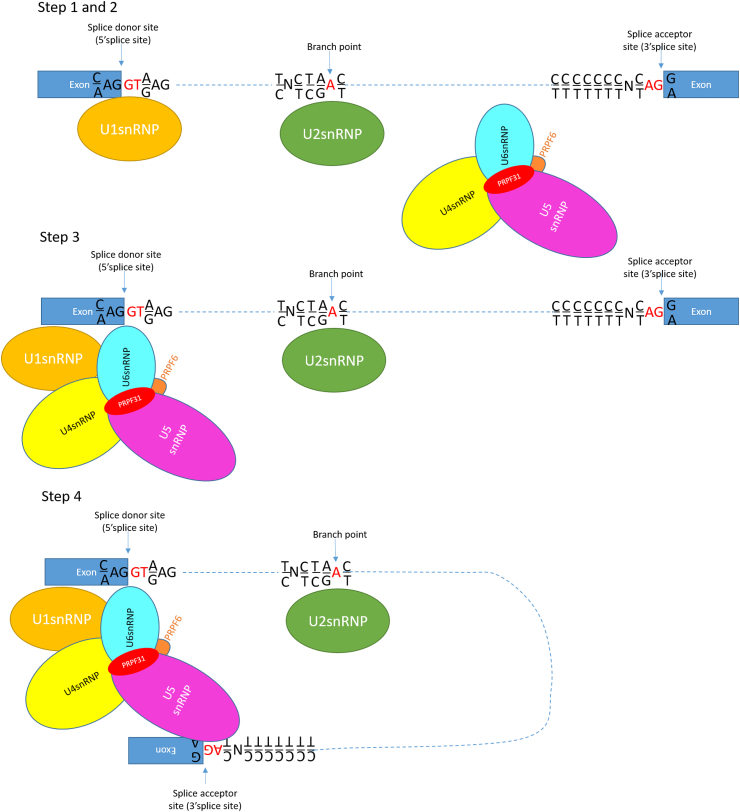
The spliceosome is composed of 5 small nuclear RNAs (snRNAs), U1–U5, and many proteins, together making 5 snRNPs. In the process of splicing, U1snRNP recognises and binds the splice donor site (the 5’ splice site), and promotes the binding of U2snRNP to the branch site. Independently of this, the U4/U6.U5 tri-snRNP forms in the cell, and is recruited to the pre-mRNA, where U6snRNP replaces U1snRNP. This forms the catalytically active spliceosome, which cuts away the intron and joins the exons through two transesterification reactions (Fig. 1).
Fig. 1.
Schematic representation of the first four steps of pre-mRNA splicing by the major spliceosome, with PRPF31 shown in red. In step 1, U1snRNP recognises and binds the splice donor site (the 5′ splice site). In step 2, binding of U1snRNP to the splice donor site promotes the binding of U2snRNP to the branch site. Independently of this, the U4/U6.U5 tri-snRNP forms in the cell. In step 3, the U4/U6.U5 tri-snRNP is recruited to the pre-mRNA, where U6snRNP replaces U1snRNP. This forms the catalytically active spliceosome, which in step 4 cuts away the intron and joins the exons through two transesterification reactions. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
1.2. PRPF31, splicing and retinal disease
The S. cerevisiae yeast homologue of PRPF31, Prp31, was cloned and identified as a key splicing factor in 1996 (Weidenhammer et al., 1996), and later was shown to be essential for the association of the U4/U6.U5 tri-snRNP with pre-spliceosomes(Weidenhammer et al., 1997). It was subsequently found to play a role in both splicing and meiosis in S. pombe(Bishop et al., 2000). Unexpectedly, in 2001, it was discovered that heterozygous pathogenic variants in PRPF31 are associated with retinitis pigmentosa (RP), an inherited retinal dystrophy affecting 1:2000 to 1:3500 people worldwide(Vithana et al., 2001). This was surprising because pre-mRNA splicing factors are highly conserved from yeast to man with a core function in all cells. Intuitively, it would be expected that a defect in a core spliceosomal protein should have an impact on all cells, not just retinal cells.
The original paper described seven different pathogenic variants in four families and three simplex cases. These included mutations in the region of the splice site, leading to inactivation of a splice acceptor site, inactivation of a splice donor site, two missense changes, three frameshift variants and an in-frame duplication(Vithana et al., 2001).
Since then, and particularly since the advent of massively parallel sequencing technologies, it has become clear that pathogenic variants in PRPF31 are a major cause of autosomal dominant RP (adRP). Indeed they are the second most common genetic cause of adRP in most populations, accounting for 6% of US cases (Sullivan et al., 2013), 8% of Spanish, French and French-Canadian cases (Martin-Merida et al., 2018; Audo et al., 2010; Coussa et al., 2015), 8.9% of cases in North America (Daiger et al., 2014), 10–11.1% of Chinese cases (Lim et al., 2009; Xu et al., 2012) and 10.5% of Belgian cases(Van Cauwenbergh et al., 2017).
However, this is likely to be an underestimate due to non-penetrance of this form of RP (Rose and Bhattacharya, 2016). It is common to see very variable severity of eye disease in different members of the same family with the same pathogenic PRPF31 variant. Furthermore, obligate carriers may be totally asymptomatic, showing complete non-penetrance. This complicates attempts to co-segregate PRPF31 variants with clinical disease and makes genetic diagnosis difficult, likely contributing to an underestimation of the prevalence of RP associated with PRPF31 variants.
The genetic mechanism controlling incomplete penetrance remains unclear, but a fairly consistent observation of correlation between expression level of the non-mutant copy of PRPF31 and disease severity has been reported.(Rio Frio et al. 2008b, 2009; Rivolta et al., 2006).
This varied expression can be explained by a number of factors including:
-
-
expression quantitative trait loci (eQTLs) (on ch.14q21-23) in trans with PRPF31(Rio Frio et al., 2008a)
-
-
variable level of expression of CNOT3, a trans-acting epistatic factor which is genetically linked to PRPF31 and regulates expression of PRPF31. CNOT3 encodes a subunit of the Ccr4-not transcription complex, which binds to the promoter of PRPF31 and represses transcription of PRPF31. An intronic variant in CNOT3 determines its level of expression and thus how efficiently PRPF31 expression is downregulated. The alleles of CNOT3 inherited determine the expression of non-mutant PRPF31 and thus whether a person will be affected by the disease(Venturini et al., 2012; Rose et al., 2014).
-
-
the number of minisatellite repeat elements (MSR1) adjacent to the PRPF31 core promoter, which determines the level of transcriptional repression of the non-mutant PRPF31.4 MSR1 copies are associated with higher non-mutant PRPF31 expression and are found in non-symptomatic carriers only(Rose et al., 2016).
On the basis of these observations, the mechanism of incomplete penetrance in this form of RP has been described as ‘variant haploinsufficiency’, in which the absence of a second wild-type PRPF31 allele is sometimes sufficient to produce disease, and sometimes is not, depending on the nature of the mutant allele inherited and the nature of the wild-type allele inherited. So the severity of the resultant disease depends on both the type of mutant allele inherited (ie complete loss-of-function, gain-of-function or hypomorphic), the level at which this allele is expressed, and the level at which the wild-type allele is expressed (Rose and Bhattacharya, 2016). This form of variant haploinsufficiency has only been described in a very few Mendelian disorders, making the mechanism of variable penetrance in this disease quite unique (Rose and Bhattacharya, 2016).
1.3. PRPF31 gene and PRPF31 protein structure
PRPF31 is a 16.3 kb gene on chromosome 19 which encodes 9 different transcripts, 6 of which are protein coding. The largest, most widely expressed transcript consists of 14 exons; 1 non-coding and 13 coding, which produces a 499 amino acid protein of 55 kDa in size, pre-mRNA splicing factor 31, PRPF31.
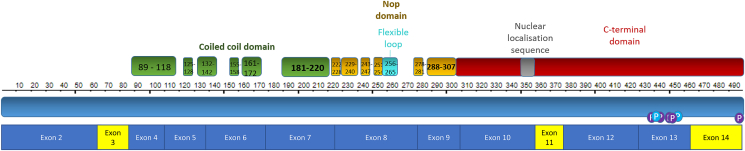
PRPF31 contains several important functional domains; the flexible loop, Nop domain, coiled-coil domain and tip. Recent advances in spectroscopy and microscopy methods such as NMR and cryo-electron microscopy have allowed accurate resolution of the crystal structure of proteins of the spliceosome, including PRPF31, in their native conformations at different points during splicing(Agafonov et al., 2016; Bertram et al. 2017a, 2017b; Haselbach et al., 2018). These studies have revealed that PRPF31 contains a conserved Nop domain (residues 222–254 and 278–307), with regions for binding protein and RNA(Liu et al., 2007). This Nop domain has relaxed sequence conservation in PRPF31, but it retains high specificity for binding U4 or U4atac and 15.5K protein (Liu et al., 2007). The flexible loop (residues 256–265) protects the exposed C4’ atoms of residues 37 and 38 from attack by free radicals, to protect the RNA without directly contacting it(Liu et al., 2007). The protein also has several phosphorylation sites, clustered in the C-terminus(Liu et al., 2007). PRPF31 contains a nuclear localisation sequence, NLS, which allows it to be targeted to the nucleus after translation (Fig. 2).
Fig. 2.
Schematic representation of the protein and cDNA structure of PRPF31, showing major structural domains encoded by each exon.
1.4. PRPF31 protein function
PRPF31 is required for tri-snRNP assembly in human cells(Makarova et al., 2002). With PRFP6, PRPF31 forms an essential connection between the U4/U6 and U5 snRNPs. siRNA knockdown of PRPF31 results in inhibition of tri-snRNP formation and nuclear accumulation of U5 mono-snRNPs and U4/U6 di-snRNPs containing U4/U6 proteins and the U4/U6 recycling factor p110(Schaffert et al., 2004).
The specific function of PRPF31 in retinal cells remains less clear. It remains unclear whether the photoreceptor cells are the primary affected cells in RP associated with PRPF31, with a number of studies suggesting that the RPE is the primary affected tissue(Farkas et al., 2014; Hamieh and Nandrot, 2019; Valdés-Sánchez et al., 2019). Retinal cells are highly metabolically active, with a high demand for ATP and protein anabolism as around 10% of protein from photoreceptor outer segments is shed every day. Rates of metabolism in photoreceptors are similar to dividing tumour cells, and undergo extensive anaerobic glycolysis rather than oxidative phosphorylation to produce energy, in what is termed the ‘Warburg effect’(Ng et al., 2015; Rajala et al., 2016). The reliance on glycolysis seems to promote efficient protein anabolism in photoreceptors(Chinchore et al., 2017). However, the photoreceptors still require mitochondria to produce a proportion of their ATP via oxidative phosphorylation(Grenell et al., 2019). It has been postulated that photoreceptor cells have a greater demand for pre-mRNA splicing factors to meet this metabolic demand, but evidence to support this hypothesis is inconsistent. Some studies have reported higher levels of PRPF31 expression in retina than in other tissues (Cao et al., 2011) but other studies show a consistent level of expression in all tissues, with no significantly higher expression in retina or any other tissue(Yuan et al., 2005).
Related to this elevated rate of oxidative phosphorylation, retinal cells are subject to much higher rates of oxidative damage, including UV-induced photooxidative damage, which may explain the retinal-specific phenotype of RP associated with pre-mRNA splicing factor mutations.(Comitato et al., 2007; Shinde et al., 2016; Jin et al., 2011; Schmidt-Kastner et al., 2008). In patients expressing mutant forms of pre-mRNA splicing factors, it has been shown that proteins have reduced solubility, which can lead to formation of protein aggregates, and it has been suggested that the environment of UV-induced photooxidative damage in the photoreceptors makes these cells specifically prone to degeneration(Wheway et al., 2019; Valdés-Sánchez et al., 2019; Wilkie et al., 2006; Yin et al., 2011; Bryant et al., 2019). This splicing-independent disease mechanism is appealing because there is inconsistent evidence of splicing defects in cells carrying PRPF31 mutations. Studies seem to suggest that expression of mutant PRPF31 affects splicing of some transcripts but not others.
Immunoprecipitation of splicing complexes from PRPF31 mutant retinal cells showed that mutant PRPF31 proteins significantly inhibited pre-mRNA splicing of intron 3 in the rhodopsin (RHO) gene(Yuan et al., 2005). In primary retinal cell cultures, expression of the mutant PRPF31 proteins reduced total RHO expression and caused apoptosis of rhodopsin-positive retinal cells(Alagramam et al., 2001). In a study of patient lymphoblastoid cell lines, splicing efficiency of RPGR intron 9 was significantly decreased in PRPF31 mutant cell lines but no consistent decrease in the splicing efficiency of U12 and noncanonical U2 introns was seen in PRPF31 mutant cells(Ivings et al., 2008). In a minigene study, assays using the RHO intron 3 minigene template revealed a direct negative effect on splicing efficiency of mutant PRPF31. However, no effect of the mutation on splicing efficiency could be detected using the longer GNAT1 minigene template or using a full-length RHO transcript, splicing of which had an efficiency of 100%. Similarly, no unspliced RHO transcripts could be detected in RNA from human retina(Wilkie et al., 2008).
Using novel stem cell technologies, recent studies in retinal organoids and retinal pigment epithelium (RPE) derived from induced pluripotent stem cells (iPSCs) from patients with PRPF31 mutations show decreased efficiency of splicing of E1A minigene(Buskin et al., 2018). RPE from patient iPSCs also show a substantial downregulation of SART1, a U5 snRNP protein important for the formation of the pre-catalytic spliceosome, but no changes in the expression of the U5 protein PRPF8 or the U4/U6 protein PRPF4(Buskin et al., 2018). In both RPE and retinal organoids derived from PRPF31 patients, the most significantly mis-spliced genes were genes involved in pre-mRNA and alternative mRNA splicing via the spliceosome(Buskin et al., 2018).
Alongside these findings, it was observed that retinal organoids from patients showed differential expression of actin cytoskeleton, ciliary membrane, primary cilium, photoreceptor inner and outer segment, axon terminal and phototransduction proteins. Furthermore, patient organoids showed an enrichment of mis-spliced centriole and microtubule organisation genes. This suggests that centriole and ciliogenesis and cilium function are all regulated by alternative splicing in the retina, and this is defective in patients carrying PRPF31 mutations(Buskin et al., 2018). These findings were confirmed in independent studies of splicing in PRPF31 siRNA-treated human organotypic retinal cultures(Azizzadeh Pormehr et al., 2019). This is in keeping with earlier studies from ourselves, and others, which showed that siRNA knockdown of pre-mRNA splicing factors including PRPF31 has a specific and significant effect on ciliogenesis(Wheway et al., 2015; Kim et al., 2016). Further investigation showed that these proteins localise to the base of the photoreceptor cilium, classifying these conditions as retinal ciliopathies(Wheway et al., 2015). Recent work developing PRPF31 gene augmentation therapy has shown rescue of ciliogenesis in PRPF31 ± RPE cells derived from human patient iPSCs after expression of wild-type PRPF31 delivered by an AAV vector, further suggesting that PRPF31 plays a key role in regulating ciliogenesis in patients(Brydon et al., 2019).
Further work is needed to understand the nature of the splicing factors’ involvement in ciliogenesis and cilium function in the retina, and this work is ongoing. It is possible that PRPF31 and other splicing factors have roles beyond splicing. Many proteins involved in splicing have multiple functions in the cell, such as the proteins of the PRP19 complex which have roles in ubiquitination (Vander Kooi et al., 2006), in DNA damage sensing (Grey et al., 1996; Marechal et al., 2014), DNA damage repair (Zhang et al., 2005), mRNA export (Chanarat et al., 2011) and in mitotic spindle assembly (Hofmann et al., 2013). PRPF31 has been shown to perform splicing-independent functions in mitotic chromosome segregation, although this would not explain disease phenotype in the post-mitotic retina. With deeper understanding of the molecular mechanism of PRPF31 disease arise greater opportunities for developing effective targeted therapies.
1.5. PRPF31 mutation spectrum
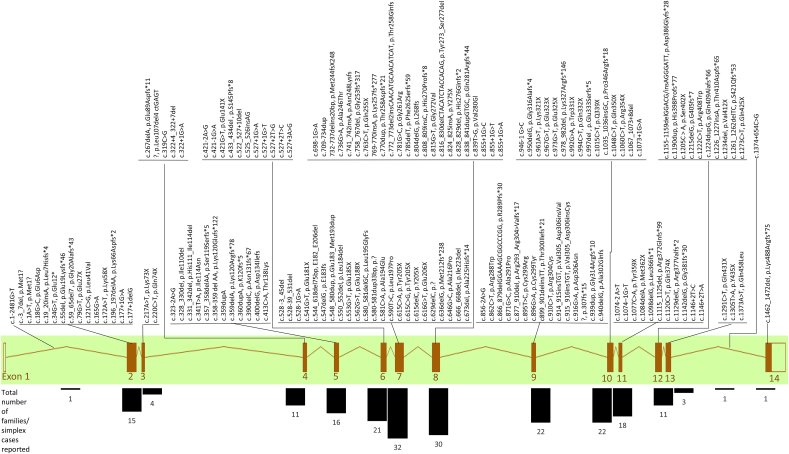
In order to fully understand the molecular mechanism of RP associated with PRPF31 variants, it is necessary to fully understand the genetics of this condition. This will aid accurate diagnostics, prognostics and development of targeted therapies. To this end, we have reviewed the literature and the major clinical variant database ClinVar to summarise all reported pathogenic variants in PRPF31 (Table 1). Mutations are spread throughout the gene, but are most common in exons 6–10, particularly exons 7 and 8 (Fig. 3).
Table 1.
All reported pathogenic variants in PRPF31 associated with adRP, from peer-reviewed publications and clinical variant database ClinVar (variants classified as pathogenic only). The location in cDNA, nature of the variant and impact on protein (if known) is included, alongside age of onset and age at diagnosis, where reported.
Fig. 3.
Schematic representation PRPF31 gene, with all reported pathogenic variants labelled above, and total numbers of variants in each intron and exon displayed as a bar chart below. This shows that exons 7 and 8 are most enriched for pathogenic variants.
The majority of reported mutations in PRPF31 are presumed loss-of-function variants including frameshift (51 different variants reported in 70 different families), splice site (30 variants in 52 families), nonsense (30 variants in 40 families) or large-scale insertions or deletions (25 variants in 32 families), which are predicted to lead to complete loss of expression of protein from the affected allele. PRPF31 is highly intolerant to loss-of-function with a probability of being loss-of-function intolerant (pLI) score of 0.98 (Lek et al., 2016). A pLI score of >0.9 indicates that a gene is intolerant of protein-truncating variation (Lek et al., 2016) and thus loss-of-function variants in PRPF31 are highly likely to cause disease through a haploinsufficiency disease mechanism (discussed in more detail later). However, it is important to note that whilst frameshift, consensus splice site, nonsense and large indel variants are often assumed to cause loss-of-function, this is not always the case, particularly when frameshift or nonsense variants are found in the final exon or C-terminal portion of the penultimate exon; transcripts from genes with such variants are likely to evade nonsense mediated decay (Ziegler et al., 2019). At least 3 frameshift or nonsense mutations in the final two exons of PRPF31 have been reported as pathogenic, but functional study is required to confirm pathogenicity (Martin-Merida et al., 2018; Huang et al., 2015). Similarly, consensus splice site mutations are often also assumed to cause complete loss of wild-type protein expression from the affected allele, when in fact the complex mechanisms of alternative splicing may lead to production of a truncated protein, particularly if the splicing change produces an in-frame transcript. In several cases where mutations are assumed to be causing loss-of-function through haploinsufficiency, in addition to presumed loss-of-function variants, at least 19 missense variants have been reported in PRPF31 as being pathogenic. Gene constraint metrics, which provide quantitative measures of the extent to which a gene can tolerate change, indicate that PRPF31 gene is highly intolerant to missense variants (Z = 3.27) (Samocha et al., 2014; Lek et al., 2016). Missense mutations in PRPF31 tend to reduce the solubility of protein so it does not translocate into nucleus efficiently after being translated in the cytoplasm (Deery et al., 2002; Bryant et al., 2019; Wheway et al., 2019), effectively leading to a loss of this protein. However, only 4 missense variants have been functionally studied in vitro, and a comprehensive study of reported missense variants is required to confirm the functional effect of pathogenic variants, and indeed the pathogenicity of reported variants. At least one variant originally described as a missense variant was later confirmed to be affecting splicing (Rio Frio et al., 2008b) and it is possible that other variants classified as missense, both recognised pathogenic and those currently considered non-pathogenic, may in fact be impacting upon splicing of PRPF31. Furthermore, non-synonymous rare variants may impact on splicing. It is therefore likely that the rate of pathogenic variants affecting splicing in PRPF31 is underestimated.
1.6. Genotype-phenotype correlation
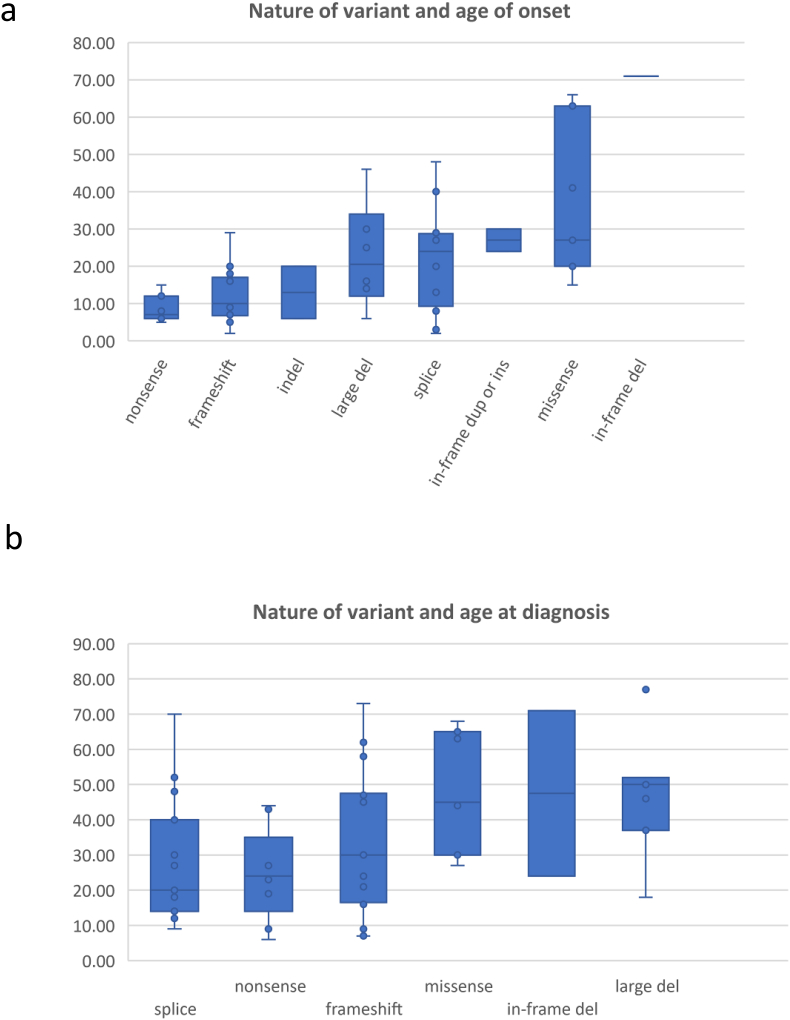
We reviewed the literature and recorded the age of onset of first symptoms, and age of diagnosis, where it was reported alongside specific genetic variants. Age of onset of first symptoms (usually night-blindness) is lowest in patients with nonsense, frameshift or indel variants, with median age of onset between 8 and 12 years of age. Patients with large deletions or splice variants tend to show first symptoms at a slightly later median age of 20–24. Patients with in-frame duplications, insertions or missense variants show the latest median age of onset of first symptoms, around 27 years of age (Fig. 4a). The difference in age of onset between the different types of mutation is statistically significant (one-way ANOVA p = 5.76 × 10−5).
Fig. 4.
(a) Box and whisker plots showing upper and lower limits, median and interquartile range of reported age of onset of RP patients with different types of variant in PRPF31 (b) Box and whisker plots showing upper and lower limits, median and interquartile range of reported age of diagnosis of RP patients with different types of variant in PRPF31.
We also recorded the age of diagnosis where it was reported alongside specific PRPF31 genetic variants. In this case, patients with nonsense, frameshift or splice variants were diagnosed at a median age of 20–30 years (usually because of loss of peripheral vision alongside night blindness), whereas patients with missense variants, in-frame deletions or large deletions tended to be diagnosed between the ages of 45 and 50 (Fig. 4b). The difference in age of diagnosis between the different types of mutation is statistically significant (one-way ANOVA p = 0.030).
There is no significant correlation between location of the variant in the gene and age of onset of symptoms or age of diagnosis.
It is an interesting observation, made in several studies and confirmed here, that patients with large-scale deletions, including multi-exon and whole gene deletions have the latest age of diagnosis. There is a clear difference in age of diagnosis of patients with large-scale deletions compared to patients with nonsense mutations or splice mutations although this is not statistically significant after correction for multiple testing (two-tailed unpaired t-test p = 0.016 and p = 0.032 respectively, p = 0.24 and p = 0.48 respectively after Bonferroni correction) (Fig. 4b). It could be postulated that there is an element of dominant negative effect at play in cases of nonsense, frameshift, indel, in-frame and missense variants compared to large deletions. This is a feature of the disease which should be considered when designing targeted therapies. The abundance of loss-of-function mutations, including complete gene deletions, in PRPF31 patients has led to a consensus view that haploinsufficiency is the disease mechanism in this form of RP(Abu-Safieh et al., 2006; Rio Frio et al., 2008b; Rose and Bhattacharya, 2016). This has influenced approaches for targeted therapies, namely gene augmentation approaches, which involve replacing a wild-type copy of the coding sequence of PRPF31 into the subretinal space of patients. This may not be fully effective in patients with genetic variants which have a dominant negative effect as well as a haploinsufficiency effect, and as a result other approaches for treatment may need to be investigated. These findings are supported by other recent work which also proposes a combined haploinsufficiency and dominant-negative disease mechanism in disease associated with PRPF31 mutations(Valdés-Sánchez et al., 2019). Study of the Prpf31p.A216P/+ mouse has shown that heterozygous missense mutations in Prpf31 lead to aggregation of both wild-type and mutant protein in the cytoplasm of the RPE cells of mice, leading to overexpression of HSP70 family proteins(Valdés-Sánchez et al., 2019). This work suggests that over-expression of these HSP70 proteins may be a target for therapy in PRPF31 patients, rather than targeting PRPF31 itself(Valdés-Sánchez et al., 2019).
2. Opportunities for therapies
2.1. Gene augmentation therapy
As a result of the abundance of loss-of-function variants in PRPF31 gene augmentation has been postulated as a potential therapeutic approach to treat this form of RP(Hafler et al., 2016). The coding sequence of PRPF31 is only 1.5 kb, well within the limits of current gene therapy vectors, and a PRPF31 heterozygous knockout mouse is available for study, although it only develops very late onset retinal degeneration which may be more characteristic of age-related macular degeneration than RP(Farkas et al., 2014). Researchers have begun preparatory work to define pre-treatment characteristics of RP associated with PRPF31 mutations in order to be able to assess the effectiveness of AAV-mediated PRPF31 gene augmentation therapy(Hafler et al., 2016). These researchers have also patented PRPF31 gene therapy by AAV2 delivery (International Publication Number WO2016144892A1) and, shown rescue of key cellular disease phenotypes including phagocytosis, ciliogenesis, cell morphology and barrier function in mutant PRPF31+/− RPE derived from patient iPSCs after deliver of PRPF31(Brydon et al., 2019).
2.2. Antisense oligonucleotide therapy
If the majority of genetic variants have some dominant negative effect, it is important to consider other potential therapeutic approaches. These include antisense oligonucleotides (ASOs) which can bind pre-mRNA or mRNA and modulate splicing of PRPF31 pre-mRNA or inhibit translation of the mRNA. In addition, siRNAs, shRNAs or gapmer-style ASOs can be used to completely silence a gene, which when combined with gene augmentation could potentially correct a disease with dominant negative effects. This approach has been successfully applied to the treatment of RP associated with dominant negative RHO mutations(Cideciyan et al., 2018). Splice-switching ASOs can be used to bind and mask deep intronic variants which introduce novel splice sites (such as the deep intronic variant in intron 13 reported in Rio Frio et al. (2009)(Rio Frio et al., 2009). Alternatively, they can be used to induce exon skipping of an in-frame exon (ie an exon with a multiple of 3 base pairs) carrying a frameshift or null variant, in order to remove this variant and restore the reading frame. Three of the fourteen exons in PRPF31 have multiples of 3 base pairs; exons 3, 11 and 14 (Fig. 2, Fig. 3). These are also relatively small exons, and do not encode functional important domains of the protein (Fig. 2) so they could be targeted for skipping without removing large or functionally important regions of the protein. This could have the effect of reverting a severe, early-onset frameshift or nonsense variant into a less severe splice or in-frame deletion variant, although the exon skipping would affect both alleles, mutant and normal, so the effect may be like having a homozygous exon deletion. According to the genotype-phenotype data in this study, this could delay age of onset from 8 to 10 years of age to 25 years of age or later. If this exon skipping approach led to a disease more like in-frame deletions, this could delay age of diagnosis (taken as a proxy for loss of peripheral vision) from 25 to 30 years of age to 47 years of age. This could potentially preserve vision in the working age of these individuals. This is a promising approach in theory, and such drugs are already being developed for a range of previously untreatable genetic conditions.(Scoles and Pulst, 2019; Levin, 2019; Khan et al., 2019). A clinically available splice-switching ASO drug (nusinersen) based on 2′O-methoxyethyl phosphorothioate chemistry has been successfully developed for the treatment of the neurodegenerative disease spinal muscular atrophy (approved by NICE) and a similar type of drug (eteplirsen) utilising phosphorodiamidate morpholino chemistry has been developed for treatment of certain forms of Duchenne muscular dystrophy(Finkel et al., 2017; Mendell et al., 2016). Intraocularly delivered ASO drugs are also currently undergoing clinical trials for a specific form of Leber congenital amaurosis caused by a CEP290 deep intronic mutation (ClinicalTrials.gov NCT03140969). ASOs are highly versatile drugs, being sequence-specific in their action, titratable in dosage, and in the setting of a well-defined and enclosed target organ such as the eye, straightforward to deliver by direct intravitreal or subretinal injection. However, there are limited numbers of affected individuals who could be treated by targeting these regions of PRPF31 (around 27 families).
2.3. Gene independent approaches
As RP associated with PRPF31 is so genetically diverse, (172 different reported variants in 240 different families or simplex cases) gene independent approaches are extremely attractive alternatives to gene therapies. These include stem cell therapies and bionic retinal implants. Stem cell therapies are both gene and disease-agnostic, and can replace lost retinal cells, whereas gene therapies can only recover function of intact cells. Stem cell therapies are closest to being effective in replacement of the retinal pigment epithelium (RPE), which has no neural connection. It is more challenging to regenerate functional neural retina. Recent studies have shown promising results in stem cell replacement of RPE for treatment of age-related macular degeneration(da Cruz et al., 2018; Kashani et al., 2018). Bioinic retinae such as the Argus II(Finn et al., 2018) are able to restore limited light and shape perception in people with end-stage retinal disease and limited to no remaining retinal function.
3. Conclusions and future perspective
Gene therapy offers real potential for treatment of a range of currently untreatable inherited retinal degenerations. As the second most common cause of adRP, and a relatively small gene, PRPF31 is becoming a focus for gene augmentation therapy(Brydon et al., 2019). This approach assumes a disease mechanism of haploinsufficiency, of which there is considerable evidence. However, new data presented here supports the recently proposed theory that, except in cases of complete exon or gene deletion, dominant negative effects may contribute to disease progression in RP associated with PRPF31 variants (Valdés-Sánchez et al., 2019), and that gene augmentation therapy may not be as effective in patients with missense, nonsense or splice mutations compared to whole exon or whole gene deletions. Whilst it is important to pursue these studies, data from knockout mice must be interpreted with caution when translating into human studies, and alternatively approaches must also be investigated. These include antisense oligonucleotide therapy targeting suitable exons, and gene-independent approaches. With several potential therapeutic approaches under investigation, there is real hope that treatment options for this disorder will be available to the next generation of patients.
Author contributions
GW undertook the literature review, collected and tabulated genotype and phenotype data and prepared figures. EG performed statistical analysis of data. GW, AD and DB wrote the manuscript.
Acknowledgments
GW is funded by a Wellcome Trust Seed Award in Science (Grant No. 204378/Z/16/Z), National Eye Research Centre Small Grant (SAC019) and a University of Southampton Faculty of Medicine Research Management Committee research grant.
DB and AD are supported by a NIHR Research Professorship to DB (RP- 2016-07-011).
References
- Abdulridha-Aboud W., Kjellstrom U., Andreasson S., Ponjavic V. Characterization of macular structure and function in two Swedish families with genetically identified autosomal dominant retinitis pigmentosa. Mol. Vis. 2016;22:362–373. [PMC free article] [PubMed] [Google Scholar]
- Abu-Safieh L., Vithana E.N., Mantel I., Holder G.E., Pelosini L., Bird A.C., Bhattacharya S.S. A large deletion in the adRP gene PRPF31: evidence that haploinsufficiency is the cause of disease. Mol. Vis. 2006;12:384–388. [PubMed] [Google Scholar]
- Agafonov D.E., Kastner B., Dybkov O., Hofele R.V., Liu W.T., Urlaub H., Luhrmann R., Stark H. Molecular architecture of the human U4/U6.U5 tri-snRNP. Science (New York, N.Y.) 2016;351:1416–1420. doi: 10.1126/science.aad2085. [DOI] [PubMed] [Google Scholar]
- Alagramam K.N., Yuan H., Kuehn M.H., Murcia C.L., Wayne S., Srisailpathy C.R., Lowry R.B., Knaus R., Van Laer L., Bernier F.P., Schwartz S., Lee C., Morton C.C., Mullins R.F., Ramesh A., Van Camp G., Hageman G.S., Woychik R.P., Smith R.J. Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum. Mol. Genet. 2001;10:1709–1718. doi: 10.1093/hmg/10.16.1709. [DOI] [PubMed] [Google Scholar]
- Aleman T.S., Lam B.L., Cideciyan A.V., Sumaroka A., Windsor E.A., Roman A.J., Schwartz S.B., Stone E.M., Jacobson S.G. Genetic heterogeneity in autosomal dominant retinitis pigmentosa with low-frequency damped electroretinographic wavelets. Eye. 2009;23:230–233. doi: 10.1038/eye.2008.264. [DOI] [PubMed] [Google Scholar]
- Almoguera B., Li J., Fernandez-San Jose P., Liu Y., March M., Pellegrino R., Golhar R., Corton M., Blanco-Kelly F., Lopez-Molina M.I., Garcia-Sandoval B., Guo Y., Tian L., Liu X., Guan L., Zhang J., Keating B., Xu X., Hakonarson H., Ayuso C. Application of whole exome sequencing in six families with an initial diagnosis of autosomal dominant retinitis pigmentosa: lessons learned. PloS One. 2015;10 doi: 10.1371/journal.pone.0133624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audo I., Bujakowska K., Mohand-Said S., Lancelot M.E., Moskova-Doumanova V., Waseem N.H., Antonio A., Sahel J.A., Bhattacharya S.S., Zeitz C. Prevalence and novelty of PRPF31 mutations in French autosomal dominant rod-cone dystrophy patients and a review of published reports. BMC Med. Genet. 2010;11:145–2350. doi: 10.1186/1471-2350-11-145. 11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizzadeh Pormehr L., Ahmadian S., Daftarian N., Mousavi S.A., Shafiezadeh M. PRPF31 reduction causes mis-splicing of the phototransduction genes in human organotypic retinal culture. Eur. J. Hum. Genet. 2019 doi: 10.1038/s41431-019-0531-1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram K., Agafonov D.E., Dybkov O., Haselbach D., Leelaram M.N., Will C.L., Urlaub H., Kastner B., Luhrmann R., Stark H. Cryo-EM structure of a pre-catalytic human spliceosome primed for activation. Cell. 2017;170:701–713. doi: 10.1016/j.cell.2017.07.011. e11. [DOI] [PubMed] [Google Scholar]
- Bertram K., Agafonov D.E., Liu W.T., Dybkov O., Will C.L., Hartmuth K., Urlaub H., Kastner B., Stark H., Luhrmann R. Cryo-EM structure of a human spliceosome activated for step 2 of splicing. Nature. 2017;542:318–323. doi: 10.1038/nature21079. [DOI] [PubMed] [Google Scholar]
- Bhatia S., Goyal S., Singh I.R., Singh D., Vanita V. 2018. A Novel Mutation in the PRPF31 in a North Indian adRP Family with Incomplete Penetrance. Documenta Ophthalmologica.Advances in Ophthalmology. [DOI] [PubMed] [Google Scholar]
- Birtel J., Eisenberger T., Gliem M., Muller P.L., Herrmann P., Betz C., Zahnleiter D., Neuhaus C., Lenzner S., Holz F.G., Mangold E., Bolz H.J., Charbel Issa P. Clinical and genetic characteristics of 251 consecutive patients with macular and cone/cone-rod dystrophy. Sci. Rep. 2018;8:4824. doi: 10.1038/s41598-018-22096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtel J., Gliem M., Mangold E., Muller P.L., Holz F.G., Neuhaus C., Lenzner S., Zahnleiter D., Betz C., Eisenberger T., Bolz H.J., Charbel Issa P. Next-generation sequencing identifies unexpected genotype-phenotype correlations in patients with retinitis pigmentosa. PloS One. 2018;13 doi: 10.1371/journal.pone.0207958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D.T., McDonald W.H., Gould K.L., Forsburg S.L. Isolation of an essential Schizosaccharomyces pombe gene, prp31(+), that links splicing and meiosis. Nucleic Acids Res. 2000;28:2214–2220. doi: 10.1093/nar/28.11.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowne S.J., Sullivan L.S., Koboldt D.C., Ding L., Fulton R., Abbott R.M., Sodergren E.J., Birch D.G., Wheaton D.H., Heckenlively J.R., Liu Q., Pierce E.A., Weinstock G.M., Daiger S.P. Identification of disease-causing mutations in autosomal dominant retinitis pigmentosa (adRP) using next-generation DNA sequencing. Invest. Ophthalmol. Vis. Sci. 2011;52:494–503. doi: 10.1167/iovs.10-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant L., Lozynska O., Marsh A., Papp T.E., van Gorder L., Serrano L.W., Gai X., Maguire Am M.D., Aleman T.S., Bennett J. Identification of a novel pathogenic missense mutation in PRPF31 using whole exome sequencing: a case report. The British Journal of Ophthalmology. 2019;103(6):761–767. doi: 10.1136/bjophthalmol-2017-311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon E.M., Bronstein R., Buskin A., Lako M., Pierce E.A., Fernandez-Godino R. AAV-mediated gene augmentation therapy restores critical functions in mutant PRPF31. Mol Ther Methods Clin Dev. 2019;15:392–402. doi: 10.1016/j.omtm.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskin A., Zhu L., Chichagova V., Basu B., Mozaffari-Jovin S., Dolan D., Droop A., Collin J., Bronstein R., Mehrotra S., Farkas M., Hilgen G., White K., Pan K.T., Treumann A., Hallam D., Bialas K., Chung G., Mellough C., Ding Y., Krasnogor N., Przyborski S., Zwolinski S., Al-Aama J., Alharthi S., Xu Y., Wheway G., Szymanska K., McKibbin M., Inglehearn C.F., Elliott D.J., Lindsay S., Ali R.R., Steel D.H., Armstrong L., Sernagor E., Urlaub H., Pierce E., Luhrmann R., Grellscheid S.N., Johnson C.A., Lako M. Disrupted alternative splicing for genes implicated in splicing and ciliogenesis causes PRPF31 retinitis pigmentosa. Nat. Commun. 2018;9:4234. doi: 10.1038/s41467-018-06448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Wu J., Lam S., Duan R., Newnham C., Molday R.S., Graziotto J.J., Pierce E.A., Hu J. Temporal and tissue specific regulation of RP-associated splicing factor genes PRPF3, PRPF31 and PRPC8--implications in the pathogenesis of RP. PloS One. 2011;6 doi: 10.1371/journal.pone.0015860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carss K.J., Arno G., Erwood M., Stephens J., Sanchis-Juan A., Hull S., Megy K., Grozeva D., Dewhurst E., Malka S., Plagnol V., Penkett C., Stirrups K., Rizzo R., Wright G., Josifova D., Bitner-Glindzicz M., Scott R.H., Clement E., Allen L., Armstrong R., Brady A.F., Carmichael J., Chitre M., Henderson R.H.H., Hurst J., MacLaren R.E., Murphy E., Paterson J., Rosser E., Thompson D.A., Wakeling E., Ouwehand W.H., Michaelides M., Moore A.T., Webster A.R., Raymond F.L. Comprehensive rare variant analysis via whole-genome sequencing to determine the molecular pathology of inherited retinal disease. Am. J. Hum. Genet. 2017;100:75–90. doi: 10.1016/j.ajhg.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakarova C.F., Cherninkova S., Tournev I., Waseem N., Kaneva R., Jordanova A., Veraitch B.K., Gill B., Colclough T., Nakova A., Oscar A., Mihaylova V., Nikolova-Hill A., Wright A.F., Black G.C., Ramsden S., Kremensky I., Bhattacharya S.S. Molecular genetics of retinitis pigmentosa in two Romani (Gypsy) families. Mol. Vis. 2006;12:909–914. [PubMed] [Google Scholar]
- Chanarat S., Seizl M., Strasser K. The Prp19 complex is a novel transcription elongation factor required for TREX occupancy at transcribed genes. Genes Dev. 2011;25:1147–1158. doi: 10.1101/gad.623411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchore Y., Begaj T., Wu D., Drokhlyansky E., Cepko C.L. Glycolytic reliance promotes anabolism in photoreceptors. Elife. 2017;6 doi: 10.7554/eLife.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan A.V., Sudharsan R., Dufour V.L., Massengill M.T., Iwabe S., Swider M., Lisi B., Sumaroka A., Marinho L.F., Appelbaum T., Rossmiller B., Hauswirth W.W., Jacobson S.G., Lewin A.S., Aguirre G.D., Beltran W.A. Mutation-independent rhodopsin gene therapy by knockdown and replacement with a single AAV vector. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E8547–e8556. doi: 10.1073/pnas.1805055115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comitato A., Spampanato C., Chakarova C., Sanges D., Bhattacharya S.S., Marigo V. Mutations in splicing factor PRPF3, causing retinal degeneration, form detrimental aggregates in photoreceptor cells. Hum. Mol. Genet. 2007;16:1699–1707. doi: 10.1093/hmg/ddm118. [DOI] [PubMed] [Google Scholar]
- Coussa R.G., Chakarova C., Ajlan R., Taha M., Kavalec C., Gomolin J., Khan A., Lopez I., Ren H., Waseem N., Kamenarova K., Bhattacharya S.S., Koenekoop R.K. Genotype and phenotype studies in autosomal dominant retinitis pigmentosa (adRP) of the French Canadian founder population. Invest. Ophthalmol. Vis. Sci. 2015;56:8297–8305. doi: 10.1167/iovs.15-17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz L., Fynes K., Georgiadis O., Kerby J., Luo Y.H., Ahmado A., Vernon A., Daniels J.T., Nommiste B., Hasan S.M., Gooljar S.B., Carr A.F., Vugler A., Ramsden C.M., Bictash M., Fenster M., Steer J., Harbinson T., Wilbrey A., Tufail A., Feng G., Whitlock M., Robson A.G., Holder G.E., Sagoo M.S., Loudon P.T., Whiting P., Coffey P.J. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018;36:328–337. doi: 10.1038/nbt.4114. [DOI] [PubMed] [Google Scholar]
- Daiger S.P., Bowne S.J., Sullivan L.S., Blanton S.H., Weinstock G.M., Koboldt D.C., Fulton R.S., Larsen D., Humphries P., Humphries M.M., Pierce E.A., Chen R., Li Y. Application of next-generation sequencing to identify genes and mutations causing autosomal dominant retinitis pigmentosa (adRP) Adv. Exp. Med. Biol. 2014;801:123–129. doi: 10.1007/978-1-4614-3209-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cerda B., Diez-Lloret A., Ponte B., Valles-Saiz L., Calado S.M., Rodriguez-Bocanegra E., Garcia-Delgado A.B., Moya-Molina M., Bhattacharya S.S., Diaz-Corrales F.J. Generation and characterization of the human iPSC line CABi001-A from a patient with retinitis pigmentosa caused by a novel mutation in PRPF31 gene. Stem Cell Res. 2019;36:101426. doi: 10.1016/j.scr.2019.101426. [DOI] [PubMed] [Google Scholar]
- de Sousa Dias M., Hernan I., Pascual B., Borras E., Mane B., Gamundi M.J., Carballo M. Detection of novel mutations that cause autosomal dominant retinitis pigmentosa in candidate genes by long-range PCR amplification and next-generation sequencing. Mol. Vis. 2013;19:654–664. [PMC free article] [PubMed] [Google Scholar]
- Deery E.C., Vithana E.N., Newbold R.J., Gallon V.A., Bhattacharya S.S., Warren M.J., Hunt D.M., Wilkie S.E. Disease mechanism for retinitis pigmentosa (RP11) caused by mutations in the splicing factor gene PRPF31. Hum. Mol. Genet. 2002;11:3209–3219. doi: 10.1093/hmg/11.25.3209. [DOI] [PubMed] [Google Scholar]
- Dong B., Chen J., Zhang X., Pan Z., Bai F., Li Y. Two novel PRP31 premessenger ribonucleic acid processing factor 31 homolog mutations including a complex insertion-deletion identified in Chinese families with retinitis pigmentosa. Mol. Vis. 2013;19:2426–2435. [PMC free article] [PubMed] [Google Scholar]
- Eisenberger T., Neuhaus C., Khan A.O., Decker C., Preising M.N., Friedburg C., Bieg A., Gliem M., Charbel Issa P., Holz F.G., Baig S.M., Hellenbroich Y., Galvez A., Platzer K., Wollnik B., Laddach N., Ghaffari S.R., Rafati M., Botzenhart E., Tinschert S., Borger D., Bohring A., Schreml J., Kortge-Jung S., Schell-Apacik C., Bakur K., Al-Aama J.Y., Neuhann T., Herkenrath P., Nurnberg G., Nurnberg P., Davis J.S., Gal A., Bergmann C., Lorenz B., Bolz H.J. Increasing the yield in targeted next-generation sequencing by implicating CNV analysis, non-coding exons and the overall variant load: the example of retinal dystrophies. PloS One. 2013;8 doi: 10.1371/journal.pone.0078496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingford J.M., Barton S., Bhaskar S., O'Sullivan J., Williams S.G., Lamb J.A., Panda B., Sergouniotis P.I., Gillespie R.L., Daiger S.P., Hall G., Gale T., Lloyd I.C., Bishop P.N., Ramsden S.C., Black G.C.M. Molecular findings from 537 individuals with inherited retinal disease. J. Med. Genet. 2016;53:761–767. doi: 10.1136/jmedgenet-2016-103837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingford J.M., Barton S., Bhaskar S., Williams S.G., Sergouniotis P.I., O'Sullivan J., Lamb J.A., Perveen R., Hall G., Newman W.G., Bishop P.N., Roberts S.A., Leach R., Tearle R., Bayliss S., Ramsden S.C., Nemeth A.H., Black G.C. Whole genome sequencing increases molecular diagnostic yield compared with current diagnostic testing for inherited retinal disease. Ophthalmology. 2016;123:1143–1150. doi: 10.1016/j.ophtha.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas M.H., Lew D.S., Sousa M.E., Bujakowska K., Chatagnon J., Bhattacharya S.S., Pierce E.A., Nandrot E.F. Mutations in pre-mRNA processing factors 3, 8, and 31 cause dysfunction of the retinal pigment epithelium. Am. J. Pathol. 2014;184:2641–2652. doi: 10.1016/j.ajpath.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-San Jose P., Corton M., Blanco-Kelly F., Avila-Fernandez A., Lopez-Martinez M.A., Sanchez-Navarro I., Sanchez-Alcudia R., Perez-Carro R., Zurita O., Sanchez-Bolivar N., Lopez-Molina M.I., Garcia-Sandoval B., Riveiro-Alvarez R., Ayuso C. Targeted next-generation sequencing improves the diagnosis of autosomal dominant retinitis pigmentosa in Spanish patients. Invest. Ophthalmol. Vis. Sci. 2015;56:2173–2182. doi: 10.1167/iovs.14-16178. [DOI] [PubMed] [Google Scholar]
- Finkel R.S., Mercuri E., Darras B.T., Connolly A.M., Kuntz N.L., Kirschner J., Chiriboga C.A., Saito K., Servais L., Tizzano E., Topaloglu H., Tulinius M., Montes J., Glanzman A.M., Bishop K., Zhong Z.J., Gheuens S., Bennett C.F., Schneider E., Farwell W., De Vivo D.C. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 2017;377:1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- Finn A.P., Grewal D.S., Vajzovic L. Argus II retinal prosthesis system: a review of patient selection criteria, surgical considerations, and post-operative outcomes. Clin. Ophthalmol. 2018;12:1089–1097. doi: 10.2147/OPTH.S137525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandra M., Anandula V., Authiappan V., Sundaramurthy S., Raman R., Bhattacharya S., Govindasamy K. Retinitis pigmentosa: mutation analysis of RHO, PRPF31, RP1, and IMPDH1 genes in patients from India. Mol. Vis. 2008;14:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- Ghazawy S., Springell K., Gauba V., McKibbin M.A., Inglehearn C.F. Dominant retinitis pigmentosa phenotype associated with a new mutation in the splicing factor PRPF31. Br. J. Ophthalmol. 2007;91:1411–1413. doi: 10.1136/bjo.2006.105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockle N., Kohl S., Mohr J., Scheurenbrand T., Sprecher A., Weisschuh N., Bernd A., Rudolph G., Schubach M., Poloschek C., Zrenner E., Biskup S., Berger W., Wissinger B., Neidhardt J. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. Eur. J. Hum. Genet. 2014;22:99–104. doi: 10.1038/ejhg.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovleva I., Kohn L., Burstedt M., Daiger S., Sandgren O. Mutation spectra in autosomal dominant and recessive retinitis pigmentosa in northern Sweden. Adv. Exp. Med. Biol. 2010;664:255–262. doi: 10.1007/978-1-4419-1399-9_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenell A., Wang Y., Yam M., Swarup A., Dilan T.L., Hauer A., Linton J.D., Philp N.J., Gregor E., Zhu S., Shi Q., Murphy J., Guan T., Lohner D., Kolandaivelu S., Ramamurthy V., Goldberg A.F.X., Hurley J.B., Du J. Loss of MPC1 reprograms retinal metabolism to impair visual function. Proc. Natl. Acad. Sci. U. S. A. 2019;116:3530–3535. doi: 10.1073/pnas.1812941116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey M., Dusterhoft A., Henriques J.A., Brendel M. Allelism of PSO4 and PRP19 links pre-mRNA processing with recombination and error-prone DNA repair in Saccharomyces cerevisiae. Nucleic Acids Res. 1996;24:4009–4014. doi: 10.1093/nar/24.20.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler B.P., Comander J., Weigel DiFranco C., Place E.M., Pierce E.A. Course of ocular function in PRPF31 retinitis pigmentosa. Semin. Ophthalmol. 2016;31:49–52. doi: 10.3109/08820538.2015.1114856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamieh A., Nandrot E.F. Retinal pigment epithelial cells: the unveiled component in the etiology of Prpf splicing factor-associated retinitis pigmentosa. Adv. Exp. Med. Biol. 2019;1185:227–231. doi: 10.1007/978-3-030-27378-1_37. [DOI] [PubMed] [Google Scholar]
- Hariri A.H., Gui W., Datoo O'Keefe G.A., Ip M.S., Sadda S.R., Gorin M.B. Ultra-widefield fundus autofluorescence imaging of patients with retinitis pigmentosa: a standardized grading system in different genotypes. Ophthalmol Retina. 2018;2:735–745. doi: 10.1016/j.oret.2017.10.018. [DOI] [PubMed] [Google Scholar]
- Haselbach D., Komarov I., Agafonov D.E., Hartmuth K., Graf B., Dybkov O., Urlaub H., Kastner B., Luhrmann R., Stark H. Structure and conformational dynamics of the human spliceosomal B(act) complex. Cell. 2018;172:454–464. doi: 10.1016/j.cell.2018.01.010. e11. [DOI] [PubMed] [Google Scholar]
- Hofmann J.C., Tegha-Dunghu J., Drager S., Will C.L., Luhrmann R., Gruss O.J. The Prp19 complex directly functions in mitotic spindle assembly. PloS One. 2013;8 doi: 10.1371/journal.pone.0074851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.F., Huang F., Wu K.C., Wu J., Chen J., Pang C.P., Lu F., Qu J., Jin Z.B. Genotype-phenotype correlation and mutation spectrum in a large cohort of patients with inherited retinal dystrophy revealed by next-generation sequencing. Genet. Med. 2015;17:271–278. doi: 10.1038/gim.2014.138. [DOI] [PubMed] [Google Scholar]
- Ivings L., Towns K.V., Matin M.A., Taylor C., Ponchel F., Grainger R.J., Ramesar R.S., Mackey D.A., Inglehearn C.F. Evaluation of splicing efficiency in lymphoblastoid cell lines from patients with splicing-factor retinitis pigmentosa. Mol. Vis. 2008;14:2357–2366. [PMC free article] [PubMed] [Google Scholar]
- Jespersgaard C., Fang M., Bertelsen M., Dang X., Jensen H., Chen Y., Bech N., Dai L., Rosenberg T., Zhang J., Moller L.B., Tumer Z., Brondum-Nielsen K., Gronskov K. Molecular genetic analysis using targeted NGS analysis of 677 individuals with retinal dystrophy. Sci. Rep. 2019;9:1219. doi: 10.1038/s41598-018-38007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z.B., Okamoto S., Osakada F., Homma K., Assawachananont J., Hirami Y., Iwata T., Takahashi M. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PloS One. 2011;6 doi: 10.1371/journal.pone.0017084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani A.H., Lebkowski J.S., Rahhal F.M., Avery R.L., Salehi-Had H., Dang W., Lin C.M., Mitra D., Zhu D., Thomas B.B., Hikita S.T., Pennington B.O., Johnson L.V., Clegg D.O., Hinton D.R., Humayun M.S. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao4097. [DOI] [PubMed] [Google Scholar]
- Khan N., Eliopoulos H., Han L., Kinane T.B., Lowes L.P., Mendell J.R., Gordish-Dressman H., Henricson E.K., McDonald C.M. Eteplirsen treatment attenuates respiratory decline in ambulatory and non-ambulatory patients with Duchenne muscular dystrophy. J. Neuromuscul. Dis. 2019;6:213–225. doi: 10.3233/JND-180351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Ki S.M., Joung J.G., Scott E., Heynen-Genel S., Aza-Blanc P., Kwon C.H., Kim J., Gleeson J.G., Lee J.E. Genome-wide screen identifies novel machineries required for both ciliogenesis and cell cycle arrest upon serum starvation. Biochim. Biophys. Acta. 2016;1863:1307–1318. doi: 10.1016/j.bbamcr.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser K., Webb-Jones K.D., Bowne S.J., Sullivan L.S., Daiger S.P., Birch D.G. Time course of disease progression of PRPF31-mediated retinitis pigmentosa. Am. J. Ophthalmol. 2019;200:76–84. doi: 10.1016/j.ajo.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L., Bowne S.J., L S.S., Daiger S.P., Burstedt M.S., Kadzhaev K., Sandgren O., Golovleva I. Breakpoint characterization of a novel approximately 59 kb genomic deletion on 19q13.42 in autosomal-dominant retinitis pigmentosa with incomplete penetrance. Eur. J. Hum. Genet. 2009;17:651–655. doi: 10.1038/ejhg.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y., Akiyama M., Nishiguchi K.M., Momozawa Y., Kamatani Y., Takata S., Inai C., Iwasaki Y., Kumano M., Murakami Y., Omodaka K., Abe T., Komori S., Gao D., Hirakata T., Kurata K., Hosono K., Ueno S., Hotta Y., Murakami A., Terasaki H., Wada Y., Nakazawa T., Ishibashi T., Ikeda Y., Kubo M., Sonoda K.H. Genetic characteristics of retinitis pigmentosa in 1204 Japanese patients. J. Med. Genet. 2019;56:662–670. doi: 10.1136/jmedgenet-2018-105691. [DOI] [PubMed] [Google Scholar]
- Kurata K., Hosono K., Hotta Y. Long-term clinical course of 2 Japanese patients with PRPF31-related retinitis pigmentosa. Jpn. J. Ophthalmol. 2018;62:186–193. doi: 10.1007/s10384-017-0560-7. [DOI] [PubMed] [Google Scholar]
- Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O'Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Tukiainen T., Birnbaum D.P., Kosmicki J.A., Duncan L.E., Estrada K., Zhao F., Zou J., Pierce-Hoffman E., Berghout J., Cooper D.N., Deflaux N., DePristo M., Do R., Flannick J., Fromer M., Gauthier L., Goldstein J., Gupta N., Howrigan D., Kiezun A., Kurki M.I., Moonshine A.L., Natarajan P., Orozco L., Peloso G.M., Poplin R., Rivas M.A., Ruano-Rubio V., Rose S.A., Ruderfer D.M., Shakir K., Stenson P.D., Stevens C., Thomas B.P., Tiao G., Tusie-Luna M.T., Weisburd B., Won H.-H., Yu D., Altshuler D.M., Ardissino D., Boehnke M., Danesh J., Donnelly S., Elosua R., Florez J.C., Gabriel S.B., Getz G., Glatt S.J., Hultman C.M., Kathiresan S., Laakso M., McCarroll S., McCarthy M.I., McGovern D., McPherson R., Neale B.M., Palotie A., Purcell S.M., Saleheen D., Scharf J.M., Sklar P., Sullivan P.F., Tuomilehto J., Tsuang M.T., Watkins H.C., Wilson J.G., Daly M.J., MacArthur D.G., Exome Aggregation C. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A.A. Treating disease at the RNA level with oligonucleotides. N. Engl. J. Med. 2019;380:57–70. doi: 10.1056/NEJMra1705346. [DOI] [PubMed] [Google Scholar]
- Lim K.P., Yip S.P., Cheung S.C., Leung K.W., Lam S.T., To C.H. Novel PRPF31 and PRPH2 mutations and co-occurrence of PRPF31 and RHO mutations in Chinese patients with retinitis pigmentosa. Arch. Ophthalmol. 2009;127:784–790. doi: 10.1001/archophthalmol.2009.112. [DOI] [PubMed] [Google Scholar]
- Liu J.Y., Dai X., Sheng J., Cui X., Wang X., Jiang X., Tu X., Tang Z., Bai Y., Liu M., Wang Q.K. Identification and functional characterization of a novel splicing mutation in RP gene PRPF31. Biochem. Biophys. Res. Commun. 2008;367:420–426. doi: 10.1016/j.bbrc.2007.12.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Li P., Dybkov O., Nottrott S., Hartmuth K., Luhrmann R., Carlomagno T., Wahl M.C. Binding of the human Prp31 Nop domain to a composite RNA-protein platform in U4 snRNP. Science (New York, N.Y.) 2007;316:115–120. doi: 10.1126/science.1137924. [DOI] [PubMed] [Google Scholar]
- Lu F., Huang L., Lei C., Sha G., Zheng H., Liu X., Yang J., Shi Y., Lin Y., Gong B., Zhu X., Ma S., Qiao L., Lin H., Cheng J., Yang Z. A novel PRPF31 mutation in a large Chinese family with autosomal dominant retinitis pigmentosa and macular degeneration. PloS One. 2013;8 doi: 10.1371/journal.pone.0078274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.S., Zhao C., Cui Y., Li N.D., Zhang X.M., Zhao K.X. [Novel splice-site mutation in the pre-mRNA splicing gene PRPF31 in a Chinese family with autosomal dominant retinitis pigmentosa] Zhonghua Yan Ke Za Zhi. 2005;41:305–311. [PubMed] [Google Scholar]
- Makarova O.V., Makarov E.M., Liu S., Vornlocher H.P., Luhrmann R. Protein 61K, encoded by a gene (PRPF31) linked to autosomal dominant retinitis pigmentosa, is required for U4/U6*U5 tri-snRNP formation and pre-mRNA splicing. EMBO J. 2002;21:1148–1157. doi: 10.1093/emboj/21.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal A., Li J.M., Ji X.Y., Wu C.S., Yazinski S.A., Nguyen H.D., Liu S., Jimenez A.E., Jin J., Zou L. PRP19 transforms into a sensor of RPA-ssDNA after DNA damage and drives ATR activation via a ubiquitin-mediated circuitry. Mol. Cell. 2014;53:235–246. doi: 10.1016/j.molcel.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Merida I., Aguilera-Garcia D., Fernandez-San Jose P., Blanco-Kelly F., Zurita O., Almoguera B., Garcia-Sandoval B., Avila-Fernandez A., Arteche A., Minguez P., Carballo M., Corton M., Ayuso C. Toward the mutational landscape of autosomal dominant retinitis pigmentosa: a comprehensive analysis of 258 Spanish families. Invest. Ophthalmol. Vis. Sci. 2018;59:2345–2354. doi: 10.1167/iovs.18-23854. [DOI] [PubMed] [Google Scholar]
- Martinez-Gimeno M., Gamundi M.J., Hernan I., Maseras M., Milla E., Ayuso C., Garcia-Sandoval B., Beneyto M., Vilela C., Baiget M., Antinolo G., Carballo M. Mutations in the pre-mRNA splicing-factor genes PRPF3, PRPF8, and PRPF31 in Spanish families with autosomal dominant retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2003;44:2171–2177. doi: 10.1167/iovs.02-0871. [DOI] [PubMed] [Google Scholar]
- McLenachan S., Zhang D., Zhang X., Chen S.C., Lamey T., Thompson J.A., McLaren T., De Roach J.N., Fletcher S., Chen F.K. Generation of two induced pluripotent stem cell lines from a patient with dominant PRPF31 mutation and a related non-penetrant carrier. Stem Cell Res. 2019;34:101357. doi: 10.1016/j.scr.2018.11.018. [DOI] [PubMed] [Google Scholar]
- Mendell J.R., Goemans N., Lowes L.P., Alfano L.N., Berry K., Shao J., Kaye E.M., Mercuri E. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol. 2016;79:257–271. doi: 10.1002/ana.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveling K., Collin R.W., Gilissen C., van Huet R.A., Visser L., Kwint M.P., Gijsen S.J., Zonneveld M.N., Wieskamp N., de Ligt J., Siemiatkowska A.M., Hoefsloot L.H., Buckley M.F., Kellner U., Branham K.E., den Hollander A.I., Hoischen A., Hoyng C., Klevering B.J., van den Born L.I., Veltman J.A., Cremers F.P., Scheffer H. Next-generation genetic testing for retinitis pigmentosa. Hum. Mutat. 2012;33:963–972. doi: 10.1002/humu.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.K., Wood J.P., Chidlow G., Han G., Kittipassorn T., Peet D.J., Casson R.J. Cancer-like metabolism of the mammalian retina. Clin. Exp. Ophthalmol. 2015;43:367–376. doi: 10.1111/ceo.12462. [DOI] [PubMed] [Google Scholar]
- Pan Q., Shai O., Lee L.J., Frey B.J., Blencowe B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- Pan X., Chen X., Liu X., Gao X., Kang X., Xu Q., Chen X., Zhao K., Zhang X., Chu Q., Wang X., Zhao C. Mutation analysis of pre-mRNA splicing genes in Chinese families with retinitis pigmentosa. Mol. Vis. 2014;20:770–779. [PMC free article] [PubMed] [Google Scholar]
- Pomares E., Riera M., Permanyer J., Mendez P., Castro-Navarro J., Andres-Gutierrez A., Marfany G., Gonzalez-Duarte R. Comprehensive SNP-chip for retinitis pigmentosa-Leber congenital amaurosis diagnosis: new mutations and detection of mutational founder effects. Eur. J. Hum. Genet. 2010;18:118–124. doi: 10.1038/ejhg.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala R.V., Rajala A., Kooker C., Wang Y., Anderson R.E. The warburg effect mediator pyruvate kinase M2 expression and regulation in the retina. Sci. Rep. 2016;6:37727. doi: 10.1038/srep37727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio Frio T., Civic N., Ransijn A., Beckmann J.S., Rivolta C. Two trans-acting eQTLs modulate the penetrance of PRPF31 mutations. Hum. Mol. Genet. 2008;17:3154–3165. doi: 10.1093/hmg/ddn212. [DOI] [PubMed] [Google Scholar]
- Rio Frio T., McGee T.L., Wade N.M., Iseli C., Beckmann J.S., Berson E.L., Rivolta C. A single-base substitution within an intronic repetitive element causes dominant retinitis pigmentosa with reduced penetrance. Hum. Mutat. 2009;30:1340–1347. doi: 10.1002/humu.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio Frio T., Wade N.M., Ransijn A., Berson E.L., Beckmann J.S., Rivolta C. Premature termination codons in PRPF31 cause retinitis pigmentosa via haploinsufficiency due to nonsense-mediated mRNA decay. J. Clin. Invest. 2008;118:1519–1531. doi: 10.1172/JCI34211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta C., McGee T.L., Rio Frio T., Jensen R.V., Berson E.L., Dryja T.P. Variation in retinitis pigmentosa-11 (PRPF31 or RP11) gene expression between symptomatic and asymptomatic patients with dominant RP11 mutations. Hum. Mutat. 2006;27:644–653. doi: 10.1002/humu.20325. [DOI] [PubMed] [Google Scholar]
- Roberts L., Ratnapriya R., du Plessis M., Chaitankar V., Ramesar R.S., Swaroop A. Molecular diagnosis of inherited retinal diseases in indigenous african populations by whole-exome sequencing. Invest. Ophthalmol. Vis. Sci. 2016;57:6374–6381. doi: 10.1167/iovs.16-19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A.M., Bhattacharya S.S. Variant haploinsufficiency and phenotypic non-penetrance in PRPF31-associated retinitis pigmentosa. Clin. Genet. 2016;90:118–126. doi: 10.1111/cge.12758. [DOI] [PubMed] [Google Scholar]
- Rose A.M., Mukhopadhyay R., Webster A.R., Bhattacharya S.S., Waseem N.H. A 112 kb deletion in chromosome 19q13.42 leads to retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2011;52:6597–6603. doi: 10.1167/iovs.11-7861. [DOI] [PubMed] [Google Scholar]
- Rose A.M., Shah A.Z., Venturini G., Krishna A., Chakravarti A., Rivolta C., Bhattacharya S.S. Transcriptional regulation of PRPF31 gene expression by MSR1 repeat elements causes incomplete penetrance in retinitis pigmentosa. Sci. Rep. 2016;6:19450. doi: 10.1038/srep19450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A.M., Shah A.Z., Venturini G., Rivolta C., Rose G.E., Bhattacharya S.S. Dominant PRPF31 mutations are hypostatic to a recessive CNOT3 polymorphism in retinitis pigmentosa: a novel phenomenon of “linked trans-acting epistasis”. Ann. Hum. Genet. 2014;78:62–71. doi: 10.1111/ahg.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A.M., Shah A.Z., Waseem N.H., Chakarova C.F., Alfano G., Coussa R.G., Ajlan R., Koenekoop R.K., Bhattacharya S.S. Expression of PRPF31 and TFPT: regulation in health and retinal disease. Hum. Mol. Genet. 2012;21:4126–4137. doi: 10.1093/hmg/dds242. [DOI] [PubMed] [Google Scholar]
- Saini S., Robinson P.N., Singh J.R., Vanita V. A novel 7 bp deletion in PRPF31 associated with autosomal dominant retinitis pigmentosa with incomplete penetrance in an Indian family. Exp. Eye Res. 2012;104:82–88. doi: 10.1016/j.exer.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Samocha K.E., Robinson E.B., Sanders S.J., Stevens C., Sabo A., McGrath L.M., Kosmicki J.A., Rehnström K., Mallick S., Kirby A., Wall D.P., MacArthur D.G., Gabriel S.B., DePristo M., Purcell S.M., Palotie A., Boerwinkle E., Buxbaum J.D., Cook E.H., Gibbs R.A., Schellenberg G.D., Sutcliffe J.S., Devlin B., Roeder K., Neale B.M., Daly M.J. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Wada Y., Itabashi T., Nakamura M., Kawamura M., Tamai M. Mutations in the pre-mRNA splicing gene, PRPF31, in Japanese families with autosomal dominant retinitis pigmentosa. Am. J. Ophthalmol. 2005;140:537–540. doi: 10.1016/j.ajo.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Schaffert N., Hossbach M., Heintzmann R., Achsel T., Luhrmann R. RNAi knockdown of hPrp31 leads to an accumulation of U4/U6 di-snRNPs in Cajal bodies. EMBO J. 2004;23:3000–3009. doi: 10.1038/sj.emboj.7600296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R., Yamamoto H., Hamasaki D., Yamamoto H., Parel J.M., Schmitz C., Dorey C.K., Blanks J.C., Preising M.N. Hypoxia-regulated components of the U4/U6.U5 tri-small nuclear riboprotein complex: possible role in autosomal dominant retinitis pigmentosa. Mol. Vis. 2008;14:125–135. [PMC free article] [PubMed] [Google Scholar]
- Scoles D.R., Pulst S.M. Antisense therapies for movement disorders. Mov. Disord. 2019;34:1112–1119. doi: 10.1002/mds.27782. [DOI] [PubMed] [Google Scholar]
- Shinde V., Kotla P., Strang C., Gorbatyuk M. Unfolded protein response-induced dysregulation of calcium homeostasis promotes retinal degeneration in rat models of autosomal dominant retinitis pigmentosa. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2015.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan L.S., Bowne S.J., Birch D.G., Hughbanks-Wheaton D., Heckenlively J.R., Lewis R.A., Garcia C.A., Ruiz R.S., Blanton S.H., Northrup H., Gire A.I., Seaman R., Duzkale H., Spellicy C.J., Zhu J., Shankar S.P., Daiger S.P. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: a screen of known genes in 200 families. Invest. Ophthalmol. Vis. Sci. 2006;47:3052–3064. doi: 10.1167/iovs.05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan L.S., Bowne S.J., Reeves M.J., Blain D., Goetz K., Ndifor V., Vitez S., Wang X., Tumminia S.J., Daiger S.P. Prevalence of mutations in eyeGENE probands with a diagnosis of autosomal dominant retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2013;54:6255–6261. doi: 10.1167/iovs.13-12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan L.S., Bowne S.J., Seaman C.R., Blanton S.H., Lewis R.A., Heckenlively J.R., Birch D.G., Hughbanks-Wheaton D., Daiger S.P. Genomic rearrangements of the PRPF31 gene account for 2.5% of autosomal dominant retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2006;47:4579–4588. doi: 10.1167/iovs.06-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira K., Nakazawa M., Sato M. Mutation c. 1142 del G in the PRPF31 gene in a family with autosomal dominant retinitis pigmentosa (RP11) and its implications. Jpn. J. Ophthalmol. 2007;51:45–48. doi: 10.1007/s10384-006-0394-1. [DOI] [PubMed] [Google Scholar]
- Terray A., Fort V., Slembrouck A., Nanteau C., Sahel J.A., Reichman S., Audo I., Goureau O. Establishment of an induced pluripotent stem (iPS) cell line from dermal fibroblasts of an asymptomatic patient with dominant PRPF31 mutation. Stem Cell Res. 2017;25:26–29. doi: 10.1016/j.scr.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Tiwari A., Lemke J., Altmueller J., Thiele H., Glaus E., Fleischhauer J., Nurnberg P., Neidhardt J., Berger W. Identification of novel and recurrent disease-causing mutations in retinal dystrophies using whole exome sequencing (WES): benefits and limitations. PloS One. 2016;11 doi: 10.1371/journal.pone.0158692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz V.M., Beight C.D., Marino M.J., Hagstrom S.A., Traboulsi E.I. Autosomal dominant retinitis pigmentosa secondary to pre-mRNA splicing-factor gene PRPF31 (RP11): review of disease mechanism and report of a family with a novel 3-base pair insertion. Ophthalmic Genet. 2013;34:183–188. doi: 10.3109/13816810.2012.762932. [DOI] [PubMed] [Google Scholar]
- Valdés-Sánchez L., Calado S.M., de la Cerda B., Aramburu A., García-Delgado A.B., Massalini S., Montero-Sánchez A., Bhatia V., Rodríguez-Bocanegra E., Diez-Lloret A., Rodríguez-Martínez D., Chakarova C., Bhattacharya S.S., Díaz-Corrales F.J. Retinal pigment epithelium degeneration caused by aggregation of PRPF31 and the role of HSP70 family of proteins. Mol. Med. 2019;26:1. doi: 10.1186/s10020-019-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenbergh C., Coppieters F., Roels D., De Jaegere S., Flipts H., De Zaeytijd J., Walraedt S., Claes C., Fransen E., Van Camp G., Depasse F., Casteels I., de Ravel T., Leroy B.P., De Baere E. Mutations in splicing factor genes are a major cause of autosomal dominant retinitis pigmentosa in Belgian families. PloS One. 2017;12 doi: 10.1371/journal.pone.0170038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huet R.A., Pierrache L.H., Meester-Smoor M.A., Klaver C.C., van den Born L.I., Hoyng C.B., de Wijs I.J., Collin R.W., Hoefsloot L.H., Klevering B.J. The efficacy of microarray screening for autosomal recessive retinitis pigmentosa in routine clinical practice. Mol. Vis. 2015;21:461–476. [PMC free article] [PubMed] [Google Scholar]
- Vander Kooi C.W., Ohi M.D., Rosenberg J.A., Oldham M.L., Newcomer M.E., Gould K.L., Chazin W.J. The Prp19 U-box crystal structure suggests a common dimeric architecture for a class of oligomeric E3 ubiquitin ligases. Biochemistry. 2006;45:121–130. doi: 10.1021/bi051787e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini G., Rose A.M., Shah A.Z., Bhattacharya S.S., Rivolta C. CNOT3 is a modifier of PRPF31 mutations in retinitis pigmentosa with incomplete penetrance. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva A., Willer J.R., Bryois J., Dermitzakis E.T., Katsanis N., Davis E.E. Whole exome sequencing of a dominant retinitis pigmentosa family identifies a novel deletion in PRPF31. Invest. Ophthalmol. Vis. Sci. 2014;55:2121–2129. doi: 10.1167/iovs.13-13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithana E.N., Abu-Safieh L., Allen M.J., Carey A., Papaioannou M., Chakarova C., Al-Maghtheh M., Ebenezer N.D., Willis C., Moore A.T., Bird A.C., Hunt D.M., Bhattacharya S.S. A human homolog of yeast pre-mRNA splicing gene, PRP31, underlies autosomal dominant retinitis pigmentosa on chromosome 19q13.4 (RP11) Mol. Cell. 2001;8:375–381. doi: 10.1016/s1097-2765(01)00305-7. [DOI] [PubMed] [Google Scholar]
- Wang F., Wang H., Tuan H.F., Nguyen D.H., Sun V., Keser V., Bowne S.J., Sullivan L.S., Luo H., Zhao L., Wang X., Zaneveld J.E., Salvo J.S., Siddiqui S., Mao L., Wheaton D.K., Birch D.G., Branham K.E., Heckenlively J.R., Wen C., Flagg K., Ferreyra H., Pei J., Khan A., Ren H., Wang K., Lopez I., Qamar R., Zenteno J.C., Ayala-Ramirez R., Buentello-Volante B., Fu Q., Simpson D.A., Li Y., Sui R., Silvestri G., Daiger S.P., Koenekoop R.K., Zhang K., Chen R. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum. Genet. 2014;133:331–345. doi: 10.1007/s00439-013-1381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ribaudo M., Zhao K., Yu N., Chen Q., Sun Q., Wang L., Wang Q. Novel deletion in the pre-mRNA splicing gene PRPF31 causes autosomal dominant retinitis pigmentosa in a large Chinese family. Am. J. Med. Genet. 2003;121a:235–239. doi: 10.1002/ajmg.a.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem N.H., Vaclavik V., Webster A., Jenkins S.A., Bird A.C., Bhattacharya S.S. Mutations in the gene coding for the pre-mRNA splicing factor, PRPF31, in patients with autosomal dominant retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2007;48:1330–1334. doi: 10.1167/iovs.06-0963. [DOI] [PubMed] [Google Scholar]
- Weidenhammer E.M., Ruiz-Noriega M., Woolford J.L., Jr. Prp31p promotes the association of the U4/U6 x U5 tri-snRNP with prespliceosomes to form spliceosomes in Saccharomyces cerevisiae. Mol. Cell Biol. 1997;17:3580–3588. doi: 10.1128/mcb.17.7.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenhammer E.M., Singh M., Ruiz-Noriega M., Woolford J.L., Jr. The PRP31 gene encodes a novel protein required for pre-mRNA splicing in Saccharomyces cerevisiae. Nucleic Acids Res. 1996;24:1164–1170. doi: 10.1093/nar/24.6.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisschuh N., Mayer A.K., Strom T.M., Kohl S., Glockle N., Schubach M., Andreasson S., Bernd A., Birch D.G., Hamel C.P., Heckenlively J.R., Jacobson S.G., Kamme C., Kellner U., Kunstmann E., Maffei P., Reiff C.M., Rohrschneider K., Rosenberg T., Rudolph G., Vamos R., Varsanyi B., Weleber R.G., Wissinger B. Mutation detection in patients with retinal dystrophies using targeted next generation sequencing. PloS One. 2016;11 doi: 10.1371/journal.pone.0145951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheway G., Nazlamova L., Meshad N., Hunt S., Jackson N., Churchill A. A combined in silico, in vitro and clinical approach to characterize novel pathogenic missense variants in PRPF31 in retinitis pigmentosa. Front. Genet. 2019;10:248. doi: 10.3389/fgene.2019.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheway G., Schmidts M., Mans D.A., Szymanska K., Nguyen T.M., Racher H., Phelps I.G., Toedt G., Kennedy J., Wunderlich K.A., Sorusch N., Abdelhamed Z.A., Natarajan S., Herridge W., van Reeuwijk J., Horn N., Boldt K., Parry D.A., Letteboer S.J., Roosing S., Adams M., Bell S.M., Bond J., Higgins J., Morrison E.E., Tomlinson D.C., Slaats G.G., van Dam T.J., Huang L., Kessler K., Giessl A., Logan C.V., Boyle E.A., Shendure J., Anazi S., Aldahmesh M., Al Hazzaa S., Hegele R.A., Ober C., Frosk P., Mhanni A.A., Chodirker B.N., Chudley A.E., Lamont R., Bernier F.P., Beaulieu C.L., Gordon P., Pon R.T., Donahue C., Barkovich A.J., Wolf L., Toomes C., Thiel C.T., Boycott K.M., McKibbin M., Inglehearn C.F., Consortium U.K., University of Washington Center for Mendelian G., Stewart F., Omran H., Huynen M.A., Sergouniotis P.I., Alkuraya F.S., Parboosingh J.S., Innes A.M., Willoughby C.E., Giles R.H., Webster A.R., Ueffing M., Blacque O., Gleeson J.G., Wolfrum U., Beales P.L., Gibson T., Doherty D., Mitchison H.M., Roepman R., Johnson C.A. An siRNA-based functional genomics screen for the identification of regulators of ciliogenesis and ciliopathy genes. Nat. Cell Biol. 2015;17:1074–1087. doi: 10.1038/ncb3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie S.E., Morris K.J., Bhattacharya S.S., Warren M.J., Hunt D.M. A study of the nuclear trafficking of the splicing factor protein PRPF31 linked to autosomal dominant retinitis pigmentosa (ADRP) Biochim. Biophys. Acta. 2006;1762:304–311. doi: 10.1016/j.bbadis.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Wilkie S.E., Vaclavik V., Wu H., Bujakowska K., Chakarova C.F., Bhattacharya S.S., Warren M.J., Hunt D.M. Disease mechanism for retinitis pigmentosa (RP11) caused by missense mutations in the splicing factor gene PRPF31. Mol. Vis. 2008;14:683–690. [PMC free article] [PubMed] [Google Scholar]
- Will C.L., Luhrmann R. Spliceosome structure and function. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Zhong M., Li M., Huang H., Liao J., Lu A., Guo K., Ma N., Lin J., Duan J., Liu L., Xu F., Zhong Z., Chen J. Mutation analysis of pre-mRNA splicing genes PRPF31, PRPF8, and SNRNP200 in Chinese families with autosomal dominant retinitis pigmentosa. Curr. Mol. Med. 2018;18:287–294. doi: 10.2174/1566524018666181024160452. [DOI] [PubMed] [Google Scholar]
- Xi X.H., Zheng D., Xia K., Pan Q., Lei L.Y., Liu Z., Tang C.Z., Xia J.H., Jiang D.Y., Deng H.X. [Splicing site mutation of D19S418 in PRPF-31 gene and its phenotypic characters with autosomal dominant retinitis pigmentosa] Zhonghua Yan Ke Za Zhi. 2005;41:1020–1026. [PubMed] [Google Scholar]
- Xia K., Zheng D., Pan Q., Liu Z., Xi X., Hu Z., Deng H., Liu X., Jiang D., Deng H., Xia J. A novel PRPF31 splice-site mutation in a Chinese family with autosomal dominant retinitis pigmentosa. Mol. Vis. 2004;10:361–365. [PubMed] [Google Scholar]
- Xiao X., Cao Y., Zhang Z., Xu Y., Zheng Y., Chen L.J., Pang C.P., Chen H. Novel mutations in PRPF31 causing retinitis pigmentosa identified using whole-exome sequencing. Invest. Ophthalmol. Vis. Sci. 2017;58:6342–6350. doi: 10.1167/iovs.17-22952. [DOI] [PubMed] [Google Scholar]
- Xie D., Peng K., Yi Q., Liu W., Yang Y., Sun K., Zhu X., Lu F. Targeted next generation sequencing revealed novel PRPF31 mutations in autosomal dominant retinitis pigmentosa. Genet. Test. Mol. Biomarkers. 2018;22:425–432. doi: 10.1089/gtmb.2018.0036. [DOI] [PubMed] [Google Scholar]
- Xu F., Sui R., Liang X., Li H., Jiang R., Dong F. Novel PRPF31 mutations associated with Chinese autosomal dominant retinitis pigmentosa patients. Mol. Vis. 2012;18:3021–xxx. [PMC free article] [PubMed] [Google Scholar]
- Yang L., Yin X., Wu L., Chen N., Zhang H., Li G., Ma Z. Targeted exome capture and sequencing identifies novel PRPF31 mutations in autosomal dominant retinitis pigmentosa in Chinese families. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Tian D., Lee J., Zeng J., Zhang H., Chen S., Guo H., Xiong Z., Xia K., Hu Z., Luo J. Clinical and genetic identification of a large Chinese family with autosomal dominant retinitis pigmentosa. Ophthalmic Genet. 2015;36:64–69. doi: 10.3109/13816810.2013.809458. [DOI] [PubMed] [Google Scholar]
- Yin J., Brocher J., Fischer U., Winkler C. Mutant Prpf31 causes pre-mRNA splicing defects and rod photoreceptor cell degeneration in a zebrafish model for Retinitis pigmentosa. Mol. Neurodegener. 2011;6 doi: 10.1186/1750-1326-6-56. 56-1326-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Kawada M., Havlioglu N., Tang H., Wu J.Y. Mutations in PRPF31 inhibit pre-mRNA splicing of rhodopsin gene and cause apoptosis of retinal cells. J. Neurosci. : the official journal of the Society for Neuroscience. 2005;25:748–757. doi: 10.1523/JNEUROSCI.2399-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Kaur R., Lu X., Shen X., Li L., Legerski R.J. The Pso4 mRNA splicing and DNA repair complex interacts with WRN for processing of DNA interstrand cross-links. J. Biol. Chem. 2005;280:40559–40567. doi: 10.1074/jbc.M508453200. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Xu M., Verriotto J.D., Li Y., Wang H., Gan L., Lam B.L., Chen R. Next-generation sequencing-based molecular diagnosis of 35 Hispanic retinitis pigmentosa probands. Sci. Rep. 2016;6:32792. doi: 10.1038/srep32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Wang F., Wang H., Li Y., Alexander S., Wang K., Willoughby C.E., Zaneveld J.E., Jiang L., Soens Z.T., Earle P., Simpson D., Silvestri G., Chen R. Next-generation sequencing-based molecular diagnosis of 82 retinitis pigmentosa probands from Northern Ireland. Hum. Genet. 2015;134:217–230. doi: 10.1007/s00439-014-1512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Wang H.-L., Li J.-K., Xu L., Tellier L., Li X.-L., Huang X.-Y., Li W., Niu T.-T., Yang H.-M., Zhang J.-G., Liu D.-N. A novel mutation in PRPF31, causative of autosomal dominant retinitis pigmentosa, using the BGISEQ-500 sequencer. Int. J. Ophthalmol. 2018;11:31–35. doi: 10.18240/ijo.2018.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A., Colin E., Goudenège D., Bonneau D. A snapshot of some pLI score pitfalls. Hum. Mutat. 2019;40:839–841. doi: 10.1002/humu.23763. [DOI] [PubMed] [Google Scholar]