Abstract
Tryptophan is an essential amino acid catabolized initially to kynurenine (kyn), an immunomodulatory metabolite that we have previously shown to promote bone loss. Kyn levels increase with aging and have also been associated with neurodegenerative disorders. Picolinic acid (PA) is another tryptophan metabolite downstream of kyn. However, in contrast to kyn, PA is reported to be neuroprotective and further, to promote osteogenesis in vitro. Thus, we hypothesized that PA might be osteoprotective in vivo. In an IACUC-approved protocol, we fed PA to aged (23-month-old) C57BL/6 mice for eight weeks. In an effort to determine potential interactions of PA with dietary protein we also fed PA in a low-protein diet (8%). The mice were divided into four groups: Control (18% dietary protein), +PA (700 ppm); Low-protein (8%), +PA (700 ppm). The PA feedings had no impact on mouse weight, body composition or bone density. At sacrifice bone and stem cells were collected for analysis, including μCT and RT-qPCR. Addition of PA to the diet had no impact on trabecular bone parameters. However, marrow adiposity was significantly increased in PA-fed mice, and in bone marrow stromal cells isolated from these mice increases in the expression of the lipid storage genes, Plin1 and Cidec, were observed. Thus, as a downstream metabolite of kyn, PA no longer showed kyn’s detrimental effects on bone but instead appears to impact energy balance.
Keywords: Bone loss, Stem cells, Aging, marrow adipocytes
Introduction:
Tryptophan is an essential amino acid that may be either utilized for protein synthesis or broken down into a series of catabolites that have been shown themselves to have biological effects. Tryptophan breakdown occurs predominantly through one of two enzymes, indoleamine 2,3-dioxygenase (IDO), expressed predominantly in inflammatory cells, or tryptophan 2,3-dioxygenase (TDO), expressed predominantly in the liver. TDO-generated kynurenine is the first catabolite in the tryptophan degradation process. TDO is inducible by glucocorticoids and is the main source of circulating kynurenine in younger animals (Ogbechi and others 2020). In contrast, IDO1 expression is inducible by inflammatory cytokines, in particular interferon γ, and may be the main source of tissue kynurenine in older animals, as part of the inflamm-aging process (Badawy 2017). We have previously shown that dietary kynurenine results in an accelerated aging phenotype in mice, with a decrease in bone mass and an increase in marrow adiposity (Refaey and others 2017). Further, in humans, high bone marrow concentrations of kynurenine are associated with an increased risk for hip fracture (Kim and others 2019).
At the other end of this degradation pathway are quinolinic acid (QA) and picolinic acid (PA). 3-hydroxyanthranilic acid (3HAA) is converted to 2-amino-3 carboxymuconic-6 semialdehyde which is then either converted to PA though ACMSD (amino-β-carboxymuconate-semialdehye-decarboxylase) or to QA through a nonenzymatic reaction. QA is a neuroexcitatory metabolite, an NMDA receptor agonist that also increases glutamate levels, lipid peroxidation and oxidative stress in the nervous system (Tan and others 2012). However, QA can be further broken down to nicotinamide adenine dinucleotide (NAD+), an important co-factor in metabolism.
Studies to date on PA have focused mainly on either its neuroprotective or immune regulatory effects. In situations where 3HAA levels exceed ACMSD’s capacity to maintain 3HAA breakdown (e.g., inflammatory states associated with greater tryptophan degradation), then more is converted to QA and there is further neurotoxicity. In fact, high QA and low PA levels have been associated with an increase in suicidal behavior (Brundin and others 2016) and neurodegenerative disorders such as Parkinson’s disease (Tan and others 2012). PA also has been shown to have immunomodulatory properties. Rapisarda et al. (Rapisarda and others 2002) showed that PA at concentrations of 4 mM stimulated macrophage inflammatory protein (MIP) 1α and 1-β synthesis and release by macrophages. Heilman et al. (Heilman and others 2019) showed that there was a negative association between PA and interleukin-6, a cytokine that positively correlated with personality disorders. These data would suggest that PA has a neuroprotective role. There are few studies examining PA effects on the musculoskeletal system but a publication by Vidal et al. (Vidal and others 2015) would suggest that it is also osteoprotective/osteogenic. These authors examined the effect of various doses of PA on mesenchymal stem cell differentiation in vitro and found that at concentrations between 50 and 100 μM, PA increased the number of osteoblastic colony forming units, osteoblastic differentiation and Runx 2 expression; at the higher PA dose of 100 μM, PA also increased osteocalcin expression. However, PA effects on the skeletal system in vivo have not been studied to date. Thus, in the present study we wished to evaluate the impact of PA addition to the diet of aged mice to determine if, similar to its neuroprotective effects, it would be protective against age-related bone loss.
Materials and Methods:
Animal experimental design:
All protocols were conducted in accordance with the guidelines established by the Augusta University Institutional Animal Care and Use Committee (AU-IACUC). For low protein and PA-supplemented diets, experiments were conducted as previously described (Refaey and others 2017). Twenty-three-month-old, male C57BL/6 mice were obtained from the aged rodent colony at the National Institute on Aging. The animals were fed either standard protein (18%) or low protein (8%) diets with or without added picolinic acid (700 ppm) for eight weeks. Picolinic acid concentration was selected based on previously published data (Grant and others 2009). Diets were prepared by Harlan-Teklad in consultation with their nutritionist and were isocaloric purified diets that contained all essential amino acids. The study groups consisted of: 1) 18% (standard) protein diet; 2) 18% protein diet + PA (700 ppm); 3) 8% (low) protein diet and (4) 8% protein + PA (700 ppm) for 8 wks. Animals were euthanized using CO2 overdose followed by thoracotomy according to an AU IACUC-approved protocol. The animals were weighed at the beginning, at the end and every 2 wks during the experiment. Body composition and bone density were measured by dual-energy x-ray absorptionmetry (DXA, PIXImus) as previously described (Refaey and others 2017). The right femora were fixed in 10% neutral buffered formalin (NBF) for 24 hours and then stored in 70% ethanol for plastic embedding (Hamrick and others 2006). The right femora were fixed in NBF for μCT imaging.
Micro-computed tomography (μ-CT):
Femora were scanned with an ex vivo μ-CT system (Skyscan 1174; Skyscan, Aartlesaar, Belgium) as previously described (Refaey and others 2017). Briefly, samples under examination were maintained in a moist environment to prevent dehydration. Four samples were placed in a plastic sample holder with the long axes oriented parallel to the image plane and scanned in air using 15-μm isotropic voxels, 400 ms integration time, 0.5° rotation step, 360° rotation, and frame averaging of 5.
Stacks of two-dimensional X-ray shadow projections were used to reconstruct cross-sectional images using NRecon software (Skyscan) and subjected to morphometric analyses using CTAn software (Skyscan). The three-dimensional morphometric parameters of bone microarchitecture were calculated using CTAn (Skyscan) software and the parameters measured included bone volume fraction [bone volume/total volume (BV/TV)], trabecular thickness (Tb.Th) and number (Tb.N) and trabecular separation (Tb.Sp). Significance was evaluated by ANOVA with Tukey’s post-hoc tests.
Marrow Adiposity
Right femora were decalcified in 4% EDTA for approximately 2–3 weeks and then dehydrated, cleared in xylene, and embedded in paraffin. Histological sections were stained with hematoxylin and eosin for quantification of marrow adipocyte area fraction (Ad.Ar/M.Ar, %) and marrow adipocyte density (N.Ad/M.Ar, #/mm^2), as previously described (Pierce and others 2019). Significance was evaluated by ANOVA followed by Tukey’s post-hoc tests.
Bone Marrow Stromal Cell (BMSC) Cultures
Bone marrow was flushed from the long bones, and adherent BMSC were cultured in osteogenic culture medium for 7 days (RNA) or 21 days (mineralized matrix production) as previously described (McGee-Lawrence and others 2016).
Isolation of RNA, synthesis of cDNA, and real-time PCR:
Total RNA was isolated from the cultured cells and tibiae of mice as previously described (Refaey and others 2017). Tibiae were ground in liquid nitrogen with a mortar and pestle and the powdered tissue was dissolved in TRIzol. RNA was isolated following the manufacturer’s instructions, and the quality of the RNA preparations was monitored by absorbance at 260 and 280 nm (Helios-Gamma, Thermo Spectronic, Rochester, NY). The RNA was reverse-transcribed into complementary deoxyribonucleic acid (cDNA) using iScript reagents from Bio-Rad on a programmable thermal cycler (PCR-Sprint, Thermo Electron, Milford, MA). The cDNA (50 ng) was amplified by real-time PCR using a Bio-Rad iCycler and ABgene reagents (Fisher Scientific, Pittsburgh, PA) and appropriate primers (Table 1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control for normalization.
Table 1:
Primers.
| Gene | Primer |
|---|---|
| Runx2 | GGAAAGGCACTGACTGACCTA |
| Cidec | TCCAAGCCCTGGCAAAAGAT |
| Plin1 | TGCTGCACGTGGAGAGTAAG |
| GAPDH | CATGGCCTCCAAGGAGTAAGA |
Alizarin red assay
Alizarin red assays were performed as previously described (Fulzele and others 2013). Briefly, BMSCs (106 cells per well) were plated in 6-well plates followed by osteogenic treatment with and without PA. Alizarin red staining was performed after 21 days as previously described (McGee-Lawrence and others 2013) Image density in individual wells was calculated using Adobe Photoshop and significance evaluated by ANOVA and Tukey’s post-hoc tests
Statistical analysis:
All the data are expressed as mean ±SD. Statistical analyses were performed as previously described (Refaey and others 2017) using Prism 8 software (Graphpad). Significance for all tests was determined at alpha=0.05.
Results:
PA did not impact body weight or body composition
Initial outcome measurements focused on body weight and composition to evaluate possible PA toxicity and effects on muscle mass as measured by lean body mass, as we had previously found for kynurenine and muscle metabolism (Dukes and others 2015). As shown in Figure 1, Panel A, mice on the low dietary protein (8%) diet tended to have lower body weights but these differences were not statistically significant and are consistent with our previous published studies (Refaey and others 2017). Further, addition of PA to the two dietary protein conditions did not have any additional effect on weight (Panel A), lean body mass (Panel B) or fat content (Panel C).
Figure 1: PA had no impact on either body weight or body composition.
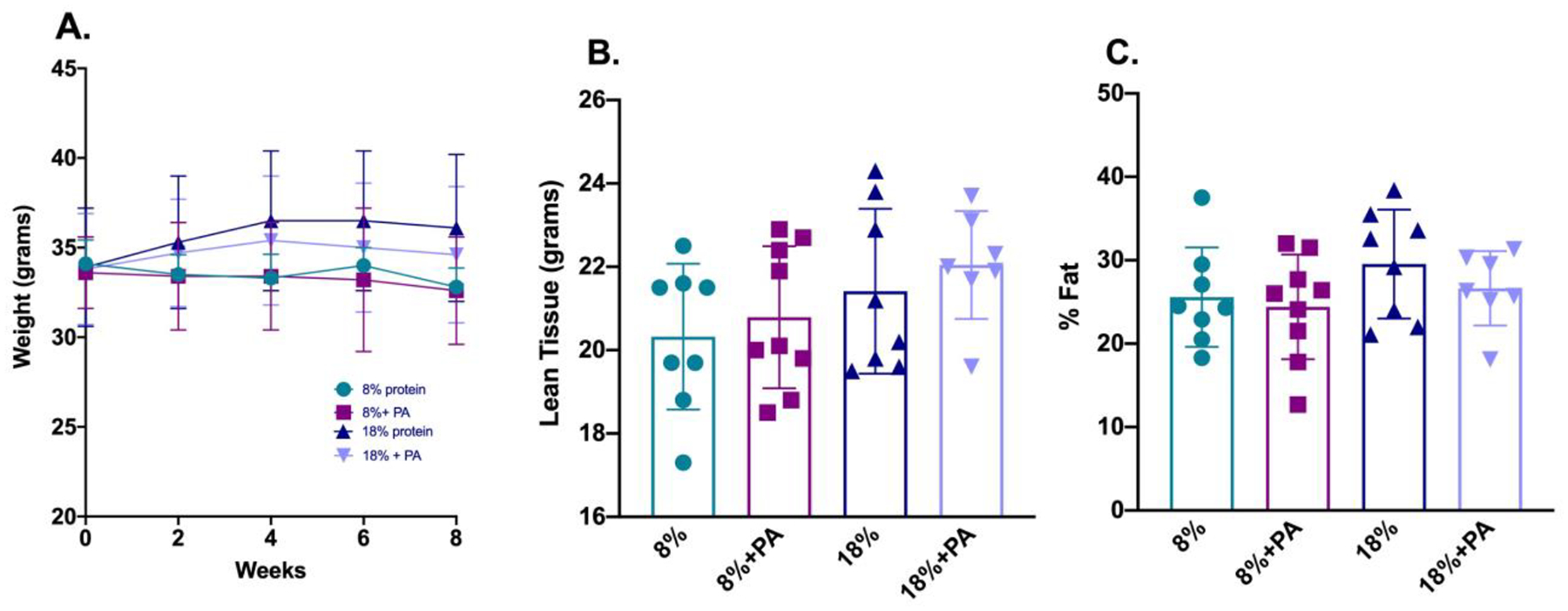
Twenty-three-month-old C57BL/6 mice from the NIA aging colony were placed on either a low (8%) or standard (18%) protein diet with or without added PA for eight weeks. Body weight was measured throughout the eight-week period (Panel A). Body composition was measured using the PixiMus densitometer. Dietary PA had no effect on either lean body mass (Panel B) or % fat (Panel C). Values are shown for each individual mouse with each bar representing the mean ± SD of the 7–9 animals per group.
PA did not impact bone mineral density
We previously showed that 12 month old C57BL/6 mice placed on a low protein diet had a lower bone mineral density, as measured by bone densitometry, than those fed a standard protein diet (Refaey and others 2017). However, dietary PA did not have any beneficial effect on bone mass in these aged mice (Figure 2) whether measured as total density (Panel A), spinal or trabecular bone density (Panel B) or femoral or cortical bone density (Panel C). These findings are in contrast to those we reported for another tryptophan catabolite, kynurenine, which decreased bone density after an eight-week dietary intervention (Refaey and others 2017).
Figure 2: PA was not osteoprotective against age-induced bone loss.
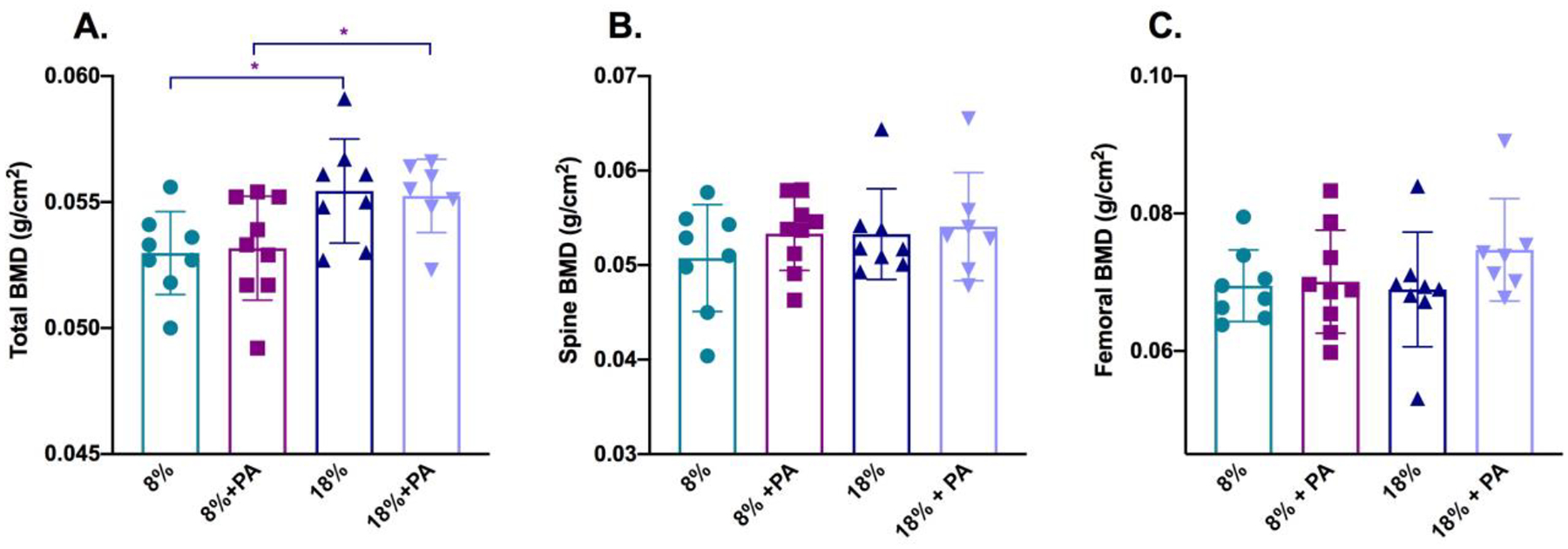
Twenty-three-month-old C57BL/6 mice were placed on a low (8%)- or standard (18%)-protein diet with or without PA for eight weeks. At the end of the two-month period, bone density measurements were made on the now 25-month-old mice using a PixiMus densitometer. Total bone mineral density was significantly reduced in the mice placed on the low-protein diet. However, PA supplementation did not have any additional effect on either total bone density (Panel A), spinal bone density (Panel B) or femoral bone density (Panel C). Data are presented as mean ± SD; n=7–9 for each group. *=p<0.05
PA did not impact trabecular bone parameters
To better evaluate possible PA effects on trabecular bone, we used μCT to evaluate the microarchitecture of the femoral head as previously described (Refaey and others 2017). As seen in Figure 3, PA had no significant effects on bone volume over total volume (BV/TV-Panel A), trabecular thickness (Tb.Th-Panel B), trabecular number (Tb.N-Panel C), or trabecular separation (Tb.Sp.-Panel D). Also shown below the graphs are representative 3D reconstructions of a femur removed from mice who received the specified treatment regimen.
Figure 3: Bone microarchitecture was not affected by dietary PA supplementation.
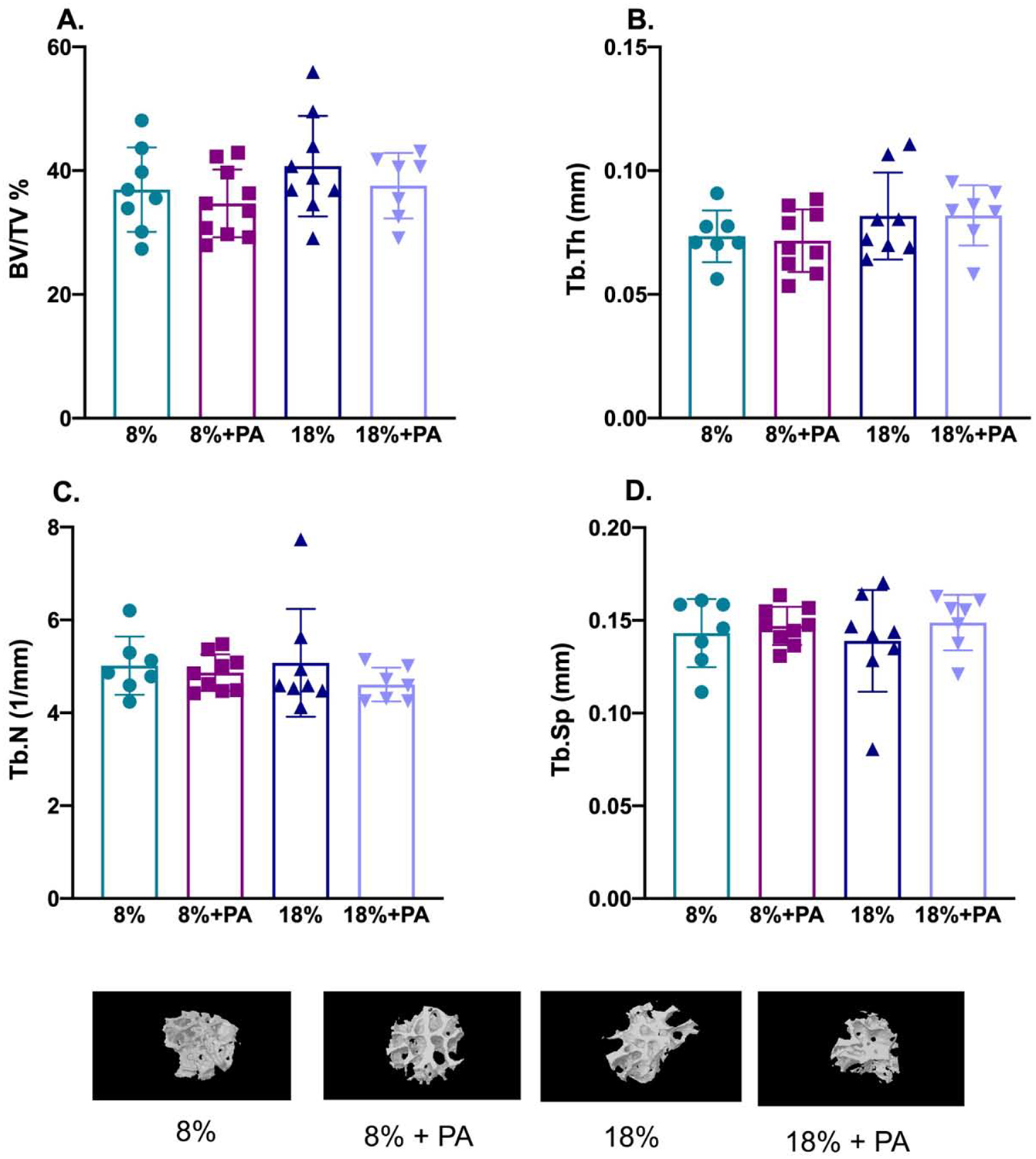
Twenty-three-month-old mice were fed the indicated diets for 8 weeks and bone microarchitectural parameters of femora monitored ex vivo by μCT after sacrifice. PA did not have any significant effect on either the percentage of bone volume/total volume (BV/TV in %; Panel A); trabecular thickness (Tb.Th in mm; Panel B); trabecular number (Tb.N, per mm; Panel C); or trabecular separation (Tb.Sp, in mm; Panel D). Also shown are representative 3-dimensional μCT reconstructions of femora from mice fed the indicated diets. Data are presented as mean ± SD; n=7–10 for each diet group.
PA increased marrow adiposity
We had previously shown that kyn feeding to mice resulted in bone loss and an increase in marrow adiposity suggestive of an accelerated aging phenotype (Refaey and others 2017). Thus, we next examined the impact of PA, a downstream kyn catabolite, on marrow adiposity (Figure 4). Modification of the dietary protein content had no impact on adiposity, which was similar whether the mice were on the 8% or 18% dietary protein regimen. However, PA addition to the standard protein diet (18%) significantly increased adipocyte number (Panel B). Of note this study used older mice (23 months) which already have higher marrow adiposity than mature mice (12 months); nevertheless, this parameter was further increased by the addition of PA. We next decided to evaluate the impact of PA on gene expression associated with lipid storage since we had previously shown these to be modulated by kynurenine (Refaey and others 2017).
Figure 4: PA supplementation increased adipocyte number for mice on the 18% dietary protein.
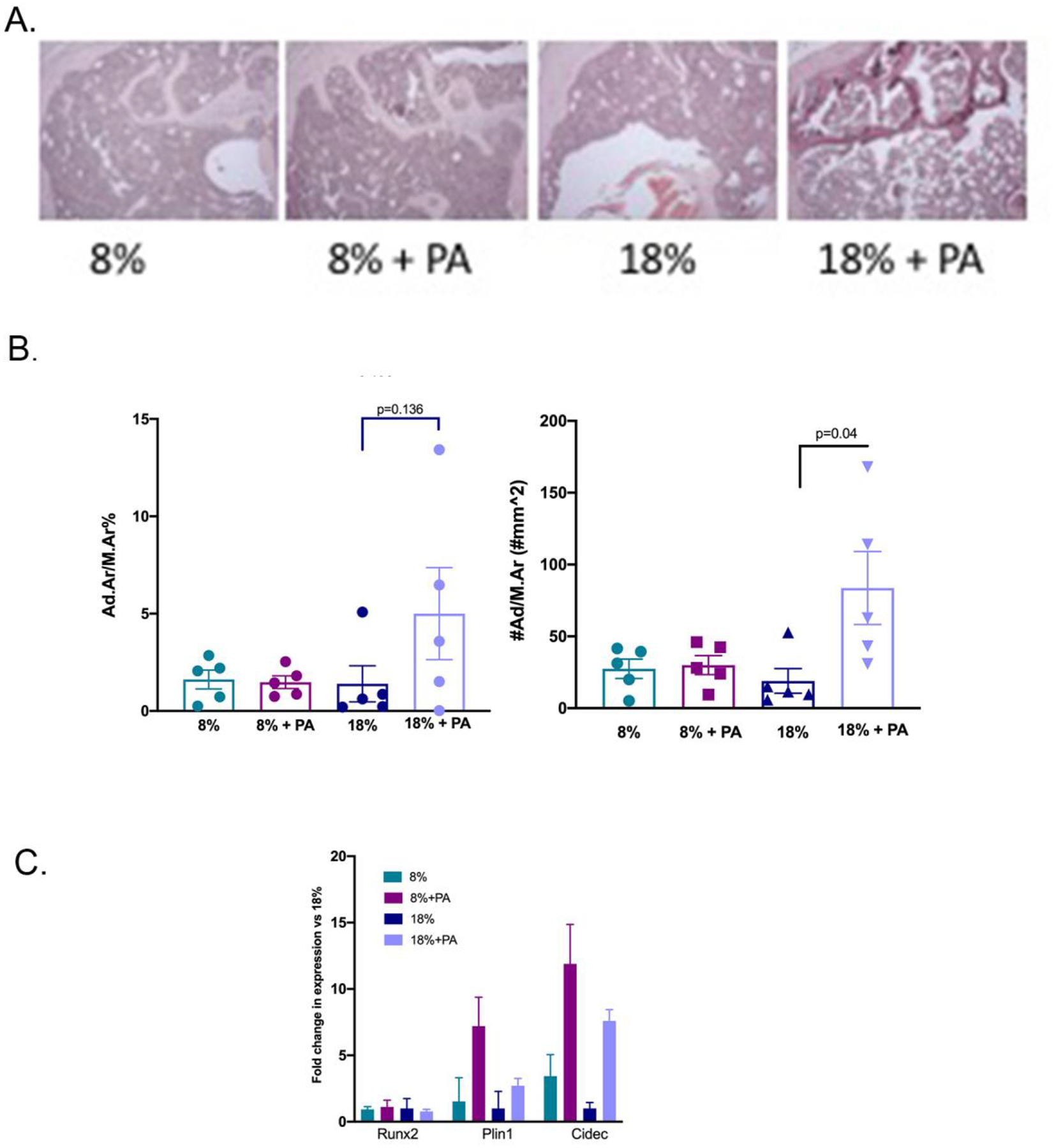
Tibiae from mice fed the four diets were isolated and the bone marrow analyzed for adiposity as described in Methods. (A) Adipocyte size was calculated using adipocyte area divided by marrow area. Although there were no significant differences between the groups, adipocyte size tended to be larger for those mice on the PA and 18% dietary protein. (B) Adipocyte number/area was counted and expressed as number/mm2. Adipocyte number was significantly increased in the mice placed on the 18% protein with PA supplementation consistent with the results from Panel A. Values represent the mean ± SD of n=5 mice per group. (C) BMSCs were isolated from the humerii of animals fed for 8 weeks with the specified diets. The expression of the lipid storage genes, Plin1 and Cidec and of Runx2, associated with osteoblastic differentiation, was determined by quantitative RT-PCR as described in Methods. Values are expressed as means ± SD of n=3.
We had previously shown an effect of kynurenine on lipid storage genes Cidec and Plin1 in early osteoblastic cell cultures (Refaey and others 2017). To explore potential causal relationships between PA and marrow adiposity, BMSCs were isolated from the bones of mice treated with the low or high dietary protein ± PA. PA treatment increased expression of the lipid storage genes, Cidec and Plin1 (Figure 4C). Interestingly, the larger effect on lipid storage gene expression was seen in the low protein (8%) conditions. We also examined RUNX2 expression and did not find any impact of PA treatment on RUNX2.
PA had no impact on bone mineralization as measured by Alizarin Red
BMSCs were isolated from mice treated with low/high protein ± PA. PA has been previously shown to promote bone mineralization (Vidal and others 2015). Thus, we next examined the impact of PA treatment on BMSCs of mice exposed to the different dietary manipulations (Figure 5). We found that the low-protein diet resulted in impaired mineralization but otherwise PA had no impact on mineralization, as measured by alizarin red.
Figure 5: PA supplementation had no effect on osteoblastic mineralization.
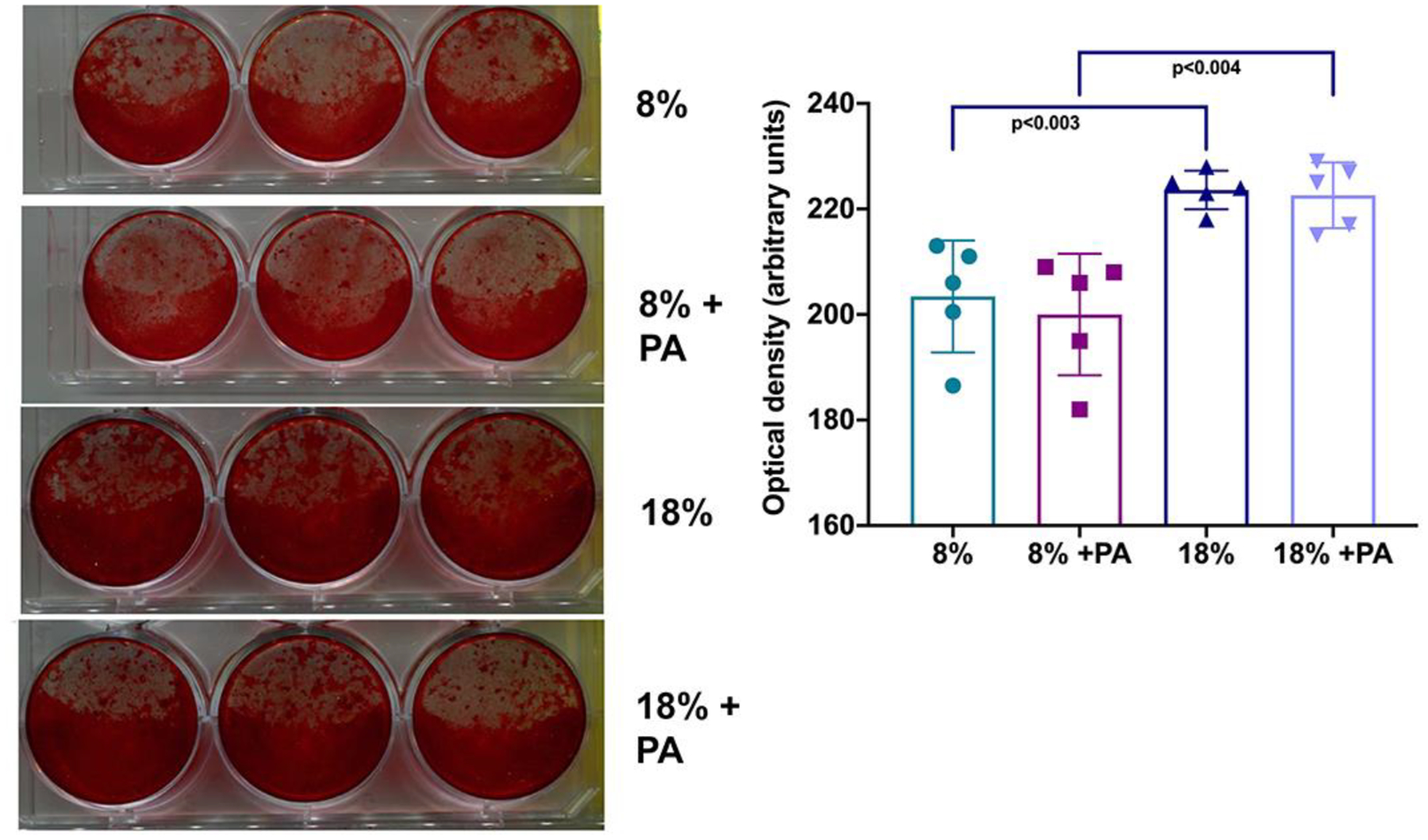
BMSCs isolated from C57BL/6 humerii were induced to differentiate using osteogenic medium, and bone mineralization was determined by alizarin red staining as described in Methods. Shown in the left panel are representative wells, and their quantification using Photoshop is illustrated in the right panel. BMSC mineralization was significantly lower in the mice that received the low-protein diet, but PA had no additional effect. Results are representative of at least three separate experiments
Discussion
To sum up, dietary PA treatment of aged mice altered their marrow adiposity but had no effect on bone mass. PA is a downstream kyn catabolite and our initial hypothesis was that PA would be osteoprotective for bone, as it appears to be for the nervous system (Lovelace and others 2017). Our current data are in contrast to those of Vidal et al. (Vidal and others 2015) who found that PA had an osteogenic effect. The cause of this discrepancy is not clear but may relate to differences between concentrations used in vivo versus in vitro, the age of the animals from which the BMSCs were derived (2-month versus 23-month old) or differences in the type of PA exposure (direct versus through dietary intake). It should also be noted that the osteogenic studies with PA in vitro were performed with either human cells or IDO knockout mouse-derived BMSCs so this might also be a contributory factor to the observed differences.
The first metabolite of tryptophan is kyn, with breakdown catalyzed by either IDO or TDO. Although the definitive receptor for kyn action has not yet been identified, at least some of its actions may be mediated by the aryl hydrocarbon receptor (AhR) (Jaronen and Quintana 2014). AhR is an evolutionarily conserved receptor belonging to the basic helix-loop-helix family of dimerizing transcription factors initially characterized as a receptor for environmental toxins such as 2,3,7,8-tetrachlorodibenzo-p-dioxin but widely expressed in normal and abnormal tissue and bound by endogenous ligands including kynurenine (Jaronen and Quintana 2014). Thus, it is possible that some of the differences in bone mass observed between PA and kyn treatment are related to differences in receptor affinity for AhR or for other tissue specific receptors. It is also possible that PA and kyn show differences in epigenetic activation. Thus, we have previously shown that kyn suppresses histone deacetylase-3 (Hdac-3) (Refaey and others 2017), an epigenetic factor known to be important in bone mineralization (McGee-Lawrence and others 2013) and impairs mineralization as measured by alizarin red staining (Refaey and others 2017). Our current study shows that PA does not inhibit bone mineralization as does kyn. This result implies that PA and kyn have intrinsically different mechanisms of action on bone cells. A potential concern is that we only utilized a single concentration of PA for our studies and that bone cell-specific effects required a higher PA dose in the study of Vidal et al. (Vidal and others 2015). Also, our studies were performed in male, rather than female, mice. As reviewed by Grant et al. (Grant and others 2009) intraperitoneal PA administration to C57BL/6 mice at 100 mg/kg showed antitumor activity (Ruffmann and others 1987) and endogenous concentrations of PA in human plasma have been reported to be 0.299 μM (Smythe and others 2003). The dose utilized for the present study was estimated to be twice the reported physiologic level but still less than the lower dose of 1 μM used by Vidal, for which they did not observe an osteogenic effect (Vidal and others 2015). Thus, although the PA dose selected for use in vivo was based on published literature (Grant and others 2009), these studies focused on its neuroprotective effect, which could be different from any potential osteoprotective effects. One additional caveat is that in these various studies, comparing the actual PA levels is difficult in view of the different modes of administration and conditions. Local tryptophan breakdown is related predominantly to IDO1 activity which is in turn activated by inflammatory cytokines such as interferon-γ. Our data, however, are consistent with increased marrow adiposity being the initial response to inflammation and perhaps with even greater inflammation and thus greater PA production, osteogenic protective mechanisms may then be activated.
We had previously shown that kyn administration resulted in increased marrow adiposity and altered gene expression of the lipid storage genes Cidec and Plin1 in early osteoblastic cultures (Refaey and others 2017), which is why they were measured in the present study. For the current experiment we used aged mice, but PA still had a similar effect to kyn on marrow adiposity. Although this might be a direct effect on BMSC differentiation, our experiments did not show any PA effects on mineralization of BMSC in later osteoblastic cultures in vitro (alizarin red), in contrast to what we observed with kyn (Refaey and others 2017). Thus, it seems more likely that PA effects on marrow adiposity are indirect. PA has a number of indirect systemic effects one of which is chelation of metal ions like iron, chromium and zinc (Michalowska and others 2015). Interestingly, PA’s ability to bind zinc has been shown to at least partially contribute to its neuroprotective effect against QA toxicity (Jhamandas and others 1998). In a rat ovariectomy model, Li et al. (Li and others 2015) showed that increased adiponectin secretion and increased marrow adiposity were prevented by zinc addition. Thus, PA could potentially increase marrow adiposity through zinc chelation. Another potential mechanism for PA effects on bone could be through its effect on iron chelation. PA has been shown to inhibit cellular iron uptake, and iron chelation under conditions of oxidative stress inhibits osteoclastic activity and increases bone mass (Balogh and others 2018; Balogh and others 2016; Chen and others 2015; Guo and others 2015). In fact, a combination of factors may be involved. PA has been shown to have antiproliferative and cytotoxicity-promoting effects on macrophages; PA effects may serve to prime the surrounding cells but require a second signal provided by inflammation (e.g., interferon gamma) to trigger a response (Varesio and others 1990).
In conclusion, although tryptophan breakdown results in generation of a number of catabolites, these breakdown products may themselves have tissue-specific bioactivity. The effects of these catabolites may depend on localization (the sites at which they are generated, e.g., central versus peripheral tissues), concentration (which may vary depending on the degree of inflammation), duration of exposure (chronic versus acute inflammation) and indirect effects (e.g., through chelation of metal ions). Thus, as discussed above determining a true “physiological” concentration may be difficult since PA effects are probably site- and context-dependent and change over time, making these conditions difficult to reproduce with feeding or injection studies. Therapeutically, however, our findings suggest that ultimately it would be beneficial to accelerate the conversion of catabolic metabolites such as kynurenine to less toxic or beneficial metabolites such as PA.
Highlights.
Tryptophan oxidation products accumulate with age and inflammation
Tryptophan breakdown products such as kynurenine result in bone loss
Picolinic Acid is a breakdown product downstream of kynurenine
Picolinic Acid administration did not negatively impact bone mass but increased marrow adiposity
Tryptophan metabolites may have varied effects on tissues in which they accumulate
Acknowledgements
This work was supported by a grant from the National Institutes of Aging P01 AG 036675. WBB is supported in part by the Veterans Administration. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Badawy AA Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology. 112:248–263; 2017 [DOI] [PubMed] [Google Scholar]
- Balogh E; Paragh G; Jeney V Influence of Iron on Bone Homeostasis. Pharmaceuticals (Basel). 11; 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh E; Tolnai E; Nagy B Jr.; Nagy B; Balla G; Balla J; Jeney V Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim Biophys Acta. 1862:1640–1649; 2016 [DOI] [PubMed] [Google Scholar]
- Brundin L; Sellgren CM; Lim CK; Grit J; Palsson E; Landen M; Samuelsson M; Lundgren K; Brundin P; Fuchs D; Postolache TT; Traskman-Bendz L; Guillemin GJ; Erhardt S An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl Psychiatry. 6:e865; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B; Li GF; Shen Y; Huang XI; Xu YJ Reducing iron accumulation: A potential approach for the prevention and treatment of postmenopausal osteoporosis. Exp Ther Med. 10:7–11; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes A; Davis C; El Refaey M; Upadhyay S; Mork S; Arounleut P; Johnson MH; Hill WD; Isales CM; Hamrick MW The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrition. 31:1018–1024; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulzele S; Chothe P; Sangani R; Chutkan N; Hamrick M; Bhattacharyya M; Prasad PD; Zakhary I; Bowser M; Isales C; Ganapathy V Sodium-dependent vitamin C transporter SVCT2: Expression and function in bone marrow stromal cells and in osteogenesis. Stem Cell Res. 10:36–47; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RS; Coggan SE; Smythe GA The physiological action of picolinic Acid in the human brain. Int J Tryptophan Res. 2:71–79; 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JP; Pan JX; Xiong L; Xia WF; Cui S; Xiong WC Iron Chelation Inhibits Osteoclastic Differentiation In Vitro and in Tg2576 Mouse Model of Alzheimer’s Disease. PLoS One. 10:e0139395; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick MW; Ding KH; Pennington C; Chao YJ; Wu YD; Howard B; Immel D; Borlongan C; McNeil PL; Bollag WB; Curl WW; Yu J; Isales CM Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 39:845–853; 2006 [DOI] [PubMed] [Google Scholar]
- Heilman P; Hill MN; Coussons-Read M; Brundin L; Coccaro EF Role of the kynurenine pathway and the endocannabinoid system as modulators of inflammation and personality traits. Psychoneuroendocrinology. 110:104434; 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaronen M; Quintana FJ Immunological Relevance of the Coevolution of IDO1 and AHR. Front Immunol. 5:521; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas KH; Boegman RJ; Beninger RJ; Flesher S Role of zinc in blockade of excitotoxic action of quinolinic acid by picolinic acid. Amino Acids. 14:257–261; 1998 [DOI] [PubMed] [Google Scholar]
- Kim BJ; Hamrick MW; Yoo HJ; Lee SH; Kim SJ; Koh JM; Isales CM The Detrimental Effects of Kynurenine, a Tryptophan Metabolite, on Human Bone Metabolism. J Clin Endocrinol Metab. 104:2334–2342; 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B; Liu H; Jia S Zinc enhances bone metabolism in ovariectomized rats and exerts anabolic osteoblastic/adipocytic marrow effects ex vivo. Biol Trace Elem Res. 163:202–207; 2015 [DOI] [PubMed] [Google Scholar]
- Lovelace MD; Varney B; Sundaram G; Lennon MJ; Lim CK; Jacobs K; Guillemin GJ; Brew BJ Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 112:373–388; 2017 [DOI] [PubMed] [Google Scholar]
- McGee-Lawrence ME; Bradley EW; Dudakovic A; Carlson SW; Ryan ZC; Kumar R; Dadsetan M; Yaszemski MJ; Chen Q; An KN; Westendorf JJ Histone deacetylase 3 is required for maintenance of bone mass during aging. Bone. 52:296–307; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Lawrence ME; Carpio LR; Schulze RJ; Pierce JL; McNiven MA; Farr JN; Khosla S; Oursler MJ; Westendorf JJ Hdac3 Deficiency Increases Marrow Adiposity and Induces Lipid Storage and Glucocorticoid Metabolism in Osteochondroprogenitor Cells. J Bone Miner Res. 31:116–128; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowska M; Znorko B; Kaminski T; Oksztulska-Kolanek E; Pawlak D New insights into tryptophan and its metabolites in the regulation of bone metabolism. J Physiol Pharmacol. 66:779–791; 2015 [PubMed] [Google Scholar]
- Ogbechi J; Clanchy FI; Huang YS; Topping LM; Stone TW; Williams RO IDO activation, inflammation and musculoskeletal disease. Exp Gerontol. 131:110820; 2020 [DOI] [PubMed] [Google Scholar]
- Pierce JL; Ding KH; Xu J; Sharma AK; Yu K; Del Mazo Arbona N; Rodriguez-Santos Z; Bernard P; Bollag WB; Johnson MH; Hamrick MW; Begun DL; Shi XM; Isales CM; McGee-Lawrence ME The glucocorticoid receptor in osteoprogenitors regulates bone mass and marrow fat. J Endocrinol; 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapisarda A; Pastorino S; Massazza S; Varesio L; Bosco MC Antagonistic effect of picolinic acid and interferon-gamma on macrophage inflammatory protein-1alpha/beta production. Cell Immunol. 220:70–80; 2002 [DOI] [PubMed] [Google Scholar]
- Refaey ME; McGee-Lawrence ME; Fulzele S; Kennedy EJ; Bollag WB; Elsalanty M; Zhong Q; Ding KH; Bendzunas NG; Shi XM; Xu J; Hill WD; Johnson MH; Hunter M; Pierce JL; Yu K; Hamrick MW; Isales CM Kynurenine, a Tryptophan Metabolite That Accumulates With Age, Induces Bone Loss. J Bone Miner Res. 32:2182–2193; 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffmann R; Schlick R; Chirigos MA; Budzynsky W; Varesio L Antiproliferative activity of picolinic acid due to macrophage activation. Drugs Exp Clin Res. 13:607–614; 1987 [PubMed] [Google Scholar]
- Smythe GA; Poljak A; Bustamante S; Braga O; Maxwell A; Grant R; Sachdev P ECNI GC-MS analysis of picolinic and quinolinic acids and their amides in human plasma, CSF, and brain tissue. Adv Exp Med Biol. 527:705–712; 2003 [DOI] [PubMed] [Google Scholar]
- Tan L; Yu JT; Tan L The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. J Neurol Sci. 323:1–8; 2012 [DOI] [PubMed] [Google Scholar]
- Varesio L; Clayton M; Blasi E; Ruffman R; Radzioch D Picolinic acid, a catabolite of tryptophan, as the second signal in the activation of IFN-gamma-primed macrophages. J Immunol. 145:4265–4271; 1990 [PubMed] [Google Scholar]
- Vidal C; Li W; Santner-Nanan B; Lim CK; Guillemin GJ; Ball HJ; Hunt NH; Nanan R; Duque G The kynurenine pathway of tryptophan degradation is activated during osteoblastogenesis. Stem Cells. 33:111–121; 2015 [DOI] [PubMed] [Google Scholar]


