Abstract
Aims
To describe the real‐world prevalence and consequences of hypertriglyceridaemia.
Materials and methods
We searched two large patient databases, the National Health and Nutrition Examination Survey (NHANES) database (2007–2014) and the Optum Research Database, as well as electronic medical records from two Kaiser Permanente regions.
Results
The NHANES data showed that ~26% of US adults, including nearly one‐third of statin users, had at least borderline hypertriglyceridaemia (triglycerides [TGs] ≥1.69 mmol/L), and ~40% of adults with diabetes had levels of ≥150 mg/dL despite statin use. The Optum analyses demonstrated that those with TG levels ≥1.69 mmol/L who were on statins had a significantly increased risk of composite initial major cardiovascular (CV) events (hazard ratio [HR] 1.26, 95% confidence interval [CI] 1.19–1.34; P < 0.001 vs. patients with TGs <150 mg/dL). This was accompanied by increased healthcare utilization and direct healthcare costs (HR 1.12, 95% CI 1.08–1.16; P < 0.001). In the analyses of the Kaiser Permanente records, patients with diabetes and TG levels 2.26–5.64 mmol/L had significantly higher adjusted incidence rates of non‐fatal myocardial infarction (rate ratio 1.30, 95% CI 1.08–1.58; P = 0.006), non‐fatal stroke (rate ratio 1.23; 95% CI 1.01–1.49; P = 0.037) and coronary revascularization (rate ratio 1.21; 95% CI 1.02–1.43; P = 0.027), but not unstable angina (rate ratio 1.33; 95% CI 0.87–2.03; P = 0.185) compared with patients with TG levels <1.69 mmol/L.
Conclusions
Real‐world analyses suggest that elevated TGs are prevalent and commonly associated with increased CV risk. CV outcomes trials in patients with established hypertriglyceridaemia will clarify whether strategies to reduce TG levels can ameliorate residual CV risk in patients taking statins.
Keywords: atherosclerosis, cardiovascular disease, cost‐effectiveness, database research, dyslipidaemia, hypertriglyceridaemia
1. INTRODUCTION
Residual cardiovascular (CV) risk in statin‐treated patients persists despite control of LDL cholesterol.1 Other atherogenic lipids and lipoproteins, such as triglycerides (TGs) and TG‐rich lipoproteins (TRLs), are also likely causal and prognostic factors as well as potential treatment targets in atherosclerotic CV disease (ASCVD).1, 2 The biological plausibility of elevated TG and TRL levels as factors in residual CV risk is supported by epidemiological, genetic and clinical evidence. TG levels correlate with heightened risk for CV events in patients with well‐controlled LDL cholesterol levels on statin therapy.3 Given that TGs are hydrophobic and do not float freely in plasma but are carried in such TG‐enriched lipoproteins as VLDL, VLDL remnants and intermediate‐density lipoprotein (IDL), the relationships observed probably reflect atherogenic effects exerted by both TGs and TRLs; this is reflected in non‐HDL cholesterol.4, 5, 6, 7, 8, 9, 10, 11 TG‐enriched remnants, which include both small VLDL and IDL, are proinflammatory, induce endothelial dysfunction, and can be isolated from atheromatous plaque.5, 11
In addition to the direct association between TG levels and CVD risk, elevated TGs also contribute to the atherogenic lipid triad. In patients with elevated TGs, there is typically a reduction in the activity of lipoprotein lipase, the enzyme primarily responsible for hydrolysing TGs in the core of TRL. As TRL levels increase in plasma, cholesteryl ester transfer protein (CETP) engages in neutral lipid exchange between TRLs and both LDL cholesterol and HDL particles. CETP catalyses a 1:1 exchange of TGs for cholesterol between TRL and HDL and LDL particles. The LDL and HDL particles become more enriched with TGs and become better substrates for lipolysis by hepatic lipase, which results in increased HDL catabolism (and corresponding reductions in serum levels of this lipoprotein) and the formation of large numbers of smaller, denser LDL particles. This atherogenic lipid triad, consisting of high concentrations of TGs/TRLs, increased numbers of small‐dense LDLs and low levels of HDL, is particularly prevalent in patients with insulin resistance and diabetes mellitus and is believed to underlie much of the accelerated atherogenesis in these patients.
Epidemiological studies have shown that elevated TGs correlate with elevated CV risk,12, 13, 14 and the American Heart Association (AHA) has long recognized that elevated TGs are a marker of CV risk.15 The role of elevated TGs in the development and progression of ASCVD has been further elucidated in genetic studies,16, 17, 18, 19, 20, 21, 22, 23 genome‐wide analysis studies,24, 25, 26, 27 and Mendelian randomization studies.14, 28, 29, 30, 31 Some common polymorphisms have been associated with higher TG levels and increased CV risk, including APOA5 and LPL variants.32 Analyses of clinical data have also demonstrated that lower on‐treatment TGs correlate with reduced CV risk.3, 33 TRLs can modulate ASCVD by becoming trapped in the subendothelial space, where they are scavenged by macrophages to form foam cells, which potentiate both inflammation and atherosclerotic plaque formation.34 There is evidence that fatty acids produced from TGs and TRLs are pro‐inflammatory and contribute to atherogenesis.35, 36, 37 Inflammatory mediators lead to increased expression of vascular cell adhesion molecule‐1, which leads to adhesion of monocytes and T‐helper cells.38 Inflammation and increased influx of leukocytes into the intima trigger atherosclerotic changes and ultimately create the pathophysiological environment for the development of ASCVD.34
As a result of the increasing prevalence of metabolic syndrome, diabetes and obesity, which induces the “atherogenic dyslipidaemia” phenotype,39, 40 many clinicians are faced with unclear therapeutic decisions when managing CVD risk in patients with LDL cholesterol controlled by statins. The objective of the present review was to describe the real‐world consequences of borderline hypertriglyceridaemia (TG levels ≥1.69–2.26 mmol/L) and hypertriglyceridaemia (TG levels ≥2.26 mmol/L) based on large patient databases, including the prevalence of hypertriglyceridaemia, distribution of TGs in the population, 10‐year ASCVD risk from the National Health and Nutrition Examination Survey (NHANES), as well as ASCVD risk, healthcare resource utilization and costs in patients with hypertriglyceridaemia with or without diabetes from the Optum Research Database and the electronic medical records of the Northwest and Southern California regions of Kaiser Permanente (Kaiser Permanente database). We chose to review results from these databases because they are large and representative of the US population.
2. HYPERTRIGLYCERIDAEMIA IN REAL‐WORLD DATABASES
2.1. NHANES database
The NHANES database includes laboratory, medical history and prescription data. Analysis of the ASCVD burden in patients with TG levels ≥1.69 mmol/L from this database included data from 23 482 adults collected over an 8‐year period from 2007 to 2014, and forms a representative sample of the US population.41 The final sample included 9593 adults aged ≥20 years (projected to represent 219.9 million in the overall US population) who had morning fasting TG data available. A subgroup of 1448 adults also had a diagnosis of diabetes.
Based on population‐weighted estimates from this analysis, 26% of US adults (~57 million individuals) and nearly one‐third of statin users have TG levels ≥1.69 mmol/L (Table 1).41 This is broadly in agreement with a similar analysis conducted in a European cohort in which 21% had TG levels ≥1.69 mmol/L.42 This means that greater proportions of patients that were not treated with statins had desirable TG levels (<1.69 mmol/L) compared with statin‐treated patients (Table 1).41 TG levels in statin‐treated patients were elevated even when LDL cholesterol was controlled at <2.59 mmol/L, with 27.6% having TG levels ≥1.69 mmol/L and 12.2% having TG levels ≥2.26 mmol/L.41 By comparison, for those not taking a statin who had LDL cholesterol <2.59 mmol/L, 16.4% had TG levels ≥1.69 mmol/L and 8.2% had hypertriglyceridaemia (TGs ≥2.26 mmol/L).41 Increased age (odds ratio [OR] 1.15, 95% confidence interval [CI] 1.09–1.23) and body mass index (OR 1.18, 95% CI 1.07–1.31), as well as female vs male gender (OR 1.17, 95% CI 0.99–1.37), lower HDL cholesterol (OR 0.26, 95% CI 0.21–0.31), higher LDL cholesterol (OR 1.48, 95% CI 1.32–1.66) and diabetes (OR 1.64, 95% CI 1.28–2.09) were all independently associated with TG levels ≥1.69 mmol/L.41 Among US adults with diabetes, approximately 40% had TG levels ≥1.69 mmol/L, and half of these had TG levels ≥2.26 mmol/L, regardless of statin use.43
Table 1.
Proportion of US adults according to triglyceride category and current statin use, NHANES 2007–201441
| TG level | All (n = 9593, 219.9 M)a | With statin use (n = 1847, 38.9 M)a | Without statin use (n = 7746, 181.0 M)a | P b |
|---|---|---|---|---|
| <1.69 mmol/L | 7070 (163.0 M, 74.1%) | 1287 (26.6 M, 68.4%) | 5783 (136.4 M, 75.3%) | <0.0001 |
| 1.69–2.25 mmol/L | 1287 (29.5 M, 13.4%) | 284 (6.3 M, 16.2%) | 1003 (23.2 M, 12.8%) | |
| 2.26–5.64 mmol/L | 1141 (25.3 M, 11.5%) | 259 (5.6 M, 14.5%) | 882 (19.7 M, 10.9%) | |
| ≥5.65 mmol/L | 95 (2.1 M, 1.0%) | 17 (0.4 M, 0.9%) | 78 (1.7 M, 1.0%) |
Abbreviations: NHANES, National Health and Nutrition Examination Survey; TG, triglyceride.
Reprinted from J Clin Lipidol, 2019;13 (1):100–108. Fan W, Philip S, Granowitz C, Toth P, Wong N. Hypertriglyceridemia in statin‐treated US adults: The National Health and Nutrition Examination Survey. Copyright 2019, with permission from Elsevier.41
Number of participants in each category (projected population in millions [M] and % of total).
P < 0.0001 across TG categories, comparing those with vs. without statin use.
Among all adults, with or without statin treatment, there was a significant (P < 0.0001) increase in the American College of Cardiology (ACC)/AHA 10‐year ASCVD risk score across increasing TG strata (Figure 1A).41 It was estimated that over the next decade, 3.4 million ASCVD events will occur among people with TG levels ≥150 mg/dL, with about one‐third of expected events to occur among statin users.
Figure 1.
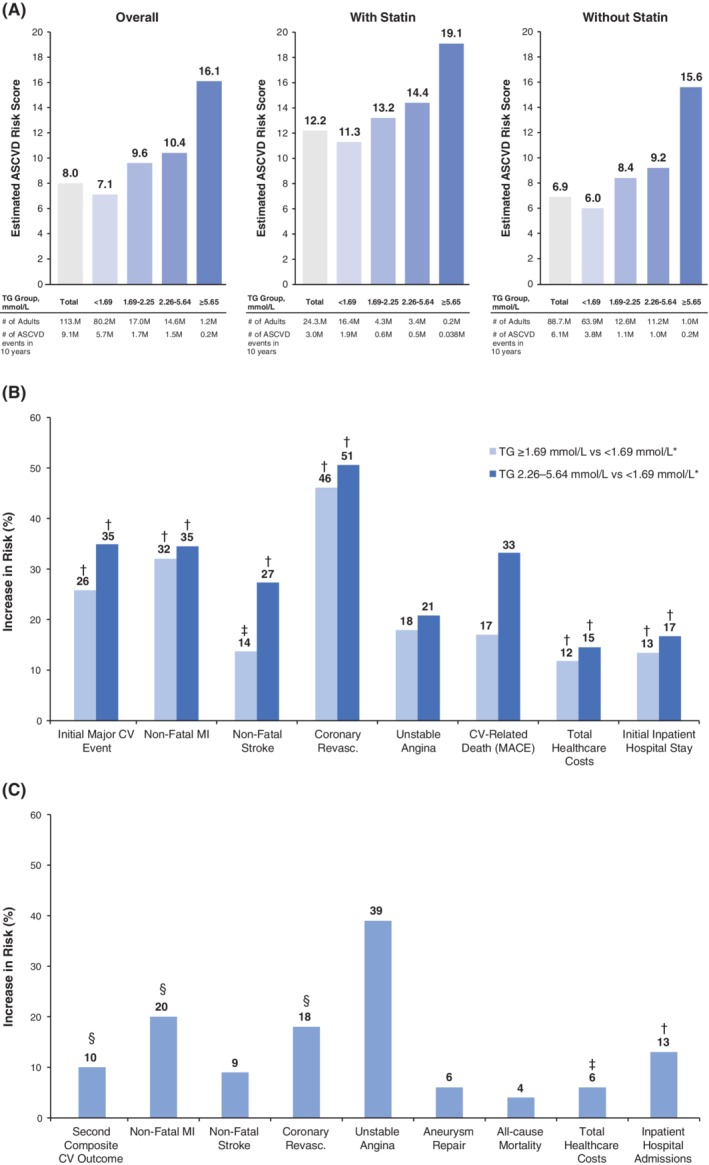
Effect of hypertriglyceridaemia on risk, outcomes, healthcare utilization and costs.41, 44, 45, 47, 50 A, Estimated number of atherosclerotic cardiovascular disease (ASCVD) events in 10 years among people aged 40–79 years, by triglyceride (TG) concentration, and stratified by statin use, based on the 9593 participants identified in the National Health and Nutrition Examination Survey (NHANES) database. The estimated number of events in 10 years was calculated by multiplying the estimated ASCVD risk score by the corresponding projected population (the estimated ASCVD risk score also indicated the proportion of events expected to occur in 10 years). The 10‐year risk of ASCVD was defined as non‐fatal myocardial infarction (MI) or coronary heart disease death, or fatal or non‐fatal stroke, over a 10‐year period among people free from ASCVD at the beginning of the period. B, Increase in risk in patients with TG levels ≥1.69 mmol/L and in the subcohort with TG levels 2.26–5.64 mmol/L versus comparators from the Optum Research Database. See Table 2 for analysis details. C, Increase in risk in patients from the Kaiser Permanente database with TG levels 2.26–5.64 mmol/L versus patients with TG levels <1.69 mmol/L. See Table 3 footnotes for analysis details. *Overall pre‐match cohort: TG ≥1.69 mmol/L (n = 25 452 patients); comparator pre‐match cohort: TG <1.69 mmol/L and HDL cholesterol >1.04 mmol/L (n = 31 805 patients); pre‐match subcohort: TG 2.26–5.64 mmol/L (n = 13 411 patients); comparator pre‐match cohort: TG <1.69 mmol/L and HDL cholesterol >1.04 mmol/L (n = 32 506 patients). † P < 0.001, ‡ P < 0.01, § P < 0.05, all others not significant. BMI, body mass index; CV, cardiovascular; TG, triglycerides
2.2. Optum Research Database
The Optum Research Database is a retrospective observational administrative claims database that includes >160 million individuals and electronic health records for >80 million patients.44, 45 The analysis of the impact of TG on health outcomes from this database included patients with ≥1 claim for statin treatment. The follow‐up period began on the index date (date of first statin claim in 2010) and ended at plan disenrolment, death, or the end of the study (March 31, 2016), whichever occurred first. The population included men or women aged ≥45 years on index date with documented ASCVD and/or diabetes. ASCVD included acute coronary syndrome, myocardial infarction (MI), angina, coronary or other arterial revascularization, stroke, transient ischaemic attack, or peripheral arterial disease. Patients were required to have 6 months of baseline data before the index date, and at least 6 months of follow‐up data, which began on the index date. Patients with niacin remaining from a recent prescription fill at the index date or having International Classification of Disease (ICD)‐9 codes indicating the presence of severe liver disease, acute or chronic pancreatitis, malabsorption syndrome or gastric/intestinal bypass surgery, and end‐stage renal disease were excluded. An overall TG cohort included individuals with TG levels ≥1.69 mmol/L (n = 23 181),45 and a subcohort had TG levels 2.26 to 5.64 mmol/L (n = 10 990)44; propensity‐matched comparator cohorts had TG levels <1.69 mmol/L and HDL cholesterol levels >1.04 mmol/L. The propensity‐score matching made it possible to control for baseline characteristics including age, sex, insurance type, region, baseline medical cost, LDL cholesterol relative to the median (if available), baseline use of statin, fibrate or omega‐3 fatty acids, and certain diagnoses (ASCVD, diabetes mellitus, stroke, hypertension, renal disease and peripheral artery disease); non‐HDL cholesterol and HDL cholesterol were added to the model in a separate analysis to evaluate their potential impact. Use of other lipid‐lowering drugs in this population was quite low, with ~7.4% of patients taking a fibrate at baseline and 1.4% a prescription omega‐3 fatty acid product (ezetimibe was grouped with low‐intensity statins).
Over the study follow‐up period, multivariate analysis showed that patients in the borderline hypertriglyceridaemia (TGs ≥1.69 mmol/L) cohort had significantly greater risk of composite initial major CV events (P < 0.001) and individual CV events versus the comparator cohort, as did patients in the hypertriglyceridaemia subcohort with TG levels 2.26–5.64 mmol/L (Table 2; Figure 1B).45 Increased CV risk was apparent, even with addition of non‐HDL cholesterol to the multivariate model and when analysing HDL cholesterol subgroups.45 In this model, diabetes (hazard ratio [HR] 1.37, 95% CI 1.26–1.48; P < 0.001), male gender (HR 1.32, 95% CI 1.24–1.40; P < 0.001) and ASCVD (HR 2.16, 95% CI 2.01–2.33; P < 0.001) were significant predictors of major CV events. Only a minority of patients in the study had any intervention for management of elevated TGs, so there was little change in TG levels over the course of the study. The mean TG level initially decreased, then increased during the first year, before stabilizing at a concentration slightly below baseline in the TG ≥1.69 mmol/L cohort; TG levels increased in the comparison group. LDL cholesterol also did not change substantially during follow‐up, despite the fact that persistence with statin therapy was poor, with ~20% continuing therapy after 5 years in both the elevated‐TG group and the control group.46
Table 2.
Optum Research Database: Effects of triglycerides on cardiovascular (CV) events and medical resource utilization in statin‐treated patients with elevated atherosclerotic CV disease risk (multivariate analysis)a , b
| Hazard or cost ratio for cohort variable (95% CI) | P | Hazard or cost ratio for cohort variable (95% CI) | P | |||
|---|---|---|---|---|---|---|
| TG ≥1.69 mmol/L vs comparatora | TG 2.26–5.64 mmol/L vs comparatora | |||||
| Initial major CV eventc | 1.26 | (1.19–1.34) | <0.001 | 1.35 | (1.23–1.49) | <0.001 |
| Non‐fatal MIc | 1.32 | (1.2–1.45) | <0.001 | 1.35 | (1.19–1.52) | <0.001 |
| Non‐fatal strokec | 1.14 | (1.04–1.24) | 0.004 | 1.27 | (1.14–1.42) | <0.001 |
| Coronary revascularizationc | 1.46 | (1.33–1.61) | <0.001 | 1.51 | (1.34–1.69) | <0.001 |
| Unstable anginac | 1.18 | (0.71–1.96) | 0.527 | 1.21 | (0.65–2.26) | 0.555 |
| CV‐related deathc , d | 1.17 | (0.90–1.52) | 0.125 | 1.33 | (0.97–1.83) | 0.076 |
| Total healthcare costse | 1.12 | (1.08–1.16) | <0.001 | 1.15 | (1.08–1.21) | <0.001 |
| Initial inpatient hospital stayc | 1.13 | (1.10–1.17) | <0.001 | 1.17 | (1.11–1.22) | <0.001 |
Abbreviations: CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; TG, triglycerides.
Overall pre‐match cohort: TG ≥1.69 mmol/L (n = 25 452 patients); comparator pre‐match cohort: TG <1.69 mmol/L and HDL cholesterol >1.04 mmol/L (n = 31 805 patients); pre‐match subcohort: TG 2.26–5.64 mmol/L (n = 13 411 patients); comparator pre‐match cohort: TG <1.69 mmol/L and HDL cholesterol >1.04 mmol/L (n = 32 506 patients).
Separate pre‐match multivariate analyses of major CV events, total healthcare costs and initial inpatient stay were performed. Covariates included TG cohort, as represented here, along with age (45–54, 55–64, ≥65 years), sex, insurance coverage type, geographic region of enrolment, baseline clinical characteristics (diabetes, ASCVD, LDL cholesterol laboratory result in relation to median), and baseline medication use (fibrates, prescription omega‐3s, both, and neither).
Multivariate analysis using Cox proportional hazards model.
Event occurred in an inpatient setting with discharge status indicating a non‐fatal event (absence of CV‐related death; CV‐related death was defined as death in the follow‐up period [as identified with discharge status or the Death Master File]) based on diagnosis code for major CV events (MI, stroke, revascularization) in the first or second position, that occurred in an emergency department setting within 1 day of a death date, or in an inpatient stay with a discharge date within 7 days of a death date.
Generalized linear model (gamma distribution, log link).
After controlling for patient characteristics, there was also a higher rate of inpatient hospital stay per unit time (all P < 0.001; Table 2, Figure 1B).44, 45 The total healthcare cost ratio was higher in the TG ≥1.69 mmol/L cohort versus the comparator cohort as well as the hypertriglyceridaemia (TGs 2.26–5.64 mmol/L) subcohort versus the comparator cohort (all P < 0.001; Table 2, Figure 1B).44, 45 Patients in the TG ≥5.64 mmol/L cohort had higher mean (SD) total monthly healthcare costs ($1438 [$3214]) versus the comparator cohort ($1270 [$2516]; P < 0.001; n = 23 181 patients for both cohorts).45 Extrapolating from the per‐patient‐per‐month average total healthcare costs across the variable follow‐up time in this study, the approximate average annual cost difference between the TG ≥1.69 mmol/L and comparator cohorts was $47 m per year; this translates to $200 m per year per 100 000 patients.45 The mean (SD) total monthly healthcare cost was $1462 ($3354) in the hypertriglyceridaemia subcohort versus $1279 ($2628) in the comparator cohort (P < 0.001).44 Again, extrapolated to the overall study population over 1 year, this difference resulted in average healthcare costs that were $24 m greater in the hypertriglyceridaemia subcohort, or an additional $220 m per year per 100 000 patients compared with the comparator cohort.44
2.3. Kaiser Permanente database
The Kaiser Permanente database contains electronic health records (rather than administrative claims) collected via an integrated clinical delivery system that provides medical care to patients in eight semi‐autonomous regions of the United States, including the Northwest (KPNW) and Southern California (KPSC) regions.47 Both organizations use an Epic‐based electronic health record (Verona, Wisconsin, USA) that is combined with enrolment, laboratory and pharmacy data to create a comprehensive dataset that can be standardized into a common data model. This longitudinal observational cohort study investigated the impact of TG levels on CVD risk using data from the KPNW and KPSC regions, which together serve ~4.5 million members. The inclusion criteria for this analysis mirrored the entry criteria for the REDUCE‐IT study.48 All patients aged ≥45 years with a charted diagnosis of MI, stroke, acute coronary syndrome or peripheral arterial disease, TG levels <5.65 mmol/L and LDL cholesterol levels 1.04–2.59 mmol/L while on statin therapy were included (use of other lipid‐lowering drugs was an exclusion criterion).47 Patients were followed from the index date for a maximum of 6.5 years. The first of two primary endpoints for the analysis was a composite of all‐cause mortality and first occurrence of non‐fatal MI, non‐fatal stroke, coronary revascularization or unstable angina; the second primary composite outcome also included peripheral revascularization and aneurysm repair.47 Secondary analyses evaluated individual components of the composites separately. Patients were categorized according to index TG value, and categorized as having hypertriglyceridaemia (TG levels 2.26–5.64 mmol/L, n = 2702) or TG levels <1.69 mmol/L (n = 14 481).47 The treatment groups differed at baseline in that the hypertriglyceridaemia group was younger, had a higher prevalence of diabetes and chronic kidney disease, and was more likely to be white or Hispanic, a current smoker, and have lower HDL cholesterol versus the TG <1.69 mmol/L group.47
The crude prevalence of the composite outcomes did not differ between the groups at any time during the study, but crude prevalence of non‐fatal MI (P = 0.023), coronary revascularization (P < 0.001) and peripheral revascularization (P = 0.026) were significantly more frequent in the hypertriglyceridaemia group versus the TG <1.69 mmol/L group.47 There was no significant difference in the rate ratio between men and women (P = 0.698).47 However, with multivariate statistical adjustment and accounting for time to event, the hypertriglyceridaemia group was found to be 10% more likely to experience a second primary composite outcome event versus the TG <1.69 mmol/L group (P = 0.041; Table 3).47 This difference was largely driven by increased likelihoods of non‐fatal MI, coronary revascularization and peripheral revascularization in the hypertriglyceridaemia group versus the TG <1.69 mmol/L group (Table 3, Figure 1C).47 Although rates were elevated in the hypertriglyceridaemia group versus the TG <1.69 mmol/L group, no significant differences were observed for other endpoints (ie, first composite outcome, non‐fatal stroke, unstable angina, aneurysm repair, all‐cause mortality; Table 3, Figure 1C).47 Similar patterns were seen in an analysis of patients with diabetes and hypertriglyceridaemia (TG levels 2.26–5.64 mmol/L; n = 5542) versus normal TG levels (TG <1.69 mmol/L; n = 22 411) from this database.49 The hypertriglyceridaemia group versus the TG <1.69 mmol/L group within the diabetes population had significantly higher adjusted incidences of non‐fatal MI (rate ratio 1.30, 95% CI 1.08–1.58; P = 0.006), non‐fatal stroke (rate ratio 1.23, 95% CI 1.01–1.49; P = 0.037) and coronary revascularization (rate ratio 1.21, 95% CI 1.02–1.43; P = 0.027), but not unstable angina (rate ratio 1.33, 95% CI 0.87–2.03; P = 0.185).49
Table 3.
Kaiser Permanente database: Effects of triglyceride level on incidence of cardiovascular (CV) events and medical resource utilization in statin‐treated patients with elevated atherosclerotic CV disease risk
| Outcome | Hypertriglyceridaemia (TGs 2.26–5.64 mmol/L) | Normal TG level (TGs <1.69 mmol/L) | Rate ratio or difference (arithmetic ratio) | P |
|---|---|---|---|---|
| Primary composite outcomesa | ||||
| Firsta , b | 45.9 (42.2–49.9) | 42.8 (41.1–44.5) | 1.07 (0.98–1.18) | 0.127 |
| Seconda , b | 50.9 (47.0–55.2) | 46.5 (44.8–48.2) | 1.10 (1.00–1.20) | 0.041 |
| Secondary outcomesa | ||||
| Non‐fatal MIa | 10.5 (8.9–12.4) | 8.7 (8.0–9.5) | 1.20 (1.00–1.45) | 0.045 |
| Non‐fatal strokea | 8.4 (7.0–10.2) | 7.8 (7.1–8.5) | 1.09 (0.89–1.33) | 0.423 |
| Unstable anginaa | 2.3 (1.6–3.3) | 1.6 (1.3–2.0) | 1.39 (0.94–2.06) | 0.101 |
| Coronary revascularizationa | 11.9 (10.2–13.9) | 10.0 (9.3–10.9) | 1.18 (1.00–1.40) | 0.045 |
| Peripheral revascularizationa | 3.4 (2.5–4.5) | 2.2 (1.8–2.6) | 1.56 (1.14–2.13) | 0.006 |
| Aneurysm repaira | 1.3 (0.8–2.0) | 1.2 (0.9–1.5) | 1.06 (0.64–1.76) | 0.817 |
| All‐cause mortalitya | 20.7 (18.4–23.2) | 19.9 (18.8–21.1) | 1.04 (0.92–1.17) | 0.533 |
| Total costsc | $17 848 ($17 224–$18 473) | $16 884 ($16 625–$17 143) | $964 (6%) | 0.006 |
| Inpatient admissiond | 0.26 (0.24–0.28) | 0.23 (0.22–0.24) | 0.03 (13%) | <0.001 |
Abbreviations: MI, myocardial infarction; TG, triglyceride.
Values represent incidence (95% CIs) of study outcomes per 1000 person‐years and rate ratios adjusted for age, sex, race/ethnicity, body mass index, smoking status, blood pressure, diabetes, use of insulin, history of MI, stroke or other ischaemic heart disease, serum creatinine and study site.
First primary composite outcome: all‐cause mortality and first occurrence of non‐fatal MI, non‐fatal stroke, coronary revascularization, or unstable angina. Secondary composite outcome: first composite plus peripheral revascularization and aneurysm repair.
Values represent mean (95% CI) annualized costs per person adjusted for age, sex, race/ethnicity, study site, baseline costs, diabetes, chronic kidney disease, obesity, hypertension, and low HDL cholesterol.
Values represent mean (95% CI) annualized utilization per person adjusted for age, sex, race/ethnicity, and study site.
Adjusting for age, gender, study site and race/ethnicity, each of the following was significantly higher in the hypertriglyceridaemia group versus the TG <1.69 mmol/L group over a mean follow‐up time of ~5.2 years: inpatient admission (P < 0.001; Table 3; Figure 1C), inpatient days (P = 0.038), emergency room visits (P < 0.001), and pharmaceutical dispenses (P < 0.001).50 In models further adjusted for baseline costs, presence of diabetes, hypertension, chronic kidney disease, obesity and low HDL cholesterol, the following mean annualized adjusted per‐capita costs were significantly higher in the hypertriglyceridaemia group versus the TG <1.69 mmol/L group: total costs (by $964; P = 0.006; Table 3, Figure 1C), emergency room visits ($70; P = 0.031), hospital ambulatory care ($199; P = 0.032), total ambulatory care ($327; P = 0.035), and pharmaceutical dispenses ($185; P = 0.012).
3. STRENGTHS AND LIMITATIONS
The real‐world experience studies included in this review have a number of strengths and limitations. The NHANES population was designed to be generalizable to the US population, with sample weighting allowing estimation of the millions of US adults with hypertriglyceridaemia. In addition, as a prospective cohort there are uniform protocols for the measurement of lipids and medical history acquisition across all study centres. A limitation of NHANES, however, is the lack of follow‐up for CV events other than CV mortality, as well as a lack of information on statin dose, duration or adherence.
Data collected at the point of care, such as those included in the Optum and Kaiser Permanente databases, provide a pragmatic examination of real‐world data in the context of clinical practice, and may be more reflective of actual use than evidence from clinical trials,51, 52 bringing a novel perspective to the healthcare costs and disease burden of this large population that is representative of the overall US population. As a result, these analyses may help place the results of ongoing CV outcomes trials into a real‐world perspective. Although analysis of smaller claims databases may be of interest, their results would probably be less representative. The propensity‐score matching in the Optum Research Database further allowed the study populations to be statistically controlled for group differences including demographic and cardiometabolic risk factors.44, 45, 47, 50 Because this approach was not taken for the Kaiser Permanente analysis, its ability to control for the same factors may not be as great. As a result, hypertriglyceridaemia was the main difference in risk factors between the groups, so it is likely that the observed differences in CV disease risk can be ascribed, at least in part, to the disparity in TG levels. A limitation of these analyses, however, is that it was not possible to control for other potential confounding factors such as alcohol use and other lifestyle factors that were not captured in these databases.
Data collected for the purpose of claims, or from electronic medical records of managed care health plans rather than for research, may contain inaccurate recording of health events, may have missing data, and there may be uncertainty about the internal validity of the database.52, 53 For instance, patient fasting status cannot be accurately determined from observational laboratory data such as those used in this study, and the data probably contain a mix of fasting and non‐fasting TG results. While fasting TG values may be preferred for diagnosing hypertriglyceridaemia,54 non‐fasting TG values may be better predictors of CV disease risk.55, 56 Non‐fasting TG values are generally higher than fasting TG,54, 57 and resulting misclassification would bias the results of this real‐world analysis toward the null hypothesis, suggesting that the estimates of excess CV disease risk due to hypertriglyceridaemia may be conservative. In addition, some costs to the patient (eg, transportation, missed work days) are not available from these databases.
In addition to the limitations discussed above, a number of other issues should be kept in mind when considering these findings. Observational studies based on registries, electronic medical records, and other non‐prospective real‐life cohorts cannot conclusively determine causality, but are more generalizable to clinical practice than results of clinical trials.51, 53 Biases and confounding due to unmeasured variables are possible outside the context of well‐controlled randomized trials.52 Furthermore, analyses of this type of database cannot be effectively used to determine cause and effect, that is, to measure the efficacy of pharmaceutical interventions.51, 53 As a result, it cannot be determined from these analyses whether risk, costs and resource utilization could be reduced by TG‐lowering intervention. Despite these limitations, real‐world evidence provides clinicians, payers and clinical guideline developers with additional information to guide decision‐making.53
The findings of the Optum Research Database and Kaiser Permanente studies are generally consistent, with some notable differences. With regard to age, sex and diabetes status, the Optum analyses (~85% had diabetes) may have been more similar to the Kaiser Permanente analysis of patients with diabetes than to the main Kaiser Permanente analysis, which had ≤50% patients with diabetes and the population was generally older and had more men than the Optum and Kaiser Permanente diabetes analyses. Accordingly, the rates for MI and stroke were similar between the Optum and the Kaiser Permanente diabetes analyses.44, 49 The Optum analysis used propensity matching whereas the Kaiser Permanente analysis used statistical controls49; therefore, the Kaiser Permanente results are more subject to residual confounding. The lower admissions and costs found in the Kaiser Permanente analyses may have been attributable to the fact that the Kaiser Permanente system is a much more controlled system than the claims data used in the Optum analysis. Finally, the findings of these studies may be suggestive of elevated TG level as a greater risk factor in women than men because the Optum analysis included more women (~50%) and generally found higher risk than the Kaiser Permanente main analysis (31%–37% women).44, 50
The increased CV risk identified in the Optum and Kaiser Permanente studies has recently been supported by an analysis of 439 019 patients from the United States Veterans Affairs Corporate Data Warehouse, which demonstrated that, in statin‐treated veterans with well‐controlled LDL cholesterol, those with TG levels 1.69–5.64 mmol/L had a significant increase in CV events versus veterans with TG levels <1.69 mmol/L (rate ratio 1.37, 95% CI 1.34–1.40; P < 0.001) even after adjusting for HDL cholesterol (rate ratio 1.19, 95% CI 1.16–1.22; P < 0.001).58
4. HYPERTRIGLYCERIDAEMIA AND ASCVD OUTCOMES TRIALS
Given the risks conferred by hypertriglyceridaemia, the potential for ASCVD risk reduction has been the subject of a number of important outcomes trials employing agents with TG‐lowering effects, such as niacin and fenofibrate, including AIM‐HIGH (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes),59 ACCORD (Action to Control Cardiovascular Risk in Diabetes),7 and HPS2‐THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events).7, 59, 60 However, many of these trials did not prospectively enroll patients with hypertriglyceridaemia; only the AIM‐HIGH trial of extended‐release niacin required participants to have TG levels >1.69 mmol/L.59 Despite this, subgroup analyses of the AIM‐HIGH and ACCORD studies did suggest that modification of TG in patients with hypertriglyceridaemia and low HDL cholesterol may lead to possible ASCVD benefit.7, 59
Results from omega‐3 fatty acid outcomes trials have also had variable results.61 Early findings conducted prior to current statin treatment guidelines that suggested possible CV benefit were either conducted with a combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)62 or with pure EPA.63 However, later studies in the modern statin era that investigated EPA + DHA combinations failed to demonstrate CV benefit.64, 65, 66, 67, 68 Notably, these studies had design issues such as the use of low‐dose omega‐3 treatment and a focus on patients with low HDL cholesterol rather than hypertriglyceridaemia.64, 65, 66, 67, 68 Other limitations included a lack of assessment of long‐chain omega‐3 fatty acid status prior to and during treatment, and an absence of a clear biological target.61 In the past year, several major CV outcomes trials of omega‐3 agents have concluded. The VITAL (Vitamin D and Omega‐3 Trial)69 and ASCEND (A Study of Cardiovascular Events in Diabetes)70 trials failed to show any reduction in major CV events with a low 1‐g/d dose of EPA + DHA compared with placebo.69, 70 These studies included use of other medications such as aspirin (ASCEND) and vitamin D (VITAL) and did not require hypertriglyceridaemia or statin treatment for participation, although patients in ASCEND were required to have a diagnosis of diabetes. REDUCE‐IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) was different from VITAL, ASCEND and prior omega‐3 trials because it investigated high‐dose (4 g/d), high‐purity EPA (icosapent ethyl) in high‐risk (71% with established CVD with or without diabetes, and 29% with diabetes without established CVD) statin‐treated patients with fasting TG 1.53–5.64 mmol/L.71 Icosapent ethyl was associated with a statistically significant and clinically meaningful 25% relative risk reduction (HR 0.75, 95% CI 0.68–0.83; P < 0.001) in the primary composite endpoint of the first occurrence of major adverse CV event (MACE; CV death, non‐fatal MI, non‐fatal stroke, coronary revascularization, or unstable angina requiring hospitalization) and a 26% reduction (HR 0.74; 95% CI 0.65–0.83; P < 0.001) in the key secondary endpoint (three‐point MACE consisting of CV death, non‐fatal MI, or non‐fatal stroke) over a median follow‐up of 4.9 years. This represented an absolute risk reduction of 4.8% and 3.6% and a number needed to treat of 21 and 28 over 4.9 years in the primary and key secondary composite endpoints, respectively. REDUCE‐IT also demonstrated a 20% relative reduction in CV death (HR 0.80, 95% CI 0.66–0.98; P = 0.03), a 31% relative reduction in MI (HR 0.69, 95% CI 0.58–0.81; P < 0.001), and a 28% relative reduction in stroke (HR 0.72, 95% CI 0.55–0.93; P = 0.01).
While hypertriglyceridaemia confers increased ASCVD risk as described by the real‐world evidence reviewed herein, it may seem paradoxical that the reduction of MACE in REDUCE‐IT was similar across baseline TG strata and appeared to occur irrespective of attained TG. These findings from REDUCE‐IT suggest that the observed risk reduction may not entirely be explained by TG‐lowering and may also be the result of other pleiotropic beneficial effects of EPA on multiple steps in the development and progression of ASCVD.71 These include anti‐inflammatory effects mediated by downstream effector molecules produced from EPA, such as resolvins and protectins.72, 73 In addition, one has to keep in mind that EPA is a long‐chain fatty acid that incorporates in membranes and other lipid structures, such as lipoproteins. It is thus plausible to speculate that cellular function and lipoprotein processing may be improved by the compositional changes. EVAPORATE (Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy) is an ongoing randomized, double‐blind, placebo‐controlled trial, designed to assess the effect of icosapent ethyl 4 g/d on low‐attenuation plaque volume in statin‐treated US patients with coronary atherosclerosis and elevated TG and may help elucidate the mechanisms by which EPA reduces CVD risk.74
Other important omega‐3 CV outcomes studies are ongoing and may help to define the role of omega‐3 fatty acids in the management of patients with hypertriglyceridaemia. RESPECT‐EPA (Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy ‐ Statin and Eicosapentaenoic Acid)75 is an open‐label study being conducted in Japan of 1.8 g/d of high‐purity EPA in 3900 statin‐treated patients with stable coronary artery disease, and STRENGTH (Long‐Term Outcomes Study to Assess Statin Residual Risk Reduction with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia) is a study of high‐dose (4 g/d) EPA + DHA in statin‐treated patients with TG levels ≥2.03 and <5.65 mmol/L.76 Finally, OMEMI (OMega‐3 fatty acids in Elderly patients with Myocardial Infarction) is an ongoing Norwegian secondary prevention study of low‐dose (1.8 g/d) omega‐3 fatty acids in elderly patients with acute MI.77 The results of these trials, along with the already released REDUCE‐IT trial, should better inform the role of omega‐3 fatty acid supplementation for the primary and secondary prevention of ASCVD in future guidelines. Indeed, findings from REDUCE‐IT have prompted recent updates to the American Diabetes Association Standards of Medical Care in Diabetes. The recommendations now include a statement that icosapent ethyl should be considered for patients with diabetes and ASCVD or other cardiac risk factors with LDL cholesterol controlled on statin therapy, but with TG levels 1.53–5.64 mmol/L.78
5. CONCLUSIONS
Approximately 57 million US adults have hypertriglyceridaemia (TG ≥1.69 mmol/L), about one‐third of whom have LDL cholesterol controlled to <100 mg/dL with statins, and nearly 40% of those with diabetes also have TG ≥1.69 mmol/L despite statin therapy. This means that a substantial number of people in the USA have residual CV risk despite the fact that their LDL cholesterol is well controlled; it is forecasted that as many as 3.4 million ASCVD events will occur in this population over the next 10 years.41 The series of real‐world evidence studies reviewed here suggests that statin‐treated patients with high CV risk and hypertriglyceridaemia have worse CV and health economic outcomes than similar patients with normal TG (<1.69 mmol/L in the Kaiser Permanente database), or with normal TG and HDL cholesterol (<1.69 mmol/L and >1.04 mmol/L, respectively, in the Optum database).41, 44, 45, 47, 50 Recently completed and ongoing intervention trials designed to assess CV outcomes in patients with elevated TG levels will help to determine if certain strategies to reduce TG will help to ameliorate the residual CV risk in these patients.
CONFLICT OF INTEREST
The authors declare the following. P.P.T.: Speakers Bureau: Amarin Pharma Inc., Amgen, Kowa, Merck, Novo‐Nordisk, Regeneron, Sanofi, and Consultant: Amarin Pharma Inc., Amgen, Kowa, Novo‐Nordisk, Resverlogix, Theravance. S.F.: Consultant: Amarin Pharma Inc., Amgen, Esperion, AstraZeneca, Novartis. N.D.W.: Research support: Amarin Pharma Inc., Amgen (through institution); Speakers Bureau: Amarin Pharma Inc., Sanofi, Novartis; and Advisory Boards: Amarin Pharma Inc., Sanofi, Novartis. M.H.: Employment: Optum. G.A.N.: Research funding: Boehringer Ingelheim, Bristol‐Myers Squibb, Merck & Co.
AUTHOR CONTRIBUTIONS
The author contributions to the study were as follows: design: P.P.T., S.F., N.D.W., M.H. and G.A.N. Conduct/data collection: S.F., N.D.W., M.H. and G.A.N. Analysis: P.P.T., S.F., N.D.W., M.H. and G.A.N. Writing manuscript: P.P.T., S.F., N.D.W., M.H. and G.A.N.
Toth PP, Fazio S, Wong ND, Hull M, Nichols GA. Risk of cardiovascular events in patients with hypertriglyceridaemia: A review of real‐world evidence. Diabetes Obes Metab. 2020;22:279–289. 10.1111/dom.13921
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13921.
Funding information Medical writing assistance was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, New Jersey, and funded by Amarin Pharma Inc., Bedminster, New Jersey.
REFERENCES
- 1. Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet need for adjunctive dyslipidemia therapy in hypertriglyderidemia management. J Am Coll Cardiol. 2018;72(3):330‐343. [DOI] [PubMed] [Google Scholar]
- 2. Rizzo M, Barylski M, Rizvi AA, Montalto G, Mikhailidis DP, Banach M. Combined dyslipidemia: should the focus be LDL cholesterol or atherogenic dyslipidemia? Curr Pharm Des. 2013;19(21):3858‐3868. [DOI] [PubMed] [Google Scholar]
- 3. Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E. Impact of triglyceride levels beyond low‐density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT‐TIMI 22 trial. J Am Coll Cardiol. 2008;51(7):724‐730. [DOI] [PubMed] [Google Scholar]
- 4. Imke C, Rodriguez BL, Grove JS, et al. Are remnant‐like particles independent predictors of coronary heart disease incidence? The Honolulu Heart study. Arterioscler Thromb Vasc Biol. 2005;25(8):1718‐1722. [DOI] [PubMed] [Google Scholar]
- 5. Kugiyama K, Doi H, Takazoe K, et al. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation. 1999;99(22):2858‐2860. [DOI] [PubMed] [Google Scholar]
- 6. Karpe F, Boquist S, Tang R, Bond GM, de Faire U, Hamsten A. Remnant lipoproteins are related to intima‐media thickness of the carotid artery independently of LDL cholesterol and plasma triglycerides. J Lipid Res. 2001;42(1):17‐21. [PubMed] [Google Scholar]
- 7. Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim JY, Park JH, Jeong SW, et al. High levels of remnant lipoprotein cholesterol is a risk factor for large artery atherosclerotic stroke. J Clin Neurol. 2011;7(4):203‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quispe R, Manalac RJ, Faridi KF, et al. Relationship of the triglyceride to high‐density lipoprotein cholesterol (TG/HDL‐C) ratio to the remainder of the lipid profile: the Very Large Database of Lipids‐4 (VLDL‐4) study. Atherosclerosis. 2015;242(1):243‐250. [DOI] [PubMed] [Google Scholar]
- 10. McNamara JR, Shah PK, Nakajima K, et al. Remnant‐like particle (RLP) cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study. Atherosclerosis. 2001;154(1):229‐236. [DOI] [PubMed] [Google Scholar]
- 11. Rapp JH, Lespine A, Hamilton RL, et al. Triglyceride‐rich lipoproteins isolated by selected‐affinity anti‐apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb. 1994;14(11):1767‐1774. [DOI] [PubMed] [Google Scholar]
- 12. Kasai T, Miyauchi K, Yanagisawa N, et al. Mortality risk of triglyceride levels in patients with coronary artery disease. Heart. 2013;99(1):22‐29. [DOI] [PubMed] [Google Scholar]
- 13. Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450‐458. [DOI] [PubMed] [Google Scholar]
- 14. Varbo A, Benn M, Tybjaerg‐Hansen A, Jorgensen AB, Frikke‐Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61(4):427‐436. [DOI] [PubMed] [Google Scholar]
- 15. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292‐2333. [DOI] [PubMed] [Google Scholar]
- 16. Budoff M. Triglycerides and triglyceride‐rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118(1):138‐145. [DOI] [PubMed] [Google Scholar]
- 17. Jorgensen AB, Frikke‐Schmidt R, Nordestgaard BG, Tybjaerg‐Hansen A. Loss‐of‐function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32‐41. [DOI] [PubMed] [Google Scholar]
- 18. Crosby J, Peloso GM, Auer PL, et al. Loss‐of‐function mutations in APOC3, triglycerides and coronary disease. N Engl J Med. 2014;371(1):22‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Natarajan P, Kohli P, Baber U, et al. Association of APOC3 loss‐of‐function mutations with plasma lipids and subclinical atherosclerosis. J Am Coll Cardiol. 2015;66(18):2053‐2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Do R, Stitziel NO, Won HH, et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518(7537):102‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarwar N, Sandhu MS, Ricketts SL, et al. Triglyceride‐mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375(9726):1634‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dewey FE, Gusarova V, O'Dushlaine C, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med. 2016;374(12):1123‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stitziel N, Stirrups K, Masca N, et al. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. 2016;374(12):1134‐1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schunkert H, Konig IR, Kathiresan S, et al. Large‐scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45(11):1345‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jorgensen AB, Frikke‐Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg‐Hansen A. Genetically elevated non‐fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34(24):1826‐1833. [DOI] [PubMed] [Google Scholar]
- 29. Varbo A, Benn M, Tybjaerg‐Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low‐grade inflammation and ischemic heart disease, whereas elevated low‐density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128(12):1298‐1309. [DOI] [PubMed] [Google Scholar]
- 30. Thomsen M, Varbo A, Tybjaerg‐Hansen A, Nordestgaard BG. Low nonfasting triglycerides and reduced all‐cause mortality: a Mendelian randomization study. Clin Chem. 2014;60(5):737‐746. [DOI] [PubMed] [Google Scholar]
- 31. Holmes MV, Asselbergs FW, Palmer TM, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36(9):539‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dron JS, Hegele RA. Genetics of triglycerides and the risk of atherosclerosis. Curr Atheroscler Rep. 2017;19(7):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwartz GG, Abt M, Bao W, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65(21):2267‐2275. [DOI] [PubMed] [Google Scholar]
- 34. Nordestgaard BG. Triglyceride‐rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118(4):547‐563. [DOI] [PubMed] [Google Scholar]
- 35. Wang YI, Schulze J, Raymond N, et al. Endothelial inflammation correlates with subject triglycerides and waist size after a high‐fat meal. Am J Physiol Heart Circ Physiol. 2011;300(3):H784‐H791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. März W, Scharnagl H, Winkler K, et al. Low‐density lipoprotein triglycerides associated with low‐grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: the Ludwigshafen risk and cardiovascular health study. Circulation. 2004;110(19):3068‐3074. [DOI] [PubMed] [Google Scholar]
- 37. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626‐635. [DOI] [PubMed] [Google Scholar]
- 38. Wang YI, Bettaieb A, Sun C, et al. Triglyceride‐rich lipoprotein modulates endothelial vascular cell adhesion molecule (VCAM)‐1 expression via differential regulation of endoplasmic reticulum stress. PLoS One. 2013;8(10):e78322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palmer MK, Toth PP. Trends in lipids, obesity, metabolic syndrome, and diabetes mellitus in the United States: An NHANES analysis (2003‐2004 to 2013‐2014). Obesity (Silver Spring, Md). 2019;27(2):309‐314. [DOI] [PubMed] [Google Scholar]
- 40. Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF. Pharmacological targeting of the atherogenic dyslipidemia complex: The next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes. 2016;65(7):1767‐1778. [DOI] [PubMed] [Google Scholar]
- 41. Fan W, Philip S, Granowitz C, Toth P, Wong N. Hypertriglyceridemia in statin‐treated US adults: The National Health and Nutrition Examination Survey. J Clin Lipidol. 2019;13:100‐108. [DOI] [PubMed] [Google Scholar]
- 42. Zdrojewski T, Solnica B, Cybulska B, et al. Prevalence of lipid abnormalities in Poland. The NATPOL 2011 survey. Kardiol Pol. 2016;74(3):213‐223. [DOI] [PubMed] [Google Scholar]
- 43. Fan W, Philip S, Granowitz C, Toth PP, Wong ND. Residual Hypertriglyceridemia and Estimated Atherosclerotic Cardiovascular Disease Risk by Statin Use in U.S. Adults With Diabetes: National Health and Nutrition Examination Survey 2007‐2014. Diabetes Care. 2019;42(12):2307‐2314. [DOI] [PubMed] [Google Scholar]
- 44. Toth PP, Granowitz C, Hull M, Liassou D, Anderson A, Philip S. High triglycerides are associated with increased cardiovascular events, medical costs, and resource utilization: a real‐world administrative claims analysis of statin‐treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7(15):e008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Toth PP, Philip S, Hull M, Granowitz C. Association of elevated triglycerides with increased cardiovascular risk and direct costs in statin‐treated patients. Mayo Clin Proc. 2019;94(9):1670‐1680. [DOI] [PubMed] [Google Scholar]
- 46. Toth PP, Granowitz C, Hull M, Anderson A, Philip S. Long‐term statin persistence is poor among high‐risk patients with dyslipidemia: a real‐world administrative claims analysis. Lipids Health Dis. 2019;18:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased cardiovascular risk in hypertriglyceridemic patients with statin‐controlled LDL cholesterol. J Clin Endocrinol Metab. 2018;103(8):3019‐3027. [DOI] [PubMed] [Google Scholar]
- 48. Bhatt DL, Steg PG, Brinton EA, et al. Rationale and design of REDUCE‐IT: reduction of cardiovascular events with icosapent ethyl‐intervention trial. Clin Cardiol. 2017;40(3):138‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased residual cardiovascular risk in patients with diabetes and high vs. normal triglycerides despite statin‐controlled LDL cholesterol. Diabetes Obes Metab. 2019;21(2):366‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nichols GA, Philip S, Reynolds K, Granowitz CB, O'Keefe‐Rosetti M, Fazio S. Comparison of medical care utilization and costs among patients with statin‐controlled low‐density lipoprotein cholesterol with versus without hypertriglyceridemia. Am J Cardiol. 2018;122(7):1128‐1132. [DOI] [PubMed] [Google Scholar]
- 51. Jarow JP, LaVange L, Woodcock J. Multidimensional evidence generation and FDA regulatory decision making: defining and using "real‐world" data. JAMA. 2017;318(8):703‐704. [DOI] [PubMed] [Google Scholar]
- 52. Sherman RE, Anderson SA, Dal Pan GJ, et al. Real‐world evidence ‐ what is it and what can it tell us? N Engl J Med. 2016;375(23):2293‐2297. [DOI] [PubMed] [Google Scholar]
- 53. Berger ML, Sox H, Willke RJ, et al. Good practices for real‐world data studies of treatment and/or comparative effectiveness: Recommendations from the Joint ISPOR‐ISPE Special Task Force on Real‐World Evidence in Health Care Decision Making. Value Health. 2017;20(8):1003‐1008. [DOI] [PubMed] [Google Scholar]
- 54. Driver SL, Martin SS, Gluckman TJ, Clary JM, Blumenthal RS, Stone NJ. Fasting or nonfasting lipid measurements: It depends on the question. J Am Coll Cardiol. 2016;67(10):1227‐1234. [DOI] [PubMed] [Google Scholar]
- 55. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309‐316. [DOI] [PubMed] [Google Scholar]
- 56. Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118(10):993‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schaefer EJ, Audelin MC, McNamara JR, et al. Comparison of fasting and postprandial plasma lipoproteins in subjects with and without coronary heart disease. Am J Cardiol. 2001;88(10):1129‐1133. [DOI] [PubMed] [Google Scholar]
- 58. Leatherman S, Ferguson R, Weir I, et al. Increased residual cardiovascular risk in US veterans and monerately‐elevated baseline triglycerides and well‐controlled LCL‐C levels on statins [abstract]. J Am Coll Cardiol. 2019;73(9[suppl 1]):1719. [DOI] [PMC free article] [PubMed]
- 59. Guyton JR, Slee AE, Anderson T, et al. Relationship of lipoproteins to cardiovascular events: the AIM‐HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes). J Am Coll Cardiol. 2013;62(17):1580‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. The HPS2‐THRIVE Collaborative Group . Effects of extended‐release niacin with laropiprant in high‐risk patients. N Engl J Med. 2014;371(3):203‐212. [DOI] [PubMed] [Google Scholar]
- 61. Maki KC, Dicklin MR. Omega‐3 fatty acid supplementation and cardiovascular disease risk: glass half full or time to nail the coffin shut? Nutrients. 2018;10(7):E864. 10.3390/nu10070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.GISSI Prevenzione Investigators. Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico Lancet. 1999;354(9177):447‐455. [PubMed] [Google Scholar]
- 63. Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open‐label, blinded endpoint analysis. Lancet. 2007;369(9567):1090‐1098. [DOI] [PubMed] [Google Scholar]
- 64. Kromhout D, Giltay EJ, Geleijnse JM. n‐3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015‐2026. [DOI] [PubMed] [Google Scholar]
- 65. Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo‐controlled trial to test the effect of highly purified omega‐3 fatty acids on top of modern guideline‐adjusted therapy after myocardial infarction. Circulation. 2010;122(21):2152‐2159. [DOI] [PubMed] [Google Scholar]
- 66. The Risk and Prevention Study Collaborative Group . n‐3 Fatty acids in patients with multiple cardiovascular risk factors: the Risk and Prevention Study Collaborative Group. N Engl J Med. 2013;368:1800‐1808. [DOI] [PubMed] [Google Scholar]
- 67. Galan P, Kesse‐Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ORIGIN Trial Investigators. n‐3 Fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309‐318. [DOI] [PubMed] [Google Scholar]
- 69. Manson JE, Cook NR, Lee IM, et al. Marine n‐3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. ASCEND Study Collaborative Group , Bowman L, Mafham M, Wallendszus K, et al. Effects of n‐3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379(16):1540‐1550. [DOI] [PubMed] [Google Scholar]
- 71. Bhatt DL, Steg G, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 72. Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol. 2009;158(4):960‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111(10):5922‐5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Budoff M, Brent Muhlestein J, Le VT, May HT, Roy S, Nelson JR. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200‐499 mg/dL) on statin therapy: Rationale and design of the EVAPORATE study. Clin Cardiol. 2018;41(1):13‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Randomized trial for evaluation in secondary prevention efficacy of combination therapy ‐ statin and eicosapentaenoic acid UMIN000012069. UMIN Clinical Trials Registry 2016. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000014051&type=summary&language=E. Accessed October 9, 2019.
- 76. Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega‐3 carboxylic acids in statin treated patients with high levels of triglycerides and low levels of high density lipoprotein cholesterol: rationale and design of the STRENGTH Trial. Clin Cardiol. 2018;41(10):1281‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Laake K, Myhre P, Nordby LM, et al. Effects of omega3 supplementation in elderly patients with acute myocardial infarction: design of a prospective randomized placebo controlled study. BMC Geriatr. 2014;14:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.American Diabetes Association® issues critical updates to the 2019. standards of medical care in diabetes. 2019. http://www.diabetes.org/newsroom/press-releases/2019/ada-issues-critical-updates-to-2019-standards-of-care.html. Accessed: October 9, 2019.


