Abstract
Background
The intestinal microbiome plays a versatile role in the etiology of arterial thrombosis. In venous thrombosis, driven chiefly by plasma coagulation, no such role has yet been established. We hypothesized that the intestinal microbiome composition affects coagulation in humans.
Methods
We used healthy donor fecal microbiota transplant (FMT) to experimentally change the microbiome composition in metabolic syndrome patients. Thirty‐five subjects were randomized in a blinded fashion to healthy donor FMT or autologous FMT as a control in a 2:1 ratio. We measured thrombin generation at baseline and after 6 weeks using automated calibrated thrombinography, and we determined plasma abundance of 32 coagulation related proteins using a targeted mass spectrometry‐based quantitative proteomics assay with heavy labeled internal standards.
Results
Healthy donor FMT prolonged the thrombinography lag time (median delta 0.0 versus 0.25 minutes, P = .039). The other thrombinography parameters showed no significant difference. Unsupervised cluster analysis suggested overall downregulation of coagulation related plasma proteins in subject clusters containing predominantly subjects that had a metabolic response to healthy donor FMT. FMT treatment status itself showed no clear clustering pattern with up‐ or downregulation, however, and proteins did not cluster according to an apparent biological grouping.
Discussion
A single healthy donor FMT tends to modestly suppress the onset thrombin generation in metabolic syndrome patients, representing initial proof‐of‐principle that the intestinal microbiota composition might affect the coagulation system in humans. The findings merit external validation as a role for intestinal microbiota in coagulation can have clinically important implications.
Keywords: coagulation, fecal microbiota transplant, intestinal microbiome, metabolic syndrome, thrombin generation, thrombosis
Essentials.
The role of the gut microbiome in human plasma coagulation and venous thrombosis has gone unexplored.
Fecal microbiota transplant experimentally changed the microbiome in metabolic syndrome patients.
Healthy donor microbiota transplant modestly delayed the onset of thrombin generation.
This represents initial proof‐of‐principle that microbiota might affect coagulation in humans.
1. INTRODUCTION
The intestinal microbiome, the microbial ecosystem occupying the intestinal tract, affects human physiology in systems ranging from metabolism to immunity. Accruing evidence reveals a pathophysiological role in arterial thrombosis.1 Intestinal microbiome alterations are associated with susceptibility to atherothrombosis in both animal models and in humans.2, 3 Meta‐organismal metabolic pathways produce signaling molecules, including trimethylamine N‐oxide, serotonin, and fatty acids, that modulate inflammation, platelet function, and cardiovascular disease manifestations.1, 4, 5, 6
In the pathophysiology of venous thrombosis, however, in which plasma coagulation plays a more prominent role, effects of the intestinal microbiome go largely unexplored. One intriguing finding in this context is that intestinal microbes locally affect glycosylation and cell‐membrane expression of tissue factor in mice.7 Intestinal tissue lysates concordantly show lower levels of thrombin‐antithrombin complexes. A direct effect on plasmatic thrombin formation has yet to be demonstrated.
Our main hypothesis was that the composition of the intestinal microbiome affects coagulation in humans. To test this we used healthy donor fecal microbiota transplants (FMT) to experimentally change the intestinal microbiome composition, and investigated its effect on coagulation in subjects with metabolic syndrome, a condition that displays a pathologic hypercoagulable state.8 FMT elicits a metabolic response in some patients but not in others.9 Metabolic response to FMT coincides with a fecal microbiota composition distinct from that in non‐responders, suggesting that in the responder subgroup an effective microbiota alteration is attained.10 A secondary hypothesis therefore was that the effects on coagulation would be most pronounced in metabolic responders, which we regarded as subjects with a beneficial intestinal microbiome engraftment.
2. MATERIALS AND METHODS
2.1. Subjects, study design, and sample collection
The current trial has previously been reported on, along with a detailed description of the methods.10 In brief, we included obese male Caucasian metabolic syndrome patients between the ages of 21 and 69 years, who were otherwise healthy. Subjects with recent weight loss, a history of cardiovascular events or cholecystectomy, or the use of medication known to influence gut microbial composition in the past 3 months were excluded. Lean healthy donors were tested for any infectious diseases.
Using computerized randomization, subjects were allocated to either allogenic FMT from healthy donors or a control treatment, which consisted of autologous but otherwise identical FMT. The allocation ratio was 2:1. Recipients were randomly assigned a donor and were blinded to treatment allocation, as were the researchers. The Institutional Review Board of the Academic Medical Center in Amsterdam, the Netherlands, approved the study and all subjects gave informed consent. Subjects in the healthy donor FMT group were classified as either metabolic responders or metabolic non‐responders based on the improvement after FMT in the main outcome in the initial study, which was peripheral insulin sensitivity (Rd) expressed as rate of glucose disappearance in hyperinsulinemic euglycemic clamp tests, as previously described.10 A glucose disappearance rate increase ≥ 10% in hyperinsulinemic conditions defined metabolic response. After an overnight fast, blood was drawn on 3.2% citrate 1:10 from a peripheral intravenous catheter at baseline and 6 weeks after FMT. Samples were centrifuged once at 1550 g for 10 minutes at 4°C and stored at −80°C. The 6‐week interval was based on earlier work showing coexistence of donor and recipient strains persisting for at least 3 months after FMT, with metabolic effects being manifest until 6 weeks.9, 10, 11
2.2. Intestinal microbiome composition
Methods for isolating and sequencing the microbiota for this study were previously described.10 In short, DNA was isolated from fecal samples using repeated‐bead beating and column purification. The microbial composition was determined using the Human Intestinal Tract Chip, containing approximately 5.500 specific oligonucleotide probes. The 16S rRNA gene was amplified and transcribed into RNA, labeled, and hybridized to the array in duplicates.
2.3. Thrombin generation
Calibrated automated thrombinography (CAT) was performed as previously described.12 Thrombin generation was measured after recalcification of the citrated platelet‐poor plasma and addition of 5PM tissue factor (Thrombinoscope BV, Maastricht, the Netherlands), using FluCa fluorogenic substrate (Thrombinoscope BV). Fluorescence measurement was obtained with a Fluoroskan Ascent fluorometer (ThermoLabsystems, Helsinki, Finland) and thrombin generation parameters were determined with Thrombinoscope® software (Thrombinoscope BV). Values were rounded to one decimal because of the measurement interval. Parameters involving thrombin concentration (peak height and endogenous thrombin potential) were normalized to pooled plasma from healthy volunteers.
2.4. Targeted quantitative proteomics
We used a multiplexed targeted mass spectrometry‐based quantitative proteomics assay to measure secondary hemostasis and fibrinolysis related proteins. The multiplexed assay was developed and validated at the University of Victoria Proteomics Centre, Victoria, BC, Canada, as part of building multiple reaction monitoring (MRM) assays for blood plasma proteins,13, 14, 15, 16 and measures the abundance of 270 proteins in total covered by 274 surrogate peptides, of which we included in this analysis the 43 proteins involved in secondary hemostasis and fibrinolysis (with Vitamin K‐dependent protein Z covered by two surrogate peptides). A list of the peptides and proteins studied is provided in Table S1 in supporting information.
All protein concentrations were determined by comparing their response in the mass spectrometer with the response of the known amount of heavy labeled internal standard peptides spiked in the sample. The surrogate peptides for the assays were selected by PeptidePicker16 and synthesized at the University of Victoria Proteomics Centre. The sample preparation protocol was developed previously13, 17 and is available from PeptideTracker.15 Briefly, for the current study, the sample digests were prepared through denaturation and reduction of the homogenate with 9M urea/20mM dithiothreitol for 30 minutes at 37ºC. Denatured proteins were alkylated with iodoacetamide (40 mM final concentration) for 30 minutes at room temperature, and then samples were diluted to reach a final urea concentration of 0.55 mM prior to tryptic digestion. Digestion was carried out at a 10:1 substrate:enzyme ratio using tosyl phenylalanyl chloromethyl ketone (TPCK)‐treated trypsin (Worthington) for 18 hours at 37ºC. After digestion, samples were acidified with aqueous 1% formic acid (FA), and a chilled stable isotope‐labeled standard (SIS) peptide mixture was added. Samples were concentrated via solid phase extraction (SPE; 10 mg Oasis HLB cartridges; Waters), using the manufacturer's recommended protocol. The SPE column was conditioned with 100% methanol (1 mL), followed by washing with 100% H2O/0.1% FA (1 mL), the sample (diluted to 1 mL using 100% H2O/0.1% FA) was then loaded onto the column, followed by washing two times with water (1 mL each). Finally, the sample was eluted with 55% acetonitrile (can)/0.1% FA (300 μL) and lyophilized to dryness. The dried samples were rehydrated in 0.1% FA to a 1 μg/μL concentration for liquid chromatography (LC)/MRM‐MS analysis. The samples were separated on‐line with a reversed phase‐ultra high performance liquid chromatography (RP‐UHPLC) column (EclipsePlusC18 RRHD 150 × 2.1 mm i.d., 1.8 μm particle diameter; Agilent) maintained at 50°C. Peptide separations were performed at 0.4 mL⁄min over a 56‐minute run, via a multi‐step LC gradient (1.5%‐81% 2%‐80%mobile phase B; mobile phase B: 0.1% FA in ACN). The exact gradient was as follows (time point in minutes, solution B %): 0 minutes, 2%; 2 minutes, 7%; 50 minutes 30%; 53 minutes, 45%, 53.5 minutes, 80%; 55.5 minutes, 80%; 56 minutes, 2%. A post‐column equilibration of 4 minutes was used after each sample analysis. The LC system was interfaced to a triple‐quadrupole mass spectrometer (Agilent 6490) via a standard‐flow electrospray ionization (ESI) source, operated in the positive ion mode. The MRM acquisition parameters employed for the quantitation were as follows: 3500 V capillary voltage, 300 V nozzle voltage, 11 L/min sheath gas flow at a temperature of 250°C, 15 L/min drying gas flow at a temperature of 150°C, 30 psi nebulizer gas pressure, 380 V fragmentor voltage, 5 V cell accelerator potential, and unit mass resolution in the first and third quadrupoles. For optimal peptide collision‐induced dissociation, peptide‐specific carbon equivalent (CE) values had previously been determined experimentally.
2.5. Data processing and analysis
For the proteomics data, Skyline18 was used to inspect the peptide response peaks and ensure accurate selection, retention time, integration, and uniformity of peak shape for the endogenous and internal standard peptide signals. For each peptide, the relative peak area ratio of the endogenous to the heavy labeled internal standard peptide was calculated. This ratio and the known concentration of internal standard peptide were used to calculate the concentration of the endogenous peptide in the sample by comparison to a standard curve generated in pooled sample.13 The criteria used for the standard curve regression analysis were 1/x2 regression weighting, <15% deviation in a given level's precision and accuracy for each concentration level, as well as 20% at the lower limit of quantification. Unsupervised cluster analysis was performed on the determined protein concentrations. We used the complete distance method to perform the clustering on the scaled and centered values. Visualization of the data was performed after centering and scaling of the determined protein concentrations.
In an attempt to deal with interindividual differences in thrombin generation and protein abundance, we used the delta at 6 weeks, meaning the value at 6 weeks after FMT minus the value at baseline, in all analyses. We also analyzed the change in lag time between baseline and 6 weeks in each group. Differences between groups in delta thrombin generation parameters and baseline characteristics were tested using Wilcoxon rank‐sum test as well as Wilcoxon signed‐rank test.
For the analysis of the intestinal microbiome composition data we converted reported intensities to percentages of total composition and generated histograms of delta change between baseline and 6 weeks within groups as well as between subjects and their corresponding donors for baseline and for 6 weeks after FMT treatment.
All data analysis and visualization were performed using our own routines written in R statistical language.
3. RESULTS
3.1. Participants
A total of 35 male metabolic syndrome patients participated in the current study. Three of the 38 subjects in the original study could not be included because adequate citrate plasma samples were not available for both time points. Eleven subjects were randomized to the control arm and 24 subjects to healthy donor FMT arm. Of the latter, 11 were metabolic responders. Baseline characteristics are displayed in Table 1. Subjects randomly assigned to the healthy donor FMT arm trended toward a lower baseline body mass index (BMI) than controls. Metabolic responders had a significantly lower BMI than the controls.
Table 1.
Baseline characteristics and thrombin generation
| Control group (n = 11) | Healthy donor FMT (n = 24) | P‐value* | Healthy donor FMT ‐ metabolic responders (n = 11) | P‐value* | |
|---|---|---|---|---|---|
| Age | 54.1 (50.2‐56.9) | 52.9 (49.5‐59.6) | 0.99 | 51.6 (48.5‐61.8) | .80 |
| Baseline BMI | 35.9 (34.1‐40.2) | 33.9 (32.5‐35.7) | 0.052 | 32.5 (31.9‐34.1) | .013 |
| Difference in thrombin generation after FMTa | |||||
| Lag time (min) | 0.0 (−0.30 to 0.10) | 0.25 (0.0‐0.53) | 0.039 | 0.40 (0.0‐0.55) | .091 |
| Time to peak (min) | 0.10 (−0.95 to 0.35) | 0.55 (−0.30 to 1.25) | 0.12 | 0.8 (−0.15 to 1.50) | .15 |
| Thrombin peak (%) | −5.0 (−16.0 to 20.0) | −12.5 (−28.5 to 14.3) | 0.17 | −21.0 (−41.0 to 20.5) | .18 |
| Endogenous thrombin potential (%) | 1.0 (−10.5 to 4.0) | 0.0 (−10.8 to 6.8) | 0.92 | −10.0 (−14.5 to 13.0) | .65 |
Values expressed as median and interquartile range.
Abbreviations: BMI, body mass index; FMT, fecal microbiota transplant.
Median of delta's (the value at 6 weeks after FMT minus the value at baseline).
Compared to the control group.
3.2. Fecal microbiota transplant
Figure 1A displays the frequency distribution of phylotype abundance differences between 0 and 6 weeks, for both controls and healthy donor FMT treated subjects. Per phylotype, all subjects in the respective groups are aggregated by taking the mean of delta change for that phylotype. As can be seen in the figure, where the controls are centered around 0, meaning no abundance difference before and after FMT, the healthy donor FMT treated subjects show a shift away from the no difference distribution.
Figure 1.
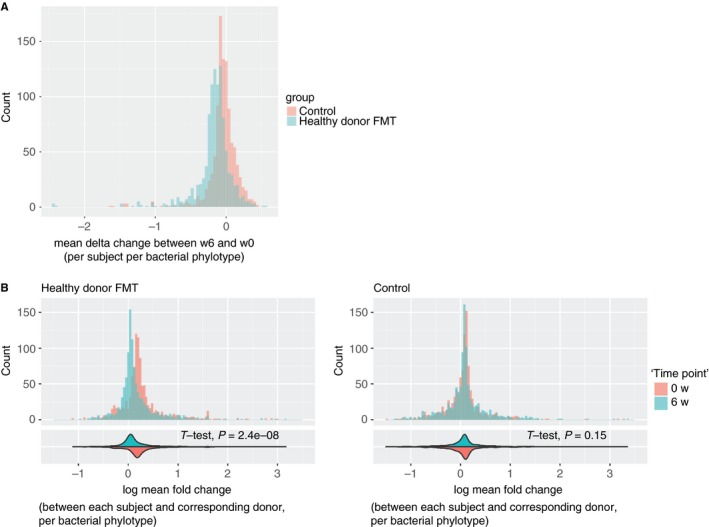
The change in the microbiome of healthy donor fecal microbiota transplant (FMT) and control subjects. A, The change in the microbiome within the treatment groups after 6 weeks in relation to baseline. B, The change over time in the difference of the microbiome between the subjects and their corresponding donors. The histograms in (B) are accompanied in the bottom with violin plots of the distributions and the student's t‐test P‐value for the difference between the two distributions. For all histograms, all subjects in the respective groups are aggregated by taking the mean of delta change per phylotype
Figure 1B presents the difference between the subjects and their corresponding donors, at both time points. Based on the measured bacterial phylotype abundances, the microbiome of the healthy donor FMT treated subjects converges toward the donor's microbiome after the transplantation. This shows the expected effect of the transplantation. In the histogram and violin plots, the value of 0 means one‐to‐one bacterial phylotype proportional presence compared to the donor, while diverting from 0 means a change in this proportion. A significant change with a P‐value of 2.4e‐8 was observed in the healthy donor FMT subjects. No change was observed in the control subjects, who were their own donor.
3.3. Thrombin generation
Calibrated automated thrombinography results are presented in Table 1. Lag time was slightly prolonged 6 weeks after healthy donor FMT when compared to controls (Figure 2). The time to peak and thrombin peak parameters showed a similar trend of modestly diminished thrombin generation but did not reach statistical significance. For both groups at both time points, group means of calibrated automated thrombinography curves are presented in Figure S1A in supporting information, and lag time values in Figure S1B in supporting information. The delta lag time did not correlate with BMI (P = .88).
Figure 2.
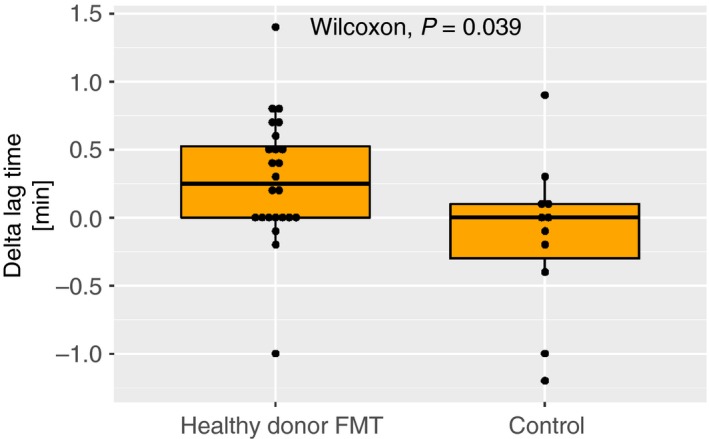
Difference in lag time between baseline and 6 weeks after fecal microbiota transplant. Horizontal line indicates median
Direction and magnitude of change in the parameters were very similar when looking at the metabolic responder subgroup only, but the changes were not significant when compared to controls.
3.4. Coagulation proteomics assay
Measurement of protein concentration was successful for 32 proteins out of the 43 coagulation and fibrinolysis related proteins we attempted to measure. Criteria for acceptance were mainly based on obtaining a good signal within the dynamic range in the corresponding standard curve for each peptide. This guarantees reproducible and trustworthy results.
The heatmaps in Figure 3 show the distribution of the change in protein abundance based on the proteomics data in subjects with different treatment and metabolic responder status. In the overall group, there was no evidence for clustering of subjects according to treatment status. Introducing a cut at a high level in the dendrogram corresponding to subjects (on the left) we observe four distinct subtrees (see Figure 3A). Subtrees 2 and 3 contain the majority of the subjects, with 13 and 16 subjects, respectively. The distribution of subjects in subtree 2 reflects the 2:1 randomization ratio between healthy donor FMT and control. Subtree 3 contains predominantly subjects treated with healthy donor FMT. This cluster showed varying proteins abundance.
Figure 3.
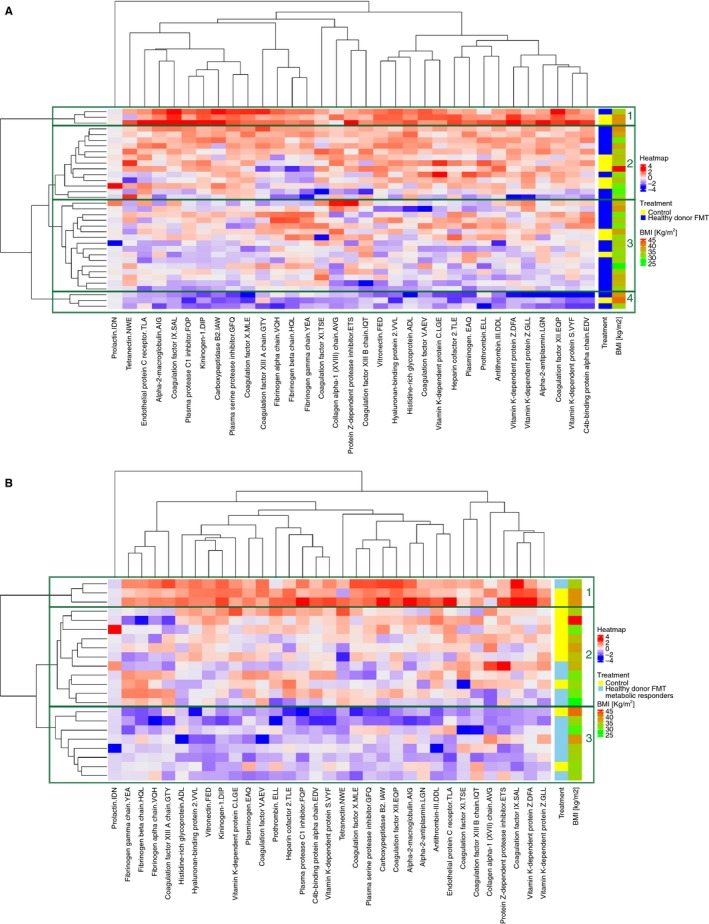
Coagulation proteome heatmaps and hierarchal clustering of subjects with different treatment and metabolic responder status. Proteins are represented as difference in abundance between baseline and 6 weeks after fecal microbiota transplant (FMT). The differential protein abundance is represented in z‐scores and displayed in continuous color levels ranging from − 4 in blue to + 4 in red. A, Healthy donor FMT versus control. The hierarchal clustering can be split with a high‐level cut into four major subtrees indicated as boxes and numbered from 1 to 4. Subtrees 2 and 3 dominate the middle part with 29 subjects. B, Healthy donor FMT metabolic responders versus control. The hierarchal clustering can be split with a high‐level cut into three major subtrees indicated as boxes and numbered from 1 to 3. Subtrees 2 and 3 dominate with 19 subjects
There is a clearer pattern when comparing the controls to the metabolic responders only, as can be seen in Figure 3B. Dividing this dendrogram around the same distance as in Figure 3A produces three subtrees. The control subjects predominate in subtree 2, with overall upregulation of the proteins. Subtree 3 consists of mostly metabolic responders and shows overall downregulation of proteins.
The clustering of proteins does not correspond to an apparent biological grouping, such as anti‐ or procoagulant factors, vitamin K dependent factors, or the intrinsic and extrinsic pathway. Subjects do not cluster according to BMI.
Absolute values of protein concentration are visualized as individual boxplots per protein, per group and per visit in Figure S2 in supporting information, and data is provided in Table S3 in supporting information.
4. DISCUSSION
The current study shows that healthy donor FMT tends to suppress the onset of thrombin generation in metabolic syndrome patients, as measured 6 weeks after a single transplantation. The effect size was small and we found a significant difference only in thrombin generation lag time, not in other measured parameters. In general, the sample size of the study was limited and external validation of our results are warranted. The double‐blinded randomized controlled trial design nevertheless supports the causality of the observed effect. The FMT was shown to elicit a change in microbiome composition toward that of the donors, supporting its use as an experimental tool to study effects of altering the gut microbiota. The study was not aimed at assessing clinical efficacy of FMT for hypercoagulability in metabolic syndrome patients. Results can be regarded as proof‐of‐principle that the intestinal microbiota composition might affect plasma coagulation, representing initial evidence for such an effect in humans.
Successful and precise determination of the abundance of 32 coagulation‐related proteins in plasma using targeted proteomics showed an overall trend of reduced abundance of these proteins in the metabolic responders. For the targeted proteomics approach, we used multiplexed measurements of the proteins of interest using mass spectrometry operated in multiple reaction monitoring mode with internal standard for each protein. This allowed for the establishment of protein signatures in plasma that were used for comparing time points and individuals.
The fact that the lag time was affected and not the other thrombin generation parameters can have several possible causes. Naturally, statistical variation could explain the discrepancy. Either a type I error on the lag time or a type II error on the other parameters might have occurred. Suggestive of the latter is the fact that also the time to peak and the peak height parameters numerically shifted toward suppressed thrombin generation after healthy donor FMT. Another explanation could be that FMT specifically affects the initiation phase of coagulation. Of the extrinsic and common pathway coagulation factors successfully measured, only prothrombin trended toward a difference, with a lower delta in the total healthy donor FMT group than in the controls (median delta 2 versus 58 fmol/μL respectively, P = .047). This was, however, a post‐hoc observation.
In the secondary analysis we investigated whether metabolic response status was a superior predictor of coagulation response than FMT treatment itself. It is important to note in this context that metabolic response, in the form of insulin resistance, was not expected to be causally related to coagulation. Rather, we regarded metabolic response status as a surrogate for successful engraftment of the transplanted microbiome. We expected a more pronounced effect on coagulation in this subgroup. This was not observed. This shows that metabolic response is in fact not an adequate surrogate for a beneficial response to FMT in terms of coagulation. One possible explanation is that the microorganisms involved in the effect on insulin resistance differ from those involved in effects on plasma coagulation. Another explanation is again the limited number of subjects. Metabolic responders had a significantly lower BMI than the control subjects. This also appeared as a trend in the study population as a whole, and given the randomized design, this difference is most likely the result of chance. Nonetheless, the difference was more pronounced in responders and therefore a relation between metabolic response and baseline BMI cannot be excluded. We observed neither a correlation between BMI and delta lag time nor an obvious clustering of proteins according to BMI.
Microbiota products reach the portal circulation, the main site of coagulation protein synthesis.19 In work by others, germ‐free mice showed reduced synthesis of von Willebrand factor in hepatic endothelial cells,20 which was mediated by toll‐like receptor‐2 signaling in the portal circulation. This finding supports the concept that the intestinal microbiome can modulate thrombosis‐related gene transcription in the liver and subsequently affect the circulation. This concept forms one possible theoretical explanation for the present observation.
An alternative mechanism of action might involve vitamin K metabolism. Vitamin K is an essential cofactor for the carboxylation of glutamate residues in the synthesis of coagulation factors II, VII, IX, and X and anticoagulant proteins C, S, and Z. Gut commensals are one source of subtype vitamin K2 and antibiotic treatment is thought to deplete vitamin K2 stores.21 An effect of FMT on thrombin generation might thus be mediated by changes in the vitamin K2 metabolism. However, in the proteomics data we observe no apparent clustering of vitamin K dependent proteins, whereas concentrations of vitamin K independent proteins are affected by healthy donor FMT. Moreover, concentrations of vitamin K dependent proteins did not uniformly change in one direction. Last, for dysbiosis as seen in metabolic syndrome to result in the observed increased thrombotic risk through altered vitamin K metabolism, a supranormal vitamin K production would have to lead to increased coagulation. This was not detected in one small experimental study.22
In summary, the present randomized study showed a small but significant prolongation of thrombin generation lag time in metabolic syndrome patients 6 weeks after changing the gut microbiome through healthy donor FMT. There also appeared to be an overall downregulation of coagulation related proteins seen in metabolic responders to FMT. Further work is needed to confirm these findings, in separate human cohorts as well as using in‐vitro and animal model tools. A possible physiological role for the gut microbiome in coagulation can have clinically important implications. Characterization of the microbiota of venous thromboembolism (VTE) patients and controls could establish certain microbiome patterns as risk factors or therapy response predictors. In the original study, for instance, baseline fecal microbiota diversity had good predictive value for response to FMT.10 If the fecal microbiota indeed has an etiological role in coagulation and VTE, then this could even provide new therapeutic options. Approaches might comprise diet interventions or anti‐ and probiotic strategies. More sophisticated still would be approaches involving luminal drugs that act on metabolic pathways within the gut microbiome. Compounds following this strategy for platelet inhibition in arterial thrombosis are already making their way toward first in‐human studies.23 Hopefully continued research into coagulation effects of the gut microbiome can contribute to future treatment of VTE in a similar manner.
CONFLICT OF INTEREST
MN is on the Scientific Advisory Board of Caelus Pharmaceuticals, the Netherlands; CHB is the chief strategy officer of MRM Proteomics, Inc; none of these are directly relevant to the current paper. There are no patents, products in development, or marketed products to declare. The other authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
Yassene Mohammed, Ruud S. Kootte, and Wil F. Kopatz collected data and performed experiments. Yassene Mohammed and Thijs E. van Mens performed analyses. Yassene Mohammed, Ruud S. Kootte, Christoph H. Borchers, Harry R. Büller, Henri H. Versteeg, Max Nieuwdorp, and Thijs E. van Mens contributed to the design of the study and interpretation of the results. Yassene Mohammed and Thijs E. van Mens wrote the manuscript with input from all authors.
Supporting information
ACKNOWLEDGMENTS
MN is supported by a ZONMW‐VIDI grant 2013 (016.146.327) and a Dutch Heart Foundation CVON IN CONTROL Young Talent Grant 2013. The study reported here was additionally supported by Le Ducq consortium grant 17CVD01 and a Novo Nordisk Foundation GUT‐MMM grant 2016. TEvM is supported by an MD/PhD grant from Amsterdam Cardiovascular Sciences. This work was supported by Genome Canada and Genome British Columbia (project codes 204PRO for operations and 214PRO for technology development).
Mohammed Y, Kootte RS, Kopatz WF, et al. The intestinal microbiome potentially affects thrombin generation in human subjects. J Thromb Haemost. 2020;18:642–650. 10.1111/jth.14699
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 27 November 2019
REFERENCES
- 1. Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14(2):79‐87. [DOI] [PubMed] [Google Scholar]
- 2. Karlsson FH, Fåk F, Nookaew I, et al Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lam V, Su J, Hsu A, Gross GJ, Salzman NH, Baker JE. Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PLoS ONE. 2016;11(8):e0160840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maslowski KM, Vieira AT, Ng A, et al Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu W, Gregory JC, Org E, et al Microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yano J, Yu K, Donaldson G, et al Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reinhardt C, Bergentall M, Greiner TU, et al Tissue factor and PAR1 promote microbiota‐induced intestinal vascular remodelling. Nature. 2012;483(7391):627‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nieuwdorp M, Stroes ESG, Meijers JCM, Büller H. Hypercoagulability in the metabolic syndrome. Curr Opin Pharmacol. 2005;5(2):155‐159. [DOI] [PubMed] [Google Scholar]
- 9. Vrieze A, Van Nood E, Holleman F, et al Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913‐916. [DOI] [PubMed] [Google Scholar]
- 10. Kootte RS, Levin E, Salojärvi J, et al Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(4):611‐619. [DOI] [PubMed] [Google Scholar]
- 11. Li SS, Zhu A, Benes V, et al Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352(6285):586‐589. [DOI] [PubMed] [Google Scholar]
- 12. Hemker HC, Giesen P, Al Dieri R, et al Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(1):4‐15. [DOI] [PubMed] [Google Scholar]
- 13. Mohammed Y, Pan J, Zhang S, Han J, Borchers CH. ExSTA: external standard addition method for accurate high‐throughput quantitation in targeted proteomics experiments. Proteomics Clin Appl. 2018;12(2):1600180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhowmick P, Mohammed Y, Borchers CH. MRMAssayDB: an integrated resource for validated targeted proteomics assays. Bioinformatics. 2018;34(20):3566‐3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohammed Y, Bhowmick P, Smith DS, et al PeptideTracker: A knowledge base for collecting and storing information on protein concentrations in biological tissues. Proteomics. 2017;17(7):1600210. [DOI] [PubMed] [Google Scholar]
- 16. Mohammed Y, Domański D, Jackson AM, et al PeptidePicker: a scientific workflow with web interface for selecting appropriate peptides for targeted proteomics experiments. J Proteomics. 2014;106:151‐161. [DOI] [PubMed] [Google Scholar]
- 17. Percy AJ, Mohammed Y, Yang J, Borchers CH. A standardized kit for automated quantitative assessment of candidate protein biomarkers in human plasma. Bioanalysis. 2015;7(23):2991‐3004. [DOI] [PubMed] [Google Scholar]
- 18. Mohammed Y, Percy AJ, Chambers AG, Borchers CH. Qualis‐SIS: automated standard curve generation and quality assessment for multiplexed targeted quantitative proteomic experiments with labeled standards. J Proteome Res. 2015;14(2):1137‐1146. [DOI] [PubMed] [Google Scholar]
- 19. Balmer ML, Slack E, de Gottardi A, et al The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6(237):237ra66. [DOI] [PubMed] [Google Scholar]
- 20. Jäckel S, Kiouptsi K, Lillich M, et al Gut microbiota regulate hepatic von Willebrand factor synthesis and arterial thrombus formation via Toll‐like receptor‐2. Blood. 2017;130(4):542‐553. [DOI] [PubMed] [Google Scholar]
- 21. Conly J, Stein K. Reduction of vitamin K2 concentrations in human liver associated with the use of broad spectrum antimicrobials. Clin Invest Med. 1994;17(6):531‐539. [PubMed] [Google Scholar]
- 22. Asakura H, Myou S, Ontachi Y, et al Vitamin K administration to elderly patients with osteoporosis induces no hemostatic activation, even in those with suspected vitamin K deficiency. Osteoporos Int. 2001;12(12):996‐1000. [DOI] [PubMed] [Google Scholar]
- 23. Roberts AB, Gu X, Buffa JA, et al Development of a gut microbe–targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


