Abstract
Chronic pain is a common condition that affects the physical, emotional, and mental well‐being of patients and can significantly diminish their quality of life. Due to growing concerns about the substantial risks of long‐term opioid use, both governmental agencies and professional societies have recommended prioritizing the use of nonpharmacologic treatments, when suitable, in order to reduce or eliminate the need for opioid use. The use of 10 kHz spinal cord stimulation (10 kHz SCS) is one such nonpharmacologic alternative for the treatment of chronic, intractable pain of the trunk and limbs. This review examines published clinical data regarding the efficacy of 10 kHz SCS for decreasing chronic pain in patients and its potential to reduce or eliminate opioid usage. Multiple prospective and retrospective studies in patients with intractable pain demonstrated that 10 kHz SCS treatment provided ≥50% pain relief in >70% patients after at least 1 year of treatment. Pain relief with 10 kHz SCS therapy ranged from 54% to 87% in the studies. More importantly, the mean daily dose of opioids required by patients in these studies was reduced after 10 kHz SCS treatment, and on average over 60% patients in studies either reduced or eliminated opioids at the last follow‐up.
Keywords: chronic pain, 10 kHz SCS, opioids
The use of 10 kHz spinal cord stimulation (10 kHz SCS) is a nonpharmacologic alternative for the treatment of chronic, intractable pain of the trunk and limbs. This review examines published clinical data regarding the efficacy of 10 kHz SCS for decreasing chronic pain in patients and its potential to reduce or eliminate opioid usage.
Introduction
Chronic pain is a common condition that affects patients’ physical and mental health, and greater pain severity is correlated with worse patient outcomes.1 The Centers for Disease Control and Prevention (CDC) estimated that 20% of adults in the United States were affected by chronic pain in 2016, which translates to about 50 million Americans,2 and the World Health Organization has reported worldwide estimates of prevalence to range from 5% to 33%, with an average of 21.5%.3 Surveys have found that patients with chronic pain often report incomplete pain relief from available treatments, and a significant proportion of patients report impacts on their general activity, mood, and enjoyment of life, as well as their ability to walk, work, or sleep.4, 5
Because of the ubiquity of chronic pain in our society, safe and effective treatments are needed in order to manage these conditions. There are several interventions available for treating chronic pain, including nerve blocks, surgeries, implantable drug delivery systems, and nerve stimulators; however, the first line of treatment for pain is most often oral analgesics, including acetaminophen, nonsteroidal anti‐inflammatory drugs (NSAIDs), and opioids.6
Opioid analgesics in chronic pain
Prior to the 1980s, pure μ‐opioids (conventional opioids) were primarily used to treat acute pain or chronic cancer pain. However, an increased emphasis on pain relief by regulators and payers, changes in prescriber attitudes about the risks and benefits of opioids, and marketing campaigns by manufacturers led to large increases in opioid use in patients with chronic noncancer pain. There was an increase in opioid prescriptions of 45 million in 2002 compared with 5 years earlier, and almost 62 million Americans filled one or more opioid prescriptions in 2016.7 Both weak opioids, such as codeine and dihydrocodeine, and strong opioids, including morphine and related drugs, are now frequently used to control chronic pain once NSAIDs alone are no longer effective.6 Although conventional opioid analgesics are routinely used to treat chronic, noncancer pain, the evidence that they are effective for this indication are sparse. In 2016, the CDC issued new guidelines for the prescription of opioid analgesics for nonterminal, noncancer chronic pain, which emphasized a preference for nonopioid therapy for chronic pain.8 The CDC noted that most randomized controlled trials (RCTs) of conventional opioids for chronic pain lasted 6 weeks or less, making the long‐term safety and efficacy difficult to determine.
Data from RCTs testing opioids (including morphine, oxycodone, fentanyl, hydromorphone, and methadone) for chronic, noncancer pain reported average pain reductions of only about 30%.9 A recent Cochrane overview concluded that there was no evidentiary basis for the use of high‐dose opioids (≥200 morphine milligram equivalents; MME) for the management of chronic, noncancer pain because all of the available data on long‐term opioids were obtained using doses substantially lower than those used clinically in chronic pain.10 Similarly, a meta‐analysis of data from over 26,000 subjects in 96 RCTs found that opioid use produced statistically significant reductions in pain, but the magnitude of these reductions was 0.79 cm on a 0–10 cm visual analog scale (VAS), which did not meet the minimum threshold (1.0 cm) of a clinically important difference.11
In contrast to the uncertainty regarding conventional opioids’ efficacy in chronic pain treatment, the risks posed by these drugs are well known. Common adverse events (AEs) include constipation, nausea, somnolence, vomiting, dizziness, itching, dry mouth, and headache.9 Opioids can also produce more serious complications, including opioid abuse/addictive behavior, and respiratory depression, which can lead to death. Long‐term opioid use in a family has further been shown to increase the risk of persistent opioid use in young people (13–21 years old) prescribed opioid analgesics after surgery and dental procedures.12 Indeed, the rise in opioid overdose deaths in the United States has been partially attributed to the rise in opioid prescribing.13 The risk of overdose is correlated with several factors common in patients with chronic pain, including severe chronic pain itself, daily opioid consumption greater than 90 MME, and opioid use or misuse over long periods of time.14
More than 165,000 Americans died from prescription opioid–related overdose from 1999 to 2014, and the rise in both opioid prescribing and opioid‐related overdoses has led to recent updates to guidelines published by the CDC as well as professional societies.8, 13, 15 These updates sought to balance patients’ need for adequate access to opioids for pain relief with the long‐term risks that have become apparent in the past several decades. However, the new guidelines have led to concerns by some providers that implementation may harm patients through the abrupt reduction or cessation of opioid therapy without reducing opioid‐related overdoses, which are primarily due to illicit opioids like heroin or fentanyl.16 Additionally, there has been an increased emphasis on physician education and safe prescribing practices,17, 18 but a study of a health care system in the state of Washington showed that new initiatives aimed at reducing opioid doses and risks had little or no impact on the prevalence of opioid use disorder among patients on chronic opioid therapy.19
Another option is to reduce the risks of long‐term opioid therapy by prioritizing the use of nonpharmacologic or nonopioid medications.8, 13, 15 Alternative treatments for chronic pain include NSAIDs, topical analgesics like lidocaine, nerve blocks, and spinal cord stimulation (SCS).
High‐frequency SCS at 10 kHz (HF10)
The use of SCS to treat human pain was first described in 1967 in a patient with severe diffuse pain in his chest and abdomen due to an inoperable bronchial carcinoma.20 Although testing lasted less than 2 days, Shealy and his colleagues reported an immediate and substantial reduction in pain and cessation of analgesics in this single patient. Further testing established SCS as a standard treatment for pain from the 1980s onward.21 Implanting SCS devices presents the risk of AEs, from incision site infections to neurologic injury, but serious AEs are uncommon.22
The goal of traditional SCS is to produce paresthesia in the affected area, and this sensation can be experienced by some patients as uncomfortable or otherwise bothersome.23, 24 Additionally, surgical placement of the epidural leads for SCS requires verbal feedback from the patient during the procedure to align the sensation of paresthesia with the region of pain. In contrast, high‐frequency SCS at 10 kHz (10 kHz SCS, Senza® System, Nevro Corp., Redwood City, CA) produces paresthesia‐free pain relief, so the epidural leads for this therapy can be placed according to anatomical landmarks alone. This simplifies the process of implantation and avoids the risk of intolerable paresthesia as well as the potential requirement for additional intraoperative radiation exposure to steer or adjust lead position and obtain optimal paresthesia coverage. The typical 10 kHz SCS waveform has a pulse width of 30 μs, an amplitude of 1–5 mA, and a frequency of 10,000 Hz, compared with pulse widths of up to 400 μs, amplitudes of 4–6 mA, and frequencies of about 40 Hz for traditional SCS.25
Devices capable of delivering 10 kHz SCS have been approved for treating chronic, intractable trunk and limb pain in Europe since 2010, Australia since 2011, and the United States since 2015.25, 26 Early prospective trials in the United States and Europe first demonstrated the efficacy and safety of 10 kHz SCS in patients with chronic, intractable low back pain,27, 28 and the pain reductions observed were durable, extending to 24 months follow‐up.29 Since then, the superiority of 10 kHz SCS to conventional SCS in treating chronic back and leg pain has been demonstrated in an RCT (SENZA‐RCT),30, 31 and clinical use of this therapy has extended into other patient populations, including neuropathic pain.32
Although these studies have been primarily focused on the outcome of pain reduction and responder rate, defined as the proportion of patients who experience at least 50% pain reduction without any neurologic deficits, many include assessments of pain medication use among reported secondary outcomes. Conventional SCS treatment has been shown to be associated with reduction in conventional opioid dose and stabilization of usage in two large retrospective studies by Sharan et al. and Simopoulos et al.33, 34 Multiple systematic reviews also demonstrated increased odds of reducing pain medication in patients with intractable pain following SCS treatment.35, 36 Considering the potential advantages of 10 kHz SCS, this review was conducted to summarize the current landscape of evidence in the medical literature regarding the efficacy of 10 kHz SCS to both treat pain symptoms and reduce the amount of conventional opioid analgesics required by patients with chronic, noncancer pain.
Current evidence
Among the various tested applications for 10 kHz SCS, the most data exist for treatment of low back pain with or without accompanying leg pain. These include the one RCT of 10 kHz SCS (known as the SENZA‐RCT study), three prospective, open‐label studies in Europe and the United States, and three real‐world studies on the efficacy/effectiveness and safety of this therapy. Study subjects had chronic low back pain that was refractory to conventional methods of treatment, including analgesic medications, physical therapy, spinal injections, or behavioral treatments. The results of studies in patients with low back and leg pain, as well as a study of patients with neuropathic pain, are summarized in Table 1.
Table 1.
Published clinical studies of 10 kHz SCS in back pain and reported outcomes for pain and opioid use
| Study | Design | Arms (n analyzed) | Pain results | Opioid results |
|---|---|---|---|---|
| Van Buyten et al.;28 Al‐Kaisy et al.29 | Prospective, multicenter, open‐label (SENZA‐EU) | 10 kHz SCS (72 6 months; 65 24 months) | aBack pain: baseline—8.4; 6 months—2.7; 24 months—3.3 | aLeg pain: baseline—5.4; 6 months—1.4; 24 months—2.3 | Use: baseline—86%; 6 months—53%; 12 months—57% | Average daily dose: baseline—84 MME/day; 24 months—27 MME/day |
| Rapcan et al.37 | Prospective, nonrandomized | 10 kHz SCS (21) | aMean pain: baseline—8.7; 3 months—4.4; 6 months—4.4; 9 months—4.0; 12 months—4.0 | Opioid use halved in 65% of patients after 12 months |
| Kapural et al.;30 Kapural et al.31 | Prospective, randomized, controlled trial (SENZA‐RCT) | 12 months: 10 kHz SCS (90); conventional SCS (81) | 24 months: 10 kHz SCS (85); conventional SCS (71) | Back pain: 12 months—10 kHz SCS ↓67%/SCS ↓44% 24 months—10 kHz SCS ↓67%/SCS ↓41% | Leg pain: 12 months—10 kHz SCS ↓70%/SCS ↓49% 24 months—10 kHz SCS ↓65%/SCS ↓46% | Average daily dose: 12 months—10 kHz SCS 19% decrease/SCS 1% decrease |
| Amirdelfan et al.38 | Post‐hoc analysis of combined SENZA‐RCT and SENZA‐EU data | 10 kHz SCS (122) | aBack pain: baseline—7.8; 12 months—2.5 | Leg pain: baseline—6.3; 12 months—2.0 | All subjects: 41% reduction in mean daily dose from 104.2 to 61.4 MME | High‐dose subjects only: 46% reduction in mean daily dose from 196.8 to 106.5 MME |
| DiBenedetto et al.40 | Retrospective case–control review | 10 kHz SCS + CMM (32) | CMM only (64) | bBack pain: 45.6% below baseline (P < 0.001) | Leg pain: 50.9% below baseline (P = 0.01) | SCS + CMM decreased from 92.2 to 66.0 MME/day (P = 0.001) | CMM only decreased from 89.1 to 83.3 MME/day (P = 0.11) |
| Stauss et al.41 | Retrospective review of real‐world outcomes | 10 kHz SCS (1603) | bMedian pain scores decreased from 8.0 (7.0–9.0) at baseline to 3.0 (2.0–4.0) at the last visit | 32.1% of all patients reduced medication use by last visit compared with baseline |
| Wilding et al.42 | Retrospective review of real‐world outcomes | 10 kHz SCS (36) | NR | 25 subjects reduced opioid use (69%); 10 maintained use at same level (28%); 1 increased opioid use (3%) |
| Al‐Kaisy et al.;43 Al‐Kaisy et al.44 | Preliminary, single‐center, prospective, proof‐of‐concept study | 10 kHz SCS (20 12 months; 17 24 months) | aBack pain: reduced from 7.9 to 1.0 at 36 months | aLeg pain: reduced from 3.3 to 0.9 at 36 months | 12 months: 64% reduction in mean daily dose from 112 to 40 MME | Abstinence from opioids increased from 10% to 88% at 36 months |
| Salmon32 | Retrospective review | 10 kHz cervical and/or thoracic SCS (35) | Reduction of mean 3.5 ± 1.6 NRS at 2.3 years post‐implant (P ≤ 0.001) | Number of patients using opioids reduced from 24 to 15; mean daily dose decreased 40.0% in 15 patients using opiates at follow‐up |
Pain scores in cm (range 0–10).
Numerically rated pain scores (range 0–10).
Low back pain
RCT in patients with back pain (SENZA‐RCT)
The SENZA‐RCT study (NCT01609972), a multicenter, randomized, controlled, pivotal trial, was undertaken to establish the efficacy of 10 kHz SCS in patients with chronic back and leg pain that was refractory to conservative treatment.30 The 171 study subjects who received permanent implants had not responded to previous conventional treatments for at least 3 months and reported average pain intensities of at least 5 cm on a 0–10 cm VAS. After randomization, subjects responding to a short‐term trial of respective SCS received permanent implants; 90 subjects received a 10 kHz SCS implant, while 81 received a traditional low‐frequency SCS implant. Treatment responders were defined as patients who reported ≥50% reduction in pain scores and experienced no treatment‐related neurological deficit. Patients who increased their opioid consumption during the study were considered as “nonresponders” regardless of their pain scores.
The SENZA‐RCT investigators reported that 88.3% of study subjects were taking conventional opioid analgesics at the time of initiating SCS therapy and 86.6% had previous back surgery, including 77.1% who were diagnosed with failed back surgery syndrome (FBSS). The authors found that 10 kHz SCS treatment significantly reduced average back and leg pain scores in all subjects through 12 months post‐implant (P < 0.001 for both). In concordance with the reduction in pain measures, the researchers reported that the average daily dose of conventional opioid analgesics, reported in morphine equivalents, fell by 18.8% (from 113 to 88 mg/day) among patients who received 10 kHz SCS compared with a 1% decrease (from 125 to 118 mg) in daily doses among those who received traditional SCS (P = 0.014). At the 12‐month assessment, the study reported that 38.2% (n = 26/68) of the subjects receiving 10 kHz SCS treatment either reduced or eliminated their opioid usage (Fig. 1A), compared with 26.4% of the subjects receiving traditional SCS treatment (P = 0.41).
Figure 1.
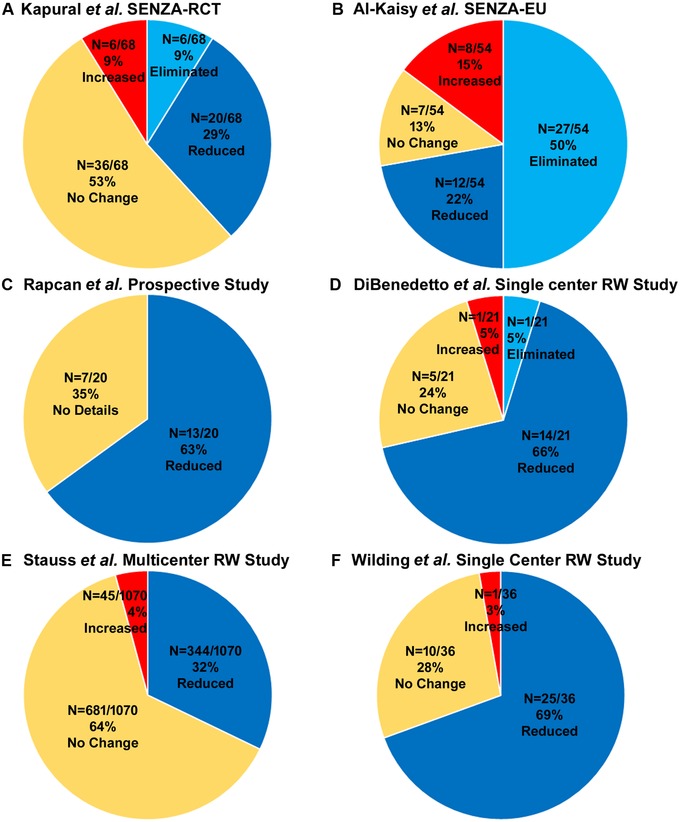
Studies reporting the opioid reduction in low back and leg pain patients. (A) SENZA‐RCT study by Kapural et al. (B) SENZA‐EU study by Al‐Kaisy et al. (C) Prospective study by Rapcan et al. (D) Retrospective case–controlled study by DeBeneditto et al. (E) Retrospective real‐world study by Stauss et al. (F) Retrospective real‐world study by Wilding et al.
Prospective studies in patients with chronic low back pain
Two prospective single‐arm, open‐label studies reported pain relief and conventional opioid reduction in patients with chronic low back pain with or without leg pain. The first prospective, open‐label clinical study of 10 kHz SCS for treating chronic back pain was undertaken at two centers in the United Kingdom and Belgium in subjects whose back pain was ≥5 cm on a 0–10 cm VAS and refractory to conventional treatment for ≥6 months.28 A total of 83 subjects were enrolled, including 67 (81%) with FBSS, of whom 72 (88%) received permanent implants after a successful trial of 10 kHz SCS. Mean subject pain scores were significantly reduced (P < 0.001) for both back and leg pain 6 months after the implantation. Opioid use was reported in 86% of study subjects at baseline, and 6 months following the procedure, 62.0% of these subjects had reduced opioid use, while 38.0% eliminated the use of these medications completely.
The 24‐month follow‐up results of this study were obtained and reported for 65 subjects,29 and these data showed continued reductions in mean pain scores as well as conventional opioid usage. Both mean back pain and leg pain scores were reduced after 24 months of stimulation compared with baseline scores (P < 0.001 for both). More importantly, the number of subjects who were not taking any opioids increased from 14% of subjects at baseline to 57% at the 24‐month time point. At the 24‐month follow‐up, 72.2% of subjects (n = 39/54) either reduced or eliminated their opioid usage (Fig. 1B). The mean daily dose of conventional opioids in the study population of 27.0 MME at 24 months was significantly reduced from the mean daily dose at baseline of 84.0 MME.
A second, prospective, nonrandomized study conducted at four sites in Slovakia also tested 10 kHz SCS in patients with FBSS.37 A total of 21 subjects were enrolled and all completed the 12‐month study. Mean VAS pain scores were significantly reduced compared with the baseline score immediately after implant and at 3, 6, and 12 months following the procedure. The authors further reported that at 12 months, 65.0% (n = 13/20) of the patient pool had halved their opioid intake (Fig. 1C).
A recently published post‐hoc analysis used combined data from the SENZA‐RCT study30 and the 12‐month results of the European prospective trial first reported by Van Buyten and colleagues.28 The combined analysis included only subjects treated with 10 kHz SCS, and baseline measures were available for 129 study subjects with low back pain and/or leg pain, while 12‐month outcomes were available for 122 subjects.38, 39 In the total study population, back pain intensity decreased from 7.8 cm on a 0–10 cm VAS at baseline to 2.5 cm at 12 months, and leg pain fell from 6.3 cm at baseline to 2.0 cm at 12 months (P < 0.001 for both measures). Mean daily opioid dose in all subjects fell from 104.2 to 61.4 MME after 12 months (P < 0.001). Among the subpopulation of subjects taking more than 90 MME of conventional opioid analgesics per day at baseline, reductions in pain scores and opioid intake were even more dramatic after a year. The average intensity of back pain in these patients fell by 5.5 cm and leg pain scores by 4.8 cm from baseline to 12 months (P < 0.001 for both measures). The opioid intake in these patients, likewise, declined from 196.8 MME per day at baseline to 106.5 MME at 12 months (P < 0.001). The authors concluded that the results of this post‐hoc analysis support the individual findings of both original trials and that 10 kHz SCS can provide a nonpharmacologic treatment option for chronic low back and leg pain by reducing both patient‐reported pain intensity and conventional opioid intake.
Real‐world results
The efficacy and safety of 10 kHz SCS were also recently evaluated in a retrospective case–control study of patients with chronic low back pain, with or without leg pain, who were treated at a single community‐based, interdisciplinary pain management center.40 In all, 32 individuals who had undergone implantation of a 10 kHz SCS system and received SCS therapy plus conventional medical management (SCS + CMM) for at least 12 months were compared with 64 matched controls who received CMM only during the treatment period. In patients receiving both 10 kHz SCS and CMM, back pain numeric rating scale (NRS) scores decreased 42.6% after 12 months compared with baseline measures (P < 0.001), and leg pain NRS scores decreased 50.9% (P = 0.01). The mean daily consumption of opioid analgesics by 21 study patients in the SCS + CMM group fell from 92.2 MME at baseline to 66.0 MME at 12 months (P = 0.001), and this effect was not observed in controls from the CMM group, indicating that the opioid‐sparing effects were specifically due to the use of 10 kHz SCS. At 12 months, 71.4% (n = 15/21) of the patients either reduced or eliminated their conventional opioid usage (Fig. 1D). Finally, there was no difference in the number of office visits made by members of either treatment group, but patients in the SCS + CMM group had a significantly greater decrease in interventional pain procedures (72.0%) than CMM‐only controls (34.6%; P = 0.03).
A larger observational study was published in 2019 using data from 1603 patients with chronic trunk or limb pain who underwent a stimulation trial and/or permanent implant for 10 kHz SCS treatment at one of eight sites in the United States, the United Kingdom, and Germany.41 The mean time from implantation to final study visit was 8.9 months (SD ± 6.7), and median pain scores decreased by 5.0 points on an 11‐point verbal numeric rating scale between baseline and the final visit. Responder rates, defined as pain reductions of 50% or more, were consistent in both 12‐month follow‐up data (77.6%; n = 326) and last visit data (74.1%; n = 1131). The study reported decreases in medication use in patients (Fig. 1E). At last follow‐up visit, 32.1% (n = 344/1070) of patients reported decrease in the use of their medications, a result that was similar to the proportion of subjects in the SENZA‐RCT trial that reduced or eliminated their consumption of pain medication (35.5%).30, 41
Most recently, the results of a retrospective review of 50 patients who underwent a trial of 10 kHz SCS in a National Health Service pain clinic were presented at the 14th World Congress of the International Neuromodulation Society in Sydney, Australia.42 The investigators reported that of the 36 subjects who completed the implantation procedure and took conventional opioids at baseline, 25 subjects (69%) reduced their opioid intake during follow‐up, while 10 subjects (28%) took the same daily dose, and one subject took more opioids after 10 kHz SCS treatment (Fig. 1F). Although pain was not reported, measures of work ability and quality of life increased in these patients after 1 year of 10 kHz SCS treatment.
Intractable low back pain patients with no history of back surgery
In order to test whether 10 kHz SCS can be efficacious in chronic refractory low back pain patients who are not candidates for back surgery, Al‐Kaisy and colleagues conducted a single‐center, prospective, proof‐of‐concept study.43 The study enrolled 21 subjects, 20 of whom underwent permanent implant and were followed up for 12 months. Mean back pain and leg pain scores were significantly decreased at 1, 3, and 12 months after the implantation. By the end of the 12‐month study period, the average daily opioid intake among all subjects decreased 64.3%, from 112 to 40 MME. More recent results from this study show the decreases in back and leg pain from 10 kHz SCS remained significant after 36 months of treatment.44 Additionally, the proportion of patients taking opioid analgesics fell from 18/20 (90%) at baseline to 2/17 (11.7%) at 36 months (Fig. 2A).
Figure 2.
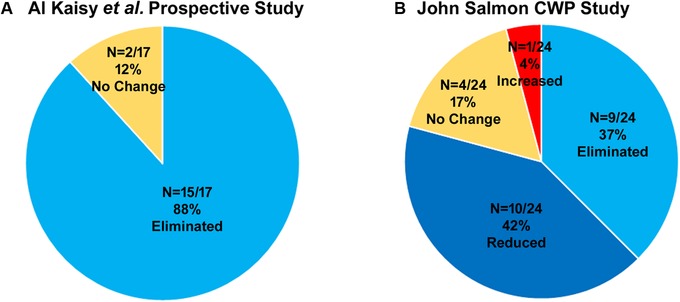
Studies reporting the opioid reduction in other neuropathic pain. (A) Prospective study in NSRBP subjects. (B) Retrospective study in chronic widespread pain patients.
Chronic widespread pain
Another retrospective review has been published using data from patients with widespread refractory neuropathic/nociplastic pain affecting the trunk and limbs.32 A total of 38 patients underwent a successful trial stimulation and received a permanent implant. Follow‐up data were available for 35 patients at a mean interval of 2.3 ± 1.7 years after implantation. Patients used a 10‐point NRS to report pain intensity, and the data showed a mean pain reduction of 3.5 ± 1.6 points from baseline (P ≤ 0.001) with 33 of 35 (94.3%) subjects reporting pain reductions of more than 40%. Twenty‐four of 35 (68.6%) patients reported daily use of one or more strong opioids, including oxycodone, morphine, methadone, buprenorphine, and tapentadol, at baseline at a mean daily dose of 118.4 ± 107.1 MME. At follow‐up, 15 (42.9%) patients were taking strong opioids, a decrease of 37.5%, and their mean daily dose was 40% lower than baseline levels, falling from 165.4 ± 109.0 to 99.3 ± 49.6 MME. A single patient increased opiate intake after 10 kHz SCS treatment, from 70 MME at baseline to 100 MME at follow‐up, and four patients did not alter their opiate use over the study period (Fig. 2B). Among 11 patients taking the highest doses of opiates, the mean daily dose at follow‐up fell to 111.8 ± 52.7 MME, a 46.9% reduction from the baseline level of 210.5 ± 92.8 MME.
Adverse events
The safety profile of implantation and treatment with 10 kHz SCS was similar to that expected for traditional SCS. None of the studies reported any neurological deficits in study subjects receiving 10 kHz SCS treatment. Stauss and colleagues examined explant rates in their review of real‐world data, and reported that of 1290 patients with safety data available, 48 had their 10 kHz SCS device explanted by 12 months (3.7%).41 The most common reasons for explant were infection in 22 patients (1.7%) and loss of efficacy in 15 patients (1.2%).
Explants
Two studies have reported explant rates of 10 kHz SCS. The first study was a European multicenter, retrospective analysis that used data from 946 implanted subjects to derive annualized rates of explants defined as those that resulted in the termination of SCS therapy.45 The authors reported that the explant rate for inadequate pain relief was 5.0% per year of follow‐up for 10 kHz SCS, 5.5% for conventional rechargeable SCS, and 2.8% for nonrechargeable SCS. It is important to understand that 37.4% of the nonrechargeable devices were explanted and replaced due to battery depletion and 8.2% were replaced with a rechargeable IPG within the mean observation time of 2.2 years. These were not considered as explants in the annualized rate analysis. Regardless, with covariate adjustment, 10 kHz SCS was not different from nonrechargeable SCS for risk of explant due to inadequate pain relief. While the study reports 5‐year data, any conclusions about long‐term rates are limited by extensive attrition in all groups: the initial sample was 946; however, only 70 are reported at 5 years.
The second international, multicenter, retrospective study by Stauss et al. reported a 1660‐consecutive patient (1603 with evaluable data), “real‐world” analysis of satisfaction and explant rates in 10 kHz SCS commercial cases for any etiology. The study included data from centers in the United States, the United Kingdom, and Germany, and reported that the overall explant rate of 10 kHz SCS for all causes was 3.7% and for loss of efficacy it was 1.2%, further indicating the lower explant rates of 10 kHz SCS devices.41
Cost‐effectiveness
The financial costs of treating patients with FBSS using 10 kHz SCS over 15 years have been compared with treatment with CMM or conventional SCS using a model of the British healthcare system.46 This analysis found incremental cost benefits to 10 kHz SCS compared with CMM and improvements in both financial costs and the number of quality‐adjusted life years compared with conventional SCS. Explant of the SCS devices can be a burden to the patient and the payers, impacting the cost‐effectiveness of the treatment. However, the study took the cost burden related to explants into account and included the variables related to explants in their model for cost‐effectiveness analysis. The study assumed complication, withdrawal, and replacement rates for 10 kHz SCS to be 9.5% for 6 months (4.8%, 0.8%, and 3.9%, respectively).
The explant rates reported by Stauss et al. for all causes (3.7%) were in similar range of values that were used in the model, further supporting the conclusions of the cost‐effectiveness study.41 Furthermore, in their analysis of the SENZA‐RCT results, the authors concluded that the significant benefits and low risks of 10 kHz SCS relative to alternative treatments suggested that economic benefits might be associated with this treatment modality.30 Likewise, other investigators suggested that improvements in quality of life measures43 or reductions in conventional opioid use40, 41 should result in lower long‐term healthcare costs. Finally, the finding by DiBenedetto and colleagues that patients treated with 10 kHz SCS required fewer interventional procedures than matched controls provides further support for the possible financial benefits of the treatment.40
Summary
Overall, 10 kHz SCS has shown potential in treating types of chronic pain, including low back pain and neuropathic pain, that are often unresponsive to conventional medical treatments. Prospective, nonrandomized trials of 10 kHz SCS in patients with chronic back pain reported pain relief ranging from 54% to 87% after at least 1 year of treatment, and leg pain scores similarly improved by 57% to 72% in these patients.28, 29, 37, 43, 44 The mean daily dose of conventional opioids required by patients in these studies was halved or more after 10 kHz SCS treatment, and the number of opioid‐abstinent subjects increased in all the studies. These results were strengthened by the SENZA‐RCT trial in subjects with low back pain, which showed that 10 kHz SCS reduced mean back pain by 67% and leg pain by 65% for at least 24 months and was associated with a 19% reduction in daily opioid consumption after 12 months, whereas traditional SCS was associated with only 1% reduction in daily opioid consumption (P = 0.014, difference between groups).30 Retrospective reviews of real‐world data in patients with chronic back pain also found pain reductions in the range of 45–63%, as well as decreases in opioid requirements and increases in opioid abstinence.40, 41, 42
Literature reviews often include a meta‐analysis, and this is a useful way to combine outcomes from multiple studies. However, meta‐analysis is only accurate when heterogeneity is low.47 Specifically, using studies that are similar in design and patient population is recommended to avoid incorrect estimation of effect size. This review included prospective and retrospective studies whose patient populations were also not homogenous. Meta‐analysis was, therefore, not performed in order to avoid further reducing the number of included studies, which might confound the results or overestimate effect size.
It is important to note that, excepting studies by DiBenedetto et al. and Al‐Kaisy et al., the reductions in opioid use reported here were spontaneous, observational outcomes of the respective studies.29, 40 It is therefore readily conceivable that pain treatment with 10 kHz SCS in conjunction with a targeted strategy to reduce conventional opioid use could achieve greater decreases and/or higher rates of abstinence than those reported here. In the development of an effective strategy to reduce opioids, it is critical to understand the association between absolute opioid dose and patient risk, and the importance of willing participation of the patient in achieving opioid reduction. Mainly, the risk of overdose rises with the average daily dose, and patients receiving 100 MME per day have a ninefold greater risk than those taking ≥20 MME per day.48 Additionally, the curve plateaus somewhat at high doses,49 and a reduction from 200 to 100 MME per day likely, reduce patient risk less than reducing from 100 MME to less than 50 MME. In patients treated with 10 kHz SCS, opioid reduction strategy could aim to wean to a preimplant dose of less than 30 MME and continue to wean further following permanent implantation. Higher use of conventional opioids before and during SCS has been shown to be associated with higher rates of explant,33 and patients who eliminate opioid use completely have been reported to have superior clinical outcomes in pain scores and disability compared with patients who remained on opioids through the study.
The pain‐reducing and opioid‐sparing effects observed in these studies are all the more notable due to the fact that the patients treated had intractable pain conditions that were unresponsive to conventional medical treatments, including, in some cases, previous treatment with conventional low‐frequency SCS. By reducing the burden of pain and opioid use in patients with chronic, intractable pain, 10 kHz SCS has the potential to reduce the health care costs associated with these patients’ care, as noted in several of these studies. The ability of 10 kHz SCS to reduce opioid requirements while also effectively reducing pain gives this treatment the potential to address two major unmet needs in these patients, and the added fact that no neurological AEs have been observed encourages the use of this treatment as an alternative to conventional medical treatments for chronic, noncancer pain.
Author contributions
A.A.K., J.P.V.B, K.A., D.C., B.G., J.S., A.R., and L.K. were involved in conceptualizing the review, preparing the outline, and reviewing the manuscript draft. A.R. worked with the medical writers in drafting the manuscript and preparing the illustrations. All authors have reviewed and approved the final manuscript.
Competing interests
A.A.K., J.P.V.B., K.A., and L.K. are consultants to Nevro Corp., Redwood City, CA. D.C., B.G., J.S., and A.R. are employees of Nevro Corp., Redwood City, CA.
Acknowledgments
The authors thank Erik J. MacLaren, PhD, Galen Medical Writing, LLC for drafting the manuscript, and Madhuri Bhandaru, PhD, for assistance in the preparation of illustrations.
References
- 1. Smith, B.H. , Elliott A.M., Chambers W.A., et al 2001. The impact of chronic pain in the community. Fam. Pract. 18: 292–299. [DOI] [PubMed] [Google Scholar]
- 2. Dahlhamer, J. , Lucas J., Zelaya C., et al 2018. Prevalence of chronic pain and high‐impact chronic pain among adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 67: 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gureje, O. , Von Korff M., Simon G.E. & Gater R.. 1998. Persistent pain and well‐being: a World Health Organization study in primary care. JAMA 280: 147–151. [DOI] [PubMed] [Google Scholar]
- 4. Torrance, N. , Smith B.H., Watson M.C. & Bennett M.I.. 2007. Medication and treatment use in primary care patients with chronic pain of predominantly neuropathic origin. Fam. Pract. 24: 481–485. [DOI] [PubMed] [Google Scholar]
- 5. Kawai, K. , Kawai A.T., Wollan P. & Yawn B.P.. 2017. Adverse impacts of chronic pain on health‐related quality of life, work productivity, depression and anxiety in a community‐based study. Fam. Pract. 34: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hylands‐White, N. , Duarte R.V. & Raphael J.H.. 2017. An overview of treatment approaches for chronic pain management. Rheumatol. Int. 37: 29–42. [DOI] [PubMed] [Google Scholar]
- 7. Rummans, T.A. , Burton M.C. & Dawson N.L.. 2018. How good intentions contributed to bad outcomes: the opioid crisis. Mayo Clin. Proc. 93: 344–350. [DOI] [PubMed] [Google Scholar]
- 8. Dowell, D. , Haegerich T.M. & Chou R.. 2016. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 315: 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalso, E. , Edwards J.E., Moore R.A. & McQuay H.J.. 2004. Opioids in chronic non‐cancer pain: systematic review of efficacy and safety. Pain 112: 372–380. [DOI] [PubMed] [Google Scholar]
- 10. Els, C. , Jackson T.D., Hagtvedt R., et al 2017. High‐dose opioids for chronic non‐cancer pain: an overview of Cochrane Reviews. Cochrane Database Syst. Rev. 10: CD012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Busse, J.W. , Wang L., Kamaleldin M., et al 2018. Opioids for chronic noncancer pain: a systematic review and meta‐analysis. JAMA 320: 2448–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harbaugh, C.M. , Lee J.S., Chua K.P., et al 2019. Association between long‐term opioid use in family members and persistent opioid use after surgery among adolescents and young adults. JAMA Surg. 54: e185838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manchikanti, L. , Kaye A.M., Knezevic N.N., et al 2017. Responsible, safe, and effective prescription of opioids for chronic non‐cancer pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 20: S3–S92. [PubMed] [Google Scholar]
- 14. Blanco, C. & Volkow N.D.. 2019. Management of opioid use disorder in the USA: present status and future directions. Lancet 393: 1760–1772. [DOI] [PubMed] [Google Scholar]
- 15. Klaess, C.C. , Urton M., Whitehead P., et al 2019. Pain management pillars for the clinical nurse specialist: summary of National Association of Clinical Nurse Specialists Opioid Pain Management Task Force. Clin. Nurse Spec. 33: 136–145. [DOI] [PubMed] [Google Scholar]
- 16. Rubin, R. 2019. Limits on opioid prescribing leave patients with chronic pain vulnerable. JAMA 321: 2059–2062. [DOI] [PubMed] [Google Scholar]
- 17. Hamnvik, O.R. , Alford D.P., Ryan C.T., et al 2019. NEJM knowledge + pain management and opioids—a new adaptive learning module. N. Engl. J. Med. 380: 1576–1577. [DOI] [PubMed] [Google Scholar]
- 18. Okie, S. 2010. A flood of opioids, a rising tide of deaths. N. Engl. J. Med. 363: 1981–1985. [DOI] [PubMed] [Google Scholar]
- 19. Von Korff, M. , Walker R.L., Saunders K., et al 2017. Prevalence of prescription opioid use disorder among chronic opioid therapy patients after health plan opioid dose and risk reduction initiatives. Int. J. Drug Policy 46: 90–98. [DOI] [PubMed] [Google Scholar]
- 20. Shealy, C.N. , Mortimer J.T. & Reswick J.B.. 1967. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth. Analg. 46: 489–491. [PubMed] [Google Scholar]
- 21. Wolter, T. 2014. Spinal cord stimulation for neuropathic pain: current perspectives. J. Pain Res. 7: 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eldabe, S. , Buchser E. & Duarte R.V.. 2016. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med. 17: 325–336. [DOI] [PubMed] [Google Scholar]
- 23. De Carolis, G. , Paroli M., Tollapi L., et al 2017. Paresthesia‐independence: an assessment of technical factors related to 10 kHz paresthesia‐free spinal cord stimulation. Pain Physician 20: 331–341. [PubMed] [Google Scholar]
- 24. Deer, T.R. , Mekhail N., Provenzano D., et al 2014. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation 17: 515–550; discussion 50. [DOI] [PubMed] [Google Scholar]
- 25. Russo, M. & Van Buyten J.P.. 2015. 10‐kHz high‐frequency SCS therapy: a clinical summary. Pain Med. 16: 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nevro Corp . 2015.. Nevro receives FDA approval for Senza® spinal cord stimulation system delivering HF10™ therapy.
- 27. Tiede, J. , Brown L., Gekht G., et al 2013. Novel spinal cord stimulation parameters in patients with predominant back pain. Neuromodulation 16: 370–375. [DOI] [PubMed] [Google Scholar]
- 28. Van Buyten, J.P. , Al‐Kaisy A., Smet I., et al 2013. High‐frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation 16: 59–65; discussion 65–66. [DOI] [PubMed] [Google Scholar]
- 29. Al‐Kaisy, A. , Van Buyten J.P., Smet I., et al 2014. Sustained effectiveness of 10 kHz high‐frequency spinal cord stimulation for patients with chronic, low back pain: 24‐month results of a prospective multicenter study. Pain Med. 15: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kapural, L. , Yu C., Doust M.W., et al 2015. Novel 10‐kHz high‐frequency therapy (HF10 therapy) is superior to traditional low‐frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA‐RCT randomized controlled trial. Anesthesiology 123: 851–860. [DOI] [PubMed] [Google Scholar]
- 31. Kapural, L. , Yu C., Doust M.W., et al 2016. Comparison of 10‐kHz high‐frequency and traditional low‐frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24‐month results from a multicenter, randomized, controlled pivotal trial . Neurosurgery 79: 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salmon, J. 2019. High‐frequency spinal cord stimulation at 10 kHz for widespread pain: a retrospective survey of outcomes from combined cervical and thoracic electrode placements. Postgrad. Med. 131: 230–238. [DOI] [PubMed] [Google Scholar]
- 33. Sharan, A.D. , Riley J., Falowski S., et al 2018. Association of opioid usage with spinal cord stimulation outcomes. Pain Med. 19: 699–707. [DOI] [PubMed] [Google Scholar]
- 34. Simopoulos, T. , Sharma S., Wootton R.J., et al 2019. Discontinuation of chronic opiate therapy after successful spinal cord stimulation is highly dependent upon the daily opioid dose. Pain Pract. 10.1111/papr.12807. [DOI] [PubMed] [Google Scholar]
- 35. Gee, L. , Smith H.C., Ghulam‐Jelani Z., et al 2019. Spinal cord stimulation for the treatment of chronic pain reduces opioid use and results in superior clinical outcomes when used without opioids. Neurosurgery 84: 217–226. [DOI] [PubMed] [Google Scholar]
- 36. Pollard, E.M. , Lamer T.J., Moeschler S.M., et al 2019. The effect of spinal cord stimulation on pain medication reduction in intractable spine and limb pain: a systematic review of randomized controlled trials and meta‐analysis. J. Pain Res. 12: 1311–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rapcan, R. , Mlaka J., Venglarcik M., et al 2015. High‐frequency—spinal cord stimulation. Bratisl. Lek. Listy 116: 354–356. [DOI] [PubMed] [Google Scholar]
- 38. Amirdelfan, K. , Al‐Kaisy A., Van Buyten J.‐P., et al 2015. 10 kHz‐SCS therapy for chronic pain, effects on opioid usage: post hoc analysis of data from two prospective studies. In American Society of Interventional Pain Physicians 21st Annual Meeting, May 3–5, Las Vegas, NV, 2019. [DOI] [PMC free article] [PubMed]
- 39. Al‐Kaisy, A. , Van Buyten J.P., Carganillo R., et al 2019. 10 kHz SCS therapy for chronic pain, effects on opioid usage: post hoc analysis of data from two prospective studies. Sci. Rep. 9: 11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DiBenedetto, D.J. , Wawrzyniak K.M., Schatman M.E., et al 2018. 10 kHz spinal cord stimulation: a retrospective analysis of real‐world data from a community‐based, interdisciplinary pain facility. J. Pain Res. 11: 2929–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stauss, T. , El Majdoub F., Sayed D., et al 2019. A multicenter real‐world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann. Clin. Transl. Neurol. 6: 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilding, R. , Barnes S., Chincholkar M. & Lalkhen A.. 2019. Spinal cord stimulation at 10 kHz is effective in reducing opioid consumption in patients with chronic pain. In International Neuromodulation Society 14th World Congress, May 25–30, Sydney, Australia.
- 43. Al‐Kaisy, A. , Palmisani S., Smith T.E., et al 2017. 10 kHz high‐frequency spinal cord stimulation for chronic axial low back pain in patients with no history of spinal surgery: a preliminary, prospective, open label and proof‐of‐concept study. Neuromodulation 20: 63–70. [DOI] [PubMed] [Google Scholar]
- 44. Al‐Kaisy, A. , Palmisani S., Smith T.E., et al 2018. Long‐term improvements in chronic axial low back pain patients without previous spinal surgery: a cohort analysis of 10‐kHz high‐frequency spinal cord stimulation over 36 months. Pain Med. 19: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 45. Van Buyten, J.P. , Wille F., Smet I., et al 2017. Therapy‐related explants after spinal cord stimulation: results of an International Retrospective Chart Review Study. Neuromodulation 20: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Annemans, L. , Van Buyten J.P., Smith T. & Al‐Kaisy A.. 2014. Cost effectiveness of a novel 10 kHz high‐frequency spinal cord stimulation system in patients with failed back surgery syndrome (FBSS). J. Long Term Eff. Med. Implants 24: 173–183. [DOI] [PubMed] [Google Scholar]
- 47. Bown, M.J. & Sutton A.J.. 2010. Quality control in systematic reviews and meta‐analyses. Eur. J. Vasc. Endovasc. Surg. 40: 669–677. [DOI] [PubMed] [Google Scholar]
- 48. Dunn, K.M. , Saunders K.W., Rutter C.M., et al 2010. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann. Intern. Med. 152: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dasgupta, N. , Funk M.J., Proescholdbell S., et al 2016. Cohort study of the impact of high‐dose opioid analgesics on overdose mortality. Pain Med. 17: 85–98. [DOI] [PubMed] [Google Scholar]


