Abstract
Objective
To systematically review the literature on the prognostic value of lymphovascular invasion (LVI) and embryonal carcinoma (EC) for occult metastatic disease in clinical stage I nonseminomatous germ cell tumour (CS I NSGCT).
Materials and methods
The PubMed, Embase (OVID) and SCOPUS databases were searched up to March 2019. Studies reporting on the association between LVI and/or EC and occult metastatic disease were considered for inclusion. The quality and risk of bias were evaluated by the Quality in Prognosis Studies tool.
Results
We screened 5287 abstracts and 207 full‐text articles. We included 35 studies in the narrative synthesis and 24 studies in a meta‐analysis. LVI showed the strongest effect. Pooled rates of occult metastasis were 47.5% and 16.9% for LVI‐positive and LVI‐negative patients, respectively (odds ratio [OR] 4.33, 95% confidence interval [CI] 3.55–5.30; P < 0.001). Pooled rates of occult metastasis were 33.2% for EC presence and 16.2% for EC absence (OR 2.49, 95% CI 1.64–3.77; P < 0.001). Pooled rates of occult metastasis were 40.0% for EC >50% and 20.0% for EC <50% (OR 2.62, 95% CI 1.93–3.56; P < 0.001).
Conclusions
LVI is the strongest risk factor for relapse. The prognostic value of EC is high, but there is no common agreement on how to define this risk factor. Both EC presence and EC >50% have similar ORs for occult metastasis. This shows that the assessment of EC presence is sufficient for the classification of EC.
Keywords: testicular germ cell tumour, nonseminomatous germ cell tumour, prognostic factors, pathology, systematic review, meta‐analysis
Abbreviations
- AS
active surveillance
- BEP
bleomycin, etoposide, and cisplatin
- CS I
clinical stage I
- EC
embryonal carcinoma
- HR
hazard ratio
- LVI
lymphovascular invasion
- MOOSE
Meta‐analysis Of Observational Studies in Epidemiology
- (NS)GCT
(nonseminomatous) germ cell tumour
- OR
odds ratio
- (N)(P)PV
(negative) (positive) predictive value
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis
- PROSPERO
International Prospective Register of Systematic Reviews
- QUIPS
Quality in Prognosis Studies
- RPLND
retroperitoneal lymph node dissection
- RR
relative risk
- SWENOTECA
Swedish and Norwegian Testicular Cancer Group
- VI
vascular invasion
Introduction
Approximately 30% of patients with nonseminomatous germ cell tumour (NSGCT) presenting with clinical stage I (CS I) have occult metastatic disease in their retroperitoneal lymph nodes 1. These patients will relapse if treated with active surveillance (AS).
Several management strategies for CS I NSGCT exist. Primary retroperitoneal lymph node dissection (RPLND) is still a standard approach in the USA 2. In Europe, its role is largely diminished, as it is associated with high morbidity and European follow‐up is generally well organised 3. Various guideline statements acknowledge non‐risk‐adapted AS as a preferred management strategy 3, 4. This approach limits overtreatment, and most relapsed patients can still be cured with salvage chemotherapy. However, salvage treatment consists of multiple cycles of bleomycin, etoposide, and cisplatin (BEP) chemotherapy and is associated with an increased risk of secondary malignancy 5 and cardiovascular disease 6.
The high survival rate and the long life‐expectancy of patients have shifted focus to minimisation of treatment‐related morbidity. This includes a reduction of treatment‐associated long‐term toxicities caused by salvage therapy. Early identification of patients who have a high risk of relapse enables adjuvant treatment at an early stage. This prevents relapse, thereby avoiding the necessity of salvage treatment and reducing toxicity 7, 8.
In order to select these high‐risk patients, several risk‐adapted strategies have been developed 7, 9. Patient selection is largely based on two histopathological features in the primary tumour: presence of lymphovascular invasion (LVI) and presence or predominance of the tumour subtype embryonal carcinoma (EC) 3, 7, 8, 10, 11.
High‐risk patients can be offered treatment with one course of BEP 3. This reduces the relapse risk by 90–95%, regardless of risk classification 7. In a prospective study by the Swedish and Norwegian Testicular Cancer Group (SWENOTECA), the relapse risk after one course of BEP was 3.2% and 1.6% for patients with and without LVI, respectively 7.
As the presence of LVI and EC are important factors that aid clinical decision‐making on adjuvant treatment in patients with CS I NSGCT, their prognostic value needs to be clarified. Several studies have investigated the association between these predictors and occult metastatic disease. However, a systematic review with meta‐analysis is necessary to quantify the strength of these predictors more precisely. The aim of the present study was to systematically review the literature to establish the prognostic value of LVI and EC in CS I NSGCT.
Materials and methods
Search strategy
This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement and the recommendations of the Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group 12, 13. The review protocol has been published in the International Prospective Register of Systematic Reviews (PROSPERO) database (registration number CRD42018107698).
A systematic PubMed, Embase (OVID) and SCOPUS literature search was conducted up to March 2019. An information scientist (E.W.) was involved in the design of the search strategy. The full search strategy is available in Appendix S1. Relevant references from selected studies were also considered. Two reviewers (J.B. and I.P.) independently screened all abstracts and full‐text articles. Disagreement was resolved by discussion.
Study eligibility
Studies reporting on the individual association of LVI and/or EC with occult metastatic disease in patients with CS I NSGCT treated with AS or primary RPLND were eligible for inclusion. Studies reporting on patients treated with adjuvant therapy or with a risk‐adapted protocol were not included. Studies reporting on patients with pure seminoma, paediatric GCT, or bilateral testicular tumours were also not included. Reviews, case reports, conference papers, editorials, commentaries, and studies not in the English language were excluded. If multiple studies reported on the same patient cohort and reported the same outcome measures, only the most recent publication was included. If multiple studies possibly included the same patients (but not the same cohort), we included both studies in the narrative synthesis but included only the most recent study in the meta‐analysis.
Studies making a distinction between vascular and lymphatic invasion were also included in the narrative synthesis but not in the meta‐analysis. If it was not explicitly stated whether LVI or strictly vascular invasion (VI) was meant, the corresponding author was contacted.
Outcome measures of interest were relapse during AS or positive nodes on primary RPLND. LVI and EC were evaluated as dichotomous variables (presence vs absence). The percentage of EC was evaluated either as a continuous variable or as a categorical variable using different cut‐off points. Studies reporting raw data were included in the meta‐analysis. If relapse rates were reported, these were converted to number of patients. AS studies with a median follow‐up of <24 months were included in the narrative synthesis but not in the meta‐analysis.
Risk of bias assessment
Two reviewers (J.B. and I.P.) independently assessed the quality and risk of bias in the included studies using the Quality in Prognosis Studies (QUIPS) tool for six domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting 14. Disagreement was resolved by discussion. The highest score on one of the domains was taken as the overall grade of bias. In addition, the sources of funding for the included studies were evaluated. Publication bias was assessed using a funnel plot.
Data extraction and statistical analysis
Data from the articles were extracted independently by two reviewers (J.B. and I.P.). Baseline characteristics were summarised using descriptive statistics. Cochrane’s Review Manager (version 5.3) was used for the meta‐analysis and construction of the Forest plots in collaboration with a biostatistician (K.J.). Statistical heterogeneity was evaluated by calculating I 2.
Results
Our search identified 9314 manuscripts (March 2019). After removal of duplicates, 5287 studies were screened. Of these, 207 studies were selected for full‐text evaluation. A total of 35 studies, reporting on 7113 patients were included in the systematic review 1, 10, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 (Fig. 1, Table 1, Appendix S2); 26 studies reported on patients treated with AS 1, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 46 and nine reported on patients treated with primary RPLND 10, 39, 40, 41, 42, 43, 44, 45, 47. Of these studies, 14 included >150 patients 1, 10, 15, 17, 19, 20, 22, 25, 28, 32, 37, 39, 42, 43.
Figure 1.
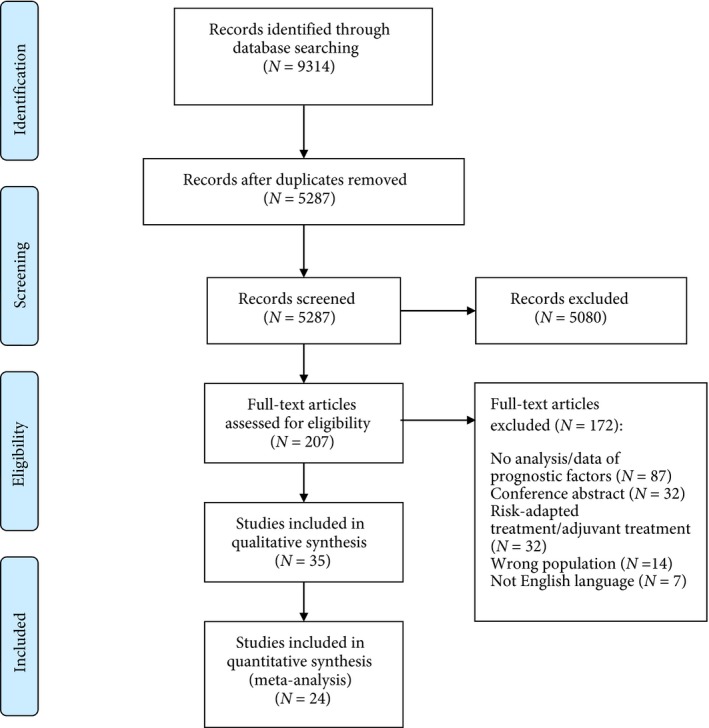
PRISMA diagram.
Table 1.
Study criteria.
| Study | Inclusion period | Country | Inclusion criteria for AS | Patients, n | Age, years, median (range) | Follow‐up, months, median (range) | Overall metastatic rate, % | Central pathology review | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| AS studies | |||||||||
| Gilbert et al. 2016 [15] | NR | UK | NR | 177 | NR | NR | NR | Yes | High |
| Li et al. 2015 [16] | 1999–2013 | China | NR | 78 | 29.5 (14–56) | 74.4 (12–180) | 23.1 | Yes | High |
| Kollmannsberger et al. 2015 [1] | 1983–2012 | Canada, Norway, Sweden, UK, USA | NR | 1139 | 30 (14–85) | 62 (1–277) | 19.4 | No | High |
| Daugaard et al. 2014 [17] | 1984–2007 | Denmark | Standard policy | 1226 | 30 (15–79) | 180 (1–346) | 31.2 | No | Moderate |
| Keskin et al. 2011 [18] | 2002–2009 | Turkey | Patient preference | 70 | 27.8 (16–67) | 18.5 (6–71) | 17.1 | Yes | High |
| Sturgeon et al. 2011 [19] | 1981–2005 | Canada | Preferred management option, no pure choriocarcinoma | 371 | Mean 30.5 (13.2–76.6) | 75.6 (0.96–310.8) | 28.0 | Yes | Moderate |
| Kollmannsberger et al. 2010 [20] | 1998–2007 | Canada | Preferred management option | 223 | 29 (15–63) | 52 (3–136) | 26.5 | Yes | Moderate |
| Atsü et al. 2003 [21] | 1978–2000 | Turkey | Normalization of markers | 132 | 28 (16–52) | 38 (6–265) | 24.2 | Yes | High |
| Daugaard et al. 2003 [22] | 1984–2002 | Denmark | Standard policy | 301 | 34 (15–72) | 60 (1–226) | 28.6 | No | High |
| Alexandre et al. 2001 [23] | 1984–1996 | France | Patient preference, not based on histopathological characteristics | 88 | 30.5 (15.9–55.7) | 51.6 (12–144) | 27.3 | Yes | Moderate |
| Roeleveld et al. 2001 [24] | 1982–1994 | The Netherlands | No pure choriocarcinoma, no history of any previous tumour | 90 | Mean 30 (16–60) | 97.2 | 25.6 | Yes | Moderate |
| Colls et al. 1999 [25] | 1980–1997 | New Zealand | Histological NSGCT, seminoma with β‐HCG ≥300 IU/L, or seminoma with elevated AFP | 248 | 29 (16–77) | 53 (1–185) | 28.2 | No | High |
| Sogani et al. 1998 [26] | 1979–1987 | USA | No T2–T4, no pure choriocarcinoma, no pure seminoma, no history of orchidopexy, no unreliability for close follow‐up | 105 | 26 (15–46) | 135.6 (28.8–201.6) | 25.7 | Yes | High |
| Maher and Lee 1998 [27] | 1980–1993 | UK | Standard policy | 42 | 28 (18–53) | 79.4 (30.6–183.2) | 31.0 | Yes | High |
| Gels et al. 1995 [28] | 1982–1992 | The Netherlands | Standard policy | 154 | 29 (15–66) | 84 (24–144) | 27.3 | No | Moderate |
| Nicolai and Pizzocaro 1995 [29] | 1981–1984 | Italy | Offered to all CS I patients, no tumour at cut end of spermatic cord | 85 | NR | 132 (114–156) | 29.4 | Yes | High |
| Ondrus and Hornak 1994 [30] | 1984–NR | Slovakia | No seminoma or choriocarcinoma component | 80 | 27 (13–58) | Mean: 83.1 (61–110) | 36.3 | NR | High |
| Tekgül et al. 1995 [31] | 1985–1994 | Turkey | No tumour at cut end of spermatic cord, eligible for close and proper AS | 58 | 31 (17–43) | 39 (14–79) | 29.3 | Yes | High |
| Read et al. 1992 [32] | 1984–1987 | UK and Norway | No tumour at cut end of spermatic cord | 373 | NR | 60 | 26.8 | Yes | Moderate |
| Sturgeon et al. 1992 [33] | 1981–NR | Canada | Preferred management option, no pure choriocarcinoma | 105 | 28 (17–76) | 60 (12–121) | 35.2 | Yes | Moderate |
| Rørth et al. 1991 [34] | 1980–1984 | Denmark | Randomisation | 83 | 30 (17–64) | 64 (33–103) | 27.7 | Yes | High |
| Wishnow et al. 1989 [46] | 1981–1987 | USA | NR | 82 | NR | NR | 29.3 | Yes | High |
| Dunphy et al. 1988 [35] | 1981–1986 | USA | NR | 93 | Mean 28 (16–54) | 34 (12–61) | 30.1 | Yes | Moderate |
| Thompson et al. 1988 [36] | 1979–NR | New Zealand | No tumour at cut end of spermatic cord | 36 | 27 (18–45) | 36 (14–92) | 33.3 | Yes | High |
| Freedman et al. 1987 [37] | 1979–1983 | UK | NR | 259 | NR | 30 (10–63) | 27.0 | Yes | Moderate |
| Hoskin et al 1986 [38] | 1979–1985 | UK | Histological NSGCT or seminoma with elevated AFP, no tumour at cut end of spermatic cord | 126 | NR | 42 | 28.6 | Yes | Moderate |
| Primary RPLND studies | |||||||||
| Nicolai et al. 2011* [39] | 2002–2007 | Italy | NR | 183 | NR | NR | 18.6 | Yes | Moderate |
| Albers et al. 2003† [10] | 1996–2002 | Germany | CS I, randomisation | 165 | Mean 31.3 (SD 8.3) | Mean 34.5 (12–64) | 37.6 | Yes | Moderate |
| Spermon et al. (2002)* [40] | 1986–1992 | The Netherlands | NR | 50 | NR | NR | 30.0 | Yes | High |
| Sweeney et al. 2000† [43] | 1990–1995 | USA | NR | 292 | NR | 46 (24–89) | 30.5 | Yes | Moderate |
| Albers et al. 1997† [47] | 1983–1994 | Germany | NR | 78 | NR | Mean 58.2 (8–149) | 35.9 | Yes | High |
| Albers et al. (1995)* [45] | 1992–1993 | USA | CS I | 90 | NR | Mean 15.9 (5–27) | 27.8 | Yes | High |
| Moul et al. 1994* [44] | 1980–1993 | USA | NR | 92 | NR | NR (1–10) | 41.3 | Yes | High |
| Klepp et al. 1990† [42] | 1981–1986 | Sweden, Norway | CS I, no previous malignancy | 279 | NR | 50 (30–90) | 37.6 | Yes | Moderate |
| Fung et al. 1988* [41] | 1979–1987 | USA | NR | 60 | 25 (15–56) | 18 (NR) | 33.3§ | Yes | Moderate |
| Total | 1978–2013 | 7113‡ | |||||||
AFP, α‐fetoprotein; β‐HCG, β‐human chorionic gonadotropin; NR, not reported. *Study endpoint is pathological Stage II. †Study endpoint is pathological Stage II or relapse after pathological Stage I. ‡Includes patients reported in multiple studies. §48.3% including patients with relapse after pathological Stage I.
The median age of the patients at time of diagnosis ranged from 25 to 31 years. In the primary RPLND studies, the percentage of patients with pathological Stage II was between 18.6% and 41.3%. In the AS studies, overall relapse rates varied between 17.1% and 36.3%. Reported median follow‐up durations ranged from 18 to 180 months.
A total of 24 studies could be included in a meta‐analysis.1, 10, 15, 16, 17, 21, 22, 24, 25, 26, 27, 28, 29, 30, 31, 34, 39, 40, 41, 42, 43, 45, 46, 47. In these studies, the rate of occult metastatic disease ranged from 18.6% to 41.3%. The median follow‐up for the 16 AS studies in the meta‐analysis varied between 38 and 180 months
In one study with an accrual period from 1982 to 1992, patients in the first 2 years underwent explorative laparotomy in conjunction with orchidectomy 28. If no palpable lymph nodes were found during surgery, the lymph nodes were not resected and the patients were classified as CS I and treated with AS.
The overall risk of bias was moderate to high for all studies (Table S1). Symmetry shown in the funnel plots for studies on LVI and EC predominance indicates that there was a low risk of publication bias (Fig. S1). The funnel plot for studies on EC presence showed asymmetry, which suggests that there may be some unpublished negative studies.
LVI as a risk factor for recurrence
All but one study reported the effect of LVI (Table 2) [1,10,15–44,46,47]. Six studies analysed VI and lymphatic invasion separately or mentioned only VI 28, 32, 36, 37, 38, 44. The proportion of patients with LVI ranged from 16.4% to 61.4%.
Table 2.
Results of studies reporting on LVI.
| Reference | Patients with LVI information, N | LVI missing, % | LVI positive, % | Metastasis LVI present, % | Metastasis LVI absent, % | Reported univariable analysis | Method multivariable analysis | Reported multivariable analysis |
|---|---|---|---|---|---|---|---|---|
| Gilbert et al. 2016 [15] | 177 | 0 | 36.7 | 2‐year RFR 58.3 | 2‐year RFR 88.3 | P < 0.001 | Stratified log‐rank test | N/A (stratified by LVI) |
| Li et al. 2015 [16] | 78 | 0 | 21.8 | 52.9 | 14.8 | OR 6.500 (1.984–21.291) P = 0.002 | Logistic regression analysis |
OR 6.521 1.872–22.721 P = 0.003 |
| Kollmannsberger et al. 2015 [1] | 1118 | 1.8 | 16.4 | 44.3 | 14.1 | NR | NR | NR |
| Daugaard et al. 2014 [17] | 683 | 44.3 | 24.9 | 42.6 | 26.4 | HR 2.62 (2.03–3.39) P < 0.001 | Cox prop. hazards model |
HR 1.57 1.64–2.99 P < 0.001 |
| Keskin et al. 2011 [18] | 70 | 0 | 32.9 | 26.1 | 12.8 | P = 0.322 | NR | NR |
| Nicolai et al. 2011 [39] | 163 | 10.9 | 38.0 | 25.8 | 11.9 | P = 0.032 | NR | NR |
| Sturgeon et al. 2011 [19] | 331 | 10.8 | 27.8 | NR | NR | NR | Cox prop. hazards model |
HR 3.22 2.17–4.78 P < 0.001* |
| Kollmannsberger et al. 2010 [20] | 206 | 7.6 | 29.1 | 50.0 | 13.0 | NR | NR | NR |
| Albers et al. 2003 [10] | 152 | 7.9 | 48.7 | 52.7 | 23.1 | P = 0.001 | Logistic regression analysis | OR 3.7143 (1.8501–7.4566) P < 0.001 |
| Atsü et al. 2003 [21] | 132 | 0 | 36.4 | 27.1 | 22.6 | P = 0.7 | Cox prop. hazards model | LVI NS |
| Daugaard et al. 2003 [22] | 145 | 51.8 | 31.7 | 54.3 | NR | NR | NR | NR |
| Spermon et al. (2002) [40] | 50 | 0 | 36.0 | 61.1 | 12.5 | P = 0.001 | Multivariate logistic model | P = 0.001 |
| Alexandre et al. 2001 [23] | 84 | 4.5 | 47.6 | NR | NR | RR 5.3 (2.0–14.2) P < 0.001 | Cox prop. hazards model |
RR 3.8 1.4–10.4 P = 0.008 |
| Roeleveld et al. 2001 [24] | 79 | 12.2 | 41.8 | 51.5 | 10.9 | P < 0.001 | Logistic regression analysis | P < 0.001 |
| Sweeney et al. 2000 [43] | 178 | 39.0 | 51.1 | 50.5 | 20.7 | P < 0.001 | NR | NR |
| Colls et al. 1999 [25] | 243 | 2.0 | 37.9 | 45.7 | 17.2 | P < 0.001 | NR | NR |
| Sogani et al. 1998 [26] | 105 | 0 | 19.0 | 60.0 | 16.5 | P < 0.001 | Cox prop. hazards model |
OR 4.2 P < 0.001 |
| Maher and Lee 1998 [27] | 41 | 2.4 | 26.8 | 54.5 | 20.0 | P = 0.025 | NR | NR |
| Albers et al. 1997 [47] | 78 | 0 | 41.0 | 62.5 | 17.4 | P < 0.001 | Maximum likelihood analysis | P = 0.010 |
| Gels et al. 1995 [28] | VI: 154 | 0 | VI: 23.4% | VI: 52.8 | VI: 19.5 | P < 0.001 | Logistic regression analysis | OR 4.24, P < 0.001 |
| Nicolai and Pizzocaro 1995 [29] | 28 | 67.1 | 35.7 | 50 | 11.1 | P = 0.069 | NR | NR |
| Moul et al. 1994 [44] | 92 | 0 |
VI: 41.3 LI: 21.7 |
VI: 76.3 LI: 85.0 |
VI: 16.7 LI: 29.2 |
VI: P < 0.001 LI: P < 0.001 |
Logistic regression analysis | VI: P < 0.001 |
| Ondrus and Hornak 1994 [30] | 80 | 0 | 40.0 | 53.1 | 18.8 | P = 0.042 | NR | NR |
| Tekgül et al. 1995 [31] | 36 | 37.9 | 27.8 | 40.0 | NR | P > 0.05 | NR | NR |
| Read et al. 1992 [32] |
LI: 362 VI: 363 |
LI: 2.9 VI: 2.7 |
LI: 16.9 VI: 47.1 |
2‐year RFR LI: 59 VI: 65 |
2‐year RFR LI: 79 VI: 86 |
LI: P < 0.001 VI: P < 0.001 |
Cox prop. hazards model | VI: P < 0.001 |
| Sturgeon et al. 1992 [33] | 103 | 1.9 | 32.0 | 60.6 | 24.3 | P < 0.001 | NR | NR |
| Rørth et al. 1991 [34] | 77 | 7.2 | 58.4 | 37.8 | 18.8 | NR | NR | NR |
| Klepp et al. 1990 [42] | 265 | 5.0 | 28.3 | 65.3 | 25.8 | P < 0.001 | Logistic regression analysis | P < 0.001 |
| Wishnow et al. 1989 [46] | 82 | 0 | 25.6 | 52.4 | 21.3 | NR | NR | NR |
| Dunphy et al. 1988 [35] | 93 | 0 | 34.4 | 53.1 | 18.0 | P < 0.01 | Cox regression analysis | P = 0.99 |
| Fung et al. 1988 [41] | 60 | 0 | 50.0 | 46.7 | 20.0 | P = 0.05 | NR | NR |
| Thompson et al. 1988 [36] | 34 | 5.6 |
VI: 29.4 LI: 52.9 |
VI: 40.0 LI: 55.6 |
VI: 29.2 LI: 6.3 |
VI: P > 0.1 LI: P < 0.005 |
Cox regression analysis | Only LI significant |
| Freedman et al. 1987 [37] |
LI: 256 VI: 259 |
LI: 1.2 VI: 2.3 |
LI: 18.7 VI: 50.6 |
2‐year RFR LI: 45 VI: 57 |
2‐year RFR LI: 80 VI: 90 |
LI: P < 0.001 VI: P < 0.001 |
Cox prop. hazards model |
LI: P < 0.001 VI: P < 0.001 |
| Hoskin et al 1986 [38] |
VI: 118 LI: 116 |
VI: 6.3 LI: 7.9 |
VI: 31.4 LI: 18.1 |
VI: 45.9 LI: 57.1 |
VI: 23.5 LI: 23.2 |
VI: P < 0.01 LI: P < 0.005 |
Cox proportional hazards model |
VI: NS LI: HR 3.7, P < 0.01 |
N/A, not applicable; LI, lymphatic invasion; NR, not reported; NS, not significant; RFR, relapse‐free rate. *With imputation of missing data.
Studies with central pathology review reported a higher rate of LVI. The weighted average percentage of LVI‐positive patients was 23.5% for studies without pathology review and 36.6% for studies with central pathology review.
The relapse rate for LVI‐positive patients varied between 26.1% and 60.6%, and was <40% in four of 28 studies that reported on it 18, 21, 34, 39. The relapse rate for LVI‐negative patients ranged from 10.9% to 37.0%. In the RPLND studies, the rate of N+ was 25.8–65.3% and 11.9–25.8% for patients with and without LVI, respectively. In all studies, the metastatic rate was higher for LVI‐positive patients.
A total of 21 studies reported the univariable analysis of LVI 10, 15, 16, 17, 18, 21, 23, 24, 25, 26, 27, 29, 30, 33, 35, 39, 40, 41, 42, 43, 47, and this was statistically significant in 18 studies 10, 15, 16, 17, 23, 24, 25, 26, 27, 30, 33, 35, 39, 40, 41, 42, 43, 47.
In all, 18 studies reported raw data and were eligible for inclusion in the meta‐analysis (Fig. 2A) 1, 10, 16, 21, 24, 25, 26, 27, 29, 30, 34, 39, 40, 41, 42, 43, 46, 47. These studies reported on 3009 patients, of which 894 (29.7%) were LVI positive. The pooled rate of occult metastatic disease for LVI‐positive patients was 47.5%, compared to 16.9% for LVI‐negative patients (odds ratio [OR] 4.33, 95% CI 3.55–5.30; P < 0.001).
Figure 2.
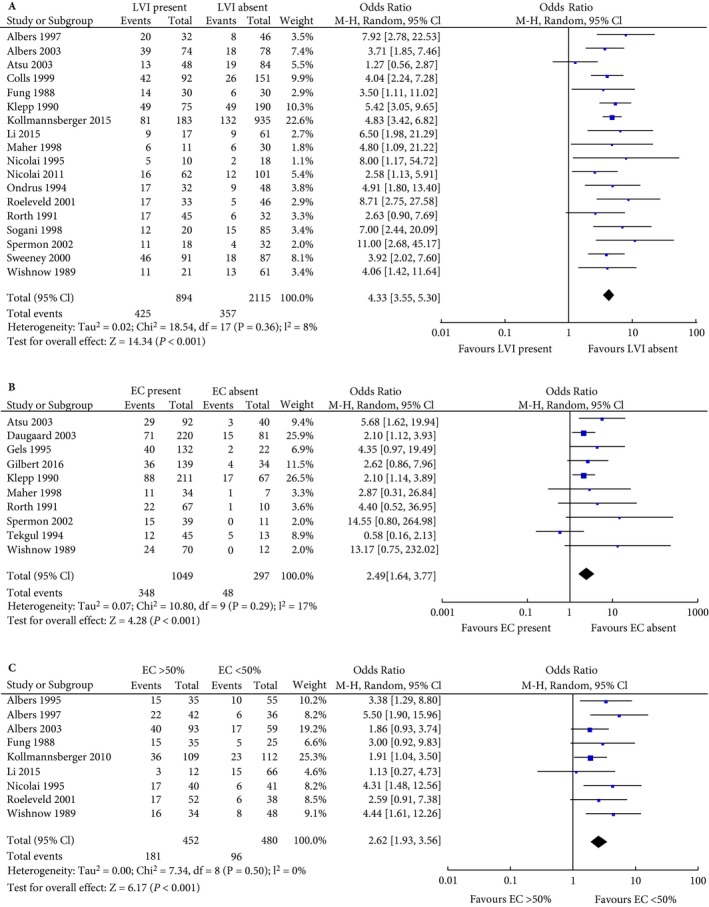
Forest plot of meta‐analysis for (A) LVI presence, (B) EC presence, (C) EC >50%.
EC as a risk factor for recurrence
A total of 27 studies analysed the association between EC and relapse (Table 3) 10, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 26, 27, 28, 29, 30, 31, 33, 34, 35, 40, 41, 42, 43, 45, 46, 47. In 12 studies, EC was analysed as present vs absent. The percentage of EC was analysed in several studies, but mostly as a categorical variable with different cut‐off values. Two studies analysed EC percentage as a continuous variable 15, 47.
Table 3.
Results of studies reporting on EC.
| Author | Patients with EC information, N | EC missing, % | Method of EC reporting | Patients per category, % | Metastases for EC present, % | Metastases for EC absent, % | Reported univariable analysis | Method multivariable analysis | Reported multivariable analysis |
|---|---|---|---|---|---|---|---|---|---|
| Present vs absent | |||||||||
| Gilbert et al. 2016 [15] | 177 | 0 | Present vs absent | Present: 78.5 |
2‐year RFR 74.3 |
2‐year RFR 89.2 |
P = 0.096 | Stratified log‐rank test (stratified by LVI) Cox regression model | P = 0.243 |
| Daugaard et al. 2014 [17] | 1226 | 0 | Present vs absent | Present: 78.1 | NR | NR | HR 3.00 (2.14–4.22) P < 0.001 | Cox prop. hazards model | HR 2.73 (1.94–3.85) P < 0.001 |
| Keskin et al. 2011 [18] | 70 | 0 | Present vs absent | Present: 71.4 | 22.0 | 5.0 | P = 0.157 | NR | NR |
| Atsü et al. 2003 [21] | 132 | 0 | Present vs absent | Present: 69.7 | 31.5 | 7.5 | P = 0.003 | Cox prop. hazards model | RR 3.7 |
| Daugaard et al. 2003 [22] | 301 | 0 | Present vs absent | Present: 73.1 | 32.3 | 18.5 | NR | NR | NR |
| Spermon et al. (2002) [40] | 50 | 0 | Present vs absent | Present: 78.0 | 38.5 | 0 | P = 0.02 | NR | NR |
| Maher and Lee 1998 [27] | 41 | 2.4 | Present vs absent | Present: 82.9 | 32.4 | 14.3 | P = 0.38 | NR | NR |
| Gels et al. 1995 [28] | 154 | 0 | Present vs absent | Present: 85.7 | 30.3 | 9.1 | P = 0.039 | Logistic regression analysis |
OR 3.49 P = 0.110 |
| Tekgül et al. 1995 [31] | 58 | 0 | Present vs absent | Present: 77.6 | 26.7 | 38.5 | NR | Cox prop. hazards model | P > 0.05 |
| Sturgeon et al. 1992 [33] | 105 | 0 | Present vs absent | Present: 27.6 | 48.3 | 30.3 | NR | NR | NR |
| Rørth et al. 1991 [34] | 77 | 7.2 | Present vs absent | Present: 87.0 | 32.8 | 10.0 | NR | NR | NR |
| Klepp et al. 1990 [42] | 278 | 0.4 | Present vs absent | Present: 75.9 | 41.7 | 25.4 | P = 0.024 | Logistic regression analysis | P = 0.11 |
| Dunphy et al. 1988 [35] | 93 | 0 | Present vs absent | Present: 87.1 | 34.6 | 0 | P = 0.05 | Cox regression analysis | P = 0.05 |
| EC percentage | |||||||||
| Li et al. 2015 [16] | 78 | 0 | >50% vs <50% | >50%: 15.4 | >50%: 25.0 | <50%: 22.7 | OR 1.133 (0.272–4.726) P = 0.864 | NR | NR |
| Kollmannsberger et al. 2010 [20] | 221 | 0.9 | ≥50% vs <50% | ≥50%: 49.3 | ≥50%: 33.0 | <50%: 20.5 | NR | NR | NR |
| Albers et al. 2003 [10] | 152 | 7.9 | ≥50% vs <50% | ≥50%: 61.2 | ≥50%: 43.0 | <50%: 28.8 | P = 0.088 | Logistic regression analysis | OR 1.8646 (0.9286–3.7440) P = 0.080 |
| Alexandre et al. 2001 [23] | 84 | 4.5 | >40% vs ≤40% | >40%: 50.0 | NR | NR | RR 3.5 (1.4–8.7) P = 0.008 | Cox prop. hazards model | EC NS |
| Albers et al. 1997 [47] | 78 | 0 | ≥50% vs <50% | ≥50%: 53.9 | ≥50%: 52.4 | <50%: 16.7 | Continuous: P = 0.001 | Maximum likelihood analysis | Continuous: P = 0.024 |
| Fung et al. 1988 [41] | 60 | 0 | ≥50% vs <50% | ≥50%: 58.3 | ≥50%: 42.9 | <50%: 20.0 | P = 0.10 | NR | NR |
| Wishnow et al. 1989 [46] | 82 | 0 | All data given | >50%: 40.2 | >50%: 47.1 | <50%: 16.7 | NR | NR | NR |
| Multiple categories | |||||||||
| Gilbert et al. 2016 [15] | 177 | 0 |
3 categories: ≤25% 26–99% 100% Continuous variable |
≤25%: 45.2 26–99%: 31.6 100%: 23.2 |
2‐year RFR ≤25%: 88.4 26–99%: 76.4 100%: 57.5 |
3 categories: ≤25%: ref 26–99%: HR 1.679 (0.736–3.831) 100%: HR 3.118 (1.391–6.988) P = 0.019 Continuous: HR 1.011 (1.002–1.019) P = 0.012 |
Stratified log‐rank test (stratified by LVI) Cox regression model | 3 categories: P = 0.006 | |
| Roeleveld et al. 2001 [24] | 90 | 0 |
4 categories: 0–25% 25–50% 50–75% 75–100% |
0–25%: 25.6 25–50%: 16.7 50–70%: 25.6 75–100%: 32.2 >50%: 57.8 |
0–25%: 21.7 25–50%: 6.6 50–70%: 47.8 75–100%: 20.7 >50%: 32.7 |
<50%: 15.8 | 4 categories: P = 0.032 | Logistic regression analysis | 4 categories: P = 0.220 |
| Nicolai and Pizzocaro 1995 [29] | 81 | 4.7 |
3 categories: <50% 50–99% 100% |
<50%: 50.6 50–99%: 37.0 100%: 12.3 >50%: 49.4 |
<50%: 14.6 50–99%: 36.7 100%: 60 >50%: 42.5 |
<50%: 14.6 | 3 categories: P = 0.008 | NR | NR |
| Albers et al. 1995 [45] | 90 | 0 |
4 categories: 0–25% 26–50% 51–75% 76–100% |
0–25%: 43.3 26–50%: 17.8 51–75%: 14.4 76–100%: 24.4 >50%: 38.9 |
0–25%: 15.4 26–50%: 25.0 51–75%: 30.8 76–100%: 50.0 >50%: 42.9 |
≤50%: 18.2 | 4 categories: NS | NR | NR |
| Other categories | |||||||||
| Sturgeon et al. 2011 [19] | 371 | 0 | Pure EC | Pure: 15.1 | NR | NR | NR | Cox prop. hazards model | HR 1.74 (1.10–2.74) P = 0.02 |
| Sweeney et al. 2000 [43] | 292 | 0 | Predominant vs not predominant | Predominant: 42.8 | 46.4 | 18.6 | P < 0.001 | NR | NR |
| Sogani et al. 1998 [26] | 105 | 0 | Predominance | 24.8 | 46 | 19 | P = 0.007 | Cox prop. hazards model |
OR 2.6 P = 0.016 |
| Ondrus and Hornak 1994 [30] | 80 | 0 | Major EC vs minor EC |
Major EC: 51.3 Minor EC: 30.0 |
58.5 | 20.8 | P = 0.096 | NR | NR |
EC, embryonal carcinoma; NR, not reported; NS, not significant; RFR, relapse‐free rate.
The percentage of EC‐positive patients ranged from 69.7% to 87.1%. Rates of occult metastatic disease were 22.0–34.6% and 0–38.5% for EC‐positive and ‐negative patients, respectively.
A total of 10 studies reported raw data on the analysis of EC present vs absent and were included in the meta‐analysis (Fig. 2B) 15, 21, 22, 27, 28, 31, 34, 40, 42, 46. These studies reported on 1346 patients of whom 1049 (77.9%) were EC positive. The pooled rates of occult metastasis were 33.2% and 16.2% for EC‐positive and ‐negative patients, respectively (OR 2.49, 95% CI 1.64–3.77; P < 0.001).
One study analysed the prognostic value of pure EC and found that it was significantly associated with recurrence (hazard ratio [HR] 1.74, 95% CI 1.10–2.74; P = 0.02) 19. Patients classified as high risk, based on the presence of pure EC and/or LVI, had a 52% risk of relapse, compared to 15.8% of patients classified as low risk.
Studies reporting on the predictive value of percentage of EC were of heterogeneous design. Four studies divided the study population into more than two categories, all using different cut‐off values 15, 24, 29, 45. The association between percentage of EC and relapse was significant on univariable analysis in three studies.
Six studies analysed EC percentage as a binary variable 10, 16, 20, 23, 41, 47. The cut‐off value was 50% in five studies 10, 16, 20, 41, 47. Three studies found no significant difference in occult metastasis between EC ≥50% and EC <50% 10, 16, 41 and two studies did not report on it, but showed a significant difference when we re‐calculated the ORs 20, 47.
Alexandre et al. 23 used 40% as a cut‐off value and reported a significant difference in relapse‐free survival on univariable analysis. The relative risk (RR) for patients with EC >40% in comparison to patients with EC ≤40 was 3.5 (95% CI 1.4–8.7; P = 0.008), but this was not statistically significant on multivariable analysis.
Three of the four studies that divided EC percentage in to more than two categories found a significant difference in occult metastatic disease occurrence 15, 24, 29. Two studies included EC percentage in a multivariable model, and this was significant only in the study by Gilbert et al. 15. However, the cut‐off values in this study (<25%; 26–99%; 100%) were data‐driven and not based on previous reports.
Gilbert et al. 15 also analysed EC percentage as a continuous variable. In their model, which also included LVI, the OR for EC percentage was 1.011 (95% CI 1.002–1.019; P = 0.012). As mentioned before, Albers et al. 47 also found a significant correlation between EC as a continuous variable and occult metastatic disease, but LVI and tumour proliferation rate were better predictors.
We included nine studies, reporting on 932 patients, in the meta‐analysis comparing EC >50% with EC <50% (Fig. 2C) 10, 16, 20, 24, 29, 41, 45, 46, 47. Four studies used 50% as a cut‐off value in their own statistical analysis 10, 16, 41, 47. The other studies reported sufficient raw data that it was possible to construct 2 × 2 tables and include them in the analysis. Pooled rates of occult metastasis were 40.0% and 20.0% for patients with EC >50% and EC <50%, respectively (OR 2.62, 95% CI 1.93–3.56; P < 0.001).
Multivariable analyses
A total of 21 studies reported multivariable analysis, but with various levels of quality. Most studies used the Cox proportional hazards model, and six studies used logistic regression analysis 10, 16, 24, 28, 42, 44. Three studies reported HRs instead of ORs 15, 17, 19.
The presence of LVI was the most studied predictor and showed the strongest effect. The largest cohort, by Daugaard et al. 17 (n = 1226), found an HR of 1.57 (95% CI 1.22–2.02; P < 0.001) for LVI alone. The Princess Margaret Cancer Center reported on a series of 371 patients treated between 1981 and 2005 19. LVI, regardless of other prognostic factors, was an independent predictor of relapse (HR 3.22, 95% CI 2.17–4.78; P < 0.001) in this cohort. Albers et al. 10 calculated the negative (NPVs) and positive predictive values (PPVs) for various combinations of histopathological risk factors. The best prediction of a low‐risk group was a combination of absent LVI and low proliferation rate. This resulted in a NPV of 86.5%. Patients with a combination of LVI presence, high proliferation rate, and EC ≥50% were the best predicted high‐risk group (PPV 63.6%).
The independent predictive value of EC was analysed in several studies, but different definitions were used. Sturgeon et al. 19 was the only study to include the presence of pure EC in a multivariable analysis and found a significant association (HR 1.74, 95% CI 1.10–2.74; P = 0.02). The cohort by Daugaard et al. 17 analysed EC presence as a single risk factor and also found a significant association (HR 2.73, 95% CI 1.94–3.85; P < 0.001). In a Turkish study of 138 patients, the presence of EC led to a 3.7‐fold increase of the relapse risk 21. Three studies reported no significant association between presence of EC and relapse 28, 31, 42.
EC ≥50% was included in a multivariable analysis in two studies, with contradictory results 10, 26. Sogani et al. 26 found that it was a significant predictor (OR 2.6; P = 0.016), but it was not significant in the study by Albers et al. 10 (P = 0.080). Gilbert et al. 15 analysed the predictive value of EC in various ways. LVI and EC, either as a continuous variable or split into the three previously mentioned categories (≤25%; 26–99%; 100%), were independent predictors of relapse. Only when EC was analysed as a binary variable (present/absent), the molecular marker C‐X‐C motif chemokine 12 (CXCL12), but not EC, was a significant negative predictor. As mentioned before, Albers et al. 47 also found a significant correlation between EC as a continuous variable and occult metastatic disease, but LVI and tumour proliferation rate were better predictors.
Discussion
Our present study confirms that the presence of LVI is the strongest predictor of occult metastatic disease in CS I NSGCT. The prognostic value of this parameter is affirmed by several large cohort studies and our present meta‐analysis.
EC is an additionally useful risk prognosticator but agreement about the definition to be used is necessary. Our meta‐analysis showed that the ORs for EC presence and EC ≥50% are quite similar (2.49 vs 2.62) and the relapse rates are approximately equal (33.2% vs 40.0%). This small difference in prognostic value between EC presence and EC ≥50% suggests that the assessment of EC presence may be sufficient to identify high‐risk patients.
A continuous correlation between EC and occult metastatic disease was found in both studies that investigated it 15, 47. The clinically most relevant cut‐off value, however, is still up for debate. It is likely that the risk of occult metastatic disease is already high in the presence of only a small amount of EC and any further increase in EC percentage does not involve a relevant increase in clinical risk.
A meta‐analysis from 2002 by Vergouwe et al. 48 also investigated the predictive value of LVI and EC. The results of that study are very much in line with our present findings. LVI had the strongest predictive value (OR 4.7) and EC presence and EC >50% showed similar ORs for metastasis (OR 2.9 and 2.8, respectively).
Risk stratification of CS I NSGCT is important for patient counselling and when adjuvant treatment is considered. Several stratifications have been proposed. Since 1995, the SWENOTECA has identified high‐risk patients on the basis of LVI presence or absence 7. Lago‐Hernandez et al. 9 developed a 0, 1, and 2 scoring system to stratify patients according to LVI presence and EC predominance (defined as EC presence at a larger level than any other histological type). Relapse rates were 25%, 41%, and 77% for 0, 1, and 2 risk factors, respectively. Daugaard et al. 17 also explored the combination of different risk factors and found that 5‐year relapse risk was highest for patients with EC + LVI + rete testis invasion (50%, HR 5.65). Risk for patients with LVI alone was 18% (HR 1.57) and 41% for patients with EC + LVI (HR 4.29).
The proportion of high‐risk patients based on LVI and/or EC differed between the included studies. This may be due to selected patient groups and is not necessarily a reflection of differences between study populations. More specifically, not all AS studies reported on truly unselected AS populations. In both studies by Sturgeon et al., 19, 33 AS was offered as the preferred management method for all men with CS I NSGCT, but patients were allowed to choose. This may have introduced bias, which is illustrated by the differences in proportion of LVI‐positive patients and relapse rates between the two studies by Kollmannsberger et al. 1, 20. The data included in Kollmannsberger et al. 1 is pooled from several institutions and almost half of the cohort comes from centres where patients can choose between AS and adjuvant therapy (SWENOTECA). Both the relapse rate (19.4%) and the proportion of LVI‐positive patients (16.4%) in that study were low. In an earlier study by the same author 20, which reports on some of the same patients as the 2015 study and is also not a strictly AS population, the relapse rate and LVI percentage were higher (26.5% and 29.1%, respectively).
We compared the weighted average of strictly AS studies with studies that reported no strict AS in a subgroup analysis. Weighted average relapse rates were 30.2% and 25.0% for strictly AS and non‐strictly AS studies, respectively. The weighted rate of LVI‐positive patients was slightly higher for strictly AS patients (27.4% vs 25.0%). Thus, studies that did not explicitly state that a strict AS protocol was followed, often reported on a selected population. This can give contradictory results.
The difference in rate of high‐risk patients could also be due to a lack of reproducibility of LVI assessment by pathologists. This is reflected by the difference in rate of LVI between reports with and without central pathology review. In a series of 221 patients by Harari et al. 49, reporting of LVI changed in 22% of cases after central pathology review. Purshouse et al. 50 reported that in 7.2% of patients with NSGCT the tumour prognostic factors were changed after central pathology review (5% for LVI status, 2.2% for EC >50% vs <50%). These discrepancies emphasise the need for pathology review by an expert genitourinary pathologist.
Most studies investigated other possible histopathological risk factors in addition to LVI and EC. Tumour size, an important prognostic factor in seminoma, was significantly associated with relapse in the study by Roeleveld et al. 24 (cut‐off value 5 cm; P = 0.039). Five other studies in our present study also assessed this factor, but none found a significant correlation with occult metastatic disease 16, 28, 35, 36, 38. In a large series of 779 patients by Beck et al. 51 (not included in our review), primary tumour size was not predictive of occult metastatic disease (P = 0.167).
Several studies reported on the tumour proliferation rate, which is one of the prognostic markers mentioned in the European Association of Urology (EAU) guidelines 3. It is commonly expressed as rate of MIB‐1‐positive tumour cells and was an independent predictor of metastatic disease in a prospective trial by the German Testicular Cancer Study Group Trial 10. In that study, MIB‐1 scores were available for 152 patients. Using a cut‐off value of 70%, the OR for metastatic disease was 2.75 (95% CI 1.28–5.91; P = 0.010). However, the PPV was relatively low at 43.0%. In an earlier study by the same author (but in a different patient cohort), the pathological stage was correctly classified in 69% of cases (NPV 88%, PPV 55%) 47. These findings are contradicted by a series of 149 specimens by Heidenreich et al. 52 in which the MIB‐1 score was not useful in predicting the pathological stage. Gilbert et al. 15 used the same cut‐off values as the German trial and found no evidence of any prognostic value. This could be explained by the fact that only five of 179 patients had MIB‐1 staining in ≥70% cells. When MIB‐1 staining was dichotomized (weak vs high), it had some prognostic value on univariable analysis, but this was reduced after stratification for LVI (P = 0.045). In the meta‐analysis by Vergouwe et al. 48, patients with MIB‐1 staining >70% were at higher risk of occult metastasis (OR 4.7). However, the authors noted that this analysis was based on a low number of patients (N = 212), the 70% cut‐off value was data‐driven, and, therefore, additional research is necessary.
One of the limitations of our present study is the heterogeneity of included studies. Studies were heterogeneous in terms of study population, year of accrual, assessment of histopathological risk factors, and methodological quality. Although studies reporting on a risk‐adapted protocol were excluded, some studies reported on selected populations. Furthermore, only a few studies performed central pathology review in the context of the study. Several single‐centre and some larger studies reported pathology review by an expert pathologist as part of standard care. Especially in low‐volume centres, however, the quality of risk factor assessment might be low. In addition, most studies did not report the definition for LVI and several studies did not report the definition for EC predominance.
Missing data of the histopathological features of interest were high in a number of studies. Some retrospective studies only included patients with complete data without reporting the total number of patients treated during the study period. Therefore, missing data were not assessable in these studies. Most studies that reported missing data excluded these patients from further analysis. Imputation of missing data was only performed in the study by Daugaard et al. 17, in which LVI status was unknown in 44% of the cohort.
In the present study, we were only able to analyse LVI and EC separately. It would be interesting to evaluate the predictive value of both factors together. For example, it was not possible to assess the difference in relapse risk between LVI‐positive patients with EC >50% and LVI‐positive patients without EC >50%. This requires an individual patient data meta‐analysis of the series included in this review.
The major strength of our present review is the systematic approach that was applied. Our methodology is in line with the Cochrane reporting standards, such as the PRISMA statement and the QUIPS tool for risk‐of‐bias assessment. Furthermore, a high number of participants have been included in our meta‐analysis and we payed special attention to avoid the inclusion of overlapping populations. Even though methodological heterogeneity might exist, statistical heterogeneity I 2 was low for all meta‐analyses.
Conclusions
Our present review and meta‐analysis show that LVI is the strongest predictor of occult metastatic disease in CS I NSGCT. The prognostic value of EC is high, but consensus on how to use this risk factor is necessary. A cut‐off value of 50% is reported in only a few studies, with contradicting results. Both EC presence and EC >50% show similar ORs for occult metastasis. This suggests that the assessment of EC presence is sufficient for the classification of EC.
Funding
None.
Conflict of Interest
None declared.
Supporting information
Fig. S1. Funnel plot for (A) LVI presence, (B) EC presence, (C) EC >50%.
Table S1. Risk‐of‐bias assessment (QUIPS).
Appendix S1. Search strategy (PubMed).
Appendix S2. Studies included after full‐text screening.
References
- 1. Kollmannsberger C, Tandstad T, Bedard PL et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol 2015; 33: 51–7 [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Jonasch E, Agarwal N et al. Testicular cancer, version 2.2015. J Natl Compr Canc Netw 2015; 13: 772–99 [DOI] [PubMed] [Google Scholar]
- 3. Albers P, Albrecht W, Algaba F et al. Guidelines on testicular cancer: 2015 update. Eur Urol 2015; 68: 1054–68 [DOI] [PubMed] [Google Scholar]
- 4. Wood L, Kollmannsberger C, Jewett M et al. Canadian consensus guidelines for the management of testicular germ cell cancer. J Can Urol Assoc 2010; 4: 19–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Groot HJ, Lubberts S, de Wit R et al. Risk of solid cancer after treatment of testicular germ cell cancer in the platinum era. J Clin Oncol 2018; 36: 2504–13 [DOI] [PubMed] [Google Scholar]
- 6. van den Belt‐Dusebout AW, Nuver J, de Wit R et al. Long‐term risk of cardiovascular disease in 5‐year survivors of testicular cancer. J Clin Oncol 2006; 24: 467–75 [DOI] [PubMed] [Google Scholar]
- 7. Tandstad T, Ståhl O, Håkansson U et al. One course of adjuvant BEP in clinical stage I nonseminoma mature and expanded results from the SWENOTECA group. Ann Oncol 2014; 25: 2167–72 [DOI] [PubMed] [Google Scholar]
- 8. Vidal AD, Thalmann GN, Karamitopoulou‐Diamantis E, Fey MF, Studer UE. Long‐term outcome of patients with clinical stage I high‐risk nonseminomatous germ‐cell tumors 15 years after one adjuvant cycle of bleomycin, etoposide, and cisplatin chemotherapy. Ann Oncol 2015; 26: 374–7 [DOI] [PubMed] [Google Scholar]
- 9. Lago‐Hernandez CA, Feldman H, O’Donnell E et al. A refined risk stratification scheme for clinical stage 1 NSGCT based on evaluation of both embryonal predominance and lymphovascular invasion. Ann Oncol 2015; 26: 1396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albers P, Siener R, Kliesch S et al. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. J Clin Oncol 2003; 21: 1505–12 [DOI] [PubMed] [Google Scholar]
- 11. Honecker F, Aparicio J, Berney D et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow‐up. Ann Oncol 2018; 29: 1658–86 [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–12 [DOI] [PubMed] [Google Scholar]
- 13. Stroup DF, Berlin JA, Morton SC et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–12 [DOI] [PubMed] [Google Scholar]
- 14. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Research and reporting methods annals of internal medicine assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158: 280–6 [DOI] [PubMed] [Google Scholar]
- 15. Gilbert DC, Al‐Saadi R, Thway K et al. Defining a new prognostic index for stage I nonseminomatous germ cell tumors using CXCL12 expression and proportion of embryonal carcinoma. Clin Cancer Res 2016; 22: 1265–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li X, Guo S, Wu Z et al. Surveillance for patients with clinical stage I nonseminomatous testicular germ cell tumors. World J Urol 2015; 33: 1351–7 [DOI] [PubMed] [Google Scholar]
- 17. Daugaard G, Gundgaard MG, Mortensen MS et al. Surveillance for stage I nonseminoma testicular cancer: outcomes and long‐term follow‐up in a population‐based cohort. J Clin Oncol 2014; 32: 3817–23 [DOI] [PubMed] [Google Scholar]
- 18. Keskin S, Ekenel M, Başaran M, Bavbek S. Surveillance results of patients with stage I nonseminomatous germ cell testicular cancer. Onkologie 2011; 34: 173–6 [DOI] [PubMed] [Google Scholar]
- 19. Sturgeon JF, Moore MJ, Kakiashvili DM et al. Non‐risk‐adapted surveillance in clinical stage I nonseminomatous germ cell tumors: the Princess Margaret Hospital’s experience. Eur Urol 2011; 59: 556–62 [DOI] [PubMed] [Google Scholar]
- 20. Kollmannsberger C, Moore C, Chi KN et al. Non‐risk‐adapted surveillance for patients with stage I nonseminomatous testicular germ‐cell tumors: diminishing treatment‐related morbidity while maintaining efficacy. Ann Oncol 2010; 21: 1296–301 [DOI] [PubMed] [Google Scholar]
- 21. Atsü N, Eskiçorapçi S, Uner A et al. A novel surveillance protocol for stage I nonseminomatous germ cell testicular tumours. BJU Int 2003; 92: 32–5 [DOI] [PubMed] [Google Scholar]
- 22. Daugaard G, Petersen PM, Rørth M. Surveillance in stage I testicular cancer. APMIS 2003; 111: 76–85 [DOI] [PubMed] [Google Scholar]
- 23. Alexandre J, Fizazi K, Mahé C et al. Stage I non‐seminomatous germ‐cell tumours of the testis: identification of a subgroup of patients with a very low risk of relapse. Eur J Cancer 2001; 37: 576–82 [DOI] [PubMed] [Google Scholar]
- 24. Roeleveld TA, Horenblas S, Meinhardt W, van de Vijver M, Kooi M, ten Bokkel Huinink WW. Surveillance can be the standard of care for stage I nonseminomatous testicular tumors and even high risk patients. J Urol 2001; 166: 2166–70 [PubMed] [Google Scholar]
- 25. Colls BM, Harvey VJ, Skelton L et al. Late results of surveillance of clinical stage I nonseminoma germ cell testicular tumours: 17 years’ experience in a national study in New Zealand. BJU Int 1999; 83: 76–82 [DOI] [PubMed] [Google Scholar]
- 26. Sogani PC, Perrotti M, Herr HW, Fair WR, Thaler HT, Bosl G. Clinical stage I testis cancer: long‐term outcome of patients on surveillance. J Urol 1998; 159: 855–8 [DOI] [PubMed] [Google Scholar]
- 27. Maher TM, Lee AH. Vascular density does not predict future metastatic disease in clinical stage 1 non‐seminomatous germ cell tumours of the testis. Histopathology 1998; 32: 217–24 [DOI] [PubMed] [Google Scholar]
- 28. Gels ME, Hoekstra HJ, Sleijfer DT et al. Detection of recurrence in patients with clinical stage I nonseminomatous testicular germ cell tumors and consequences for further follow‐up: a single‐center 10‐year experience. J Clin Oncol 1995; 13: 1188–94 [DOI] [PubMed] [Google Scholar]
- 29. Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10‐year followup. J Urol 1995; 154: 1045–9 [PubMed] [Google Scholar]
- 30. Ondrus D, Hornak M. Orchiectomy alone for clinical stage I nonseminomatous germ cell tumors of the testis (NSGCTT): a minimum follow‐up period of 5 years. Tumori 1994; 80: 362–4 [DOI] [PubMed] [Google Scholar]
- 31. Tekgül S, Ozen H, Ozgü I, Sahin A, Ergen A, Remzi D. Surveillance‐only policy in clinical stage‐I non‐seminomatous germ‐cell tumors of the testis. Bull Cancer 1995; 82: 162–6 [PubMed] [Google Scholar]
- 32. Read G, Stenning SP, Cullen MH et al. Medical Research Council prospective study of surveillance for stage I testicular teratoma. Medical Research Council Testicular Tumors Working Party. J Clin Oncol 1992; 10: 1762–8 [DOI] [PubMed] [Google Scholar]
- 33. Sturgeon JF, Jewett MA, Alison RE et al. Surveillance after orchidectomy for patients with clinical stage I nonseminomatous testis tumors. J Clin Oncol 1992; 10: 564–8 [DOI] [PubMed] [Google Scholar]
- 34. Rørth M, Jacobsen GK, von der Maase H et al. Surveillance alone versus radiotherapy after orchiectomy for clinical stage I nonseminomatous testicular cancer. Danish Testicular Cancer Study Group. J Clin Oncol 1991; 9: 1543–8 [DOI] [PubMed] [Google Scholar]
- 35. Dunphy CH, Ayala AG, Swanson DA, Ro JY, Logothetis C. Clinical stage I nonseminomatous and mixed germ cell tumors of the testis. A clinicopathologic study of 93 patients on a surveillance protocol after orchiectomy alone. Cancer 1988; 62: 1202–6 [DOI] [PubMed] [Google Scholar]
- 36. Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol 1988; 6: 1597–603 [DOI] [PubMed] [Google Scholar]
- 37. Freedman LS, Parkinson MC, Jones WG et al. Histopathology in the prediction of relapse of patients with stage I testicular teratoma treated by orchidectomy alone. Lancet 1987; 2: 294–8 [DOI] [PubMed] [Google Scholar]
- 38. Hoskin P, Dilly S, Easton D, Horwich A, Hendry W, Peckham MJ. Prognostic factors in stage I non‐seminomatous germ‐cell testicular tumors managed by orchiectomy and surveillance: implications for adjuvant chemotherapy. J Clin Oncol 1986; 4: 1031–6 [DOI] [PubMed] [Google Scholar]
- 39. Nicolai N, Colecchia M, Biasoni D et al. Concordance and prediction ability of original and reviewed vascular invasion and other prognostic parameters of clinical stage I nonseminomatous germ cell testicular tumors after retroperitoneal lymph node dissection. J Urol 2011; 186: 1298–302 [DOI] [PubMed] [Google Scholar]
- 40. Spermon JR, De Wilde PC, Hanselaar AG et al. alpha‐Catenin expression pattern and DNA image‐analysis cytometry have no additional value over primary histology in clinical stage I nonseminomatous testicular cancer. BJU Int 2002; 89: 278–84 [DOI] [PubMed] [Google Scholar]
- 41. Fung CY, Kalish LA, Brodsky GL, Richie JP, Garnick MB. Stage I nonseminomatous germ cell testicular tumor: prediction of metastatic potential by primary histopathology. J Clin Oncol 1988; 6: 1467–73 [DOI] [PubMed] [Google Scholar]
- 42. Klepp O, Olsson AM, Henrikson H et al. Prognostic factors in clinical stage I nonseminomatous germ cell tumors of the testis: multivariate analysis of a prospective multicenter study. Swedish‐Norwegian Testicular Cancer Group. J Clin Oncol 1990; 8: 509–18 [DOI] [PubMed] [Google Scholar]
- 43. Sweeney CJ, Hermans BP, Heilman DK, Foster RS, Donohue JP, Einhorn LH. Results and outcome of retroperitoneal lymph node dissection for clinical stage I embryonal carcinoma–predominant testis cancer. J Clin Oncol 2000; 18: 358–62 [DOI] [PubMed] [Google Scholar]
- 44. Moul JW, McCarthy WF, Fernandez EB, Sesterhenn IA. Percentage of embryonal carcinoma and of vascular invasion predicts pathological stage in clinical stage I nonseminomatous testicular cancer. Cancer Res 1994; 54: 362–4 [PubMed] [Google Scholar]
- 45. Albers P, Miller GA, Orazi A et al. Immunohistochemical assessment of tumor proliferation and volume of embryonal carcinoma identify patients with clinical stage A nonseminomatous testicular germ cell tumor at low risk for occult metastasis. Cancer 1995; 75: 844–50 [DOI] [PubMed] [Google Scholar]
- 46. Wishnow KI, Johnson DE, Swanson DA et al. Identifying patients with low‐risk clinical stage I nonseminomatous testicular tumors who should be treated by surveillance. Urology 1989; 34: 339–43 [DOI] [PubMed] [Google Scholar]
- 47. Albers P, Bierhoff E, Neu D, Fimmers R, Wernert N, Müller SC. MIB‐1 immunohistochemistry in clinical stage I nonseminomatous testicular germ cell tumors predicts patients at low risk for metastasis. Cancer 1997; 79: 1710–6 [PubMed] [Google Scholar]
- 48. Vergouwe Y, Steyerberg EW, Eijkemans MJ, Albers P, Habbema JD. Predictors of occult metastasis in clinical stage I nonseminoma: a systematic review. J Clin Oncol 2003; 21: 4092–9 [DOI] [PubMed] [Google Scholar]
- 49. Harari SE, Sassoon DJ, Priemer DS et al. Testicular cancer: The usage of central review for pathology diagnosis of orchiectomy specimens. Urol Oncol 2017; 35: 605.e9–e16 [DOI] [PubMed] [Google Scholar]
- 50. Purshouse K, Watson RA, Church DN et al. Value of supraregional multidisciplinary review for the contemporary management of testicular tumors. Clin Genitourin Cancer 2017; 15: 152–6 [DOI] [PubMed] [Google Scholar]
- 51. Beck SD, Foster RS, Bihrle R, Donohue JP. Significance of primary tumor size and preorchiectomy serum tumor marker level in predicting pathologic stage at retroperitoneal lymph node dissection in clinical stage A nonseminomatous germ cell tumors. Urology 2007; 69: 557–9 [DOI] [PubMed] [Google Scholar]
- 52. Heidenreich A, Sesterhenn IA, Mostofi FK, Moul JW. Prognostic risk factors that identify patients with clinical stage I nonseminomatous germ cell tumors at low risk and high risk for metastasis. Cancer 1998; 83: 1002–11 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Funnel plot for (A) LVI presence, (B) EC presence, (C) EC >50%.
Table S1. Risk‐of‐bias assessment (QUIPS).
Appendix S1. Search strategy (PubMed).
Appendix S2. Studies included after full‐text screening.


