Abstract
Aim
Oxidative stress is strongly implicated in many psychiatric disorders, which has resulted in the development of new interventions to attempt to perturb this pathology. A great deal of attention has been paid to glutathione, which is the brain's dominant antioxidant and plays a fundamental role in removing free radicals and other reactive oxygen species. Measurement of glutathione concentration in the brain in vivo can provide information on redox status and potential for oxidative stress to develop. Glutathione might also represent a marker to assess treatment response.
Methods
This paper systematically reviews studies that assess glutathione concentration (measured using magnetic resonance spectroscopy) in various mental health conditions.
Results
There is limited evidence showing altered brain glutathione concentration in mental disorders; the best evidence suggests glutathione is decreased in depression, but is not altered in bipolar disorder. The review then outlines the various methodological options for acquiring glutathione data using spectroscopy.
Conclusions
Analysis of the minimum effect size measurable in existing studies indicates that increased number of participants is required to measure subtle but possibly important differences and move the field forward.
Keywords: glutathione, magnetic resonance spectroscopy, youth mental health
1. INTRODUCTION
There is now extensive evidence that oxidative stress, defined as a disturbance in the balance between the production of reactive oxygen species and antioxidant defences (Betteridge, 2000) plays a role in the pathophysiology of many psychiatric disorders, including depression, bipolar disorder, anxiety and schizophrenia (Smaga et al., 2015). For example, depressed patients display increased markers of oxidative stress in plasma with an associated decrease in total antioxidant capacity (Gałecki, Szemraj, Bieńkiewicz, Florkowski, & Gałecka, 2009) (Yumru et al., 2009), and similar findings have been reported in anxiety (Atmaca, Kuloglu, Tezcan, & Ustundag, 2008) and schizophrenia (Akiibinu, Ogundahunsi, & Ogunyemi, 2012; Dietrich‐Muszalska, Olas, Głowacki, & Bald, 2009). The result of this oxidative stress is cellular damage, with consequent impacts on cell function or even cell death. The presence of markers of oxidative stress across diagnostic groups suggests some common pathophysiological process that might be amenable to universal treatment, an approach that would be particularly helpful in the early stages of mental illness when specific diagnoses have not yet crystalized (McGorry, Hickie, Yung, Pantelis, & Jackson, 2006).
The primary focus of much research in this area has been glutathione (GSH). GSH is the brain's dominant antioxidant, and plays a fundamental role in removing free radicals and other reactive oxygen species (Wood, Yücel, Pantelis, & Berk, 2009). Both reduced (GSH) and oxidized (GSSG) GSH are present in cerebral tissue. Reduced GSH is converted to GSSG either directly or through catalysis by glutathione peroxidase (GPx) (Xin et al., 2016), upon interaction with a radical species. This process is protective for cells and intracellular cellular components against damage. GSSG is subsequently reduced back to GSH, a reaction catalysed by glutathione reductase (GR), and in this way GSH contributes to regulation and maintenance of cellular redox status (Lushchak, 2012). The ratio of the redox active couple (GSH‐GSSG) can be used directly to measure oxidative stress, but precise quantification of this ratio is challenging, even in blood measures, so that GPx and GR assessment are frequently used instead (Xin et al., 2016). In post‐mortem studies of the prefrontal cortex (PFC) of patients and non‐psychiatric controls, GSH, GPx and GR alterations were associated with bipolar disorder, major depressive disorder and schizophrenia (Gawryluk, Wang, Andreazza, Shao, Yatham et al., 2011; Gawryluk, Wang, Andreazza, Shao, & Young, 2011). While direct measurement of these metabolites can be conducted in brain post‐mortem, in vivo quantification generally remains extremely challenging; however, GSH concentration in the brain can be assessed using magnetic resonance spectroscopy (MRS).
This review therefore aims to integrate the results of published studies employing MRS measurement of GSH in the brains of those presenting with mental illness, particularly young people. Two main questions will be addressed in this review:
Is perturbed GSH reliably associated with indicators of mental illness in young people, such as diagnosis, symptoms or functioning?
What are the strengths and weaknesses of the various approaches to the in vivo quantification of GSH concentration using MRS?
These two questions are of importance to ongoing and planned investigations regarding aetiology and prognosis in youth mental health. Simultaneously, the optimal GSH measurement protocols that utilize MRS are paramount. In order to address these questions, a systematic review was conducted selecting those publications that contained human in vivo neurological MRS studies in which cerebral GSH concentration was a target metabolite considered as a possible marker of mental health conditions.
2. METHODS
This systematic review followed PRISMA guidelines (Moher et al., 2009) (Figure 1). However, due to the recent and emerging nature of the outcome measures, no quantitative meta‐analysis was conducted on the resulting data.
Figure 1.
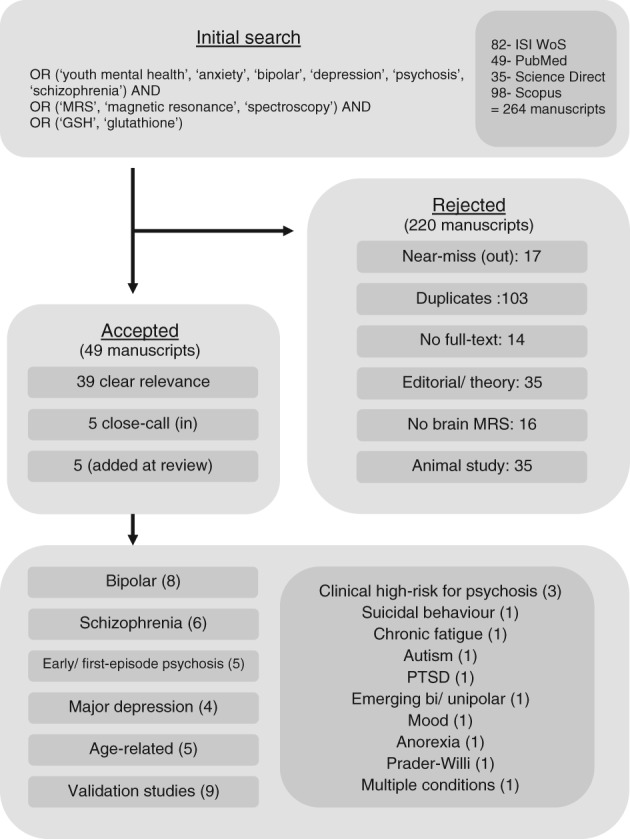
Flow diagram representing the database search with relevant accepted manuscripts considered in this investigation. PTSD, post traumatic stress disorder
2.1. Databases and search terms
The review aims to provide information that will inform the design and parameters of an investigation into the role of GSH in the quantification of neurological markers associated with conditions relating to youth mental health. Such a study would require that optimal parameters to assess GSH concentration in small regions of interest within the brain, using standard, routine, clinical infrastructure currently available. Studies considered within the present review were limited to those published after 2000 in order to restrict results to technically relevant procedures.
Four databases were identified for search, three of which provided general coverage (Web of Science, ScienceDirect and Scopus) and one of which was medically focussed (PubMed). Records were requested from each database (as of July 16, 2018) using the same search terminology and using a breadth‐first approach to ensure sufficient coverage. Three sub‐strings were required, each arising from a range of alternatives: the population of interest (“youth mental health” OR “anxiety” OR “bipolar” OR “depression” OR “psychosis” OR “schizophrenia”), the measurement technique (“MRS” OR “magnetic resonance” OR “spectroscopy”) and the comparative measure, in this case a target metabolite (“GSH” OR “Glutathione”), were required (AND) in the title or abstract (where possible) of the publications returned.
The database search returned 264 studies in total (WoS:82, Sd:35, Sc:98, PM:49) 103 of which were identified as duplicate studies (ie, the same paper was present within the studies returned from two or more data bases). The remaining studies were assessed for relevance. Inclusion criteria were reasonably broad, requiring human study‐participants undergoing in vivo MRS of brain GSH with quantitative outcomes and accessible full‐text material. From the considered studies, 108 were rejected as they did not meet these criteria, while nine were not assessed (as they were not appropriate for the analysis) leaving 49 studies of relevance to this review.
3. RESULTS
Relevant features (deemed important variables for the review) extracted from studies are shown in Table 1.
Table 1.
In vivo MRS of GSH for mental health: summary of findings
| Condition | Brain region | MR‐Sq | Study and main finding | #Part. | d min | Other GSH‐specific correlations |
|---|---|---|---|---|---|---|
| BD | ACC | 3 T PRESS | (Lagopoulos et al., 2013)
|
53 (BD) 51 (HC) |
0.6 |
GSH vs YMRS: (ρ = −0.198; P = .214; N = 41) GSH vs HDRS: (ρ = 0.127; P = .385; N = 49) GSH vs age of onset: (ρ = −0.09; P = .522; N = 53) |
| GSH vs duration (ρ = −0.125; P = .374; N = 53). | ||||||
| 3 T PRESS | (Chitty, Lagopoulos, et al., 2013)
|
33 (BD) 17 (HC) |
0.9 | High alcohol use disorders identification test score negatively correlated with GSH in BD subjects (r = −0.478, P = .005). | ||
| 3 T PRESS | (Chitty, Lagopoulos, Hickie, & Hermens, 2015a, 2015b)
|
30 (BD) | 0.5 | GSH vs alcohol frequency: r = −0.381, P < .05 GSH vs smoking frequency: r = −0.367, P < .05 | ||
| 3 T J‐PRESS | (Soeiro‐de‐Souza et al., 2016)
|
50 (BD) 38 (HC) |
0.6 |
Lac vs GSH Patients: (B = 0.20, t = 3.2, P = .003 [0.07, 0.33]) Controls:(B = 0.17, t = 0.64, P = .11 [0.04, 0.39]) |
||
| ACC + Hip | 3 T PRESS | (Chitty, Lagopoulos, Hickie, & Hermens, 2014)
|
64 (BD) 49 (HC) |
0.5 | GSHHip vs risky drinking (BD): (r = 0.489, P < .021) GSHACC vs smoking (BD): (t(53) = 4.162, P < .001) | |
| l‐Hip | 3 T PRESS | (Chitty et al., 2015b)
|
28 (BD) 22 (HC) |
0.8 | GSH vs left‐MMN (r = 0.068, P = .74, 95% [−0.36, 0.69]) | |
| GSH vs right‐MMN (r = −0.057, P = .78, 95% [−0.52, 0.73]). | ||||||
| OCC + mPFC | 3 T SPECIAL | (Godlewska, Yip, Near, Goodwin, & Cowen, 2014)
|
13 (BD)11 (HC) |
1.2 | ||
| ACC + OCC | 7 T STEAM | (Masaki et al., 2016) After treatment:
|
20 (HC) | 0.6 | ||
| Schiz. (SZ) | ACC | 4 T STEAM | (Terpstra et al., 2005)
|
13 (SZ)9 (HC) | 1.3 |
GSHpat = 1.6土0.2 GSHcont. = 1.5土0.3 |
| MEGA‐ PRESS | ||||||
| 7 T STEAM | (Brandt et al., 2016)
|
24 (SZ) 24 (HC) |
0.8 |
GSH not correlated with age Overall no GSH difference between patients and controls. |
||
| ACC + LI + VC | 7 T STEAM | (Kumar et al., 2018)
|
28 (SCH) 45 (HC) |
0.7 |
GSH and glutamine correlated in all three voxels GSH vs ACC: r = 0.56 GSH vs LI: r = 0.80 GSH vs VC: r = 0.65 |
|
| mPFC | 1.5 T PRESS | (Do, Trabesinger et al.)
|
14 (SZ) 14 (HC) |
1.1 | ||
| pMFC | 3 T MEGA‐ PRESS | (Matsuzawa et al., 2008)
|
20 (SZ) 16 (HC) |
1.0 | For patients GSH correlated to negative symptoms SANS and BPRS (r = −0.68, P < .001) and related to trail making test A (P < .05). | |
| Imag. | 4 T proton echo planar spectroscopic imaging | (Bustillo et al., 2011)
|
30 (SZ) 28 (HC) |
0.8 | ||
| Major Depression (MD) |
OCC, bilat. |
3 T MEGA‐ PRESS | (Lapidus et al., 2014)
|
11 (MD) 10 (HC) |
1.3 |
MDD sample in isolation showed associations between anhedonia and GSH: (r = −0.53, P = .09). No associations between fatigue severity and GSH |
| OCC | 3 T SPECIAL | (Godlewska, Near, & Cowen, 2015)
|
39 (MD) 31 (HC) |
0.7 | ||
| 3 T PRESS | (Freed et al., 2017)
|
19 (MD) 8 (HC) |
1.3 | No correlation between GSH and anhedonia, MD severity, or onset | ||
| Imag. | 3 T MRSI | (Li et al., 2016)
|
16 (MD) 10 (HC) |
1.2 |
GSH/tCrpat. = 0.23士0.06 GSH/tCrcont. = 0.28士0.05 |
|
| Early Psych. (FEP/EP) | Temp | 3 T PRESS | (Berger et al., 2008)
|
24 (FEP) | 0.6 |
PANSS negative symptom change negatively correlated with GSH (r = −0.57, P = .041). Percent change in GSH and Glutamate/Glutamine correlated: (r = 0.64, P = .01) |
| 3 T PRESS | (Wood et al., 2009)
|
30(FEP) 18(HC) |
0.9 | Patients not responding to topical niacin show 35% higher GSH than responders (F 1,28 = 5.1, P = .007). | ||
| mPFC | 3 T SPECIAL | (Monin et al., 2015)
|
30 (EP) 40 (HC) |
0.7 |
Controls: GSH correlated to general FA (r = 0.34, P = .03) and functional connectivity (r = 0.40, P = .01). Patients controlled for medication and duration: GSH correlated to general FA (0.31, P = .01). |
|
| 3 T SPECIAL | (Xin et al., 2016)
|
25 (EP) 33 (HC) |
0.8 | GSHmPFC correlated to GSHblood in controls (P = .021) but not in patients (P = .39). | ||
| CHR for psychosis | mPFC | 3 T PRESS | (Hafizi et al., 2018)
|
27 (CHR) 21 (HC) |
0.9 | mPFC GSH and [18F]FEPPA VT (radioligand of TSPO) not sig. Different between groups. |
| 3 T PRESS | (Da Silva, Hafizi et al. 2018a)
|
30 (CHR) 27 (HC) |
0.8 |
No sig correlations between cerebral GSH and clinical and neuropsychological measures No sig difference between GPx activity and CHR vs HC (F = 0.15, P = .70) |
||
| Significant effect lifetime cannabis use in GPx activity (F = 7.41, P = .01) | ||||||
| 3 T PRESS | (Da Silva et al., 2018)
|
27 (CHR) 16 (HC) |
0.9 | No differences between microglial activation and GSH between groups | ||
|
ACC + Striat. |
3 T PRESS | (Demro et al., 2017)
|
12 (CHR) | 0.7 |
GSH correlation with SIPS: P1: r ACC = −0.578 (0.062), r STR = −0.566 (0.088) P2: r ACC = −0.074 (0.828), r STR = −0.474 (0.167) P3 r ACC = −0.673 (0.023), r STR = −0.775 (0.009) P4: r ACC = −0.259 (0.441), r STR = −0.409 (0.241) P5: r ACC = 0.645 (0.032), r STR = 0.138 (0.704) Positive symptom sum: r ACC = −0.134 (0.695), r ACC = −0.550 (0.099) |
|
| Age‐related (AD, Deprs‐ at.risk, sleep‐ apnea, and MCI) | Th | 3 T PRESS | (Duffy et al., 2015)
|
51 (DEP) (28 treat+ 23 plac.) |
0.8 | Increased GSHTh associated with worsening symptoms (r = 0.43, P = .043) |
| ACC | 3 T PRESS | (Duffy et al., 2015)
|
58 (DEP) 12 (HC) |
0.9 |
Depressed patients showed a correlation between HADS symptoms and GSH/Cr (r = 0.28, P = .035). Depressed patients showed a negative correlation between verbal learning and GSH/Cr (r = −0.28, P = .04) |
|
| 3 T PRESS | (Duffy et al., 2016)
|
24 (ARD) | 0.6 | GSHACC vs Oxygen desat: r = −0.54, P = .007 GSHACC vs apnea‐hypopnea: r = .42, P = .050 GSHACC vs response inhib: r = −.49, P = .015. GSHACC vs set shifting: r = −0.43, P = .37. | ||
| ACC + PCC | 3 T PRESS | (Duffy et al., 2014)
|
54 (MCI) 41 (HC) |
0.6 | MCI GSHACC: 0.47 土 0.15 MCI GSHPCC: 0.37土 0.07 | |
| Control GSHACC: 0.41土 0.10 | ||||||
| Control GSHPCC: 0.29土 0.05 | ||||||
| Vari. | 3 T MEGA‐ PRESS | (Mandal, Tripathi, & Sugunan, 2012)
|
25 (ym) 20 (yf) 9 (om) 6 (of) |
~1.3 |
GSHLFC different from GSHRFC in young female (P = .02) and male (P = .001) subjects. GSHLFC vs GSHRFC: young females (r = 0.641, P = .004) |
|
(P = .05) compared to young controls.
|
7 (fAD) |
GSHLPC vs GSHRpC: young females (r = 0.797, P = .000) GSHLFC vs GSHLPC: young males (r = 0.481, P = .032) (Healthy young males/females (ym/yf); healthy older males/females (om/of); males/females with mild cognitive impairment and Alzheimer's disease.) |
||||
| Mult. | ACC | 3 T PRESS |
(Hermens, Lagopoulos, Naismith, Tobias‐Webb, & Hickie, 2012) Clustering of patients based on metabolites: 3 subgroups. GSH responsible for cluster 2. |
37 (DD) 29 (BP) 22 (PD) 25 (HC) |
N/A | Discriminant function 2 (40% variance) characterized by GSH/Cr (r = −0.753). |
| Suicidal behaviour | dPFC | 3 T SPECIAL | (Jollant, Near, Turecki, & Richard‐Devantoy, 2016)
|
15 (SA) 10 (PC) 33 (HC) |
~1.0 | GSHdPFC Suicide Attempters: 0.24土0.03 GSHdPFC Patient Controls: 0.23 土 0.02 GSHdPFC Healthy Controls: 0.23土0.03 |
| Chronic fatigue |
OCC + Imag. |
3 T MEGA‐ PRESS/ MRSI | (Shungu et al., 2012)
|
15 (CFS) 15 (MD) 13 (HC) |
1.0 | GSH was inversely correlated with ventricular lactate (r = −0.545, P = .001) and a range of key indices of physical health. |
| Autism |
Basal ganglia + dPFC |
3 T PRESS | (Durieux et al., 2016)
|
21 (ASD) 29 (HC) |
0.8 | Correlation between GSH and Autism spectrum disorder was observed in either region. |
| PTSD |
ACC + dLPFC |
3 T MEGA‐ PRESS | (Michels et al., 2014)
|
12 (PTS) 17 (HC) |
1.1 |
GSHACC: PTSD = 0.15 ± 0.03, Non = 0.11 ± 0.03 (d = 1.33) GSHdLPFC: = 0.14 ± 0.03, Non = 0.11 ± 0.03 (d = 1.00) |
| Emerging Unipolar/Bi polar | ACC | 3 T PRESS | (Naismith et al., 2014)
|
53 (EBD) | 0.4 | GSH not associated with sleep midpoint (r = 0.211, P = .151) |
| Mood |
ACC + HIPP |
3 T PRESS | (Hermens et al., 2018)
|
94 (DEP) 76 (BD) 59 (HC) |
0.2 |
Decreased white matter integrity was associated with decreased GSHHIPP. |
| Anorexia Nervosa |
ACC + OC + PUT |
7 T STEAM | (Godlewska et al., 2017)
|
13 (AN) 12 (HC) |
1.2 |
AN (SEM) HC (SEM) p‐value GSHACC 1.19 (0.07) 1.27 (0.10) 0.38 CRLB 10.2 (2.9) 8.9 (2.8) GSHOCC 0.95 (0.03) 0.94 (0.04) 0.85 CRLB 10.5 (3.0) 10.67 (3.08) GSHPUT 1.51 (0.45) 1.10 (0.05) 0.43 CRLB 15.0 (4.5) 15.4 (4.6) |
| Prader‐Willi Synd. |
ACC + P‐OCC |
‐ MEGA‐ PRESS | (Rice, Lagopoulos, Brammer, & Einfeld, 2016)
|
15(PWS) 15 (HC) |
1.0 | |
| .Validation studies | mPFC | 7 T PRESS | (Choi et al., 2010) | Optimized PRESS with sub‐TE pair showed improved selectability of coupled metabolites (eg, Glu, Gln, GSH). | ||
|
mPFC + rPFC |
7 T J‐PRESS | (An et al., 2015) | TE‐optimized J‐PRESS was shown to minimize NAA signals while retaining GSH peak resolution. | |||
| Hipp |
3 T semi‐LASER |
(Bednařík et al., 2015) | Using a short‐echo sequence with 5 minutes averaging the GSH CRLB was kept below 30% in a 4 mL voxel at 3 T. | |||
| ACC | 7 T PRESS | (Lally et al., 2016) | Intra Class Correlation (ICC) using TE‐optimized PRESS both within sessions (ICC > 0.7) and between sessions (ICC > 0.6) showed good repeatability. GSH negatively associated with age (r = −0.37, P < .05). | |||
|
ACC + PCC |
3 T STEAM | (Wijtenburg et al., 2014) | Short‐TE phase rotation STEAM at 3 T showed excellent reproducibility for GSH: absolute reliability: SEM < 9.9%, relative reliability: ICCs 0.42‐0.51 | |||
| Midline parietal | 3 T HERMES | (Saleh et al., 2016) | HERMES scanning protocol showed excellent separation of GABA and GSH. Results agree with MEGA‐PRESS, achieving similar signal‐to‐noise ratio in half the time. | |||
| mPFC | 7 T PRESS | (Choi et al., 2010) | Optimized PRESS sequence showed lower CRLBs of Gln and GSH than with STEAM. | |||
| 3 T SPECIAL | (Schubert, Kühn, Gallinat, Mekle, & Ittermann, 2017) | Short‐TE SPECIAL sequence was used to measure MRS spectra at 3 T in 21 healthy adults. GSH was detected with low uncertainty (CRLB < 30%) in only 16 cases. | ||||
|
3 T J‐PRESS |
(Jensen, Auerbach, Pisoni, & Pizzagalli, 2017) |
Test–retest reliability of metabolite quantification was assessed in a 3 T shortened J‐resolved MRS sequence in healthy adolecents. GSH demonstrated satisfactory reliability with a score of 8.8–4.1%. |
Abbreviations: ACC, anterior cingulate cortex; AD, Alzheimer's disease; AN, anorexia nervosa; ARD, age‐related disorder; ASD, autism spectrum disorder;BD, bipolar disorder; BP, bipolar disorder; BPRS, brief psychiatric rating scale; CAT, catalase; CFS, chronic fatigue syndrome; CRLB, cramer‐rao lower bound; CHR, clinical high risk; CRLB, Cramer Rao lower bound; DD, depressive disorder; DEP, depression; dLPFC, dorsal left prefrontal cortex; dPFC, dorsolateral prefrontal cortex; EBD, emerging bipolar disorder; EP, early psychosis; EPI, echo‐planar imaging; FA, fractional anisotropy; fAD, females with Alzheimer's disease; FEPPA, tracer; fMCI, females with mild cognitive impairment; FSL, FMRIB software library; GSH, glutathione; GCL, glutamate cysteine ligase; GABA, gamma‐aminobutyric acid; GSSG, glutathione disulphide; HADS, hospital anxiety and depression scale; HC, healthy control; HDRS, hamilton depression rating scale; HERMES, Hadamard encoding and reconstruction of mega‐edited spectroscopy; HIPP, hippocampus; ICC, inferior colliculus; J‐PRESS, J‐resolved spectroscopy; LFC, left frontal cortex; LI, left insular; mAD, males with Alzheimer's disease; MCI, mild cognitive impairment; MD, major depression; MDA, malondialdehyde; MDD, major depressive disorder; mMCI, males with mild cognitive impairment; MMN, mismatch negativity; MRS, magnetic resonance spectroscopy; MRSI, magnetic resonance spectroscopy imaging; mPFC, medial prefrontal cortex; NAA, n‐acetyl aspartate; OCC, occipital cortex; OCD, obsessive compulsive disorder; PANSS, positive and negative symptom scale; PC, patient controls; PD, psychotic disorder; PFC, prefrontal cortex; pMFC, posterior medial frontal cortex; PTS, post traumatic stress; PTSD, post traumatic stress disorder; PRESS, point‐resolved spectroscopy; PUT, putamen; PWS, prader‐willi syndrome; RFC, right frontal cortex; SA, suicide attempters; SANS, scale for assessment of negative symptoms; SOD, superoxide dismutase; SEM, standard error of the mean; SIPS, structured interview for psychosis‐risk syndromes; STEAM, stimulated echo acquisition model; SZ, schizophrenia; TBARS, thiobarbituric acid; TE, echo time; THC, tetrahydrocannabinol; TM, mixing time; TSPO, translocator protein; VC, visual cortex; VT, total distribution volume; YMRS, young mania rating scale.
Summary of findings, describing the clinical group, brain region of interest, magnetic resonance sequence used, study reference and main findings, number of control vs clinical participants, dmin (the minimum effect size that could be founds significant given the cohorts in each study), and other GSH‐specific correlations that are not directly related to the outcomes of the paper, but important nonetheless.
Those studies that included quantitative comparisons (either tests for significant differences or regression analysis) were rated according to the minimum effect size required to achieve a power of 0.8, given a significance value of less than 0.05. Using a simplified simulation,1 multiple realizations of participants were drawn from either:
Two unit‐variance normal distributions with mean separation dμ = μ1 − μ1, or
A two‐element, zero‐mean, multivariate normal with covariance .
The effect size (dμ) or covariance (dσ) was then increased from zero until at least 80% of 1 × 104 trials returned a result with a two‐tailed significance of less than 0.05. Results are shown in Table 1 as d min (where dσ is only shown for single‐group studies).
3.1. Non‐quantitative outcomes
3.1.1. GSH and cerebral GSH Concentration
Given that GSH is the major free radical scavenger within the brain, that the GSH redox couple have been associated with psychiatric disorders, and that GSH may be directly measured using sophisticated MRS sequences in vivo within patient brains, a number of studies have been performed that probe this connection. However, due to the fact that there is considerable heterogeneity in methods for MRS data acquisition, most studies differ in voxel size and placement, parameters and post‐processing, and also the quantitative measures used to assess study outcomes. Therefore, it is not feasible to perform quantitative analysis on acquired results. However, there are a number of conclusions that can be drawn from the data by summarizing the main findings of the studies conducted in this domain. Here attention is divided between discussion of the evidence regarding the role of GSH in the context of mental illness and the utility of MRS in the measurement of this metabolite in vivo.
3.1.2. Findings of in vivo GSH and mental illness
The majority of the studies (37) were aimed at determining if GSH concentration significantly differed between healthy cohorts and those with diagnosed mental disorders, or if there were specific alterations by diagnostic sub‐group. A second group of studies (7) were targeted at novel approaches to spectral measurement and quantification yet used in vivo measurements of GSH concentration both as a test‐bed for novel techniques as well as a means of confirmation of earlier findings. Eighteen of the studies concentrated (at least in part) on the anterior cingulate cortex (ACC), while the remainder contained a diverse range of regions of interest from the medial (m), dorsal (d) and posterior (p) PFC (11 studies), hippocampus (Hip: three), occipital cortex (OCC: four), temporal lobes (Temp: two) and one study each from the basal ganglia (BG), the thalamus (Th), the midline parietal, the precuneus (Pre), the left insular LI) and the visual cortex (VI) with additional studies that performed spectroscopy over an array of voxels, or chemical shift imaging (CSI). With the exception of CSI, regions were studied using a single large voxel (of varying size, but generally around 2 × 2 × 2 cm3). Voxels were placed and oriented by a researcher, guided by a scout scan over the region of interest, and multiple regions were studied with separate voxels acquired sequentially.
Any patterns of GSH perturbation are difficult to identify, since there are only a few relevant studies that met our criteria for this review.
Overall, evidence of altered GSH concentration was inconsistent. While there were some indications of a decrease in GSH for major depression, treatments did not alter GSH levels—even when those treatments were associated with decreased symptoms. However, these changes are consistent with the changes seen in blood GSH levels (Maes, Galecki, Chang, & Berk, 2011), providing some support for a true alteration of GSH systemically. While generally there was some evidence that GSH levels are perturbed in first‐episode psychosis, there was no consistency in the direction of change, and no changes were observed in either chronic schizophrenia patients or individuals at clinical high risk for psychosis.
None of the studies of BD included in this review show a change in brain GSH compared with controls. This may reflect a medication effect, since lithium (a commonly prescribed mood‐stabilizer) has antioxidant properties and most notably can increase GSH in rat cerebral cells (Cui, Shao, Young, & Wang, 2007). Another interesting point to consider is the difference between brain GSH and blood GSH in BD. Studies present conflicting blood and brain GSH data, suggesting that blood GSH levels are abnormal or perturbed (Gu, Chauhan, & Chauhan, 2015). This may represent a compensatory response of GSH production in the brain to balance the depleted peripheral GSH pool, or may suggest that the two systems are locally regulated via the GSH‐GSSG cycle.
There were some correlational findings between GSH concentration and clinical variables. For example, in early psychosis, GSH concentration in the posterior medial frontal cortex was negatively correlated with negative symptoms of schizophrenia (Berger et al., 2008; Demro et al., 2017). Negative symptoms are typically more difficult to treat with traditional anti‐psychotic medication, and this relationship suggests that an intervention to increase GSH may be a promising alternative treatment. Similarly, in MD, there was a negative relationship between GSH and anhedonia. While there were relationships in bipolar disorder between GSH and risky drinking (based on the alcohol use disorders identification test; Chitty, Kaur, Lagopoulos, Hickie, & Hermens, 2014; Chitty, Lagopoulos, , Hickie, & Hermens, 2014; Saunders et al., 1993) or smoking. Finally, as a function of age, a positive relationship was observed between GSH and AD, MCI and cognitive decline, yet these observations may simply be due to other age‐related effects.
3.1.3. Heterogeneity of studies and the strength of a brain GSH measurement
The complex nature of psychiatric illness, as well as the difficulty in GSH measurement, leaves a heterogeneous evidence base that makes comparison challenging. Contradictions between studies assessing brain GSH make it difficult to compare brain and blood GSH, where studies assessing blood GSH present consistent findings in perturbation in GSH metabolism for first‐episode psychosis (Fraguas, Díaz‐Caneja, Rodríguez‐Quiroga, & Arango, 2017), schizophrenia (Ng, Berk, Dean, & Bush, 2008), bipolar disorder and autism (Gu et al., 2015).
Medication exposure
Medication status is important to consider as a confounder when assessing oxidative stress and GSH antioxidant action within youth mental health. In bipolar disorder, lithium and valproate (commonly prescribed mood stabilizing drugs) results in a dose‐dependent GSH increase in rat cortical cells. After 1‐week chronic treatment, the cells demonstrated reduced oxidative stress, including increased GSH and GCL expression (upstream GSH synthesis; Cui et al., 2007). This presents a new issue, here GSH is elevated as a result of drug administration, rather than as a result of illness. It is possible the cessation of the medication would result in a return to the baseline levels of GSH. Another study looking at first‐episode mania in bipolar disorder (Machado‐Vieira et al., 2007) assessed the effects of lithium treatment vs no‐treatment. Antioxidants SOD and CAT were elevated in unmedicated patients, indicative of generation of reactive species intrinsic to the illness. Lithium reduced markers of oxidative stress (TBARS‐lipid damage), as well as the SOD/CAT ratio. There is a small body of evidence associating anti‐psychotic and mood‐stabilizing medication with protective effects such as increased antioxidant enzyme activity or expression, and increased GSH (Cui et al., 2007; Wang, Xu, Dyck, & Li, 2005). This may have a profound effect on studies in early psychosis, since not all studies control for drug‐naïve vs medicated patients. The use of a first generation dopamine antagonist like haloperidol may contribute to increased oxidative injury (Lohr, Kuczenski, Bracha, Moir, & Jeste, 1990). In patients with chronic schizophrenia treated with haloperidol, increased lipid peroxidation markers were observed (TBARS), as well as the antioxidant enzyme SOD, presumably in response to the peroxidative injury to membrane phospholipids (Gama, Salvador, Andreazza, Kapczinski, & Silva Belmonte‐de‐Abreu, 2006). There are some second‐generation dopamine antagonists that demonstrate protective properties. Olanzapine, clozapine, quetiapine and risperidone all play a role in upregulating SOD1 gene expression (Bai et al., 2002), indicative of an antioxidant response against radical species. The most common treatment for depression, selective‐serotonin reuptake inhibitor prescription, has resulted in significant reductions in lipid peroxidation as a marker of oxidative stress (Khanzode, Dakhale, Khanzode, Saoji, & Palasodkar, 2003). This study found that treatment with fluoxetine and citalopram decreased serum SOD (antioxidant) and MDA (lipid peroxidation marker).
Voxel placement
Because there are few studies analysing GSH levels in mental illness using MRS, there appears to be no “gold standard” voxel location. There is rationale for the use of the medial frontal cortex due to association of this region with schizophrenia (Pomarol‐Clotet et al., 2010). It has been argued that the medial temporal lobe is a more appropriate location for spectroscopy since there are reported links with schizophrenia, as well as the regions vulnerability to insult, particularly in the context of oxidative stress (Wood, Yücel, et al., 2009). The concentration of GSH in different regions of the brain varies significantly. The studies that have considered brain GSH have focused on the cortex, which represents the greatest concentration of brain GSH, with the cerebellum, hippocampus and striatum following in descending order of GSH concentration (Kang et al., 1999). The location of GSH in the brain is highly tissue specific (Rae & Williams, 2017), with a report of 30% higher GSH in cortical white matter (WM) compared with grey matter dominated PFC (An et al., 2015). Grey matter (GM) demonstrates increased metabolite concentrations (Srinivasan, Ratiney, Hammond‐Rosenbluth, Pelletier, & Nelson, 2010), and this tissue also consumes oxygen in a 4:1 ratio to WM, despite only comprising 40% of the total brain volume (Mintun et al., 2001), rendering the GM more susceptible to oxidative insult. When comparing the frontal cortex of young healthy participants, there was a significantly greater concentration of GSH in females compared with males (Mandal et al., 2012), which could provide a confounding factor when comparing both genders in youth mental health. These results indicate a significant gender bias towards GSH concentration in the brain, which may be important considering the greater incidence of schizophrenia and first‐episode psychosis (Ochoa, Usall, Cobo, Labad, & Kulkarni, 2012) in males.
Tobacco and cannabis
The prevalence of smoking in people with mental illness far outweighs that in the general population. It is estimated in the United Kingdom that 16% of the general population smoke, whereas in psychosis incidence is 56%, major depression 40%, anxiety 37% and OCD 40% (Szatkowski & McNeill, 2013). Exogenous administration of nicotine to isolated cell lines in vivo reduces antioxidant constituents (Yildiz, Liu, Ercal, & Armstrong, 1999), including GSH. Within a chronic schizophrenia population, tobacco smoke induces the oxidation of lipids and proteins (Yao, Leonard, & Reddy, 2006). In bipolar disorder, GSH concentration is reduced in the ACC of smokers, but not the non‐smoking patients (Chitty, Lagopoulos, et al., 2014), and in a longitudinal study assessing tobacco consumption, a reduction in smoking was a significant predictor of increased GSH (Chitty, Lagopoulos, Hickie, & Hermens, 2015a, 2015b).
In marijuana research, it has been reported that brief exposure of an endothelial cell line to marijuana (3.95%) stimulated increased oxidative stress by 80%, as well as an 81% reduction in GSH concentration (Sarafian, Magallanes, Shau, Tashkin, & Roth, 1999). In the same study, exposure to smoke containing no THC (psychoactive component of cannabis) resulted in no change in oxidative species, but a decline in GSH of 70%. This is interesting since, as with tobacco, marijuana use in mental illness is far greater than in the general population (6.6% (UNDOC, World Drug Report, 2011) compared with 23% in psychosis (Green, Young, & Kavanagh, 2005), 9.5% in major depression (Chen, Wagner, & Anthony, 2002), 19% in bipolar disorder (Marken et al., 1992) and 17% in anxiety (Degenhardt, Hall, & Lynskey, 2001)).
3.2. Strength of a brain GSH measure over periphery
A number of studies have tried to draw comparisons between GSH in the brain and peripheral measures such as blood components or cerebrospinal fluid (CSF). Due to the variable nature of study design in the tissue assessed and the brain region of interest, the concentration of GSH across differing compartments of the human body is not well characterized (Richie, Skowronski, Abraham, & Leutzinger, 1996; Samuelsson, Vainikka, & Ollinger, 2011; Sanaei Nezhad, Anton, Parkes, Deakin, & Williams, 2017)).
Many studies, when investigating GSH, do not differentiate between reduced, oxidized and total GSH, rendering the measure comparable only within its own intervention, and difficult to use in comparison to other studies. This makes the use of MRS as a tool for GSH quantification useful since the measure is direct, and informative of brain GSH status.
Blood measures of GSH are the most commonly reported methods, since assays are cheaper and more accessible than MRS. Plasma measures of GSH are commonly reported, but report a wide range of concentrations of GSH (0.5‐759 μM) (Nuttall, Martin, Sinclair, & Kendall, 1998; Raffa et al., 2009). Plasma acts as a medium for metabolic waste products in the body, where cells excrete partial protein and lipid components for clearance. In addition, the role of GSH is to provide redox balance within a cellular system. Therefore, it is unlikely that plasma GSH offers a reliable and accurate representation of peripheral GSH, and even more unlikely that it would be a representation of brain GSH.
3.3. MRS methods
MRS provides a means to explore novel chemical environments and analyse known molecular systems. Through the analysis of the free induction decay (FID) spectra of a target nucleus, the local chemical environment may be inferred. While exotic nuclei, such as 31P and 13C, are possible for targeting in clinical environments, 1H is the most widely available (Blüml, 2013). However, accurate quantification of some metabolites is hampered by both the low spatial resolution, due to the low chemical concentrations and the decreased spectral resolution of clinical scanners with lower field strengths. Generally, in vivo MRS is conducted over a single voxel placed within a target brain region (identified using a preliminary scout scan); while imaging protocols that perform spectroscopy over an array of large voxels are possible they are less common.
3.4. GSH measurement in vivo
In vivo quantification of GSH in humans is particularly challenging due to the low concentration (1.5‐3 mmol/L), and the fact that all resonances overlap with stronger signals from alternate metabolites (De Graaf, 2013). However, it is possible to quantitatively isolate the spectral contribution from GSH through both refinement and calibration of standard approaches to MRS measurement as well as the development of novel sequences. Additionally, in order to accurately quantify the contribution of GSH to the measured spectra, a range of post‐processing techniques, from spectral decomposition to partial volume correction, are required. While similarities and trends do exist, studies conducted within the past 10 years vary in voxel placement, measurement sequence, acceptance bounds on signal quality and post‐processing methodologies. Here the predominant approaches are described.
3.5. Acquisition modalities
In order to accurately estimate the concentration of GSH within a volume of interest, a range of different tradeoffs must be considered. The method of spatial localisation utilized is, perhaps, the first consideration, while voxel size and placement, which is affected by field and tissue homogeneity, are of related concern as are the methods used to isolate GSH from other contributors.
3.5.1. Spectroscopy
The FID spectrum exhibits a range of peaks unique to the local electronic environment of contributing chemical systems. In order to quantify low concentration metabolites, the extracted spectra must be of high quality, predominantly assessed through the signal‐to‐noise ratio (SNR) and the spectral linewidth (usually expressed in terms of the full width at half maximum—FWHM). While quantification accuracy is usually dependant on the spectral SNR, small linewidths allow for good separation of distinct peaks. Averaging multiple signals can increase the SNR, yet patient motion means that shorter scan times are preferred (both in terms of field homogeneity and partial volume correction). Approaches to decomposition using basis vectors determined by contributing metabolites can decrease the importance of linewidth (removing any requirement of spectral separation), yet the accuracy to which each contribution can be estimated still relies on this parameter.
A high‐quality shim can be used to provide field (B0) homogeneity over some region of interest, reducing the expected spectral linewidth sufficiently for GSH analysis. However, specific sequences are required in order to localize the induced FID to this region. A number of different approaches exist, each with competing properties in terms of measured spectral quality.
3.5.2. Spatial selectivity
While a range of spatial localisation methods exist, the studies considered here used point ‐resolved spectroscopy (PRESS) or stimulated echo acquisition mode (STEAM), and variants or extensions to these sequences, almost exclusively.2 PRESS and STEAM both use three slice selective pulses (Figure 2):
PRESS: 1 × 90o + 2 × 180o
STEAM: 3 × 90o
Figure 2.
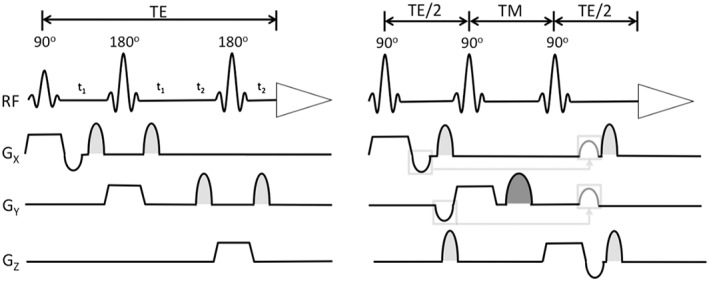
Point‐resolved spectroscopy (right) and stimulated echo acquisition mode (left) sequences, taken from “In Vivo NMR Spectroscopy,” (De Graaf, 2013)
STEAM decreases the specific absorption and because there is no T2 decay in the TM period is preferred for short T2 metabolites, but this approach does sacrifice the SNR of each acquired spectra.
3.5.3. Water suppression and spectral editing
Because of the local environment, water suppression is required in order to resolve the much smaller spectral peaks induced by the target metabolites. Chemical shift selective and variable pulse powers and optimized relaxation delays (VAPOUR) are commonly used for water suppression, while it is also possible to include water into the macromolecular baseline utilized during molecular decomposition.
With an accurate high‐order shim, it is possible to resolve GSH peaks directly. However, should the spectral linewidth be too large (or simply to enhance quantitative accuracy) spectral editing may also be used to extract specific metabolic signals. The MEscher‐GArwood (MEGA) technique uses editing pulses aimed at spins which are J‐coupled with the spin of interest, for instance, an editing pulse is applied at 4.56 ppm which targets a α‐CH resonance J‐coupled to the desired GSH spins at 2.95 ppm (Saleh et al., 2016). Sequences with and without editing are subtracted to produce a final spectrum that contains greatly decreases cross‐talk between the spins of interest and other peaks within the spectra.
3.5.4. Modifications and extensions
The predominant approach to spectral measurement in the studies considered here used highly optimized PRESS and STEAM sequences directly with, in some cases, editing sequences such as MEGA to home in on specific metabolites. However, a range of those studies considered also investigated novel spectroscopy acquisition protocols. Such approaches used relationships involving GSH, tested in vivo, to not only validate and test the novel protocol, but also to validate and explore the metabolic relationships.
Hadamard encoding and reconstruction of mega‐edited spectroscopy: The use of a Hadamard encoding in editing allows the simultaneous acquisition of two different voxels and two different metabolites simultaneously, quartering acquisition time (or doubling SNR).
Phase rotation: Phase cycling is introduced into the radio frequency signal to remove undesired spin echo signals, and has been used with STEAM to isolate the stimulated echo.
2D J‐differences editing: Two dimensionally resolved MRS provides spectra over a range of editing frequencies and as such can be used to perform accurate MRS with no expectation as to the target metabolite.
MRS imaging: While direct repetition of PRESS or STEAM for multiple voxels is possible, imaging (or spatial encoding of the spectroscopic signal) is generally conducted using phase encoded gradient fields.
Proton echo planar spectroscopic imaging (PEPSI): PEPSI uses an approach to echo planar imaging, yet one dimension of echos are encoded with chemical shift information, rather than spatial information as in standard EPI. In studies considered that employ PEPSI large outer volume suppression slabs and advanced approaches to shimming (FAST(EST)MAP) were required.
3.6. Data acquisition post‐processing
Once the spectra have been acquired, extraction of the GSH signal is required. Signal extraction can take a number of directions and may be dependent on the approach taken to measurement. Should sufficient and accurate editing have been conducted, the GSH peak may then be measured directly (often using simple summation over the GSH peak). However, in the case that there is residual overlap between GSH and spectra from other metabolites present in the sample more sophisticated processing is required.
3.6.1. Spectral deconvolution
A standard approach, in the case of overlapping peaks, is linear decomposition of the acquired spectra into metabolic basis functions. Standard software packages (such as LCModel) are available, or in‐house software may also be used for this approach. In all cases, a database of metabolic basis functions must be available that are either carefully measured (with the same system and sequence used for measurement), simulated using advanced modelling tools such as GAMMA, VERSI, FID‐A (or in‐house developed tools) or even a combination of the two where measurements of the macromolecular spectra may be measured and used in concert with simulated spectra of specific metabolites.
Once the basis metabolites are identified, they are used to estimate a linear decomposition of the measured spectra (based on some cost function such as least‐squares). The decomposition provides an estimate of the contribution from each metabolite, while the residual allows for the presence of missing features to be assessed (ie, it should represent noise). Generally, such fits also provide a measure of uncertainty, as a percentage of the Cramer Rao lower bound (CRLB) in the case of LCModel, which can be used to accept or reject the measurement. Where acceptance levels were quoted as CRLB, most commonly >20% uncertainty measurements were rejected, but in some cases rejection was reserved only for >50% uncertainty. While it is not clear as to the impact on final values of the estimated value or its uncertainty, nor rejection levels utilized, using the %CRLB as a rejection threshold has been shown to be unreliable, particularly for low concentration metabolites (Kreis, 2016). Kreis (2016) argued that despite the unreliability of these widely used threshold levels of 20% to 50%, CRLB is a valuable tool to give an idea of minimal uncertainties in MRS, when the obtained error is understood to be an estimate of the lower bound of the fitting error.
Finally, once the metabolite concentration has been identified, the voxel heterogeneity was accounted for (in a similar way for most studies). The MRS voxel (or voxels) used in measurement is segmented based on the original magnetic resonance imaging image used for positioning. The contributing volume fractions from GM, WM and CSF are calculated (often using the FAST4 algorithm from FSL) and used to correct the measured concentration (or understood as covariates with this concentration).
3.6.2. Measurement normalization and post‐processing
Some note should be made regarding the comparison of spectroscopy techniques across the studies considered here. However, this is made difficult not only by the broad range of sequences used but also the range of parameters explored within each sequence. In each study different spectral bandwidths, sampling rates, and number of averages were considered. Each of these parameters will affect the FWHM and SNR of the recovered spectra, and hence the reliability of the spectral decomposition (as will the methods used to generate the basis metabolites). Even the units used in measurement of the metabolic concentration differed. While in many studies the absolute GSH concentration was used as the final measurement, in other studies GSH as a fraction of total Creatine (tCr) or Water peaks were used. Finally, comparison across studies that use different scanners is a potential confound, while the pooling of MRS data is becoming more important in terms of providing a larger sample size to increase confidence in effect size, or to facilitate collaborative work, the risk of systematic error is increased, since there is always variability in participant positioning, partial volume effects and image intensity inhomogeneity (Stonnington et al., 2008).
Such diversity may not impact the overall neuroscientific findings, yet each choice will impact the accuracy of each finding, hindering comparison. No study regarding the impact on quantification of the relevant tradeoffs has been conducted, so that generally in‐house optimisations and conveniences have been used. While useful for immediate application, this approach may hinder a standardized approach for quantitative exploration.
4. SUMMARY
There is evidence of perturbations in the oxidative stress/antioxidant response system across youth metal health, with emerging evidence implicating GSH in the eitiology of mental illness. However, the field is hampered by highly variable methodology, from basic sequence and voxel selection to analysis approach and choice of measurement units. This is before problems surrounding diagnostic heterogeneity are considered and the simple issue of statistical power. If this area of study is to progress, studies with larger participant numbers are required, most probably involving multiple collaborating sites where very close attention is paid to minimizing between‐site variability in both clinical and spectroscopic data collection.
Fisher E, Gillam J, Upthegrove R, Aldred S, Wood SJ. Role of magnetic resonance spectroscopy in cerebral glutathione quantification for youth mental health: A systematic review. Early Intervention in Psychiatry. 2020;14:147–162. 10.1111/eip.12833
ENDNOTES
Exceptions include spin‐echo full‐intensity acquired localized spectroscopy (SPECIAL) and imaging studies.
REFERENCES
- Akiibinu, M. O. , Ogundahunsi, O. A. , & Ogunyemi, E. O. (2012). Inter‐relationship of plasma markers of oxidative stress and thyroid hormones in schizophrenics. BMC Research Notes, 5, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, L. , Li, S. , Murdoch, J. B. , Araneta, M. F. , Johnson, C. , & Shen, J. (2015). Detection of glutamate, glutamine, and glutathione by radiofrequency suppression and echo time optimization at 7 tesla. Magnetic Resonance in Medicine, 73(2), 451–458. [DOI] [PubMed] [Google Scholar]
- Atmaca, M. , Kuloglu, M. , Tezcan, E. , & Ustundag, B. (2008). Antioxidant enzyme and malondialdehyde levels in patients with social phobia. Psychiatry Research, 159(1), 95–100. [DOI] [PubMed] [Google Scholar]
- Bai, O. , Wei, Z. , Lu, W. , Bowen, R. , Keegan, D. , & Li, X. M. (2002). Protective effects of atypical antipsychotic drugs on PC12 cells after serum withdrawal. Journal of Neuroscience Research, 69(2), 278–283. [DOI] [PubMed] [Google Scholar]
- Bednařík, P. , Moheet, A. , Deelchand, D. K. , Emir, U. E. , Eberly, L. E. , Bareš, M. , … Öz, G. (2015). Feasibility and reproducibility of neurochemical profile quantification in the human hippocampus at 3 T. NMR in Biomedicine, 28(6), 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, G. E. , Wood, S. J. , Wellard, R. M. , Proffitt, T. M. , McConchie, M. , Amminger, G. P. , … McGorry, P. D. (2008). Ethyl‐eicosapentaenoic acid in first‐episode psychosis. A 1H‐MRS study. Neuropsychopharmacology, 33(10), 2467–2473. [DOI] [PubMed] [Google Scholar]
- Betteridge, D. J. (2000). What is oxidative stress? Metabolism, 49(2 Suppl 1), 3–8. [DOI] [PubMed] [Google Scholar]
- Blüml, S. (2013). Magnetic resonance spectroscopy: Basics In Blüml S, Panigrahy A, eds MR spectroscopy of pediatric brain disorders (pp. 11–23). New York, NY: Springer. [Google Scholar]
- Brandt, A. S. , Unschuld, P. G. , Pradhan, S. , Lim, I. A. , Churchill, G. , Harris, A. D. , … Margolis, R. L. (2016). Age‐related changes in anterior cingulate cortex glutamate in schizophrenia: A (1)H MRS Study at 7 Tesla. Schizophrenia Research, 172(1–3), 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo, J. R. , Chen, H. , Gasparovic, C. , Mullins, P. , Caprihan, A. , Qualls, C. , … Posse, S. (2011). Glutamate as a marker of cognitive function in schizophrenia: A proton spectroscopic imaging study at 4 Tesla. Biological Psychiatry, 69(1), 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. Y. , Wagner, F. A. , & Anthony, J. C. (2002). Marijuana use and the risk of major depressive episode. Epidemiological evidence from the United States National Comorbidity Survey. Social Psychiatry and Psychiatric Epidemiology, 37(5), 199–206. [DOI] [PubMed] [Google Scholar]
- Chitty, K. M. , Kaur, M. , Lagopoulos, J. , Hickie, I. B. , & Hermens, D. F. (2014). Risky alcohol use predicts temporal mismatch negativity impairments in young people with bipolar disorder. Biological Psychology, 99, 60–68. [DOI] [PubMed] [Google Scholar]
- Chitty, K. M. , Lagopoulos, J. , Hickie, I. B. , & Hermens, D. F. (2013). Risky alcohol use in young persons with emerging bipolar disorder is associated with increased oxidative stress. Journal of Affective Disorders, 150(3), 1238–1241. [DOI] [PubMed] [Google Scholar]
- Chitty, K. M. , Lagopoulos, J. , Hickie, I. B. , & Hermens, D. F. (2014). The impact of alcohol and tobacco use on in vivo glutathione in youth with bipolar disorder: An exploratory study. Journal of Psychiatric Research, 55, 59–67. [DOI] [PubMed] [Google Scholar]
- Chitty, K. M. , Lagopoulos, J. , Hickie, I. B. , & Hermens, D. F. (2015a). A longitudinal proton magnetic resonance spectroscopy study investigating oxidative stress as a result of alcohol and tobacco use in youth with bipolar disorder. Journal of Affective Disorders, 175, 481–487. [DOI] [PubMed] [Google Scholar]
- Chitty, K. M. , Lagopoulos, J. , Hickie, I. B. , & Hermens, D. F. (2015b). Investigating the role of glutathione in mismatch negativity: An insight into NMDA receptor disturbances in bipolar disorder. Clinical Neurophysiology, 126, 1178–1184. [DOI] [PubMed] [Google Scholar]
- Choi, C. , Dimitrov, I. E. , Douglas, D. , Patel, A. , Kaiser, L. G. , Amezcua, C. A. , & Maher, E. A. (2010). Improvement of resolution for brain coupled metabolites by optimized (1)H MRS at 7T. NMR in Biomedicine, 23(9), 1044–1052. [DOI] [PubMed] [Google Scholar]
- Cui, J. , Shao, L. , Young, L. T. , & Wang, J. F. (2007). Role of glutathione in neuroprotective effects of mood stabilizing drugs lithium and valproate. Neuroscience, 144(4), 1447–1453. [DOI] [PubMed] [Google Scholar]
- Da Silva, T. , Hafizi, S. , Andreazza, A. C. , Kiang, M. , Bagby, R. M. , Navas, E. , … Mizrahi, R. (2018). Glutathione, the major redox regulator, in the prefrontal cortex of individuals at clinical high risk for psychosis. The International Journal of Neuropsychopharmacology, 21(4), 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva, T. , Wu, A. , Laksono, I. , Prce, I. , Maheandiran, M. , Kiang, M. , … Mizrahi, R. (2018). Mitochondrial function in individuals at clinical high risk for psychosis. Scientific Reports, 8(1), 6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf, R. A. (2013). In vivo NMR spectroscopy: Principles and techniques. Hoboken, New Jersey: John Wiley and Sons. [Google Scholar]
- Degenhardt, L. , Hall, W. , & Lynskey, M. (2001). The relationship between cannabis use, depression and anxiety among Australian adults: Findings from the National Survey of Mental Health and Well‐Being. Social Psychiatry and Psychiatric Epidemiology, 36(5), 219–227. [DOI] [PubMed] [Google Scholar]
- Demro, C. , Rowland, L. , Wijtenburg, S. A. , Waltz, J. , Gold, J. , Kline, E. , … Schiffman, J. (2017). Glutamatergic metabolites among adolescents at risk for psychosis. Psychiatry Research, 257, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich‐Muszalska, A. , Olas, B. , Głowacki, R. , & Bald, E. (2009). Oxidative/nitrative modifications of plasma proteins and thiols from patients with schizophrenia. Neuropsychobiology, 59(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Duffy, S. L. , Lagopoulos, J. , Cockayne, N. , Hermens, D. F. , Hickie, I. B. , & Naismith, S. L. (2015). Oxidative stress and depressive symptoms in older adults: A magnetic resonance spectroscopy study. Journal of Affective Disorders, 180, 29–35. [DOI] [PubMed] [Google Scholar]
- Duffy, S. L. , Lagopoulos, J. , Cockayne, N. , Lewis, S. J. , Hickie, I. B. , Hermens, D. F. , & Naismith, S. L. (2015). The effect of 12‐wk ω‐3 fatty acid supplementation on in vivo thalamus glutathione concentration in patients "at risk" for major depression. Nutrition, 31(10), 1247–1254. [DOI] [PubMed] [Google Scholar]
- Duffy, S. L. , Lagopoulos, J. , Hickie, I. B. , Diamond, K. , Graeber, M. B. , Lewis, S. J. , & Naismith, S. L. (2014). Glutathione relates to neuropsychological functioning in mild cognitive impairment. Alzheimers Dement, 10(1), 67–75. [DOI] [PubMed] [Google Scholar]
- Duffy, S. L. , Lagopoulos, J. , Terpening, Z. , Lewis, S. J. , Grunstein, R. , Mowszowski, L. , … Naismith, S. L. (2016). Association of anterior cingulate glutathione with sleep apnea in older adults at‐risk for dementia. Sleep, 39(4), 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux, A. M. S. , Horder, J. , Mendez, M. A. , Egerton, A. , Williams, S. C. R. , Wilson, C. E. , … McAlonan, G. M. (2016). Cortical and subcortical glutathione levels in adults with autism spectrum disorder. Autism Research, 9(4), 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraguas, D. , Díaz‐Caneja, C. M. , Rodríguez‐Quiroga, A. , & Arango, C. (2017). Oxidative stress and inflammation in early onset first episode psychosis: A systematic review and meta‐analysis. The International Journal of Neuropsychopharmacology, 20(6), 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed, R. D. , Hollenhorst, C. N. , Weiduschat, N. , Mao, X. , Kang, G. , Shungu, D. C. , & Gabbay, V. (2017). A pilot study of cortical glutathione in youth with depression. Psychiatry Research: Neuroimaging, 270, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gałecki, P. , Szemraj, J. , Bieńkiewicz, M. , Florkowski, A. , & Gałecka, E. (2009). Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacological Reports, 61(3), 436–447. [DOI] [PubMed] [Google Scholar]
- Gama, C. S. , Salvador, M. , Andreazza, A. C. , Kapczinski, F. , & Silva Belmonte‐de‐Abreu, P. (2006). Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in schizophrenia: A study of patients treated with haloperidol or clozapine. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 30(3), 512–515. [DOI] [PubMed] [Google Scholar]
- Gawryluk, J. W. , Wang, J.‐F. , Andreazza, A. C. , Shao, L. , Yatham, L. N. , & Young, L. T. (2011). Prefrontal cortex glutathione S‐transferase levels in patients with bipolar disorder, major depression and schizophrenia. International Journal of Neuropsychopharmacology, 14(8), 1069–1074. [DOI] [PubMed] [Google Scholar]
- Gawryluk, J. W. , Wang, J.‐F. , Andreazza, A. C. , Shao, L. , & Young, L. T. (2011). Decreased levels of glutathione, the major brain antioxidant, in post‐mortem prefrontal cortex from patients with psychiatric disorders. International Journal of Neuropsychopharmacology, 14(1), 123–130. [DOI] [PubMed] [Google Scholar]
- Godlewska, B. R. , Near, J. , & Cowen, P. J. (2015). Neurochemistry of major depression: A study using magnetic resonance spectroscopy. Psychopharmacology, 232(3), 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska, B. R. , Pike, A. , Sharpley, A. L. , Ayton, A. , Park, R. J. , Cowen, P. J. , & Emir, U. E. (2017). Brain glutamate in anorexia nervosa: A magnetic resonance spectroscopy case control study at 7 Tesla. Psychopharmacology, 234(3), 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska, B. R. , Yip, S. W. , Near, J. , Goodwin, G. M. , & Cowen, P. J. (2014). Cortical glutathione levels in young people with bipolar disorder: A pilot study using magnetic resonance spectroscopy. Psychopharmacology, 231(2), 327–332. [DOI] [PubMed] [Google Scholar]
- Green, B. , Young, R. , & Kavanagh, D. (2005). Cannabis use and misuse prevalence among people with psychosis. The British Journal of Psychiatry, 187, 306–313. [DOI] [PubMed] [Google Scholar]
- Gu, F. , Chauhan, V. , & Chauhan, A. (2015). Glutathione redox imbalance in brain disorders. Current Opinion in Clinical Nutrition and Metabolic Care, 18(1), 89–95. [DOI] [PubMed] [Google Scholar]
- Hafizi, S. , Da Silva, T. , Meyer, J. H. , Kiang, M. , Houle, S. , Remington, G. , … Mizrahi, R. (2018). Interaction between TSPO‐a neuroimmune marker‐and redox status in clinical high risk for psychosis: A PET‐MRS study. Neuropsychopharmacology, 43, 1700–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens, D. F. , Hatton, S. N. , Lee, R. S. C. , Naismith, S. L. , Duffy, S. L. , Paul Amminger, G. , … Hickie, I. B. (2018). In vivo imaging of oxidative stress and fronto‐limbic white matter integrity in young adults with mood disorders. European Archives of Psychiatry and Clinical Neuroscience, 268(2), 145–156. [DOI] [PubMed] [Google Scholar]
- Hermens, D. F. , Lagopoulos, J. , Naismith, S. L. , Tobias‐Webb, J. , & Hickie, I. B. (2012). Distinct neurometabolic profiles are evident in the anterior cingulate of young people with major psychiatric disorders. Translational Psychiatry, 2, e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, J. E. , Auerbach, R. P. , Pisoni, A. , & Pizzagalli, D. A. (2017). Localized MRS reliability of in vivo glutamate at 3 T in shortened scan times: A feasibility study. NMR in Biomedicine, 30(11), e3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant, F. , Near, J. , Turecki, G. , & Richard‐Devantoy, S. (2016). Spectroscopy markers of suicidal risk and mental pain in depressed patients. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 73, 63–71. [DOI] [PubMed] [Google Scholar]
- Kang, Y. , Viswanath, V. , Jha, N. , Qiao, X. , Mo, J. Q. , & Andersen, J. K. (1999). Brain gamma‐glutamyl cysteine synthetase (GCS) mRNA expression patterns correlate with regional‐specific enzyme activities and glutathione levels. Journal of Neuroscience Research, 58(3), 436–441. [PubMed] [Google Scholar]
- Khanzode, S. D. , Dakhale, G. N. , Khanzode, S. S. , Saoji, A. , & Palasodkar, R. (2003). Oxidative damage and major depression: The potential antioxidant action of selective serotonin re‐uptake inhibitors. Redox Report, 8(6), 365–370. [DOI] [PubMed] [Google Scholar]
- Kreis, R. (2016). The trouble with quality filtering based on relative Cramér‐Rao lower bounds. Magnetic Resonance in Medicine, 75(1), 15–18. [DOI] [PubMed] [Google Scholar]
- Kumar, J. , Liddle, E. B. , Fernandes, C. C. , Palaniyappan, L. , Hall, E. L. , Robson, S. E. , … Liddle, P. F. (2018). Glutathione and glutamate in schizophrenia: A 7T MRS study. Molecular Psychiatry. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagopoulos, J. , Hermens, D. F. , Hatton, S. N. , Tobias‐Webb, J. , Griffiths, K. , Naismith, S. L. , … Hickie, I. B. (2013). Microstructural white matter changes in the corpus callosum of young people with bipolar disorder: A diffusion tensor imaging study. PLoS One, 8(3), e59108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally, N. , An, L. , Banerjee, D. , Niciu, M. J. , Luckenbaugh, D. A. , Richards, E. M. , … Nugent, A. C. (2016). Reliability of 7T (1) H‐MRS measured human prefrontal cortex glutamate, glutamine, and glutathione signals using an adapted echo time optimized PRESS sequence: A between‐ and within‐sessions investigation. Journal of Magnetic Resonance Imaging, 43(1), 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus, K. A. , Gabbay, V. , Mao, X. , Johnson, A. , Murrough, J. W. , Mathew, S. J. , & Shungu, D. C. (2014). In vivo (1)H MRS study of potential associations between glutathione, oxidative stress and anhedonia in major depressive disorder. Neuroscience Letters, 569, 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Jakary, A. , Gillung, E. , Eisendrath, S. , Nelson, S. J. , Mukherjee, P. , & Luks, T. (2016). Evaluating metabolites in patients with major depressive disorder who received mindfulness‐based cognitive therapy and healthy controls using short echo MRSI at 7 Tesla. Magma, 29(3), 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr, J. B. , Kuczenski, R. , Bracha, H. S. , Moir, M. , & Jeste, D. V. (1990). Increased indices of free radical activity in the cerebrospinal fluid of patients with tardive dyskinesia. Biological Psychiatry, 28(6), 535–539. [DOI] [PubMed] [Google Scholar]
- Lushchak, V. I. (2012). Glutathione homeostasis and functions: Potential targets for medical interventions. Journal of Amino Acids, 2012, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado‐Vieira, R. , Andreazza, A. C. , Viale, C. I. , Zanatto, V. , Cereser, V. , da Silva Vargas, R. , … Gentil, V. (2007). Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: A possible role for lithium antioxidant effects. Neuroscience Letters, 421(1), 33–36. [DOI] [PubMed] [Google Scholar]
- Maes, M. , Galecki, P. , Chang, Y. S. , & Berk, M. (2011). A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 35(3), 676–692. [DOI] [PubMed] [Google Scholar]
- Mandal, P. K. , Tripathi, M. , & Sugunan, S. (2012). Brain oxidative stress: Detection and mapping of anti‐oxidant marker 'Glutathione' in different brain regions of healthy male/female, MCI and Alzheimer patients using non‐invasive magnetic resonance spectroscopy. Biochemical and Biophysical Research Communications, 417(1), 43–48. [DOI] [PubMed] [Google Scholar]
- Marken, P. A. , Stanislav, S. W. , Lacombe, S. , Pierce, C. , Hornstra, R. , & Sommi, R. W. (1992). Profile of a sample of subjects admitted to an acute care psychiatric facility with manic symptoms. Psychopharmacology Bulletin, 28(2), 201–205. [PubMed] [Google Scholar]
- Masaki, C. , Sharpley, A. L. , Godlewska, B. R. , Berrington, A. , Hashimoto, T. , Singh, N. , … Cowen, P. J. (2016). Effects of the potential lithium‐mimetic, ebselen, on brain neurochemistry: A magnetic resonance spectroscopy study at 7 tesla. Psychopharmacology, 233(6), 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa, D. , Obata, T. , Shirayama, Y. , Nonaka, H. , Kanazawa, Y. , Yoshitome, E. , … Hashimoto, K. (2008). Negative correlation between brain glutathione level and negative symptoms in schizophrenia: A 3T 1H‐MRS study. PLoS One, 3(4), e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry, P. D. , Hickie, I. B. , Yung, A. R. , Pantelis, C. , & Jackson, H. J. (2006). Clinical staging of psychiatric disorders: A heuristic framework for choosing earlier, safer and more effective interventions. Australian and New Zealand Journal of Psychiatry, 40(8), 616–622. [DOI] [PubMed] [Google Scholar]
- Michels, L. , Schulte‐Vels, T. , Schick, M. , O'Gorman, R. L. , Zeffiro, T. , Hasler, G. , & Mueller‐Pfeiffer, C. (2014). Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: Preliminary findings. Psychiatry Research, 224(3), 288–295. [DOI] [PubMed] [Google Scholar]
- Mintun, M. A. , Lundstrom, B. N. , Snyder, A. Z. , Vlassenko, A. G. , Shulman, G. L. , & Raichle, M. E. (2001). Blood flow and oxygen delivery to human brain during functional activity: Theoretical modeling and experimental data. Proceedings of the National Academy of Sciences of the United States of America, 98(12), 6859–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin, A. , Baumann, P. S. , Griffa, A. , Xin, L. , Mekle, R. , Fournier, M. , … Do, K. Q. (2015). Glutathione deficit impairs myelin maturation: Relevance for white matter integrity in schizophrenia patients. Molecular Psychiatry, 20(7), 827–838. [DOI] [PubMed] [Google Scholar]
- Naismith, S. L. , Lagopoulos, J. , Hermens, D. F. , White, D. , Duffy, S. L. , Robillard, R. , … Hickie, I. B. (2014). Delayed circadian phase is linked to glutamatergic functions in young people with affective disorders: A proton magnetic resonance spectroscopy study. BMC Psychiatry, 14, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, F. , Berk, M. , Dean, O. , & Bush, A. I. (2008). Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. The International Journal of Neuropsychopharmacology, 11(6), 851–876. [DOI] [PubMed] [Google Scholar]
- Nuttall, S. L. , Martin, U. , Sinclair, A. J. , & Kendall, M. J. (1998). Glutathione: In sickness and in health. Lancet, 351(9103), 645–646. [DOI] [PubMed] [Google Scholar]
- Ochoa, S. , Usall, J. , Cobo, J. , Labad, X. , & Kulkarni, J. (2012). Gender differences in schizophrenia and first‐episode psychosis: A comprehensive literature review. Schizophrenia Research and Treatment, 2012, 916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol‐Clotet, E. , Canales‐Rodríguez, E. J. , Salvador, R. , Sarró, S. , Gomar, J. J. , Vila, F. , … McKenna, P. J. (2010). Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Molecular Psychiatry, 15(8), 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae, C. D. , & Williams, S. R. (2017). Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Analytical Biochemistry, 529, 127–143. [DOI] [PubMed] [Google Scholar]
- Raffa, M. , Mechri, A. , Othman, L. B. , Fendri, C. , Gaha, L. , & Kerkeni, A. (2009). Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 33(7), 1178–1183. [DOI] [PubMed] [Google Scholar]
- Rice, L. J. , Lagopoulos, J. , Brammer, M. , & Einfeld, S. L. (2016). Reduced gamma‐aminobutyric acid is associated with emotional and behavioral problems in Prader‐Willi syndrome. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 171(8), 1041–1048. [DOI] [PubMed] [Google Scholar]
- Richie, J. P. , Skowronski, L. , Abraham, P. , & Leutzinger, Y. (1996). Blood glutathione concentrations in a large‐scale human study. Clinical Chemistry, 42(1), 64–70. [PubMed] [Google Scholar]
- Saleh, M. G. , Oeltzschner, G. , Chan, K. L. , Puts, N. A. , Mikkelsen, M. , Schär, M. , … Edden, R. A. (2016). Simultaneous edited MRS of GABA and glutathione. NeuroImage, 142, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson, M. , Vainikka, L. , & Ollinger, K. (2011). Glutathione in the blood and cerebrospinal fluid: A study in healthy male volunteers. Neuropeptides, 45(4), 287–292. [DOI] [PubMed] [Google Scholar]
- Sanaei Nezhad, F. , Anton, A. , Parkes, L. M. , Deakin, B. , & Williams, S. R. (2017). Quantification of glutathione in the human brain by MR spectroscopy at 3 tesla: Comparison of PRESS and MEGA‐PRESS. Magnetic Resonance in Medicine, 78(4), 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian, T. A. , Magallanes, J. A. , Shau, H. , Tashkin, D. , & Roth, M. D. (1999). Oxidative stress produced by marijuana smoke. An adverse effect enhanced by cannabinoids. American Journal of Respiratory Cell and Molecular Biology, 20(6), 1286–1293. [DOI] [PubMed] [Google Scholar]
- Saunders, J. B. , Aasland, O. G. , Babor, T. F. , de la Fuente, J. R. , & Grant, M. (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction, 88(6), 791–804. [DOI] [PubMed] [Google Scholar]
- Schubert, F. , Kühn, S. , Gallinat, J. , Mekle, R. , & Ittermann, B. (2017). Towards a neurochemical profile of the amygdala using short‐TE. NMR in Biomedicine, 30(5). (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Shungu, D. C. , Weiduschat, N. , Murrough, J. W. , Mao, X. , Pillemer, S. , Dyke, J. P. , … Mathew, S. J. (2012). Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR in Biomedicine, 25(9), 1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaga, I. , Niedzielska, E. , Gawlik, M. , Moniczewski, A. , Krzek, J. , Przegaliński, E. , … Filip, M. (2015). Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacological Reports, 67(3), 569–580. [DOI] [PubMed] [Google Scholar]
- Soeiro‐de‐Souza, M. G. , Pastorello B. F., Leite C. A. C., Henning A., Moreno R. A. and Garcia Otaduy M. C. (2016). "Dorsal anterior cingulate lactate and glutathione levels in euthymic bipolar I disorder: 1H‐MRS study." The International Journal of Neuropsychopharmacology 19(8), pyw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, R. , Ratiney, H. , Hammond‐Rosenbluth, K. E. , Pelletier, D. , & Nelson, S. J. (2010). MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magnetic Resonance Imaging, 28(2), 163–170. [DOI] [PubMed] [Google Scholar]
- Stonnington, C. M. , Tan, G. , Klöppel, S. , Chu, C. , Draganski, B. , Jack, C. R. , … Frackowiak, R. S. (2008). Interpreting scan data acquired from multiple scanners: A study with Alzheimer's disease. NeuroImage, 39(3), 1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowski, L. , & McNeill, A. (2013). The prevalence of smoking in people with mental health problems: Evidence from UK data sources. NICE, 1–5. [Google Scholar]
- Terpstra, M. , Vaughan, T. J. , Ugurbil, K. , Lim, K. O. , Schulz, S. C. , & Gruetter, R. (2005). Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: Application to schizophrenia. Magma, 18(5), 276–282. [DOI] [PubMed] [Google Scholar]
- United Nations Office of Drugs and Crime (UNDOC), World Drug Report (2011). United Nations Publication, Sales No. E.11.XI.10.
- Wang, H. , Xu, H. , Dyck, L. E. , & Li, X. M. (2005). Olanzapine and quetiapine protect PC12 cells from beta‐amyloid peptide(25‐35)‐induced oxidative stress and the ensuing apoptosis. Journal of Neuroscience Research, 81(4), 572–580. [DOI] [PubMed] [Google Scholar]
- Wijtenburg, S. A. , Gaston, F. E. , Spieker, E. A. , Korenic, S. A. , Kochunov, P. , Hong, L. E. , & Rowland, L. M. (2014). Reproducibility of phase rotation STEAM at 3T: Focus on glutathione. Magnetic Resonance in Medicine, 72(3), 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, S. J. , Berger, G. E. , Wellard, R. M. , Proffitt, T. M. , McConchie, M. , Berk, M. , … Pantelis, C. (2009). Medial temporal lobe glutathione concentration in first episode psychosis: A 1H‐MRS investigation. Neurobiology of Disease, 33(3), 354–357. [DOI] [PubMed] [Google Scholar]
- Wood, S. J. , Yücel, M. , Pantelis, C. , & Berk, M. (2009). Neurobiology of schizophrenia spectrum disorders: The role of oxidative stress. Annals of the Academy of Medicine, Singapore, 38(5), 396–396. [PubMed] [Google Scholar]
- Xin, L. , Mekle, R. , Fournier, M. , Baumann, P. S. , Ferrari, C. , Alameda, L. , … Cuenod, M. (2016). Genetic polymorphism associated prefrontal glutathione and its coupling with brain glutamate and peripheral redox status in early psychosis. Schizophrenia Bulletin, 42(5), 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, J. K. , Leonard, S. , & Reddy, R. (2006). Altered glutathione redox state in schizophrenia. Disease Markers, 22(1–2), 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz, D. , Liu, Y. S. , Ercal, N. , & Armstrong, D. W. (1999). Comparison of pure nicotine‐ and smokeless tobacco extract‐induced toxicities and oxidative stress. Archives of Environmental Contamination and Toxicology, 37(4), 434–439. [DOI] [PubMed] [Google Scholar]
- Yumru, M. , Savas, H. A. , Kalenderoglu, A. , Bulut, M. , Celik, H. , & Erel, O. (2009). Oxidative imbalance in bipolar disorder subtypes: A comparative study. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 33(6), 1070–1074. [DOI] [PubMed] [Google Scholar]


