Abstract
Background
Stimulation of the dorsal root ganglion (DRG) in the treatment of chronic, intractable pain has shown excellent clinical results in multiple published studies, including a large prospective, randomized, controlled trial. Both safety and efficacy have been demonstrated utilizing this therapeutic approach for many chronic complaints. Continued assessment of neuromodulation therapies, such as DRG stimulation, are not only an important aspect of vigilant care, but are also necessary for the evaluation for safety.
Materials and Methods
Safety and complaint records for DRG and spinal cord stimulation (SCS) stimulation were obtained from the manufacturer, analyzed and compiled to further assess ongoing device safety. Complaint event data were stratified according to complain type as well as overall rates. Data from similar time periods were compared between epidural neurostimulation devices by the same manufacturer as well as rates reported in the literature.
Results
Overall, DRG stimulation device event rates were lower or comparable to similar epidurally placed neurostimulation devices. Rates of events varied from 0 to 1.0% for DRG stimulation (n >500+ implants) which was similar to the event rate for SCS by the same manufacturer (n >2000+ implants). In comparison, complaints and adverse events ranged from 0 to 14% for SCS in the literature.
Discussions
The current results from a large consecutive cohort obtained from manufacturer records indicates that DRG stimulation demonstrates an excellent safety profile. Reported event rates are similar to previously reported adverse event and complaint rates in the literature for this therapy. Similarly, safety events rates were lower or similar to previously reported rates for SCS, further demonstrating the comparative safety of this neuromodulation technique for chronic pain treatment.
Keywords: chronic pain, epidural, implant, Neurostimulation, safety
Introduction
The dorsal root ganglion is a sensory neural structure located within the intervertebral foramen that contains the primary sensory neuron (PSN) somata 1, 2, 3. Several reviews have outlined the importance that the PSN plays in the development and maintenance of chronic pain 1, 4, 5. Many of the pathophysiologic changes in neuronal function of PSNs observed in models of chronic pain are specifically located in the neuronal cell body, and these can include increased membrane excitability as well as the generation of ectopic action potentials 1, 6, 7, 8. The PSNs also contain a t‐junction where the distal and primary axons combine with a stem axon that connects the soma. This junction in the pseudounipolar neurons acts as a junctional failure point for centrally projecting neural traffic. It also serves as a modulatory area for controlling sensory information originating from both the periphery and cell body to more central neural pathways 9, 10. Given these functional considerations and the anatomic accessibility of the dorsal root ganglion (DRG) in the spine, neurostimulation techniques have been developed to therapeutically target this spinal structure 3, 11, 12, 13.
Clinically, it has been shown in multiple, published studies that DRG stimulation produces significant analgesia in patients suffering from chronic pain 11, 14, 15, 16, 17. This includes results from a large, prospective, multi‐center, randomized controlled trial (ACCURATE study) in which DRG stimulation was shown to be safe and effective 11. The ACCURATE study also demonstrated that DRG stimulation is superior to spinal cord stimulation (SCS) in the treatment of CRPS types I and II (causalgia). Subsequent studies have not only shown utility of this neuromodulation target in other pain conditions outside CRPS, but also have continued to document the clinically efficacy and safety of this therapeutic approach in “real‐world” settings 12, 14, 16, 18, 19, 20, 21. After regulatory approvals (both CE Mark and FDA) commercialization of the only product approved for DRG stimulation in the treatment of chronic pain has allowed increased patient access to this important neuromodulation therapy.
One important aspect of medical device use is ongoing safety assessments and vigilance efforts by both physicians and manufacturers. There are multiple ways to adequately assess postmarket safety including running specific postmarket safety studies in large patient populations (often a requirement of device regulatory approval), systematic reviews of peer‐reviewed published data, analysis of public safety databases as well as review of manufacturer internal complaint and safety records. Each of these approaches have their strengths and weaknesses 22, but given the regulatory requirements for device vigilance reporting, internal company records are generally the most accurate reporting methods for very large cohorts. Quite often, however, these records and results are not made public. Many of these approaches have been used to assess ongoing safety of neuromodulation devices 12, 22, 23, 24, 25 and are synthesized in clinical consensus recommendations, such as those published by the Neuromodulation Appropriate Use Consensus Committee (NACC) 26, 27, 28.
To assess the ongoing performance and safety of DRG stimulation, we have compiled and analyzed device specific manufacturer safety and complaint records. For comparative purposes, the records analyzed included both DRG and SCS systems from the same manufacturer.
Methods
A postmarket surveillance analysis was conducted to generate performance and safety data for both DRG and SCS stimulation. Data, generously provided by Abbott Neuromodulation (Chicago, IL, USA), were systematically collected from an internal complaint reporting and handling database and utilized in the current analysis. The product experiences reported and recorded in this type of database are used to collect any written, electronic, or oral communication that alleges deficiencies related to the physical characteristics, identity, quality, purity, potency, durability, reliability, safety, effectiveness, or performance of a distributed product. The time frame selected was April 2016 to March 2018 based on DRG stimulation FDA approval. Comparative safety data were also acquired through a review of the DRG and SCS published literature (data sources: Medline and EMBASE).
The comparison between the two therapies was limited only to products implanted within this time frame to provide a direct comparison of product performance utilizing the same associated implant durations. Data were validated through the manufacturer's quality assurance system for complaint handling, and both a unique patient identifier as well as specific data fields for device identification and the specific complaint descriptor/category were compiled. The sources of potentially reportable events included, but were not limited to, the following: Customer complaints (primary source), contact with manufacturer employees or contract personnel, field service records, device malfunctions, advertising and promotion materials, professional meetings, congresses, seminars, clinical studies using manufacturer marketed products and clinical research, product actions, legal actions, regulatory affairs, manufacturer employees of different divisions, other companies with manufacturer/distributor relationships with manufacturer, regulatory agencies, and other telecommunications (internet), website postings, web logs (blogs) and published literature. Each of the patient implant records were characterized into groups based on the implantable pulse generator (IPG) and leads that were implanted together. Only implanted systems where both an IPG and a lead were implanted were included for this evaluation. Implant records were excluded from the investigation in rare cases where there were multiple IPGs and leads implanted involving both DRG and SCS devices in the same patient. Records from any associated accessories (eg, lead anchors, lead extensions, etc.) were not included in this analysis.
Based on the implant data records, the “Implant Therapy” was determined based on the IPG model utilized. Products were further grouped into “Implant Family” based on the IPG model (SMI‐Axium [DRG] Proclaim DRG or Proclaim SCS) and the implanted lead models (DRG lead, SCS percutaneous lead, SCS surgical/paddle lead). Information (patient ID, model, serial/lot number, implant date) from the patient implant data was used to identify the related complaint records. Once matched, the complaint record details were then associated with the patient implant event and the associated “Implant Therapy” and “Implant Family,” after which the patient implant record was marked to indicate whether there was a complaint and/or explant associated with the implant event. Comparative rates of complaint and explant were calculated using either the “Implant Therapy,” “Implant Family,” individual product model, or specific complaint record variable by summing the number of associated complaints and explant records and normalizing by the number of overall implant records for the “Implant Therapy,” “Implant Family,” or individual product model.
Data were compiled and stratified according to general event categories as has been previously published 11, 25, 29. Event categories ranged from biological/physical descriptor to device events (device malfunction or related events). A literature review was also conducted to retrieve safety reporting from the published literature. Multiple databases (Medline and EMBASE) were searched with relevant search terms (“Dorsal root ganglion stimulation,” “DRG stimulation,” “spinal cord stimulation,” “safety,” etc.) in order to produce comparative data from peer‐reviewed publications.
Results
Manufacturer records yielded data from over 500 DRG stimulator and 2000 spinal cord stimulator implants. Primary results and outcomes from the manufacturer records are presented in Table 1. Overall, DRG stimulation reported safety event rates were 3.2%. This compares to an event rate during the same time frame of 3.1% in SCS. Infection was the most frequent event noted, with an overall rate of approximately 1% for both DRG and SCS systems. This was one‐third of overall event incidence. All other biologically classified event rates were less than 1%. Comparatively, both the DRG and SCS systems demonstrated equivalent event rates from the manufacturer records with slight variations in individual categories. Table 2 lists the comparative incident rates of the highest occurring events (infection, pain at implant site and CSF leaks) in the current data set and literature for both DRG and SCS. In all cases, the incident rates reported in the literature are either higher or the same as reported from the manufacturer records in the current analysis.
Table 1.
Rates of Reported Events From Both DRG and SCS Systems.
| Event description | SCS incidence rate | DRG incidence rate |
|---|---|---|
| Allergic reaction | 0.09% | 0.18% |
| Cardiovascular changes | 0.04% | 0 |
| CSF leaks | 0.30% | 0.54% |
| Device related pain | 0.30% | 0.54% |
| Diminished or loss of motor or musculoskeletal symptom control | 0.09% | 0 |
| Gastroesophageal or gastrointestinal changes | 0 | 0.18% |
| Headache | 0.04% | 0 |
| Hematoma | 0.17% | 0 |
| Infection | 1.12% | 1.08% |
| Neurological deficit/dysfunction (NDD) | 0.13% | 0 |
| Persistent pain at the implant site | 0.56% | 0.18% |
| Pocket heating | 0.04% | 0 |
| Post Op pain | 0 | 0 |
| Pulmonary changes | 0.04% | 0 |
| Reduced surgical would healing | 0.17% | 0.18% |
| Seizure | 0.04% | 0 |
| Skin erosion | 0.04% | 0.36% |
| Total incidence rate | 3.09% | 3.24% |
N = >500 systems for DRG and n = >2000 systems for SCS.
Table 2.
Most Common Events Reported From DRG and SCS Systems.
| Event description | Nerve root incidence rate* | Published SCS incidence rates | SCS incidence rate | DRG incidence rate |
|---|---|---|---|---|
| CSF leaks | 12% | 0.3%‐7% | 0.30% | 0.54% |
| Infection | 12% | 2.5%‐14% | 1.12% | 1.08% |
| Persistent pain at the implant site | N/A | 0.9%‐12% | 0.56% | 0.18% |
Comparison between events reported in current analysis and published rates from SCS and nerve root stimulation.
Reference 31.
Event rates were also comparable or better than the published literature for both SCS and DRG systems. Table 3 shows the manufacturer event rates compared to the event rates reported in the ACCURATE study. The manufacturer postmarket events rates were either comparable or less than the rates reported in the ACCURATE clinical trial demonstrating continued or improved safety of the DRG stimulation system. Results from a literature review demonstrated that the current event rates compared favorably to published SCS clinical event rates.
Table 3.
Comparison Between Reported Adverse Event Rates (by Subject) in the ACCURATE Clinical Trial and the Current Manufacturer Safety Surveillance Data.
| Event description | Accurate DRG | Incidence rate |
|---|---|---|
| Allergic reaction | 2.7% | 0.18% |
| Cardiovascular changes | 1.4% | 0% |
| CSF leaks | 2.7% | 0.54% |
| Device related pain | 1.4% | 0.54% |
| Diminished or loss of motor or musculoskeletal symptom control | 3.9% | 0% |
| Gastroesophageal or gastrointestinal changes | 1.3% | 0.18% |
| Headache | 1.4% | 0% |
| Hematoma | 0% | 0% |
| Infection | 1.3% | 1.08% |
| Neurological deficit/dysfunction (NDD) | 0% | 0% |
| Persistent pain at the implant site | 1.4% | 0.18% |
| Pocket heating | 0% | 0% |
| Post Op pain | 1.4% | 0% |
| Pulmonary changes | 1.3% | 0% |
| Reduced surgical would healing | 0% | 0.18% |
| Seizure | 0% | 0% |
Note the event rate calculations and specific categorical definitions differ between the ACCURATE study and the current analysis.
Discussion
The findings from this safety analysis, including >500 DRG system implants from a 2‐year time period following commercial approval, demonstrate that clinical adverse events and device complaint rates were comparably or less frequent than those reported for, 1) similar epidural SCS neurostimulation systems in the literature, 2) similar SCS systems from the same manufacturer in the same time frame as DRG stimulation, 3) a similar DRG system as reported in the results from a large, prospective, multi‐center, randomized controlled trial (ACCURATE), and, 4) a similar DRG stimulation system as reported in the literature. These findings represent the most complete postmarket safety analysis completed for DRG stimulation and consider all reported events from a manufacturer quality system for commercially implanted systems within a 2‐year time frame. This approach helped to avoid biases of event reporting in public databases and also allows for a larger sample size of device reporting than all published clinical studies combined.
The overall incidence rate of 3.2% for DRG stimulation was comparable to the event rate for fully‐implantable SCS systems (3.1%) from the same manufacturer and so represents a true comparison of rates given that the same requirements for reporting and methods for data collection were taken as a part of the required device vigilance monitoring. These rates are similar to those observed during the ACCURATE clinical trial conducted for FDA approval as well as those reported in the literature 11, 12, 15, 17, 18, 30. The latter of these two data collection methods (clinical studies) yield fairly large ranges of events, mostly due to the heterogeneity in data collection methods, reporting decisions and the fact that data was obtained from different geographies as well as different clinical sites. This approach, however, provides event rates from different sources, and in so doing yields data from larger patient samples than available through single clinical studies.
To that extent, the results from the current analysis are also in agreement with event rates published from the ACCURATE study 11. Safety event rates published from large clinical studies are another good source for comparative data. Generally, the events reported from high‐quality clinical trials involve smaller and more homogeneous patient populations than larger postmarket patient cohorts. The data is also collected within a highly controlled environment, generally with very experienced physicians participating as investigators. It is very encouraging to see that, in the current analysis, data collected from a “real‐world” setting, such as clinical practice across multiple locations and physicians, matches or exceeds the safety rates from controlled clinical studies. Not only do the postmarket results substantiate those findings from the approval study, but also demonstrate increased external validity of the initial safety results within a more varied patient group in variable practice environments. Refined placement techniques and safety considerations, including neuromonitoring and awake placement of leads, will help continue to maintain safety and efficacy in the hands of practicing physicians.
The DRG stimulation system examined in the current analysis was specifically designed, tested and validated for the clinical use and safety of epidural access and placement in the lateral epidural space around the DRG. While other approaches have been utilized for lateral epidural lead placement as well, the published results from the use of these systems have been typically substandard (Fig. 1) 31, 32, 33. Generally, the systems utilized have either been standard SCS systems or systems not generally designed or intended to be anatomically placed for any appreciable amount of time near the DRG 31, 32, 34. As a result, lead designs and delivery approaches are inadequate for long‐term, effective use in stimulating the DRG. The use of these approaches results in relative lack of long‐term efficacy of stimulation as well as higher safety event incidence 31, 32. For example, Weigel and colleagues published a case series attempting to repurpose standard SCS hardware for DRG stimulation 32. Ultimately, this group found that stimulating the DRG with this system did not result in long‐term clinical benefit and also resulted in overstimulation producing uncomfortable paresthesias. Similarly, a recent report by Levine and colleagues reported higher clinical event rates when placing leads in the lateral recess, or “gutter” of the epidural space. Presumably the size and flexibility of the leads as well as other aspects of the system resulted in the clinical performance noted.
Figure 1.
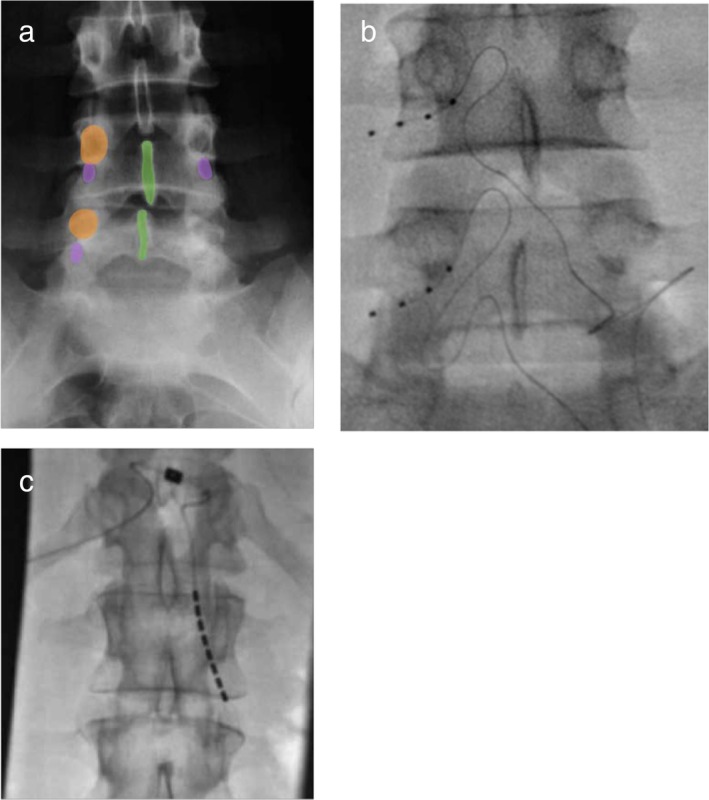
Fluoroscopic images of spine anatomy relating location of the dorsal root ganglia as well as implanted DRG leads in the foramen and SCS lead in the lateral epidural space. Panel A depicts relevant spinal anatomy and location of the DRG within the dorsal aspect of the neural foramen just under the spinal pedicle. Panel B shows a lead specifically designed for DRG stimulation in the dorsal intervertebral foramen adjacent to the DRG. Note the flexibility and outer diameter of the lead. Panel C shows a lead deigned for spinal cord stimulation in the lateral epidural space partly extending into the ventral spinal foramen. Note the difference in lead approaches and locations in the lateral recess.
It is not surprising to see that leads and systems not specifically designed to be anatomically located, and stably positioned, in the lateral epidural space do not perform as well as systems that do take these design considerations into account. Similar findings have been observed when SCS leads have been repurposed to be positioned in other anatomies 27. As it would be expected, the design engineering of specialized leads can result in better overall performance and clinical outcomes, especially when the intended neural target is relatively small. It is also not surprising to find that these results manifest themselves over longer time periods as well. Clinically, the results from nerve root stimulation were very different than those published for dorsal root ganglion stimulation 11, 31, 32. Specifically, Levine and colleagues demonstrated no difference between nerve root stimulation and SCS when treating neuropathic pain 31. The response rates of patients treated with nerve root stimulation was less than 50%, as opposed to the responder rate of greater than 90% observed in the ACCURATE trial 11. While anatomically connected, critical cytoarchitectonic structures such as the cell bodies and T‐junction of the PSNs that are housed in the ganglia are distinct from the nerve roots. As it has been shown that these structures play a large role in the putative mechanisms underlying DRG stimulation 1, 9, 35 and so it is not surprising that clinical results would show differences between nerve root and DRG stimulation.
The analysis presented are not without limitations. The data collected represent safety findings soon after FDA approval. Most of the data were collected from sites with experienced implanters, so it is unclear how results may or may not differ from sites with less experienced implanters. Currently, there are comprehensive training programs required by the manufacturer in order for physicians to begin utilizing the therapy. This also might be a partial explanation for the safety results obtained. Data were obtained from manufacturer records and so may face bias issues, similar to other forms of data collection (results from published clinical studies, public databases, etc.). We feel that this method of data collection offers benefits such as comprehensive collection of events that avoids underreporting often encountered from public databases as well as large sample sizes for analysis.
Conclusion
Following review and analysis of manufacturer safety records, DRG stimulation continues to demonstrate an excellent safety profile with low adverse event rates. The current analysis has reinforced the initial findings that DRG stimulation demonstrates an excellent safety profile that is equal or better to, 1) the ACCURATE pivotal trial results, 2) SCS devices, 3) results from published literature on both DRG and SCS therapies.
These event rates are also consistent or lower with rates previously deemed acceptable by neuromodulation consensus committees and regulatory agencies. Ongoing device vigilance and safety reporting by physicians will continue to be a valued assessment of the safety and performance attributed to DRG stimulation therapy as well as other neuromodulation approaches.
Authorship Statements
Drs. Deer and Kramer conducted the data analysis and assembly for presentation in the document. Dr. Kramer prepared the manuscript draft with important intellectual input from Drs. Deer, Levy, Falowski, Pope, Kapural, and Hunter. All authors provided editorial input. Abbott provided safety and device diligence data from internal databases for analysis and inclusion in the manuscript.
COMMENT
This article demonstrates that the incidence of complications between DRG and conventional SCS are similar and should be in experienced hands.
Timothy Lubenow, MD
Chicago, IL, USA
Comments not included in the Early View version of this paper.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Conflict of Interest: Dr. Deer is a consultant for Abbott, Axonics, Nalu, Saluda, Vertos, Vertiflex, Flowonix, and SpineThera, and has funded research from Abbott, Saluda, Vertiflex, and Vertos and Mainstay. He has minority equity in Axonics, Bioness, Ethos, Flowonix, Saluda, Nalu, Cornerloc, Spinethera, Vertos, and Vertiflex. Dr. Pope is a consultant for Abbott, Saluda, VertiFlex, Vertos, SpineThera, Jazz Pharmaceuticals, Flowonix, and SPR Therapeutics, has funded research with Abbott, Flowonix, VertiFlex, Saluda, and has minority equity in AGR, SPR Therapeutics, Celeri Health. Dr. Kramer is a consultant for Abbott, Autonomic Technologies, Nalu Medical, Circuit Therapeutics, CereVu and ENSO. Dr. Levy––Abbott (consultant, Medical Advisory Board), Mainstay Medical (consultant, Medical Advisory Board), Nalu (consultant, Medical Advisory Board), Nuvectra (consultant, Medical Advisory Board), Saluda (consultant, Medical Advisory Board). Dr. Falowski is a consultant for Abbott, Medtronic, Nevro, Vertiflex, Saluda, and SPR therapeutics; funded research from Abbott, Medtronic, Nuvectra, Biotronik; has minority equity in SpineThera, SPR therapeutics, Saluda, AGR, and Suture Concepts. Dr. Kapural is consultant for Nevro, Abbott, Best Doctors and has funded research from Biotronik, SPR Therapeutics, Medtronic, Boston Scientific, Neuros, Gimer, Sollis Medical. Dr. Hunter is a consultant for Abbott, Saluda, Nuvectra, Flowonix and serves on the Medical Advisory Board for Vertiflex.
Source(s) of financial support: Abbott Neuromodulation provided data from their internal records on safety diligence reporting for inclusion into the manuscript. Abbott provided support for preparation of the manuscript.
[The copyright line for this article was changed on 27 April 2019 after original online publication.]
References
- 1. Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation 2015;18:24–32, discussion 32. [DOI] [PubMed] [Google Scholar]
- 2. Vancamp T, Levy RM, Peña I, Pajuelo A. Relevant anatomy, morphology, and implantation techniques of the dorsal root ganglia at the lumbar levels. Neuromodulation 2017;20:690–702. [DOI] [PubMed] [Google Scholar]
- 3. Liem L, van Dongen E, Huygen FJ, Staats P, Kramer J. The dorsal root ganglion as a therapeutic target for chronic pain. Reg Anesth Pain Med 2016;41:511–519. [DOI] [PubMed] [Google Scholar]
- 4. Hogan QH. Role of decreased sensory neuron membrane calcium currents in the genesis of neuropathic pain. Croat Med J 2007;48:9–21. [PMC free article] [PubMed] [Google Scholar]
- 5. Pope JE, Deer TR, Kramer J. A systematic review: current and future directions of dorsal root ganglion therapeutics to treat chronic pain. Pain Med 2013;14:1477–1496. [DOI] [PubMed] [Google Scholar]
- 6. Koplovitch P, Devor M. Dilute lidocaine suppresses ectopic neuropathic discharge in dorsal root ganglia without blocking axonal propagation: A new approach to selective pain control. Pain 2018;159:1244–1256. [DOI] [PubMed] [Google Scholar]
- 7. Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009;196:115–128. [DOI] [PubMed] [Google Scholar]
- 8. Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain 1999;Suppl 6:S27–S35. [DOI] [PubMed] [Google Scholar]
- 9. Kent AR, Min X, Hogan QH, Kramer JM. Mechanisms of dorsal root ganglion stimulation in pain suppression: a computational modeling analysis. Neuromodulation 2018;21:234–246. [DOI] [PubMed] [Google Scholar]
- 10. Gemes G, Koopmeiners A, Rigaud M et al. Failure of action potential propagation in sensory neurons: mechanisms and loss of afferent filtering in C‐type units after painful nerve injury. J Physiol 2013;591:1111–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deer TR, Levy RM, Kramer J et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain 2017;158:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrison C, Epton S, Bojanic S, Green AL, FitzGerald JJ. The efficacy and safety of dorsal root ganglion stimulation as a treatment for neuropathic pain: a literature review. Neuromodulation 2018;21:225–233. [DOI] [PubMed] [Google Scholar]
- 13. Vuka I, Vučić K, Repić T, Ferhatović Hamzić L, Sapunar D, Puljak L. Electrical stimulation of dorsal root ganglion in the context of pain: a systematic review of in vitro and in vivo animal model studies. Neuromodulation 2018;21:213–224. [DOI] [PubMed] [Google Scholar]
- 14. Liem L, Russo M, Huygen FJPM et al. One‐year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation 2015;18:41–48, discussion 48‐49. [DOI] [PubMed] [Google Scholar]
- 15. Liem L, Russo M, Huygen FJPM et al. A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation 2013;16:471–482, discussion 482. [DOI] [PubMed] [Google Scholar]
- 16. Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation 2013;16:67–71, discussion 71‐72. [DOI] [PubMed] [Google Scholar]
- 17. Morgalla MH, Fortunato M, Lepski G, Chander BS. Dorsal root ganglion stimulation (DRGS) for the treatment of chronic neuropathic pain: a single‐center study with long‐term prospective results in 62 cases. Pain Physician 2018;21:E377–E387. [PubMed] [Google Scholar]
- 18. Huygen F, Liem L, Nijhuis H, Cusack W, Kramer J. Evaluating dorsal root ganglion stimulation in a prospective Dutch cohort. Neuromodulation 2018;22:80–86. [DOI] [PubMed] [Google Scholar]
- 19. Eldabe S, Espinet A, Wahlstedt A et al. Retrospective case series on the treatment of painful diabetic peripheral neuropathy with dorsal root ganglion stimulation. Neuromodulation 2018;21:787–792. [DOI] [PubMed] [Google Scholar]
- 20. Huygen F, Liem L, Cusack W, Kramer J. Stimulation of the L2‐L3 dorsal root ganglia induces effective pain relief in the low Back. Pain Pract 2018;18:205–213. [DOI] [PubMed] [Google Scholar]
- 21. Eldabe S, Burger K, Moser H et al. Dorsal root ganglion (DRG) stimulation in the treatment of phantom limb pain (PLP). Neuromodulation 2015;18:610–616, discussion 616‐617. [DOI] [PubMed] [Google Scholar]
- 22. Levy R, Henderson J, Slavin K et al. Incidence and avoidance of neurologic complications with paddle type spinal cord stimulation leads. Neuromodulation 2011;14:412–422, discussion 422. [DOI] [PubMed] [Google Scholar]
- 23. Deer T, Skaribas I, McJunkin T et al. Results from the partnership for advancement in neuromodulation registry: a 24‐month follow‐up. Neuromodulation 2016;19:179–187. [DOI] [PubMed] [Google Scholar]
- 24. Kumsa D, Steinke GK, Molnar GF et al. Public regulatory databases as a source of insight for neuromodulation devices stimulation parameters. Neuromodulation 2018;21:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bendersky D, Yampolsky C. Is spinal cord stimulation safe? A review of its complications. World Neurosurg 2014;82:1359–1368. [DOI] [PubMed] [Google Scholar]
- 26. Deer TR, Lamer TJ, Pope JE et al. The Neurostimulation Appropriateness Consensus Committee (NACC) safety guidelines for the reduction of severe neurological injury. Neuromodulation 2017;20:15–30. [DOI] [PubMed] [Google Scholar]
- 27. Deer TR, Mekhail N, Provenzano D et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014;17:515–550, discussion 550. [DOI] [PubMed] [Google Scholar]
- 28. Deer TR, Mekhail N, Provenzano D et al. The appropriate use of neurostimulation: avoidance and treatment of complications of neurostimulation therapies for the treatment of chronic pain. Neuromodulation 2014;17:571–597, discussion 597‐598. [DOI] [PubMed] [Google Scholar]
- 29. Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20‐year literature review. J Neurosurg 2004;100:254–267. [DOI] [PubMed] [Google Scholar]
- 30. Schu S, Gulve A, ElDabe S et al. Spinal cord stimulation of the dorsal root ganglion for groin pain‐a retrospective review. Pain Pract 2015;15:293–299. [DOI] [PubMed] [Google Scholar]
- 31. Levine AB, Steven DA, Parrent AG, MacDougall K. Successful long‐term nerve root stimulation for chronic neuropathic pain: a real world, Single Center Canadian Experience. Pain Physician 2017;20:95–106. [PubMed] [Google Scholar]
- 32. Weigel R, Capelle HH, Krauss JK. Failure of long‐term nerve root stimulation to improve neuropathic pain. J Neurosurg 2008;108:921–925. [DOI] [PubMed] [Google Scholar]
- 33. Levine AB, Parrent AG, MacDougall KW. Cervical spinal cord and dorsal nerve root stimulation for neuropathic upper limb pain. Can J Neurol Sci 2017;44:83–89. [DOI] [PubMed] [Google Scholar]
- 34. Levine AB, Parrent AG, MacDougall KW. Stimulation of the spinal cord and dorsal nerve roots for chronic groin, pelvic, and abdominal pain. Pain Physician 2016;19:405–412. [PubMed] [Google Scholar]
- 35. Koopmeiners AS, Mueller S, Kramer J, Hogan QH. Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation 2013;16:304–311, discussion 310‐311. [DOI] [PubMed] [Google Scholar]


