Abstract
Objective
Previous studies have shown additive weight loss when intensive behavioral therapy (IBT) was combined with weight‐loss medication. The present multisite study provides the first evaluation, in primary care, of the effect of the Centers for Medicare and Medicaid Services–based IBT benefit, delivered alone (with placebo) or in combination with liraglutide 3.0 mg.
Methods
The Satiety and Clinical Adiposity—Liraglutide Evidence in individuals with and without diabetes (SCALE) IBT was a 56‐week, randomized, double‐blind, placebo‐controlled, multicenter trial in individuals with obesity who received liraglutide 3.0 mg (n = 142) or placebo (n = 140) as an adjunct to IBT.
Results
At week 56, mean weight loss with liraglutide 3.0 mg plus IBT was 7.5% and 4.0% with placebo combined with IBT (estimated treatment difference [95% CI]–3.4% [–5.3% to –1.6%], P = 0.0003). Significantly more individuals on liraglutide 3.0 mg than placebo achieved ≥ 5% weight loss (61.5% vs. 38.8%; odds ratio [OR] 2.5% [1.5% to 4.1%], P = 0.0003), > 10% weight loss (30.5% vs. 19.8%; OR 1.8% [1.0% to 3.1%], P = 0.0469), and > 15% weight loss (18.1% vs. 8.9%; OR 2.3% [1.1% to 4.7%], P = 0.0311). Liraglutide 3.0 mg in combination with IBT was well tolerated, with no new safety signals identified.
Conclusions
In a primary care setting, Centers for Medicare and Medicaid Services–based IBT produced clinically meaningful weight loss at 56 weeks, enhanced by the addition of liraglutide 3.0 mg.
Study Importance.
What is already known?
-
►
A previous 52‐week, open‐label, single‐site trial assessed the efficacy of intensive behavioral therapy (IBT), as delivered in a specialist setting, either alone or in combination with liraglutide 3.0 mg.
What does this study add?
-
►
The present study provides the first randomized, placebo‐controlled assessment of IBT, in combination with liraglutide 3.0 mg, as tested in a multisite trial with patients from principally primary care practices.
-
►
The study found that IBT with placebo produced clinically meaningful weight loss at 56 weeks in nearly 40% of participants and that weight loss was significantly enhanced by the addition of liraglutide 3.0 mg.
How might these results change the direction of research?
-
►
The results from this study raise questions concerning the extent to which high‐intensity behavioral counseling, as compared with less intensive lifestyle intervention, contributes to additional weight loss with liraglutide 3.0 mg.
Introduction
Expert panels from multiple nations have recommended that individuals with obesity receive comprehensive lifestyle modification to induce a loss of 5% to 10% of baseline body weight, with its associated improvements in cardiometabolic disease risk factors 1, 2, 3, 4. Such programs include a hypocaloric diet, increased physical activity, and behavioral therapy 1, 5. High‐intensity interventions that provide ≥ 14 treatment contacts in the first 6 months produce significantly larger losses than moderate‐ or low‐intensity programs, providing ≤ 1 contact monthly 1, 6.
Based on these findings, the Centers for Medicare and Medicaid Services (CMS) in the United States now reimburses intensive behavioral therapy (IBT) for eligible beneficiaries treated in primary care settings 7. Patients are provided 14 to 15 brief (15‐minute) counseling sessions in the first 6 months. Those who lose ≥ 3 kg are eligible for six additional monthly visits. Under CMS provisions, counseling must be provided by a physician, nurse practitioner, nurse specialist, or physician assistant or by an auxiliary health care professional (e.g., registered dietitian [RD]) who works “incident to” these providers in primary care 8.
To date, there has been only one randomized evaluation of IBT as proposed by CMS 9. It observed a mean loss of 6.1% of baseline weight at 1 year, and 44% of participants lost ≥ 5% of baseline weight. Participants in a second treatment arm in this open‐label trial received IBT combined with liraglutide 3.0 mg, a glucagon‐like peptide‐1 receptor agonist approved as an adjunct to diet and exercise for chronic weight management 10, 11. These participants lost a mean 11.5% of initial weight at 1 year, and 70% lost ≥ 5% of baseline weight, confirming the additive benefits of combining lifestyle modification and weight‐loss medication 12, 13, 14.
The present study also examined the effect of combining IBT and liraglutide 3.0 mg, and it was initiated just a few months after the Wadden et al. 9 investigation began. This study, however, differs in important ways from the prior trial, which was a single‐site investigation conducted in an obesity‐specialty practice, in which the lifestyle interventionists had prior experience with weight management, factors that could limit the generalizability of the findings. By contrast, the present trial was a multisite investigation conducted largely in primary care settings, and it employed interventionists with varying amounts of weight‐management experience.
The present study thus provides the first evaluation, in primary care, of the effect of the CMS‐based IBT benefit delivered alone (with placebo) or combined with liraglutide 3.0 mg. We hypothesized that the combination of IBT and liraglutide would produce significantly greater weight loss at 56 weeks post randomization than would IBT alone.
Methods
Study overview
The Satiety and Clinical Adiposity—Liraglutide Evidence in individuals with and without diabetes (SCALE) IBT trial (NCT02963935) was conducted from February 2017 to June 2018 at 17 sites in the United States. The trial protocol was approved by local ethics committees or institutional review boards, and the study was conducted in accordance with the principles of the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice guidelines 15. The sponsor, Novo Nordisk, developed the study protocol, supplied the trial drugs, planned and performed the statistical analyses, and provided writing assistance.
Study objectives
The primary objective of the trial was to compare the effect of liraglutide 3.0 mg versus placebo, as an adjunct to CMS‐based IBT, on weight loss in individuals with obesity. Secondary objectives were to investigate the effects of these interventions on cardiometabolic and other efficacy end points, as well as to evaluate the safety and tolerability of liraglutide 3.0 mg versus placebo as an adjunct to CMS‐based IBT.
Participants
All individuals provided written informed consent before participation. Eligible participants were aged ≥ 18 years, with stable body weight (maximum 5‐kg self‐reported weight change within 90 days before screening) and BMI ≥ 30 kg/m2. Key exclusion criteria were glycated hemoglobin (HbA1c) ≥ 6.5%, type 1 or 2 diabetes, use of medications (in the past 90 days) known to induce significant weight loss or gain, inadequately treated hypertension, pregnancy or breastfeeding, history of cardiovascular disease, severe congestive heart failure, second‐degree or greater heart block, medullary thyroid carcinoma, multiple endocrine neoplasia type 2, pancreatitis, major depressive disorder within the past 2 years, history of suicide attempt, or malignancy within the past 5 years.
Study design
SCALE IBT was a 56‐week, randomized, double‐blind, placebo‐controlled, two‐armed, multicenter phase 3b trial. Individuals were randomized centrally, using an interactive voice/Web response system, to either liraglutide 3.0 mg or placebo (1:1) as an adjunct to IBT (Figure 1). The trial product was self‐administered once daily by subcutaneous injection. During the first 4 weeks post randomization, the dose was escalated in weekly increments of 0.6 mg to reach the final dose (see online Supporting Information for detailed description). A 30‐day observational follow‐up period was included after the 56 weeks of treatment in accordance with the Food and Drug Administration guidance.
Figure 1.
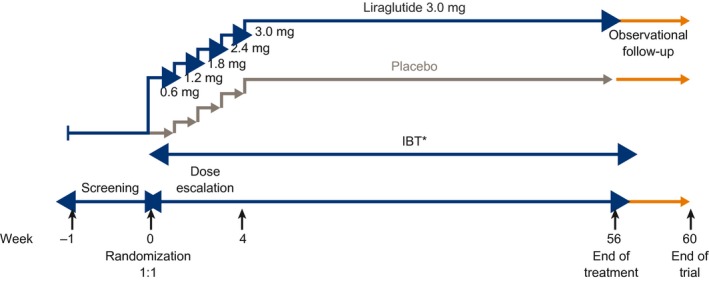
SCALE IBT study design. *IBT, intensive behavioral therapy, comprising behavioral counseling, a hypocaloric diet, and physical activity (building up from 100 to 250 minutes/week).
Throughout the 56 weeks, participants had clinic visits to monitor their response to treatment and received 23 brief (~15‐minute) CMS‐based IBT counseling sessions. Visits were weekly for the first month, every 2 weeks in months 2 to 6, and monthly from months 7 to 13, regardless of whether participants lost ≥ 3 kg during the first 6 months (the CMS requirement for continued treatment after month 6).
The CMS‐based IBT program followed an abbreviated lifestyle counseling protocol adapted from the Diabetes Prevention Program (DPP) 16 for delivery in primary care settings 9, 17, 18. The program was delivered by RDs, which is permitted by CMS if they work “incident to” the primary care providers described previously 8. The RDs were either contractors hired for this specific study or they were already employed at the individual sites. The program included recommendations for diet, physical activity, and behavior change. Participants were encouraged to attend the counseling visits regardless of whether they discontinued study medication. Participants who weighed <91 kg (< 200 lb) at randomization were prescribed 1,200 kcal/d; the caloric prescription for those who weighed 91 to 136 kg (200‐300 lb) was calculated by body weight (pounds) × 6 (kilocalories per pound), and participants who weighed >136 kg (> 300 lb) were prescribed 1,800 kcal/d 1. Diet recommendations were based on current guidance from the US Department of Agriculture, including approximately 15% to 20% of kilocalories from protein, 20% to 35% from fat, and the remainder from carbohydrates 19. All participants were initially prescribed 100 min/wk of moderate‐intensity physical activity (e.g., brisk walking). They were encouraged to be physically active in bouts of 10 minutes or more 20 and to spread their activity across 4 to 5 days each week. Physical activity was increased by 25 minutes every 4 weeks, with an ultimate goal of 250 min/wk.
Before the study began, all RDs attended an in‐person 2.5‐hour training that reviewed key IBT principles and practices, as presented in the provider and participant treatment manuals used in this study 18. Thereafter, RDs had approximately monthly 1‐hour group conference calls that reviewed participants' adherence to the IBT program. Most RDs had experience with weight management but not with a structured IBT protocol.
Study end points
Coprimary end points were change in body weight (percent) from baseline to week 56 and the proportion of participants who lost ≥ 5% of baseline body weight. Secondary confirmatory end points included the proportion of participants who lost > 10% or > 15% of baseline body weight at week 56 and the proportion who lost ≥ 4% of baseline body weight at week 16 (i.e., the US label for liraglutide recommends treatment discontinuation if patients do not lose ≥ 4% of baseline body weight by week 16). A post hoc analysis of the proportion of liraglutide‐IBT participants who met the ≥ 4% weight‐loss‐stopping rule at week 16 and then went on to achieve ≥ 5% weight loss at week 56 is included. Additional post hoc exploratory weight‐related outcomes reported here include mean weight loss and the proportion of participants who lost ≥ 5% of baseline body weight at 6 months (week 28), the proportion of participants who lost ≥ 3 kg at 6 months (CMS criterion for continued behavioral therapy), and, of these patients, how many achieved ≥ 5% weight loss at end of trial (week 56).
Other secondary confirmatory end points were changes from baseline to week 56 in waist circumference and in self‐reported quality of life related to physical function, as measured by the Short Form‐36 v2 Health Survey (Acute Version 2.0 [SF‐36]) physical functioning score and by the physical function scale of the Impact of Weight on Quality of Life‐Lite Clinical Trial version (IWQOL‐Lite‐CT) 21, 22. Change in objective physical capacity was measured by a 6‐minute walk test (6MWT) 23.
Supportive secondary end points included change from baseline to week 56 in cardiometabolic parameters (HbA1c, fasting plasma glucose, systolic and diastolic blood pressure, and lipids). Changes from baseline to week 56 were also evaluated for other domains of health‐related quality of life, as measured by the SF‐36 and IWQOL‐Lite‐CT. Safety was assessed by adverse events, physical examination, resting pulse, electrocardiogram, and laboratory measurements. Safety was assessed using two different observation periods. The in‐trial period included time from randomization to the final follow‐up visit (or date of last contact) regardless of trial product discontinuation. The on‐drug period was the date of the first trial product administration to 14 days after the final trial product administration, and it excluded off‐drug time intervals triggered by at least 14 days off trial product.
Statistical considerations
The planned sample size of 282 participants, with a 1:1 randomization and assumption of 30% discontinuation rate, resulted in a combined power of 88.5%, estimated to be adequate to evaluate the two coprimary end points. Power for the continuous end point, percentage weight change, was calculated with a two‐sided t test, whereas power for the categorical end point, 5% responders, was calculated using a two‐sided χ2 test, both at a 5% significance level. The two coprimary end points were tested in hierarchal order.
To estimate the intervention effect, a treatment policy estimand (primary estimand using the intention‐to‐treat [ITT] principle) was defined for each efficacy end point, consistent with the updated regulatory guidelines of the International Council for Harmonisation 24. The treatment policy estimand evaluated the effect of liraglutide 3.0 mg versus placebo at week 56 (or week 16) for all randomized individuals regardless of premature discontinuation of trial product. Missing values at week 56 were imputed from the placebo arm using a jump‐to‐reference multiple imputation approach 25. For the coprimary end points and confirmatory secondary end points, a secondary trial product estimand was calculated (using an if‐all‐adhered‐to‐treatment principle). Using a mixed model for repeated measurements, this analysis evaluated the treatment effect of liraglutide 3.0 mg versus placebo at week 56 for all randomized individuals, with the assumption that all participants remained on trial product 24. Continuous end points were analyzed with an ANCOVA and categorical end points with logistic regression (see Supporting Information for additional information).
Results
Trial population
In total, 328 individuals were screened and 282 randomized, with 142 assigned to liraglutide 3.0 mg combined with IBT (liraglutide‐IBT) and 140 to placebo combined with IBT (placebo‐IBT). Baseline demographics were well matched between treatment arms (Table 1).
Table 1.
Participants' baseline demographics and anthropometry
| Liraglutide‐IBT | Placebo‐IBT | |
|---|---|---|
| Number of participants, n (N) | 142 (142) | 140 (140) |
| Male sex, n (%) | 23 (16.2) | 24 (17.1) |
| Age, years, mean (SD) | 45.4 (11.6) | 49.0 (11.2) |
| Race, n (%) | ||
| White | 112 (78.9) | 115 (82.1) |
| Black | 27 (19.0) | 22 (15.7) |
| Asian | 2 (1.4) | 3 (2.1) |
| Ethnicity: not Hispanic or Latino, n (%) | 118 (83.1) | 131 (93.6) |
| Body weight, kg, mean (SD) | 108.5 (22.1) | 106.7 (22.0) |
| BMI, kg/m2, mean (SD) | 39.3 (6.8) | 38.7 (7.2) |
| Waist circumference, cm, mean (SD) | 116 (14.4) | 115 (15.6) |
| HbA1c, %, mean (SD) | 5.5 (0.4) | 5.5 (0.4) |
| FPG, mmol/L, mean (SD) | 5.4 (0.5) | 5.4 (0.6) |
| Heart rate, beats/min, mean (SD) | 72 (10) | 73 (9) |
| SBP, mmHg, mean (SD) | 125 (15) | 127 (14) |
| DBP, mmHg, mean (SD) | 80 (9) | 81 (8) |
| Total cholesterol, mmol/L, mean (SD) | 4.9 (0.9) | 5.1 (1.0) |
| LDL cholesterol, mmol/L, mean (SD) | 2.9 (0.8) | 3.1 (0.9) |
| HDL cholesterol, mmol/L, mean (SD) | 1.3 (0.3) | 1.4 (0.4) |
| VLDL cholesterol, mmol/L, mean (SD) | 1.4 (0.4) | 0.6 (0.3) |
| Triglycerides, mmol/L, mean (SD) | 1.5 (1.2) | 1.4 (0.7) |
| Free fatty acids, mmol/L, mean (SD) | 0.5 (0.3) | 0.5 (0.3) |
Data from the “all randomized” analysis set.
DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; IBT, intensive behavioral therapy; LDL, low‐density lipoprotein; SBP, systolic blood pressure; VLDL, very low‐density lipoprotein.
A high proportion of participants completed the trial (99% in the liraglutide‐IBT and 93% in the placebo‐IBT arm) and remained on the study drug at week 56 (80.3% and 73.6%, respectively) (Figure 2; Supporting Information Table S1). Liraglutide‐IBT and placebo‐IBT participants attended a mean of 22.5 and 21.2 visits, respectively, of 23 possible treatment visits, corresponding to mean (SD) adherence rates of 97.8% (9.7%) and 92.1% (18.1%). In the liraglutide‐IBT group, 89% of participants attended all visits, compared with 74% in the placebo‐IBT group.
Figure 2.
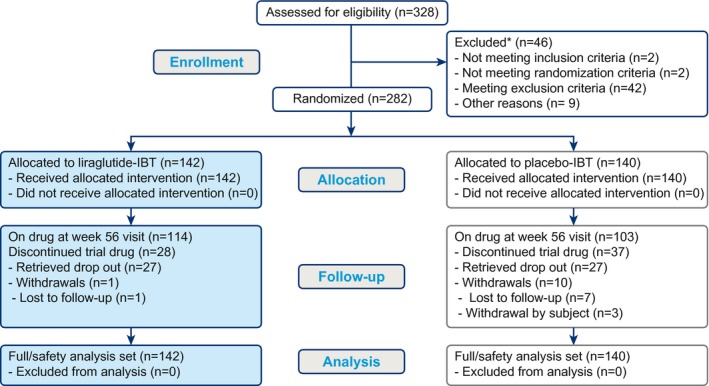
Patient disposition. *A given individual may be excluded for more than one reason. IBT, intensive behavioral therapy.
Body weight
Figure 3 shows the observed mean weight loss over time in the two groups. The estimated mean weight change at week 56 (treatment policy estimand, ITT principle) was −7.5% for liraglutide‐IBT and −4.0% for placebo‐IBT (estimated treatment difference [ETD] [95% CI] −3.4% [−5.3% to −1.6%], P = 0.0003; Supporting Information Figure S1A). For the trial product estimand (if‐all‐adhered principle), estimated mean weight change at 56 weeks was −8.4% for liraglutide‐IBT and −3.8% for placebo‐IBT (ETD −4.6% [−6.5% to −2.6%], P < 0.0001; Supporting Information Figure S1B). Weight change in individuals who remained on the trial product at 56 weeks was −9.1% (n = 114) and −4.8% (n = 103), respectively (Figure 3).
Figure 3.
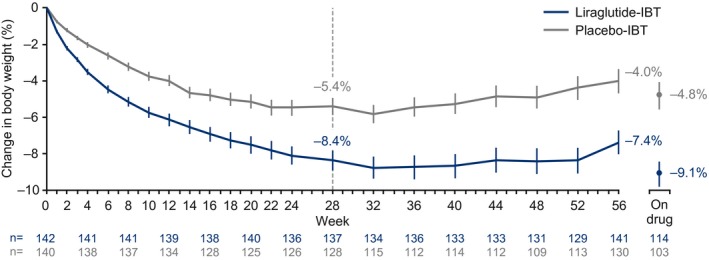
Change in body weight over time. Observed mean data ± SEM based on all in‐trial observations. Data from individuals who discontinued the trial product and returned for week 56 assessments are included. IBT, intensive behavioral therapy.
For the remaining end points, only the treatment policy estimand data (ITT principle) are reported in the main text (i.e., Figure 4 and Table 2). The corresponding trial product estimand data (if‐all‐adhered principle) for the secondary confirmatory end points are shown in Supporting Information Table S2.
Figure 4.
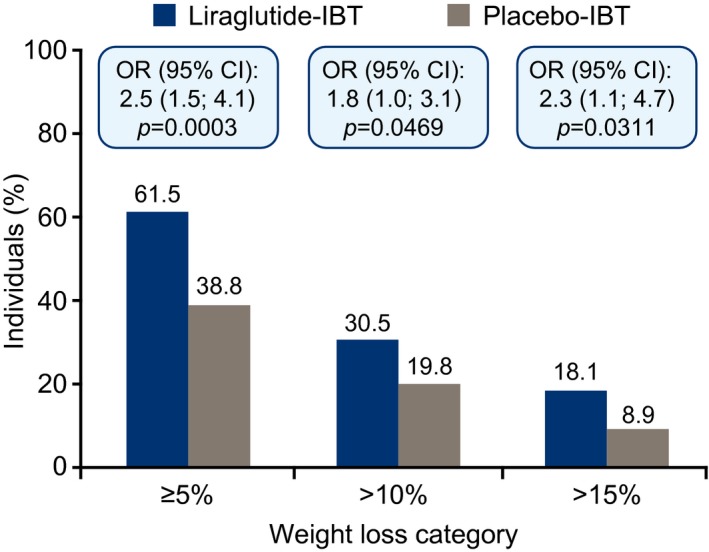
Weight loss of ≥ 5%, > 10%, or > 15% of baseline body weight at week 56. Data are for primary treatment policy estimand, logistic regression, J2R‐MI. IBT, intensive behavioral therapy; J2R‐MI, jump‐to‐reference multiple imputation.
Table 2.
Changes in secondary end points from baseline to week 56: treatment policy estimand
| Liraglutide‐IBT | Placebo‐IBT | ETD (95% CI) | P | |
|---|---|---|---|---|
| Waist circumference (cm) | −9.4 | −6.7 | −2.7 (−4.7 to −0.8) | 0.0063 |
| SF‐36 physical functioning score | 4.0 | 3.8 | 0.2 (−1.2 to 1.5) | 0.8137 |
| IWQOL‐Lite‐CT Physical Function score | 14.9 | 14.1 | 0.9 (−3.4 to 5.1) | 0.6916 |
| 6MWT (m) | 49.5 | 46.3 | 3.1 (−12.7 to 18.9) | 0.6986 |
| HbA1c (%) | −0.16 | −0.06 | −0.10 (−0.2 to −0.04) | 0.0008 |
| Fasting plasma glucose (mmol/L) | −0.23 | 0.01 | −0.23 (−0.36 to −0.11) | 0.0002 |
| Heart rate (beats/min) | 1.9 | 0.6 | 1.3 (−0.8 to 3.4) | 0.2287 |
| SBP (mmHg) | −2.8 | −0.6 | −2.2 (−4.9 to 0.5) | 0.1119 |
| DBP (mmHg) | −1.0 | −0.8 | −0.2 (−2.12 to 1.8) | 0.8691 |
| Total cholesterol (mmol/L) | −0.04 | 0.06 | −0.10 (−0.26 to 0.06) | 0.2163 |
| LDL cholesterol (mmol/L) | −0.04 | 0.04 | −0.07 (−0.21 to 0.06) | 0.2700 |
| HDL cholesterol (mmol/L) | 0.05 | 0.03 | 0.02 (−0.02 to 0.07) | 0.3323 |
| VLDL cholesterol (mmol/L) | −0.06 | −0.01 | −0.05 (−0.11 to 0.01) | 0.1355 |
| Triglycerides (mmol/L) | −0.17 | −0.05 | −0.12 (−0.26 to 0.02) | 0.0951 |
| Free fatty acids (mmol/L) | −0.08 | −0.06 | −0.02 (−0.08 to 0.04) | 0.4753 |
Baseline to week 56 vs. placebo. ANCOVA‐jump‐to‐reference multiple imputation, full analysis set.
DBP, diastolic blood pressure; ETD, estimated treatment difference; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; IBT, intensive behavioral therapy; IWQOL‐Lite‐CT, Impact of Weight on Quality of Life‐Lite Clinical Trials Version; LDL, low‐density lipoprotein; SBP, systolic blood pressure; SF‐36, 36‐Item Short Form Health Survey; VLDL, very low‐density lipoprotein; 6MWT, 6‐minute walk test.
The proportion of participants who achieved ≥ 5% weight loss at 56 weeks was 61.5% with liraglutide‐IBT and 38.8% with placebo‐IBT (OR 2.5% [1.5%‐4.1%], P = 0.0003) (Figure 4). The proportions who lost > 10% were 30.5% and 19.8%, respectively, and > 15% were 18.1% and 8.9%, respectively (statistical comparisons shown in Figure 4).
A greater proportion of participants treated with liraglutide‐IBT than placebo‐IBT (78.7% vs. 52.7%, respectively) lost ≥ 4% of baseline weight at week 16 (Supporting Information Figure S2). This criterion qualifies patients as early responders, eligible for long‐term liraglutide treatment, according to the US label. The majority of individuals on liraglutide‐IBT who achieved this criterion achieved ≥ 5% weight loss at week 56 (72.2%).
Observed mean weight change at week 28 was −8.4% in individuals receiving liraglutide‐IBT and −5.4% for placebo‐IBT (Figure 3). Post hoc exploratory analyses showed that 82.4% and 57.9% of these individuals, respectively, met the CMS criterion for continued behavioral treatment (weight loss ≥ 3 kg at 6 months); 69.0% and 44.3%, respectively, lost ≥ 5% of baseline weight at this time (Supporting Information Figure S3). The majority of individuals who achieved the CMS criterion for continued treatment achieved ≥ 5% weight loss at week 56 (68.4% on liraglutide‐IBT vs. 58.0% on placebo‐IBT).
Cardiometabolic parameters and assessments of physical function and capacity
Changes in secondary end points from baseline to week 56 are summarized in Table 2. Waist circumference decreased significantly more in liraglutide‐IBT than in the placebo‐IBT participants (−9.4 vs. −6.7 cm [ETD −2.7 cm, P = 0.0063]). Significant differences between groups in favor of liraglutide‐IBT also were observed for changes in both HbA1c (−0.2% vs. −0.1% [ETD −0.1%, P = 0.0008]) and fasting plasma glucose (−0.2 vs. 0.01 mmol/L [ETD −0.2 mmol/L, P = 0.0002]).
Improvements in lipids and systolic and diastolic blood pressure were also observed in both groups at week 56 but with no significant differences between treatment arms (Table 2). Heart rate increased in both groups, with a numerically larger increase with liraglutide‐IBT compared with placebo‐IBT.
Improvements in physical function were observed for both groups, as measured by the IWQOL‐Lite‐CT physical function score (14.9 for liraglutide‐IBT vs. 14.1 for placebo‐IBT; ETD 0.9 [95% CI: −3.4 to 5.1; P = 0.6916]) and the SF‐36 physical functioning score (4.0 vs. 3.8; ETD 0.2 [95% CI: −1.2 to 1.5; P = 0.8137]) (Table 2; Supporting Information Figure S4). These changes met the criteria for a clinically meaningful improvement 21. Both groups also improved on the 6MWT (49.5 m vs. 46.3 m; ETD 3.1 [95% CI: −12.7 to 18.9; P = 0.6986]) but with no significant difference between groups.
Safety
Liraglutide 3.0 mg combined with IBT was generally well tolerated, and adverse events (AEs) were consistent with the established safety profile for liraglutide 3.0 mg as used for weight management 26, 27, 28. No new safety signals were identified. The most frequent AEs were gastrointestinal (71% with liraglutide‐IBT vs. 49% with placebo‐IBT) (Table 3; Supporting Information Table S3).
Table 3.
Adverse events (on drug)
| Liraglutide‐IBT | Placebo‐IBT | |||||||
|---|---|---|---|---|---|---|---|---|
| n | % | E | R | n | % | E | R | |
| Number of participants | 142 | 140 | ||||||
| Total adverse events | 136 | 95.8 | 867 | 609.0 | 124 | 88.6 | 601 | 452.1 |
| Serious adverse events | 6 | 4.2 | 7 | 4.9 | 2 | 1.4 | 2 | 1.5 |
| Fatal adverse events | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Events leading to treatment discontinuation | 12 | 8.5 | 20 | 14.0 | 6 | 4.3 | 15 | 11.3 |
| GI adverse events | 101 | 71.1 | 341 | 239.5 | 68 | 48.6 | 149 | 112.1 |
| Nausea | 68 | 47.9 | 102 | 71.6 | 25 | 17.9 | 33 | 24.8 |
| Constipation | 43 | 30.3 | 57 | 40.0 | 26 | 18.6 | 34 | 25.6 |
| Diarrhea | 31 | 21.8 | 47 | 33.0 | 23 | 16.4 | 26 | 19.6 |
| Vomiting | 33 | 23.2 | 47 | 33.0 | 7 | 5.0 | 7 | 5.3 |
| Abdominal discomfort | 8 | 5.6 | 9 | 6.3 | 4 | 2.9 | 4 | 3.0 |
Safety analysis set. Adverse event considered “on‐drug” if any dose of trial product administered within prior 14 days.
E, number of events; IBT, intensive behavioral therapy; GI, gastrointestinal; n, number of individuals; R, event rate per 100 years of exposure time.
The incidence of nausea was greater with liraglutide‐IBT (47.9%) than with placebo‐IBT (17.9%) (Table 3). Most events were mild or moderate in severity and occurred primarily within the first 4 to 8 weeks of treatment, as shown for nausea in Supporting Information Figure S5.
Three acute gallstone disease events (one cholelithiasis, two cholecystitis) occurred with liraglutide‐IBT and two (one cholelithiasis, one gallbladder hypofunction) with placebo‐IBT. No events of pancreatitis were observed in the study.
The proportion of participants with reported serious AEs was 4.2% (seven events in six participants) in liraglutide‐IBT and 1.4% (two events in two participants) with placebo‐IBT (Table 3; Supporting Information Table S4). These events were distributed across seven “System Organ Classes.” There were no deaths.
More participants reported neoplasms in the liraglutide‐IBT (16 AEs in 15 participants) than placebo‐IBT group (seven AEs in seven participants). Two events in the liraglutide‐IBT group were serious (one case of in situ/premalignant papillary thyroid cancer and one benign ovarian cyst). The remaining events were nonserious and, of these, all except one (pulmonary mass in the placebo group) were reported as benign. No cases of breast cancer or medullary thyroid carcinoma were reported. No participants randomized to liraglutide‐IBT reported AEs related to depression or suicidal ideation or behavior.
Discussion
In individuals with obesity, liraglutide‐IBT was superior to placebo‐IBT in reducing baseline body weight and producing a clinically meaningful ≥ 5% weight loss at week 56. Thus, the trial's primary objective was met.
Participants treated with liraglutide‐IBT, compared with placebo, also achieved significantly greater reductions in cardiometabolic risk factors, including waist circumference, HbA1c and fasting plasma glucose. The greater reductions in the latter two variables are likely attributable to the direct effects of liraglutide on glycemic control.
Clinically relevant improvements in physical function were observed in both groups, as measured by IWQoL‐Lite‐CT and SF‐36. Both groups also improved on the 6MWT. However, differences between the two groups were not significant, perhaps because the same physical activity interventions were included in both treatment arms.
Placebo‐adjusted weight loss in the present SCALE IBT study (3.4% [5.3%−1.6%]) was smaller than in the SCALE Obesity and Prediabetes trial (5.4% [5.8% −5.0%]) 27. However, different statistical analyses were applied in the two studies, making the estimated treatment differences difficult to compare. It may be more appropriate to compare weight loss in those individuals still on trial product at week 56, although differences in attrition in the studies could lead to a potential overestimation of efficacy in SCALE Obesity and Prediabetes. In SCALE IBT, liraglutide‐treated participants who remained on the trial product lost 9.1% of their baseline body weight at week 56 versus 4.8% with placebo. In the SCALE Obesity and Prediabetes study, trial completers achieved a 9.2% loss with liraglutide versus 3.5% with placebo. Thus, the placebo‐IBT group in SCALE IBT appeared to induce a slightly greater weight loss than the placebo group in SCALE Obesity and Prediabetes, whereas weight losses in the liraglutide‐treated arms were very similar in the two trials.
The lifestyle intervention was less intensive in the SCALE Obesity and Prediabetes trial; participants were instructed to increase physical activity to at least 150 minutes/week and reduce daily energy intake by 500 kcal. All participants received standardized lifestyle counseling approximately monthly, with 15 sessions in 56 weeks. Thus, we had expected somewhat greater absolute weight loss in SCALE IBT given the more intensive diet and physical activity intervention and the provision of 23 counseling visits in 56 weeks. Placebo‐IBT participants, in fact, lost a mean 5.4% of baseline at week 28 (supported by 15 brief counseling sessions) but maintained a loss of only 4.0% at week 56 (potentially because of the reduced frequency of IBT visits during the second half of the study).
In SCALE IBT, 78.7% of participants treated with liraglutide‐IBT met the US label stopping rule of ≥ 4% weight loss by week 16, and of these individuals, almost three‐quarters (72.2%) achieved ≥ 5% weight loss at week 56. These numbers are similar to those observed in SCALE Obesity and Prediabetes, in which 77.3% of individuals treated with liraglutide 3.0 mg, as an adjunct to “standard” diet and exercise recommendations, met the week 16 ≥ 4% weight‐loss criterion 29. Fully 84.1% of these qualifying individuals went on to achieve ≥ 5% weight loss at week 56 29. Taken together, these data demonstrate the usefulness of the US label stopping rule in predicting long‐term weight loss.
To our knowledge, SCALE IBT represents only the second randomized controlled evaluation of the CMS‐based IBT benefit alone and combined with liraglutide 3.0 mg. The previously described trial by Wadden et al. 9 observed a significantly greater mean 1‐year weight loss of 11.5% in participants who received IBT‐liraglutide (n = 50) compared with a loss of 6.1% for those treated with IBT alone (n = 50). At week 24, participants in the two groups lost 10.1% and 5.4% of their baseline body weight, respectively, with 86% and 56% losing ≥ 3 kg and thus being eligible for additional IBT visits according to CMS criteria. A total of 78% and 46% of participants in the two groups, respectively, lost ≥ 5% of baseline weight at week 24. In the present SCALE IBT trial, at week 28, participants in the liraglutide‐IBT arm lost 8.4% of baseline weight versus 5.4% for placebo‐IBT; 82.4% and 57.9% of these participants, respectively, lost ≥ 3 kg, and 69.0% and 44.3%, respectively, lost ≥ 5% of baseline weight. Moreover, the majority of participants who met the CMS criterion went on to achieve weight losses ≥ 5% at week 56 (68.4% and 58.0% for liraglutide‐IBT and placebo‐IBT, respectively), supporting the clinical utility of this stopping rule.
The percentage of participants who attended all treatment visits was higher in SCALE IBT (89% in liraglutide‐IBT and 74% in placebo‐IBT) than in the Wadden et al. trial 9 (60% in liraglutide‐IBT and 40% in IBT alone). Similarly, mean adherence to scheduled visits was also higher in SCALE IBT (97.8% in liraglutide‐IBT; 92.1% in placebo‐IBT) versus the Wadden et al. trial 9 (91.2% in liraglutide‐IBT; 72.4% in IBT alone). Thus, differences in participants' adherence would not appear to explain the smaller weight losses in the SCALE IBT trial.
There were, however, several major differences in experimental design between the two studies in addition to their sample sizes. Wadden et al. 9 used an open‐label (rather than double‐blind) design, and the study was conducted at a single site in an obesity‐specialty practice, where all interventionists had weight‐management experience. By contrast, the present SCALE IBT multisite trial was conducted largely at primary‐care–oriented sites, and interventionists had limited exposure to structured IBT. Of the 17 sites in this trial, four were obesity‐specialty practices, with the remaining 13 characterized as family‐, general‐, or internal‐medicine practices. Participants recruited at obesity‐specialty practices may be more motivated to lose weight than those recruited in more general medical practices. All of these factors could have contributed to the larger weight losses observed in the Wadden et al. study 9.
Findings from the present SCALE IBT study provide the best available estimate of the effectiveness of CMS‐based IBT delivered in a primary care setting and show that approximately half of participants can achieve the CMS‐IBT weight‐loss criterion (≥ 3 kg) at 6 months with brief counseling, which can be further increased when combined with the addition of liraglutide 3.0 mg. We note, however, that SCALE IBT used an adapted version of the highly successful DPP 16, 17. The CMS‐IBT program 7 does not recommend a specific IBT intervention for primary care practitioners to use, except for noting that it should be consistent with the 5A approach to health counseling (assess, advise, agree, assist, and arrange) 30. We believe that the CMS‐IBT program will be most effective when used with a structured weight‐management protocol, such as that employed by Wadden et al. 9, 18 and by the present study.
Strengths of our study, relative to previous trials, include the large sample of participants, recruited at 17 principally primary care sites, and use of a double‐blind placebo‐controlled design. Study retention was outstanding, with 96% of participants providing a measured weight at 56 weeks. A major limitation is the inability to determine the precise amount of IBT needed to achieve clinically meaningful weight loss when combined with liraglutide 3.0 mg.
Results from this study raise questions concerning the extent to which high‐intensity behavioral counseling (i.e., 14‐15 sessions in 6 months) contributes to additional weight loss with liraglutide 3.0 mg given the comparable weight losses achieved with approximately monthly lifestyle counseling provided in the SCALE Obesity and Prediabetes study 27. The latter approach may provide a less resource‐demanding and less costly means of providing the lifestyle counseling needed in a nonspecialist setting. Additional trials, currently being conducted or planned, should yield further insight on this question.
Clinical trial registration
https://www.ClinicalTrials.gov identifier NCT02963935.
Funding agencies
The study was sponsored by Novo Nordisk.
Disclosure
TW has received grants, on behalf of the University of Pennsylvania, from Novo Nordisk as well as honoraria from Novo Nordisk and WW (formerly Weight Watchers) for serving on scientific advisory boards. JST has received consulting fees/honoraria from Novo Nordisk. DS has received research grants from Novo Nordisk. DR has received grants from Obesinov SARL as well as consulting fees/honoraria from Novo Nordisk. MTL is an employee of Novo Nordisk and owns stock in the company. PA is an employee of Novo Nordisk. CJ is an employee of Novo Nordisk.
Supporting information
Acknowledgments
The authors are grateful to the patients who participated in this study; to Lars Endahl and Lisa von Huth Smith, Novo Nordisk, for review of and input to the manuscript; and to Adam Dagnall, Watermeadow Medical, an Ashfield company (supported by Novo Nordisk), for writing assistance.
Individual participant data will be shared in data sets in deidentified/ anonymized format. Study protocol and redacted Clinical Study Report are available according to Novo Nordisk data sharing commitments. Data will be shared with bona fide researchers submitting a research proposal requesting data access.
References
- 1. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129(suppl 2):S102‐S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lau DC; Obesity Canada Clinical Practice Guidelines Steering Committee and Expert Panel . Synopsis of the 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ 2007;176:1103‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/. Published February 16, 2018. Accessed August 8, 2019.
- 4. National Institute for Health and Care Excellence . Obesity: identification, assessment and management. Clinical guideline [CG189]. https://www.nice.org.uk/guidance/cg189. Published November 2014. Accessed August 8, 2019.
- 5. Webb VL, Wadden TA. Intensive lifestyle intervention for obesity: principles, practices, and results. Gastroenterology 2017;152:1752‐1764. [DOI] [PubMed] [Google Scholar]
- 6. Leblanc ES, O'Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care‐relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:434‐447. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Medicare and Medicaid Services (CMS) . Decision memo for intensive behavioral therapy for obesity (CAG‐00423N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=253. Published November 29, 2011. Accessed August 8, 2019.
- 8. Centers for Medicare and Medicaid Services (CMS) . Services and supplies incident to a physician's professional services: conditions, 42 CFR §410.26. https://www.govinfo.gov/app/details/CFR-2011-title42-vol2/CFR-2011-title42-vol2-sec410-26. Published 2011. Accessed August 8, 2019.
- 9. Wadden TA, Walsh OA, Berkowitz RI, et al. Intensive behavioral therapy for obesity combined with liraglutide 3.0 mg: a randomized controlled trial. Obesity (Silver Spring) 2019;21:75‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Food and Drug Administration (FDA) . Drugs. https://www.fda.gov/Drugs/default.htm. Accessed August 8, 2019.
- 11. European Medicines Agency . Medicines. https://www.ema.europa.eu/. Accessed August 8, 2019.
- 12. Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med 2005;353:2111‐2120. [DOI] [PubMed] [Google Scholar]
- 13. Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med 2009;150:255‐262. [DOI] [PubMed] [Google Scholar]
- 14. Craighead LW, Stunkard AJ, O'Brien RM. Behavior therapy and pharmacotherapy for obesity. Arch Gen Psychiatry 1981;38:763‐768. [DOI] [PubMed] [Google Scholar]
- 15. World Medical Association . Declaration of Helsinki. Helsinki, Finland: WMA General Assembly; 1964. [Google Scholar]
- 16. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wadden TA, Volger S, Sarwer DB, et al. A two‐year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011;365:1969‐1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wadden TA, Tsai AG, Tronieri JS. A protocol for delivering intensive behavioral therapy (IBT) for obesity in primary care settings: the MODEL‐IBT Program. Obesity (Silver Spring) 2019;27:1562‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US Department of Health and Human Services, US Department of Agriculture . 2015‐2020 Dietary Guidelines for Americans. 8th ed. http://health.gov/dietaryguidelines/2015/guidelines/. Published December 2015. Accessed August 9, 2019. [Google Scholar]
- 20. Jakicic JM, Rogers RJ, Sherman SA, Kovacs SJ. Physical activity and weight management In: Wadden TA, Bray GA, eds. Handbook of Obesity Treatment. 2nd ed New York: Guilford Press; 2018:322‐335. [Google Scholar]
- 21. Maruish ME, ed. User's Manual for the SF‐36v2 Health Survey. Lincoln, RI: QualityMetric Incorporated; 2011. [Google Scholar]
- 22. Kolotkin RL, Williams VSL, Ervin CM, et al. Validation of a new measure of quality of life in obesity trials: Impact of Weight on Quality of Life‐Lite Clinical Trials Version. Clin Obes 2019;9:e12310. doi: 10.1111/cob.12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Thoracic Society (ATS) Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002;166:111‐117. [DOI] [PubMed] [Google Scholar]
- 24. US Food and Drug Administration. International Council for Harmonisation (ICH) . ICH harmonised guideline: estimands and sensitivity analysis in clinical trials. E9(R1). https://www.fda.gov/media/108698/download. Published June 16, 2017. Accessed August 8, 2019.
- 25. Carpenter JR, Roger JH, Kenward MG. Analysis of longitudinal trials with protocol deviation: a framework for relevant, accessible assumptions, and inference via multiple imputation. J Biopharm Stat 2013;23:1352‐1371. [DOI] [PubMed] [Google Scholar]
- 26. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015;314:687‐699. [DOI] [PubMed] [Google Scholar]
- 27. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11‐22. [DOI] [PubMed] [Google Scholar]
- 28. le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double‐blind trial. Lancet 2017;389:1399‐1409. [DOI] [PubMed] [Google Scholar]
- 29. Fujioka K, O'Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1‐year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring) 2016;24:2278‐2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glasgow RE, Emont S, Miller DC. Assessing delivery of the five 'As' for patient‐centered counseling. Health Promot Int 2006;21:245‐255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


