Abstract
Background and objective
Dorsal root ganglion stimulation (DRGS) has recently emerged as a neuromodulation modality in the treatment of chronic neuropathic pain. The objective of this study was to compare the efficacy of different Burst‐DRGS amplitudes in an experimental model of painful diabetic peripheral neuropathy (PDPN).
Methods
Diabetes mellitus was induced in female Sprague–Dawley rats by intraperitoneal injection of streptozotocin (STZ, n = 28). Animals were tested for mechanical hypersensitivity (von Frey paw withdrawal test) before, and four weeks after STZ injection. PDPN rats (n = 13) were implanted with a unilateral bipolar electrode at the L5 DRG. Animals received Burst‐DRGS at 0%, 10%, 33%, 50%, 66%, and 80% of motor threshold (MT) in a randomized crossover design on post‐implantation days 2–7 (n = 9). Mechanical hypersensitivity was assessed before stimulation onset, 15 and 30 min during stimulation, and 15 and 30 min after stimulation.
Results
Burst‐DRGS at amplitudes of 33%, 50%, 66%, and 80% MT resulted in significant attenuation of STZ‐induced mechanical hypersensitivity at 15 and 30 min during stimulation, as well as 15 min after cessation of stimulation. No effect on mechanical hypersensitivity was observed for Burst‐DRGS at 0% MT and 10% MT. Optimal pain relief and highest responder rates were achieved with Burst‐DRGS at 50–66% MT, with an estimated optimum at 52% MT.
Conclusion
Our findings indicate a nonlinear relationship between Burst‐DRGS amplitude and behavioral outcome, with an estimated optimal amplitude of 52% MT. Further optimization and analysis of DRGS driven by insights into the underlying mechanisms related to the various stimulation paradigms is warranted.
Keywords: burst stimulation, dorsal root ganglion stimulation, neuromodulation, neuropathic pain, painful diabetic peripheral neuropathy, Von Frey
INTRODUCTION
Painful diabetic peripheral neuropathy (PDPN) is a frequent and disabling complication of diabetes mellitus (DM) 1, 2, 3. In PDPN, small fibers of the Aδ and C type are damaged, leading to subsequent neuropathic pain in extremities and often starting in the lower limbs 4. As the effectiveness of pharmacological interventions in PDPN is limited 5, there is an urgent need for new nonpharmacological approaches. Spinal cord stimulation of the dorsal columns with conventional settings (Con‐SCS) is an established last‐resort treatment for PDPN patients who are refractory to pharmacological interventions. The effectiveness of Con‐SCS in patients with PDPN has been demonstrated in two randomized clinical trials (RCTs) 6, 7. However, despite considerable improvements with Con‐SCS, Con‐SCS has limitations with regard to effect size, responder rate, specificity, stability, and energy consumption 6, 7, 8, 9, 10, 11. Recently, dorsal root ganglion stimulation (DRGS) was developed and expected to overcome some of the limitations observed with Con‐SCS.
Since the implantation of the first fully implantable DRGS system in 2011 9, DRGS has shown clinical success in groin pain, axial back pain 12, leg and foot pain,9 complex regional pain syndrome (CRPS) 13, chest wall pain14 and postamputation pain syndromes 15. Interestingly, a recent study by Eldabe and colleagues showed DRGS to be an effective and safe neuromodulation technique to improve painful symptoms in PDPN patients 16. However, despite considerable improvements in terms of pain scores using DRGS, many PDPN patients still experience unsatisfactory pain relief 16.
Besides changes in the anatomical target of stimulation, the introduction of novel stimulation waveforms has also aided to the field of spinal cord stimulation (SCS) in recent years. SCS paradigms that use bursting patterns 17 offer a paresthesia‐free solution to conventional Con‐SCS, and decrease pain intensity to a greater degree than Con‐SCS in some studies 18, 19. Nevertheless, only few studies have investigated the combination of both novel anatomical targeting such as DRGS, and the use of novel stimulation waveforms, such as burst stimulation.
In a previous experimental study, we showed that Burst‐DRGS in a rat model of PDPN showed signs of a residual effect after cessation of stimulation, which was accompanied with higher responder rates when compared to conventional DRGS (Con‐DRGS) 20. Although the results of this study already seem to favor Burst‐DRGS over Con‐DRGS, there is still room for improvement in terms of the optimal stimulation settings of Burst‐DRGS. Previous experimental electrophysiological and behavioral studies suggest that Burst‐SCS can be optimized by changing stimulation settings that are related to the amount of energy delivered to the system, such as amplitude and pulse width 21, 22, 23. This study aims to investigate the effect of stimulation intensity on the therapeutic efficacy or pain relief of Burst‐DRGS in an experimental rat model of PDPN.
METHODS
Ethical Statement
All experiments were performed in accordance with the European Directive for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (86/609/EU). The study was approved by the Central Authority for Scientific Procedures on Animals, The Netherlands (project license 2017‐022).
Animals
Experiments were conducted in female Sprague–Dawley rats (170–210 g at study onset; n = 28). Animals were housed in pairs in polycarbonate cages placed in a controlled environment (temperature 21 ± 1°C, humidity 55 ± 15%) in a reversed day/night cycle. Animals had ad libitum access to distilled water and food. Before onset of the experiments, the animals were habituated to the housing facility for one week without experimenter contact.
Induction of DM
DM was induced by intraperitoneal injection of Streptozotocin (STZ; Sigma‐Aldrich, Schnelldorf, Germany). Animals were weighed and fasted overnight, after which STZ was freshly dissolved in 0.9% NaCl to achieve a solution of 65 mg/mL. 65 mg/kg STZ was then injected in order to induce DM. One week following STZ injection, animals were tested for blood glucose levels via blood derived from the saphenous vein (Accu‐Chek Aviva®, Roche Diagnostics GmbH, Mannheim, Germany). Rats with a blood glucose level of ≥15 mmol/L were considered diabetic 24. In case of excessively high glucose values (>31.4 mmol/L), one‐third of a slow releasing insulin pellet (LinShin Canada, Inc.) was placed subcutaneously in the trunk of the animal.
Assessment of Mechanical Hypersensitivity
Mechanical hypersensitivity was assessed by determining the paw withdrawal threshold of the hind paw of the animals to calibrated Von Frey filaments. In short, animals were placed on an elevated mesh floor in a transparent box. Animals were allowed to acclimatize to the testing environment for 15 min. A series of calibrated Von Frey filaments (0.6, 1.2, 2.0, 3.6, 5.5, 8.5, 15.1, and 28.84 g) were then applied to the plantar surface of the hind paw for 5 sec using the “up‐down” method 25. The 50% withdrawal threshold (WT) was calculated after completion of a sequence of six consecutive responses. A cutoff value of 28.84 g was defined to prevent tissue damage. Lastly, the 50% WT was multiplied by 10,000 and logarithmically transformed to account for Weber's law and obtain a linear scale 26.
Only animals displaying mechanical hypersensitivity at four weeks after STZ injection were implanted and treated with DRGS. Animals without mechanical hypersensitivity were excluded from the study. The presence of mechanical hypersensitivity was defined as a decrease of ≥0.2 unit in log10 (10,000 x 50% WT) when compared to pre‐STZ values 27, 28, 29.
DRGS
For DRGS, the lead fashioned out of two platinum‐iridium wires (diameter 0.010 and 0.005 in.) custom made for experimental studies, was unilaterally implanted adjacent to the left L5 DRG as previously described 20, 30. Via paravertebral incision the intervertebral foramen was exposed at the level of the L5 spinal nerve. The entry of the lead into the foramen was ensured by a blunt nerve hook gently probing inside the foramen. Then the lead was inserted into the foramen and secured to the transverse process of L6 using a small stainless steel wire and screw (diameter 0.86 mm, length 3.2 mm). Lastly, the lead was subcutaneously tunneled through the neck of the animal, after which the wounds were closed in layers. Animals were allowed to recover for two days before starting the stimulation protocol.
Animals received Burst‐DRGS using an Abbott Inc. Proclaim implantable pulse generator (IPG) at 0%, 10%, 33%, 50%, 66%, and 80% of motor threshold (MT) at days 2–7 following implantation (one amplitude each day) using a randomized crossover design. Randomization was performed by an independent researcher using the website http://randomize.org. The experimenter was blinded for the applied amplitude. On stimulation days, animals were externally connected to the IPG and tested for MT after which the amplitude was set accordingly for each animal. The MT was determined using an interburst frequency of 10 Hz, intraburst frequency of 500 Hz, pulse width of 1000 μsec, interpulse interval of 1000 μsec, and a burst pulse count of 5. MT was defined as the current inducing contractions of the lower trunk or hind limb. Animals with an MT ≥1 mA were excluded from analysis. Settings for Burst‐DRGS were as follows: monophasic stimulation with interburst frequency = 40 Hz, intraburst frequency = 500 Hz, pulse width = 1000 μsec, interpulse interval = 1000 μsec, burst pulse count = 517, 18, 19 (Fig. 1). Animals were not restrained during Burst‐DRGS.
Figure 1.
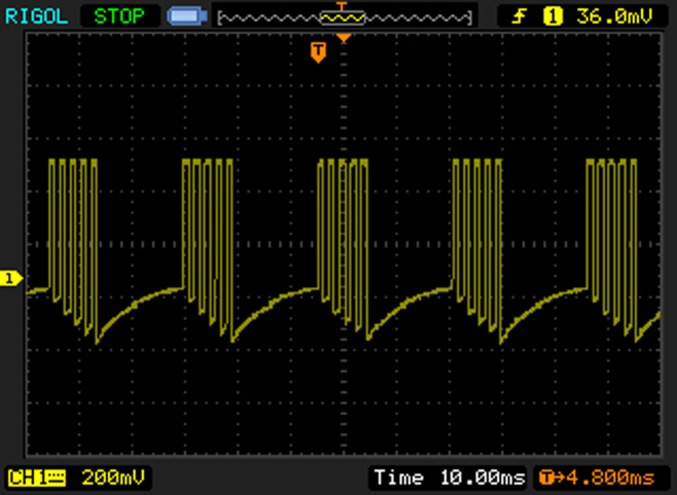
Oscilloscope recording of Burst‐DRGS waveform. Settings for Burst‐DRGS were as follows: monophasic stimulation with interburst frequency = 40 Hz, intraburst frequency = 500 Hz, pulse width = 1000 μsec, interpulse interval = 1000 μsec, burst pulse count = 5. [Color figure can be viewed at http://wileyonlinelibrary.com]
Timeline of Experiments
Animals were tested for mechanical hypersensitivity (Von Frey) at baseline (week −1), after which animals were injected with STZ at week 0. Blood was taken from the animals at week 1 to confirm induction of DM (defined as blood glucose level ≥ 15 mmol/L24). Animals were again tested for mechanical hypersensitivity (Von Frey) at week 4, in order to select animals that developed PDPN (defined as ≥0.2 decrease in log10 (10,000 x 50% WT) on Von Frey test when compared to pre‐STZ baseline27, 28, 29). Animals that developed PDPN were then implanted with a DRGS lead at week 5, and stimulated on days 2–7 following implantation using different amplitudes (0%, 10%, 33%, 50%, 66%, and 80% MT). Animals were each day tested for mechanical hypersensitivity on Von Frey just before Burst‐DRGS onset (baseline), 15 and 30 min during Burst‐DRGS, and 15 and 30 min after Burst‐DRGS (45 and 60 min) (Fig. 2).
Figure 2.
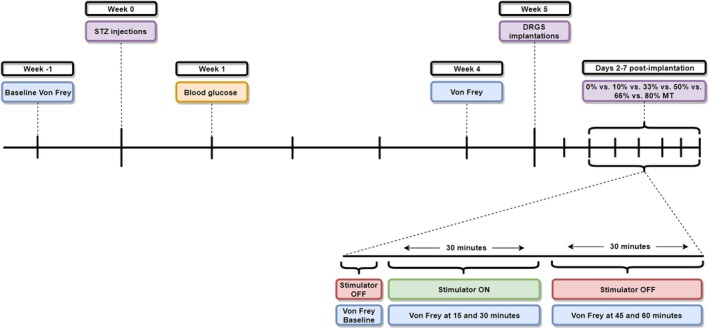
Study design. [Color figure can be viewed at http://wileyonlinelibrary.com]
Data Analysis
Values are presented as mean ± standard error of the mean (SEM). For statistical analysis, Von Frey data were logarithmically transformed to account for Weber's Law and obtain a linear scale 26. For analysis of the effect of Burst‐DRGS over time and intragroup changes in WTs over time, two‐way repeated measures analysis of variance (ANOVA) was performed, followed by Dunnett's multiple comparison test. For between‐groups analyses, a two‐way ANOVA followed by Tukey's multiple comparisons test was used. For comparisons between pre‐STZ WTs and preimplant WTs, a paired‐samples t‐test was used. For the analysis of MTs over time, a one‐way repeated measures ANOVA with Dunnett's multiple comparisons test was used. For calculation of the amplitude‐mechanical hypersensitivity relationship at each specific time point (baseline, 15 min, 30 min, 45 min, and 60 min), mean log10 (10,000 x 50% WT) values at each time point were expressed as a function of Burst‐DRGS amplitude. Nonlinear regression was then performed for each time point, and the X‐value corresponding to the vertex of the resulting parabola‐shaped curve was defined as the optimal DRGS amplitude for that time point. For calculation of the overall optimal DRGS amplitude, area under the curve analysis was first performed for the effect of Burst‐DRGS over time for each specific animal, at all tested Burst‐DRGS amplitudes. The mean AUC values ± SEM were then expressed as a function of Burst‐DRGS amplitude, after which nonlinear regression was performed, and the X‐value corresponding to the vertex of the parabola was identified as the overall optimal Burst‐DRGS amplitude.
RESULTS
Flowchart of Animals
Out of the 28 STZ‐injected animals, 27 animals developed diabetes mellitus (96%; blood glucose level ≥ 15 mmol/L). Seven animals required insulin treatment (blood glucose level ≥ 31.4 mmol/L). Thirteen out of these 27 diabetic animals developed subsequent PDPN (48%; ≥0.2 decrease in log10 [10,000 x 50% WT] on Von Frey when compared to the pre‐STZ injection baseline). All PDPN animals (n = 13) were then implanted with a unilateral DRGS device at the L5 lumbar level. Two animals were withdrawn from the study due to excessively high MT (>1 mA) and two animals were withdrawn due to not displaying neuropathic symptoms following implantation. In the end, nine of these 13 implanted animals successfully finished the six‐day stimulation protocol.
Development of STZ‐Induced Mechanical Hypersensitivity
The mean log10 (10,000 x 50% WT) of the 13 implanted animals dropped from 5.1 ± 0.05 g at pre‐STZ‐baseline to 4.7 ± 0.05 g preimplantation (four weeks after STZ injection) (p < 0.0001) (Fig. 3).
Figure 3.
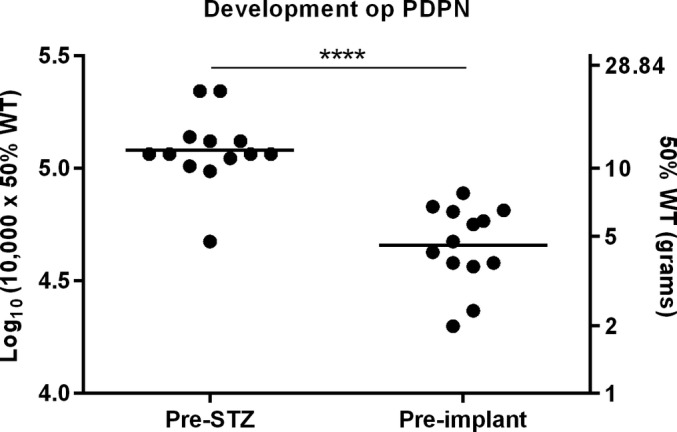
Development of mechanical hypersensitivity after STZ injection of all implanted rats (n = 13). ****p < 0.0001 compared to pre‐STZ baseline.
Intragroup Analyses: Effect of Burst‐DRGS Over Time
Burst‐DRGS significantly attenuated STZ‐induced mechanical hypersensitivity over time (p < 0.0001, effect of factor time; two‐way repeated measures ANOVA) (Fig. 4a). Burst‐DRGS at 33% MT, 50% MT, 66% MT, and 80% MT significantly reduced mechanical hypersensitivity at 15 min, 30 min, and 45 min as compared to the corresponding baseline (p < 0.05) (Table 1). As expected, no significant effects over time were observed for very low amplitudes of 0% MT and 10% MT (p > 0.05) (Table 1).
Figure 4.
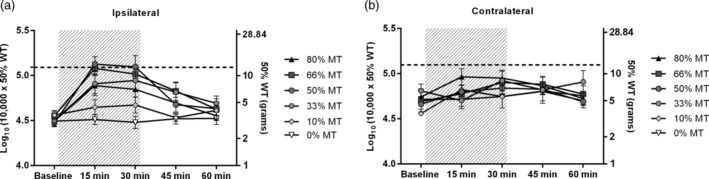
A combined presentation of the effect of Burst‐DRGS for different amplitudes. Dotted line = the mean pre‐STZ baseline of all stimulated animals. Gray area = period of DRGS.
Table 1.
Significance Values of Within and Between Stimulation Amplitude Comparisons.
| 0% MT | 10% MT | 33% MT | 50% MT | 66% MT | 80% MT | |
|---|---|---|---|---|---|---|
| Baseline | 4.49 ± 0.04 | 4.56 ± 0.05 | 4.50 ± 0.05 | 4.47 + 0.04 | 4.55 ± 0.05 | 4.49 ± 0.06 |
| 15 min | 4.51 ± 0.06 | 4.65 ± 0.08 | 4.91 ± 0.11 | 5.13 ± 0.08 | 5.08 ± 0.07 | 4.89 ± 0.11 |
| * p<0.0001 | * p<0.0001 | * p<0.0001 | * p<0.0001 | |||
| # p<0.0001 | # p<0.0001 | # p<0.0001 | # p<0.001 | |||
| $ p<0.05 | $ p<0.0001 | $ p<0.0001 | $ p<0.05 | |||
| † p<0.05 | ||||||
| 30 min | 4.47 ± 0.07 | 4.67 ± 0.09 | 4.95 ± 0.12 | 5.10 ± 0.12 | 5.02 ± 0.08 | 4.85 ± 0.09 |
| * p<0.0001 | * p<0.0001 | * p<0.0001 | * p<0.0001 | |||
| # p<0.0001 | # p<0.0001 | # p<0.0001 | # p<0.001 | |||
| $ p<0.05 | $ p<0.0001 | $ p<0.001 | ||||
| † p<0.05 | ||||||
| 45 min | 4.52 ± 0.06 | 4.53 ± 0.04 | 4.82 ± 0.11 | 4.68 ± 0.08 | 4.83 ± 0.10 | 4.69 ± 0.10 |
| * p<0.001 | * p<0.05 | * p<0.01 | * p<0.05 | |||
| # p<0.01 | # p<0.01 | |||||
| $ p<0.01 | $ p<0.01 | |||||
| 60 min | 4.52 ± 0.07 | 4.61 ± 0.06 | 4.69 ± 0.08 | 4.63 ± 0.07 | 4.63 ± 0.11 | 4.55 ± 0.05 |
p < 0.05 vs. baseline.
p < 0.05 vs. 0% MT.
p < 0.05 vs. 10% MT.
p < 0.05 vs. 80% MT.
Intergroup Analyses: Effect Between Stimulation Amplitudes
A significant effect of amplitude was found (p < 0.01, effect of factor amplitude; two‐way repeated measures ANOVA) (Fig. 4a). Significant differences were observed between Burst‐DRGS at 33% MT, 50% MT, 66% MT, and 80% MT when compared to 10% MT and 0% MT (p < 0.05) (Table 1). Additionally, Burst‐DRGS at 50% MT was significantly more effective in normalizing STZ‐induced mechanical hypersensitivity as compared to Burst‐DRGS at 80% MT while the stimulator was turned on (15 and 30 min) (p < 0.05) (Table 1). As expected, no significant effect of amplitude was observed in the contralateral hind paw (p = 0.69, effect of factor amplitude; two‐way repeated measures ANOVA) (Fig. 4b).
Responder Rates
The percentage of animals responding to Burst‐DRGS (responder defined as increase of the log10 [10,000 x 50% WT] ≥0.2 compared to prestimulation baseline) was highest in the 50% MT and 66% MT group at 15 min (100%), the 66% MT group at 30 min (89%), the 66% MT and 80% MT group at 45 min (56%), and the 50% MT group at 60 min (56%) (Table 2).
Table 2.
Responder Rate for Each Stimulation Amplitude.
| 0% MT | 10% MT | 33% MT | 50% MT | 66% MT | 80% MT | |
|---|---|---|---|---|---|---|
| 15 min | 0/9 (0%) | 4/9 (44%) | 7/9 (78%) | 9/9 (100%) | 9/9 (100%) | 6/9 (67%) |
| 30 min | 0/9 (0%) | 3/9 (33%) | 7/9 (78%) | 7/9 (78%) | 8/9 (89%) | 6/9 (67%) |
| 45 min | 0/9 (0%) | 1/9 (11%) | 4/9 (44%) | 4/9 (44%) | 5/9 (56%) | 5/9 (56%) |
| 60 min | 1/9 (11%) | 3/9 (33%) | 4/9 (44%) | 5/9 (56%) | 2/9 (22%) | 2/9 (22%) |
A responder to DRGS is defined as an animal with an increase of the log10 (10,000 × 50% WT) ≥0.2 compared to prestimulation.
Relationship Between Stimulation Amplitude and Mechanical Hypersensitivity
Calculation of the Optimal Burst‐DRGS Amplitude at Each Specific Time Point
In order to investigate the relationship between amplitude and mechanical hypersensitivity, log10 (10,000 x 50% WT) responses were expressed as a function of Burst‐DRGS amplitude. At prestimulation baseline (Fig. 5a), no substantial relationship was present between amplitude and mechanical hypersensitivity (R 2 < 0.001). With the stimulator turned on (15 and 30 min), log10 (10,000 x 50% WT) values could be expressed by a polynomial function of the second order (y = c + bx − ax2) (R 2 = 0.42 at 15 min, R 2 = 0.37 at 30 min). The resulting plot was parabolic in shape, with the peak of the graph (Ymax) showing the estimated maximum log10 (10,000 x 50% WT) response and the corresponding amplitude (Xmax). The calculated optimal DRGS amplitudes (Xmax) at 15 and 30 min were 54% MT and 51% MT, respectively (Fig. 5b,c). At 45 and 60 min (after the stimulator was turned off), this nonlinear relationship was less obvious (R 2 = 0.14 at 45 min and R 2 = 0.05 at 60 min). Optimal DRGS amplitudes were observed at an Xmax of 54% MT and 64% MT for 45 min and 60 min, respectively (Fig. 5d,e).
Figure 5.
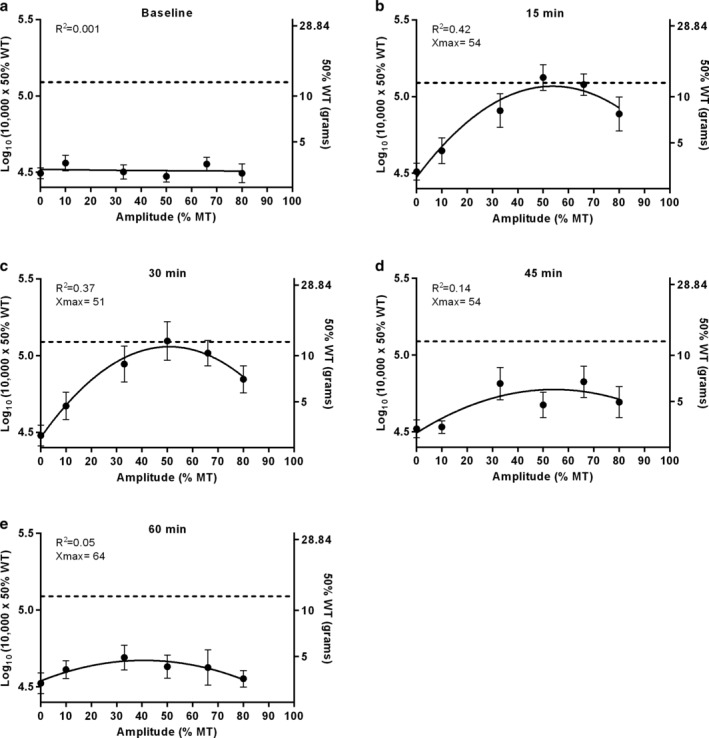
Plots resulting from nonlinear curve‐fitting for the amplitude‐mechanical hypersensitivity interaction for each time point. a. At baseline, no substantial relationship was present between amplitude and mechanical hypersensitivity. b,c. When the stimulator was turned on at 15 and 30 min, the curves describing the amplitude‐mechanical hypersensitivity relationship were downward opening parabolas that can be described by the formula y = c + bx − ax2. Optimal DRGS amplitudes were estimated at 51%–54% MT. d,e. When the stimulator was turned off at 45 and 60 min, the nonlinear relationship became less obvious. Here, the optimal DRGS amplitude was estimated at 54%–64% MT.
Calculation of the Overall Optimal Burst‐DRGS Amplitude
Also when area under the curves (AUC) from Figs. 4a and 6 were plotted, the nonlinear relationship between amplitude and mechanical hypersensitivity was clearly visible (R 2 = 0.35), with an estimated optimal pain relieving effect of Burst‐DRGS amplitude at Xmax = 52% MT (Fig. 6).
Figure 6.
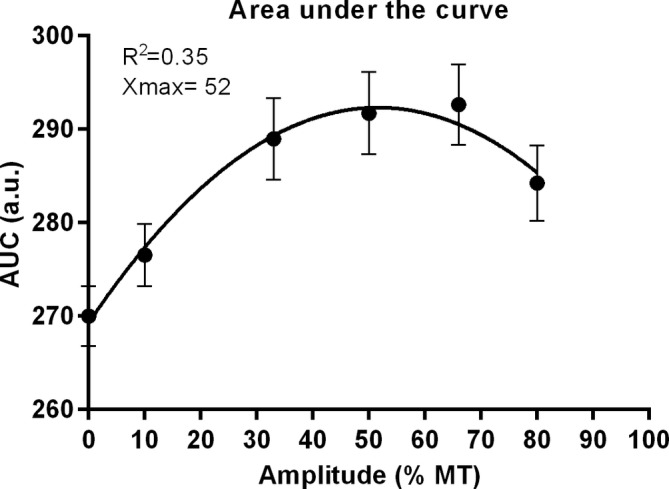
Plot resulting from nonlinear curve fitting for the amplitude‐AUC interaction. AUCs were based on outcomes as depicted in Fig. 4a and were calculated for each animal individually before they were averaged. The resulting plot shows a clear nonlinear relationship between amplitude and AUC with an optimal DRGS amplitude at Xmax = 52% MT.
Motor Thresholds
Lastly, MTs remained stable throughout the experiment (day 1: 0.12 ± 0.008 mA, day 2: 0.14 ± 0.01 mA, day 3: 0.18 ± 0.03 mA, day 4: 0.18 ± 0.02 mA, day 5: 0.15 ± 0.02 mA. p = 0.07) (Fig. 7).
Figure 7.
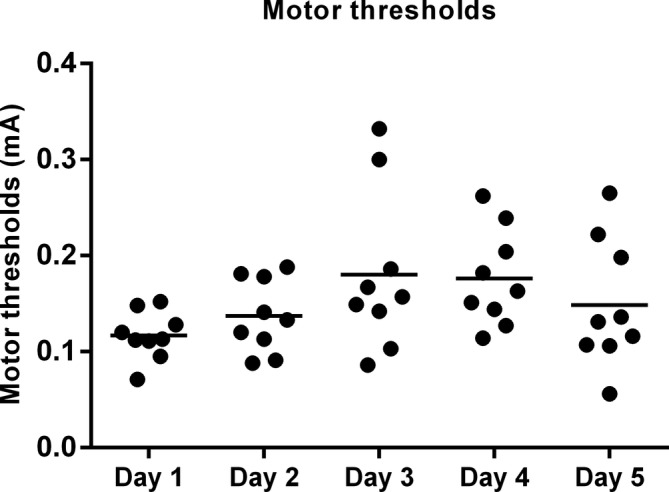
Observed motor thresholds (MT) throughout the study period. MT was assessed using a pulse width of 1000 μsec, and five pulses (500 Hz intraburst frequency) administered at an interburst frequency of 10 Hz.
DISCUSSION
The present study demonstrates that: 1) Burst‐DRGS at 33%–80% MT is capable of attenuating STZ‐induced mechanical hypersensitivity in rats not only during stimulation, but also 15 min after stimulation cessation. 2) There is a nonlinear relation between Burst‐DRGS amplitude and observed behavioral outcome with the best range of stimulation amplitude being 50%–66% MT, and an estimated optimal pain relieving effect at 52% MT.
Burst‐DRGS at amplitudes of 33% MT, 50% MT, 66% MT, and 80% MT all significantly attenuated STZ‐induced mechanical hypersensitivity during stimulation (15 min and 30 min) as well as 15 min after stimulation (45 min), after which mechanical hypersensitivity values returned to prestimulation baseline values (60 min). These behavioral effects validate an earlier study published by our group on Burst‐DRGS at 66% MT, which indicated that Burst‐DRGS is capable of treating STZ‐induced mechanical hypersensitivity, with the advantage of a significant residual effect after turning off the stimulator 20. Along these lines, results from a recent randomized controlled trial found that Burst‐SCS microdosing, a paradigm that relies on the introduction of stimulation‐off phases in‐between stimulation‐on phases, is as effective as standard Burst‐SCS, while having significantly lower battery consumption 31. Moreover, the analgesic efficacy of Burst‐SCS microdosing was found to be equal as compared to standard Burst‐SCS, as measured by rat fMRI brain responses following noxious stimulation 32. Together, these results strongly indicate a beneficial carryover effect of Burst stimulation, both when delivered at the dorsal column and at the DRG, and suggest that some form of plasticity is induced following each stimulation‐on phase. It has recently been shown that the amplitude of spinal neuronal responses in rats can be potentiated for several minutes following a short burst of high‐frequency tetanic pulses (555 Hz) 33. As Burst stimulation uses a similar intraburst frequency, it is reasonable to assume that Burst stimulation follows a similar mechanism, explaining the persistence of pain relief when the stimulator is off (stimulation‐off phase). Furthermore, high‐frequency stimulation is known to induce long‐term potentiation in lamina I of spinal projection neurons 34, and signs of short‐term plasticity have been found in response to electrical stimulation, including those modulated by Burst stimulation 35.
Previous studies have indicated that Burst stimulation can be optimized by adjusting relevant stimulation parameters to modulate the charge delivered to the spinal cord during stimulation, such as amplitude 22. In our study, there seems to be no linear correlation between Burst‐DRGS amplitude and the observed behavioral outcome, as the highest amplitude (80% MT) did not result in optimal pain relief and highest responder rates. Significant differences were observed between 33% MT, 50% MT, and 66% MT (at 15 min, 30 min, and 45 min) when compared to 10% MT and 0% MT, and Burst‐DRGS at 50% MT was significantly more effective in normalizing STZ‐induced mechanical hypersensitivity as compared to Burst‐DRGS at 80% MT while the stimulator was turned on (15 and 30 min). Moreover, Burst‐DRGS at 50% MT and 66% MT (15 min) and Burst‐DRGS at 66% MT (30 min) yielded the highest responder rates, seemingly favoring amplitudes of 50%–66% over the higher (80% MT) and lower (0%–33% MT) stimulation amplitudes. Lastly, the estimated optimal amplitude of DRGS based on nonlinear regression of AUCs was found to be 52% MT. Interestingly, previous work on high frequency SCS suggests the sensation threshold, defined as the amplitude at which animals start to show signs of disturbance of their normal behavior, to be around 50% MT 36. The gradual decrease in therapy efficacy with stimulation amplitudes beyond this optimum might be explained by the fact that sensory, potential uncomfortable, sensations induced by the stimulation might have taken place. Interestingly, a recent study by Meuwissen et al. showed that Burst‐SCS also followed this nonlinear course between amplitude and behavioral outcome 23. The optimal amplitude for Burst‐SCS in that study was reported to be 50% MT, and the behavioral outcome was shown to decline rather rapidly when this optimal amplitude was surpassed 23. This is in line with the findings of Courtney et al. who demonstrated that the absolute therapeutic window of Burst‐SCS in terms of amplitude is considerably smaller when compared to Con‐SCS 37. In contrast, the Burst‐DRGS paradigm as used in the present study demonstrated a larger optimal therapeutic window (50%–66% MT). Differences in terms of this optimal therapeutic window between our study and the study of Meuwissen and colleagues might be attributed to the experimental model used (PDPN vs. Seltzer lesion), the location of stimulation (spinal cord vs. DRG), the type of stimulation used (quadripolar vs. bipolar), and/or the Burst waveform used (biphasic with active recharge balance vs. monophasic with passive recharge balance) 23. Additionally, a study by Tang et al. found that while spinal neuronal responses to colorectal distension and pinch were reduced similarly using tonic SCS and Burst‐SCS at 90% MT, Burst, but not tonic SCS significantly decreased the nociceptive somatic response after colorectal distension or pinch using lower amplitudes of 60% MT.21 Combined, these data suggest that Burst stimulation is effective at lower amplitudes relative to the observed MT as compared to tonic stimulation. The combination of this relatively low optimal Burst stimulation amplitude, both in DRGS and SCS studies, together with the earlier described increased carryover effect of Burst stimulation, could have important implications for optimal stimulation delivery as well as battery life of the IPG in clinical practice.
To date, few studies have investigated the mechanisms underlying (Burst‐)DRGS and the question remains how (Burst‐)DRGS might affect the nociceptive afferents and firing of these afferents. The DRG consists of a unique pseudo unipolar design, in which the T‐junction of the DRG can act as a selective filter for stimuli that is, action potentials traveling toward the spinal cord.38 A study by Koopmeiners et al. reported DRGS to reduce the amplitude and/or amount of action potentials arising from the DRG, thereby inhibiting neuronal excitability 39. Interestingly, it was recently suggested that the outcome of dorsal column SCS is correlated to the active stimulation period or duty cycle 40, and that SCS can attenuate aberrant, hyperactive firing of pain transmission neurons 40, 41. Along these lines, a stimulation mode with a high duty cycle, like Burst‐SCS, but also Burst‐DRGS, is more likely to counteract this aberrant firing of pain transmission neurons. The impact of the high duty cycle of Burst‐DRGS may therefore preclude the strength–duration and charge–duration relationship from defining the relationship between single pulse parameters and neural activation thresholds. Besides these electrophysiological findings, DRGS has been linked to attenuation of brain areas that are considered to be part of the pain matrix like the contralateral thalamic VPL/VPM nuclei, and cortical S1 and S2 42, and one can argue that also spinal mechanisms may underlie DRGS as modulating firing rates of DRG neurons by DRGS may also affect interneurons and GABAergic systems in the dorsal horn as is the case in traditional SCS 43, 44, 45. However, more research is needed to fully understand the underlying mechanisms of DRGS and its relation to specific waveforms, like Burst‐DRGS and/or individual DRGS‐stimulation parameters, like amplitude.
Limitations of this study include the timeframe of the used stimulation protocol. In clinical practice, patients typically receive DRGS for a longer period of time as compared to the short‐term protocol used in this study. Second, it should be stressed that using the MT for determining the desired stimulation amplitude in rodents may have several shortcomings. In preclinical studies, the MT often replaces the perception threshold (PT) as used in humans, as it is a quick, easy to use, and objective outcome measure based on visible contractions of the lower trunk and/or hind limbs. In contrast, the PT is relatively difficult to observe in rodents, as the experimenter has to “determine” whether or not an animal perceives the stimulation or not, which can lead to subjectivity. The PT has been estimated to be approximately 30%–50% MT in rats 36, 46, and a study by Koyama et al. found that this PT/MT ratio varies across rats 46. The latter might interfere with direct translation of our results to the clinic, as there might have been differences in the amount of stimulation relative to the PT (% PT) delivered to the system across animals. However, it is worth noting that the MTs in the study of Koyama and colleagues were measured under anesthesia, which might significantly influence the (variation between) observed MTs in their study 46. We are convinced that future studies should try to objectively establish the PT in rodent models that would then allow for an even more precise translation of findings to the human situation. Additionally, it should also be noted that there might be differences in the exact MT amplitude when stimulating with an interburst frequency of 10 Hz (which was used to determine the MT used in the experiment) as compared to an interburst frequency of 40 Hz (which was used for final delivery of Burst‐DRGS), given the lower duty cycle of 10 Hz stimulation. As it is near impossible to objectively assess MTs using higher frequencies, including 40 Hz, preclinical studies tend to use lower frequencies for assessment of MT (both in DRGS and [Burst‐]SCS studies). The latter is needed to obtain a clear and easy to observe MT, even though final delivery of stimulation happens at higher frequencies 23, 30, 47. Third, in the present study we chose to only include female Sprague–Dawley rats. Female rats reach their mature body weight and nerve conduction status faster as compared to their male counterpart, or either sex of other strains 24. As such, one should be cautious when extrapolating these results to the male sex 48. Fourth, there are fundamental differences in the clinical and preclinical manifestation of PDPN. In patients, diabetes is a chronic disease where complications often only arise after many years. STZ injection in rats relies on destruction of beta cells in the pancreas 24. The injection leads therefore to a very swift development of diabetes (within one week), and subsequent development of mechanical allodynia (within four weeks) 20, 27, 29, 49. Although the injection of streptozotocin is the most common used diabetes model in rats, one should be cautious when extrapolating these results to a human situation. Lastly, the results presented here are limited to reflex‐based outcome measures (Von Frey) in experimental PDPN. Future studies using spontaneous or operant behavioral tests and/or different animal models should be conducted to verify these results, as it is very well possible that Burst stimulation only targets specific components of pain, not observable by the Von Frey test 50.
Altogether, our findings indicate that there is a nonlinear relationship between Burst‐DRGS amplitude and behavioral outcome, as Burst‐DRGS at 50% MT and 66% MT resulted in optimal pain relief and highest responder rates, with an estimated optimal pain relief at 52% MT. A further increase in Burst‐DRGS amplitude up to 80% MT did not result in better pain relief, even though Burst‐DRGS at amplitudes of 33% MT–80% MT significantly attenuated mechanical hypersensitivity in PDPN animals. Also, a significant wash‐out of Burst‐DRGS was observed 15 min following Burst‐DRGS cessation at these amplitudes. Further optimization and analysis of DRGS driven by insights into the underlying mechanisms related to the various stimulation paradigms is warranted.
Authorship Statements
Glenn Franken performed the experiments, analyzed the data, and wrote the manuscript. Elbert A.J. Joosten and Glenn Franken conceived and designed the experiment. Jacques Debets performed the DRGS implantations. All authors have approved the final version of the manuscript.
Source(s) of financial support: This work was supported by a sponsored research contract titled “Dorsal Root Ganglion Stimulation in neuropathic pain: Effect of stimulation settings and pain relief in an experimental model of painful diabetic polyneuropathy” from Abbott Inc., granted to Elbert A.J. Joosten.
Conflict of Interest: Elbert A. J. Joosten is a consultant for Abbott Inc. Glenn Franken and Jacques Debets have no conflicts of interest to disclose.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
REFERENCES
- 1. Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community‐based diabetic population in the U.K. Diabet Care Oct 2011;34:2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: A controlled comparison of people with and without diabetes. Diabet Med Sep 2004;21:976–982. [DOI] [PubMed] [Google Scholar]
- 3. Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care Jul 2006;29:1518–1522. [DOI] [PubMed] [Google Scholar]
- 4. Kaur S, Pandhi P, Dutta P. Painful diabetic neuropathy: An update. Ann Neurosci Oct 2011;18:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finnerup NB, Otto M, Jensen TS, Sindrup SH. An evidence‐based algorithm for the treatment of neuropathic pain. Med Gen Med May 15 2007;9:36. [PMC free article] [PubMed] [Google Scholar]
- 6. de Vos CC, Meier K, Zaalberg PB et al. Spinal cord stimulation in patients with painful diabetic neuropathy: A multicentre randomized clinical trial. Pain Nov 2014;155:2426–2431. [DOI] [PubMed] [Google Scholar]
- 7. Slangen R, Schaper NC, Faber CG et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: A prospective two‐center randomized controlled trial. Diabet Care Nov 2014;37:3016–3024. [DOI] [PubMed] [Google Scholar]
- 8. van Beek M, Slangen R, Schaper NC et al. Sustained treatment effect of spinal cord stimulation in painful diabetic peripheral neuropathy: 24‐month follow‐up of a prospective two‐Center randomized controlled trial. Diabet Care Sep 2015;38:e132–e134. [DOI] [PubMed] [Google Scholar]
- 9. Liem L, Russo M, Huygen FJ et al. A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation. Sep‐Oct 2013;16:471–482. [DOI] [PubMed] [Google Scholar]
- 10. Liem L, Russo M, Huygen FJ et al. One‐year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation. Jan 2015;18:41–48. [DOI] [PubMed] [Google Scholar]
- 11. Kramer J, Liem L, Russo M, Smet I, Van Buyten JP, Huygen F. Lack of body positional effects on paresthesias when stimulating the dorsal root ganglion (DRG) in the treatment of chronic pain. Neuromodulation. Jan 2015;18:50–57. discussion 57. [DOI] [PubMed] [Google Scholar]
- 12. Huygen F, Liem L, Cusack W, Kramer J. Stimulation of the L2‐L3 dorsal root ganglia induces effective pain relief in the low back. Pain Pract Feb 2018;18:205–213. [DOI] [PubMed] [Google Scholar]
- 13. Deer TR, Levy RM, Kramer J et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: A randomized comparative trial. Pain Apr 2017;158:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: Meta‐analysis. J Pain Sep 2014;15:887–897. [DOI] [PubMed] [Google Scholar]
- 15. Eldabe S, Burger K, Moser H et al. Dorsal root ganglion (DRG) stimulation in the treatment of phantom limb pain (PLP). Neuromodulation. Oct 2015;18:610–616. [DOI] [PubMed] [Google Scholar]
- 16. Eldabe S, Espinet A, Wahlstedt A et al. Retrospective case series on the treatment of painful diabetic peripheral neuropathy with dorsal root ganglion stimulation. Neuromodulation. Mar 2018;21:787–792. [DOI] [PubMed] [Google Scholar]
- 17. De Ridder D, Vanneste S, Plazier M, van der Loo E, Menovsky T. Burst spinal cord stimulation: Toward paresthesia‐free pain suppression. Neurosurgery May 2010;66:986–990. [DOI] [PubMed] [Google Scholar]
- 18. De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg. Nov 2013;80:642–649. [DOI] [PubMed] [Google Scholar]
- 19. De Ridder D, Lenders MW, De Vos CC et al. A 2‐center comparative study on tonic versus burst spinal cord stimulation: Amount of responders and amount of pain suppression. Clin J Pain May 2015;31:433–437. [DOI] [PubMed] [Google Scholar]
- 20. Franken G, Debets J, Joosten EAJ. Dorsal root ganglion stimulation in experimental painful diabetic peripheral neuropathy: Burst vs. conventional stimulation paradigm. Neuromodulation. Dec 2018;20. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang R, Martinez M, Goodman‐Keiser M, Farber JP, Qin C, Foreman RD. Comparison of burst and tonic spinal cord stimulation on spinal neural processing in an animal model. Neuromodulation Feb 2014;17:143–151. [DOI] [PubMed] [Google Scholar]
- 22. Crosby ND, Goodman Keiser MD, Smith JR, Zeeman ME, Winkelstein BA. Stimulation parameters define the effectiveness of burst spinal cord stimulation in a rat model of neuropathic pain. Neuromodulation Jan 2015;18:1–8. discussion 8. [DOI] [PubMed] [Google Scholar]
- 23. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Conventional‐SCS vs. burst‐SCS and the Behavioral effect on mechanical hypersensitivity in a rat model of chronic neuropathic pain: Effect of amplitude. Neuromodulation Jan 2018;21:19–30. [DOI] [PubMed] [Google Scholar]
- 24. Calcutt NA. Modeling diabetic sensory neuropathy in rats. Methods Mol Med 2004;99:55–65. [DOI] [PubMed] [Google Scholar]
- 25. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods Jul 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 26. Mills C, Leblond D, Joshi S et al. Estimating efficacy and drug ED50's using von Frey thresholds: Impact of Weber's law and log transformation. J Pain Jun 2012;13:519–523. [DOI] [PubMed] [Google Scholar]
- 27. Pluijms WA, van Kleef M, Honig WM, Janssen SP, Joosten EA. The effect of spinal cord stimulation frequency in experimental painful diabetic polyneuropathy. Eur J Pain Oct 2013;17:1338–1346. [DOI] [PubMed] [Google Scholar]
- 28. van Beek M, Hermes D, Honig WM et al. Long‐term spinal cord stimulation alleviates mechanical hypersensitivity and increases peripheral cutaneous blood perfusion in experimental painful diabetic polyneuropathy. Neuromodulation. Mar 2018;21:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Beek M, van Kleef M, Linderoth B, van Kuijk SM, Honig WM, Joosten EA. Spinal cord stimulation in experimental chronic painful diabetic polyneuropathy: Delayed effect of high‐frequency stimulation. Eur J Pain May 2017;21:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pan B, Yu H, Fischer GJ, Kramer JM, Hogan QH. Dorsal root ganglionic field stimulation relieves spontaneous and induced neuropathic pain in rats. J Pain Dec 2016;17:1349–1358. [DOI] [PubMed] [Google Scholar]
- 31. Vesper J, Slotty P, Schu S et al. Burst SCS microdosing is as efficacious as standard burst SCS in treating chronic back and leg pain: Results from a randomized controlled trial. Neuromodulation Feb 2019;22:190–193. [DOI] [PubMed] [Google Scholar]
- 32. Saber M SD, Tessmer J, Khan Z et al. Rat fMRI brain responses to noxious stimulation during tonic, burst, and burst‐microdosing spinal cord stimulation. Poster, International Neuromodulation Society 2019.
- 33. Lloyd DP. Post‐tetanic potentiation of response in monosynaptic reflex pathways of the spinal cord. J Gen Physiol Nov 1949;33:147–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. Feb 21 2003;299:1237–1240. [DOI] [PubMed] [Google Scholar]
- 35. Shyu BC, Vogt BA. Short‐term synaptic plasticity in the nociceptive thalamic‐anterior cingulate pathway. Mol Pain Sep 4 2009;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song Z, Viisanen H, Meyerson BA, Pertovaara A, Linderoth B. Efficacy of kilohertz‐frequency and conventional spinal cord stimulation in rat models of different pain conditions. Neuromodulation Apr 2014;17:226–234. discussion 234‐225. [DOI] [PubMed] [Google Scholar]
- 37. Courtney P, Espinet A, Mitchell B et al. Improved pain relief with burst spinal cord stimulation for two weeks in patients using tonic stimulation: Results from a small clinical study. Neuromodulation Jul 2015;18:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: A review. Neuromodulation Jan 2015;18:24–32. [DOI] [PubMed] [Google Scholar]
- 39. Koopmeiners AS, Mueller S, Kramer J, Hogan QH. Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation Jul‐Aug 2013;16:304–311. [DOI] [PubMed] [Google Scholar]
- 40. Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: A review. Neuromodulation Jun 2016;19:373–384. [DOI] [PubMed] [Google Scholar]
- 41. Elbasiouny SM, Mushahwar VK. Suppressing the excitability of spinal motoneurons by extracellularly applied electrical fields: Insights from computer simulations. J Appl Physiol (1985). Nov 2007;103:1824–1836. [DOI] [PubMed] [Google Scholar]
- 42. Pawela CP, Kramer JM, Hogan QH. Dorsal root ganglion stimulation attenuates the BOLD signal response to noxious sensory input in specific brain regions: Insights into a possible mechanism for analgesia. Neuroimage Feb 15 2017;147:10–18. [DOI] [PubMed] [Google Scholar]
- 43. Stiller CO, Cui JG, O'Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of gamma‐aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery Aug 1996;39:367–374. discussion 374‐365. [DOI] [PubMed] [Google Scholar]
- 44. Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain Oct 1997;73:87–95. [DOI] [PubMed] [Google Scholar]
- 45. Janssen SP, Gerard S, Raijmakers ME, Truin M, Van Kleef M, Joosten EA. Decreased intracellular GABA levels contribute to spinal cord stimulation‐induced analgesia in rats suffering from painful peripheral neuropathy: The role of KCC2 and GABA(a) receptor‐mediated inhibition. Neurochem Int Jan 2012;60:21–30. [DOI] [PubMed] [Google Scholar]
- 46. Koyama S, Xia J, Leblanc BW, Gu JW, Saab CY. Sub‐paresthesia spinal cord stimulation reverses thermal hyperalgesia and modulates low frequency EEG in a rat model of neuropathic pain. Sci Rep. May 8 2018;8:7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Burst spinal cord stimulation in peripherally injured chronic neuropathic rats: A delayed effect. Pain Pract. Apr 2018;18:988–996. [DOI] [PubMed] [Google Scholar]
- 48. Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: The role of gonadal hormones. Eur J Pain Oct 2004;8:397–411. [DOI] [PubMed] [Google Scholar]
- 49. Koetsier E, Franken G, Debets J et al. Effectiveness of dorsal root ganglion stimulation and dorsal column spinal cord stimulation in a model of experimental painful diabetic polyneuropathy. CNS Neurosci Ther 2019;25(3):367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meuwissen KPV, van Beek M, EAJ J. Burst and tonic spinal cord stimulation in the mechanical conflict‐avoidance system: Cognitive‐motivational aspects. Neuromodulation. Apr 11 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


