Abstract
Objectives
Despite the availability of HIV testing guidelines to facilitate prompt diagnosis, late HIV diagnosis remains high across Europe. The study synthesizes recent evidence on HIV testing strategies adopted in health care settings in the European Union/European Economic Area (EU/EEA).
Methods
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines were followed and systematic searches were run in five databases (2010–2017) to identify studies describing HIV testing interventions in health care settings in the EU/EEA. The grey literature was searched for unpublished studies (2014–2017). Two reviewers independently performed study selection, data extraction and critical appraisal.
Results
One hundred and thirty intervention and/or feasibility studies on HIV testing in health care settings were identified. Interventions included testing provision (n = 94), campaigns (n = 14) and education and training for staff and patients (n = 20). HIV test coverage achieved through testing provision varied: 2.9–94% in primary care compared to 3.9–66% in emergency departments. HIV test positivity was lower in emergency departments (0–1.3%) and antenatal services (0–0.05%) than in other hospital departments (e.g. inpatients: 0–5.3%). Indicator condition testing programmes increased HIV test coverage from 3.9–72% before to 12–85% after their implementation, with most studies reporting a 10–20% increase. There were 51 feasibility and/or acceptability studies that demonstrated that HIV testing interventions were generally acceptable to patients and providers in health care settings (e.g. general practitioner testing acceptable: 77–93%).
Conclusions
This review has identified several strategies that could be adopted to achieve high HIV testing coverage across a variety of health care settings and populations in the EU/EEA. Very few studies compared the intervention under investigation to a baseline, but, where this was assessed, data suggested increases in testing.
Keywords: adults, Europe, health care, HIV diagnosis and adults, HIV testing
Introduction
In 2017, 49% of people diagnosed with HIV infection were first identified at a late stage of infection (CD4 count < 350 cells/µL) in Europe 1. Late diagnosis is associated with increased risk of morbidity and mortality 2, 3 as well as increased risk of onward transmission of HIV as a consequence of delayed initiation of treatment 4. The Joint United Nations Programme on HIV/AIDS (UNAIDS) set the global 90‐90‐90 target where 90% of all people with HIV infection should be diagnosed, 90% of those diagnosed should receive HIV treatment and 90% of those on treatment should have a suppressed viral load by 2020 5. HIV testing is therefore a vital first step in the HIV care continuum and in Europe it has historically been offered in traditional health care settings, such as sexual health clinics, antenatal services and voluntary counselling and testing sites. Testing guidance for sexually transmitted infection (STI)/genitourinary/dermato‐venereology clinics exists at national, European and international levels promoting universal testing offer 6, 7, 8, 9, 10. However, other health care settings that are nonspecialist for HIV and where patients are presenting for the management of other conditions present opportunities to increase HIV testing, thereby reducing undiagnosed infections. In 2016, an estimated 101 400 people were living with undiagnosed HIV infection in the European Union/European Economic Area (EU/EEA), and, although this represents a decline in the number since 2012, it highlights the continued need for effective HIV testing programmes to improve HIV test coverage 11.
The World Health Organization (WHO) consolidated guidelines on HIV testing services, recommending that HIV testing services should be integrated with other relevant clinical services such as those for tuberculosis (TB), maternal health, sexual and reproductive health and harm reduction programmes, especially as these services attract populations considered to be at higher risk for HIV infection 9. The guidelines endorse the use of provider‐initiated testing and counselling when the epidemic is generalized and the routine offer of testing for all clients in all health facilities (including primary care, inpatient and outpatient services and all services for key populations) is recommended as an effective way to identify people with HIV infection.
Although guidance is available from international organizations and national public health bodies to inform service provision for HIV testing 12, health care providers within European countries need to be able to operationalize these into clinical practice so as to diagnose HIV infection at an earlier stage of infection. An evidence synthesis published in 2010 by the European Centre for Disease Prevention and Control (ECDC) showed that a number of strategies could reduce missed opportunities for HIV testing, including indicator condition (IC)‐guided testing, which involves offering testing to all patients presenting to care with an AIDS‐defining illness or with an HIV ‘indicator’ condition (IC) 8. An HIV IC is a condition associated with an undiagnosed HIV prevalence of at least 1 per 1000 13. Other strategies were routine HIV testing implemented as part of routine care in health care settings and the use of rapid tests that offer immediate results.
A recent evaluation of the 2010 ECDC guidance found that, although the document was considered important for policy and guideline development, an update to the guidance was necessary to incorporate new approaches and technologies that have been adopted to increase testing offer and coverage in recent years 14. The purpose of this paper is to present the recent body of evidence on HIV testing strategies employed in health care settings in Europe. Additionally, the paper reviews the evidence on testing provision strategies that increase HIV testing coverage and on the feasibility and acceptability of HIV testing strategies. The systematic review described here was conducted as part of a wider review of the evidence on HIV testing in the EU/EEA and barriers to testing to update the 2010 ECDC HIV testing guidance.
Methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement for the reporting of systematic reviews 15. A full description of the methodology is described elsewhere 16. Briefly, five electronic databases (OVID Medline, Embase, PsycINFO, Scopus and the Cochrane Library of Systematic Reviews) (January 2010 to March 2017) and the proceedings of six conferences (2014–2017) were searched using key search terms covering concepts including ‘HIV’, ‘HIV testing’ and ‘Europe’ (Tables S1–S5). Only studies pertaining to adults and set in the 30 EU/EEA countries (Table S6) and outside occupational settings were included in the review. No language restrictions were applied.
Two reviewers independently undertook title review, full‐text review and data extraction. ECDC completed all reviews for non‐English studies, with data extraction in English. Data on qualitative and quantitative outcome indicators were extracted, including information on HIV coverage, test positivity and intervention feasibility and acceptability. Two reviewers carried out quality assessment and risk of bias assignment for published studies based on National Institute for Health and Clinical Excellence (NICE) checklists and the AXIS quality assessment tool (Table S7). Studies were rated as being high, medium or low quality and having high, medium or low bias. Conference proceedings were not appraised for quality and bias. Critical appraisal results can be found in Table S8.
This paper focuses on studies of HIV testing in health care settings including STI clinics, primary care, hospitals, pharmacies, prisons, drug services and TB services. HIV testing strategies involved interventions categorized as testing provision, education programmes, campaigns, use of communication technologies, use of clinical decision‐making tools and other interventions. Data from HIV testing provision studies were examined for the impact of testing on HIV testing coverage. Studies documenting the feasibility and/or acceptability of HIV testing interventions studies were also included. The remaining studies covering barriers to testing, economic evaluations, audits and non‐health care settings are included in the wider systematic review findings that informed the guidance (n = 238) 17. European regions referred to are based on the United Nations geoscheme for Europe.
Results
Study identification and overview
The searches yielded 15 004 records after de‐duplication; after full‐text review, 368 studies were included in the overall systematic review (Figure 1). Of the 368 studies, 130 are described in this paper, exploring interventions and feasibility of HIV testing in health care settings in Europe, including 84 peer‐reviewed articles and 46 conference proceedings (Table S8) 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147.
Figure 1.
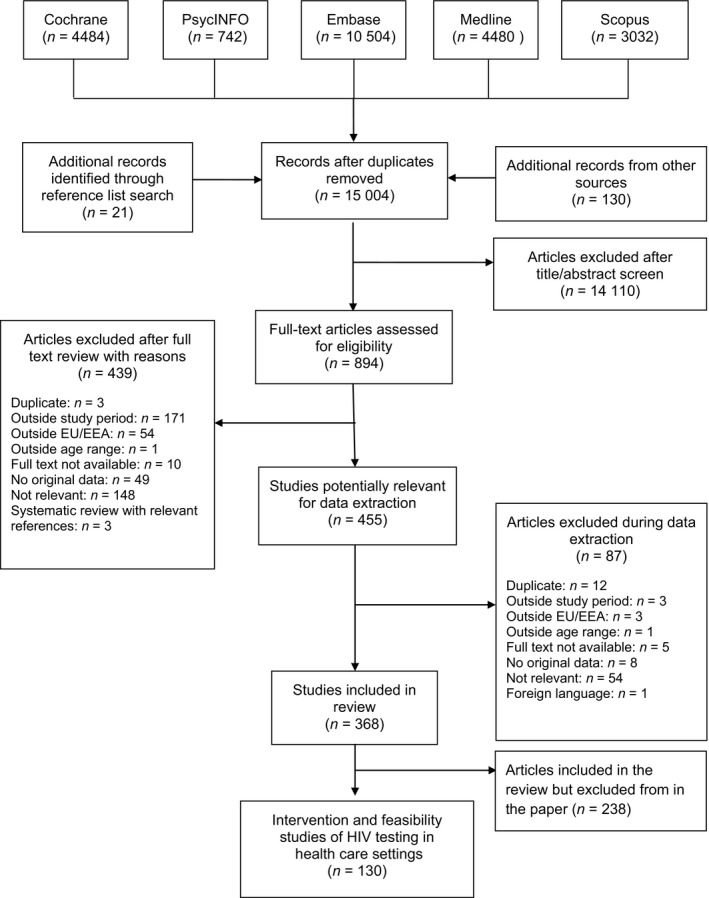
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram. EU/EEA, European Union/European Economic Area.
Studies were from 13 of the 30 EU/EEA countries. Most studies were from Northern Europe (n = 78; 64%), followed by Western (n = 23) and Southern Europe (n = 24). There was only one study from Eastern Europe 109 and four studies set across multiple European countries 110, 114, 127, 128. The majority of the studies from Northern Europe were from the UK (92%). Other than the UK, there were two countries with more than five studies (Spain and France).
Studies were set in a range of health care facilities, the most common being primary care (n = 45) followed by inpatient services (n = 25), STI clinics (n = 24) and emergency departments (n = 23). Other health care testing sites for HIV included outpatient services (n = 16), prisons (n = 4) and pharmacies (n = 4). Almost a fifth of studies were conducted in more than one setting type.
There were a number of interventions implemented to increase HIV testing in health care settings, including innovative/improved testing provision (n = 94), use of testing campaigns (n = 14), use of communication technologies (n = 4), education and training for staff and patients (n = 20), use of tools to aid clinical decision‐making (n = 10) and relocation of a clinic to a higher men who have sex with men (MSM) density area (n = 1). Twenty‐two studies applied strategies with multiple interventions to increase testing.
The quality of the peer‐reviewed studies was variable; of the 84 articles, 70% were of high quality, 19 were of medium quality (23%) and six were of low quality (7%) (Table S8). Risk of bias was low in 42 studies (50%), medium in 36 studies (43%) and high in six studies (7%) (Table S8). Risk of bias was low in 42 studies (50%), medium in 36 studies (43%) and high in six studies (7%) (Table S8).
Testing provision strategies
Novel HIV testing technologies were employed by 40 studies in a wide range of clinical settings to increase testing coverage; the majority utilized rapid testing (n = 36) 18, 19, 22, 24, 25, 34, 36, 45, 46, 48, 49, 50, 56, 57, 59, 65, 75, 77, 81, 83, 85, 88, 89, 99, 100, 101, 107, 113, 117, 129, 132, 134, 137, 139, 141 while four utilized self‐sampling (n = 3) 44, 52, 102 and self‐testing strategies (n = 1) 108. Two of the four self‐sampling studies used oral fluid sampling while the self‐test required a blood sample. Novel testing approaches were particularly applied to improve testing coverage in HIV risk groups including MSM (n = 9) 44, 45, 46, 52, 57, 81, 85, 129, 134, migrants (n = 7) 22, 45, 46, 57, 85, 129, 132, and people who use/inject drugs (PWUD/PWID) (n = 5) 50, 57, 85, 89, 129.
Other testing strategies included routine testing (n = 32) 20, 21, 24, 25, 32, 35, 36, 43, 61, 64, 65, 69, 74, 77, 78, 92, 93, 94, 95, 96, 98, 104, 111, 115, 116, 117, 120, 125, 130, 135, 137, 147, provision of HIV testing as a component of an integrated testing programme (n = 29) 22, 23, 32, 35, 37, 38, 47, 50, 52, 55, 62, 63, 70, 72, 74, 79, 92, 93, 94, 96, 97, 102, 103, 119, 122, 123, 134, 137, 143, IC testing (n = 14) 26, 38, 47, 57, 88, 110, 114, 116, 120, 125, 126, 127, 128, 135 and partner notification (n = 4) 54, 60, 104, 133. Routine testing was most commonly implemented in hospital departments including emergency departments (n = 15) 20, 21, 25, 35, 36, 64, 69, 78, 92, 93, 115, 116, 117, 137, 147, inpatient units (n = 10) 24, 43, 61, 95, 98, 104, 111, 125, 130, 135 and outpatient departments (n = 4) 21, 74, 113, 116. Similarly, IC testing programmes were predominantly instigated in hospitals (n = 8) 109, 114, 116, 125, 126, 127, 128, 135 and primary care (n = 10) 26, 38, 47, 57, 88, 110, 114, 116, 127, 128. In contrast, integrated testing for HIV with other infections such as hepatitis B and C and STIs was adopted in diverse settings including prisons (n = 3) 70, 103, 122, STI clinics (n = 4) 23, 52, 63, 134, drug services (n = 2) 50, 79 and pharmacies (n = 1) 102.
The majority of the 94 testing interventions were directed to the general population (74%). Testing strategies directed to risk groups included studies among migrants and black and minority ethnic groups (n = 12) 22, 23, 32, 45, 46, 57, 62, 80, 85, 129, 132, 143, MSM (n = 11) 44, 45, 46, 52, 57, 63, 81, 85, 129, 134, 136, young people (n = 1) 102, PWUD/PWID (n = 7) 50, 57, 79, 85, 89, 103, 129, and mental health patients (n = 1) 123. Often, these studies targeted multiple risk groups without presenting group‐specific results.
HIV test coverage and positivity differed considerably between health care settings (Table 1). HIV test coverage varied from 2.9 to 94% in primary care and from 3.9 to 66% in emergency departments. HIV positivity ranged from 0 to 25% in STI clinics, with the higher rates achieved when partner notification was used to identify cases. In general, positivity rates were lower in studies set in emergency departments (0–1.3%) and antenatal services (0–0.05%) than in those set in other hospital departments (e.g. up to 5.3% in inpatient units).
Table 1.
HIV testing and positivity rates by health care testing venue
| Testing venue | Number of people tested | Test offered (%) | Test accepted (%) | Test coverage (%) | Positivity rate (%) | References |
|---|---|---|---|---|---|---|
| Primary care | 3–7706* | 12–97 | 45–99.7 | 2.9–94 | 0–4.7 | 25, 32, 37, 44, 45, 46, 54, 55, 56, 61, 71, 74, 76, 79, 81, 86, 87, 93, 98, 100, 106, 115 |
| STI clinic | 4–3738, 15–62 kits returned | 63 | 7–78 |
0.6–4.5 21–25 (PN) |
22, 33, 43, 51, 59, 62, 99, 132, 135 | |
| Inpatient services | 10–4122* | 48–80 | 70–100 | 17–73 | 0–5.3 | 23, 27, 42, 60, 94, 97, 103, 110, 115, 122, 124, 125, 129, 134 |
| Emergency department | 275–27 632 | 6.2–74 | 30–95 | 3.9–66 | 0–1.3 | 19, 24, 34, 35, 63, 68, 77, 91, 92, 96, 112, 115, 116, 146 |
| Outpatient services | 55–166 | 53 | 32–68 | 35–98 | 0–1.9 | 21, 73, 82, 142 |
| Prison | 357–1932 | 51–67 | 0.3–3.9 | 18, 69, 102, 121 | ||
| Pharmacies | 2168*–24 151, 96 kits returned | 45 | 0.9 | 47, 48, 58, 101 | ||
| Drug services | 146–211 | 33–69 | 40–99 | 13–52 | 0–2.5 | 49, 88 |
| Antenatal services | 430–561 158* | 100 | 35–99 | 18 | 0–0.05 | 36, 95 |
| Other health care sites (e.g. TB services) | 71–3881 | 31–100 | 76–99 | 24–99 | 0–2.0 | 31, 64, 118, 119, 131, 140 |
| Combined health care settings† | 141–9471 | 14 | 63 | 56–89 |
0.3–5.4 12–21 (PN) |
20, 53, 109, 112, 113, 126, 127, 133, 138 |
| Combined health care and non‐health care settings† | 119–11 549 | 54 | 0.7–2.5 | 80, 84, 99, 107, 128 |
PN, partner notification; TB, tuberculosis; STI, sexually transmitted infection.
Includes number of tests performed, where there is more than 1 test per person.
Where combined health care and non‐health care settings could not be separated.
Testing provision strategies that increase testing
Thirty studies evaluated the impact of a testing intervention by comparing the intervention data with baseline data (n = 24) 20, 26, 34, 44, 47, 65, 72, 75, 78, 80, 83, 87, 95, 96, 98, 102, 111, 113, 125, 126, 130, 133, 135, 141 or with a control group (n = 6) 22, 77, 81, 82, 120, 136 (Table 2). Twelve studies employed novel testing (10 rapid testing and two self‐sampling) in diverse settings, of which one reported an increase in HIV test coverage from 2% before the intervention to 45% after 65, while others reported increases in HIV diagnoses 77, 141, testing 44, 75, 83, 102 and test acceptance 81 and higher positivity rates 34 after the intervention. The use of rapid tests also resulted in 98% of people obtaining their results compared to 64% in the standard serology group 22. One study reported a decline in numbers of tests performed 113. A further six studies conducted in inpatient services, TB services and primary care reported the impact of IC testing: HIV test coverage changed from 3.9–72% before to 12–85% after its implementation, with most studies reporting a 10–20% increase 26, 120, 125, 126, 135 and the median number of tests also increased 47. Twelve studies, of which half were set in inpatient services, examined the impact of universal routine testing on test coverage. These studies reported an increase in coverage from 2–28% before to 17–80% after the intervention 65, 78, 98, 111, 125, 130, 135 and higher coverage in the intervention group compared to the control group (85% versus 72%, respectively) 120. Other indicators included increases in test acceptance 20, numbers tested (although the increase was small) 95 and numbers diagnosed 77 and a reduction in vertical transmission 96. Only six studies measured the impact of the intervention in at least one risk group; five in MSM 44, 81, 133, 136, 141, one in young people 102 and one in migrants 141.
Table 2.
Impact of HIV testing interventions on HIV tests, coverage and positivity
| Author (year) | Testing venue | Testing strategy | Number of people tested | Tests performed | Offered and/or accepted a test (%) | Testing coverage (%) | Positivity (%) and/or HIV diagnoses | QA score | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Bath et al. (2015) 19 | Emergency department | NTS; UT | Before, 72; after, 2828 |
Accepted before, 2.4%; after, 30% |
High | Low | |||
| Bottero et al. (2015) 21 | Outpatient services | BR | Control, 115; intervention, 159 | Control, 71%; intervention, 98% | High | Low | |||
| Cayuelas‐Redondo et al. (2015) 25 | Primary care | ICT; NTS | Before, 22; after, 78 | Before, 3.9%; after, 12% | Before, 12 diagnoses; after, 13 | High | Medium | ||
| Cuesta et al. (2012) 33 | STI clinic | RT | After, 1011 |
Before, 0.33%; after, 1.1% |
Medium | medium | |||
| Elmahdi et al. (2014) 43 | STI clinic | OFSS; risk group (MSM) | Men returning kits, 15 | Median tests per person per year prior, 1 (1–2); during study, 2 (1–3) | NA | NA | |||
| Fagard et al. (2014) 46 | Primary care | I; ICT; NTS | 1338 | Median GP prescribed tests week before, 2; week of, 16 | Offered after, 68%; accepted, 76% | NA | NA | ||
| Herbert et al. (2012) 64 | Emergency clinic, hospital for tropical diseases | BR; NTS; UT | Before, 38; post‐UT, 183; post‐BR, 1261 | Before, 2%; post‐UT, 23%; post‐BR, 45% | Before, 0%; post‐UT, 1.1%; post‐BR, 0.4% | Medium | Medium | ||
| Kelly et al. (2014) 71 | Primary care | NTS; I | Before, 5; after, 61 | Before, 4.8%; after, 43% | Before & after, 0% | Low | High | ||
| Kuttner‐May (2015) 74 | Primary care | R; NTS | Increase in HIV testing rates: 13% | Before, 1.0%; after, 1.2% | NA | NA | |||
| Lascar et al. (2015) 82 | Outpatient services | OFR; NTS | 148 | Before, 420; after, 676 | Medium | Low | |||
| Leber et al. (2015) 76 | Primary care | BR; UT; NTS | Control GP practices, 2465; intervention, 7706 (BR, 4978) | Rate of HIV diagnosis/10 000 patients/years: control, 0.07; intervention, 0.3 | Medium | Medium | |||
| Leber et al. (2017) 81 | Primary care | NTS; BR | Increase in HIV testing rates: 85% | NA | NA | ||||
| Lim et al. (2014) 77 | Emergency department | NTS; UT | Before, 16%; after: 33% | NA | NA | ||||
| Loos et al. (2014) 79 | Primary care | NTS | GPs testing no sub‐Saharan Africa migrant patients: before, 28%; after, 8% | High | Medium | ||||
| Lorente et al. (2013) 80 |
VCT community testing services |
BR; NTS; risk group (MSM) | Control (VCT): 119; intervention: 211 | Accepted: control, 54%; intervention, 89% | Control, 2.5%; intervention, 1.4% | Medium | Medium | ||
| Martín‐Cabo et al. (2012) 86 | Primary care | NTS; UT | Before, 22; after, 212 | Before, 3.7%; after, 27% | High | Medium | |||
| Onen et al. (2015) 94 | Inpatient services | NTS; UT | Before, 1; post‐education, 0; post‐UT, 4 | NA | NA | ||||
| Op de Coul et al. (2011) 95 | Antenatal services | UT, I | 561 158 | Children born with HIV annually: before, 5–10; after, 1 | High | Low | |||
| Palfreeman et al. (2013) 97 | Inpatient services | NTS; UT | Before, 205; during, 938; after, 1399 | Before, 3.7%; during, 17%; after, 23% |
Before, 2.0%; during, 1.1%; after, 1.1% |
Medium | Medium | ||
| Peacham et al. (2015) 101 | Pharmacy | SS; risk group (YP); I |
Kits returned, 96; increase in STI tests (incl. HIV) from before to after, 700% |
Kit return rate, 45% | NA | NA | |||
| Raman et al. (2015) 110 | Inpatient services | NTS; UT | Before, 20; during, 49; after, 34 | Before, 9%; during, 28%; after, 17% | NA | NA | |||
| Rayment et al. (2013) 112 | Emergency department | OFR; NTS | Pre‐automation of OF testing, 3721; after, 2960 | Pre‐automation, 0.1%; after, 0.1% | High | High | |||
| Roy et al. (2013) 119 | TB services | UT; ICT |
Offered: group A control, 76%; intervention, 87%; group B control, 89%; intervention, 96%; accepted: group A control, 84%; intervention, 87%; group B control, 81%; intervention, 87% |
Group A control, 72%; intervention, 82%; group B control, 76%; intervention, 85% |
High | Low | |||
| Sharvill et al. (2015) 124 | Inpatient services | NTS; UT; ICT | Before, 19; after, 43 | Before, 28% within 24 h; after, 73% | Medium | Low | |||
| Sokhi et al. (2015) 125 | Inpatient services | NTS; ICT |
Pre‐protocol, 9; post, 11; post‐proforma, 20; after 1 year, 17 |
Pre‐protocol, 22%; post, 37%; post‐proforma, 83%; after 1 year, 65% | High | Medium | |||
| Thornhill et al. (2014) 129 | Inpatient services | NTS, UT | After, 465 | Before, 6%; after, 52% | High | Low | |||
| van Aar et al. (2015) 132 | STI clinic | PN; risk group (MSM) | MSM partners, 136 | 21%; of all HIV infections, those detected through PN before, 19%: after, 34% | High | Medium | |||
| Wallis et al. (2015) 134 | Inpatient services | NTS; UT; ICT | Before, 4; after, 22 | Before, 5%; after, 26% | Medium | Low | |||
| Whitlock et al. (2015) 135 | STI clinic | RT; risk group (MSM) |
Control, 19; intervention (SMS reminder), 44 |
Control, 19%; intervention, 44% |
Control, 5.3%; intervention, 4.5% |
NA | NA | ||
| Wouters et al. (2014) 140 | Low threshold centre | BR; NTS; risk groups (MSM, African migrants) | 3881 (MSM, 1173; migrants, 454) | Pilot, 219; implementation, 4806 |
Before, 0%; after, 1.5% (MSM†, 4.0%; migrants†, 2.2%) |
High | Low |
IQR, interquartile range; GP, general practitioner; RT, rapid test; NTS, nontraditional settings; BR, blood rapid test; UT, universal testing; ICT, indicator condition testing; MSM, men who have sex with men; PN, partner notification; SMS, short message service; SS, self‐sampling; OFR, oral fluid rapid test; OFSS, oral fluid self‐sample; I, integrated test; QA, quality assessment; VCT, voluntary counselling and testing; YP, young people; R, rapid test.
NA, not applicable: conference proceedings were not assessed for quality and bias.
Group A, centres using selective testing; group B, centres using UT.
Denominator includes pilot.
Other HIV testing strategies targeted to providers
There were 30 studies using other strategies (campaigns, education and use of clinical decision‐making tools) directed to providers. Three campaigns targeted providers to increase awareness using posters, social media (e.g. Twitter) and promotional materials 21, 111, 146. A significant number of educational intervention studies targeted providers (n = 19), with the majority providing HIV testing training sessions to health care professionals including hospital doctors, general practitioners (GPs), medical students and nurses 30, 47, 67, 68, 77, 82, 83, 95, 105, 112, 126, 133, 138, 139, 146 and pharmacists 48, 49. One employed the plan, do, study, act (PDSA) methodology, which increased physicians’ willingness to test but did not increase testing 84. Another used serious incident reporting to improve testing awareness within clinics 140. Where assessed, education provision resulted in an increase in the offer of HIV testing from 2 to 11% 146 and in the number of individuals tested from 11–13 before to 16–20 after the intervention in two smaller studies, and from 420–1056 before to 676–2333 after the intervention in two larger studies 83, 105, 112, 126. Two studies reported a decline in test offer from 8–15% to 0–10%, which may have been attributable to the small numbers of patients included in the studies (n = 4–26) 68, 95. There were eight studies using clinical decision‐making tools to aid providers in identifying populations that should be tested for HIV. A variety of tools were developed: addition of HIV tests to the blood test ‘set’ requests or checklist 31, 39, 76, 77, computer prompts for higher risk populations 27, 38 and risk assessments 118, 144. Where recorded, the above interventions were successful at increasing testing 27, 31, 39.
Other HIV testing strategies targeted to patients
Other than interventions where testing was provided, there were 23 studies using other interventions directed to patients. The majority of these interventions were campaigns that promoted local testing using social media, posters, digital media and websites 19, 78, 83, 89, 146, campaigns to promote National HIV Testing Week 21, 71, 111, 124 and other regional campaigns to promote testing 48, 49, 85, 97, 142. National HIV Testing Week increased testing from 4–9% before to 8–28% during the week 111, 124, and it also resulted in half of those having blood samples collected at a hospital being tested for HIV 21. During the regional Go Viral campaign, 27% of patients were tested for blood‐borne viruses (BBVs) 97.
There were two educational interventions targeting patients; one for pregnant women 90, which resulted in an increase in testing coverage (from 87 to 92%) after provision of a patient information leaflet and one for patients admitted to a hospital inpatient unit, where there was a decline in test offer (from 8 to 0%) 95. All four communication technology studies were directed to patients, with two providing videos on HIV testing 24, 106, one utilizing text messages to recall MSM for testing 136 and one using online platforms for partner notification 60. The only study measuring intervention impact reported an increase in the re‐testing rate among MSM who were actively recalled (from 19 to 44%) 136. Two studies adopted decision‐making tools that helped individuals determine if an HIV test would be recommended 42, 118. One of these examined whether computer‐assisted self‐interviewing resulted in increased HIV testing when compared to interviews with clinicians 118 and found significantly less testing in the self‐interviewing population (63% versus 69%, respectively). Finally, in one study, the STI clinic moved location to be in a higher MSM density area, which resulted in a large increase in the number of HIV diagnoses from 175 in 2008 to 381 in 2013 after relocation 73.
Feasibility and acceptability
HIV testing interventions were generally acceptable to patients and providers in health care settings (Table 3). Results also suggest that rapid testing is acceptable to both groups (patient studies, n = 5; provider studies, n = 6), with providers willing to use rapid tests 24, 53, 56, 57, 107 and finding their interpretation easy 50. Some feasibility studies highlighted that nontraditional health care settings can target populations not previously tested for HIV (n = 4) 18, 85, 132, 145, with the reported percentage of first‐time testers in such settings ranging from 51 to 75%. One study suggested that provider‐initiated testing is unlikely to be acceptable when specific populations are targeted (in this case, sub‐Saharan African patients) 86.
Table 3.
Selection of feasibility and acceptability indicators for HIV testing in health care settings by testing venue
| Testing venue | Sample size | Feasibility/acceptability (patient) | Feasibility/acceptability (provider) | References |
|---|---|---|---|---|
| Primary care | 62–3314 |
GP testing acceptable, 77–93% First time testers, 75% MSM who strongly agreed that the clinic environment was friendly to MSM, 66% |
GPs willing to use/continue to use rapid HIV testing in their daily practice, 59–77% | 17, 46, 52, 55, 56, 57, 61, 65, 79, 87, 98, 106, 130 |
| STI clinic | 50–337 |
Self‐sampling kits acceptable, 30–62.5% (MSM) Reported self‐sampling really easy, 66% First time testers, 94% MSM who would recommend service to a friend, 100% |
‐ | 28, 43, 50, 51, 62, 144 |
| Inpatient services | 10–478 |
Inpatient testing acceptable, 84–100% Rapid HIV testing in inpatients acceptable, 97% |
Clinical staff who thought HIV rapid testing disrupted their job, 0% | 23, 42, 94, 103 |
| Emergency department | 19–5657 | Emergency department testing acceptable, 50–96% |
Routine HIV testing should be rolled out permanently in the emergency department, 95% Patients not offered testing because the physician forgot to ask, 6.5% |
19, 35, 63, 92, 116 |
| Outpatient services | 166–246 |
Outpatient service testing acceptable to migrants, 72% Preference for rapid tests over standard serological tests, 76% |
‐ | 21, 142 |
| Pharmacies | 806–2168 |
Pharmacy testing acceptable, 100% Pharmacy quick and convenient, 31–71% Pharmacy accessible, 4.7–20% Young people who were very or quite satisfied with the service and were very or quite likely to use the service again, 100% |
‐ | 47, 58, 101 |
| Drug services | 12 | ‐ | Rapid test and test interpretation easy or very easy, 100% | 49 |
| Antenatal services | 1243–2123 |
Antenatal screening acceptable, 81% HIV testing of partners of pregnant women acceptable, 35% |
‐ | 36, 108 |
| Other single sites combined | 21–825 |
Rapid HIV testing acceptable to migrants, 99% First‐time testers among migrants, 71% |
Health care providers often felt untrained and unconfident giving the result Not acceptable to include behavioural survey as part of HIV test for migrants |
90, 131 |
| Combined health care settings*, * | 20–5329 |
Testing as part of routine care acceptable, 71–92% Acceptability of providing: mouth swab, 95%; blood test, 89%; finger prick blood test, 90% IC testing coverage in: primary care, 12%; hospitals, 92% PN case‐finding effectiveness, 18% |
Provider‐initiated testing of sub‐Saharan African migrants acceptable, 35% Identified need for training for physicians, 72% |
39, 40, 53, 85, 112, 115, 120, 127, 133 |
| Combined health care and non‐health care settings*, * | 128–264 |
First time testers: 51% Preference for rapid tests over standard serological tests, 84% Self‐test acceptable, 92% Successful performance of a finger‐stick whole‐blood HIV self‐test, 99% |
‐ | 84, 107 |
GP, general practitioner; IC: indicator condition; MSM, men who have sex with men; PN, partner notification.
Where combined health care and non‐health care settings could not be separated.
Discussion
This systematic review has identified a significant body of evidence on HIV testing in health care settings in Europe. Testing has been provided in a range of clinical settings with results suggesting that it is feasible to achieve high testing coverage, and that it is acceptable for providers and service users. HIV testing positivity ranged widely, from 0 to 25%, with higher positivity rates observed with certain strategies such as partner notification, IC testing and testing among risk groups compared to strategies that offered testing to the general population, including lower risk groups. However, the data also highlight that there is considerable room to increase the offer of testing in health care settings, particularly in primary care and emergency departments.
Evidence from systematic reviews shows that, in primary care, barriers to testing are related to the clinician’s knowledge 17, 148, as well as the clinician’s anxiety associated with raising the topic of HIV testing with patients 149. Improving testing in primary care is important because in many countries more testing takes place in primary care than in specialized services, as those countries have a testing strategy that primarily uses GPs (e.g. the Netherlands and Germany) 12. Furthermore, an evaluation of the impact of the guidance highlighted that, while over half the respondent countries reported that their national testing guidelines were closely aligned with the 2010 European guidance, only 35% included the relevant recommendations on routine offering in primary care 150. Testing in primary care is highly acceptable to all patients, with one study among MSM also reporting primary care as an acceptable setting, and therefore interventions that increase knowledge and provide training for primary care staff could be successful at increasing test coverage. The knowledge and capability of health care staff could be enhanced through education interventions. One study in this review gave GPs training in sexual health clinical skills and achieved large increases in testing rates from 1056 tests before to 2333 after the intervention 105, which emphasizes the impact on testing once GPs are enabled to offer testing.
In addition to testing in primary care, there are other strategies identified in this review that could improve testing coverage in health care settings in Europe, including: scaling up IC testing across all settings; introducing testing in high‐prevalence areas, although the majority of studies implementing this strategy are from the UK; and implementing integrated testing for BBVs in settings such as drug services and prisons and HIV and STI testing in STI clinics. Although IC testing is an effective strategy to diagnose HIV infection and results in high positivity rates, it is not always included in national or speciality testing guidelines for specific ICs and, where it is included, the scale of implementation is quite variable 151, 152. The concept of testing based on high diagnosed sero‐prevalence areas assumes that areas of high diagnosed prevalence are likely to also have high rates of undiagnosed infections. This strategy removes the need to target specific populations, which was found to be unacceptable to primary care providers in one study in this review. The adopted strategy will, however, depend on the health care setting. Finally, integrated testing for BBVs was recently recommended in prisons by the ECDC/ European Monitoring Centre for Drugs and Drug Addiction guidance 153.
There were only three studies set in community‐based drug services and pharmacies. These venues potentially have an important role in HIV testing by reaching populations that do not necessarily attend traditional health care venues and by acting as a bridge between health care and the community. Community‐based pharmacies are acceptable venues for HIV testing where testing can be implemented with basic teaching and skills provision to the pharmacist 48, 49. Pharmacies are already an established model of delivery for chlamydia testing 154, which only highlights the growing role of pharmacies in public health provision. Rapid testing or self‐sampling kits were the adopted testing strategies in this setting. The added benefit of self‐sampling kits is the possibility of providing integrated testing for BBVs and STIs 102.
The distribution of self‐sampling and self‐testing kits for HIV from other health care settings (e.g. STI clinics) has been found to be acceptable among MSM 44, 52 and people attending free and anonymous testing services 108. However, further evidence is needed to understand whether this strategy can increase testing frequency in high‐risk groups including MSM.
There were some limitations to this systematic review. There was only one study from Eastern Europe, which could have implications for the applicability and reproducibility of the review findings to this region. There were methodological concerns relating to measuring increases in test coverage. Although this review aimed to document the impact of intervention on increasing test coverage, a very small proportion of studies included a baseline measure or a comparator group to allow assessment of improvements in testing. Where comparisons could be made, the timeframe over which the impact of the intervention was assessed varied between studies, with most being assessed immediately after the intervention. Future studies should consider implementing interventions over longer timeframes. We restricted the review to the EU/EEA, so we may have missed studies that were applicable to and reproducible in the European setting. Finally, studies with positive findings or using novel approaches are more likely to be published. The inclusion of conference abstracts and reports (35%; 46 of 130) is therefore important to reduce publication bias; however, the quality of these studies has not been assessed. Without this assessment, we cannot know the reliability and reproducibility of the presented results. It is important that all findings including those from conference abstracts are published in peer‐reviewed journals. There are three important strengths to this review. The review employed the robust PRISMA methodology which is standardized and reproducible. Secondly, the scope of the questions facilitated a broad and all‐encompassing review of the literature on HIV testing in health care settings. Thirdly, the review included papers not in English, which were translated.
Conclusions
HIV testing is an integral component of combination HIV prevention, treatment and care. HIV testing is the first step in the global 90‐90‐90 target set by UNAIDS, where 90% of all people with HIV infection should be diagnosed, 90% of those diagnosed should receive HIV treatment and 90% of those on treatment should have a suppressed viral load by 2020 5. Therefore, strategies that diagnose individuals as early as possible are essential to achieve the ultimate goal of ending the HIV epidemic. This review has identified that, although there are considerable missed opportunities for earlier HIV diagnosis, there are also several acceptable and feasible strategies to achieve high HIV testing coverage across a variety of health care settings and populations in Europe.
Author contributions
All authors were involved in the evidence synthesis and contributed important intellectual content to this paper. SD drafted the manuscript and was responsible for submission; all authors commented on the manuscript and approved the final draft. SD and SC coordinated the systematic review process: developed the protocol and search terms (with VD, DR and LT), ran the searches and compiled the results. SC, SD, LT, AKS, LC, DR, SFJ and VD contributed to systematic review study screening, data extraction and quality assessment along with the wider review working group (as listed in the Acknowledgements). AJAG and LT provided ECDC quality control and directed the ECDC‐PHE‐CHIP collaboration.
Supporting information
Table S1 Search terms for OVID Medline – (17/03/2017).
Table S2 Search terms for OVID Embase (20/03/2017).
Table S3 Search terms for OVID PsycINFO (20/03/2017).
Table S4 Search terms for the Cochrane library (17/03/2017).
Table S5 Search terms for Scopus (20/03//2017).
Table S6 List of the 30 EU/EEA countries included in the systematic review.
Table S7 Systematic review quality assessment.
Table S8 Overview of included studies and their approach to HIV testing in healthcare settings.
Acknowledgements
This paper was funded by the European Centre for Disease Prevention and Control (ECDC) as part of a project to update the European HIV testing guidelines (Framework Contract Reference Number: ECDC/2016/035). We would like to thank the wider evidence synthesis working group: from Public Health England (PHE): Peter Kirwan, Cuong Chau, Matthew Hibbert, Meaghan Kall, Alison Brown, Zheng Yin, Nicola Pearce‐Smith and Anh Tran; and from the Centre of Excellence for Health, Immunity and Infections (CHIP): Anne Louise Grevsen, Anne Raahauge, Jeff Lazarus, Maiken Mansfeld and Ida Sperle. We would like to thank Carole Kelly (PHE) not only for her involvement in screening studies, but for her input into developing the initial review protocol. We would also like to acknowledge Csaba Kodmon and other colleagues from ECDC involved in reviewing non‐English articles.
References
- 1. European Centre for Disease Prevention and Control . HIV infection and AIDS In ECDC. Annual epidemiological report for 2017. Stockholm, ECDC, 2019. [Google Scholar]
- 2. Egger M, May M, Chene G et al Prognosis of HIV‐1‐infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 2002; 360: 119–129. [DOI] [PubMed] [Google Scholar]
- 3. Siegfried N, Uthman OA, Rutherford GW. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV‐infected, treatment‐naive adults. Cochrane Database Syst Rev 2010; (3); CD008272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20: 1447–1450. [DOI] [PubMed] [Google Scholar]
- 5. Joint United Nations Programme on HIV/AIDS . 90–90‐90. An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva, UNAIDS, 2014. [Google Scholar]
- 6. Gokengin D, Geretti AM, Begovac J et al 2014 European guideline on HIV testing. Int J STD AIDS 2014; 25: 695–704. [DOI] [PubMed] [Google Scholar]
- 7. British HIV Association . British Association of Sexual Health and HIV, British Infection Society. UK National Guidelines for HIV Testing. London, BHIVA, 2008. [Google Scholar]
- 8. European Centre for Disease Prevention and Control . HIV Testing: Increasing Uptake and Effectiveness in the European Union. Stockholm, ECDC, 2010. [Google Scholar]
- 9. World Health Organisation . Consolidated Guidelines on HIV Testing Services. Geneva, WHO, 2015. [PubMed] [Google Scholar]
- 10. Ministerio de Sanidad, Igualdad SSe . Plan Nacional sobre Sida, Guía de recomendaciones para el diagnóstico precoz de VIH en el ámbito sanitario. Madrid, Minsterio de Sanidad, 2014. [Google Scholar]
- 11. van Sighem A, Pharris A, Quinten C et al Reduction in undiagnosed HIV infection in the European Union/European Economic Area, 2012 to 2016. Euro Surveill 2017;22 10.2807/15607917.ES.2017.22.48.17-00771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deblonde J, Meulemans H, Callens S, Luchters S, Temmerman M, Hamers FF. HIV testing in Europe: mapping policies. Health Policy 2011; 103: 101–110. [DOI] [PubMed] [Google Scholar]
- 13. HIV in Europe . HIV Indicator Conditions: Guidance for Implementing HIV Testing in Adults in Health Care Settings. Copenhagen, HIV in Europe, 2015. [Google Scholar]
- 14. Sullivan AK, Sperle I, Raben D et al HIV testing in Europe: evaluating the impact, added value, relevance and usability of the European Centre for Disease Prevention and Control (ECDC)'s 2010 HIV testing guidance. Euro Surveill. 2017;22 10.2807/15607917.ES.2017.22.48.17-00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Croxford S, Tavoschi L, Sullivan AK et al Strategies to increase HIV testing outside of healthcare settings in Europe: an evidence synthesis to inform European Centre for Disease Prevention and Control guidance. Submitted to Eurosurveillance 2018. [Google Scholar]
- 17. European Centre for Disease Prevention and Control . Public Health Guidance on HIV, Hepatitis B and C Testing in the EU/EEA. An Integrated Approach. Stockholm, ECDC, 2018. [Google Scholar]
- 18. Ashby J, Braithewaite B, Walsh J, Gnani S, Fidler S, Cooke G. HIV testing uptake and acceptability in an inner city polyclinic. AIDS Care 2012; 24: 905–909. [DOI] [PubMed] [Google Scholar]
- 19. Bannan CL, Lynch PA, Conroy EP et al Point‐of‐care testing for HIV in an Irish prison setting: results from three major Irish prisons. Int J STD AIDS 2016; 27: 950–954. [DOI] [PubMed] [Google Scholar]
- 20. Bath R, Ahmad K, Orkin C. Routine HIV testing within the emergency department of a major trauma centre: a pilot study. HIV Med 2015; 16: 326–328. [DOI] [PubMed] [Google Scholar]
- 21. Bath R, O'Connell R, Lascar M et al TestMeEast: a campaign to increase HIV testing in hospitals and to reduce late diagnosis. AIDS Care 2016; 28: 608–611. [DOI] [PubMed] [Google Scholar]
- 22. Bottero J, Boyd A, Gozlan J et al Simultaneous human immunodeficiency virus‐hepatitis B‐hepatitis C point‐of‐care tests improve outcomes in linkage‐to‐care: results of a randomized control trial in persons without healthcare coverage. Open Forum Infect Dis 2015; 2: ofv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bulman J, Goode D, Evans A, eds. HIV‐testing african service users within a newly integrated sexual health service‐our experience. Sex Transm Infect 2016; 92: A87. [Google Scholar]
- 24. Burns F, Edwards SG, Woods J et al Acceptability, feasibility and costs of universal offer of rapid point of care testing for HIV in an acute admissions unit: results of the RAPID project. HIV Med 2013; 14(Suppl 3): 10–14. [DOI] [PubMed] [Google Scholar]
- 25. Casalino E, Bernot B, Bouchaud O et al Twelve months of routine HIV screening in 6 emergency departments in the Paris area: results from the ANRS URDEP study. PLoS ONE 2012; 7: e46437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cayuelas‐Redondo L, Menacho‐Pascual I, Noguera‐Sanchez P et al Indicator condition guided human immunodeficiency virus requesting in primary health care: results of a collaboration. Enferm Infecc Microbiol Clin 2015; 33: 656–662. [DOI] [PubMed] [Google Scholar]
- 27. Chadwick DR, Hall C, Rae C et al A feasibility study for a clinical decision support system prompting HIV testing. HIV Med 2016; 21: 21. [DOI] [PubMed] [Google Scholar]
- 28. Chan SY, Hill‐Tout R, Rodgers M, Cormack I. Acceptance of HIV testing in medical inpatients: a local acceptability study. Int J STD AIDS 2011; 22: 187–189. [DOI] [PubMed] [Google Scholar]
- 29. Chislett L, Clarke J, eds. Which elements of a novel self‐directed rapid asymptomatic sexually transmitted infection screening service are most important to users? Sex Transm Infect 2015; 91: A195. [Google Scholar]
- 30. Chong M, Cartledge J, Waters LJ. Significant benefit of a targeted HIV testing module on medical students' knowledge and confidence. Int J STD AIDS 2016; 27: 1326–1329. [DOI] [PubMed] [Google Scholar]
- 31. Clifford S, Mutch C, eds. Improving inpatient HIV screening on an infectious disease ward in an area of high HIV prevalence. HIV Med 2015; 17: 57. [Google Scholar]
- 32. Coppola N, Alessio L, Gualdieri L et al., eds. A strategy to favor the access of irregular and refugee migrants to a screening program for HBV, HCV and HIV infection. J Hepatol 2015; 62: S530. [Google Scholar]
- 33. Cuadrado JM, ed. HIV infection early diagnosis experience in primary care. J Int AIDS Soc 2014; 17(Suppl 3): 19597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cuesta Mdel M, Lopez Mdel C, Nieto P, Junquera ML, Varela JA, Vazquez F. [Introduction of a rapid HIV test in sexually transmitted infections units]. Enferm Infecc Microbiol Clin 2012; 30: 189–191. [DOI] [PubMed] [Google Scholar]
- 35. Bradshaw D, Rae C, Turner R et al, eds. Testing for blood borne viruses in the emergency department of a large London hospital. HepHIV Conference. Malta, January-February 2017.
- 36. d'Almeida KW, Pateron D, Kierzek G et al Understanding providers' offering and patients' acceptance of HIV screening in emergency departments: a multilevel analysis. ANRS 95008, Paris, France. PLoS ONE 2013; 8: e62686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dhairyawan R, Creighton S, Sivyour L, Anderson J. Testing the fathers: carrying out HIV and STI tests on partners of pregnant women. Sex Transm Infect 2012; 88: 184–186. [DOI] [PubMed] [Google Scholar]
- 38. Brigstock‐Barron O, Logan L, Sowerbutts H et al, eds. Using epidemiology and collaborative funding to enable innovation in opportunistic screening to reduce the late diagnosis of HIV: interim results from a targeted primary care project in England (UK). J. Int. AIDS Soc 2016; 19(Suppl 5): 69. [Google Scholar]
- 39. Douthwaite S, O'Shea S, Palmer S et al. London initiative for glandular fever HIV testing for diagnosis of primary HIV infection: initial results. HIV Med 2015; 16: 46–47.25711323 [Google Scholar]
- 40. Drayton R, Keane F, Prentice E. Patients' attitudes towards increasing the offer of HIV testing in primary and secondary care. Int J STD AIDS 2010; 21: 563–566. [DOI] [PubMed] [Google Scholar]
- 41. Elias MJ, Gomez‐Ayerbe C, Elias PP et al. Development and validation of an HIV risk exposure and indicator conditions questionnaire to support targeted HIV screening. Medicine 2016; 95: e2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elías M, ed. Coverage and acceptability of a targeted testing strategy, resourced by an external program intervention, DRIVE study (rapid diagnosis of HIV infection in Spain). European AIDS Conference (EACS). Barcelona, Spain, October 2015.
- 43. Ellis S, Graham L, Price DA, Ong EL. Offering HIV testing in an acute medical admissions unit in Newcastle upon Tyne. Clin Med 2011; 11: 541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elmahdi R, Fidler S, Ward H, Smith A. SPIT (Saliva Patient Initiated Testing for HIV) study: feasibility and acceptability of repeat home‐based HIV saliva testing using self‐sampling amongst men who have sex with men. HIV Med 2014; 15: 101. [Google Scholar]
- 45. Esteban‐Vasallo MD, Dominguez‐Berjon MF, Garcia‐Riolobos C et al Factors associated to a reactive result of rapid‐HIV test in socio‐culturally adapted services in primary care in Spain. AIDS Behav 2015; 19: 2370–2379. [DOI] [PubMed] [Google Scholar]
- 46. Esteban‐Vasallo MD, Moran‐Arribas M, Garcia‐Riolobos C et al Targeted rapid HIV testing in public primary care services in Madrid. Are we reaching the vulnerable populations? Int J Infect Dis 2014; 19: 39–45. [DOI] [PubMed] [Google Scholar]
- 47. Fagard C, ed. Feasibility of joint HIV, HBV and HCV testing offered routinely by general practitioners during one week in two French counties in 2012. HepHIV Conference. Barcelona, Spain, October 2014.
- 48. Fernandez‐Balbuena S, Belza MJ, Zulaica D et al Widening the access to HIV testing: the contribution of three in‐pharmacy testing programmes in Spain. PLoS ONE 2015; 10: e0134631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fernandez‐Balbuena S, Marcos H, Perez‐Rubio A, Hoyos J, Belza MJ, de la Fuente L. The rapid test in Spanish pharmacies: a novel programme to reach heterosexual men? HIV Med 2015; 16: 362–369. [DOI] [PubMed] [Google Scholar]
- 50. Fernandez‐Lopez L, Folch C, Majo X, Gasulla L, Casabona J. Implementation of rapid HIV and HCV testing within harm reduction programmes for people who inject drugs: a pilot study. AIDS Care 2016; 28: 712–716. [DOI] [PubMed] [Google Scholar]
- 51. Fernando I, Thompson C. Testing times: testing patient acceptance and ability to self‐screen for a No‐Talk Testing service. Int J STD AIDS 2013; 24: 341–344. [DOI] [PubMed] [Google Scholar]
- 52. Fisher M, Wayal S, Smith H et al Home sampling for sexually transmitted infections and HIV in men who have sex with men: a prospective observational study. PLoS ONE 2015; 10: e0120810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fraisse T, Fourcade C, Brazes‐Sanz J et al A cross sectional survey of the barriers for implementing rapid HIV testing among French general practitioners. Int J STD AIDS 2016; 27: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 54. Garcia de Olalla P, Molas E, Barbera MJ et al Effectiveness of a pilot partner notification program for new HIV cases in Barcelona, Spain. PLoS ONE 2015; 10: e0121536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Garner A, Gibbons N, Williams N, Olufunso O, Gupta N, Dewsnap C. Sexual health outreach clinic in a deprived GP setting: a service evaluation. HIV Med 2014; 15: 43. [Google Scholar]
- 56. Gauthier R, Livrozet JM, Prevoteau du Clary F et al Feasibility and acceptability of rapid HIV test screening (DEPIVIH) by French family physicians. Med Mal Infect 2012; 42: 553–560. [DOI] [PubMed] [Google Scholar]
- 57. Gennotte AF, Semaille P, Ellis C et al Feasibility and acceptability of HIV screening through the use of rapid tests by general practitioners in a Brussels area with a substantial African community. HIV Med 2013; 14(Suppl 3): 57–60. [DOI] [PubMed] [Google Scholar]
- 58. Gonah T, Scrivener J, Gando I, Sangha R, Richardson D. Walk‐in primary‐care centres are acceptable to men who have sex with men (MSM). Sex Transm Infect 2015; 91: A56. [Google Scholar]
- 59. Gorostiza I. Elizondo Lopez de Landache I, Braceras Izagirre L. [HIV/AIDS screening program in community pharmacies in the Basque Country (Spain)]. Gac Sanit 2013; 27: 164–166. [DOI] [PubMed] [Google Scholar]
- 60. Gotz HM, van Rooijen MS, Vriens P et al Initial evaluation of use of an online partner notification tool for STI, called 'suggest a test': a cross sectional pilot study.[Erratum appears in Sex Transm Infect. 2015 Feb;91(1):74; PMID: 25609474]. Sex Transm Infect 2014; 90: 195–200. [DOI] [PubMed] [Google Scholar]
- 61. Haidari G, Navin R, Wood D, Larbalestier N. Opt‐out testing for HIV is flawed: it's time for change. HIV Med 2014; 15: 94–95. [Google Scholar]
- 62. Hargreaves S, Seedat F, Car J et al Screening for latent TB, HIV, and hepatitis B/C in new migrants in a high prevalence area of London, UK: a cross‐sectional study. BMC Infect Dis 2014; 14: 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Harte D, Jarman J, Mercey D, Copas A, Benn P. Recall of men who have sex with men diagnosed with bacterial sexually transmitted infections for retesting: a feasible and effective strategy? HIV Med 2010; 11: 17–18. [DOI] [PubMed] [Google Scholar]
- 64. Hempling MC, Zielicka‐Hardy A, Ellis JP, Majewska W, Fida G. Routine HIV testing in the emergency department: feasible and acceptable? Int J STD AIDS 2016; 27: 1267–1274. [DOI] [PubMed] [Google Scholar]
- 65. Herbert R, Ashraf AN, Yates TA et al. Nurse‐delivered universal point‐of‐care testing for HIV in an open‐access returning traveller clinic. HIV Med 2012; 13: 499–504. [DOI] [PubMed] [Google Scholar]
- 66. Hindocha S, Charlton T, Rayment M, Theobald N. Feasibility and acceptability of routine human immunodeficiency virus testing in general practice: your views. Prim Health Care Res Dev 2013; 14: 212–216. [DOI] [PubMed] [Google Scholar]
- 67. Hine P, Wolujewicz A, Chalwa A, Atkin M, Chaponda M. The effect of hospital‐wide and departmental teaching events on HIV testing rates over time. HIV Med 2015; 16: 55.25711324 [Google Scholar]
- 68. Hulley JL, Nurse K, eds. HIV: are we testing appropriately? HepHIV Conference. Malta, January-February 2017.
- 69. Hunter L, Larbalestier N, Paparello J, eds. Routine HIV testing in an inner city emergency department‐ avoiding missed opportunities for testing. HepHIV Conference. Malta, January-February 2017.
- 70. Jacomet C, Guyot‐Lenat A, Bonny C et al Addressing the challenges of chronic viral infections and addiction in prisons: the PRODEPIST study. Eur J Public Health. 2016; 26: 122–128. [DOI] [PubMed] [Google Scholar]
- 71. James C. National HIV testing week: how ambitious expansion is being achieved through widening stakeholder engagement. HIV Med 2015; 16: 54–55. [Google Scholar]
- 72. Kelly C, Johnston J, Carey F. Evaluation of a partnership between primary and secondary care providing an accessible Level 1 sexual health service in the community. Int J STD AIDS 2014; 25: 751–757. [DOI] [PubMed] [Google Scholar]
- 73. Kershaw E. "If you build it, they will come": how the targeted location of a sexual health clinic within the social heart of an at risk community can significantly increase the detection and management of infections. Sex Transm Infect 2015; 91: A70–A71. [Google Scholar]
- 74. Kumar V, Ohizua O, Acharya S, Arumainayagam J. Outcomes of HIV testing in a NHS community abortion counselling clinic in West Midlands, United Kingdom (UK). Eur J Contracept Reprod Health Care 2014; 19: S105–S106. [Google Scholar]
- 75. Kuttner‐May S, Kroenke S, Muenstermann D, Lucht A. HIV‐and syphilis‐counselling and‐testing in the public health service in North Rhine‐Westphalia (NRW). Int J Med Microbiol 2015; 305: 8–9. [Google Scholar]
- 76. Lander M, Tohani A, Dias A, O'Connell R. Assessing HIV testing in hepatitis: an audit of HIV testing uptake in a specialist hepatology clinic in an area of high prevalence for hepatitis B and C. HIV Med 2014; 15: 96–97. [Google Scholar]
- 77. Leber W, McMullen H, Anderson J et al Promotion of rapid testing for HIV in primary care (RHIVA2): a cluster‐randomised controlled trial. Lancet HIV 2015; 2: e229–e235. [DOI] [PubMed] [Google Scholar]
- 78. Lim K, Burns M, Hardie J et al HIV testing in the ED is effective and sustainable. HIV Med 2014; 15: 101. [Google Scholar]
- 79. Linnet M, ed. Organizational barriers as an explanation for differences in offer and uptake rates for hepatitis B/C and HIV testing in three drug addiction centres in Copenhagen. HepHIV Conference. Malta, January-February 2017.
- 80. Loos J, Manirankunda L, Hendrickx K, Remmen R, Nostlinger C. HIV testing in primary care: feasibility and acceptability of provider initiated HIV testing and counseling for sub‐Saharan African migrants. AIDS Educ Prev 2014; 26: 81–93. [DOI] [PubMed] [Google Scholar]
- 81. Lorente N, Preau M, Vernay‐Vaisse C et al Expanding access to non‐medicalized community‐based rapid testing to men who have sex with men: an urgent HIV prevention intervention (the ANRS‐DRAG study).[Erratum appears in PLoS One. 2013;8(6). doi:10.1371/annotation/73d8610b‐c201‐4889‐889e‐db0fba5a3ddc]. PLoS ONE 2013; 8: e61225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Leber W, Anderson J, Figueroa J et al., eds. Promotion of HIV testing in primary care in East London through a research programme is effective. An MRC phase IV implementation study. HepHIV Conference. Malta, January-February 2017.
- 83. Freer J, Lascar M, Phiri E. Tailoring HIV testing in a setting of late HIV diagnosis: is the tide turning?.[Erratum appears in Br J Hosp Med (Lond). 2015 Nov;76(11):668; PMID: 26551504]. Br J Hosp Med 2015; 76: 592–595. [DOI] [PubMed] [Google Scholar]
- 84. Lugo R, Sullivan A, Rae C et al, eds. HIV testing improvement in primary care through opt‐TEST´s indicator condition guided testing: the Tool‐1 and Plan‐Do‐Study‐Act experience in Catalonia, 2016. HepHIV Conference. Malta, January-February 2017.
- 85. MacPherson P, Chawla A, Jones K et al. Feasibility and acceptability of point of care HIV testing in community outreach and GUM drop‐in services in the North West of England: a programmatic evaluation. BMC Public Health 2011; 11: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Manirankunda L, Loos J, Debackaere P, Nostlinger C. "It is not easy": challenges for provider‐initiated HIV testing and counseling in Flanders, Belgium. AIDS Educ Prev 2012; 24: 456–468. [DOI] [PubMed] [Google Scholar]
- 87. Martin‐Cabo R, Losa‐Garcia JE, Iglesias‐Franco H, Iglesias‐Gonzalez R, Fajardo‐Alcantara A, Jimenez‐Moreno A. [Promoting routine human immunodeficiency virus testing in primary care]. Gac Sanit 2012; 26: 116–122. [DOI] [PubMed] [Google Scholar]
- 88. Menacho I, Sequeira E, Muns M et al Comparison of two HIV testing strategies in primary care centres: indicator‐condition‐guided testing vs. testing of those with non‐indicator conditions. HIV Med 2013; 14(Suppl 3): 33–37. [DOI] [PubMed] [Google Scholar]
- 89. Middleton L, ed. HIV testing in the community: responding to the Glasgow outbreak. International Congress of Drug Therapy in HIV infection. Glasgow, Scotland, October 2016
- 90. Morin M, Potin J, Perrin C, Thiercelin N, Perrotin F. [Antenatal screening for HIV: knowledge, attitudes, beliefs and practices of pregnant women. Analysis of current practices and the impact of setting up an informative brochure]. J Gynecol Obstet Biol Reprod (Paris) 2011; 40: 216–224. [DOI] [PubMed] [Google Scholar]
- 91. Navaza B, Abarca B, Bisoffi F, Pool R, Roura M. Provider‐initiated HIV testing for migrants in Spain: a qualitative study with health care workers and foreign‐born sexual minorities. PLoS ONE 2016; 11: e0150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. O'Connell S, Lillis D, Cotter A et al Opt‐out panel testing for HIV, hepatitis B and hepatitis C in an urban emergency department: a pilot study. PLoS ONE 2016; 11: e0150546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. O'Connell S, Lillis D, O'Dea S et al A universal testing programme for blood borne viruses in an urban emergency department‐a call for widespread ED testing in Ireland. Hepatology 2014; 60: 961A. [Google Scholar]
- 94. O'Kelly M, Byrne D, Naughten E, Bergin C, Williams C. Opt‐out testing for blood‐borne viruses in primary care: a multicentre, prospective study. Br J Gen Pract 2016; 66: e392–e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Onen BL, Sinha A, Ratnaike T, Smith B, Mital D. HIV testing on the medical admissions unit. HIV Med 2015; 16: 55.25711324 [Google Scholar]
- 96. Op de Coul EL, Hahne S, van Weert YW et al Antenatal screening for HIV, hepatitis B and syphilis in the Netherlands is effective. BMC Infect Dis 2011; 11: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Orkin C, Flanagan S, Wallis E et al. Incorporating HIV/hepatitis B virus/hepatitis C virus combined testing into routine blood tests in nine UK Emergency Departments: the "Going Viral" campaign. HIV Med 2016; 17: 222–230. [DOI] [PubMed] [Google Scholar]
- 98. Palfreeman A, Nyatsanza F, Farn H, McKinnon G, Schober P, McNally P. HIV testing for acute medical admissions: evaluation of a pilot study in Leicester, England. Sex Transm Infect 2013; 89: 308–310. [DOI] [PubMed] [Google Scholar]
- 99. Papadima AD, ed. Use of 3 HIV testing methods in French primary care: classical ELISA laboratory screening versus 2 rapid finger‐stick HIV tests with result under 5 minutes (INSTI) and up to 30 minutes (VIKIA). European AIDS Conference (EACS). Barcelona, Spain, October 2015.
- 100. Parisi MR, Soldini L, Negri S et al Early diagnosis and retention in care of HIV‐infected patients through rapid salivary testing: a test‐and‐treat fast track pilot study. New Microbiol 2015; 38: 20. [PubMed] [Google Scholar]
- 101. Parisi MR, Soldini L, Vidoni G et al Cross‐sectional study of community serostatus to highlight undiagnosed HIV infections with oral fluid HIV‐1/2 rapid test in non‐conventional settings. New Microbiol 2013; 36: 121–132. [PubMed] [Google Scholar]
- 102. Peacham A, Symonds M. Enhanced sexual health services in community pharmacies‐pilot. Sex Transm Infect 2015; 91: A21. [Google Scholar]
- 103. Peters S, Cassells Y, Paton J, Aitken C, eds. HIV testing and care in prisoners: the first year results of opt‐out BBV testing in Glasgow, UK. International Congress of Drug Therapy in HIV infection. Glasgow, Scotland, October 2016.
- 104. Phillips D, Barbour A, Stevenson J, Draper S, Motazed R, Elgalib A. Implementation of a routine HIV testing policy in an acute medical setting in a UK general hospital: a cross‐sectional study. Sex Transm Infect 2014; 90: 185–187. [DOI] [PubMed] [Google Scholar]
- 105. Pillay TD, Mullineux J, Smith CJ, Matthews P. Republished: unlocking the potential: longitudinal audit finds multifaceted education for general practice increases HIV testing and diagnosis.[Reprint of Sex Transm Infect. 2013 May;89(3):191‐6; PMID: 23044438]. Postgrad Med J 2014; 90: 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pinzone MR, ed. Unselected, emergency department‐based rapid HIV screening detects high proportion of early, asymptomatic HIV infection. European AIDS Conference (EACS). Barcelona, Spain, October 2015.
- 107. Poirier C, Aymeric S, Grammatico‐Guillon L et al Rapid HIV test in family practice. Med Mal Infect 2015; 45: 207–214. [DOI] [PubMed] [Google Scholar]
- 108. Prazuck T, Karon S, Gubavu C et al A finger‐stick whole‐blood HIV self‐test as an HIV screening tool adapted to the general public. PLoS ONE 2016; 11: e0146755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Raba G, Skret‐Magierlo J, Skret A. Knowledge about HIV infection and acceptability of HIV testing among women delivered in Podkarpackie Province, Poland. Int J Gynaecol Obstet 2010; 108: 108–110. [DOI] [PubMed] [Google Scholar]
- 110. Raben D, ed. Ongoing Mononucleosis‐like Illness – a clear indicator condition for HIV testing: results from the HIDES 2 study – single arm extension. European AIDS Conference (EACS). Barcelona, Spain, October 2015.
- 111. Raman L, Duschl J, Wallis E, Orkin C. Effectiveness of opt‐out HIV testing on a medical assessment unit in a high‐prevalence area. HIV Med 2015; 16: 49. [Google Scholar]
- 112. Randhawa G, Powell M, Sloan B. Testing times: increasing HIV testing rates in two tertiary intensive care units. Anaesthesia 2015; 70: 13.25489610 [Google Scholar]
- 113. Rayment M, Doku E, Thornton A et al Automatic oral fluid‐based HIV testing in HIV screening programmes: automatic for the people. HIV Med 2013; 14(Suppl 3): 49–52. [DOI] [PubMed] [Google Scholar]
- 114. Rayment M, Kutsyna G, Mocroft A et al The effectiveness of indicator disease‐based HIV testing across Europe‐results from a prospective multi‐centre study. HIV Med 2015; 16: 1. [Google Scholar]
- 115. Rayment M, Rae C, Ghooloo F et al Routine HIV testing in the emergency department: tough lessons in sustainability. HIV Med 2013; 14(Suppl 3): 6–9. [DOI] [PubMed] [Google Scholar]
- 116. Rayment M, Thornton A, Mandalia S et al HIV testing in non‐traditional settings–the HINTS study: a multi‐centre observational study of feasibility and acceptability. PLoS ONE 2012; 7: e39530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Reyes‐Uruena J, Fernandez‐Lopez L, Force L, Daza M, Agusti C, Casabona J. Level of impact on the public health of universal human immunodeficiency virus screening in an emergency department. Enferm Infecc Microbiol Clin 2015; 01: 01. [DOI] [PubMed] [Google Scholar]
- 118. Richens J, Copas A, Sadiq ST et al A randomised controlled trial of computer‐assisted interviewing in sexual health clinics. Sex Transm Infect 2010; 86: 310–314. [DOI] [PubMed] [Google Scholar]
- 119. Matkovic Puljic V, Kosanovic Licina ML, Kavic M, Nemeth Blazic T. Repeat HIV testing at voluntary testing and counseling centers in Croatia: successful HIV prevention or failure to modify risk behaviors? PLoS ONE 2014; 9: e93734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Roy A, Anaraki S, Hardelid P et al. Universal HIV testing in London tuberculosis clinics: a cluster randomised controlled trial. Eur Respir J 2013; 41: 627–634. [DOI] [PubMed] [Google Scholar]
- 121. Sadler KE, Low N, Mercer CH et al. Testing for sexually transmitted infections in general practice: cross‐sectional study. BMC Public Health 2010; 10: 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sagnelli E, Starnini G, Sagnelli C et al Blood born viral infections, sexually transmitted diseases and latent tuberculosis in italian prisons: a preliminary report of a large multicenter study. Eur Rev Med Pharmacol Sci 2012; 16: 2142–2146. [PubMed] [Google Scholar]
- 123. Sanger C, Hayward J, Patel G et al Acceptability and necessity of HIV and other blood‐borne virus testing in a psychiatric setting. Br J Psychiatry 2013; 202: 307–308. [DOI] [PubMed] [Google Scholar]
- 124. Seneviratne K, Porter C, Taylor R. Increasing HIV testing uptake in an inner city sexual and reproductive health clinic: a simple and effective method. J Fam Plann Reprod Health Care 2014; 40: 314–315. [DOI] [PubMed] [Google Scholar]
- 125. Sharvill R, Fernandes A, Allen K, Astin J. Adopting universal testing for HIV in intensive care for patients admitted with severe pneumonia: results from our change in practice. Int J STD AIDS 2015; 28: 88–90. [DOI] [PubMed] [Google Scholar]
- 126. Sokhi DS, Oxenham C, Coates R, Forbes M, Gupta NK, Blackburn DJ. Four‐stage audit demonstrating increased uptake of HIV testing in acute neurology admissions using staged practical interventions. PLoS ONE 2015; 10: e0134574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sullivan A, ed. Condition guided HIV testing – progress and challenges. HepHIV Conference. Malta, January-February 2017.
- 128. Sullivan AK, Raben D, Reekie J et al Feasibility and effectiveness of indicator condition‐guided testing for HIV: results from HIDES I (HIV indicator diseases across Europe study). PLoS ONE 2013; 8: e52845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Taegtmeyer M, MacPherson P, Jones K et al Programmatic evaluation of a combined antigen and antibody test for rapid HIV diagnosis in a community and sexual health clinic screening programme. PLoS ONE 2011; 6: e28019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Thornhill J, Mandersloot G, Bath R, Orkin C. Opt‐out HIV testing in adult critical care units.[Erratum appears in Lancet. 2014 May 17;383(9930):1720]. Lancet 2014; 383: 1460. [DOI] [PubMed] [Google Scholar]
- 131. Puentes Torres RC, Aguado Taberne C, Perula de Torres LA, Espejo Espejo J, Castro Fernandez C, Fransi Galiana L. [Acceptability of the opportunistic search for human immunodeficiency virus infection by serology in patients recruited in Primary Care Centres in Spain]. Aten Primaria 2016; 48: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Uccella I, Petrelli A, Vescio MF et al HIV rapid testing in the framework of an STI prevention project on a cohort of vulnerable Italians and immigrants. AIDS Care 2017; 29: 996–1002. [DOI] [PubMed] [Google Scholar]
- 133. van Aar F, van Weert Y, Spijker R, Gotz H, Op de Coul E, Partner Notification G . Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS. 2015; 26: 565–573. [DOI] [PubMed] [Google Scholar]
- 134. Van Loo I, Muijers ND, Heuts R, Van Der Sande M, Hoebe C. Serological testing for sexually transmitted diseases on dried blood spots: Are results as reliable as for blood drawn by venous puncture? Int J STD AIDS 2015; 1: 96. [Google Scholar]
- 135. Wallis E, Thornhill J, Saunders J, Orkin C. Introducing opt‐out HIV testing in an acute medical admissions unit: does it improve testing uptake in those with lobar pneumonia? Sex Transm Infect 2015; 91: 153. [DOI] [PubMed] [Google Scholar]
- 136. Whitlock G, Duke O, Nwokolo N, McOwan A. Active recall of high‐risk MSM by text message Sex Transm Infect 2015; 91(Suppl 1): A72. [Google Scholar]
- 137. Wicker S, Rabenau HF, Scheller B, Marzi I, Wutzler S. [Prevalence of blood‐borne pathogens among 275 trauma patients: a prospective observational study]. Unfallchirurg 2016; 119: 648–653. [DOI] [PubMed] [Google Scholar]
- 138. Wikeley S, Bradbury R, Bath R et al 'Legion' of medical students participates in largest hospitalbased HIV testing week initiative‐breaking down testing barriers in the doctors of tomorrow. HIV Med 2014; 15: 91. [Google Scholar]
- 139. Wilkin‐Crowe H, Majewska W, Lau R, Webb H, Pakianathan M. Changing trends in HIV diagnosis in an inner city London teaching hospital 2007–2011. Int J STD AIDS 2013; 24: 269–272. [DOI] [PubMed] [Google Scholar]
- 140. Womack J, O'Brien N, Herieka E et al Reducing very late diagnosis of HIV infection in south west England using serious incident reporting (SIR). HIV Med 2015; 16: 51–52. [Google Scholar]
- 141. Wouters K, Fransen K, Beelaert G et al Use of rapid HIV testing in a low threshold centre in Antwerp, Belgium, 2007–2012. Int J STD AIDS 2014; 25: 936–942. [DOI] [PubMed] [Google Scholar]
- 142. Wressell A, Twaites H, Taylor S, Hartland D, Gove‐Humphries T. Saving Lives through visual health communication: a multidisciplinary team approach. J Vis Commun Med 2014; 37: 81–90. [DOI] [PubMed] [Google Scholar]
- 143. Aparicio C, Mourez T, Simoneau G et al. Proposal of HIV, HBV and HCV targeted screening: short period feasibility study in a free‐access outpatient medical structure. Presse Med 2012; 41: e517–e523. [DOI] [PubMed] [Google Scholar]
- 144. Ahmed A, Fattah S, McCallum A et al, eds. HIV testing in acute medicine; assessing the rates and barriers to testing in a busy Scottish acute medical unit. HIV Med 2016; 17(Suppl 1): 60. [Google Scholar]
- 145. Allstaff S, Bird C, Ditcham K et al., eds. Co‐production in practice: HIV prevention services for men who have sex with men. HIV Med 2014; 15(Suppl 3): 34. [Google Scholar]
- 146. Baillie S. HIV testing in primary care. HIV Med 2014: 15(Suppl 3): 108.24025147 [Google Scholar]
- 147. Ellis J, Hempling M, Zielicka‐Hardy A, Fida G, Majewska W. Routine opt‐out HIV testing in the emergency department: Feasible and acceptable. HIV Med 2015; 16: 50. [DOI] [PubMed] [Google Scholar]
- 148. Deblonde J, Van Beckhoven D, Loos J et al HIV testing within general practices in Europe: a mixed‐methods systematic review. BMC Public Health 2018; 18: 1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Deblonde J, De Koker P, Hamers FF, Fontaine J, Luchters S, Temmerman M. Barriers to HIV testing in Europe: a systematic review. Eur J Public Health 2010; 20: 422–432. [DOI] [PubMed] [Google Scholar]
- 150. European Centre for Disease Prevention and Control . HIV Testing in Europe ‐ Evaluation of the Impact of the ECDC Guidance on HIV Testing: Increasing Uptake and Effectiveness in the European Union. Stockholm, ECDC, 2016. [Google Scholar]
- 151. European Centre for Disease Prevention and Control . HIV Testing – Monitoring Implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2017 Progress Report. Stockholm, ECDC, 2017. [Google Scholar]
- 152. Lord E, Stockdale AJ, Malek R et al Evaluation of HIV testing recommendations in specialty guidelines for the management of HIV indicator conditions. HIV Med 2017; 18: 300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tavoschi L, Vroling H, Madeddu G et al Active case finding for communicable diseases in prison settings: increasing testing coverage and uptake among the prison population in the European Union/European Economic area. Epidemiol Rev 2018; 40: 105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Gudka S, Afuwape FE, Wong B, Yow XL, Anderson C, Clifford RM. Chlamydia screening interventions from community pharmacies: a systematic review. Sex Health 2013; 10: 229–239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search terms for OVID Medline – (17/03/2017).
Table S2 Search terms for OVID Embase (20/03/2017).
Table S3 Search terms for OVID PsycINFO (20/03/2017).
Table S4 Search terms for the Cochrane library (17/03/2017).
Table S5 Search terms for Scopus (20/03//2017).
Table S6 List of the 30 EU/EEA countries included in the systematic review.
Table S7 Systematic review quality assessment.
Table S8 Overview of included studies and their approach to HIV testing in healthcare settings.


