Abstract
Background
δ‐storage pool disease (δ‐SPD) is a bleeding disorder characterized by a reduced number of platelet‐dense granules. The diagnosis of δ‐SPD depends on the measurement of platelet ADP content, but this test is time consuming and requires a relatively large blood volume. Flow cytometric analysis of platelet mepacrine uptake is a potential alternative, but this approach lacks validation, which precludes its use in a diagnostic setting.
Objectives
To evaluate the performance of platelet mepacrine uptake as a diagnostic test for δ‐SPD.
Patients/Methods
Mepacrine fluorescence was determined with flow cytometry before and after platelet activation in 156 patients with a suspected platelet function disorder and compared with platelet ADP content as a reference test. Performance was analyzed with a receiver operating characteristic (ROC) curve.
Results
Eleven of 156 patients had δ‐SPD based on platelet ADP content. Mepacrine fluorescence was inferior to platelet ADP content in identifying patients with δ‐SPD, but both mepacrine uptake (area under the ROC curve [AUC] 0.87) and mepacrine release after platelet activation (AUC 0.80) had good discriminative ability. In our tertiary reference center, mepacrine uptake showed high negative predicitive value (97%) with low positive predictive value (35%). Combined with a negative likelihood ratio of 0.1, these data indicate that mepacrine uptake can be used to exclude δ‐SPD in patients with a bleeding tendency.
Conclusion
Mepacrine fluorescence can be used as a screening tool to exclude δ‐SPD in a large number of patients with a suspected platelet function disorder.
Keywords: blood platelets, platelet function testing, quinacrine, flow cytometry, platelet storage pool deficiency
Essentials.
Flow cytometry is recommended in the diagnosistic approach of platelet function disorders.
Prospective validation of mepacrine fluorescence for storage pool disease.
High negative predictive value of mepacrine fluorescence in diagnosis storage pool disease.
Mepacrine fluorescence is suitable for exclusion of storage pool disease.
1. INTRODUCTION
Platelets play an important role in hemostasis by forming a platelet plug upon vascular injury. When platelets are activated, they secrete the content of their storage organelles, alpha‐ and dense granules, to promote further platelet activation and coagulation.1, 2 One of the molecules secreted from dense granules is ADP, which promotes secondary platelet activation via the P2Y12 receptor and is essential for thrombus stability.3
Defects in platelet‐dense granules can be classified into storage pool disease (δ‐SPD) and secretion defects. δ‐SPD results from either a decreased number or complete absence of dense granules or a decreased granule content, such as the empty sack syndrome.4 Secretion defects are associated with a defective release mechanism resulting from impaired signal transduction or granule trafficking.5
Platelet secretion disorders, in particular dense granule disorders, are the most common inherited platelet function disorders and may be more prevalent than von Willebrand disease.6 Nonetheless, there is no consensus on the best laboratory practice to detect these disorders, and the methodology is poorly standardized.7, 8 The current approach to evaluate platelet‐dense granule secretion includes lumi‐aggregometry and the measurement of ADP and ATP in platelet lysate using bioluminescence.9, 10 Lumi‐aggregometry is currently the most often used method, but cannot distinguish between a decreased granule number or a secretion defect.9 Measuring ADP and ATP content in platelet lysates will diagnose patients with storage pool deficiency,11 but is insensitive for secretion defects.12 Interestingly, many diagnostic laboratories do not measure platelet nucleotide content, resulting in potential underdiagnosis of δ‐SPD.13, 14 In addition, none of these tests can be performed in patients with thrombocytopenia. Another method used to diagnose δ‐SPD is to count the total number of dense granules per platelet with whole mount transmission electron microscopy (TEM)15, 16. However, this technique is challenging and not widely available. Therefore, there is an unmet need for an easy and rapid diagnostic tool to evaluate platelet dense granule secretion.
Flow cytometry has been recommended by the International Society on Thrombosis and Haemostasis/Scientific and Standardization Committee guidelines as a tool to diagnose patients with a platelet function disorder, and has been shown to have added value to light transmission aggregometry in diagnosing platelet function disorders.9, 17 Platelet granule markers, such as CD63 and P‐selectin, have also been used in the screening of mild platelet function disorders on the flow cytometer, but require platelet stimulation before analysis. Mepacrine, a fluorescent acridine derivative that binds adenosine nucleotides,18 has been used to measure platelet dense granule content. Several studies showed decreased platelet mepacrine fluorescence in patients with δ‐SPD,19, 20, 21, 22 and implementation of mepacrine fluorescence in a diagnostic algorithm for platelet function disorders has been proposed.23 However, although current data on mepacrine fluorescence are promising, the performance of mepacrine fluorescence has not yet been compared with routine diagnostic tests for δ‐SPD in a real‐life clinical setting.24, 25 In the present study, we validated a flow cytometric mepacrine fluorescence assay for dense granule content in patients with a suspected platelet function disorder.
2. METHODS
2.1. Participants
2.1.1. Healthy volunteers
Blood from healthy individuals was obtained via the Mini Donor Service, a blood donation facility for research purposes that is approved by the medical ethics committee of the University Medical Center (UMC) Utrecht. All donors provided written informed consent, in accordance with the declaration of Helsinki, and self‐reported to be free from antiplatelet drugs or nonsteroidal anti‐inflammatory drugs for at least 10 days before blood donation.
2.1.2. Patients
Two different patient cohorts were used in this study. Cohort 1 consisted of seven patients with a previously diagnosed δ‐SPD (ADP content < 1.7 µmol/1011 platelets) and was used to provide proof of principle for diagnostic mepacrine fluorescence. Cohort 2 included patients from the Thrombocytopathy in the Netherlands (TiN) study and was used to validate the flow cytometric mepacrine uptake. The TiN study is a nationwide cross‐sectional study to collect data on clinical characteristics, functional assays, and genetics in a population of patients with a suspected platelet disorder. Patients were included when von Willebrand disease or a coagulation factor deficiency was excluded and (a) they had previously abnormal platelet counts or platelet function test results or (b) they exhibited a predominantly mucocutaneous bleeding tendency compatible with a platelet function disorder. After a single hospital visit, laboratory tests were performed for platelet count, aggregation in response to four agonists, nucleotide content, surface receptor expression via flow cytometry, and genetic analysis with a selected primary hemostasis gene panel. Platelet mepacrine content and release were measured at the time of inclusion as well. In total, the TiN cohort included 173 patients with a bleeding tendency in whom a platelet function disorder was suspected, and in 156 patients both mepacrine fluorescence and platelet ADP content was measured. All patients were aged > 18 years and were referred to the Van Creveldkliniek for platelet function testing. Donors and patients declared to be free from any antiplatelet drugs. The medical ethics review board of the UMC Utrecht approved this study and patients provided written informed consent in accordance with the declaration of Helsinki.
2.2. Blood collection and platelet preparation
Peripheral venous blood from patients and controls was collected with venipuncture into 3.2% sodium citrate Vacutainer tubes (BD Biosciences). Flow cytometric assays were performed in whole blood, whereas the other tests required platelet‐rich plasma (PRP). PRP was obtained by centrifugation of whole blood at 160 g without brake for 15 minutes at 20°C. Platelet‐poor plasma was obtained by centrifugation of whole blood at 2000 g for 10 minutes and was used to adjust PRP concentration to 250 × 109 platelets/L. All experiments were performed within 1 to 6 hours after blood collection.
2.3. Flow cytometric determination of dense granule content
Five microliters whole blood was diluted 1:10 (v:v) in HEPES buffered saline (10 mM HEPES, 150 mM NaCl, 1 mM MgSO4, 5 mM KCl, pH 7.4), which contained 100 µM mepacrine (Sigma Aldrich) and 15 µg/mL in‐house developed PE‐conjugated anti‐GP1b nanobodies (clone 17), with or without 25 µM protease activating receptor (PAR)‐1 activating peptide SFLLRN (PAR1‐AP; Bachem). Whole blood was incubated for 10 minutes at 37°C, after which samples were fixed with 0.148% formaldehyde, 137 mM NaCl, 2.7 mM KCl, 1.12 mM NaH2PO4, 10.2 mM Na2HPO4, 1.15 mM KH2PO4, 4 mM EDTA, pH 6.8 for 20 minutes at room temperature and analyzed on a BD FACSCanto II (BD Biosciences). The flow cytometer was calibrated every week to maintain stable fluorescent intensity. Platelets were identified based on forward and sideward scatter, as well as GPIbα‐expression. Mepacrine fluorescence was normalized on the median fluorescence of the healthy control population and was expressed as normalized median fluorescent intensity. The coefficient of variation for mepacrine uptake was 2.3%. Flow cytometric analysis was reproducible within 6 hours after blood collection (data not shown).
2.4. Platelet ADP concentration
One milliliter PRP with a platelet count between 100 and 250 × 109/L was diluted 1:3 (v:v) in ice cold 86.4% ethanol, 10 mM EDTA, pH 7.4. Platelets were lysed by vortex and one freeze/thaw cycle and samples were stored at −80°C until further processing. Platelet lysates were split into two fractions. The first fraction was incubated with 95 µM phosphoenolpyruvate and 25 µg/mL pyruvate kinase in 0.2 M Tris‐Maleate, 10 mM KCl, 15 mM MgSO4, pH 7.4 at 37°C for 15 minutes to convert all ADP to ATP. Reactions were stopped by heating the samples for 10 minutes at 80°C. The second fraction was used without prior treatment. ATP levels in both fractions were determined with the ATPLite 1 step kit (Perkin Elmer) on a Spectramax L luminometer (Molecular Devices) according to the protocol of the manufacturer. ATP levels were derived from an ATP calibration curve. ADP concentrations were calculated by subtracting the ATP concentration of the second fraction from the first. ADP levels were expressed in µmol/1011 platelets.
2.5. Quantification of dense granules with TEM
Platelet‐dense granule numbers were counted using TEM images made with the Jeol1010 microscope (Jeol). Formvar‐coated grids were stabilized with carbon (Edwards Auto306) and coated with 100 μg/mL fibrinogen for 20 minutes at room temperature. Coated grids were blocked with 1% bovine serum albumin in HEPES buffered saline. Platelets were allowed to adhere to the grids for 1 minute, after which the grids were rinsed with demi water and air dried. Images of 10 platelets at 12,000× magnification were taken for every subject. Six independent individuals were instructed to quantify the dense granule number in all images according to the guidelines for dense granule identification.26 Observers were blinded to the case or control status of the sample.
2.6. Data analysis
Statistical analysis was performed with GraphPad Prism software, version 6, and IBM SPSS Statistics 21. Variables were analyzed for a normal distribution with the Shapiro‐Wilk test. Non‐normally distributed variables were transformed with a Box‐Cox power transformation, after which normality was checked again. Cutoff values for mepacrine fluorescence and mepacrine release were determined in normally distributed data using the 2.5th percentile from 89 healthy controls. The lower cutoff value for platelet ADP content (<1.4 µmol/1011 platelets) was based on the 2.5th percentile of 49 healthy controls. Intertest agreement between the different tests was expressed as Cohen's kappa coefficient. The R 2 Pearson's correlation coefficient was calculated for the correlation between platelet size or platelet count and mepacrine uptake. The discriminative ability of mepacrine fluorescence and mepacrine release was determined with the area under a receiver operator characteristic curve (AUC) in patients with a bleeding tendency without prior diagnosis in whom a platelet function disorder was suspected. Researchers were blinded for the δ‐SPD diagnosis during analysis.
3. RESULTS
3.1. Mepacrine fluorescence and mepacrine release show good agreement in patients with previously diagnosed with δ‐SPD
Seven patients with previously diagnosed with δ‐SPD were enrolled in this study and were compared with healthy controls. This population included four female and three male patients with an ADP concentration ranging from 0.3 to 1.21 µmol/1011 platelets and an average dense granule number per platelet ranging from 0.3 to 1.15 (Table 1). Platelet ADP content (Figure 1A) was decreased in all patients with δ‐SPD, whereas the platelet ATP/ADP ratio (Figure 1B) was increased in all patients with δ‐SPD. In the seven SPD patients, platelet‐dense granule content was also determined with flow cytometry by measuring mepacrine uptake in resting platelets, or mepacrine release after PAR1‐AP stimulation. The lower limit of normal (2.5th percentile) was 75.1%. Patients with δ‐SPD had reduced mepacrine uptake compared with healthy controls (P < .05) (Figure 1C). Platelet activation with 25 µM PAR1‐AP resulted in decreased mepacrine release in patients with δ‐SPD (Figure 1D). Mepacrine uptake did not correlate with platelet size (R 2 = 0.02; P = .43) or platelet count (R 2 = 0.003: P = .78). Mepacrine fluorescence was in perfect agreement (ƙ = 1) with platelet ADP content (Figure 2A) and mepacrine release after PAR1‐AP activation was in good agreement (ƙ = 0.93) with platelet ADP content (Figure 2B). Mepacrine fluorescence was also in good agreement (ƙ = 0.93) with PAR1‐AP‐induced mepacrine release (Figure 2C).
Table 1.
Baseline characteristics of δ‐storage pool disease patients
| SPD patients | n = 7 | Reference value |
|---|---|---|
| Male | 3 | |
| Age (years), median (IQR) | 48 (23.5‐63) | |
| Platelet count (109/L), median (IQR) | 232 (183‐306) | 150‐450 |
| MPV (fL), median (IQR) | 7.2 (6.8‐7.5) | 7.0‐9.5 |
| Platelet ADP content (µmol/1011 platelets), median (IQR) | 1 (0.5‐1.43) | 1.7‐3.8 |
| Number of dense granules, median (IQR) | 0.85 (0.5‐1.15) | 4‐6 |
Abbreviations: IQR, interquartile range; MPV, mean platelet volume.
Figure 1.
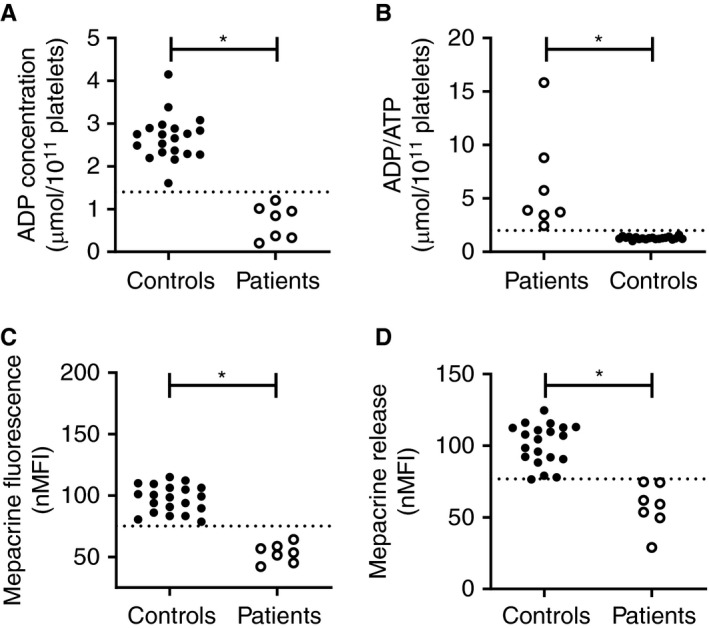
High discriminative ability of flow cytometric measurement of platelet‐dense granule content in patients with previously diagnosed δ‐SPD. (A) Platelet ADP content, expressed as µmol/1011 platelets measured with luminescence, (B) platelet ADP/ATP ratio, (C) normalized mepacrine fluorescence, and (D) mepacrine release in 20 healthy controls (closed symbols) and 7 δ‐SPD patients measured with flow cytometry. The dotted line represents the 2.5th percentile of the healthy control population. *P value < .05
Figure 2.
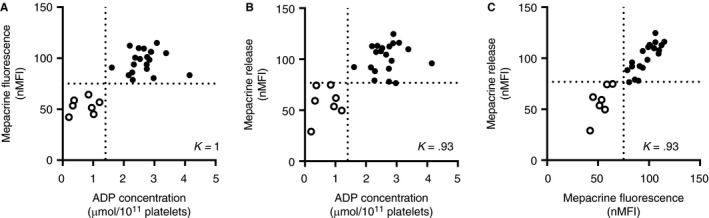
Statistically relevant agreement between mepacrine fluorescence and platelet ADP content in diagnostic testing of δ‐SPD. Cohen's kappa was calculated to determine the agreement between (A) mepacrine fluorescence and platelet ADP content (B) mepacrine release and platelet ADP content (C) mepacrine release and mepacrine fluorescence in 20 healthy controls and 7 δ‐SPD patients. Platelet ADP content is expressed in µmol/1011 platelets. Mepacrine fluorescence and release are normalized on the median fluorescence of the healthy control group. The dotted line represents the 2.5th percentile of the healthy control population
3.2. Validation of mepacrine fluorescence in patients with suspected platelet function disorders
To prospectively validate mepacrine fluorescence and mepacrine release in a relevant patient population, we compared these parameters with platelet ADP content as the gold standard in the TiN cohort. This cohort included 173 patients with a bleeding tendency, in whom a platelet function disorder was suspected. Mepacrine fluorescence data were not obtained in 17 patients. Consequently, 156 patients were used in the current analyses. Based on the 2.5th percentile of 49 healthy controls, the cutoff for normal platelet ADP content was set at 1.4 µmol/1011 platelets. In total, 17 of 156 patients (Table 2) had a platelet ADP content below 1.4 µmol/1011 platelets and were diagnosed with δ‐SPD (Figure 3A). Both mepacrine fluorescence (Figure 3B) and mepacrine release (Figure 3C) were decreased in patients with δ‐SPD. Mepacrine fluorescence (Figure 3D; R 2 = 0.18) and mepacrine release (Figure 3E; R 2 = 0.10) correlated with platelet ADP content. The discriminative ability of mepacrine fluorescence and mepacrine release were determined with the area under a receiver‐operator curve (AUC) (Figure 3F). Mepacrine fluorescence (AUC 0.87; 95% confidence interval [CI] 0.76‐0.96) and mepacrine release (AUC 0.79; 95% CI 0.67‐0.91) showed good discriminative ability for diagnosing δ‐SPD. When a more stringent definition of δ‐SPD, based on both platelet ADP content < 1.4 µmol/1011 platelets and an ATP/ADP ratio > 2, was used, 15 patients met the diagnostic criteria for δ‐SPD. This did not affect the AUC for mepacrine fluorescence (AUC 0.90, 95% CI 0.81‐0.996, P = .42).
Table 2.
Characteristics of patients with a suspected platelet function disorder
| Non‐SPD (n = 139) | SPD (n = 17) | Reference value | |
|---|---|---|---|
| Male (%) | 22 (16) | 7 (41%) | |
| Age (years), median (IQR) | 38 (29‐52) | 41 (31‐56) | |
| Bleeding score, median (IQR) | 9 (7‐12) | 10 (8‐14) | Male < 4, female < 629 |
| Platelet count (109/L), median (IQR) | 235 (190‐282) | 200 (69‐272) | 150‐450 |
| MPV (fL), median, (IQR) | 8 (7.2‐8.7) | 7.4 (6.5‐8.2) | 7.0‐9.5 |
| Platelet ADP content (µmol/1011 platelets), median (IQR) | 2.5 (2.1‐2.8) | 0.9 (0.5‐1.2) | 1.4‐3.8 |
Abbreviations: IQR, interquartile range; MPV, mean platelet volume.
Figure 3.
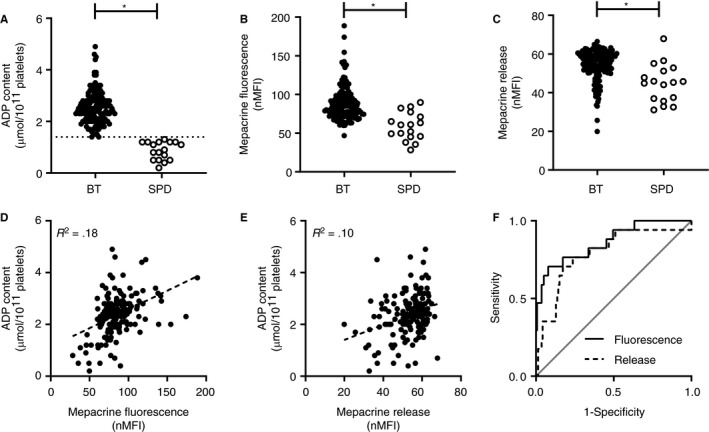
Diagnostic accuracy of flow cytometric mepacrine uptake and mepacrine release in patients with suspected platelet function disorders. (A) Platelet ADP content, expressed as µmol/1011 platelets, (B) mepacrine fluorescence expressed as normalized mean florescence intensity, (C) normalized mepacrine release for all patients included in the validation cohort. Patients were classified as bleeding tendency (BT) without δ‐SPD (BT; n = 139), or patients with a bleeding tendency and δ‐SPD (SPD; n = 17). The cutoff value for ADP content was 1.4 µmol ADP/1011 platelets. The correlation of (D) mepacrine fluorescence and (E) mepacrine release with platelet ADP content in all 156 patients with suspected platelet function disorder. (F) The discriminative ability of mepacrine fluorescence (area under the curve [AUC] 0.87) and mepacrine release (AUC 0.79) with platelet ADP as reference test plotted in a ROC curve. *P value < .05
Based on the area under the receiver operating characteristic (ROC) curve, the optimal diagnostic cutoff for mepacrine fluorescence was 71.2% of normal and 51.3% of normal for mepacrine release. Based on these cutoff values, the diagnostic accuracy for both mepacrine fluorescence and mepacrine release was determined (Table 3). With a sensitivity of 76.5% (95% CI 50.1‐93.2) and a specificity of 82.7% (95% CI 75.4‐86.6), mepacrine fluorescence showed moderate diagnostic accuracy. The diagnostic accuracy for mepacrine release was similar. The 2.5th percentile of healthy controls, a commonly used cutoff value in clinical laboratories, showed a similar diagnostic accuracy for mepacrine fluorescence, but the sensitivity of mepacrine release decreased to 35% (P = .02). To evaluate the potential use of mepacrine fluorescence as a screening test for δ‐SPD, diagnostic accuracy was determined at several cutoff values. At a cutoff value below 84.3% of normal, the sensitivity of mepacrine fluorescence was 94.1% and specificity was 50.4%. The positive likelihood ratio was 1.9, indicating mepacrine fluorescence is a poor predictor of δ‐SPD, but the negative likelihood ratio was 0.1, indicating that mepacrine fluorescence can be used to exclude δ‐SPD.
Table 3.
Diagnostic accuracy of mepacrine fluorescence and mepacrine release at different cutoff values compared with platelet ADP content as gold standard
| Cutoff (normalized median fluorescent intensity) | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Cohen's ĸ | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|
| Mepacrine fluorescence | |||||||
| 67.8a | 70.6 (44.0‐89.7) | 88.5 (82.0‐93.3) | 42.9 (30.1‐56.6) | 96.1 (92.2‐98.1) | 0.46 | 6.1 (3.5‐10.7) | 0.3 (0.2‐0.7) |
| 71.2b | 76.5 (50.1‐93.2) | 82.7 (75.4‐86.6) | 35.1 (25.7‐45.9) | 96.6 (92.4‐98.60) | 0.39 | 4.4 (2.8‐6.9) | 0.3 (0.1‐0.7) |
| 84.3c | 94.1 (71.3‐99.9) | 50.4 (41.8‐59.0) | 18.8 (15.9‐22.2) | 98.6 (91.2‐99.8) | 0.16 | 1.9 (1.5‐2.3) | 0.1 (0.02‐0.8) |
| Mepacrine release | |||||||
| 43.5a | 35.3 (14.2‐61.7) | 87.8 (81.1‐92.7) | 26.1 (13.9‐43.6) | 91.7 (88.6‐94.1) | 0.20 | 2.9 (1.3‐6.3) | 0.7 (0.5‐1.1) |
| 51.3b | 76.5 (50.1‐93.2) | 76.3 (68.3‐83.1) | 28.3 (20.9‐37.0) | 96.4 (91.8‐98.4) | 0.30 | 3.2 (2.2‐4.8) | 0.3 (0.1‐0.7) |
aCutoff value derived from the 2.5th percentile of mepacrine fluorescence or release in healthy controls.
bCutoff value derived from the ROC curve.
cOptimal cutoff value as screening test for δ‐SPD.
4. DISCUSSION
In the present study, we show that patients with δ‐SPD have both decreased mepacrine uptake and decreased mepacrine release after platelet stimulation compared with healthy controls. Because of the high negative predictive value (NPV), but low positive predictive value, mepacrine fluorescence can be used for exclusion of δ‐SPD in patients with a suspected platelet function disorder.
Flow cytometry has been recommended by the International Society on Thrombosis and Haemostasis/Scientific and Standardization Committee guidelines in the diagnostic workup of patients with platelet function disorders.9 It has been shown that flow cytometry has added value to the current diagnostic workup of patients with suspected platelet function disorders, but its value in diagnosing δ‐SPD in particular has not been validated.19 We are the first to prospectively evaluate the diagnostic accuracy of flow cytometric mepacrine fluorescence in patients with a suspected platelet function disorder. Previous studies already showed that mepacrine uptake is decreased in patients with δ‐SPD.22, 24, 25 One of these studies compared flow cytometric mepacrine assays with routine diagnostic tests in patients with δ‐SPD.22 Similar to these studies, we found that mepacrine fluorescence allows perfect discrimination between patients with confirmed δ‐SPD and healthy controls. In contrast to these findings, the performance of mepacrine fluorescence was inferior to platelet ADP measurements in the prospective evaluation of δ‐SPD in unselected patients with a bleeding tendency in whom a platelet function disorder was suspected.
We evaluated both mepacrine fluorescence in resting platelets and mepacrine release after platelet stimulation. Of these two parameters, mepacrine fluorescence seems more specific, because it provides a direct measure of platelet dense granule content without the necessity of platelet stimulation. Mepacrine release requires platelet stimulation and therefore cannot discriminate between impaired platelet activation and a secretion defect, because both result in decreased mepacrine release. This is reflected by the superior diagnostic accuracy of mepacrine fluorescence compared with mepacrine release in unselected patients with a bleeding tendency, in whom other platelet function disorders are common.
The strength of this study is that a selected cohort of patients with a suspected platelet function disorder was used for validation of mepacrine fluorescence. All tests were performed simultaneously and researchers were blinded for the diagnosis; therefore, there was no selection bias in this cohort. A potential weakness of our study is that we used platelet ADP content as a reference test. As a consequence, we could have missed δ‐SPD caused by a secretion defect. Moreover, we could have falsely diagnosed δ‐SPD in patients with a decreased metabolic adenosine nucleotide concentration, which is characterized by a low ATP/ADP ratio. This is not likely to have influenced the outcome of our study because application of a more stringent definition of δ‐SPD that includes an ATP/ADP ratio > 2 resulted in a similar performance of mepacrine fluorescence. Another limitation of this study is that the diagnosis of δ‐SPD was not confirmed in a second visit to our diagnostic center. This may have led to an overestimation of the number of patients with SPD in our study.
We report a high NPV for mepacrine fluorescence, which suggests mepacrine fluorescence can be used to exclude δ‐SPD. Our validation cohort consisted of undiagnosed patients with a bleeding tendency in whom a platelet function disorder was suspected and therefore reflects the real‐life patient population seen at a tertiary referral center. As a result, the prevalence of δ‐SPD in our study population was relatively high (11%) compared with the expected prevalence of δ‐SPD in the general population. This might have caused an overestimation of the NPV of mepacrine fluorescence. However, we also found a low negative likelihood of mepacrine fluorescence for δ‐SPD (negative likelihood ratio 0.1), which is independent of the prevalence and supports the ability of mepacrine fluorescence to exclude δ‐SPD.
The current diagnostic approach for δ‐SPD does not include a rapid screening test and could benefit from an additional test such as flow cytometry. Unlike the currently available diagnostic tools, flow cytometry is applicable in thrombocytopenic samples and requires only a small sample volume, allowing rapid exclusion of δ‐SPD in children.27, 28 Our data indicate that flow cytometric analysis is very reproducible, even immediately after blood collection.
Taken together, these data indicate that flow cytometric measurement of dense granule parameters is a potential tool for the screening of δ‐SPD. The presented method requires a minimal amount of whole blood and can be used to for the exclusion of δ‐SPD and for the selection of patients that require further extensive testing.
CONFLICT OF INTEREST
S.J.A. Korporaal and R.T. Urbanus are stockholders in U‐PACT BV, a spinoff company from UMC Utrecht. The rest of the authors state that they have no conflict of interest.
ADDENDUM
H.F. Heijnen, R.E.G. Schutgens, and R.T. Urbanus designed the study. I. van Asten and L.E.C. Granneman performed the experiments. I. van Asten, L.E.C. Granneman, S.J.A. Korporaal, and R.T. Urbanus analyzed the data. I. van Asten, M.W. Blaauwgeers, L.E.C. Granneman, H.F. Heijnen, G. Pasterkamp, M.J.H.A. Kruip, E.A.M. Beckers, M. Coppens, J. Eikenboom, R.Y.J. Tamminga, K.P.M. van Galen, A. Huisman, S.J.A. Korporaal, R.E.G. Schutgens, and R.T. Urbanus wrote the manuscript.
van Asten I, Blaauwgeers M, Granneman L, et al. Flow cytometric mepacrine fluorescence can be used for the exclusion of platelet dense granule deficiency. J Thromb Haemost. 2020;18:706–713. 10.1111/jth.14698
van Asten and Blaauwgeers are contributed equally.
Manuscript handled by: Pierre Toulon
Final decision: Pierre Toulon and 27 November 2019
REFERENCES
- 1. Holmsen H, Weiss HJ. Secretable storage pools in platelets. Annu Rev Med. 1979;30:119‐134. [DOI] [PubMed] [Google Scholar]
- 2. King SM, Reed GL. Development of platelet secretory granules. Semin Cell Dev Biol. 2002;13:293‐302. [DOI] [PubMed] [Google Scholar]
- 3. McNicol A, Israels SJ. Platelet dense granules: structure, function and implications for haemostasis. Thromb Res. 1999;95:1‐18. [DOI] [PubMed] [Google Scholar]
- 4. McNicol A, Israels SJ, Robertson C, Gerrard JM. The empty sack syndrome: a platelet storage pool deficiency associated with empty dense granules. Br J Haematol. 1994;86:574‐582. [DOI] [PubMed] [Google Scholar]
- 5. Heijnen H, van der Sluijs P. Platelet secretory behaviour: as diverse as the granules… or not? J Thromb Haemost. 2015;13(12):2141‐2151. [DOI] [PubMed] [Google Scholar]
- 6. Quiroga T, Goycoolea M, Panes O, et al High prevalence of bleeders of unknown cause among patients with inherited mucocutaneous bleeding. A prospective study of 280 patients and 299 controls. Heamatologica. 2007;92(03):357‐365. [DOI] [PubMed] [Google Scholar]
- 7. Gresele P, Harrison P, Bury L, et al Diagnosis of suspected inherited platelet function disorders: results of a worldwide survey. J Thromb Haemost. 2014;12(9):1562‐1569. [DOI] [PubMed] [Google Scholar]
- 8. Mezzano D, Quiroga T, Pereira J. The level of laboratory testing required for diagnosis or exclusion of a platelet function disorder using platelet aggregation and secretion assays. Semin Thromb Haemost. 2009;35:242‐254. [DOI] [PubMed] [Google Scholar]
- 9. Gresele P. Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13(2):314‐322. [DOI] [PubMed] [Google Scholar]
- 10. Summerfield GP, Keenan JP, Brodie NJ, Bellingham AJ. Bioluminescent assay of adenine nucleotides: rapid analysis of ATP and ADP in red cells and platelets using the LKB luminometer. Clin Lab Haematol. 1981;3:257‐271. [DOI] [PubMed] [Google Scholar]
- 11. Harrison P, Mackie I, Mumford A, et al British committee for standards in haematology. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br J Haematol. 2011;155(1):30‐44. [DOI] [PubMed] [Google Scholar]
- 12. Mumford AD, Frelinger A, Gachet C, et al A review of platelet secretion assays for the diagnosis of inherited platelet secretion disorders. Thromb Haemost. 2015;113:1‐12. [DOI] [PubMed] [Google Scholar]
- 13. Moffat KA, Ledford‐Kraemer MR, Nichols WLHC. Variability in clinical laboratory practive in testing for disorders of platelet function: results of two surveys of the North American Specialized Coagulation Laboratory Association. Thromb Haemost. 2005;93:549‐553. [DOI] [PubMed] [Google Scholar]
- 14. Jennings I, Woods TAL, Kitchen S, Walker ID. Platelet function testing: practice among UK National External Quality Assessment Scheme for Blood Coagulation participants, 2006. J Clin Pathol. 2008;61(8):950‐954. [DOI] [PubMed] [Google Scholar]
- 15. van Asten I, Schutgens REG, Baaij M, et al Validation of flow cytometric analysis of platelet function in patients with a suspected platelet function defect. J Thromb Haemost. 2018;16(16):689‐698. [DOI] [PubMed] [Google Scholar]
- 16. Logan J, Irvin E. The interaction of quinacrine nucleotides. J Biol Chem. 1954;210:45‐56. [PubMed] [Google Scholar]
- 17. Ramström AS, Fagerberga IH, Lindahl TL. A flow cytometric assay for the study of dense granule storage and release in human platelets. Platelets. 1999;10:153‐158. [DOI] [PubMed] [Google Scholar]
- 18. Billio A, Moeseneder C, Donazzan G, Triani A, Pescosta N, Coser P. Hermansky‐Pudlak syndrome: clinical presentation and confirmation of the value of the mepacrine‐based cytofluorimetry test in the diagnosis of delta granule deficiency. Haematologica. 2001;86(220):2001. [PubMed] [Google Scholar]
- 19. Gordon N, Thom J, Cole C, Baker R. Rapid detection of hereditary and acquired platelet storage pool deficiency by flow cytometry. Br J Haematol. 1995;89:117‐123. [DOI] [PubMed] [Google Scholar]
- 20. Manukjan G, Eilenberger J, Andres O, Schambeck C, Eber S, Schulze H. Functional classification of paediatric patients with non‐syndromic delta‐storage pool deficiency. Hamostaseologie. 2018;39(4):383‐391. [DOI] [PubMed] [Google Scholar]
- 21. Andres O, Henning K, Strauss G, Pflug A, Manukjan G, Schulze H. Diagnosis of platelet function disorders: a standardized, rational, and modular flow cytometric approach. Platelets. 2018;29:347‐356. [DOI] [PubMed] [Google Scholar]
- 22. Cai H, Mullier F, Frotscher B, et al Usefulness of flow cytometric mepacrine uptake/release combined with CD63 assay in diagnosis of patients with suspected platelet dense granule disorder. Semin Thromb Hemost. 2016;42(3):282‐291. [DOI] [PubMed] [Google Scholar]
- 23. Dovlatova N, Lordkipanidzé M, Lowe GC, et al Evaluation of a whole blood remote platelet function test for the diagnosis of mild bleeding disorders. J Thromb Haemost. 2014;12(5):660‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. White JG. Electron opaque structures in human platelets: which are or are not dense bodies? Platelets. 2008;19:455‐466. [DOI] [PubMed] [Google Scholar]
- 25. Israels SJ, Kahr WHA, Blanchette VS, Luban NLC, Rivard GE, Rand ML. Platelet disorders in children: a diagnostic approach. Pediatr Blood Cancer. 2011;56:975‐983. [DOI] [PubMed] [Google Scholar]
- 26. Maurer‐Spurej E, Pittendreigh C, Wu JK. Diagnosing platelet δ‐storage pool disease in children by flow cytometry. Am J Clin Pathol. 2007;127(4):626‐632. [DOI] [PubMed] [Google Scholar]
- 27. White JG. Use of the electron microscope for diagnosis of platelet disorders. Semin Thromb Haemost. 1998;24:163‐168. [DOI] [PubMed] [Google Scholar]
- 28. Brunet JG, Iyer JK, Badin MS, et al Electron microscopy examination of platelet whole mount preparations to quantitate platelet dense granule numbers: implications for diagnosing suspected platelet function disorders due to dense granule deficiency. Int J Lab Hematol. 2018;40(4):400‐407. [DOI] [PubMed] [Google Scholar]
- 29. Elbatarny M, Mollah S, Grabell J, et al Normal range of bleeding scores for the ISTH‐BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20(6):831‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]


