Abstract
Background
Drug‐induced photosensitivity refers to the development of cutaneous adverse events due to interaction between a pharmaceutical compound and sunlight. Although photosensitivity is a very commonly listed side‐effect of systemic drugs, reliable data on its actual incidence are lacking so far.
Objectives
A possible approach to evaluate the real‐life extent of drug‐induced photosensitivity would be an analysis of the frequency of exposure to a given photosensitizing drug combined with an indicator of its photosensitizing potential. This could serve as a basis for developing a pharmaceutical ‘heatmap’ of photosensitivity.
Methods
The present study investigated the number of reimbursed dispensed packages of potentially photosensitizing drugs in Germany (DE) and Austria (AT) between 2010 and 2017 based on nationwide health insurance‐based databases. In addition, an indicator for the photosensitizing potential was established for each drug based on the number of reports on photosensitivity in the literature.
Results
This analysis includes means of 632 826 944 (+/−14 894 918) drug dispensings per year in DE and 113 270 754 (+/−1 964 690) in AT. Out of these, the mean percentage of drugs that enlist photosensitivity as a potential side‐effect was 49.5% (±0.7) in DE and 48.2% (±1.2) in AT. When plotting the number of reimbursed dispensed packages vs. the number of reports on photosensitivity, two categories of drugs show high numbers for both parameters, that is diuretics and non‐steroidal anti‐inflammatory drugs (NSAIDs).
Conclusions
Diuretics and NSAIDs appear to be responsible for the greatest part of exposure to photosensitizing drugs with potential implication on public health.
Introduction
Drug‐induced photosensitivity refers to the development of cutaneous adverse events due to interaction between a given pharmaceutical compound and sunlight. Photosensitizing drugs act as exogenous chromophores that are transformed by absorption of photons into a photochemically active excited state and subsequently undergo chemical reactions.1 Manifestations of drug‐induced photosensitivity can be subdivided into phototoxic and photoallergic reactions. The action spectrum mostly lies within the UVA range although some drugs are activated by visible light or UVB radiation or a combination of different wavelengths.2 The mechanisms for tissue damage in phototoxicity involve oxidation of cellular lipids, proteins and DNA, often mediated by reactive oxygen species (ROS),3, 4 or formation of stable photoadducts.5 Photoallergy represents a T‐cell‐mediated delayed‐type hypersensitivity reaction that involves covalent binding of a pharmaceutical compound to an endogenous protein in the presence of solar radiation.2 The great majority of reactions to systemic drugs are phototoxic in nature, whereas topical agents such as chemical UV filters or non‐steroidal antirheumatics are the main causes of photoallergic reactions.6, 7
Up to the present, a vast number of drugs have been associated with photosensitivity and the number is increasing every year.8 In particular, antimicrobials, non‐steroidal anti‐inflammatory drugs (NSAIDs), cardiovascular agents and psychotropics have been implicated in photosensitive reactions.1 In general, the elderly population is at higher risk given its greater consumption of different drugs for the treatment of chronic diseases.9 Besides acute cutaneous adverse effects, an increasing number of reports also suggest chronic sequelae such as increased photoaging or photocarcinogenesis.8, 10 However, as yet this is still a matter of controversial scientific discussions.
Although drug‐induced photosensitivity is considered to account for up to 8% of reported cutaneous adverse events from drugs,1, 11 reliable data on its actual incidence are lacking so far. It is generally assumed that drug‐induced photosensitivity is heavily underreported owing to a lack of clinical recognition and a lack of reporting to databases.2 This most probably results in an appreciable number of underdiagnosed and underreported cases hampering retrospective analyses of the true incidence of drug‐induced photosensitivity. A possible approach to evaluate the actual incidence of drug‐induced photosensitive reactions would be an analysis of the ‘societal exposure’ of photosensitizing drugs combined with an indicator of their photosensitizing potential. This could serve as a basis for delineating the photosensitizing potential of currently prescribed systemic drugs.
In the present study, we investigated the number of reimbursed, dispensed packages of photosensitizing drugs in Germany and Austria between 2010 and 2017 based on nationwide health databases on drug dispensings. These data were directly correlated with an indicator of the photosensitizing potential of each drug. With this approach, we aimed at identifying drugs that have both, high dispensing rates and a high photosensitizing potential, and thus the greatest bearing on drug‐induced photosensitivity reactions.
Methods
Study design and source of data
The present work was designed as a drug utilization study targeting at the analysis of the dispensings of photosensitizing drugs within defined timely and regional boundaries. The German data set was provided by the German Institute for Drug Use Evaluation (DAPI, Berlin, Germany) which collects anonymous claims data of drugs prescribed and subsequently dispensed at community pharmacies at the expense of the Statutory Health Insurance (SHI) Funds.12 Nearly 87% of Germany's (DE's) population is insured by the SHI system.13, 14 The DAPI data cover 80% of the community pharmacies in DE and were extrapolated by regional factors to 100% of the SHI insured population. The Austrian data set was provided by The Main Association of Austrian Social Security Institutions which serves as the umbrella organization of the statutory health insurances in Austria (AT). About 98% of the Austrian population is covered by the statutory health insurances. All costs exceeding the copayment are covered by the health insurance.15, 16 Both data sets contained longitudinal data concerning the overall number of rates of dispensed drugs for single (or combined) agents within a given time frame in the respective country. Both data sets do not include drugs dispensed in hospital (inpatient) settings. For additional information, please refer to the Appendix S1 (Supporting Information).
Compilation of photosensitizing agents
To create a most complete and actual list of photosensitizing drugs, we first referred back to the compilations published in review articles by Moore,8 Drucker and Rosen 17 as well as Monteiro et al. 1 since they seemed to provide the most complete recent overviews on that subject. In addition, Litt's Drug Eruption & Reaction Database was consulted and further drugs (not mentioned in the review articles) were added to the list. Drugs listed in the database under the label ‘photosensitivity’ were added to the list of photosensitizing drugs. Finally, a MEDLINE search for the terms ‘photosensitivity’ as well as ‘phototoxic’/‘photoallergic’ was performed in November 2017 in order to complement the initial overview. For additional information, please refer to the Appendix S1 (Supporting Information).
Development of an indicator for photosensitivity
The incidence of photosensitive adverse reactions of a given pharmaceutical compound is the product of its prescription frequency and its photosensitizing potential (though further factors such as the UV spectrum, exposure dose, climate area or season of exposure may also have an influence). With regard to the latter, an indicator for each drugs’ photosensitizing potential had to be established. So far no comparative clinical or experimental studies have been performed addressing the difference in the photosensitizing potential across a large series of different pharmaceutical compounds. This may be due to the many factors influencing the clinical manifestation as well as the magnitude of different compounds. Based on the assumption that there is a correlation between the number of reported photosensitive side‐effects and the photosensitizing potential of a given drug, we established the number of publications reporting a photosensitive adverse drug reaction as an indicator for the photosensitizing potential of a drug. To this purpose, a MEDLINE search was performed between April 2018 and December 2018 to systematically identify all reports for every drug mentioned in the list above. Importantly, this analysis was only performed for drugs that were actually prescribed in DE and/or AT within the observational period. For this analysis, the search term ‘photosensitivity’ in addition to each single drug name was used since this would address both, reports on phototoxic and photoallergic side‐effects. Inclusion and exclusion criteria were formulated to determine which publications qualified to be included into the analysis. In a second step, Litt's Drug Eruption & Reaction Database was again used to complement the findings derived from the MEDLINE search in order to achieve a maximum number of reports for each compound listed. The according study flow chart is shown in Fig. 1.
Figure 1.
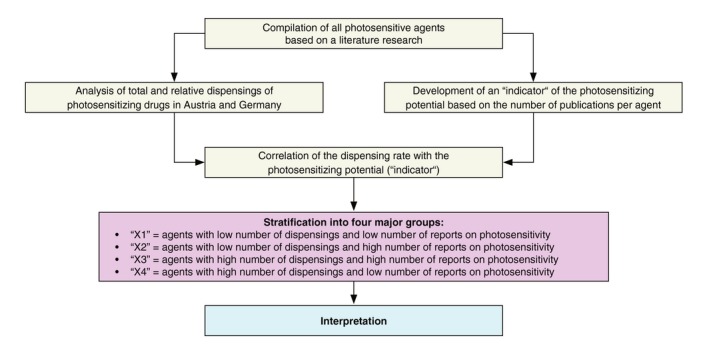
Study flow chart. First, a compilation of all photosensitive pharmaceutical agents was established based on an extensive literature research. In parallel, the total and relative number of reimbursed dispensings of photosensitizing drugs in Austria and Germany was performed. In addition, an ‘indicator’ of the photosensitizing potential of each drug was determined based on the number of reports on photosensitivity. Finally, the dispensing rate was graphically correlated with the ‘indicator’ resulting in four different groups of photosensitizing drugs ‘X1’–‘X4’, which served as a basis for the interpretation of the results.
Statistical analysis
The numbers of reimbursed dispensed packages were analysed with regard to overall yearly prescriptions to assess general trends. The agents of interest were categorized according to a classification adapted from the World Health Organization's Anatomical Therapeutic Chemical (ATC) Classification System.18 Descriptive analyses for both – drug groups and single agents – were performed concerning reimbursed drug dispensings, potential of photosensitive adverse reactions and the combination of both features using Microsoft Excel (2016, Microsoft Corp., Redmond, WA, USA). For details on the statistical analyses, please also refer to the Appendix S1 (Supporting Information).
Results
Compilation of photosensitizing agents
Based on published literature and an adverse drug reaction database, a compilation of photosensitizing medications has been established (see Table 1 summarizing all drugs). In total, 387 pharmaceutical compounds could be identified that have been associated with causing photosensitivity either from literature or by the database. The largest group containing the highest number of compounds was the group ‘nervous system’, while the group ‘anti‐infectious’ showed the second most and the group ‘cardiovascular’ the third most compounds. Out of the 387 agents with photosensitizing potential, 291 agents (75.2%) were dispensed and reimbursed in DE and 220 (56.9%) in AT during the study period. These drugs are highlighted in Table 1 with indications of their use in DE (#) and/or AT (+). Therefore, Table 1 provides both information on which drugs are more likely to cause photosensitivity since they have actually been dispensed (written in bold letters in Table 1) and information on other drugs with photosensitizing potential (non‐dispensed/reimbursed).
Table 1.
Compilation of pharmaceutical compounds with photosensitizing potential
| 1 Cardiovascular | |||
|---|---|---|---|
| Diuretics | Hydrochlorothiazide #+ | Bendroflumethiazide #+ | Indapamide #+ |
| Benzylhydrochlorothiazide | Benzthiazide | Triamterene # | |
| Chlorothiazide | Bumetanide #+ | Furosemide #+ | |
| Hydroflumethiazide | Butizide + | Amiloride # | |
| Methyclothiazide | Cyclothiazide | Torasemide #+ | |
| Piretanide # | Chlorthalidone #+ | Xipamide #+ | |
| Polythiazide | Metolazone | Ethacrynic acid | |
| Trichlormethiazide | Quinethazone | Acetazolamide #+ | |
| Bemetizide # | Spironolacton #+ | ||
| Agents acting on the renin–angiotensin system | Enalapril #+ | Benazepril # | Losartan #+ |
| Ramipril #+ | Lisinopril #+ | Olmesartan #+ | |
| Quinapril #+ | Moexipril # | Telmisartan #+ | |
| Captopril #+ | Valsartan #+ | Irbesartan #+ | |
| Fosinopril #+ | Candesartan #+ | ||
| Antiarrhythmics | Amiodarone #+ | Disopyramide | |
| Dronedarone #+ | Procainamide | ||
| Beta blocking agents | Propranolol | Carvedilol #+ | |
| Sotalol #+ | Tilisolol | ||
| Calcium channel blocking agents | Amlodipine #+ | Diltiazem #+ | |
| Nifedipine #+ | Verapamil #+ | ||
| Other antihypertensives | Hydralazine # | Methyldopa #+ | Diazoxide # |
| Rilmenidine + | |||
| Antithrombotic agents | Clopidogrel #+ | ||
| Others | Oxerutins # | Quinidine # | |
| 2 Anti‐inflammatory and antirheumatic products | |||
|---|---|---|---|
| Anti‐inflammatory and antirheumatic products, non‐steroids (excluding Coxibs) | Naproxen #+ | Benoxaprofen | Benoxaprofen |
| Ketoprofen #+ | Diflunisal | Indoprofen | |
| Tiaprofenic acid # | Nabumetone # | Indomethacin #+ | |
| Piroxicam #+ | Benzydamine #+ | Fenoprofen | |
| Carprofen | Flurbiprofen + | Sulindac | |
| Aceclofenac # | Ketorolac #+ | Suprofen | |
| Diclofenac #+ | Meclofenamate | Ibuprofen #+ | |
| Mefenamic acid + | Oxaprozin | Tolmetin | |
| Phenylbutazone #+ | Meloxicam #+ | Nimesulide + | |
| Gold # | Etodolac | ||
| Coxibs | Celecoxib #+ | Rofecoxib | Valdecoxib |
| Others | Heroin | Pentosan polysulphate Nalidixic acid | Achillea millefolium |
| Mesalazine #+ | |||
| Sulphasalazine #+ | |||
| 3 Antineoplastic and immunomodulating agents | |||
|---|---|---|---|
| Alkylating | Dacarbazine #+ | Chlorambucil #+ | |
| Antimetabolite | Fluorouracil #+ Mercaptopurine #+ | Pentostatin | Thioguanine #+ |
| Capecitabine #+ | Tegafur/Uracil # | Tegafur/Gimeracil/ | |
| Tegafur + | Oteracil # | ||
| Methotrexate #+ | |||
| Plant alkaloids and other natural products | Vinblastine #+ | Docetaxel #+ | Paclitaxel #+ |
| Anthracyclines and related substances | Epirubicin #+ | ||
| Protein kinase inhibitors | Vemurafenib #+ | Cobimetinib #+ | Dabrafenib #+ |
| Trametinib #+ | Crizotinib #+ | Erlotinib #+ | |
| Regorafenib #+ | Dasatinib #+ | Imatinib #+ | |
| Gefitinib #+ | Canertinib | Alectinib + | |
| Lapatinib #+ | Vandetanib #+ | ||
| Topoisomerase inhibitor | Irinotecan #+ | ||
| Monoclonal antibodies | Nivolumab #+ | Cetuximab # | Trastuzumab #+ |
| Eculizumab #+ | Panitumumab #+ | ||
| Others | Hydroxyurea # | Flutamide #+ | Rucaparib |
| Procarbazine # | Bicalutamide #+ | Anagrelide # | |
| PEG interferon #+ | Midostaurin + | Arsenic # | |
| Interferon alpha #+ | |||
| 4 Anti‐infectives | |||
|---|---|---|---|
| Fluoroquinolones | Lomefloxacin #+ | Ulifloxacin + | Ofloxacin #+ |
| Fleroxacin | Ciprofloxacin #+ | Trovafloxacin | |
| Clinafloxacin | Grepafloxacin | Gatifloxacin | |
| Sparfloxacin | Gemifloxacin | Moxifloxacin #+ | |
| Enoxacin # | Levofloxacin #+ | Norfloxacin #+ | |
| Pefloxacin | |||
| Tetracyclines | Tetracycline #+ | Doxycycline #+ | Chlortetracycline # |
| Oxytetracycline # | Minocycline #+ | Lymecycline + | |
| Demeclocycline # | |||
| Sulfonamides | Sulphamethoxazole | Sulphadiazine #+ | |
| Cotrimoxazol #+ | Sulphisoxazole | ||
| Cephalosporins | Cefazolin #+ | Ceftazidime #+ | Cefotaxime #+ |
| Aminoglycosides | Kanamycin # | Streptomycin # | Gentamicin #+ |
| Antimycotics | Griseofulvin # | Terbinafine #+ | Itraconazole #+ |
| Voriconazole #+ | Ketoconazole #+ | Rosemary # | |
| Antimycobacterials | Isoniazid #+ | Ethionamide | Clofazimine |
| Pyrazinamide #+ | Ethambutol #+ | Aminosalicylate sodium #+ | |
| Antivirals | Efavirenz #+ | Daclatasvir #+ | Acyclovir / Valaciclovir #+ |
| Ritonavir #+ | Amantadine #+ | Simeprevir #+ | |
| Saquinavir #+ | Ganciclovir/ | Ribavirin #+ | |
| Zalcitabine | Valganciclovir #+ | ||
| Others | Quinine # | Mefloquine #+ | Dapsone # |
| Chloroquine # | Pyrimethamine # | Furazolidone | |
| Hydroxychloroquine # | Quinacrine | Methenamine # | |
| Azithromycin #+ | Sulphadoxine | Flucytosine # | |
| 5 Nervous system | |||
|---|---|---|---|
| Antidepressants | Protriptyline | Escitalopram #+ | Duloxetine #+ |
| Amitriptyline #+ | Paroxetine #+ | Isocarboxazid | |
| Imipramine # | Hypericum #+ | Phenelzine | |
| Clomipramine #+ | Fluvoxamine #+ | Tranylcypromine #+ | |
| Desipramine # | Fluoxetine #+ | Amoxapine | |
| Trimipramine # | Sertraline #+ | Trazodone #+ | |
| Nortriptyline # | Citalopram #+ | Nefazodone | |
| Doxepin #+ | Venlafaxine #+ | ||
| Bupropion #+ | |||
| Antipsychotics | Chlorpromazine | Olanzapine #+ | Chlorprothixene #+ |
| Thioridazine # | Clozapine #+ | Perazin # | |
| Fluphenazine # | Haloperidol #+ | Loxapine # | |
| Perphenazine #+ | Mesoridazine | ||
| Flupentixol #+ | Trimeprazine | Quetiapine #+ | |
| Molindone | Prochlorperazine | Risperidone #+ | |
| Pimozide #+ | Trifluoperazine | Maprotiline #+ | |
| Thiothixene | Ziprasidone #+ | ||
| Anxiolytics | Meprobamate + | Alprazolam #+ | |
| Clorazepate # | Chlordiazepoxide # | ||
| Hypnotics and sedatives | Zolpidem #+ | Triazolam #+ | Promethazine # |
| Eszopiclone | Butobarbital | Pentobarbital | |
| Zaleplon # | |||
| Anticonvulsants/Barbiturates | Carbamazepine #+ | Topiramate #+ | Butabarbital |
| Lamotrigine #+ | Valproic acid #+ | Butalbital | |
| Phenytoin #+ | Trimethadione | Pentobarbital | |
| Felbamate #+ | Phenobarbital # | ||
| Selective serotonin (5HT1) agonists | Sumatriptan #+ | Zolmitriptan #+ | Almotriptan # |
| Naratriptan #+ | |||
| Others | Acamprosate #+ | Carisoprodol | Procyclidine #+ |
| Methylphenidate #+ | Cevimeline # | Trihexyphenidyl # | |
| Ropinirole #+ | |||
| 6 Metabolism/endocrine therapy | |||
|---|---|---|---|
| HMG‐CoA reductase inhibitors | Simvastatin #+ | Pravastatin #+ | Rosuvastatin #+ |
| Atorvastatin #+ | Pitavastatin # | ||
| Fibrates | Clofibrate | Bezafibrate #+ | Fenofibrate #+ |
| Drugs used in diabetes | Chlorpropamide | Gliquidone #+ | Canagliflozin #+ |
| Glyburide (Glibenclamide) #+ | Glymidine | Sitagliptin #+ | |
| Glipizide + | Acetohexamide | Metformin #+ | |
| Tolbutamide | Glimepiride #+ | Tolazamide | |
| Proton‐pump inhibitors | Esomeprazole #+ | Pantoprazole #+ | Rabeprazole #+ |
| Antigout preparations | Allopurinol #+ | Febuxostat #+ | Colchicine #+ |
| Hormones | Melatonin #+ | Oestrogen #+ | Progesterone #+ |
| Hydrocortisone #+ | Epoetin alpha # | Ethinyl estradiol #+ | |
| Danazol + | |||
| Antihistamines | Mequitazine # | Clemastine # | Dimenhydrinate # |
| Repirinast | Dexchlorpheniramine # | Cyproheptadine #+ | |
| Astemizole | Hydroxyzine #+ | Diphenhydramine #+ | |
| Azatadine | Meclizine | Loratadine #+ | |
| Brompheniramine | Tripelennamine # | Cetirizine #+ | |
| Chlorpheniramine # | Triprolidine | ||
| Ranitidine #+ | Terfenadine # | ||
| Thyroid therapy | Propylthiouracil #+ | ||
| Others | Bergamot # | ||
| 7 Others | |||
|---|---|---|---|
| Antiseptic | Thimerosal # | ||
| Anticholinergic | Scopolamine # | Benzatropine | Atropin sulphate # |
| Hyoscyamine # | Glycopyrrolate #+ | Tiotropium #+ | |
| Cholinergic | Pilocarpine #+ | ||
| PDE5 inhibitors | Sildenafil #+ | Vardenafil #+ | |
| Photosensitizers | Verteporfin #+ | Aminolevulinic acid #+ | Dihaematoporhphyrin ether |
| Protoporphyrin | Porfimer sodium # | Trioxsalen | |
| 5‐Methoxypsoralen + | 8‐Methoxypsoralen #+ | Haematophyrin | |
| Anthracene | |||
| Retinoids | Isotretinoin #+ | Acitretin #+ | Etretinate |
| Tretinoin #+ | |||
| Immunostimulants | Aldesleukin #+ | ||
| Immunosuppressants | Tacrolimus #+ | Omalizumab #+ | Interferon beta #+ |
| Azathioprine #+ | Tocilizumab #+ | Pirfenidone #+ | |
| Leflunomide #+ | |||
| Phytotherapeutics | Ginseng # | Hydrastis canadensis # | Angelica sinensis |
| Ruta # | |||
| Additives | Cyclamate # | Saccharin # | Tartrazine # |
| Antidot | Tiopronin # | Acetylcysteine #+ | |
| Vitamins | Pyridoxine #+ | ||
| Vaccines | Smallpox # | ||
# indicates that the compound was dispensed and reimbursed in DE between 2010 and 2017; + indicates that the compound was reimbursed in Austria between 2010 and 2017; and compounds printed in bold were reimbursed in both countries between 2010 and 2017.
Frequency of photosensitizing drug dispensings
In the period between 2010 and 2017, the mean total number of reimbursed drug packages dispensed in DE was 632.827 mio. [±14.895 mio.] per year and in AT 113.271 mio. [±1.965 mio.] per year. In DE, the total number of dispensed drug packages rose from 617.54 mio. in 2010 to a maximum of 655.351 mio. in 2016 and slightly decreased thereafter to 648.093 mio. in 2017 as presented in Fig. 2a. This corresponds to an increase in drug dispensings of + 4.95% during the study period. In AT, the total number of packages of pharmaceuticals reimbursed in the outpatient sector remained relatively stable over the years [increasing from 112.931 mio. in 2010 to a maximum of 115.761 mio. in 2014 and decreasing to 109.073 mio. in 2017 (see Fig. 2b)]. When relating these numbers to the amount of the total number of insured persons per year, a similar picture evolves (Fig. 3c,d).
Figure 2.
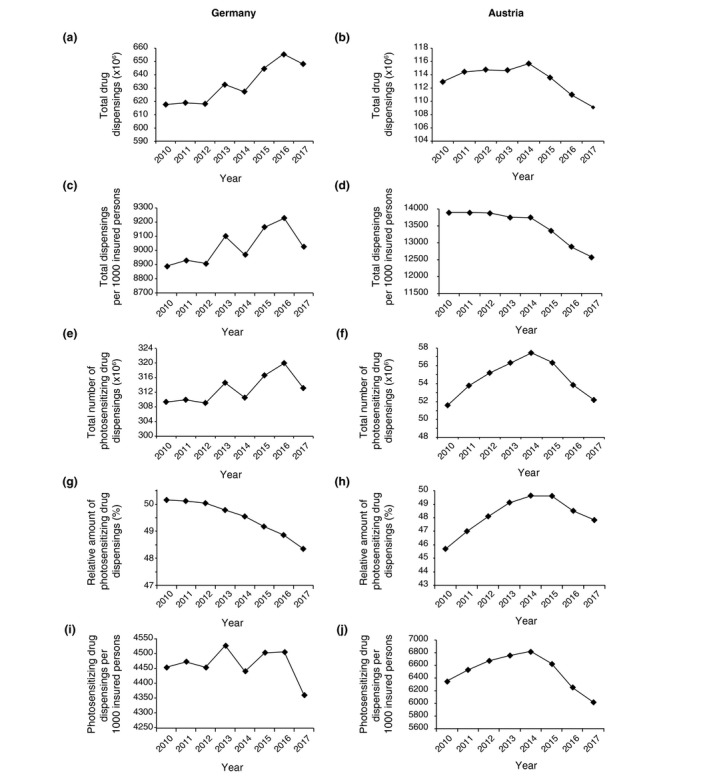
Total and relative number of drug dispensings in Germany (DE) and Austria (AT) between 2010 and 2017. Total amount of all (a–d) and photosensitizing (e–f) drug dispensings. Relative amount of photosensitizing drug dispensings out of all dispensings (in %, g, h) in DE and AT. Total amount of photosensitizing drug dispensings per 1000 insured persons in DE and AT (i, j).
Figure 3.
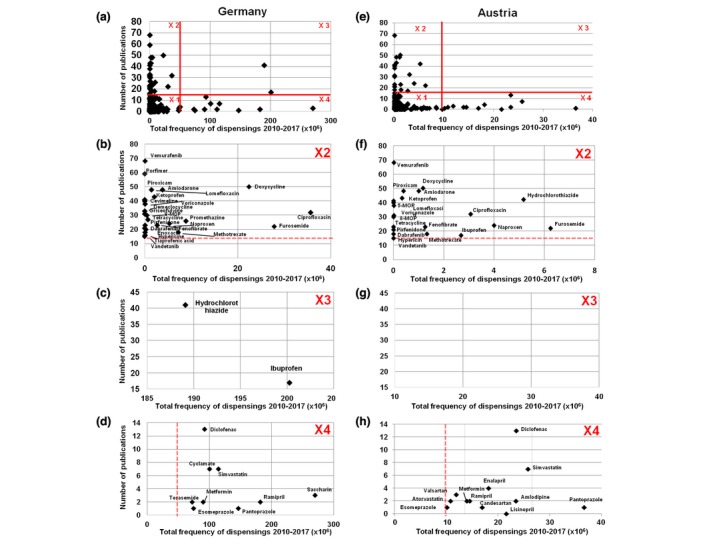
Combined analysis of drug dispensings and reference score. Scatterplot of the total number of dispensings of each single agent plotted against the number of publications (‘reference score’) in Germany (DE) (a–d) and Austria (AT) (e–h). The resulting diagram in (a) and (e) is subdivided into four segments each (‘X1’–‘X4’). In the subfigures b–d and f–h, a detailed analysis of ‘X2’–‘X4’ is shown.
Out of these total drug dispensings, the mean percentage of photosensitizing agents was 49.5% (±0.7) in DE and 48.2% (±1.2) in AT over the entire study period. The overall use of photosensitizing drugs rose from 309.416 mio. in 2010 to 313.161 mio. in 2017 in DE, representing an increase of 3.745 mio, including a peak of 320.021 mio. in 2016 (see Fig. 2e,g,i). In AT, the overall number of photosensitizing drug dispensings was 51 600 780 in 2010 that rose to 57 426 060 in 2014 and decreased to 52 176 413 in 2017 (see Fig. 2f,h,j). These findings reveal that the absolute dispensing rates of photosensitizing drugs were increasing during the initial phase of the observational period in both countries. However, in both countries a decrease in total photosensitizing drug dispensings was observed over the last years (which was much more profound in AT). Interestingly, the relative amount of photosensitizing drug prescriptions (in %) showed a continuous decrease in DE over years, while the number in AT only decreased during the last 3 years (Fig. 3g,h).
Distribution of photosensitizing agents and number of dispensings across drug classes
Photosensitizing drugs differ across drug classes both with respect to the total number of photosensitizing agents per drug class and the frequency of prescriptions per year. The category ‘nervous system’ contains the highest number of photosensitizing drugs within a category (20.7%), followed by the categories ‘anti‐infectious’ and ‘cardiovascular’ drugs (Fig. S1a, Supporting Information). However, when looking at the mean number of photosensitizing drug prescriptions per year in DE, the highest dispensing numbers are found in the category ‘cardiovascular’ (117.1 mio. dispensings, 33.0% of all dispensings) followed by the category ‘metabolism’ (85.5 mio. dispensings, 24.1% of all dispensings; see Fig. S1b,c (Supporting Information), number per 1000 insured persons – Fig. 1d). The respective absolute and relative numbers in the AT population are shown in Fig. S1e–g (Supporting Information). Interestingly, the relative distribution in AT is almost identical to the German population (also showing the highest number of dispensings in the category ‘cardiovascular’, see Fig. S1g, Supporting Information).
The example of the drug class ‘cardiovascular’ demonstrates that there is often a discrepancy between the number of photosensitive agents and their effective rate of dispensings. While the drug class ‘cardiovascular’ accounts for 15.2% of all photosensitive drugs, their mean share of all dispensings per year in DE was found to be 33.0%. A similar trend was evident for the drug class ‘metabolism’ as well as the class ‘anti‐inflammatory’, while other classes showed an inverse trend with a higher share among photosensitive drugs vs. a lower dispensing rate (see Fig. S1a–g, Supporting Information).
Development of an indicator for the photosensitizing potential
A systematic MEDLINE search identified 1697 reports on photosensitivity linked to the 291 agents prescribed in DE and the 220 agents prescribed in AT, respectively, during the observational period. In the Table S1 (Supporting Information), all references are summarized and linked to the specific pharmaceutical agent. The highest number of drug‐induced photosensitivity reactions was reported for ‘anti‐infectious’ drugs (Fig. S2a, Supporting Information). In Fig. S2b (Supporting Information), the timely evolution of the reports on photosensitivity is summarized.
Correlation analysis of the dispensing rate with the publication number
In order to determine the relevance of the absolute prescription number, a correlation analysis with the aforementioned indicator, the number of publications per drug according to the systematic literature search, was performed. Plotting the number of prescriptions vs. the number of reports on cutaneous photosensitivity reactions per agent identifies a small number of drugs with the highest potential for causing photosensitivity in the real‐life setting. For a further in‐depth analysis, these scatter plots were subdivided into four segments ‘X1’–‘X4’ (see Fig. 3a–h) as described in the Methods. ‘X1’ represents those drugs with the lowest number of dispensings (<50 000 000 over the study period) and the lowest number of reports on photosensitivity (<15 in DE and AT), thus the group with the lowest significance for photosensitive adverse events (Fig. 3a).
The segment ‘X2’ (Fig. 3b) comprises agents with a well‐documented photosensitizing potential (≥15 publications) but a limited dispensing rate (<50 000 000 over the study period). This category represents the prototypical drugs of well‐known photosensitizing potential such as vemurafenib, doxycycline or amiodarone. The extracted segment ‘X2’ labelled with the respective drug names is shown in Fig. 3b, and all agents are summarized in Table 2.
Table 2.
All photosensitizing agents (n = 26) in segment ‘X2’ of Fig. 3 with the number of reports on photosensitivity in brackets, used as indicator of the photosensitizing potential
| Furosemide (22) | Amiodarone (48) |
| Naproxen (24) | Vandetanib (15) |
| Ketoprofen (43) | Lomefloxacin (41) |
| Tiaprofenic acid (16) | Enoxacin (20) |
| Piroxicam (48) | Ciprofloxacin (32) |
| Methotrexate (18) | Tetracycline (30) |
| Vemurafenib (68) | Demeclocycline (33) |
| Dabrafenib (21) | Doxycycline (50) |
| Griseofulvin (32) | Quinine (27) |
| Voriconazole (38) | Hypericin (18) |
| Promethazine (26) | Cevimeline (40) |
| Fenofibrate (23) | 8‐Methoxypsoralen (31) |
| Porfimer (59) | Pirfenidone (23) |
Segment ‘X3’ (Fig. 3c) contains drugs with a well‐documented photosensitizing potential (≥15 publications) and high dispensing rates (≥50 000 000 over the study period). In DE, only two agents fall into this category, hydrochlorothiazide and ibuprofen. In AT, none of the drugs falls into this segment; however, several drugs are close to fit into this segment such as hydrochlorothiazide, furosemide, ciprofloxacin, naproxen and ibuprofen. These two categories of drugs, namely diuretics and NSAIDs, are most likely to cause photosensitivity reactions and are therefore most important from a public health perspective (see Fig. 3c). In addition, prescription of several agents out of these drug groups is increasing between 2010 and 2017 (data not shown).
The segment ‘X4’ (Fig. 3d) corresponds to drugs with a low number of publications (<15) but a large number of annual dispensings (≥50 000 000 over the study period). Given the limited published evidence for causing photosensitivity, their role as potential photosensitizers seems rather low. The data for the AT study population are shown in Fig. 3e–h.
Discussion
Drug‐induced photosensitivity reactions are rarely documented in the published literature. This is partly due to underreporting of affected subjects who might simply stop taking the causative drug or attributing the ‘exaggerated sunburn’ to other causes, such as excessive sun exposure. In addition, the lack of adequate pharmacovigilance and a publication bias towards publishing only extravagant cases may also play a role.
As a result, physicians without a particular interest in photodermatology may only occasionally see and report cases of drug‐induced photosensitivity.10 Due to underreporting as well as the diversity and abundance of drugs with photosensitizing potential, there are only rough approximations as to the actual incidence of photosensitive reactions. In a report by Chaabane et al.19 on patients presenting with drug‐induced skin adverse reactions, photosensitivity was the third commonest cause. In specialized photodiagnostic units, systemic drug‐induced photosensitivity is reported to account for up to 15% of photodermatoses.20, 21, 22, 23, 24, 25 However, these cases only represent those actually referred for investigation of suspected photosensitivity and as such do not reflect the true incidence.10
Up to the present, a large quantity of drugs has been associated with photosensitivity and this number is increasing constantly.8 As part of our analysis, we have identified 387 drugs with photosensitivity reported in the literature or in drug databases. To our knowledge, this is the largest compilation of potentially photosensitive drugs published so far. Of note, only 75.2% out of these agents were effectively prescribed in DE and 56.9% in AT, respectively. These data also suggest that, even if there are some disparities between nations, a large part of pharmaceuticals available for prescription might be comparable among European countries.
When further analysing the dispensed drugs, it becomes evident that only a minority of all dispensed agents have a photosensitizing potential. However, when looking at the percentage of photosensitizers of all dispensed drugs, a completely different picture evolves. Out of all drug dispensings, the mean cumulative proportion of photosensitive drugs was 49.5% in DE and 48.2% in AT, respectively. These data indicate that although the number of photosensitizers accounts for only a small portion of all drugs available, almost half of all drug dispensings contained a potentially photosensitive pharmaceutical agent. The importance of photosensitizing drugs is further underlined by the fact that the number of their dispensings was increasing during the study period, even if a plateau phase (or even decrease) was observed over the more recent years.
Assuming that the incidence of photosensitive adverse reactions is determined by the frequency of its use and its photosensitizing potential a correlation of these two parameters was performed. The resulting scatter plot was subdivided into four segments revealing two segments that contain compounds with a higher probability for causing cutaneous photosensitivity reactions. The segment ‘X2’ contains drugs with a low number of prescriptions but a high photosensitizing potential, such as vemurafenib, or amiodarone. Given their rather narrow indications with limited total prescriptions in combination with their well‐known photosensitizing potential, the number of cases of photosensitivity due to these drugs is expected to be rather low. The segment ‘X3’ represents the segment with both a well‐documented photosensitizing potential and a high prescription rate. In DE, only two agents fall into this category, hydrochlorothiazide and ibuprofen. In AT, none of the drugs falls into this category but several drugs are close to it including hydrochlorothiazide, furosemide, ciprofloxacin, naproxen and ibuprofen. Generally, compounds in the ‘X3’ segment represent those drugs being most important from a public health perspective as they will account for most (and potentially undiagnosed) cases of photosensitivity.
The category ‘X3’ becomes even more important when considering the potential long‐term effects of photosensitizing drugs. Besides acute cutaneous adverse effects, an increasing number of reports also suggest chronic sequelae such as increased photoaging or photocarcinogenesis.26 While there is overwhelming evidence for the photocarcinogenic effects of psoralens,25, 26, 27, 28, 29, 30, 31, 32 several other phototoxic drugs have been associated with photocarcinogenesis.33, 34, 35, 36, 37, 38 This includes fluoroquinolones36, 38, 39, 40, 41, 42 such as ciprofloxacin or lomefloxacin, NSAIDs,36 thiazide diuretics,34, 35, 37, 43 amiloride,34 amiodarone,44, 45, 46, 47 tetracyclines,34 azathioprine,48, 49, 50, 51 vemurafenib 52, 53, 54 or voriconazole.55 In general, these drugs may increase the risk for spinocellular carcinoma and melanoma although there are also reports on an increased risk for basal cell carcinoma with amiodarone, ciprofloxacin or tetracycline.34, 43, 44, 45, 46 However, this aspect remains to be fully elucidated as some drugs such as NSAIDs that may induce photosensitivity can even prevent photocarcinogenesis.56 Moreover, epidemiological data do not unequivocally support the association between drug‐induced photosensitivity and an elevated risk of skin cancer; instead, there are also data on reduced risk.33 The complexity of this topic is further exemplified by the different mechanisms involved in drug‐induced photosensitivity. Besides the classical mechanism via exogenous chromophores and absorption, some drugs induce photosensitivity by affecting DNA repair (e.g. in case of PARP inhibitors57), by interacting with a signalling pathway (e.g. in case of protein kinase inhibitors58) or by DNA adducts and interstrand cross‐linking (e.g. in case of psoralens5, 59). These diverse mechanisms of photosensitivity do not only complicate our understanding, they also explain why drug‐induced photosensitivity does not automatically correlate with photocarcinogenicity. However, the issue of drug‐induced photocarcinogenicity was beyond the scope of the current investigation.
A main limitation of the present study is the fact that it did not include over‐the‐counter drugs and drugs dispensed to patients without public insurance coverage. In addition, drugs with a price below the copayment rate were also not factored in the analysis since their dispensing is not registered by the statutory health insurances. In the end, it would be desirable to determine the actual number of individuals which were exposed to the photosensitive risk. However, from an epidemiological point of view, an extrapolation from the number of dispensed photosensitive drugs to the number of individuals affected is not possible. From the available data set, it is not possible to determine how many packages of the individual drugs were prescribed for how many patients and whether these medications were actually taken by these individuals (and for how long). These questions could only be clarified satisfactorily within the context of a prospective patient‐centred study. In addition, the ‘indicator’ of the photosensitizing potential of a drug as established in our study only reflects current published knowledge on drug‐induced photosensitivity. Unpublished cases or cases not published in MEDLINE or in Litt's Drug Eruption & Reaction Database were not captured by the current analysis.
In summary, our findings indicate that photosensitive drugs represent about half of all dispensings of reimbursed drugs in DE and AT. Besides the potential of causing acute cutaneous photosensitivity reactions, the recent reports on the association of phototoxic drugs with photocarcinogenesis cause particular concern. In the latter regard, special attention should be given to drugs with high dispensing numbers and a high phototoxic potential, such as hydrochlorothiazide.
Supporting information
Figure S1. Analysis of the distribution of photosensitizing drug dispensings according to drug classes.
Figure S2. Analysis of the references on photosensitivity.
Table S1. Compilation of reports on photosensitivity per pharmaceutical compound based on a MEDLINE literature search (n = 1697).
Appendix S1. Methods.
Acknowledgements
We would like to thank Dr. Robert Sauermann from the Main Association of Austrian Social Security Institutions for providing the data set on the drug dispensings in Austria and his scientific input.
Conflict of interest
The authors declare no conflicts of interest.
Funding sources
None.
References
- 1. Monteiro AF, Rato M, Martins C. Drug‐induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol 2016; 34: 571–581. [DOI] [PubMed] [Google Scholar]
- 2. Khandpur S, Porter RM, Boulton SJ et al Drug‐induced photosensitivity: new insights into pathomechanisms and clinical variation through basic and applied science. Br J Dermatol 2017; 176: 902–909. [DOI] [PubMed] [Google Scholar]
- 3. Bracchitta G, Catalfo A, Martineau S et al Investigation of the phototoxicity and cytotoxicity of naproxen, a non‐steroidal anti‐inflammatory drug, in human fibroblasts. Photochem Photobiol Sci 2013; 12: 911–922. [DOI] [PubMed] [Google Scholar]
- 4. Shimoda K, Kato M. Involvement of reactive oxygen species, protein kinase C, and tyrosine kinase in prostaglandin E2 production in Balb/c 3T3 mouse fibroblast cells by quinolone phototoxicity. Arch Toxicol 1998; 72: 251–256. [DOI] [PubMed] [Google Scholar]
- 5. Cimino GD, Gamper HB, Isaacs ST et al Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem 1985; 54: 1151–1193. [DOI] [PubMed] [Google Scholar]
- 6. Gould JW, Mercurio MG, Elmets CA. Cutaneous photosensitivity diseases induced by exogenous agents. J Am Acad Dermatol 1995; 33: 551–573. [DOI] [PubMed] [Google Scholar]
- 7. Stein KR, Scheinfeld NS. Drug‐induced photoallergic and phototoxic reactions. Expert Opin Drug Saf 2007; 6: 431–443. [DOI] [PubMed] [Google Scholar]
- 8. Moore DE. Drug‐induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf 2002; 25: 345–372. [DOI] [PubMed] [Google Scholar]
- 9. Trakatelli M, Charalampidis S, Novakovic LB et al Photodermatoses with onset in the elderly. Br J Dermatol 2009; 3: 69–77. [DOI] [PubMed] [Google Scholar]
- 10. Ibbotson S. Drug and chemical induced photosensitivity from a clinical perspective. Photochem Photobiol Sci 2018; 17: 1885–1903. [DOI] [PubMed] [Google Scholar]
- 11. Selvaag E. Clinical drug photosensitivity. A retrospective analysis of the Norwegian Adverse Drug Reactions Committee from the years 1970‐1994. Photodermatol Photoimmunol Photomed 1997; 13: 21–23. [DOI] [PubMed] [Google Scholar]
- 12. Das Deutsche Arzneiprüfungsinstitut e.V . About Us, 2017. URL http://www.dapi.de/en/the-dapi/the-dapi/ (last accessed: 16 October 2019).
- 13. Federal Ministry of Health, KM6‐statistics. URL http://www.bmg.bund.de (last accessed: 30 May 2018).
- 14. Statistisches Bundesamt (Destatis), Genesis‐Online, dl‐de/by‐2‐0. URL https://www-genesis.destatis.de/genesis/online (last accessed: 30 May 2018).
- 15. Hauptverband der österreichischen Sozialversicherungsträger . Statistische Daten aus der Sozialversicherung ‐ Versicherte, Pensionen, Renten – Jahresergebnisse, 2017. URL http://www.hauptverband.at/cdscontent/?contentid = 10007.754024%26viewmode=content (last accessed: 19 January 2019).
- 16. Statistik Austria. URL http://statistik.at/web_de/statistiken/menschen_und_gesellschaft/bevoelkerung/volkszaehlungen_registerzaehlungen_abgestimmte_erwerbsstatistik/bevoelkerungsstand/078392.html (last accessed: 19 January 2019).
- 17. Drucker AM, Rosen CF. Drug‐induced photosensitivity: culprit drugs, management and prevention. Drug Saf 2011; 34: 821–837. [DOI] [PubMed] [Google Scholar]
- 18. WHO Collaborating Centre for Drug Statistics Methodology. ATC/ DDD Index. URL https://www.whocc.no/atc_ddd_index/ (last accessed: 19 January 2019).
- 19. Chaabane H, Masmoudi A, Amouri M et al Cutaneous adverse drug reaction: prospective study of 118 cases. Tunis Med 2013; 91: 514–520. [PubMed] [Google Scholar]
- 20. Kerr HA, Lim HW. Photodermatoses in African Americans: a retrospective analysis of 135 patients over a 7‐year period. J Am Acad Dermatol 2007; 57: 638–643. [DOI] [PubMed] [Google Scholar]
- 21. Khoo SW, Tay YK, Tham SN. Photodermatoses in a Singapore skin referral centre. Clin Exp Dermatol 1996; 21: 263–268. [DOI] [PubMed] [Google Scholar]
- 22. Stratigos AJ, Antoniou C, Papathanakou E et al Spectrum of idiopathic photodermatoses in a Mediterranean country. Int J Dermatol 2003; 42: 449–454. [DOI] [PubMed] [Google Scholar]
- 23. Wadhwani AR, Sharma VK, Ramam M et al A clinical study of the spectrum of photodermatoses in dark‐skinned populations. Clin Exp Dermatol 2013; 38: 823–829. [DOI] [PubMed] [Google Scholar]
- 24. Wong SN, Khoo LS. Analysis of photodermatoses seen in a predominantly Asian population at a photodermatology clinic in Singapore. Photodermatol Photoimmunol Photomed 2005; 21: 40–44. [DOI] [PubMed] [Google Scholar]
- 25. Tolland JP, Murphy BP, Boyle J et al Ciprofloxacin‐induced phototoxicity in an adult cystic fibrosis population. Photodermatol Photoimmunol Photomed 2012; 28: 258–260. [DOI] [PubMed] [Google Scholar]
- 26. O'Gorman SM, Murphy GM. Photosensitizing medications and photocarcinogenesis. Photodermatol Photoimmunol Photomed 2014; 30: 8–14. [DOI] [PubMed] [Google Scholar]
- 27. Stern RS, Laird N, Melski J et al Cutaneous squamous‐cell carcinoma in patients treated with PUVA. N Engl J Med 1984; 310: 1156–1161. [DOI] [PubMed] [Google Scholar]
- 28. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy Follow‐up Study. Cancer 1994; 73: 2759–2764. [DOI] [PubMed] [Google Scholar]
- 29. Stern RS, Nichols KT, Väkevä LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). The PUVA Follow‐Up Study. N Engl J Med 1997; 336: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 30. Stern RS, Bolshakov S, Nataraj AJ et al p53 mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet a‐treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol 2002; 119: 522–526. [DOI] [PubMed] [Google Scholar]
- 31. Nijsten TE, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen+ultraviolet A: a cohort study. J Invest Dermatol 2003; 121: 252–258. [DOI] [PubMed] [Google Scholar]
- 32. Lindelöf B, Sigurgeirsson B, Tegner E et al PUVA and cancer risk: the Swedish follow‐up study. Br J Dermatol 1999; 141: 108–112. [DOI] [PubMed] [Google Scholar]
- 33. de Vries E, Trakatelli M, Kalabalikis D et al Known and potential new risk factors for skin cancer in European populations: a multicentre case‐control study. Br J Dermatol 2012; 2: 1–13. [DOI] [PubMed] [Google Scholar]
- 34. Jensen AØ, Thomsen HF, Engebjerg MC et al Use of photosensitising diuretics and risk of skin cancer: a population‐based case‐control study. Br J Cancer 2008; 99: 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaae J, Boyd HA, Hansen AV et al Photosensitizing medication use and risk of skin cancer. Cancer Epidemiol Biomarkers Prev 2010; 19: 2942–2949. [DOI] [PubMed] [Google Scholar]
- 36. Siiskonen SJ, Koomen ER, Visser LE et al Exposure to phototoxic NSAIDs and quinolones is associated with an increased risk of melanoma. Eur J Clin Pharmacol 2013; 69: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 37. Friedman GD, Asgari MM, Warton EM et al Antihypertensive drugs and lip cancer in non‐Hispanic whites. Arch Intern Med 2012; 172: 1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cadet J, Mouret S, Ravanat JL et al Photoinduced damage to cellular DNA: direct and photosensitized reactions. Photochem Photobiol 2012; 88: 1048–1065. [DOI] [PubMed] [Google Scholar]
- 39. Marrot L, Belaïdi JP, Jones C et al Molecular responses to stress induced in normal human caucasian melanocytes in culture by exposure to simulated solar UV. Photochem Photobiol 2005; 81: 367–375. [DOI] [PubMed] [Google Scholar]
- 40. Mäkinen M, Forbes PD, Stenbäck F. Quinolone antibacterials: a new class of photochemical carcinogens. J Photochem Photobiol, B 1997; 37: 182–187. [DOI] [PubMed] [Google Scholar]
- 41. Klecak G, Urbach F, Urwyler H. Fluoroquinolone antibacterials enhance UVA‐induced skin tumors. J Photochem Photobiol, B 1997; 37: 174–181. [DOI] [PubMed] [Google Scholar]
- 42. Johnson BE, Gibbs NK, Ferguson J. Quinolone antibiotic with potential to photosensitize skin tumorigenesis. J Photochem Photobiol, B 1997; 37: 171–173. [DOI] [PubMed] [Google Scholar]
- 43. Ruiter R, Visser LE, Eijgelsheim M et al High‐ceiling diuretics are associated with an increased risk of basal cell carcinoma in a population‐based follow‐up study. Eur J Cancer 2010; 46: 2467–2472. [DOI] [PubMed] [Google Scholar]
- 44. Monk B. Amiodarone‐induced photosensitivity and basal‐cell carcinoma. Clin Exp Dermatol 1990; 15: 319–320. [DOI] [PubMed] [Google Scholar]
- 45. Monk BE. Basal cell carcinoma following amiodarone therapy. Br J Dermatol 1995; 133: 148–149. [DOI] [PubMed] [Google Scholar]
- 46. Hall MA, Annas A, Nyman K et al Basalioma after amiodarone therapy‐not only in Britain. Br J Dermatol 2004; 151: 932–933. [DOI] [PubMed] [Google Scholar]
- 47. Maoz KB, Dvash S, Brenner S et al Amiodarone‐induced skin pigmentation and multiple basal‐cell carcinomas. Int J Dermatol 2009; 48: 1398–1400. [DOI] [PubMed] [Google Scholar]
- 48. Perrett CM, Walker SL, O'Donovan P et al Azathioprine treatment photosensitizes human skin to ultraviolet A radiation. Br J Dermatol 2008; 159: 198–204. [DOI] [PubMed] [Google Scholar]
- 49. Molina BD, Leiro MG, Pulpón LA et al Incidence and risk factors for nonmelanoma skin cancer after heart transplantation. Transplant Proc 2010; 42: 3001–3005. [DOI] [PubMed] [Google Scholar]
- 50. O'Donovan P, Perrett CM, Zhang X et al Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science 2005; 309: 1871–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hofbauer GF, Attard NR, Harwood CA et al Reversal of UVA skin photosensitivity and DNA damage in kidney transplant recipients by replacing azathioprine. Am J Transplant 2012; 12: 218–225. [DOI] [PubMed] [Google Scholar]
- 52. Anforth R, Tembe V, Blumetti T et al Mutational analysis of cutaneous squamous cell carcinomas and verrucal keratosis in patients taking BRAF inhibitors. Pigment Cell Melanoma Res 2012; 25: 569–572. [DOI] [PubMed] [Google Scholar]
- 53. Oberholzer PA, Kee D, Dziunycz P et al RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol 2012; 30: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Su F, Viros A, Milagre C et al RAS mutations in cutaneous squamous‐cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 2012; 366: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Williams K, Mansh M, Chin‐Hong P et al Voriconazole‐associated cutaneous malignancy: a literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis 2014; 58: 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bosch R, Philips N, Suárez‐Pérez JA et al Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel) 2015. Jun; 4: 248–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lakatos P, Szabo E, Hegedus C et al 3‐Aminobenzamide protects primary human keratinocytes from UV‐induced cell death by a poly(ADP‐ribosyl)ation independent mechanism. Biochim Biophys Acta 2013; 3: 743–751. [DOI] [PubMed] [Google Scholar]
- 58. Mealey KL, Dassanayake S, Burke NS. Tyrosine kinase inhibitors enhance ciprofloxacin‐induced phototoxicity by inhibiting ABCG2. Oncology 2014; 87: 364–370. [DOI] [PubMed] [Google Scholar]
- 59. Laskin JD, Lee E, Yurkow EJ, Laskin DL, Gallo MA. A possible mechanism of psoralen phototoxicity not involving direct interaction with DNA. Proc Natl Acad Sci USA 1985; 82: 6158–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Analysis of the distribution of photosensitizing drug dispensings according to drug classes.
Figure S2. Analysis of the references on photosensitivity.
Table S1. Compilation of reports on photosensitivity per pharmaceutical compound based on a MEDLINE literature search (n = 1697).
Appendix S1. Methods.


