Summary
The quantification of donor‐derived cell‐free DNA (ddcfDNA) in recipient's plasma is a novel, but technically challenging noninvasive method to assist the diagnosis of acute rejection (AR). A quantitative real‐time PCR (qPCR) approach targeting insertion/deletion polymorphisms (INDEL) was adapted to measure ddcfNA in plasma samples from 29 kidney transplant recipients obtained at time of clinically indicated biopsies (eight patients with a histologically verified AR, nine with borderline rejection and 12 without evidence of rejection). Measured ddcfDNA levels of smaller INDEL amplicon targets differed significantly (P = 0.016, Kruskal–Wallis H test) between recipients with biopsy‐proven AR (median 5.24%; range 1.00–9.03), patients without (1.50%; 0.41–6.50) and patients with borderline AR (1.91%; 0.58–5.38). Similarly, pairwise testing by Mann–Whitney U‐tests revealed significant differences between recipients with AR and without AR (P = 0.012) as well as patients with AR and borderline histology (P = 0.015). Receiver operating characteristic (ROC) analysis revealed an area under the ROC curve for discriminating AR and non‐AR biopsies of 0.84 (95% CI: 0.66–1.00). The determined cutoff value of 2.7% ddcfDNA showed a sensitivity of 0.88 (95% CI: 0.63–1.00) and specificity of 0.81 (95% CI: 0.64–0.98). INDEL qPCR represents a novel method to quantify ddcfDNA on standard qPCR instruments within 6–8 h with high sensitivity and specificity to detect AR.
Keywords: acute rejection, cell‐free DNA, donor‐derived cell‐free DNA, INDEL, qPCR
Introduction
Renal allograft biopsy represents the standard of care for differential diagnosis of acute rejection (AR) and other reasons of organ dysfunction early after kidney transplantation (KTx). Despite its good diagnostic yield, this invasive procedure involves a certain risk of complications and is highly dependent on adequate sampling. It is generally agreed that noninvasive markers would be useful to monitor patients post‐transplant and thus reduce the number of biopsies 1, 2. One of the most promising biomarker candidate is the detection of circulating donor‐specific cell‐free DNA (cfDNA) 3, 4. CfDNA is released during apoptosis, necrosis or active secretion mostly of hematopoietic cells, but also of solid organ tissues 5. In the setting of solid organ transplantation, donor‐derived cfDNA (ddcfDNA) can be detected in plasma samples during phases of graft damage 6, 7. However, there is an ongoing discussion on the optimal method of measuring cfDNA 8.
Previous studies investigated Y‐chromosomal cfDNA in plasma and/or urine of female recipients of male organs 3, 9, 10. Another approach was to determine donor/recipient differential single‐nucleotide polymorphisms (SNP) 11, 12, 13, 14, 15, 16, 17, 18 or HLA mismatches 19, 20 by classical quantitative real‐time PCR (qPCR) 6, 9, 19, digital PCR 10, 11, 15, 18, 20 or massively parallel sequencing (MPS) 12, 13, 14, 16, 17, 21. CfDNA studies have been performed in heart 13, 14, 16, 21, kidney 9, 10, 17, 18, 22, liver 11, 15, 23, lung 12 and pancreas/kidney 19 transplantation, and an association of increased levels of ddcfDNA in recipient plasma with episodes of AR has been found in most of those studies, however, with varying results for sensitivity and specificity. Lee et al. 22 recently published a study, analyzing ddcfDNA in urine and plasma of kidney transplant recipients by digital PCR of nuclear and mitochondrial SNPs. The authors only found elevated levels of ddcfDNA in urine, but not in plasma, where it was not detectable.
Insertion/deletion (INDEL) polymorphisms, another class of genome‐wide markers, have been recently used for quantitative assessment of ddcfDNA by digital PCR in three liver transplant recipients 23. Short INDELs are bi‐allelic markers exhibiting insertions/deletions of 1–10 base pairs (bp) 24. INDELs differing by at least two consecutive variable bases enable a more specific annealing of primers than classical SNPs, which are restricted to one variable base only. Real‐time quantitative PCR (qPCR) assays of INDELs already proved to outperform conventional PCR of short tandem repeat (STR) polymorphisms in monitoring donor cell engraftment after hematopoietic stem cell transplantation (HSCT). In the HSCT setting, INDELs evidenced a much higher sensitivity and better linearity compared with conventional techniques 25, 26.
The aim of the present study was to analyze whether cfDNA detection by qPCR of INDELs is feasible in KTx and whether it can be used to assist the diagnosis of AR.
Material and methods
Study participants and sample collection
In total, 29 patients that received a KTx at the Division of Transplantation, Medical University of Vienna, were included in the study. The study was approved by the Ethics Committee of the Medical University of Vienna (ECS 2261/2016). Clinical data were prospectively collected in the institutional database and included: reason for transplantation, comorbidities, preoperative DSA levels, date of transplantation, postoperative course and laboratory values, graft function and date of graft loss, rejection and biopsy results. Histopathological diagnosis of the renal biopsies was performed according to the BANFF classification 27. Nucleic DNA extracted from pretransplant EDTA‐anticoagulated venous blood samples of all donor/recipient pairs were used for INDEL genotyping.
For extraction of total cfDNA from post‐transplant plasma samples, blood was drawn into VACUETTE® TUBE 8 ml K2E K2EDTA Separator (Greiner BioOne International, Kremsmünster, Austria) within 120 min before clinically indicated renal biopsies because of allograft dysfunction for diagnosis of potential AR. cfDNA was extracted with the QIAamp® Circulating DNA Kit (Qiagen, Hilden, Germany). CfDNA quantity and quality were assessed with the Quantifiler Trio® DNA Quantification Kit on an ABI 7500 Fast Real‐Time PCR System (Applied Biosystem by Thermo Fisher Scientific, Waltham, MA, USA). More details are described in Appendix S1.
INDEL genotyping
The screening assays for informative differential markers (recipient negative/donor positive) were performed by qPCR with a panel of 34 INDEL markers according to the manufacturer's instructions (AlleleSEQR® Chimerism Assay by Celera, Alameda, CA, USA). For second transplant recipients, the informative differential markers were negative for the recipient and the first transplant donor, but positive for the second organ donor.
Relative quantification of ddcfDNA
Relative quantification of ddcfDNA was performed by INDEL qPCR in a reaction volume of 30 μl by adding 18 μl cfDNA: A donor‐specific target assay chosen from the INDEL marker panel and a reference target assay (107 bp) specific for total DNA (Ribonuclease P, a constitutive enzyme) were tested in triplicates including no template controls (NTCs) to disclose possible contamination in all participants. The donor‐specific target assay with the amplicon size closest to the reference assay (107 bp) was chosen to minimize amplicon size‐related artefacts. If enough cfDNA was available, a second donor‐specific target assay was included. The comparative cycle of threshold (C T, where the fluorescence passes the threshold) method was used to calculate the amount of ddcfDNA relative to the total cfDNA in the recipient's plasma 28. Pure pretransplant nucleic donor DNA was used as a reference for 100% donor DNA.
Statistical analysis
Statistical tests and ROC curve analysis were performed with the ibm spss Statistics 22 software (IBM). For non‐normally distributed data, nonparametric tests were performed (Kruskal–Wallis test for multiple groups and Mann–Whitney U‐test for pairwise comparisons of independent groups, Wilcoxon signed‐rank test for dependent samples). For survival analysis, the Kaplan–Meier analysis was performed. Patient survival was calculated from date of transplantation to date of death or last follow‐up, and graft survival was censored for death and was calculated from date of transplantation to date of graft failure or last follow‐up. To compare the clinical characteristics between patients with acute rejection, no rejection and borderline rejection, chi‐squared test was used for categorical variables and one‐way anova for continuous variables. ROC analysis was performed between patients with histologically proven AR and non‐AR biopsies (cases with borderline histology were excluded). P‐values <0.05 were considered as statistically significant.
Results
Patient characteristics
The study population included 27 first transplant and two second transplant recipients. Five patients received donor organs from living and 24 from deceased donors (Table 1). Kidney biopsies were performed between postoperative day 6 and 39 (median 8). The reasons for the biopsy included delayed graft function (DGF), an increase in creatinine or a reduction in urine production. In total, eight patients were diagnosed with AR, nine patients with BANFF classification borderline and twelve patients did not show any signs of rejection in histological analysis. Histological findings and postoperative complications within three months are summarized in Table 1. Table 2 provides a group‐wise comparison of clinical characteristics of patients diagnosed with AR versus borderline rejection versus no rejection. There was no difference in age (P = 0.895), gender (P = 0.145) or indication for transplantation between the three groups. Patients experiencing post‐transplant AR or borderline rejection had more HLA mismatches compared to those without any rejection. Post‐transplant creatinine levels were comparable between the three groups. Furthermore, graft and patient survival after 6 months were similar between patients with AR, borderline rejection or no rejection (Table 2).
Table 1.
Clinical characteristics of 29 kidney transplant recipients receiving post‐transplant kidney biopsy.
| Patient | Age | Recipient/donor sex | Type of Tx* | Biopsy (days after Tx*) | Pretransplant DSA† | Clinical reason for biopsy | Acute rejection | Histological finding | Other complications within 3 months |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | Male/male | Deceased | 7 | No | DGF‡ | Yes | TCMR§, BANFF 2a | – |
| 2 | 56 | Male/female | Living | 6 | No | ↑ creatinine | Yes | TCMR§, BANFF 2b | Hematoma |
| 3 | 27 | Female/male | Deceased | 7 | No | DGF‡ | Yes | TCMR§, BANFF 2a | UTI¶ |
| 4 | 58 | Male/1st female/2nd male | 2nd Tx*; deceased | 8 | Yes | DGF‡ | Yes | ABMR** | UTI¶ |
| 5 | 52 | Female/male | Deceased | 6 | No | DGF‡ | Yes | TCMR§, BANFF 2a | Ureter necrosis |
| 6 | 44 | female/male | Deceased | 17 | No | DGF‡ | Yes | TCMR§, BANFF 2a | UTI¶ |
| 7 | 61 | Female/2x male | 2nd Tx*; deceased | 21 | Yes | DGF‡ | Yes | ABMR** | Ureter fistula, UTI¶ |
| 8 | 48 | Female/male | Deceased | 8 | No | DGF‡ | Yes | ABMR** | Hematoma, UTI¶ |
| 9 | 59 | Male/female | Deceased | 9 | No | DGF‡ | No | Pyelonephritis | Hydronephrosis, UTI¶ |
| 10 | 49 | Male/male | Living | 8 | No | ↓ urine production | No | No rejection | – |
| 11 | 49 | Male/male | Deceased | 8 | No | DGF‡ | No | No rejection | CMV |
| 12 | 49 | Female/female | Deceased | 39 | No | PNF†† | No | Ischemic injury | PNF††, post‐Bx‡‡ bleeding, UTI¶ |
| 13 | 54 | Male/female | Deceased | 7 | No | ↓ urine production | No | Focal tubulus cell necrosis | – |
| 14 | 60 | Male/male | Living | 7 | No | ↑ creatinine | No | Thrombotic microangiopathy | Lymphocele |
| 15 | 67 | Male/female | Deceased | 13 | No | DGF‡ | No | No rejection | UTI¶ |
| 16 | 54 | Female/female | Deceased | 16 | Yes | DGF‡ | No | Small thrombi | Venous thrombosis—Nephrectomy |
| 17 | 53 | Female/female | Deceased | 11 | No | DGF‡ | No | Pyelonephritis | Wound infection |
| 18 | 47 | Male/male | Deceased | 7 | No | DGF‡ | No | Pyelonephritis | Polyomavirus infection |
| 19 | 37 | Male/male | Deceased | 7 | No | DGF‡ | No | No rejection | Post‐transplant bleeding, revision venous anastomosis |
| 20 | 74 | Male/male | Deceased | 15 | No | DGF‡ | No | Thrombotic microangiopathy | UTI¶ |
| 21 | 65 | Male/female | Deceased | 7 | No | DGF‡ | Borderline | TCMR§, BANFF 0–1 | – |
| 22 | 40 | Female/female | Deceased | 7 | No | ↑ creatinine | Borderline | TCMR§, BANFF 0–1 | – |
| 23 | 65 | Male/male | Deceased | 8 | No | DGF‡ | Borderline | TCMR§, BANFF 0–1 | UTI¶ |
| 24 | 68 | Male/male | Deceased | 7 | No | ↑ creatinine | Borderline | TCMR§, BANFF 0–1 | UTI¶ |
| 25 | 31 | Male/female | Deceased | 6 | No | DGF‡ | Borderline | TCMR§, BANFF 0–1 | – |
| 26 | 47 | Male/female | Living | 13 | No | ↑ creatinine | Borderline | TCMR§, BANFF 0–1 | – |
| 27 | 32 | Female/male | Deceased | 7 | No | DGF‡ | Borderline | TCMR§, BANFF 0–1 | – |
| 28 | 66 | Male/female | Living | 12 | No | ↓ urine production | Borderline | TCMR§, BANFF 0–1 | – |
| 29 | 74 | Male/female | Deceased | 7 | No | DGF‡ | Borderline | TCMR§, BANFF 0–1 | UTI¶ |
*Transplantation.
†Donor‐specific antibodies.
‡Delayed graft function.
§T‐cell mediated rejection.
¶Urinary tract infection.
**Antibody‐medicated rejection.
††Primary nonfunction.
‡‡Biopsy.
Table 2.
Comparison of clinical characteristics between patients with acute rejection (n = 8), borderline rejection (n = 9) and no rejection (n = 12) after kidney transplantation
| Clinical characteristics | Acute rejection | Borderline rejection | No rejection | P‐value |
|---|---|---|---|---|
| Number of patients | 8 | 9 | 12 | |
| Age at transplant | 52 ± 13 | 54 ± 17 | 54 ± 10 | 0.895 |
| Men, n (%) | 3 (37.5%) | 7 (77.8%) | 9 (75%) | 0.145 |
| Indication | ||||
| Diabetes | 2 (25%) | 3 (33.3%) | – | 0.088 |
| Polycystic kidney disease | 1 (12.5%) | – | – | |
| Hypertension | 1 (12.5%) | 2 (22.2%) | 2 (16.7%) | |
| Pyelonephritis/glomerulonephritis | 3 (37.5%) | 1 (11.1%) | – | |
| Alport's disease | 1 (12.5%) | – | – | |
| Focal segmental glomerulosclerosis | – | – | 1 (8.3%) | |
| IgA nephropathy | – | – | 1 (8.3%) | |
| Amyloidosis | – | – | 1 (8.3%) | |
| Other | – | – | 4 (33.3%) | |
| Unknown | – | 3 (33.3%) | 3 (25%) | |
| Matched donor/recipient sex | 2 (25%) | 3 (33.3%) | 9 (75%) | 0.05 |
| CMV* serologic status | ||||
| D‐/R+ | 4 (50%) | 3 (33.3%) | 2 (16.7%) | 0.129 |
| D+/R+ | 4 (50%) | 2 (22.2%) | 5 (41.7%) | |
| D−/R− | 0 | 3 (33.3%) | 1 (8.3%) | |
| D+/R− | 0 | 1 (11.1%) | 4 (33.3%) | |
| Donor type, n (%) | ||||
| Deceased | 7 (87.5%) | 7 (77.8%) | 10 (83.3%) | 0.867 |
| Living | 1 (12.5%) | 2 (22.2%) | 2 (16.7%) | |
| Cold Ischemia time (h) | 12 ± 5 | 16 ± 8 | 13 ± 7 | 0.539 |
| DSA† positive, n (%) | 2 (25%) | ‐ | 1 (8.3%) | 0.230 |
| HLA‡‐A no. of mismatches | 1.5 ± 0.5 | 1.1 ± 0.3 | 0.7 ± 0.7 | 0.008 |
| HLA‡‐B no. of mismatches | 1.5 ± 0.5 | 1.6 ± 0.7 | 0.9 ± 0.5 | 0.036 |
| HLA‡‐DR no. of mismatches | 1.3 ± 0.5 | 1.7 ± 0.5 | 0.9 ± 0.7 | 0.058 |
| Time of biopsy (Days after Tx§) | 10 ± 5.7 | 8.2 ± 2.5 | 12.3 ± 9 | 0.403 |
| Creatinine day 1 after Tx§ | 6.7 ± 1.1 | 5.8 ± 2.4 | 6.6 ± 2.4 | 0.589 |
| Creatinine day 7 after Tx§ | 7.4 ± 3 | 5.6 ± 3.2 | 7.4 ± 3.7 | 0.403 |
| Creatinine day 14 after Tx§ | 5.7 ± 2.1 | 3.7 ± 1.8 | 6.1 ± 3.6 | 0.149 |
| DGF¶, n (%) | 7 (87.5%) | 5 (55.6%) | 10 (83.3%) | 0.225 |
| Mean duration DGF¶ (days) | 9.8 ± 6.8 | 9.4 ± 2.2 | 18 ± 13.7 | 0.175 |
| Dialysis after Tx§, n (%) | 6 (75%) | 4 (44.4%) | 8 (66.7%) | 0.394 |
| Graft survival at 6 months | 87.5% | 100% | 83.3% | 0.148 |
| Patient survival at 6 months | 87.5% | 100% | 91.7% | 0.899 |
*Cytomegalovirus.
†Donor‐specific antibodies.
‡Human leukocyte antigen.
§Transplantation.
¶Delayed graft function.
Absolute quantification and degradation level of total cfDNA in plasma
The amount of total cfDNA was quantified by multiplex‐qPCR in 28 out of 29 KTx recipients (in one case remaining cfDNA volume was insufficient). The DNA concentrations ranged from 0.61 to 15.31 ng/μl (median 3.84 ng/μl) targeting small (80 bp) and from 0.08 to 4.11 ng/μl (median 0.71) targeting larger autosomal (214 bp) amplicons, respectively (Table 3). The median DI (degradation index; ratio of the DNA concentration of the smaller to larger amplicon) was 4.84 (range 1.92–12.35) indicating that the median amount of smaller amplifiable DNA fragments (80 bp) was 4.84 times higher compared with larger DNA fragments (214 bp). This is typical for slightly to moderately degraded DNA (DI 1–10), as only values >10 characterize significantly degraded DNA.
Table 3.
Absolute quantification of total DNA in plasma (ng/μl): target on small (80 bp) and large (214 bp) autosomal amplicons and degradation index (ratio of quantity of small to large amplicon)
| Patient | Acute rejection | Amplicon | Degradation index | |
|---|---|---|---|---|
| Small | Large | |||
| 1 | Yes | 0.61 | 0.08 | 7.63 |
| 2 | Yes | 5.69 | 0.65 | 8.75 |
| 3 | Yes | 0.83 | 0.13 | 6.38 |
| 4 | Yes | 4.86 | 0.76 | 6.39 |
| 5 | Yes | 4.31 | 0.77 | 5.60 |
| 6 | Yes | 15.31 | 1.24 | 12.35 |
| 7 | Yes | 1.71 | 0.42 | 4.07 |
| 8 | Yes | 5.03 | 1.33 | 3.78 |
| 9 | No | 1.37 | 0.35 | 3.91 |
| 10 | No | 3.37 | 1.00 | 3.37 |
| 11 | No | n.t.* | n.t. | n.t. |
| 12 | No | 4.57 | 0.92 | 4.97 |
| 13 | No | 6.86 | 0.70 | 9.80 |
| 14 | No | 9.41 | 1.34 | 7.02 |
| 15 | No | 4.87 | 0.55 | 8.85 |
| 16 | No | 1.10 | 0.17 | 6.67 |
| 17 | No | 11.33 | 1.87 | 6.06 |
| 18 | No | 4.62 | 0.63 | 7.33 |
| 19 | No | 9.86 | 1.67 | 5.90 |
| 20 | No | 13.77 | 4.11 | 3.35 |
| 21 | Borderline | 2.12 | 0.45 | 4.71 |
| 22 | Borderline | 1.45 | 0.50 | 2.90 |
| 23 | Borderline | 2.9 | 0.72 | 4.03 |
| 24 | Borderline | 2.64 | 0.67 | 3.94 |
| 25 | Borderline | 7.06 | 1.74 | 4.06 |
| 26 | Borderline | 1.57 | 0.71 | 2.21 |
| 27 | Borderline | 1.75 | 0.91 | 1.92 |
| 28 | Borderline | 1.11 | 0.40 | 2.78 |
| 29 | Borderline | 1.76 | 0.56 | 3.14 |
| Median | 3.84 | 0.71 | 4.84 | |
*Not tested.
Y‐chromosomal DNA profiling
In the cfDNA samples of three female transplant recipients who had received kidneys from male donors, a Y‐chromosomal 17‐locus STR profile was generated. This facilitated to evaluate the degradation pattern of donor specific, mostly nonhematopoietically derived DNA over a more continuous size range between 90 and 323 bp in four parallel fluorescent dye channels. The ratio of the allelic signal heights of the smallest to the largest amplifiable Y‐STR loci (DYS456, allelic size range 90–114 bp and DYS389II, allelic size range 255–295 bp) was much higher in the ddcfDNA (median 20.8; range 19.5–35.3) than in the nucleic DNA samples (median 1.4; range 1.3–1.6) of their corresponding donors. Multiplex‐PCR of the largest amplicon STR locus (DYS392; allelic size range 293–326 bp) did not yield an allelic product in all three cfDNA samples, likewise the loci DYS390 and DYS635 in one cfDNA sample each (allelic size ranges 192–228 bp and 246–270 bp, respectively).
The Y‐STR profiles from cfDNA showed, that the fluorescence signal intensity of the male ddcfDNA fragments decreased more rapidly with increasing size compared with nucleic DNA of the respective male kidney donors (Fig. S1, Table S1).
Additionally, the same test was performed in the reverse situation, in cfDNA samples of four male transplant recipients transplanted with kidneys from female donors, to determine the amount of recipient‐specific degradation. The ratio of the allelic signal heights of the smallest to the largest amplifiable STR loci was moderately higher in male recipients’ specific cfDNA (median 6.7; range 3.2–9.4) than in their own nucleic DNA samples (median 1.2; range 1.0–1.2, respectively).
Taken together the different donor and recipient‐specific degradation levels of seven sex‐mismatched donor/recipient pairs (median ratios for male‐to‐female KTx: 20.8 and for female‐to‐male KTx 6.7, respectively), the nonhematopoietically derived ddcfDNA showed an approximately threefold higher degradation compared with mostly hematopoietically derived recipient‐specific cfDNA.
Assay performance of INDEL qPCR
Mixtures of two nucleic DNA samples (10%, 2%, 1%, 0.2%, 0.1%, 0.02% and 0.01% minor component) were tested with two differential target assays (86 and 169 bp), which proved correlations of R 2 > 0.999 for each target assay (Fig. S2).
An artificial mixture of two cfDNA samples from healthy sex‐mismatched individuals was prepared (3.3% volume male/total volume male and female). The unmixed minor and major components were retrospectively quantified and exhibited 0.90 and 0.17 ng/μl cfDNA, respectively, with the 80 bp amplicon target. The effective percentage of the minor component amounted to 15.4% (mass male/total male and female mass). The relative quantification by INDEL qPCR exhibited 18.1%, 15.8% and 1.5% for target assays with increasing amplicon sizes (87, 123 and 143 bp) in the 15.4% mixture with coefficient of variation (CV) values of 16.7%, 1.9% and 91%, respectively. This was acceptable for the two smaller amplicon targets (87 and 123 bp), but not for the largest amplicon target (143 bp). Taking the DI of the unmixed female and male cfDNA components into consideration (2.32 and 4.37, respectively), we could demonstrate that reliable results can only be obtained with smaller amplicon sizes in qPCR of cfDNA.
Assessment of ddcfDNA in recipient plasma by INDEL qPCR
All measurements of cfDNA samples in KTx recipients and their corresponding reference samples (100% donor DNA) met the quality requirements for accurate INDEL qPCR in all 29 assays: (i) The mean C T of the reference assay was <32 in all reference samples, which assures that enough reference DNA was added to the reactions for 100% donor reference, (ii) the mean C T value of the reference assays was <28 in all cfDNA samples, indicating that enough cfDNA was added for a detection limit of <1%. In addition, 14 out of 29 recipients met the stricter requirements (mean C T value of reference assays <25) for a <0.1% detection limit, (iii) the mean C T value of all target assays was <38 (<37 in two or more wells) in reference and cfDNA samples indicating that sufficient target copies were present in the reaction and (iv) the C T of the NTC (no template control) was >38 indicating that no DNA contamination had occurred.
In 21 out of 29 kidney recipients enough plasma was available to assess the quantity of ddcfDNA with two donor‐specific target assays. The other eight patients were tested in a smaller target assay only. In all of those double‐tested 21 cases the relative quantity of ddcfDNA was significantly lower in larger than in smaller amplicons (P < 0.001, Wilcoxon test) except in two cases (Table 4): (i) in patient 12 the target of the smaller amplicon (94 bp) revealed 0.50%, those of the larger amplicon (106 bp) 0.58% and (ii) in patient 21 the target of the smaller amplicon (106 bp) revealed 0.58%, those of the larger amplicon (114 bp) 1.12%. Additionally, the assays targeting larger amplicons showed a higher CV than those targeting smaller amplicons (14% and 10%, respectively).
Table 4.
Relative quantification of ddcfDNA in plasma: donor/recipient differential targets on smaller (Marker 1) and larger (Marker 2) amplicons including range of ±1 SD of the qPCR test performed in triplicates
| Patient | Acute rejection | Marker 1 (smaller) | Marker 2 (larger) | ||||
|---|---|---|---|---|---|---|---|
| Size (bp) | % | −1 SD/+1 SD | Size (bp) | % | −1 SD/+1 SD | ||
| 1 | Yes | 114 | 4.21 | 3.83–4.62 | 123 | 2.05 | 1.89–2.23 |
| 2 | Yes | 126 | 9.03 | 8.60–9.48 | 128 | 4.56 | 4.19–4.96 |
| 3 | Yes | 123 | 3.28 | 2.69–4.00 | n.t.* | ||
| 4 | Yes | 100 | 6.07 | 5.66–6.51 | 124 | 0.84 | 0.79–0.89 |
| 5 | Yes | 86 | 5.87 | 5.65–6.09 | 128 | 2.09 | 1.81–2.41 |
| 6 | Yes | 114 | 5.76 | 5.55–5.96 | 124 | 0.32 | 0.28–0.35 |
| 7 | Yes | 86 | 4.71 | 4.05–5.48 | n.t.* | ||
| 8 | Yes | 100 | 1.00 | 0.93–1.04 | 127 | 0.90 | 0.84–0.98 |
| Median | 5.24 | Median | 1.48 | ||||
| 9 | No | 94 | 2.12 | 1.97–2.27 | 134 | 0.79 | 0.67–0.95 |
| 10 | No | 94 | 0.41 | 0.36–0.46 | 127 | 0.27 | 0.25–0.28 |
| 11 | No | 124 | 1.31 | 1.16–1.48 | n.t.* | ||
| 12 | No | 94 | 0.50 | 0.40–0.62 | 106 | 0.58 | 0.51–0.66 |
| 13 | No | 114 | 6.50 | 6.31–6.68 | 128 | 1.57 | 1.30–1.90 |
| 14 | No | 96 | 1.69 | 1.56–1.83 | 156 | 0.80 | 0.70–0.90 |
| 15 | No | 90 | 1.82 | 1.50–2.22 | 116 | 1.26 | 1.17–1.36 |
| 16 | No | 106 | 1.16 | 1.02–1.31 | 116 | 0.68 | 0.56–0.82 |
| 17 | No | 86 | 3.52 | 3.21–3.86 | 128 | 1.22 | 1.06–1.41 |
| 18 | No | 126 | 0.64 | 0.58–0.71 | n.t.* | ||
| 19 | No | 114 | 1.79 | 1.73–1.79 | 128 | 0.67 | 0.58–0.78 |
| 20 | No | 87 | 1.29 | 1.18–1.40 | 127 | 0.73 | 0.66–0.81 |
| Median | 1.50 | Median | 0.76 | ||||
| 21 | Borderline | 106 | 0.58 | 0.53–0.63 | 114 | 1.12 | 0.84–1.50 |
| 22 | Borderline | 94 | 5.38 | 4.79–6.04) | n.t.* | ||
| 23 | Borderline | 106 | 1.62 | 1.52–1.73 | 128 | 0.76 | 0.65–0.88 |
| 24 | Borderline | 94 | 1.91 | 1.79–2.05 | 106 | 1.71 | 1.48–1.97 |
| 25 | Borderline | 100 | 3.63 | 3.42–3.84 | 123 | 2.47 | 2.01–3.05 |
| 26 | Borderline | 128 | 0.76 | 0.59–0.98 | n.t.* | ||
| 27 | Borderline | 116 | 1.54 | 1.38–1.71 | 126 | 1.20 | 1.08–1.34 |
| 28 | Borderline | 114 | 2.19 | 1.74–2.75 | n.t.* | ||
| 29 | Borderline | 114 | 2.56 | 2.19–3.00 | n.t.* | ||
| Median | 1.91 | Median | 1.20 | ||||
*Not tested.
Therefore, only the target assays with the smaller amplicon sizes have been taken into further consideration for all patients.
Different levels of donor‐specific targets in kidney recipients with and without AR
The relative quantity of ddcfDNA in plasma differed significantly between recipients with biopsy‐proven AR (median ddcfDNA levels 5.24% range 1.00–9.03), those without (1.50%;0.41–6.50) and those with borderline AR (1.91%;0.58–5.38) in smaller amplicon target assays (P = 0.016, Kruskal–Wallis H test). Pairwise testing in Mann–Whitney U‐test revealed significantly higher levels of ddcfDNA in the recipients with AR compared to those without AR (P = 0.012) or borderline AR (P = 0.015). There was no significant difference comparing patients without AR to those with borderline AR (P = 0.723). One statistical outlier was observed in the group of recipients without AR (patient 13) and with borderline (patient 22) AR, respectively (Fig. 1). In ROC curve analysis, AR patients were compared with non‐AR patients (Fig. 2). The area under curve (AUC) amounted to 0.84 (95% confidence interval 0.66–1.00). A cutoff value of 2.7% ddcfDNA was determined from simultaneous maximization of sensitivity (0.88; 95% C.I. 0.63–1.00) and specificity (0.81; 95% C.I. 0.64–0.98) resulting in an accuracy of 0.83 (95% C.I. 0.69–0.97). Positive (PPV) and negative predictive values (NPV) amounted to 0.64 (95% C.I. 0.34–0.93) and 0.94 (95% C.I. 0.84–1.00), respectively.
Figure 1.
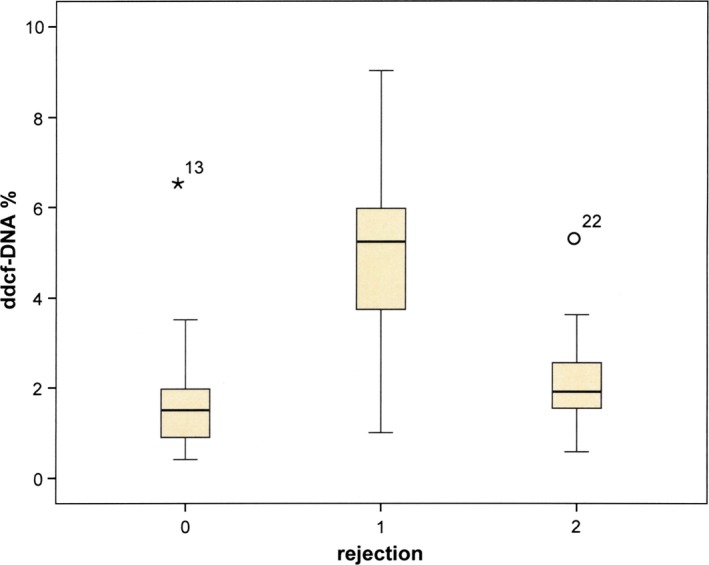
Box‐and‐whisker plots of the ddcfDNA levels. Box‐and‐whisker plots of the ddcfDNA levels in the plasma of recipients without (0), with (1) or with borderline (2) biopsy‐proven acute rejection, showing the median, 25th and 75th percentiles and the range, stars and open circles represent outlier observations (points that are beyond the quartiles by one and a half the interquartile range).
Figure 2.
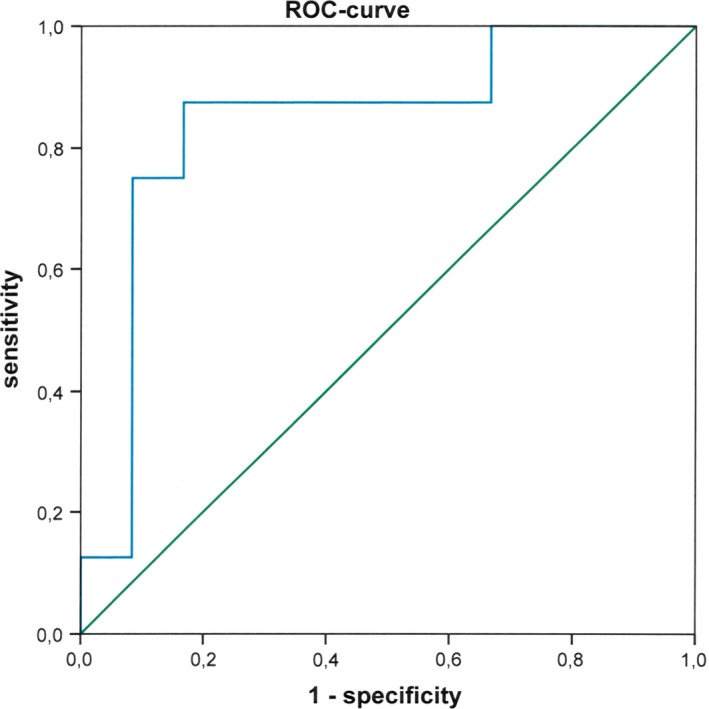
Receiver operating characteristic (ROC) curve analysis of ddcfDNA level. ROC curve analysis of the ddcfDNA level plots the true positive rate (sensitivity) versus the false‐positive rate (1‐specificity) at different discrimination thresholds. The area under curve amounted to 0.84.
Discussion
This study showed that the analysis of ddcfDNA based on INDELs was feasible for the diagnosis of AR in kidney transplant patients. We could demonstrate that smaller target amplicons are preferable to quantify ddcfDNA. Analysis of smaller amplicons showed significantly higher ddcfDNA levels in recipients with AR compared to those without and those with borderline AR.
The precise quantitation of ddcfDNA is technically challenging due to the low quantity and often limited quality of template DNA. Measured total cfDNA largely derives from hematopoietic cells which is larger in size than cfDNA originating from tissues including allograft transplants 29. Both types of cfDNA are fragmented with a majority of fragments below 200 bp, but a higher amount of fragments below 150 bp is nonhematopoietically derived tissue‐specific DNA. An approximately 10 bp periodicity in size has been observed in fragments of 142 bp and shorter, probably related to the distance of nuclease‐sensitive sites in the DNA double helix 29. The absolute quantification results of our study, the assessment of the degradation level and the donor or recipient‐specific Y‐STR profiles of the cfDNA samples confirm these findings in kidney transplant recipients.
In the transplant setting, a low amount of ddcfDNA has to be detected in a precise and reproducible manner despite a high background of recipient‐specific cfDNA. This detection is often complicated by lysis of blood cells, which leads to a further increase of recipient's DNA in the plasma and a further increase of background noise. Therefore, the preanalytical treatment of blood samples is critical in ddcfDNA testing as has already been shown for fetal cfDNA in maternal plasma 30. Cells have to be separated from plasma within a short period of time after venepuncture (mean of 150 min in this study). Preferably, blood should be drawn into special tubes with a gel plug or cell‐stabilization tubes to minimize increase of background cfDNA 31. The sensitivity and accuracy of qPCR highly depend on the amount of target DNA present in the well of the reaction plate. Approximately, ten cell equivalents are minimally required per well for a reproducible qPCR assay to avoid stochastical error 32. Previous studies investigating patients after KTx 9, 22 extracted DNA from 0.4 to 1 ml plasma and eluted it into 50–200 μl with a standard DNA extraction kit which is designed to extract mainly large fragments of genomic DNA 33. In contrast, in this study 5 ml plasma was eluted into 50 μl with a special kit designed for the extraction of small fragments of cfDNA, which resulted in a theoretically 12.5–20 times more concentrated DNA and more ddcfDNA target copies.
The degradation index (DI) strongly varies between recipients, and therefore no algorithm can be used to “normalize” the results to a common virtual target amplicon size. As demonstrated by Y‐STR profiling, a median threefold higher degraded ddcfDNA, mostly nonhematopoietically derived DNA, has to be quantified in the background of a lesser degraded recipient specific, mostly hematopoietically derived DNA. This influences the results of both the donor‐specific target assay and the reference assay. Choosing the target amplicon sizes close to those of the reference assay (107 bp) was a strategy to minimize additional artefacts due to different amplicon sizes of reference and target assays, which proved practicability in this study. Only in two recipients the larger target amplicon sizes revealed slightly higher amounts of ddcfDNA than the smaller ones: in patient 12 the result of the larger amplicon was within 1 SD, in patient 21, who exhibited the highest SD, it was within 2 SD of the smaller amplicon size target (Table 3). The reason for this observation is not known; however, the 10 bp periodicity in size of cfDNA fragments 29 may contribute as mentioned above.
Two outliers were found (one in each group) in patients without AR and borderline AR (patients 13 and 22, respectively). Patient 13 had no evidence of AR in his biopsy, however, had a high amount of ddcfDNA (6.5%) in the lower amplicon size assay (114 bp), but a very low amount (1.57%) in the larger amplicon size assay (128 bp). This corresponded to his very high DI of 9.80, which is the second highest of all recipients. The clinical follow‐up of this patient was inconspicuous, but his biopsy revealed also a moderate tubulus defect, focal tubulus cell necrosis and a mild arterio‐arteriolosclerosis. The second outlier was a borderline AR female recipient (patient 22) with 5.38% ddcfDNA in her plasma with glomerulitis and peritubular capillaritis in her biopsy. As a C4d‐negative humoral rejection was ruled out (no donor‐specific antibodies detected), another biopsy was taken 8 weeks later which unfortunately revealed a meanwhile developed thrombotic microangiopathy.
The INDEL qPCR method offers a fast, simple and cost‐effective technology. All steps (including cfDNA extraction and parallel donor/recipient typing) can be performed within 6–8 h on standard qPCR instruments, which are well‐established in most laboratories. Furthermore, it does not require gender mismatched donor/recipient pairs and can also be performed in multiple organ transplantations. The availability of pretransplant DNA of both donor and recipient is not a limitation, as it can be planned timely in advance. Genomic DNA can also be extracted from fresh or frozen cells from spleen or lymph nodes and amplified by whole genome amplification from input DNA of good quality. In contrast, the total process of massively parallel sequencing (MPS; target generation, library preparation, sequencing and data analysis) for the platform of the main manufacturers (Illumina, Thermo Fisher Scientific, Pacific Biosciences); however, needs 2–3 working days, special equipment, skilled staff and is rarely carried out on a daily basis, because cost‐effectiveness of these technologies can only be achieved by testing many samples simultaneously. On the other hand, digital PCR is a promising technology with the advantage, that pretransplant donor DNA is not necessary for quantification of ddcfDNA. However, a special digital PCR instrument is needed.
The determination of ddcfDNA as a noninvasive marker for graft rejection after kidney transplantation has been previously tested in a number of clinical studies. A recently published multicentre study by Bloom and colleagues evaluated ddcfDNA in 102 kidney recipients using SNPs 17. The authors reported a PPV and NPV for active rejection of 61% and 84%, respectively, at a cutoff of 1% ddcfDNA. This is comparable to the PPV and NPV of 64% and 94% obtained in our study by using the method‐adapted cutoff of 2.7% ddcfDNA. In contrast to Bloom's study, we were able to obtain ddcfDNA test results within 6–8 h using standard laboratory equipment. In 2018, Whitlam and colleagues reported on 61 kidney transplant recipients with for cause biopsies in the long‐term follow‐up (minimum 3 weeks after transplantation and up to 10 years), and determined ddcfDNA based on heterozygous copy number variation DNA sequences and droplet digital PCR 34. The group focused on chronic and acute ABMR, which was diagnosed in 13/61 patients. For the diagnosis of ABMR, the AUC using ddcfDNA reached 0.91 (95% CI 0.82–0.98). Similarly, Huang and colleagues just recently evaluated ddcfDNA levels in 63 patients with suspicion of rejection and reported higher levels in patients with ABMR compared to those with no rejection (P < 0.001) or cell‐mediated rejection (P = 0.01) with an AUC for ABMR of 0.82 (95% CI: 0.71–0.93) 35. In contrast to the abovementioned studies, we focused on the early post‐transplant phase with a median time to sample collection of 8 days after transplantation. This guaranteed a relatively homogenous patient group with the main clinical differential diagnosis being delayed graft function and acute AR.
There are important limitations to our study. First, this study was designed as a pilot study mainly concentrating on technical aspects of using qPCR INDEL detection for quantification of ddcfDNA. The patients were prospectively selected KTx recipients who received biopsies for clinical suspicion of AR. Therefore, they exhibit a higher likelihood of AR compared with a standard surveillance cohort. As a consequence, PPV and NPV must not be extrapolated to a general KTx population. Only future large prospective surveillance studies will be able to provide reliable PPV and NPV.
In conclusion, our results demonstrate feasibility of quantitation of ddcfDNA with smaller amplicon INDEL targets in recipient plasma of KTx patients. The present method is a useful tool to differentiate AR from other causes (non‐AR) of graft dysfunction. Future studies are warranted to confirm our results and test INDEL qPCR in a larger cohort of KTx patients.
Authorship
EMD, DK, GAB and WRM: were responsible for all aspects of the study, including the study design, analysis and interpretation of results, and writing of the article. NK, SR and TS: contributed and analyzed clinical data.
Funding
The authors have declared no funding.
Conflicts of interest
The authors have declared no conflicts of interest.
Supporting information
Appendix S1. Materials and methods.
Figure S1. Y‐STR profiles of the male ddcfDNA in the female recipient's plasma.
Figure S2. INDEL qPCR assay performance.
Table S1. A Y‐chromosomal 17‐locus STR profile was used to evaluate the donor and recipient specific degradation by the ratio of the allelic signal heights of smallest to the largest amplifiable.
Acknowledgements
The authors are grateful to A. Szymanska‐Stieger for sample collection, to D.W.M. Schwartz and H. Speckl for sharing their expertise in fetal cfDNA analysis, to M. Schönbacher for standing in for preanalytical treatment, to S. Wenda and I. Faé from G. Fischer's team for aliquoting pretransplant nucleic DNA samples and to F.F. Roch for discussing statistics.
References
- 1. Gielis EM, Ledeganck KJ, De Winter BY, et al Cell‐free DNA: an upcoming biomarker in transplantation. Am J Transplant 2015; 15: 2541. [DOI] [PubMed] [Google Scholar]
- 2. Racusen LC. Molecular techniques in transplantation. Transpl Proc 2004; 36: 731. [DOI] [PubMed] [Google Scholar]
- 3. Lo YM. Transplantation monitoring by plasma DNA sequencing. Clin Chem 2011; 57: 941. [DOI] [PubMed] [Google Scholar]
- 4. Lo YMD, Tein MSC, Pang CCP, Yeung CK, Tong KL, Hjelm NM. Presence of donor‐specific DNA in plasma of kidney and liver‐transplant recipients. Lancet 1998; 351: 1329. [DOI] [PubMed] [Google Scholar]
- 5. Tong YK, Lo YM. Diagnostic developments involving cell‐free (circulating) nucleic acids. Clin Chim Acta 2006; 363: 187. [DOI] [PubMed] [Google Scholar]
- 6. Lui YYN, Woo KS, Wang AYM, et al Origin of plasma cell‐free DNA after solid organ transplantation. Clin Chem 2003; 49: 495. [DOI] [PubMed] [Google Scholar]
- 7. Oellerich M, Walson PD, Beck J, Schmitz J, Kollmar O, Schutz E. Graft‐derived cell‐free DNA as a marker of transplant graft injury. Ther Drug Monit 2016; 38(Suppl. 1): S75. [DOI] [PubMed] [Google Scholar]
- 8. Knight SR, Thorne A, Lo Faro ML. Donor‐specific cell‐free DNA as a biomarker in solid organ transplantation. A systematic review. Transplantation 2019; 103: 273. [DOI] [PubMed] [Google Scholar]
- 9. Garcia Moreira V, Prieto Garcia B, Baltar Martin JM, Ortega Suarez F, Alvarez FV. Cell‐free DNA as a noninvasive acute rejection marker in renal transplantation. Clin Chem 2009; 55: 1958. [DOI] [PubMed] [Google Scholar]
- 10. Sigdel TK, Vitalone MJ, Tran TQ, et al A rapid noninvasive assay for the detection of renal transplant injury. Transplantation 2013; 96: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beck J, Bierau S, Balzer S, et al Digital droplet PCR for rapid quantification of donor DNA in the circulation of transplant recipients as a potential universal biomarker of graft injury. Clin Chem 2013; 59: 1732. [DOI] [PubMed] [Google Scholar]
- 12. De Vlaminck I, Martin L, Kertesz M, et al Noninvasive monitoring of infection and rejection after lung transplantation. Proc Natl Acad Sci USA 2015; 112: 13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Vlaminck I, Valantine HA, Snyder TM, et al Circulating cell‐free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med 2014; 6: 241ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon PM, Khan A, Sajid U, et al An algorithm measuring donor cell‐free DNA in plasma of cellular and solid organ transplant recipients that does not require donor or recipient genotyping. Front Cardiovasc Med 2016; 3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schutz E, Fischer A, Beck J, et al Graft‐derived cell‐free DNA, a noninvasive early rejection and graft damage marker in liver transplantation: a prospective, observational, multicenter cohort study. PLoS Med 2017; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grskovic M, Hiller DJ, Eubank LA, et al Validation of a clinical‐grade assay to measure donor‐derived cell‐free DNA in solid organ transplant recipients. J Mol Diagn 2016; 18: 890. [DOI] [PubMed] [Google Scholar]
- 17. Bloom RD, Bromberg JS, Poggio ED, et al Cell‐free DNA and active rejection in kidney allografts. J Am Soc Nephrol 2017; 28: 2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oellerich M, Shipkova M, Asendorf T, et al Absolute quantification of donor‐derived cell‐free DNA as a marker of rejection and graft injury in kidney transplantation: results from a prospective observational study. Am J Transplant 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gadi VK, Nelson JL, Boespflug ND, Guthrie KA, Kuhr CS. Soluble donor DNA concentrations in recipient serum correlate with pancreas‐kidney rejection. Clin Chem 2006; 52: 379. [DOI] [PubMed] [Google Scholar]
- 20. Zou J, Duffy B, Slade M, et al Rapid detection of donor cell free DNA in lung transplant recipients with rejections using donor‐recipient HLA mismatch. Hum Immunol 2017; 78: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Snyder TM, Khush KK, Valantine HA, Quake SR. Universal noninvasive detection of solid organ transplant rejection. Proc Natl Acad Sci USA 2011; 108: 6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee H, Park YM, We YM, et al Evaluation of digital PCR as a technique for monitoring acute rejection in kidney transplantation. Genomics Inform 2017; 15: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goh SK, Muralidharan V, Christophi C, Do H, Dobrovic A. Probe‐free digital PCR quantitative methodology to measure donor‐specific cell‐free DNA after solid‐organ transplantation. Clin Chem 2017; 63: 742. [DOI] [PubMed] [Google Scholar]
- 24. Mills RE, Luttig CT, Larkins CE, et al An initial map of insertion and deletion (INDEL) variation in the human genome. Genome Res 2006; 16: 1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alizadeh M, Bernard M, Danic B, et al Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real‐time quantitative polymerase chain reaction. Blood 2002; 99: 4618. [DOI] [PubMed] [Google Scholar]
- 26. Kletzel M, Huang W, Olszewski M, Khan S. Validation of chimerism in pediatric recipients of allogeneic hematopoietic stem cell transplantation (HSCT) a comparison between two methods: real‐time PCR (qPCR) vs. variable number tandem repeats PCR (VNTR PCR). Chimerism 2013; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loupy A, Haas M, Solez K, et al The Banff 2015 kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant 2017; 17: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) method. Methods 2001; 25: 402. [DOI] [PubMed] [Google Scholar]
- 29. Zheng YWL, Chan KCA, Sun H, et al Nonhematopoietically derived DNA is shorter than hematopoietically derived DNA in plasma: a transplantation model. Clin Chem 2012; 58: 549. [DOI] [PubMed] [Google Scholar]
- 30. Barrett AN, Zimmermann BG, Wang D, Holloway A, Chitty LS. Implementing prenatal diagnosis based on cell‐free fetal DNA: accurate identification of factors affecting fetal DNA yield. PLoS ONE 2011; 6: e25202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warton K, Yuwono NL, Cowley MJ, McCabe MJ, So A, Ford CE. Evaluation of streck BCT and PAXgene stabilised blood collection tubes for cell‐free circulating DNA studies in plasma. Mol Diagn Ther 2017; 21: 563. [DOI] [PubMed] [Google Scholar]
- 32. Stenman J, Orpana A. Accuracy in amplification. Nat Biotechnol 2001; 19: 1011. [DOI] [PubMed] [Google Scholar]
- 33. Legler TJ, Liu Z, Mavrou A, et al Workshop report on the extraction of foetal DNA from maternal plasma. Prenat Diagn 2007; 27: 824. [DOI] [PubMed] [Google Scholar]
- 34. Whitlam JB, Ling L, Skene A, et al Diagnostic application of kidney allograft‐derived absolute cell‐free DNA levels during transplant dysfunction. Am J Transplant 2019; 19: 1037. [DOI] [PubMed] [Google Scholar]
- 35. Huang E, Sethi S, Peng A, et al Early clinical experience using donor‐derived cell‐free DNA to detect rejection in kidney transplant recipients. Am J Transplant 2019; 19: 1663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Materials and methods.
Figure S1. Y‐STR profiles of the male ddcfDNA in the female recipient's plasma.
Figure S2. INDEL qPCR assay performance.
Table S1. A Y‐chromosomal 17‐locus STR profile was used to evaluate the donor and recipient specific degradation by the ratio of the allelic signal heights of smallest to the largest amplifiable.


