Abstract
Aim
To evaluate the impact of relevant patient‐level characteristics on the efficacy and safety of subcutaneous, once‐weekly semaglutide in subjects with type 2 diabetes.
Materials and Methods
Exploratory post hoc analyses of pooled SUSTAIN 1‐5 (phase 3a) randomized, controlled trials examined the change from baseline in HbA1c and body weight (BW), and the proportions of subjects achieving the composite endpoint (HbA1c < 7.0% [53 mmol/mol]), without weight gain or severe/blood glucose‐confirmed symptomatic hypoglycaemia at week 30 with semaglutide (0.5/1.0 mg) across clinically relevant patient subgroups: baseline HbA1c (≤7.5%, >7.5%‐8.0%, >8.0%‐8.5%, >8.5%‐9.0% and > 9.0%), background medications, diabetes duration and pancreatic beta‐cell function.
Results
Mean HbA1c (% point) reductions increased from lowest to highest HbA1c subgroups (−0.9%, −1.2%,‐1.5%, −1.7% and −2.3% [effect of subgroup within treatment: P = 0.247] for semaglutide 0.5 mg, and −1.1%, −1.4%, −1.9%, −2.1% and −2.7% [P = 0.045] for semaglutide 1.0 mg), with mean HbA1c ranges at week 30 of 6.3%‐7.3% and 6.1%‐6.9%, respectively. The corresponding BW reductions generally decreased with increasing baseline HbA1c (−4.4, −3.9, −3.9, −3.3 and −2.9 kg [P = 0.004], and −6.4, −5.9, −5.2, −4.5 and −4.8 kg [P < 0.001], respectively). HbA1c and BW reductions were consistently greater for semaglutide 1.0 mg versus 0.5 mg across background medication, diabetes duration and pancreatic beta‐cell function subgroups. Adverse events with semaglutide were consistent with the glucagon‐like peptide‐1 receptor agonist class, with gastrointestinal events the most common.
Conclusions
Semaglutide was consistently efficacious across the continuum of diabetes care in a broad spectrum of patient subgroups with a range of clinical characteristics.
Keywords: antidiabetic drug, glucagon‐like peptide‐1, glucagon‐like peptide‐1 analogue, glycaemic control, type 2 diabetes, weight control
1. INTRODUCTION
Current guidelines for type 2 diabetes (T2D) management prioritize the use of glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) in specific populations and as first injectable therapy before insulin.1, 2 The emphasis is on patient‐centred care and individualized treatment, including consideration of patients' clinical characteristics and co‐morbidities.1, 2 Clinical indicators of disease status in heterogeneous populations of adults with T2D, including glycaemic control (HbA1c), duration of disease, background antidiabetes medications and pancreatic beta‐cell function, may impact the efficacy and safety of GLP‐1RA therapy. An in‐depth evaluation of patient subgroups provides insight into whether distinct populations respond differently, and further guides the individualization of therapy.
Semaglutide (Novo Nordisk, Bagsværd, Denmark) is a subcutaneous (s.c.), once‐weekly (OW) GLP‐1 analogue for the treatment of T2D,3, 4 the efficacy and safety of which has been established in the global phase 3a and 3b Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) clinical trial development programme.5, 6, 7, 8, 9, 10, 11 Semaglutide s.c. OW showed superior reductions in HbA1c and body weight (BW) compared with placebo and active comparators.5, 6, 7, 8, 9 The SUSTAIN 1‐5 trials (n = 3918) represented the full continuum of diabetes care, including treatment‐naïve subjects, those on a background of oral antidiabetes drugs (OADs) and on insulin, with differences in baseline characteristics.5, 6, 7, 8, 9
The present post hoc exploratory analyses of data from the SUSTAIN trials aimed to assess the impact of clinical indicators of disease status (baseline HbA1c, background antidiabetes medications, diabetes duration and pancreatic beta‐cell function) on the efficacy and safety of semaglutide s.c. OW in subjects with inadequately controlled T2D.
2. MATERIALS AND METHODS
2.1. Individual trial designs
The phase 3a, multinational, randomized, controlled SUSTAIN 1‐5 trials compared semaglutide s.c. OW (0.5 mg and/or 1.0 mg) with placebo (SUSTAIN 1, monotherapy; SUSTAIN 5, add‐on to basal insulin) or active comparators (SUSTAIN 2, sitagliptin 100 mg; SUSTAIN 3, exenatide extended‐release 2.0 mg; SUSTAIN 4, insulin glargine titrated‐to‐target) in subjects with inadequately controlled T2D (comparator data were not included in this post hoc analysis) over 30 weeks (SUSTAIN 1, 4 and 5) or 56 weeks (SUSTAIN 2 and 3). The trials have been previously published (Table 1).5, 6, 7, 8, 9
Table 1.
Trial designs and baseline characteristics of subjects receiving semaglutide (SUSTAIN 1‐5)5, 6, 7, 8, 9
| Trial | SUSTAIN 1 | SUSTAIN 2 | SUSTAIN 3 | SUSTAIN 4 | SUSTAIN 5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Semaglutide | 0.5 mg/1.0 mg | 0.5 mg/1.0 mg | 1.0 mg | 0.5 mg/1.0 mg | 0.5 mg/1.0 mg | ||||
| Comparator | Placebo | Sitagliptin 100 mg | Exenatide ER 2.0 mg | Insulin glargine | Placebo (add‐on to basal insulin) | ||||
| Trial duration | 30 wk | 56 wk | 56 wk | 30 wk | 30 wk | ||||
| RCT design | Double‐blind, placebo‐controlled | Double‐blind, double‐dummy | Open‐label | Open‐label | Double‐blind, placebo‐controlled | ||||
| Previous therapy | Drug‐naïve | OAD | OAD | OAD | Insulin ± MET | ||||
| Therapy type | Monotherapy | Add‐on: MET ± TZD | Add‐on: 1‐2 OADs (MET ± TZD ± SU) | Add‐on: MET ± SU | Add‐on: Basal insulin ± MET | ||||
| Semaglutide | 0.5 mg | 1.0 mg | 0.5 mg | 1.0 mg | 1.0 mg | 0.5 mg | 1.0 mg | 0.5 mg | 1.0 mg |
| Subjects, n | 128 | 130 | 409 | 409 | 404 | 362 | 360 | 132 | 131 |
| Age, y | 54.6 ± 11.1 | 52.7 ± 11.9 | 54.8 ± 10.2 | 56.0 ± 9.4 | 56.4 ± 10.3 | 56.5 ± 10.3 | 56.7 ± 10.4 | 59.1 ± 10.3 | 58.5 ± 9.0 |
| Male, % | 47 | 62 | 51 | 50 | 54 | 54 | 51 | 56 | 59 |
| Body weight, kg | 89.8 ± 23.0 | 96.9 ± 25.6 | 89.9 ± 20.4 | 89.2 ± 20.7 | 96.2 ± 22.5 | 93.7 ± 21.4 | 94.0 ± 22.5 | 92.7 ± 19.6 | 92.5 ± 22.2 |
| BMI, kg/m2 | 32.5 ± 7.6 | 33.9 ± 8.4 | 32.4 ± 6.2 | 32.5 ± 6.6 | 34.0 ± 7.2 | 33.1 ± 6.5 | 33.0 ± 6.5 | 32.8 ± 6.0 | 32.0 ± 6.4 |
| HbA1c, % | 8.1 ± 0.9 | 8.1 ± 0.8 | 8.0 ± 0.9 | 8.0 ± 0.9 | 8.4 ± 0.9 | 8.1 ± 0.8 | 8.3 ± 0.9 | 8.4 ± 0.8 | 8.3 ± 0.8 |
| Diabetes duration, y | 4.8 ± 6.1 | 3.6 ± 4.9 | 6.4 ± 4.7 | 6.7 ± 5.6 | 9.0 ± 6.0 | 7.8 ± 5.1 | 9.3 ± 7.2 | 12.9 ± 7.6 | 13.7 ± 7.8 |
| HOMA‐B, %a | 39.7 ± 118.8 | 43.3 ± 114.4 | 32.8 ± 93.6 | 33.7 ± 98.1 | 39.0 ± 97.2 | ‐ | ‐ | ‐ | ‐ |
Note: Data were sourced from original trial reports if not publicly available. Data are means, or mean ± standard deviation, unless otherwise stated.
Abbreviations: BMI, body mass index; exenatide ER, exenatide extended‐release; HOMA‐B, homeostatic model assessment of beta‐cell function; MET, metformin; OAD, oral antidiabetes drug; RCT, randomized controlled trial; SU, sulphonylurea; TZD, thiazolidinedione.
HOMA‐B data are geometric mean ± coefficient of variation and were evaluated in SUSTAIN 1, 2 and 3 only.
2.2. Patient population
Key inclusion and exclusion criteria were similar across the SUSTAIN 1‐5 trials.5, 6, 7, 8, 9 Briefly, adult subjects (aged ≥18 years) with T2D (HbA1c: ≥7.0%‐10.0% [53‐86 mmol/mol] for SUSTAIN 1, 4 and 5 or ≥ 7.0%‐10.5% [53‐91 mmol/mol] for SUSTAIN 2 and 3) and an estimated glomerular filtration rate of ≥30 mL/min/1.73 m2 (SUSTAIN 1, 4 and 5) or ≥ 60 mL/min/1.73 m2 (SUSTAIN 2 and 3) were eligible for inclusion.
All trials were registered with http://clinicaltrials.gov (NCT02054897, NCT01930188, NCT01885208, NCT02128932 and NCT02305381) and conducted according to the International Conference on Harmonisation Good Clinical Practice guidelines12 and the Declaration of Helsinki.13 Trial protocols were approved by the institutional review boards and ethics committees at participating centres. Subjects provided written informed consent before trial‐related activities commenced.
2.3. Subgroup analyses
Key indicators of disease status were selected for post hoc analyses: baseline HbA1c, background antidiabetes medication, baseline diabetes duration and pancreatic beta‐cell function.
For baseline HbA1c analyses, subjects were divided into HbA1c categories (≤7.5% [≤58 mmol/mol], >7.5%‐8.0% [>58‐64 mmol/mol], >8.0%‐8.5% [>64‐69 mmol/mol], >8.5%‐9.0% [>69‐75 mmol/mol] and > 9.0% [>75 mmol/mol]). For diabetes duration analyses, diabetes duration categories (≤5 years, >5‐10 years and > 10 years) were assessed. Both HbA1c and diabetes duration subgroup analyses used pooled SUSTAIN 1‐5 data. Categories for HbA1c and diabetes duration analyses were selected based on clinical relevance, with HbA1c categories also reflecting the targets utilized in current clinical guidelines for diabetes management.1, 2, 14
Supporting the diabetes duration analyses, pancreatic beta‐cell function (glucose‐stimulated insulin secretion) was assessed using the homeostatic model assessment of beta‐cell function (HOMA‐B),15, 16 including pooled data from SUSTAIN 1‐3 only (HOMA‐B cannot be applied in subjects taking exogenous insulin, as in SUSTAIN 4 [insulin glargine comparator] and 5 [add‐on to basal insulin]).16 No specific thresholds for beta‐cell function were used and subjects were categorized into HOMA‐B tertiles: low (≤27.21%), intermediate (>27.21% to 51.71%) and high (>51.71%) endogenous beta‐cell function.
For background antidiabetes medication analyses, subjects were divided into subgroups (no background medication, metformin monotherapy, other OADs and basal insulin ± metformin). There were differences in background medications across trials: semaglutide was assessed in drug‐naïve subjects (SUSTAIN 1); as add‐on to existing stable background antidiabetes treatments (metformin, thiazolidinedione or both [SUSTAIN 2]; maximum two of metformin, thiazolidinedione and/or sulphonylurea [SUSTAIN 3]; metformin ± sulphonylurea [SUSTAIN 4]); and as add‐on to basal insulin ± metformin (SUSTAIN 5). Pooled SUSTAIN 2‐4 data were used for the metformin monotherapy and other OADs subgroups; data by trial were used for no background medication (SUSTAIN 1) and for basal insulin ± metformin (SUSTAIN 5).
2.4. Endpoints and assessments
Efficacy endpoints were similar across all trials in the pre‐planned analyses; the primary and secondary confirmatory endpoints were change in HbA1c (% point, hereafter referred to as %) and BW (kg), respectively, from baseline to end of treatment. Week 30 was the latest, common on treatment time point across the SUSTAIN 1‐5 trials and was selected as cut‐off for these analyses, allowing for inter‐trial comparisons. Supportive secondary endpoints included subjects achieving a triple composite endpoint of HbA1c < 7.0% (<53 mmol/mol) without weight gain or severe (according to the American Diabetes Association [ADA] classification)17 or blood glucose (BG)‐confirmed symptomatic hypoglycaemia (plasma glucose <3.1 mmol/L [56 mg/dL] with symptoms consistent with hypoglycaemia).5, 6, 7, 8, 9
Safety was assessed as the numbers of adverse events (AEs), serious AEs and AEs leading to premature treatment discontinuation in the subgroups within each treatment group. Specific AEs of clinical interest analyzed included gastrointestinal (GI) disorders and hypoglycaemic events.
2.5. Statistical analyses
Post hoc analyses were performed using pooled or by trial data as described in section 2.3. Efficacy analyses included on‐treatment without rescue medication data from all subjects contributing to the full analysis sets (randomized and exposed to at least one dose of the trial product) across the SUSTAIN 1‐5 trials. Changes from baseline analyses were adjusted for trial, country, treatment, baseline value and subgroup, using mixed model for repeated measurements (MMRM) imputations for missing data. Interaction effects were included for country and baseline value by trial and subgroup by treatment. Outcome values are presented as mean (standard error) for each of the categories analyzed, unless otherwise stated. The proportions of subjects achieving the composite endpoint (HbA1c < 7.0% [<53 mmol/mol], without weight gain or severe/BG‐confirmed symptomatic hypoglycaemia) were based on observed data and MMRM imputations for subjects with missing data.
The safety analysis set included data from subjects who were exposed to at least one dose of semaglutide and was based on on‐treatment data. The proportions of subjects experiencing at least one AE were adjusted per trial using the Cochran‐Mantel‐Haenszel method.
3. RESULTS
3.1. Subject disposition and baseline characteristics
Of the 3918 subjects with T2D who were randomized to semaglutide s.c. OW or comparator treatment in the SUSTAIN 1‐5 trials, 2465 were assigned to semaglutide and received at least one dose of the trial medication (0.5 mg, n = 1031 and 1.0 mg, n = 1434). Baseline characteristics by trial and treatment group (Table 1) and according to each subgroup analysis (Table S1) were broadly similar, with differences reflecting trial eligibility criteria and heterogeneity of the population with respect to the continuum of T2D care.
3.2. Efficacy by subgroup
3.2.1. Effect by baseline HbA1c (SUSTAIN 1─5 pooled)
Overall, the magnitude of the reductions in HbA1c was greater in subgroups with higher baseline HbA1c levels for both semaglutide 0.5 mg and 1.0 mg (Figure 1A). Reductions in HbA1c for semaglutide 0.5 mg ranged from −0.9% (baseline HbA1c ≤ 7.5%) to −2.3% (baseline HbA1c > 9.0%), and for semaglutide 1.0 mg ranged from −1.1% (baseline HbA1c ≤ 7.5%) to −2.7% (baseline HbA1c > 9.0%). There was a significant effect of the HbA1c subgroup within treatment for semaglutide 1.0 mg (P = 0.045), but not for 0.5 mg (P = 0.247). Similar HbA1c concentrations were achieved in all HbA1c subgroup categories, with estimated mean HbA1c levels at week 30 close to or less than 7.0% (53 mmol/mol).
Figure 1.

Efficacy endpoints at week 30 by baseline HbA1c subgroups (≤7.5%, >7.5%‐8.0%, >8.0%‐8.5%, >8.5%‐9.0% and > 9.0%): A, change from baseline in HbA1c and B, change from baseline in body weight. Abbreviations: BW, body weight; SEM, standard error of the mean. Data shown are mean ± SEM for the categories analyzed. Subgroups are categorized as ≤7.5% (≤58 mmol/mol); >7.5%‐8.0% (>58‐64 mmol/mol), >8.0%‐8.5% (>64‐69 mmol/mol), >8.5%‐9.0% (>69‐75 mmol/mol) and > 9.0% (>75 mmol/mol). Estimated changes are based on pooled data from the SUSTAIN 1‐5 trials
Conversely, the magnitude of the reductions in BW was generally lower in subgroups with higher baseline HbA1c (Figure 1B). Reductions in BW from baseline to week 30 were observed across all baseline HbA1c subgroups, ranging from −2.9 kg (baseline HbA1c > 9.0%) to −4.4 kg (baseline HbA1c ≤ 7.5%) with semaglutide 0.5 mg, and from −4.5 kg (baseline HbA1c > 8.5%‐9.0%) to −6.4 kg (baseline HbA1c ≤ 7.5%) with semaglutide 1.0 mg. There was a significant effect of the HbA1c subgroup within treatment for both semaglutide 0.5 mg (P = 0.004), and semaglutide 1.0 mg (P < 0.001). The proportions of subjects achieving the composite endpoint (HbA1c < 7.0% [<53 mmol/mol], without weight gain or severe/BG‐confirmed symptomatic hypoglycaemia) were consistently lower in subgroups with higher baseline HbA1c (Figure S1A), with ranges of 34.5%‐76.2% for semaglutide 0.5 mg and 44.9%‐80.2% for semaglutide 1.0 mg from the highest to the lowest baseline HbA1c subgroups.
3.2.2. Effect by background medication (SUSTAIN 1, SUSTAIN 2‐4 pooled, SUSTAIN 5)
Overall, reductions in HbA1c (Figure 2A) and BW (Figure 2B) were consistent across the four background antidiabetes medication subgroups (no background medication, metformin monotherapy, other OADs and basal insulin plus metformin), with slight variations in the other OADs subgroup category for both semaglutide doses. Reductions in HbA1c for semaglutide 0.5 mg ranged from −1.4% (background of other OADs) to −1.6% (treatment‐naïve), and for semaglutide 1.0 mg ranged from −1.7% (metformin monotherapy) to −1.9% (basal insulin ± metformin).
Figure 2.
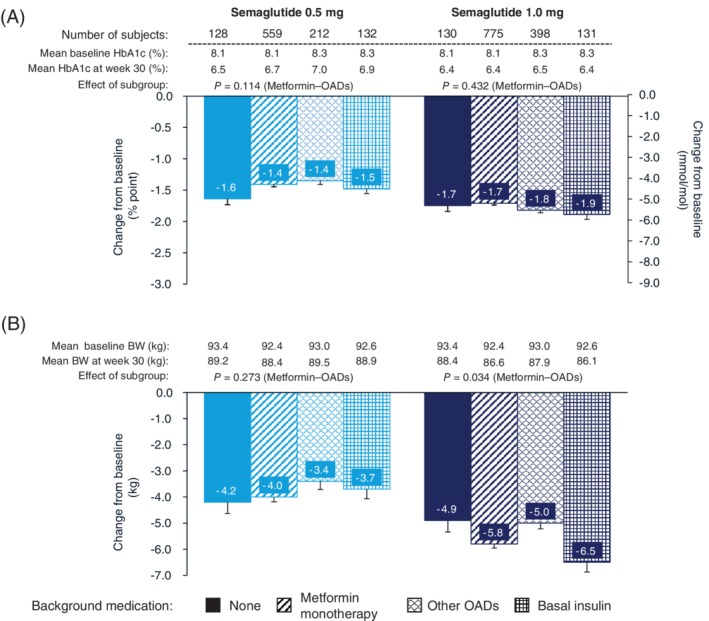
Efficacy endpoints at week 30 by background medication subgroups (no background medication [SUSTAIN 1], metformin monotherapy [pooled data from SUSTAIN 2‐4], other oral antidiabetes therapy [pooled data from SUSTAIN 2‐4] and basal insulin plus metformin [SUSTAIN 5]): A, change from baseline in HbA1c and B, change from baseline in body weight. Abbreviations: BW, body weight; OAD, oral antidiabetes drug; SEM, standard error of the mean. Data shown are mean ± SEM for the categories analyzed. Estimated changes are based on data from the SUSTAIN 1‐5 trials, with analyses performed on SUSTAIN 2‐4 collectively, but individually for SUSTAIN 1 and 5, so that P‐values are provided only for the comparison of metformin monotherapy and other OADs
Reductions in BW for semaglutide 0.5 mg ranged from −3.4 kg (other OADs) to −4.2 kg (treatment‐naïve), and for semaglutide 1.0 mg ranged from −4.9 kg (treatment‐naïve) to −6.5 kg (basal insulin plus metformin).
Because of the limitations of comparisons using pooled data and data from individual trials (as described in section 2.3), subgroup effects were only analyzed in the background medication subgroups of metformin monotherapy and other OADs (SUSTAIN 2‐4). There were no significant effects on the change from baseline in HbA1c (P = 0.114) or BW loss (P = 0.273) for the 0.5 mg semaglutide dose. For the 1.0 mg dose, a significant difference was observed for BW loss (P = 0.034), but not for change from baseline in HbA1c (P = 0.432), between metformin monotherapy and other OADs.
The proportions of subjects achieving the composite endpoint ranged from 42.0% to 66.4% for semaglutide 0.5 mg and 56.8% to 69.5% for semaglutide 1.0 mg (Figure S1B). The lowest proportions of subjects achieving the composite endpoint were observed in those receiving other OADs for both semaglutide doses.
3.2.3. Effect by baseline diabetes duration (SUSTAIN 1‐5 pooled)
Reductions in both HbA1c (Figure 3A) and BW (Figure 3B) were consistent, but with no clear pattern across the three diabetes duration subgroups (≤5, >5‐10, >10 years at baseline), with greater reductions observed for semaglutide 1.0 mg versus 0.5 mg. Mean reductions were − 1.7% to −1.8% and −5.4 to −5.7 kg with semaglutide 1.0 mg, and − 1.4% and − 3.7 to −3.8 kg with semaglutide 0.5 mg.
Figure 3.
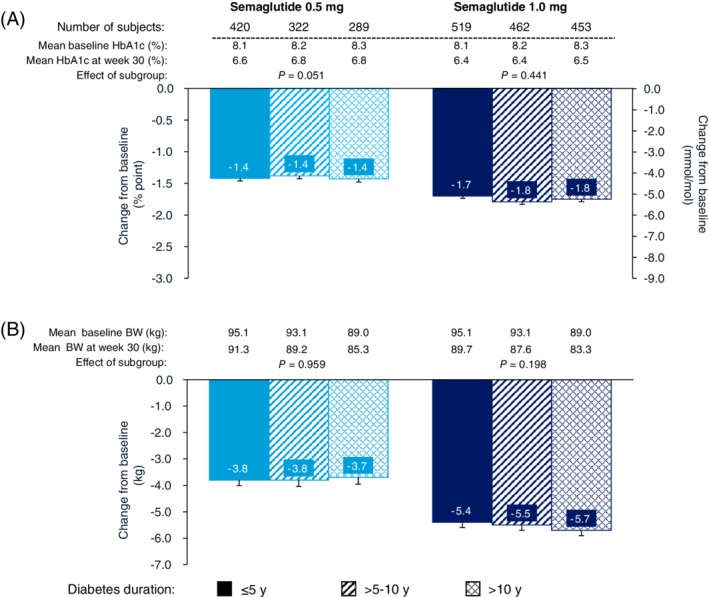
Efficacy endpoints at week 30 by diabetes duration subgroups (≤5 years, >5‐10 years and > 10 years): A, change from baseline in HbA1c and B, change from baseline in body weight. Abbreviations: BW, body weight; SEM, standard error of the mean. Data shown are mean ± SEM for the categories analyzed. Estimated changes are based on pooled data from the SUSTAIN 1‐5 trials
There were no significant effects of diabetes duration for either semaglutide dose on the changes in HbA1c (0.5 mg: P = 0.051; 1.0 mg: P = 0.441) or BW loss (0.5 mg: P = 0.959; 1.0 mg: P = 0.198).
For semaglutide 1.0 mg, the proportions of subjects achieving the composite endpoint were comparable across diabetes duration subgroups (62.5%‐67.8%). For semaglutide 0.5 mg, the lowest proportion of subjects achieving the composite endpoint was observed with a baseline diabetes duration of >10 years (49.5%) compared with those with a disease duration of <10 years (≥58.7%) (Figure S1C).
3.2.4. Baseline HOMA‐B (SUSTAIN 1‐3 pooled)
Reductions in HbA1c were observed in all baseline HOMA‐B tertiles for both semaglutide 0.5 mg and 1.0 mg (Figure S2A), with the magnitude of reductions decreasing from low to high HOMA‐B tertile (ranging from −1.3% to −1.7% and from −1.5% to −2.0%, respectively, for each semaglutide dose). There was no significant effect of HOMA‐B on HbA1c reductions with either semaglutide dose (0.5 mg: P = 0.948; 1.0 mg: P = 0.190).
In general, BW was reduced in all baseline HOMA‐B tertiles (Figure S2B). There was no apparent difference in the reduction in BW by tertile, ranging from −3.6 to −4.3 kg for semaglutide 0.5 mg, and from −4.9 to −6.1 kg for semaglutide 1.0 mg. The greatest reduction (−6.1 kg) was observed in the intermediate tertile group for semaglutide 1.0 mg; however, the effect of subgroup within treatment was non‐significant for both semaglutide doses (0.5 mg: P = 0.982; 1.0 mg: P = 0.116).
The proportions of subjects achieving the composite endpoint were similar across the baseline HOMA‐B tertiles (Figure S2C).
In each of the subgroup analyses, mean HbA1c and mean BW reductions, as well as the proportions of subjects achieving the composite endpoint, were greater with the higher dose (1.0 mg) than with the lower dose (0.5 mg) of semaglutide (Figures 1, 2, 3).
3.3. Safety outcomes
An overview of AEs, including GI AEs, is presented in Table 2. For each semaglutide dose, the proportions of subjects reporting AEs were generally similar across subgroups. The proportions of subjects reporting serious AEs were greater with longer versus shorter duration of diabetes at baseline (Table 2) and were greater in the highest baseline HOMA‐B tertile compared with the other two tertiles for semaglutide 1.0 mg (Table S2); no trend was observed for baseline HbA1c and the background medication subgroups.
Table 2.
Adverse events by subgroup (SUSTAIN 1‐5)
| Semaglutide 0.5 mg | Semaglutide 1.0 mg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 1031 n (%) | n = 1434 n (%) | |||||||||
| HbA1c, % | ≤7.5 | >7.5‐8.0 | >8.0‐8.5 | >8.5‐9.0 | >9.0 | ≤7.5 | >7.5‐8.0 | >8.0‐8.5 | >8.5‐9.0 | >9.0 |
| HbA1c, mmol/Mol | ≤58 | >58‐64 | >64‐69 | >69‐75 | >75 | ≤58 | >58‐64 | >64‐69 | >69‐75 | >75 |
| n | 332 | 216 | 178 | 134 | 171 | 409 | 300 | 250 | 179 | 296 |
| Total AEs (any grade) | 240 (72.3) | 155 (71.7) | 124 (69.6) | 93 (69.7) | 120 (70.6) | 302 (73.8) | 202 (67.9) | 172 (68.7) | 125 (70.2) | 215 (72.6) |
| Serious AEs | 19 (5.7) | 16 (7.4) | 12 (6.8) | 8 (5.9) | 12 (7.1) | 30 (7.2) | 19 (6.5) | 21 (8.5) | 16 (9.1) | 18 (6.2) |
| AEs leading to premature treatment discontinuation | 25 (7.5) | 12 (5.7) | 7 (3.9) | 12 (9.3) | 11 (6.4) | 43 (10.4) | 26 (8.8) | 16 (6.6) | 15 (8.5) | 19 (6.4) |
| Gastrointestinal AEs | 133 (40.1) | 88 (40.4) | 69 (38.8) | 51 (39.3) | 71 (41.7) | 186 (45.5) | 116 (39.0) | 88 (35.1) | 76 (42.0) | 117 (39.2) |
| Nausea | 63 (19.0) | 41 (18.7) | 26 (14.6) | 29 (22.1) | 32 (18.7) | 101 (24.8) | 63 (21.0) | 40 (16.0) | 34 (18.0) | 57 (18.9) |
| Diarrhoea | 35 (10.6) | 32 (14.7) | 26 (14.7) | 22 (17.2) | 20 (11.8) | 51 (12.6) | 43 (14.5) | 30 (12.0) | 25 (13.8) | 42 (14.0) |
| Vomiting | 23 (6.9) | 12 (5.7) | 10 (5.6) | 12 (9.2) | 13 (7.8) | 44 (10.8) | 21 (7.1) | 18 (7.2) | 20 (11.3) | 28 (9.3) |
| Severe or blood glucose‐confirmed hypoglycaemia | 8 (2.4) | 6 (3.0) | 6 (3.3) | 7 (4.9) | 7 (4.2) | 20 (4.9) | 13 (4.2) | 8 (3.3) | 10 (5.3) | 14 (4.7) |
| Background medication | None | MET monotherapy | Other OADs a | Basal insulin | None | MET monotherapy | Other OADs a | Basal insulin | ||
| n | 128 | 559 | 212 | 132 | 130 | 775 | 398 | 131 | ||
| Total AEs (any grade) | 82 (64.1) | 413 (73.9) | 146 (68.8) | 91 (68.9) | 73 (56.2) | 559 (72.1) | 300 (75.5) | 84 (64.1) | ||
| Serious AEs | 7 (5.5) | 41 (7.3) | 11 (5.2) | 8 (6.1) | 7 (5.4) | 63 (8.1) | 22 (5.5) | 12 (9.2) | ||
| AEs leading to premature treatment discontinuation | 8 (6.3) | 44 (7.9) | 9 (4.2) | 6 (4.5) | 7 (5.4) | 70 (9.0) | 34 (8.6) | 8 (6.1) | ||
| Gastrointestinal AEs | 49 (38.3) | 238 (42.6) | 89 (41.8) | 23 (17.4) | 50 (38.5) | 314 (40.6) | 174 (43.9) | 37 (28.2) | ||
| Nausea | 26 (20.3) | 108 (19.3) | 42 (19.8) | 15 (11.4) | 31 (23.8) | 164 (21.2) | 78 (19.7) | 22 (16.8) | ||
| Diarrhoea | 16 (12.5) | 77 (13.8) | 36 (16.9) | 6 (4.5) | 14 (10.8) | 98 (12.7) | 70 (17.6) | 9 (6.9) | ||
| Vomiting | 5 (3.9) | 46 (8.2) | 11 (5.2) | 8 (6.1) | 9 (6.9) | 69 (8.9) | 38 (9.6) | 15 (11.5) | ||
| Severe or blood glucose‐confirmed hypoglycaemia | 0 (0) | 8 (1.4) | 15 (7.1) | 11 (8.3) | 0 (0) | 11 (1.4) | 40 (10.0) | 14 (10.7) | ||
| Diabetes duration, y | ≤5 | >5‐10 | >10 | ≤5 | >5‐10 | >10 | ||||
| n | 420 | 322 | 289 | 519 | 462 | 453 | ||||
| Total AEs (any grade) | 301 (71.5) | 226 (70.4) | 205 (70.9) | 360 (69.6) | 319 (68.9) | 337 (74.6) | ||||
| Serious AEs | 27 (6.4) | 17 (5.3) | 23 (7.9) | 31 (5.9) | 37 (8.0) | 36 (8.1) | ||||
| AEs leading to premature treatment discontinuation | 26 (6.3) | 21 (6.5) | 20 (7.1) | 41 (7.8) | 38 (8.2) | 40 (8.9) | ||||
| Gastrointestinal AEs | 179 (42.4) | 126 (39.4) | 107 (37.2) | 205 (39.6) | 174 (37.6) | 204 (45.6) | ||||
| Nausea | 86 (20.5) | 59 (18.6) | 46 (15.9) | 106 (20.4) | 96 (20.7) | 93 (20.8) | ||||
| Diarrhoea | 56 (13.3) | 40 (12.5) | 39 (13.9) | 70 (13.5) | 64 (13.9) | 57 (12.5) | ||||
| Vomiting | 31 (7.3) | 17 (5.3) | 22 (7.5) | 46 (8.9) | 40 (8.7) | 45 (9.9) | ||||
| Severe or blood glucose‐confirmed hypoglycaemia | 11 (2.5) | 4 (1.2) | 19 (6.7) | 14 (2.8) | 20 (4.4) | 31 (6.8) | ||||
Note: %, proportion of subjects experiencing at least one event. Severe or blood glucose‐confirmed hypoglycaemia was defined as an episode that was severe according to the ADA classification or blood glucose‐confirmed by a plasma glucose value below 3.1 mmol/L (56 mg/dL) with symptoms consistent with hypoglycaemia.
Abbreviations: ADA, American Diabetes Association; AE, adverse event; MET, metformin; n, number of subjects in the safety analysis set; OAD, oral antidiabetes drug; SU, sulphonylurea; TZD, thiazolidinedione.
Other OADs included TZD monotherapy, MET+TZD or SU+TZD.
The proportions of subjects reporting treatment discontinuations because of AEs and GI AEs were generally similar across diabetes duration subgroups (Table 2) and baseline HOMA‐B tertiles (Table S2), but varied with no distinctive pattern across baseline HbA1c and background medication subgroups.
Overall, nausea was the most common GI AE across all subgroups; nausea was highest in treatment‐naïve subjects (not receiving background medication) and lowest in those on basal insulin ± metformin. The proportions of subjects reporting nausea were similar across diabetes duration subgroups with semaglutide 1.0 mg, but decreased with increasing diabetes duration for semaglutide 0.5 mg. There was no consistent pattern in the proportions of subjects reporting nausea across the baseline HbA1c subgroups and HOMA‐B tertiles. The incidences of severe/BG glucose‐confirmed hypoglycaemia were overall low and similar across HbA1c subgroups and diabetes duration; for background treatment, no incidences of hypoglycaemia were observed in treatment‐naïve subjects (ie, semaglutide monotherapy), while the highest rates were observed in those on a background of other OADs (7.1%‐10.0%) and basal insulin ± metformin (8.3%‐10.7%).
4. DISCUSSION
Semaglutide s.c. OW demonstrated consistently robust, clinically significant reductions in HbA1c and BW in the SUSTAIN 1‐5 trials.5, 6, 7, 8, 9 Encompassing patients from across the continuum of diabetes care, the SUSTAIN trials represent the heterogeneity of patients with T2D observed in everyday clinical practice, including wide‐ranging baseline HbA1c levels, background antidiabetes medications and diabetes durations.5, 6, 7, 8, 9 Understanding the impact these different clinical and disease characteristics have on the efficacy and safety of semaglutide can help clinical decision‐making and individualization of treatment. This exploratory post hoc analysis evaluated the effect of semaglutide in the context of selected subgroup characteristics.
Disease progression in diabetes is associated with worsening hyperglycaemia, indicated by increasing levels of HbA1c. Across the range of baseline HbA1c subgroups in this analysis, subjects with higher HbA1c values at baseline had the greatest reductions in HbA1c, with a statistically significant effect of baseline HbA1c in the semaglutide 1.0 mg treatment arm (P < 0.05). Baseline HbA1c is a well‐established predictor of glycaemic response for all antidiabetes treatments, even for non‐pharmacological interventions18, 19; this finding is consistent with published findings for other GLP‐1RAs, including dulaglutide,20, 21 liraglutide22 and lixisenatide.23 Importantly, from the clinical perspective, these observed differences in the magnitude of change from baseline in HbA1c across HbA1c subgroups resulted in a similar mean end‐of‐treatment HbA1c level. Indeed, irrespective of baseline HbA1c subgroup, HbA1c levels at week 30 were either close to, or less than, the ADA‐recommended target of <7.0% (<53 mmol/mol), reflecting the glucose‐dependent, antihyperglycaemic action of semaglutide and other GLP‐1RAs.24
The GLP‐1RA class promotes weight loss,25 primarily via central, appetite‐regulating mechanisms.24, 25 Subjects with the highest HbA1c at baseline lost less weight than those with lower HbA1c at baseline, albeit with clinically relevant absolute weight loss. A similar pattern, with less weight loss in subjects with higher baseline HbA1c levels, was also observed with dulaglutide in the AWARD programme,20, 21 and with liraglutide.26 These findings may be a result of treatment‐related increases in glycaemic control. In patients with particularly poor glycaemic control at baseline and associated caloric loss because of glucosuria, improving glycaemic control and reducing glucosuria may lead to mitigation of weight reduction.26, 27 Decreased protein turnover and reduced energy expenditure with improved glycaemic control can also lead to weight gain.27, 28 Furthermore, energy expenditure and resting metabolic rate decrease with weight loss, often accompanied by increased appetite.27, 28, 29 Contradicting a previous suggestion that modest weight loss in subjects with higher HbA1c may be related to concomitant insulin treatment and accompanying insulin‐related weight gain,21 the greatest weight loss was observed in these analyses in subjects on a background of basal insulin ± metformin.
GLP‐1RAs, such as semaglutide, are recommended as add‐on therapy to metformin and other OADs and as first injectable therapy in preference to insulin, unless contraindicated.1, 2 Clinically relevant reductions in HbA1c and BW were observed in all assessed background medication subgroups of the SUSTAIN 1‐5 trials and considerable weight loss was observed with semaglutide 1.0 mg, even in patients on a background of stable basal insulin ± metformin. The proportion of subjects achieving the composite endpoint of HbA1c < 7.0% (53 mmol/mol), without weight gain or severe/BG‐confirmed symptomatic hypoglycaemia has previously been reported for the SUSTAIN 1‐5 trials.30 In the analysis presented here, the proportion of subjects achieving the triple composite endpoint was lowest in subjects on a background medication of other OADs (including thiazolidinedione monotherapy, metformin plus thiazolidinedione or sulphonylurea plus thiazolidinedione), which may be a result of preceding weight gain associated with thiazolidinediones and sulphonylureas,31 or to an increased risk of hypoglycaemia associated with concomitant treatment with sulphonylureas.3
The intensification of antidiabetes therapy required over time is considered to be an indicator of diabetes severity and progression, and may be closely associated with diabetes duration. A number of published findings for other GLP‐1RAs suggest that patients with severe diabetes (ie, high baseline HbA1c levels20, 32) or a shorter disease duration (eg, less than 3 years32) may particularly benefit from GLP‐1RA treatment. In the analyses presented here, clinically relevant reductions in HbA1c and BW were consistently observed, even in subjects with long‐standing diabetes (ie, those with a duration of diabetes beyond 10 years) and the proportions of subjects achieving the triple composite endpoint were comparable across diabetes duration subgroups. There was a clear dose response in favour of semaglutide 1.0 mg versus 0.5 mg, in particular for subjects with a baseline diabetes duration of >10 years. A diminished insulinotropic effect of GLP‐1 in long‐standing diabetes is considered to be potentially a result of poor beta‐cell function,33, 34 which may result from secondary effects from other hormonal, metabolic or treatment‐related factors33, 34; this may explain the additional benefits that these subjects derive from the higher dose of semaglutide maximizing the agonistic effect of the GLP‐1RA. Notably in the present analysis, despite the recognized association of diabetes duration with progressively decreasing beta‐cell function,35 similar reductions in HbA1c and BW with semaglutide were generally observed across all levels of beta‐cell function (assessed by HOMA‐B). Consistent with previously reported semaglutide‐mediated improvements in beta‐cell function and glycaemic control,36 this finding suggests that even in patients with low beta‐cell function, clinically relevant reductions in HbA1c can be achieved with semaglutide treatment.
The safety profile of semaglutide was generally similar across all subgroups, indicating no clear association between baseline profile and risk of AEs. Overall, semaglutide was well‐tolerated regardless of baseline HbA1c, background medication, diabetes duration or baseline HOMA‐B, with no or low incidences of hypoglycaemia. As reported with other GLP‐1RAs,37 and for the SUSTAIN 1‐5 trials,5, 6, 7, 8, 9 the most common AEs leading to treatment discontinuation with semaglutide were GI5, 6, 7, 8, 9, 37; a known class effect of GLP‐1RA therapies,38, 39 these GI events typically occur during treatment initiation/escalation, but are transient, mild to moderate in severity, and diminish over time.5, 6, 7, 8, 9, 37 In these analyses, the proportions of subjects reporting GI AEs were similar across all analyzed subgroups. The safety findings with semaglutide are comparable with those previously reported for semaglutide and other GLP‐1RAs.38, 39 Lower rates of nausea were observed for subjects on a background of basal insulin ± metformin, possibly related to compound‐specific variation in GLP‐1RA‐associated GI AEs.37, 38
The strengths of these analyses include the large number of subjects in SUSTAIN 1─5 phase 3a trials from across the continuum of T2D care, representing patients on a range of treatments, from drug‐naïve to insulin therapy, with a broad spectrum of baseline characteristics. Although these analyses enable further understanding of the impact of clinical indicators of disease status on the efficacy and safety of semaglutide across different trials, limitations include the nature of pooled semaglutide data, which were analyzed without the inclusion of comparator data. As such, this is not a randomized comparison, but a comparison across post hoc‐defined subgroups. Confounding effects from underlying differences, including additive effects of different background therapies, may also be present. Similarly, although HOMA‐B analyses can be considered suitable for use in the presence of insulinotropic compounds,16 the data should be interpreted with caution and complementary to the diabetes duration subgroup analyses, as no adjustments were made for potential confounders, such as differences in baseline characteristics across HOMA‐B tertiles. Furthermore, some selection bias may result from trial inclusion/exclusion criteria. The duration of treatment (30 weeks) examined here may not accurately reflect longer term treatment, but provides an initial insight into treatment effects.
In conclusion, treatment with semaglutide s.c. OW was consistently efficacious, reducing HbA1c and BW to a clinically important extent, across subgroups by disease severity and progression in subjects with uncontrolled T2D. Semaglutide was well‐tolerated, with a low risk of hypoglycaemia, across the continuum of diabetes care and a broad range of clinical characteristics. These analyses show that the efficacy and safety of semaglutide is preserved, regardless of these patient characteristics and disease severity, and further support patient‐centred decision‐making in the treatment of T2D.
CONFLICT OF INTEREST
VRA received consultant fees from Adocia, AstraZeneca/Bristol Myers‐Squibb, Boehringer Ingelheim, Novo Nordisk, Sanofi and Zafgen, and grants from AstraZeneca/Bristol Myers‐Squibb, Calibra, Eisai, Fractyl, Janssen, Novo Nordisk, Sanofi and Theracos, and is a partner of an employee of Merck. MSC received honoraria from Novo Nordisk, Boehringer Ingelheim, Eli Lilly, Janssen and Merck Sharp & Dohme, and grants from Novo Nordisk, Novartis, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Leo, and Abbott, and served as an expert advisor to the National Institute for Health and Care Excellence (NICE), an unpaid board member of the Association for the Study of Obesity (ASO) and the Primary Care Academy of Diabetes Specialists (PCADS), a part‐time Medical Director at LighterLife (a commercial weight‐loss company), a partner at Clifton Medical Centre, and a director at RIO Weight Management, Ltd. JPF received honoraria from Eli Lilly, Merck, Novo Nordisk, Sanofi and grants from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, Pfizer and Theracos. S Madsbad received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk and Sanofi and grants from Boehringer Ingelheim and Novo Nordisk. JL served as a consultant for Eli Lilly and Novo Nordisk. JR received honoraria from Boehringer Ingelheim, Intarcia, Janssen, Novo Nordisk and Sanofi, and grants from Allergan, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Enanta, Genentech, GlaxoSmithKline, Intarcia, Janssen, Lexicon, Melior, Merck, Novo Nordisk, Oramed, PegBio, Pfizer and Sanofi. SCB received honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, Cellnovo, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Sanofi, received funding for the development of educational programmes from Cardiff University, http://doctors.net, Elsevier, Onmedica, Omnia‐Med, Medscape, provided expert advice for All‐Wales Medicines Strategy Group and NICE, UK, and is a shareholder of Glycosmedia. NLL, S Macura, and ST are employees of Novo Nordisk. LC and OT have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
All authors contributed to the analysis and interpretation of data, the writing and critical revision of the manuscript at all stages of development. All authors approved the final version.
Supporting information
Table S1. Baseline characteristics by subgroup (SUSTAIN 1‐5).
Table S2. Adverse events by HOMA‐B tertile (SUSTAIN 1‐3 trials).
Figure S1. Proportions of subjects achieving composite endpoint (HbA1c <7.0% [53 mmol/mol], with no weight gain and without severe or BG‐confirmed symptomatic hypoglycaemia) at week 30 by (A) baseline HbA1c subgroups, (B) background medication subgroups and (C) diabetes duration subgroups.
Figure S2. Efficacy endpoints at week 30 for HOMA‐B tertiles: (A) change from baseline in HbA1c, (B) change from baseline in body weight and (C) proportions of subjects achieving composite endpoint (HbA1c <7.0% [53 mmol/mol], without weight gain or severe or BG‐confirmed symptomatic hypoglycaemia).
ACKNOWLEDGMENTS
We thank all the participants, investigators and trial‐site staff who were involved in conducting the SUSTAIN 1‐5 trials. We thank Stacy Carl‐McGrath, PhD (AXON Communications), for medical writing and editorial assistance, who received compensation from Novo Nordisk.
Aroda VR, Capehorn MS, Chaykin L, et al. Impact of baseline characteristics and beta‐cell function on the efficacy and safety of subcutaneous once‐weekly semaglutide: A patient‐level, pooled analysis of the SUSTAIN 1‐5 trials. Diabetes Obes Metab. 2020;22:303–314. 10.1111/dom.13896
Funding information Novo Nordisk
REFERENCES
- 1. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of Medical Care in Diabetes‐2019. Diabetes Care. 2019;42:S90‐S102. [DOI] [PubMed] [Google Scholar]
- 2. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Novo Nordisk . Ozempic (semaglutide) Prescribing Information. 2019. https://www.novo-pi.com/ozempic.pdf. Accessed July 1, 2019.
- 4. Lau J, Bloch P, Schäffer L, et al. Discovery of the once‐weekly glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide. J Med Chem. 2015;58:7370‐7380. [DOI] [PubMed] [Google Scholar]
- 5. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251‐260. [DOI] [PubMed] [Google Scholar]
- 6. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341‐354. [DOI] [PubMed] [Google Scholar]
- 7. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2018;41:258‐266. [DOI] [PubMed] [Google Scholar]
- 8. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:355‐366. [DOI] [PubMed] [Google Scholar]
- 9. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103:2291‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 11. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275‐286. [DOI] [PubMed] [Google Scholar]
- 12. European Agency for the Evaluation of Medicinal Products . International Conference on Harmonisation‐World Health Organization Guideline for Good Clinical Practice [EMEA Web site] ICH Harmonised Tripartite Guideline Good Clinical Practice 2016. Accessed July 1, 2019.
- 13. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 14. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2019 executive summary. Endocr Pract. 2019;25:69‐100. [DOI] [PubMed] [Google Scholar]
- 15. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412‐419. [DOI] [PubMed] [Google Scholar]
- 16. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487‐1495. [DOI] [PubMed] [Google Scholar]
- 17. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose‐lowering therapies: a meta‐analysis of randomized clinical trials. Diabet Med. 2010;27:309‐317. [DOI] [PubMed] [Google Scholar]
- 19. Jones AG, Lonergan M, Henley WE, Pearson ER, Hattersley AT, Shields BM. Should studies of diabetes treatment stratification correct for baseline HbA1c? PLoS One. 2016;11:e0152428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gallwitz B, Dagogo‐Jack S, Thieu V, et al. Effect of once‐weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20:409‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pantalone KM, Patel H, Yu M, Fernandez Lando L. Dulaglutide 1.5 mg as an add‐on option for patients uncontrolled on insulin: subgroup analysis by age, duration of diabetes and baseline glycated haemoglobin concentration. Diabetes Obes Metab. 2018;20:1461‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henry RR, Buse JB, Sesti G, et al. Efficacy of antihyperglycemic therapies and the influence of baseline hemoglobin a(1C): a meta‐analysis of the liraglutide development program. Endocr Pract. 2011;17:906‐913. [DOI] [PubMed] [Google Scholar]
- 23. Blonde L, Chava P, Dex T, Lin J, Nikonova EV, Goldenberg RM. Predictors of outcomes in patients with type 2 diabetes in the lixisenatide GetGoal clinical trials. Diabetes Obes Metab. 2017;19:275‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819‐837. [DOI] [PubMed] [Google Scholar]
- 25. Shah M, Vella A. Effects of GLP‐1 on appetite and weight. Rev Endocr Metab Disord. 2014;15:181‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dahlqvist S, Ahlén E, Filipsson K, et al. Variables associated with HbA1c and weight reductions when adding liraglutide to multiple daily insulin injections in persons with type 2 diabetes (MDI Liraglutide trial 3). BMJ Open Diabetes Res Care. 2018;6:e000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pi‐Sunyer FX. Weight loss in type 2 diabetic patients. Diabetes Care. 2005;28:1526‐1527. [DOI] [PubMed] [Google Scholar]
- 28. Nair KS, Halliday D, Garrow JS. Increased energy expenditure in poorly controlled Type 1 (insulin‐dependent) diabetic patients. Diabetologia. 1984;27:13‐16. [DOI] [PubMed] [Google Scholar]
- 29. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621‐628. [DOI] [PubMed] [Google Scholar]
- 30. DeVries JH, Desouza C, Bellary S, et al. Achieving glycaemic control without weight gain, hypoglycaemia, or gastrointestinal adverse events in type 2 diabetes in the SUSTAIN clinical trial programme. Diabetes Obes Metab. 2018;20:2426‐2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng V, Kashyap SR. Weight considerations in pharmacotherapy for type 2 diabetes. J Obes. 2011;2011:984245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frías JP, Hardy E, Ahmed A, et al. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly alone, or dapagliflozin alone added to metformin monotherapy in subgroups of patients with type 2 diabetes in the DURATION‐8 randomized controlled trial. Diabetes Obes Metab. 2018;20:1520‐1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi‐phenomenon of impaired beta‐cell function? Diabetes. 2010;59:1117‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones AG, McDonald TJ, Shields BM, et al. Markers of beta cell failure predict poor glycemic response to GLP‐1 receptor agonist therapy in type 2 diabetes. Diabetes Care. 2016;39:250‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. UK Prospective Diabetes Study Group . U.K. prospective diabetes study 16: overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249‐1258. [PubMed] [Google Scholar]
- 36. Kapitza C, Dahl K, Jacobsen JB, Axelsen MB, Flint A. Effects of semaglutide on beta cell function and glycaemic control in participants with type 2 diabetes: a randomised, double‐blind, placebo‐controlled trial. Diabetologia. 2017;60:1390‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raccah D. Safety and tolerability of glucagon‐like peptide‐1 receptor agonists: unresolved and emerging issues. Expert Opin Drug Saf. 2017;16:227‐236. [DOI] [PubMed] [Google Scholar]
- 38. Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon‐like peptide‐1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19:336‐347. [DOI] [PubMed] [Google Scholar]
- 39. Madsbad S. Review of head‐to‐head comparisons of glucagon‐like peptide‐1 receptor agonists. Diabetes Obes Metab. 2016;18:317‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics by subgroup (SUSTAIN 1‐5).
Table S2. Adverse events by HOMA‐B tertile (SUSTAIN 1‐3 trials).
Figure S1. Proportions of subjects achieving composite endpoint (HbA1c <7.0% [53 mmol/mol], with no weight gain and without severe or BG‐confirmed symptomatic hypoglycaemia) at week 30 by (A) baseline HbA1c subgroups, (B) background medication subgroups and (C) diabetes duration subgroups.
Figure S2. Efficacy endpoints at week 30 for HOMA‐B tertiles: (A) change from baseline in HbA1c, (B) change from baseline in body weight and (C) proportions of subjects achieving composite endpoint (HbA1c <7.0% [53 mmol/mol], without weight gain or severe or BG‐confirmed symptomatic hypoglycaemia).


