Abstract
Dimethyl itaconate (DI) is a membrane‐permeable itaconate derivative with anti‐inflammatory functions. However, the anti‐inflammatory effect of DI has never been studied in fungal keratitis. In this study, we tested the protective effect of DI against fungal keratitis and assessed the role of NF‐E2‐related factor‐2 (Nrf2)/heme oxygenase‐1 (HO‐1) signaling in this process. Eyes of C57BL/6 (B6) mice were treated with 2 mm DI after infection with Aspergillus fumigatus. Human corneal epithelial cells (HCECs) were pretreated with 0.25 mm DI and then incubated with A. fumigatus. Clinical scoring, slit‐lamp photography, myeloperoxidase determination, flow cytometry and immunostaining were used to assess the disease response and treatment efficacy. PCR, Western blot and ELISA were used to assess the expression of interleukin‐1β (IL‐1β), chemokine (C–X–C motif) ligand 1, IL‐6, IL‐8, Nrf2 and HO‐1. In addition, quantification of viable fungi, absorbance assays and fluorimetry were used to measure DI fungistatic activity. We observed that DI‐treated eyes showed decreased clinical scores, fungal loads, polymorphonuclear neutrophil (PMN) infiltration and cytokine expression, compared with phosphate‐buffered saline‐treated infected eyes. DI treatment decreased the cytokine levels in infected corneas and in HCECs stimulated with A. fumigatus. Moreover, DI treatment increased Nrf2 and HO‐1 expression in corneas and nuclear Nrf2 accumulation in HCECs. DI‐induced cytokine downregulation was inhibited by pretreatment with an Nrf2 or HO‐1 inhibitor. Finally, DI treatment reduced the A. fumigatus absorbance and fungal mass. These data indicate that DI protects against fungal keratitis by limiting inflammation via the Nrf2/HO‐1 signaling pathway and that DI inhibits the growth of A. fumigatus.
Keywords: Dimethyl itaconate, fungal keratitis, inflammation, treatment
In this study, we showed that dimethyl itaconate (DI) exerts protective effects in fungal keratitis. On the one hand, DI alleviates inflammation by activating the NF‐E2‐related factor‐2 (Nrf2)/heme oxygenase‐1 (HO‐1) signaling pathway in Aspergillus fumigatus‐induced keratitis. On the other hand, DI reduces the fungal load and inhibits the growth of A. fumigatus.
Introduction
Fungal keratitis is a sight‐threatening disorder associated with pathogenic fungi. Agricultural trauma, extended contact lens usage and excessive use of broad‐spectrum antibiotics and corticosteroids have resulted in the increased incidence of this disease.1 Among pathogens, Fusarium and Aspergillus remain the leading causal factors of fungal keratitis.2 Excessive inflammation accelerates the course of fungal keratitis, which results in corneal ulceration and even blindness. However, current treatments, such as antifungal agents, are insufficient for alleviating disease outcomes and have severe adverse effects. Thus, finding novel helpful approaches toward fungal keratitis is desirable.
Itaconate is a metabolite produced by immune‐responsive gene 1 (Irg1) and has been found in lipopolysaccharide (LPS)‐treated macrophages and dendritic cells.3 Itaconate is involved in regulating immunity and limiting inflammation.3, 4, 5 Similarly, the membrane‐permeable derivatives of itaconate, including dimethyl itaconate (DI) and 4‐octyl itaconate (OI), exhibit anti‐inflammatory activity and DI has been used in several studies.6, 7 DI treatment suppresses interleukin‐6 (IL‐6) and inducible nitric oxide synthase expression in LPS‐stimulated bone marrow‐derived macrophages and protects against hypoxia‐induced cell death and cardiac ischemia‐reperfusion injury.6 DI limits cytokine production by activating NF‐E2‐related factor‐2 (Nrf2)/heme oxygenase‐1 (HO‐1).4 However, the anti‐inflammatory effect of DI has never been studied in fungal keratitis.
The Nrf2/HO‐1 signaling pathway plays a striking role in anti‐inflammatory activity.8, 9 Nonactivated Nrf2 remains in the cytoplasm, whereas activated Nrf2 accumulates in the nucleus.10, 11 Then, Nrf2 combines with antioxidant response elements and increases the production of downstream anti‐inflammatory and antioxidant enzymes, including HO‐1.12 The expression of cytokines and adherence of leukocytes to the vasculature are suppressed by Nrf2 and HO‐1 activation in LPS‐stimulated mouse corneas, while no change is observed in Nrf−/− mice.13 Nrf2 deficiency results in the inhibition of HO‐1 production and the aggravation of inflammatory disease outcomes.14, 15 In vitro, Nrf2 suppresses inflammation by inhibiting the transcription of genes.16 In addition, Nrf2 protects corneal epithelial cells against oxidative stress and accelerates corneal epithelial wound healing.17 These data confirm that the Nrf2/HO‐1 axis protects against inflammation‐associated diseases.
Based on the ability to impair isocitrate lyase (ICL), itaconate has been postulated to exhibit antimicrobial defense activity.18 ICL is a pivotal enzyme in glyoxylate bypass, which determines the survival and virulence of Aspergillus fumigatus and Candida albicans, as well as Mycobacterium tuberculosis in glucose‐poor environments.19, 20, 21 ICL has been observed in the hyphae of A. fumigatus and swollen conidia within macrophages in the aforesaid medium.21 Moreover, homologous protein is absent in humans, which allows drugs targeting ICL specific to microorganisms to be safely used in people. In this study, we demonstrate that DI can inhibit the growth of A. fumigatus.
We confirmed the anti‐inflammatory activity of DI in fungal keratitis and explored the mechanisms underlying the effects of DI. Our results show that DI treatment alleviated the outcome of fungal keratitis and inhibited polymorphonuclear neutrophil (PMN) infiltration. DI effectively downregulated the expression of cytokines in vitro and in vivo. In addition, DI treatment increased the production of Nrf2 and HO‐1 during the anti‐inflammatory process. Pretreatment with brusatol (BT, an inhibitor of Nrf2) or zinc protoporphyrin (ZnPP, an inhibitor of HO‐1) decreased the anti‐inflammatory effect of DI. Moreover, DI treatment inhibited the growth of A. fumigatus in vitro. Collectively, these results show that DI may play a protective role against fungal keratitis.
Results
DI treatment alleviated corneal disease in fungal keratitis
To test the functional role of DI in fungal keratitis, DI and phosphate‐buffered saline (PBS) treatment was initiated at 24 h postinjection (p.i.). Photographs taken with a slit‐lamp camera showed that DI‐treated corneas exhibited less opacity than PBS‐treated corneas at 3 and 5 days p.i. (Figure 1a). Clinical scores showed that compared with PBS treatment, 2 mm DI treatment reduced the corneal disease scores at both 3 and 5 days p.i. (Figure 1b). Fluorescein sodium was used to evaluate whether 2 mm DI could damage the corneal epithelium and the photographs showed no staining in DI‐treated corneas (Supplementary figure 1a, b). Because the severity of mouse fungal keratitis was the greatest at 3 days p.i., the corneas at 3 days p.i. were selected as the subject of follow‐up experiments. The fungal loads of infected corneas were measured by recording the fungal plate counts. Compared with PBS treatment, DI treatment effectively decreased the number of colony‐forming units (CFUs; Figure 1c) in infected corneas at 3 days p.i.
Figure 1.
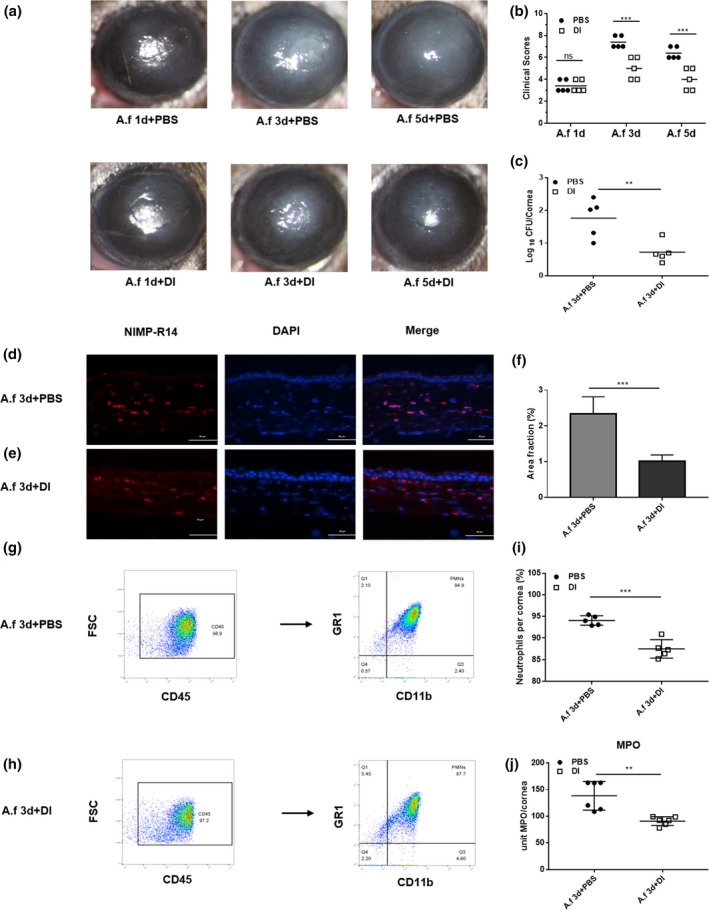
Dimethyl itaconate (DI) treatment of B6 mice. (a) Photographs of DI‐ and phosphate‐buffered saline (PBS)‐treated corneas (n = 5) taken with a slit lamp at 3 and 5 days postinfection (p.i.) showing reduced corneal opacity after DI treatment. Magnification, 25×. (b) A significant reduction in clinical scores was observed at 3 and 5 days p.i. in DI‐treated corneas (n = 5) compared with PBS‐treated corneas (n = 5). (c) The viable fungal plate count (n = 5) was reduced significantly at 3 days p.i. after DI versus PBS treatment. (d, e) Polymorphonuclear neutrophils (PMNs) were stained with NIMP‐R14, and fewer PMNs accumulated in the stroma of DI‐treated infected corneas (n = 3) than in that of PBS‐treated infected corneas (n = 3). Magnification, 400×. (f) Quantitative analysis of immunostaining images also demonstrated a reduction in PMN accumulation with DI treatment. (g–i) Flow cytometry showed that compared with PBS treatment, DI treatment decreased the percentage of PMNs in infected corneas. (j) Infected DI‐treated corneas (n = 6) showed a significantly reduced level of myeloperoxidase (MPO) at 3 days p.i. compared with PBS‐treated corneas (n = 6). The experiments were repeated two times to ensure reproducibility. P‐values are presented as the mean ± s.e.m. **P < 0.01, ***P < 0.001. A.f, Aspergillus fumigatus; DAPI, 4′,6‐diamidino‐2‐phenylindole; FSC, forward scatter; MPO, myeloperoxidase; ns, no significance.
To investigate whether DI treatment affects the infiltration of PMNs, we stained for NIMP‐R14 (a neutrophil marker) and measured the levels of myeloperoxidase (MPO) in infected corneas. Immunostaining demonstrated that fewer PMNs accumulated in the stroma of DI‐treated infected corneas than in that of PBS‐treated infected corneas (Figure 1d, e). Quantitative analysis of the images showed less staining in terms of the percent area in DI‐treated corneas (Figure 1f). Compared with PBS‐treated corneas, DI‐treated corneas displayed reduced MPO activity at 3 days p.i. (Figure 1j). In addition, flow cytometry was used to quantify PMN accumulation in corneas. Compared with PBS treatment, DI treatment significantly decreased the percentage of neutrophils in corneas infected with A. fumigatus (Figure 1g–i).
DI inhibited A. fumigatus‐induced cytokine production
Compared with PBS treatment, 2 mm DI treatment markedly reduced the messenger RNA (mRNA) levels of cytokines including IL‐1β (Figure 2a) and chemokine (C–X–C motif) ligand 1 (CXCL1; Figure 2b), in infected corneas of mice at 3 days p.i. The protein expression levels of IL‐1β p17 (Figure 2c, d) and CXCL1 (Figure 2e, f) in corneas were quantified using Western blot. Compared with PBS‐treated infected mouse corneas, DI‐treated infected corneas displayed decreased protein levels of IL‐1β p17 (Figure 2d) and CXCL1 (Figure 2f) at 3 days p.i.
Figure 2.
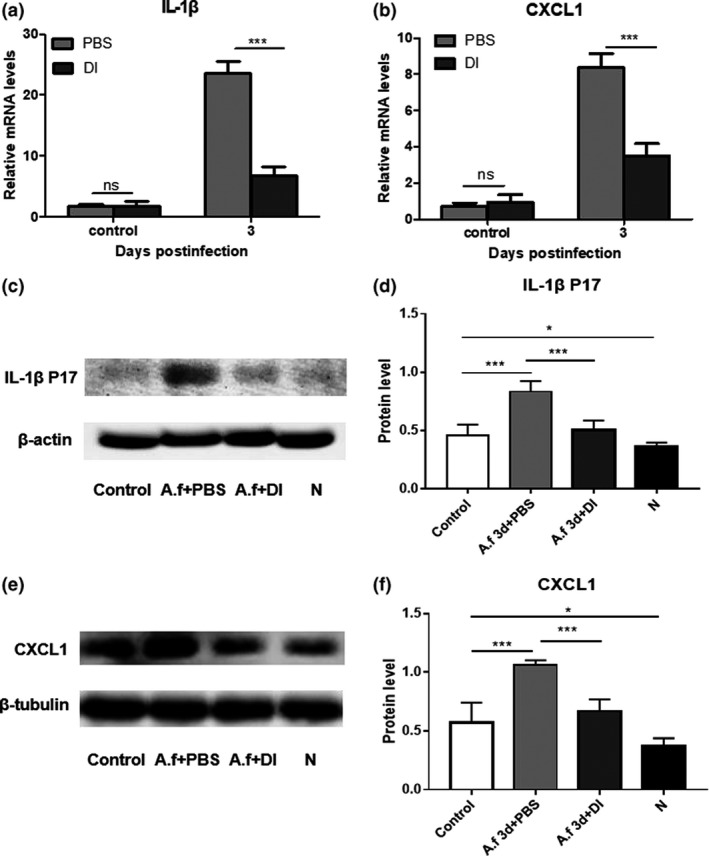
Dimethyl itaconate (DI) treatment inhibited the production of cytokines in infected corneas. Compared with phosphate‐buffered saline (PBS) treatment, DI treatment markedly decreased the relative messenger RNA (mRNA) levels of (a) interleukin‐1β (IL‐1β) and (b) chemokine (C–X–C motif) ligand 1 (CXCL1) in corneas (n = 5) infected with Aspergillus fumigatus at 3 days postinfection (p.i.). (c–f) Western blot analysis showed that compared with PBS treatment, DI treatment reduced the protein expression levels of IL‐1β p17 and CXCL1 in corneas (n = 5) at 3 days p.i. N indicates normal corneas without any treatment. The experiments were repeated two times to ensure reproducibility. P‐values are presented as the mean ± s.e.m. *P < 0.05, ***P < 0.001. A.f, Aspergillus fumigatus; N, normal corneas; ns, no significance.
Pretreatment with 0.25 mm DI largely inhibited A. fumigatus‐induced IL‐1β (Figure 3a), IL‐8 (Figure 3c) and IL‐6 (Figure 3e) mRNA expression in human corneal epithelial cells (HCECs). In addition, ELISA results demonstrated that A. fumigatus‐induced production of IL‐1β (Figure 3b), IL‐8 (Figure 3d) and IL‐6 (Figure 3f) protein was significantly inhibited by DI pretreatment. These results show that DI decreased the expression of these cytokines in infected HCECs.
Figure 3.
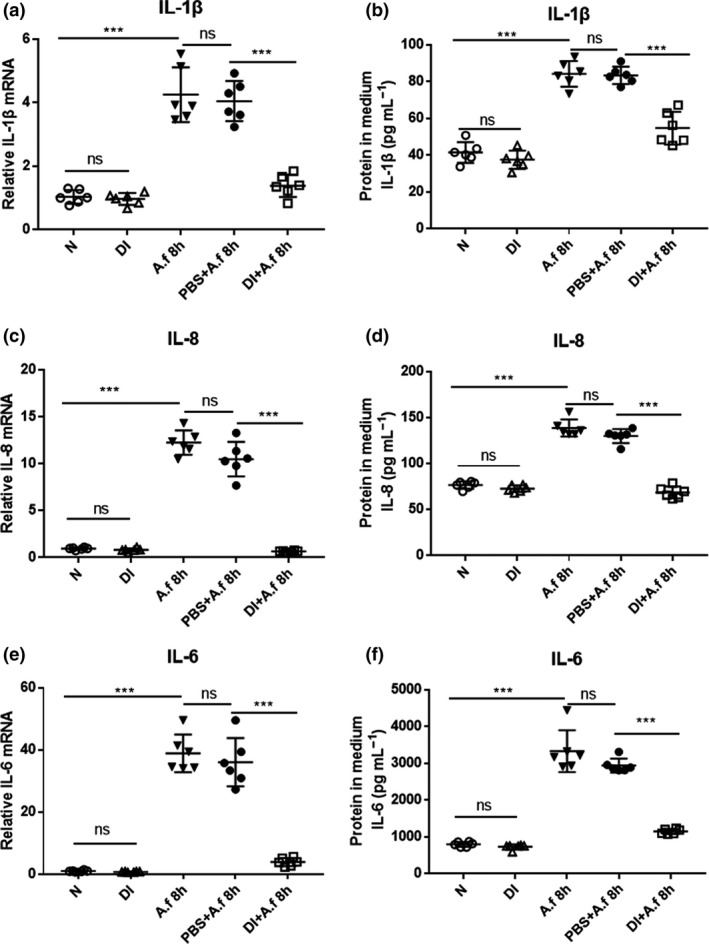
Dimethyl itaconate (DI) inhibited the production of cytokines in infected human corneal epithelial cells (HCECs). Compared with phosphate‐buffered saline (PBS) treatment, DI treatment notably decreased the relative messenger RNA (mRNA) levels of (a) interleukin‐1β (IL‐1β), (c) IL‐8 and (e) IL‐6 at 8 h postinfection (p.i.). ELISA results showed that compared with PBS treatment, DI treatment suppressed the relative protein levels of (b) IL‐1β, (d) IL‐8 and (f) IL‐6 at 24 h p.i. in HCECs (n = 6). The experiments were repeated two times to ensure reproducibility. P‐values are presented as the mean ± s.e.m. ***P < 0.001. A.f, Aspergillus fumigatus; N, normal HCECs; ns, no significance.
To assess whether DI is cytotoxic in mammalian cells, a Cell Counting Kit 8 (CCK‐8) assay was conducted on HCECs incubated with different concentrations of DI for 24 h. No difference was detected between 0.25 mm DI‐treated and control HCECs (Supplementary figure 1c).
DI exerted anti‐inflammatory effects by activating the Nrf2/HO‐1 signaling pathway
To explore the anti‐inflammatory mechanisms underlying the effects of DI in the A. fumigatus‐induced model of fungal keratitis, we analyzed the levels of Nrf2 and HO‐1 in vivo in mouse corneas (Figure 4a–f), and detected the location of Nrf2 in vitro in HCECs (Figure 4g–i). The results indicate that the expression levels of Nrf2 (Figure 4a–c) and HO‐1 (Figure 4d–f) were increased in infected corneas at 3 days p.i. compared with control corneas, and the levels were further upregulated by treatment with DI. Immunostaining showed that Nrf2 (green) was mainly located in the cytoplasm of infected control cells, whereas Nrf2 accumulated and was predominantly localized in the nucleus of DI‐pretreated HCECs (Figure 4g–i).
Figure 4.
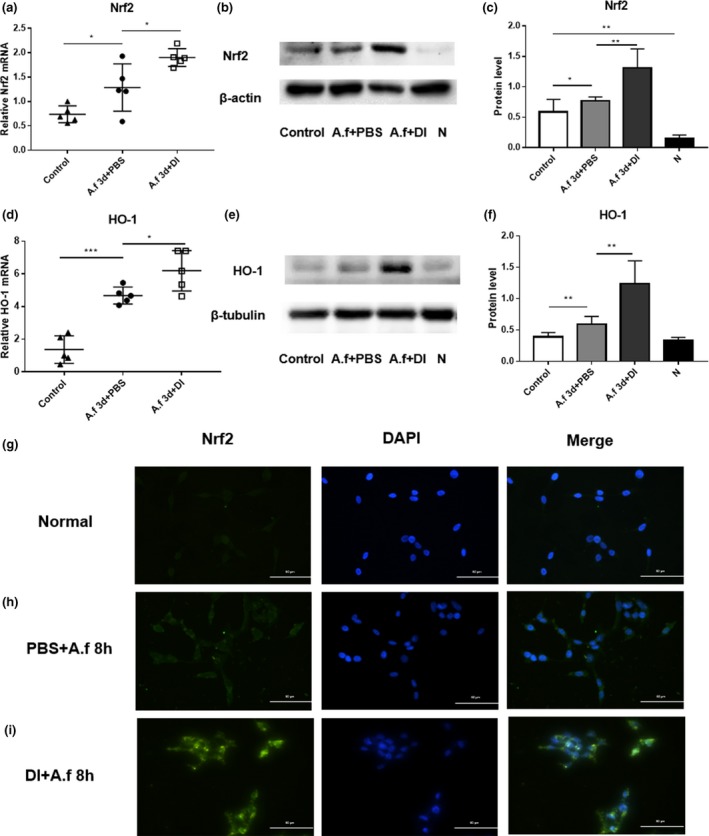
Dimethyl itaconate (DI) treatment increased the expression of NF‐E2‐related factor‐2 (Nrf2) and heme oxygenase‐1 (HO‐1). Panels a–f show data obtained in vivo from eyes, whereas the experiments described in panels g–i were performed in vitro with human cells. The messenger RNA and protein levels of (a–c) Nrf2 and (d–f) HO‐1 were increased in infected corneas (n = 5) at 3 days postinfection (p.i.) compared with those in uninfected control corneas (n = 5). Compared with phosphate‐buffered saline (PBS) treatment, DI treatment increased the expression of (a–c) Nrf2 and (d–f) HO‐1 in infected corneas. (g–i) Immunofluorescence staining showed that compared with PBS treatment, DI treatment induced the nuclear accumulation of Nrf2 in infected human corneal epithelial cells (n = 6) at 8 h p.i. Magnification, 400×. The experiments were repeated two times to ensure reproducibility. P‐values are presented as the mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. A.f, Aspergillus fumigatus; N, normal corneas or HCECs; DAPI, 4′,6‐diamidino‐2‐phenylindole.
HCECs were pretreated with BT (a specific Nrf2 inhibitor) before DI treatment and then incubated with A. fumigatus. The DI‐induced increase in the HO‐1 protein level was inhibited by BT treatment (Figure 5a, b). Reverse transcriptase‐PCR and ELISA results showed that the addition of BT or ZnPP reduced the effect of DI‐induced downregulation of cytokines, including IL‐1β (Figure 5c), IL‐8 (Figure 5e) and IL‐6 (Figure 5g) at the mRNA level and IL‐1β (Figure 5d), IL‐8 (Figure 5f) and IL‐6 (Figure 5h) at the protein level. Furthermore, the levels of IL‐1β, IL‐8 and IL‐6 were upregulated in HCECs pretreated with a combination of BT and ZnPP before DI treatment compared with HCECs pretreated with either BT or ZnPP (Supplementary figure 2a–c).
Figure 5.
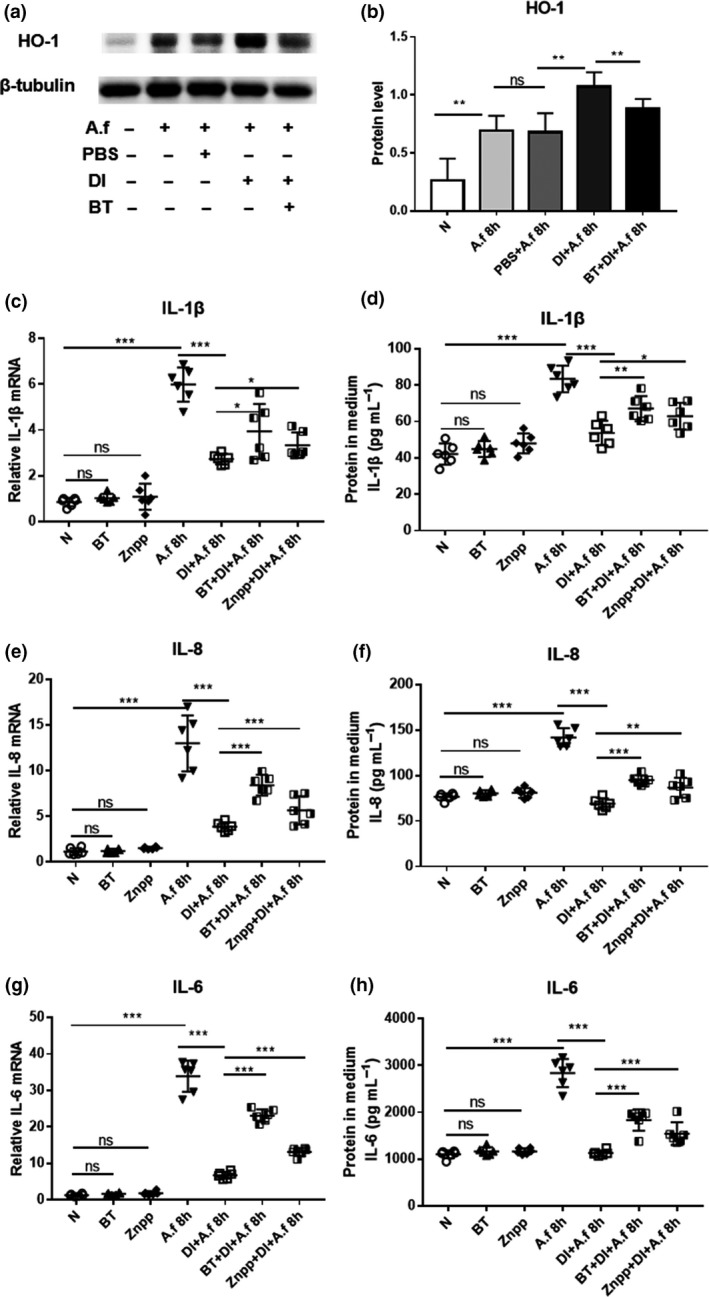
Human corneal epithelial cells (HCECs) were pretreated with brusatol (BT) or zinc protoporphyrin (ZnPP) in the presence of dimethyl itaconate (DI) and then incubated with Aspergillus fumigatus. (a, b) The upregulation of heme oxygenase‐1 (HO‐1) protein expression by DI was inhibited at 8 h postinfection (p.i.) by BT pretreatment. Reverse transcriptase (RT)‐PCR and ELISA results demonstrated that the DI treatment induced downregulation of (c, d) interleukin‐1β (IL‐1β), (e, f) IL‐8 and (g, h) IL‐6 messenger RNA (mRNA) and protein expression at 8 and 24 h p.i. in HCECs (n = 6) was inhibited by BT or ZnPP pretreatment. The experiments were repeated two times to ensure reproducibility. P‐values are presented as the mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. A.f, Aspergillus fumigatus; N, normal HCECs; ns, no significance; PBS, phosphate‐buffered saline.
DI inhibited the growth of A. fumigatus
To force fungal metabolism to use the glyoxylate shunt, A. fumigatus was cultivated in minimal defined medium supplemented with 2% glycerol as the sole carbon source. In this medium, the absorbance values of A. fumigatus incubated with different concentrations of DI for 48 h were measured. The absorbance values approximately represent the amount of A. fumigatus. With 2, 5 and 10 mm DI treatment for 48 h, the absorbance was reduced compared with that of A. fumigatus in medium alone (Figure 6a). Absorbance values indicating fungal growth were completely absent after DI treatment at 20 mm for 48 h (Figure 6a).
Figure 6.
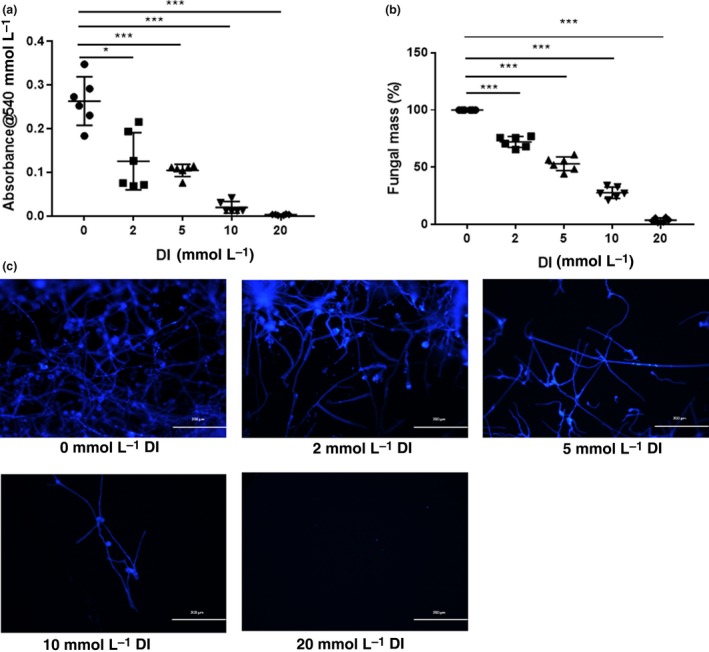
Dimethyl itaconate (DI) inhibited the growth of Aspergillus fumigatus. (a) Treatment with DI for 48 h inhibited A. fumigatus (n = 6) growth in a concentration‐dependent manner and completely inhibited visible growth at a 20 mm concentration. (b) DI decreased the fungal mass (n = 6), as measured by Calcofluor white staining, in a concentration‐dependent manner at 48 h. (c) Fluorescence imaging demonstrated that compared with no treatment, treatment with 2, 5, 10 and 20 mm DI for 48 h significantly reduced the amount of A. fumigatus. Magnification, 100×. The experiments were repeated two times to ensure reproducibility. P‐values are presented as the mean ± s.e.m. *P < 0.05, ***P < 0.001.
In addition, A. fumigatus was stained with Calcofluor white after incubation with 2, 5, 10 and 20 mm DI for 48 h. Calcofluor white is a fluorochrome that binds with chitin contained in the cell walls of fungi. The images of stained hyphae intuitively demonstrated that DI reduced the amount of A. fumigatus, and few hyphae were present after incubation with 20 mm DI for 48 h (Figure 6c). The fungal mass was quantified by analyzing the fluorescence intensity. DI treatment decreased the fungal mass in a concentration‐dependent manner (Figure 6b).
Discussion
DI, a cell‐permeable itaconate analog, exhibits anti‐inflammatory activity, and the Nrf2/HO‐1 signaling pathway plays a vital role in the process. In addition, itaconate reduces ICL activity and dampens the growth of microorganisms in ICL‐dependent environments.19, 20 Studies of DI and itaconate derivatives in immunity have indicated their therapeutic effects in multiple diseases, including psoriasis and systemic lupus erythematosus.7, 22 In our study, DI exhibited anti‐inflammatory effects during A. fumigatus infection and inhibited the growth of A. fumigatus, indicating the protective potential of DI in fungal keratitis.
We found that DI treatment alleviated inflammation and improved the outcome of fungal keratitis. Clinical scores and photographs taken with a slit lamp showed less corneal opacity in DI‐treated corneas than in PBS‐treated corneas. The viable fungal plate count was lower in DI‐treated corneas than in PBS‐treated corneas, which suggests that DI might alleviate corneal disease in fungal keratitis by suppressing fungal growth. Neutrophils are the predominant infiltrating cell type in keratitis. Neutrophils function by phagocytizing and killing invading microorganisms, but excessive neutrophil recruitment is associated with the progression of fungal keratitis. Itaconate is produced in response to the induction of Irg1.19 Nair et al. tested the effect of Irg1 on the accumulation of PMNs in the lungs during M. tuberculosis infection.23 Their results demonstrated that the lungs from Irg1−/− mice had increased percentages of infiltrated PMNs, indicating that Irg1 and its product itaconate function to limit inflammation by diminishing the accumulation of PMNs during M. tuberculosis infection.23 Therefore, we hypothesized that the anti‐inflammatory effects of DI treatment correlate with PMN infiltration. In this regard, our results show that treatment with DI effectively reduced MPO activity as well as PMN infiltration in infected corneas. Reduced PMN infiltration helps to impair microorganism growth by reducing bystander damage and limiting the nutrients available to microbes.24 These results provide evidence that DI treatment is beneficial to disease outcomes.
Our results indicate that DI treatment notably inhibited the production of IL‐1β and CXCL1, which was also correlated with the accumulation of PMNs, in corneas infected with A. fumigatus. This finding is consistent with findings of other studies showing that DI reduces cytokine production in LPS‐induced mastitis.25 In vitro tests showed that compared with PBS treatment, DI treatment significantly suppressed the A. fumigatus‐induced expression of IL‐1β, IL‐8 and IL‐6 in HCECs. Our results are in accordance with those of other work indicating that DI limits the production of cytokines in LPS‐treated macrophages.6 In addition, OI, another itaconate derivative, downregulates the levels of cytokines in mononuclear cells.22
Nrf2 and HO‐1 are activated in injured corneal epithelia and play a protective role in the wound healing process.17 In our study, we observed that the expression levels of Nrf2 and HO‐1 were upregulated in A. fumigatus‐infected corneas, suggesting that Nrf2 and HO‐1 are involved in the immune process of fungal keratitis. Our findings are in accordance with those of a previous study showing that LPS enhances Nrf2 and HO‐1 expression in lung tissues.26 Mills et al. demonstrated that itaconate and OI limit inflammation by activating Nrf2 and HO‐1.4 In addition, Nrf2 has been shown to regulate the innate immune response in an LPS‐induced uveitis model.13 Pretreatment with an Nrf2 activator reduces inflammatory mediator expression and increases antioxidant gene expression, indicating that the Nrf2/HO‐1 pathway has therapeutic value for uveitis, allergen‐induced asthma and neuroinflammation.13, 27, 28 In addition, the Nrf2/HO‐1 signaling pathway contributes to the anti‐inflammatory effects by regulating the recruitment of inflammatory cells.4, 29 Therefore, we aimed to identify the anti‐inflammatory mechanisms of DI. Consistent with previous studies, we observed that compared with PBS treatment, DI treatment enhanced the Nrf2 and HO‐1 protein levels in infected corneas. To further investigate the role of Nrf2 in the DI‐induced anti‐inflammatory effects, we observed the localization of Nrf2 protein in DI‐treated infected HCECs. Nonactivated Nrf2 remains in the cytoplasm. Once activated, Nrf2 translocates and accumulates in the cell nucleus where it elicits anti‐inflammatory effects by enhancing the transcription of anti‐inflammatory enzymes, including HO‐1.9, 29 Immunostaining showed that DI treatment increased the accumulation of Nrf2 in the nuclei of infected HCECs. Our results are consistent with those of studies showing that a derivative of itaconate induces the nuclear accumulation of Nrf2 in human macrophages.22
Our results further demonstrate that the activation of Nrf2 contributes to the DI‐induced anti‐inflammatory effects. Infected HCECs were pretreated with BT or ZnPP before DI treatment. We found that the DI‐induced upregulation of HO‐1 expression was inhibited by BT pretreatment in HCECs. These data are consistent with those of studies showing that OI‐induced HO‐1 expression is blocked in Nrf2‐deficient macrophages or when Nrf2 is silenced.22 We show here that in infected HCECs, BT or ZnPP treatment separately blocked the DI‐induced inhibition of IL‐1β, IL‐8 and IL‐6. These results are consistent with those of other work showing that the downregulation of cytokines induced by OI is almost completely reversed in Nrf2‐deficient macrophages.4, 22 Furthermore, the effect of blocking the DI‐induced inhibition of cytokines was much greater in HCECs pretreated with a combination of BT and ZnPP than in HCECs treated with either BT or ZnPP separately. These findings indicate that DI exerts anti‐inflammatory effects via the Nrf2/HO‐1 axis during A. fumigatus infection.
In addition to its anti‐inflammatory activity, DI treatment inhibited the growth of A. fumigatus. Previous studies have indicated that itaconate exhibits antimicrobial activity by inhibiting ICL, a key glyoxylate bypass enzyme.18 The glyoxylate shunt is absent in animals but determines the survival and growth of pathogens in low‐glucose environments.30 A growing body of evidence has highlighted the antimicrobial role of itaconate against pathogens during inflammation.31 We hypothesized that DI would elicit antifungal effects in low‐glucose environments. We found that DI treatment inhibited the growth of A. fumigatus in the aforementioned medium in a concentration‐dependent manner. Similarly, Michelucci et al. demonstrated that itaconate effectively inhibits the growth of M. tuberculosis.19 These data indicate the antifungal effect of DI in vitro.
In summary, our results show that DI treatment alleviates inflammation by activating the Nrf2/HO‐1 signaling pathway in A. fumigatus‐induced keratitis. Furthermore, DI reduces the fungal load and inhibits the growth of A. fumigatus. These data suggest that DI exerts protective effects in fungal keratitis.
Methods
Animals and corneal infection
C57BL/6 mice (female, 8‐week old) were purchased from Jinan Pengyue Laboratory Animal Co., Ltd. (Jinan, China). The standard A. fumigatus strain 3.0772 was purchased from the China General Microbiological Culture Collection Center (Beijing, China). All mouse treatments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Mice were anesthetized by intraperitoneal injection with 8% chloral hydrate. The central corneal epithelium of the left eye was removed within a 2‐mm diameter. A 5 μL aliquot containing 1 × 108 CFU mL−1 of A. fumigatus was coated on the ocular surface, and a soft contact lens was placed. Subsequently, the eyelids of the left eye were sutured.
DI treatment of mice
The left eye of each C57BL/6 mouse (n = 5/group) was injected with 5 μL of DI (2 mm; Sigma, Santa Clara, CA, USA) or sterile PBS (Solarbio, Beijing, China) subconjunctivally at 1 day p.i., and treated topically with 3 μL of DI (2 mm; Sigma) or PBS two times daily from day 2 p.i. The epithelium of the control corneas was removed without A. fumigatus stimulation. No treatment was administered to normal corneas.
Cell culture and A. fumigatusstimulation
HCECs were cultured in Dulbecco's Modified Eagle Medium (Gibco, San Diego, CA, USA) containing 10% fetal bovine serum (Gibco) at 37°C with 5% CO2. When HCECs reached 80% confluence, they were transferred into serum‐free Dulbecco's Modified Eagle Medium and stimulated with 75% ethanol‐killed A. fumigatus hyphae (5 × 106 CFU mL−1) for 8 or 24 h. HCECs were cultured in 6‐ or 12‐well plates for real‐time reverse transcriptase‐PCR, Western blot, immunofluorescence and ELISA. HCECs were plated in 96‐well plates for CCK‐8 assays. The mRNA levels of IL‐1β, IL‐8 and IL‐6 in HCECs were assessed by reverse transcriptase‐PCR at 8 h p.i., Nrf2 protein in HCECs was detected by immunofluorescence at 8 h p.i. and HO‐1 expression was tested by Western blot at 8 h p.i. The protein levels of cytokines were detected by ELISA at 24 h p.i. The optical density of the medium was measured by CCK‐8 assay at 24 h.
DI treatment of HCECs
HCECs were pretreated with DI (0.25 mm; Sigma) or PBS (control) for 2 h before stimulation with A. fumigatus hyphae. The mRNA levels of IL‐1β, IL‐8 and IL‐6 in HCECs were tested at 8 h p.i. and the protein levels of these cytokines were detected at 24 h p.i.
Quantitation of corneal PMNs
Mouse corneas (n = 6/group) were removed at 3 days p.i. and homogenized in 1.0 mL of the second reagent of an MPO test kit (Njjcbio, Nanjing, China). After being mixed and then incubated in a water bath, the absorbance was measured immediately at 460 nm. The units of MPO per cornea were calculated by analyzing the slope of the line.
Quantification of viable fungi
Mice were killed at 3 days p.i., and DI‐ or PBS‐treated infected corneas were harvested (n = 5/group). Each mouse cornea was homogenized in sterile saline and plated in triplicate on Sabouraud dextrose agar plates (Hopebio, Beijing, China). The plates were incubated overnight at 37°C, and then the number of visible fungal colonies on the plates was counted. The results are reported as log10 CFU/cornea ± s.e.m.
Immunofluorescence staining
Mouse eyeballs were removed (n = 3/group) at 3 days p.i. and frozen in optimal cutting temperature compound (Sakura Tissue‐Tek, Torrance, CA, USA). The immunofluorescence protocol for corneal tissue has been described in previous publications.32 Anti‐NIMP‐R14 antibody (1:100; Santa Cruz, CA, USA) was used as the primary antibody. Cy3‐conjugated goat antirat antibody (1:300; CWBiotech, Beijing, China) was used as the secondary antibody.
HCECs were seeded onto poly‐l‐lysine‐coated slips. After treatment with DI (0.25 mm) or PBS for 2 h, HCECs were incubated with A. fumigatus at 37°C for 8 h. The immunocytochemistry protocol has been described in previous studies.33
The anti‐Nrf2 antibody (Abcam, Cambridge, MA, USA) was used as the primary antibody. Fluorescein isothiocyanate‐conjugated donkey antirabbit antibody (1:100; Bioss, Beijing, China) was used as the secondary antibody.
Flow cytometry
Mouse eyeballs were collected (n = 5) at 3 days p.i., and the corneas were dissected around the scleral–limbal region. The protocol of corneal flow cytometry has been previously described.34 The gate was set on the CD45+ population. The primary antibodies used in this experiment were CD45‐PercP, CD11b‐fluorescein isothiocyanate and Ly6G‐PE (BioLegend, San Diego, CA, USA).
Real‐time reverse transcriptase‐PCR
Total RNA from mouse corneas and HCECs was extracted. The PCR method was based on previous studies.32 The primer details are listed in Supplementary table 1.
Western blot analysis
The total protein from mouse corneas and HCECs was extracted and prepared in a standardized manner for Western blotting. The Western blot protocol has been described in previous publications.32 The primary antibodies used in this study were antibodies against β‐tubulin (1:500; Elabscience, Wuhan, China), β‐actin (1:5000; Elabscience), IL‐1β (1:500; R&D Systems, Minneapolis, MN, USA), CXCL1 (1:2000; Thermo Fisher Scientific, Waltham, MA, USA), Nrf2 (1:1000; Abcam) and HO‐1 (1:250; Abcam). The corresponding secondary antibody (1:2000, Elabscience) was used to stain the blots. Full‐size images are presented in Supplementary figure 3.
ELISA
HCEC supernatants and mouse corneas were collected and centrifuged. ELISA kits (R&D Systems) were used to detect IL‐1β, IL‐8 and IL‐6 protein levels, and 100 μL of sample in the appropriate diluent was added per well. The optical density of each well was determined immediately at 450 nm with a reference wavelength of 570 nm.
HCEC Nrf2 and HO‐1 inhibitor treatments
HCECs were pretreated with the Nrf2 inhibitor BT (15 nm; Sigma) or HO‐1 inhibitor ZnPP (10 μm; Sigma) for 1 h prior to DI treatment. Then, HCECs were incubated with A. fumigatus for 8 h.
A. fumigatus growth analysis
A. fumigatus was cultivated in minimal defined medium supplemented with 2% glycerol as the sole carbon source in a black‐walled 96‐well plate with a clear bottom (Corning Inc., Corning, NY, USA) at 37°C. A. fumigatus was incubated with different concentrations of DI (2, 5, 10 and 20 mm) for 48 h. Growth was measured by spectrophotometric reading at 540 nm. The supernatant of each well was removed, followed by treatment with 50 μL of the chitin‐binding stain Calcofluor white (Sigma) for 10 min at room temperature. The fluorescence intensity of each well was quantified. Images were captured by a Zeiss Axio Vert microscope (Zeiss, Oberkochen, Baden‐Württemberg, Germany).
CCK‐8 assay
HCECs were incubated with different concentrations of DI (0.125, 0.25 and 0.5 mm) or PBS vehicle in 96‐well plates, and cell death was measured by CCK‐8 assay (Solarbio). After incubation with DI for 24 h, the CCK‐8 solution was added and incubated for 2 h. The absorbance was measured at a wavelength of 490 nm.
Statistical analysis
The Student's t‐test was applied to evaluate differences between two groups. One‐way ANOVA was used to analyze differences among three or more groups. Differences were considered significant at P ≤ 0.05. All data were obtained from repeated experiments and are shown as the mean ± s.e.m.
Conflict of Interest
The authors declare no competing financial interests.
Supporting information
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos 81870632, 81500695, 81700800 and 81800800) and the Natural Science Foundation of Shandong Province (ZR2017MH008 and ZR2017BH025).
References
- 1. Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology 2006; 113: 1943–1948. [DOI] [PubMed] [Google Scholar]
- 2. Jiang N, Zhao G, Lin J, et al Indoleamine 2,3‐dioxygenase is involved in the inflammation response of corneal epithelial cells to Aspergillus fumigatus infections. PLoS One 2015; 10: e0137423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams NC, O'Neill LAJ. A role for the krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol 2018; 9: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mills EL, Ryan DG, Prag HA, et al Itaconate is an anti‐inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018; 556: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu XH, Zhang DW, Zheng XL, Tang CK. Itaconate: an emerging determinant of inflammation in activated macrophages. Immunol Cell Biol 2019; 97: 134–141. [DOI] [PubMed] [Google Scholar]
- 6. Lampropoulou V, Sergushichev A, Bambouskova M, et al Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab 2016; 24: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bambouskova M, Gorvel L, Lampropoulou V, et al Electrophilic properties of itaconate and derivatives regulate the IκBζ‐ATF3 inflammatory axis. Nature 2018; 556: 501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nikam A, Ollivier A, Rivard M, et al Diverse Nrf2 activators coordinated to cobalt carbonyls induce heme oxygenase‐1 and release carbon monoxide in vitro and in vivo . J Med Chem 2016; 59: 756–762. [DOI] [PubMed] [Google Scholar]
- 9. Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 585–597. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki T, Yamamoto M. Molecular basis of the Keap1‐Nrf2 system. Free Radic Biol Med 2015; 88: 93–100. [DOI] [PubMed] [Google Scholar]
- 11. Li W, Kong AN. Molecular mechanisms of Nrf2‐mediated antioxidant response. Mol Carcinog 2009; 48: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross‐talk between Nrf2 and NF‐κB response pathways. Biochem Soc Trans 2015; 43: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagai N, Thimmulappa RK, Cano M, et al Nrf2 is a critical modulator of the innate immune response in a model of uveitis. Free Radic Biol Med 2009; 47: 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishii Y, Itoh K, Morishima Y, et al Transcription factor Nrf2 plays a pivotal role in protection against elastase‐induced pulmonary inflammation and emphysema. J Immunol 2005; 175: 6968–6975. [DOI] [PubMed] [Google Scholar]
- 15. Ma Q, Battelli L, Hubbs AF. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant‐activated transcription factor Nrf2. Am J Pathol 2006; 168: 1960–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobayashi EH, Suzuki T, Funayama R, et al Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 2016; 7: 11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayashi R, Himori N, Taguchi K, et al The role of the Nrf2‐mediated defense system in corneal epithelial wound healing. Free Radic Biol Med 2013; 61: 333–342. [DOI] [PubMed] [Google Scholar]
- 18. McFadden BA, Purohit S. Itaconate, an isocitrate lyase‐directed inhibitor in Pseudomonas indigofera. J Bacteriol 1977; 131: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michelucci A, Cordes T, Ghelfi J, et al Immune‐responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA 2013; 110: 7820–7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheah HL, Lim V, Sandai D. Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS One 2014; 9: e95951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ebel F, Schwienbacher M, Beyer J, Heesemann J, Brakhage AA, Brock M. Analysis of the regulation, expression, and localisation of the isocitrate lyase from Aspergillus fumigatus, a potential target for antifungal drug development. Fungal Genet Biol 2006; 43: 476–489. [DOI] [PubMed] [Google Scholar]
- 22. Tang C, Wang X, Xie Y, et al 4‐Octyl itaconate activates Nrf2 signaling to inhibit pro‐inflammatory cytokine production in peripheral blood mononuclear cells of systemic lupus erythematosus patients. Cell Physiol Biochem 2018; 51: 979–990. [DOI] [PubMed] [Google Scholar]
- 23. Nair S, Huynh JP, Lampropoulou V, et al Irg1 expression in myeloid cells prevents immunopathology during M. tuberculosis infection. J Exp Med 2018; 215: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McClellan SA, Ekanayaka SA, Li C, Jiang X, Barrett RP, Hazlett LD. Thrombomodulin protects against bacterial keratitis, is anti‐inflammatory, but not angiogenic. Invest Ophthalmol Vis Sci 2015; 56: 8091–8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao C, Jiang P, He Z, et al Dimethyl itaconate protects against lippolysacchride‐induced mastitis in mice by activating MAPKs and Nrf2 and inhibiting NF‐κB signaling pathways. Microb pathog 2019; 133: 103541. [DOI] [PubMed] [Google Scholar]
- 26. Feng G, Sun B, Liu HX, Liu QH, Zhao L, Wang TL. EphA2 antagonism alleviates LPS‐induced acute lung injury via Nrf2/HO‐1, TLR4/MyD88 and RhoA/ROCK pathways. Int Immunopharmacol 2019; 72: 176–185. [DOI] [PubMed] [Google Scholar]
- 27. Rangasamy T, Guo J, Mitzner WA, et al Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 2005; 202: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Innamorato NG, Rojo AI, García‐Yagüe AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol 2008; 181: 680–689. [DOI] [PubMed] [Google Scholar]
- 29. Kundu JK, Surh YJ. Nrf2‐Keap1 signaling as a potential target for chemoprevention of inflammation‐associated carcinogenesis. Pharm Res 2010; 27: 999–1013. [DOI] [PubMed] [Google Scholar]
- 30. Luan HH, Medzhitov R. Food fight: role of itaconate and other metabolites in antimicrobial defense. Cell Metab 2016; 24: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cordes T, Michelucci A, Hiller K. Itaconic acid: the surprising role of an industrial compound as a mammalian antimicrobial metabolite. Annu Rev Nutr 2015; 35: 451–473. [DOI] [PubMed] [Google Scholar]
- 32. Niu Y, Zhao G, Li C, et al Aspergillus fumigatus increased PAR‐2 expression and elevated proinflammatory cytokines expression through the pathway of PAR‐2/ERK1/2 in cornea. Invest Ophthalmol Vis Sci 2018; 59: 166–175. [DOI] [PubMed] [Google Scholar]
- 33. Zhou T, Zong R, Zhang Z, et al SERPINA3K protects against oxidative stress via modulating ROS generation/degradation and KEAP1‐NRF2 pathway in the corneal epithelium. Invest Ophthalmol Vis Sci 2012; 53: 5033–5043. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Chen P, Di G, Qi X, Zhou Q, Gao H. Netrin‐1 promotes diabetic corneal wound healing through molecular mechanisms mediated via the adenosine 2B receptor. Sci Rep 2018; 8: 5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


