Abstract
Diagnosis and monitoring of psychiatric disorders rely heavily on subjective self-reports of clinical symptoms, which are complicated by the varying consistency of accounts reported by patients with an impaired mental state. Hence, more objective and quantifiable measures have been sought to provide clinicians with more robust methods to evaluate symptomology and track progression of disease in response to treatments. Owing to the shared origins of the retina and the brain, it has been suggested that changes in the retina may correlate with structural and functional changes in the brain. Vast improvements in retinal imaging, namely optical coherence tomography (OCT) and electrodiagnostic technology, have made it possible to investigate the eye at a microscopic level, allowing for the investigation of potential biomarkers in vivo. This review provides a summary of retinal biomarkers associated with schizophrenia, bipolar disorder and major depression, demonstrating how retinal biomarkers may be used to complement existing methods and provide structural markers of pathophysiological mechanisms that underpin brain dysfunction in psychiatric disorders.
Keywords: retina, optical coherence tomography (OCT), electroretinogram (ERG), schizophrenia, bipolar disorder, major depression, biomarker
Introduction
Mental disorders now comprise the leading cause of disability globally.1 They afflict people without discrimination of age, geography or income level,2 with an estimated one in three people affected by a mental illness during their lifetime.3,4 Mental disorders are associated with a lower quality of life and significantly correlated with chronic disease (i.e. cardiovascular disease, diabetes) development.5,6 The Diagnostic and Statistical Manual of Mental Disorders (DSM) introduced a standard and operationalised diagnostic framework within which to make psychiatric diagnoses. Although its initial intent was to increase reliability of diagnoses and ensure continuity of care, clinical diagnosis and monitoring of psychiatric disorders often remain dependent on patient reports of clinical symptoms.7 These subjective accounts may also be compromised if patients are in an impaired mental state.
In such cases, family member reports play an important role in clinical diagnosis, such as in the case of patients with Asperger syndrome and other autism spectrum disorders.8 Moreover, clinicians differ in the process of making diagnoses as recommended by diagnostic manuals,9 which could jeopardise the patient’s treatment and care. Clinically, the functional elements of the conditions are likely to remain the therapeutic focus; however, retinal biomarkers may be a useful adjunct in detecting or predicting any structural or functional progression of neuropsychiatric disease, although longitudinal studies are needed to validate if indeed psychiatric structural changes in the brain are reflected in the retina.
Diagnosing schizophrenia, bipolar disorder and major depression commonly involves screening scales and patient-reported outcome measures. For major depression disorder, the Patient Health Questionnaire (PHQ-9) is a commonly used tool for diagnosis,10 but has been found not to provide a strong index for severity of disease.11 According to a study by Kjaergaard and colleagues, other commonly used screening tools, such as the Beck Depression Inventory-II, the Montgomery and Åsberg Depression Rating Scale, were concluded to be useful for screening a major depression episode but not reliable in accurately diagnosing depression, hence necessitating the need for a comprehensive tool to guide and monitor treatment response.12 In one study observing the reliability of patient-reported outcome measures (PROMs) in schizophrenia, differences were found in PROM self-ratings provided consequently by patients with schizophrenia.13 Greater differences were associated with higher degrees of disorganisation and cognitive impairment, thus suggesting the lack of reliability in patient reports. It is important to note that symptoms of schizophrenia can change over time, thus making it challenging to use symptomology screening scales as the mainstay.14 Further to this, screening tools such as the Mood Disorder Questionnaire (MDQ) for bipolar disorder have been found to have a low positive predictive value, suggesting that such scales may not be reliable tools in clinical practice.15 Lastly, Svendsen and colleagues concluded that subjectively experienced cognitive function fails to predict objective function.16 Therefore, in order to gain a better understanding of the neurological changes that occur, objective measures must be sought. The gap in diagnostics and monitoring has called for more objective and quantifiable measures to equip clinicians with reliable tools to assess symptomology and to track disease progression in response to treatments.
The retina’s development from the same tissue as the brain, the neuroectoderm, suggests that retinal changes may parallel structural and functions changes in the brain.17 Noninvasive, retinal imaging technology, such as optical coherence tomography (OCT) and electrodiagnostics, have made it possible to determine potential biomarkers of neural tissue structure changes and disorder progression.17 OCT is a quick, noninvasive imaging device that captures cross-sectional retinal images down to the individual layers through high resolution using near-infrared light (Figure 1).18,19
Figure 1.
An SD-OCT cross-sectional macula scan of retinal layers.
BM, Bruch’s membrane; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PR-IS/OS, photoreceptor inner segment/outer segment; RNFL, retinal nerve fibre layer; RPE, retinal pigment epithelium; SD-OCT, spectral-domain optical coherence tomography.
With its power to achieve high resolution imaging, SD-OCT has allowed for a number of previously unseen changes to be assessed, such as reductions in retinal nerve fibre layer thickness in patients with schizophrenia and decreased choroidal thickness in patients with psychosis compared with healthy controls.20,21 Oberwahrenbrock and colleagues investigated the inter-rater reliability of semiautomated segmentation of OCT scans across five centres in the United States and Europe, and concluded that OCT scans are highly reliable in a neurodegenerative population. Thus, OCT scans can potentially serve as a reliable tool in clinical trials assessing retinal injury in psychiatric disorders.22 Thus, retinal imaging allows for the possibility of surrogate biomarkers to guide clinicians and researchers and provide greater insight on diseases that have historically relied on subjective self-reports and contradictory screening scales.
Determining biomarkers may provide researchers with an opportunity to reduce cost and time in clinical trials and allow clinicians to diagnose earlier, monitor disorder progression and guide treatment. The studies in this review examine the correlation between retinal markers and disease presence, severity and duration in schizophrenia, bipolar disorder and major depression. The aims of this review are to demonstrate how retinal biomarkers may be utilised to enhance current clinical diagnostic methods and to discuss how changes in OCT measures may serve as surrogate markers of pathophysiological mechanisms that contribute to brain abnormalities in psychiatric disorders.
Schizophrenia
Schizophrenia is a chronic, debilitating psychiatric disorder that affects an estimated 4.0 per 1000 people over a lifetime,23 and is ranked the fifth leading cause of disability worldwide in 15- to 44-year olds.24 Diagnosis is complex and relies primarily on clinical judgement of positive symptoms (e.g. hallucinations, delusions, disorganised speech) or negative symptoms (e.g. avolition, flat affect).25,26 Symptoms may also fluctuate over time, with several other conditions acting as possible mimics including infectious,14 neurological, metabolic and other psychological disorders.27–29 These factors make diagnosing schizophrenia challenging.
Diminished total brain volume seen on MRI is a well-established characteristic of schizophrenia.30 In a large (352 studies) meta-analysis collating cross-sectional volume indices, longer illness duration and high doses of antipsychotics were negatively correlated with grey matter volume. Similarly, with severity of disease, grey matter volumes of the superior temporal gyrus and gyrus rectus have been found to correlate negatively with the positive scales of the Positive and Negative Syndrome Scale (PANSS), and superior temporal gyrus and anterior cingulate cortex correlated negatively with negative scales.31
There is evidence that early identification and treatment of psychotic symptoms markedly improves clinical outcomes.32,33 Given the weak predictive value of clinical features, diagnoses based on objective biomarkers would be of paramount value.26,34 Visual impairments, including deficits in visual acuity and contrast sensitivity,35,36 are both common and have a high sensitivity for converting to psychosis compared with other symptoms.37 Visual impairments have been associated with psychosis from the prodromal phase,38 to first episodes,39–41 in addition to higher rates of psychosis,42,43 increased severity of illness and chronicity.42,44–46 Visual deficits may also be secondary to reduced access to healthcare in that patients’ deficits may stem from underutilising healthcare services regardless of visual changes.47 It is uncertain to what extent visual impairments derive from the retina versus the brain. It has been suggested that structural and functional retinal changes may indicate progressive brain tissue loss in addition to explaining clinical features and perceptual visual processing deficits,17 as established in multiple sclerosis and Alzheimer’s disease (AD).48–53
Retinal neurotransmitters in schizophrenia
N-methyl-D-aspartate (NMDA) glutamatergic receptor hypoactivity and hyperactivity, also referred to as the glutamate hypothesis of schizophrenia, has been linked to the pathogenesis of both negative and positive symptoms in addition to neural excitotoxicity,54–56 which can induce neurodegeneration.57–59 Additionally, the same receptors mediate glutamatergic cone photoreceptor-bipolar cell pathways at the fovea.60 Photoreceptor complex thinning may represent NMDA dysfunction54; however, there is no direct evidence to affirm that a glutamate surplus directly causes structural changes to the retina.61,62 The dopamine hypothesis of schizophrenia proposes that dopamine abnormalities in the mesolimbic and mesocortical pathways contribute to the positive and negative symptoms of schizophrenia.63,64 In the retina, dopamine is synthesised and released from amacrine cells (ACs) and is involved in several aspects of visual function including modulating visual acuity and contrast sensitivity.65 Reduced retinal dopamine has led to excessively strong coupling of ACs and horizontal cells (HCs), associated with increased inhibition, reduced contrast sensitivity and poorer visual acuity as a possible consequence.66
Electroretinogram changes in schizophrenia
Changes in retinal function over time, or how they compare to healthy controls, can serve as an important biomarker for monitoring disease progression. Electroretinogram (ERG) assesses retinal function by measuring electrical activity of neuronal populations in response to light exposure.46,67 In schizophrenia, abnormal ERG findings are well established, and have been reported in several recent studies.68–70 Using portable hand-held flash ERG (fERG), Demmin and colleagues reported diminished a-wave and b-wave amplitude and latency in both photopic (light-adapted) and scotopic (dark adapted) conditions, suggesting weakened photoreceptor and bipolar cell activity.68 In contrast, Moghimi and colleagues saw a trend of decreasing b-wave amplitude in schizophrenia patients and healthy controls with regards to a-wave and latency, despite observing a trend towards reduced b-wave amplitude.69 Looking at the findings from a longitudinal standpoint, Hébert reported that cone a-wave and rod b-wave amplitude reductions seen in childhood were present in adults with schizophrenia.71 Finally, in a large study comparing medicated and stable 150 schizophrenia patients with 151 bipolar patients, both patient groups exhibited diminished cone a-wave amplitude and extended b-wave latency, whereas only schizophrenia patients exhibited diminished cone b-wave amplitude. Furthermore, bipolar and schizophrenia patients could be differentiated with 80% sensitivity and 82% specificity.70 These studies suggest ERG could be useful to clinicians in a number of ways, including making differential diagnoses and objectively tracking neurodevelopment of the condition and use and effectiveness of antipsychotic medications.
OCT in schizophrenia
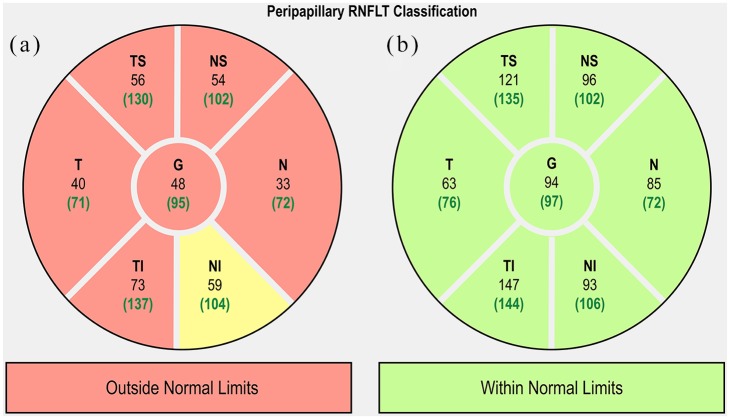
The use of OCT in schizophrenia is fairly novel, yet studies have revealed common themes, most notably retinal thinning and reduced macular thickness and macular volume (MV). Global retinal thinning has been reported in several studies.61,62,72–74 Specifically, peripapillary RNFL thinning has been observed in the nasal, inferior and, predominately, in the superior quadrants (Figure 2).74–77 In contrast, Silverstein and colleagues reported no difference in RNFL thickness between schizophrenia patients and controls,78 and Ascaso and colleagues found these significant differences only amongst right eyes, the clinical relevance of which remains unclear (Table 1). Macular thinning and reduced MV are another reported, yet debated, finding in patients with schizophrenia compared with age-matched controls. Whereas several studies observed overall macular thinning in patients compared with age-matched controls,61,62,75,79,80 one study observed macular thinning isolated to the nasal and inferior outer macular regions.81 In contrast, Silverstein and colleagues reported no difference in macular thinning between schizophrenia patients and age-matched controls.78 Additionally, Chu and colleagues observed no macular thinning in schizophrenia and schizoaffective patients compared with healthy controls. However, it is unclear how the results were affected by the inclusion of schizoaffective disorder, given that the majority of studies excluding schizoaffective patients report significant macular thinning.82
Figure 2.
Comparison of SD-OCT pRNFL thicknesses of the right eyes in a patient with schizophrenia (a) and a healthy patient (b). Yellow sectors indicate borderline limits.
pRNFL, peripapillary RNFL; RNFL, retinal nerve fibre layer; SD-OCT, spectral-domain optical coherence tomography.
Table 1.
A summary of studies reporting sector analysis of peripapillary RNFL changes in schizophrenia, bipolar disorder and major depression. Effect size has been calculated as mean percentage reduction in disease versus healthy controls.
| Schizophrenia |
OCT type | Global | Superior |
Temporal | Inferior |
Nasal | ||
|---|---|---|---|---|---|---|---|---|
| Study | ST | SN | IT | IN | ||||
| Ascaso80 | TD | 7.8% | 6.3% | 7.9% | 9.4% | 10.7% | ||
| Ascaso75 | SD | 9.8%b | 10.7%b | 5.8% | 9.2% | 15.5%b | ||
| Celik72 | SD | 4.8% | 5.1% | 2.3% | 4.7% | 10.1% | 6.7% | 4.9% |
| Chu82a | TD | 1.0% | 0.9% | 0.9% | −0.8% | −0.1% | ||
| Lee62 | SD | 8.9% | 11.7% | 9.9% | 7.4% | |||
| Topcu-Yilmaz79 | SD | 0.0% | 0.8% | −2.2% | 1.4% | 2.3% | −1.4% | −1.2% |
| Yilmaz81 | SD | 5.9% | 1.7% | 3.5% | 3.8% | 7.9% | ||
| Bipolar disorder |
OCT type | Global | Superior |
Temporal | Inferior |
Nasal | ||
| Study | ST | SN | IT | IN | ||||
| Mehraban83 | SD | 6.8% | 4.8% | −1.3% | 8.0% | 10.5% | ||
| Kalenderoglu84 | SD | 5.9% | 3.9% | 5.3% | −0.1% | 3.5% | 7.8% | 3.1% |
| Khalil85 | SD | 14.8% | 11.3% | 7.4% | 9.3% | 3.6% | ||
| Polo86 | SS | 8.1% | 16.5% | 11.2% | 4.3% | 9.4% | 4.7% | 4.9% |
| Major depression |
OCT type | Global | Superior |
Temporal | Inferior |
Nasal | ||
| Study | ST | SN | IT | IN | ||||
| Yildiz87 | SD | −0.1% | 0.1% | 4.2% | –0.4% | −5.4% | ||
| Sönmez88a | SD | 1.7% | 1.2% | −0.9% | 0.2% | −0.1% | 2.6% | 4.6% |
Grey shading indicates statistical significance as chosen by each study investigator.
Mean of right and left eyes.
OD significant only.
IN, inferonasal; IT, inferotemporal; OCT, optical coherence tomography; RNFL, retinal nerve fibre layer; SD, spectral domain, SN, superonasal; ST, superotemporal; TD, time domain.
On examination of the individual layers of the macula with OCT, one comprehensive study by Samani and colleagues found that total retinal thickness was significantly reduced in the foveal, nasal parafoveal, and temporal parafoveal regions, with all three zones having outer nuclear layer (ONL) and inner segment layer (ISL) thinning in common.61 Furthermore, thinning was observed to affect the parafoveal ganglion cell layer (GCL) temporally, and the inner plexiform layer (IPL) and retinal nerve fibre layer (RNFL) nasally.61 Of relevance to treatment potential in schizophrenia, lower macular volumes have been observed in treatment-refractory patients compared with treatment-responsive patients, possibly reflecting the duration of disease, but also with potential as a prognostic biomarker.72 In contrast, other studies have observed no difference in global mRNFL or choroidal thickness in schizophrenia and schizoaffective disorder when compared with healthy controls (Table 2).79,82 These contrasting findings have been attributed to small sample sizes, varying illness duration and lack of OCT resolution to detect loss of unmyelinated axons in early disease. In the latter study, Chu and colleagues found specifically that the right nasal RNFL in schizoaffective patients was significantly thinner (p = 0.02) compared with schizophrenia patients82; however, the clinical relevance of this finding, if any, would need to be proven with further studies.
Table 2.
Structural retinal characteristics of patients diagnosed with schizophrenia, BD and depression.
| Schizophrenia |
n | Mean age, years (SD) | Mean illness duration, years (SD) | Anti-psychotic medication status | Global RNFL thinning | Global Macular thinning | Choroidal thinning |
|---|---|---|---|---|---|---|---|
| Study | |||||||
| Ascaso80 | 20a | 39.2 (13.5) | + | − | |||
| Ascaso75 | 37 | 45.1 (10.4) | 16.3 (11.2) | Medicated | + | + | |
| Celik72 | 81 | 35.6 (10.2) | 13.3 (9.1) | Medicated | + | − | |
| Chu82 | 49 | 29.9 (8.7) | 4.4 (3.6) | Medicated and unmedicated | − | ||
| Lee62 | 30 | 37.2 (10.7) | Medicated | + | + | ||
| Samani61 | 35 | 40.6 (12.9) | 16.3 (9.1) | Medicated | + | + | |
| Topcu-Yilmaz79 | 22 | 34.6 (9.5) | 10.3b | Medicated and unmedicated | − | + | − |
| Yilmaz81 | 68a | 39.9 (10.3) | + | − | |||
| Bipolar disorder |
|||||||
| Study | |||||||
| Mehraban83 | 60a | 33.8 (9.2) | 10.6 (8.6) | + | |||
| Kalenderoglu84 | 43 | 35.6 (10.5) | 6.8 (10.6) | Medicated and unmedicated | + | ||
| Khalil85 | 40 | 30.9 (9.3) | Medicated | + | |||
| Polo86 | 23a | 49.7 (8.75) | 16.2 (6.7) | Medicated and unmedicated | + | + | − |
| Major depression |
|||||||
| Study | |||||||
| Yildiz87 | 58 | 44.6 (13.1) | Medicated and unmedicated | − | |||
| Sönmez88 | 30 | 34.6 (8.8) | 5.7 (7.3) | Medicated | − |
Positive (+) sign denotes statistically significant thinning compared with healthy controls. Negative (−) sign refers to no difference in thickness between those with disease and healthy controls. ‘n’ refers to number of patients.
Eyes.
Not reported.
RNFL, retinal nerve fibre layer; SD, standard deviation.
Wide retinal venules in schizophrenia
There is growing evidence that people with schizophrenia have higher rates of cardiovascular disease (CVD),9,89–94 and the calibre of retinal microvasculature assessed through colour fundus photography is increasingly recognised as an important biomarker of subclinical CVD. Indeed, metabolic syndrome (MetS) has been well studied in schizophrenia.95,96 MetS prevalence ranges from 5.6% in untreated schizophrenia inpatients in Brazil up to 74% in Hispanic outpatients.97,98 Patients with schizophrenia are already at elevated risk of morbidity and mortality due to CVD, so given that antipsychotic medications may potentiate the damage, especially at young ages,99,100 it is important to be able to monitor and anticipate cardiovascular changes to allay further injury from CVD.
Several familial studies have examined the association between schizophrenia or psychosis and retinal venule calibre with positive associations. The Dunedin Longitudinal Study, a birth cohort originally consisting of 1037 children, described how patients who developed schizophrenia by age 38 had distinctly wider retinal venules compared with healthy cohort members.101 Similarly, in the Twins Eye Study in Tasmania and the Brisbane Longitudinal Twin Study, individuals who experienced psychosis had wider venules compared with unaffected co-twins and controls; however, unaffected co-twins also exhibited wide venules compared with controls.102 These findings suggest widened retinal venules may reflect familial vulnerability to psychosis. Accordingly, genetic variants associated with both retinal vascular abnormalities and schizophrenia were described in the Dunedin study and revealed a strong genetic correlation between arterial diameter and weak correlation in venular diameter with a schizophrenia diagnosis.103 More recently, in a study examining the relationship between adult vascular-ischemia and the neurodevelopmental hypothesis, Moises, Wollschläger and Binder described an overwhelming number of schizophrenia-associated genes also involved with vascular function, regulation and repair.104 These findings suggest that vascular abnormalities evidenced by widened retinal venules may reflect a genotypic vulnerability to psychosis.
Magnocellular pathway dysfunction in schizophrenia
Beginning in the M-ganglion cells of the retina, the magnocellular pathway branches out into the M-layers of the lateral geniculate nucleus (LGN) of the thalamus then into the dorsal stream and visual cortex located in the parietal lobe.105 Visual pathway deficits in schizophrenia are thought to stem from disturbances in this pathway, as evidenced by reduced visual field sensitivity,106–108 eye-tracking,109,110 motion-contrast sensitivity,111 reading performance and smooth pursuit eye movement compared with healthy controls.105 Deficits in the magnocellular pathway have also been implicated in some of the cognitive impairments seen,112,113 including attention and memory.107,114 Given the multiple visual symptomology in schizophrenia, various methods have been used to investigate the potential impact of magnocellular pathway deficits on vision. Functional magnetic resonance imaging (fMRI) studies have revealed right hemisphere magnocellular pathway hypoactivation in first-degree nonpsychotic relatives and patients with schizophrenia,115 but otherwise normal pathway functioning.116 Electroencephalography (EEG) studies measuring event-related potentials (ERPs) have shown diminished amplitudes and poor detection of low spatial frequency targets.117 Regarding contrast sensitivity loss, a review by Skottun and Skyoles concluded that contrast sensitivity deficits were not consistent with magnocellular deficiencies.118 However, they did acknowledge the difficulty of drawing a clear relationship between clinical symptoms of schizophrenia to deficits in the magnocellular pathway,118 such as linking poor reading performance in schizophrenia to dyslexia, which is weakly associated.105
More notably used in glaucoma, frequency doubling perimetry (FDT) is a small, transportable device that assesses visual field defects as well as changes in contrast sensitivity.114 FDT works by creating an illusion at a high temporal frequency of a counter-phased flicker of a sinusoidal grating with a low spatial frequency.114 Since processing this type of visual stimuli is believed to involve areas of the brain that process visual information that activate the magnocellular pathway, FDT is seen as a way to assess retinal ganglion cell (RGC) function.115,119,120 FDT has been used in glaucoma patients to assess the relationship between ganglion cell-inner plexiform layer (GCIPL) thickness and mean sensitivities and showed strong statistically significant positive correlations between the two, most notably in the inferotemporal and inferior sectors.121 Several studies have explored neurological dysfunction of this pathway in patients with schizophrenia using FDT as well. Khosravani and Goodarzi reported diminished visual field sensitivity in patients with schizophrenia compared with normal controls.106 Gracitelli and colleagues reported statistically significantly lower global mean deviation in both eyes of patients with schizophrenia compared with their parents and normal controls.107 Similarly, Lima and colleagues showed lower global mean sensitivity in patients compared with healthy controls.108 Indeed, these abnormalities may reflect deeper damage to neural pathways within the brain, specifically the cortex and brainstem, as similarly proposed in AD,122 in order to offer a potential biomarker for diagnosis and monitoring of cognitive impairment in schizophrenia, thus an area of great interest for future research.
Severity and duration of schizophrenia
The correlations reported between duration of disease with retinal thickness and macular volume are mixed. Significant inverse correlations have been reported between duration of disease and thickness of the RNFL, temporal parafoveal ISL and temporal parafoveal ONL, as well as macular thinning and volume.61,62 These correlations with macula thickness and volume suggest progressive neurodegeneration seen in parallel with MRI changes of grey matter volume loss.123 However, studies containing similar numbers of patients and controls have reported no significant correlation between RNFL thickness or macular thickness with duration of illness.74,79 The Ascaso study (n = 60) found no correlation between duration and retinal abnormalities; however, the finding of macular thinning in patients with nonrecent illness episodes suggests that changes in retinal structures may be conditional as opposed to progressive.75 Similarly, Chu and colleagues reported no significant retinal changes between early stage schizophrenia patients and schizoaffective disorder to age and gender-matched controls.82 The lack of disease-related retinal changes seen in these studies may be explained by the theory that lower levels of neuroinflammation in the retina can be caused by antipsychotic drug use, particularly in patients with recent illness episodes.124,125 This has been supported by a meta-analysis that included 23 studies (n = 762), which suggested antipsychotics express a systemic anti-inflammatory effect.126 Only one study we reviewed actively assessed the role of antipsychotics on OCT abnormalities, and found no significant correlation with treatment.61
The relationship between the severity of symptoms and retinal abnormalities is also unclear. Thinning of the RNFL, GCL and IPL have been found to significantly correlate with disease severity, measured by the Positive and Negative Symptom Scale (PANSS).72,127 Moreover, GCL and IPL volumes have been found to be lower in treatment refractory patients with schizophrenia compared to treatment responsive patients,72 suggesting an inverse relationship with advanced disease. A more recent study observed that ONL and foveal photoreceptor thinning significantly correlated with the severity of negative symptoms, in addition to a significant inverse correlation with nasal parafoveal ONL thickness.61 Smaller macular volume has also been related to higher severity of symptoms using the Schedules for the Assessment of Positive and Negative Symptoms (SAPS and SANS) scales.82,128 However, similar studies with respect to number of patients and age or gender-matched controls, have reported no significant correlation between RNFL thickness or macular thickness with severity measured by the Clinical Global Impression Scale-Severity (CGI-S)129 and PANSS scores, respectively.74,79
Future possibilities in schizophrenia
Firstly, animal models representing negative symptoms are lacking. It has proven difficult to model schizophrenia in animals given that the disease impairs higher brain function resulting in human-specific symptoms such as delusions, hallucinations and disorganised speech.130 Animal models in schizophrenia are few, and tend to focus on dopamine dysfunction driving pathophysiological changes.131,132 These have shown that retinal dopaminergic deficiency may lead to loss of a subset of retinal amacrine cells.133 Treatments for negative and cognitive symptoms of schizophrenia are lacking, hence more work is needed to develop comprehensive models that will adequately reproduce the deficits created by these symptoms. Secondly, given the contentious nature of findings related to retinal thinning, macular thinning and macular volume deficits, larger studies that evaluate more detailed and comprehensive outcome measures are necessary. This would include higher resolution OCT scans adequate enough to visualise subtle abnormalities present in early schizophrenia. Chu and colleagues acknowledged lower resolution time-domain OCT (axial resolution 10 μm) as the main limitation of their study, whereas studies that offered better axial resolution of 5 μm yielded more significant macular thickness measurements.61,62,82 Thirdly, most schizophrenia patients in these studies were receiving antipsychotic medications at the time of data collection, therefore it was not possible to exclude the potential anti-inflammatory and neuroprotective effects of these medications that may have masked minor differences in RNFL between the different groups.134 Finally, tobacco smoking is very common in schizophrenia and has been suggested to have a procognitive effect on positive symptoms,135 although a recent study by Yokoyama and colleagues discovered an association between length of smoking and severe symptoms with reduced prefrontal volume on MRI.136 Additionally, chronic tobacco smokers have been shown to have a thinner ganglion cell complex and choroid compared with age and sex-matched nonsmoking controls, suggesting that smoking may confound or modify the effect of the relationship between retinal thinning and schizophrenia (Table 2).136,137
Bipolar disorder
Bipolar disorder (BD), formerly known as manic depression, is an episodic, chronic psychiatric illness that can cause severe disability and declines in quality of life.138 Patients with BD experience severe mood swings from intense highs (mania) to extreme lows (depression) that last for several weeks or months. An estimated 1% of the global population is affected by BD,138 and it has been estimated that between 30% and 50% of patients with BD have been misdiagnosed with unipolar major depression because of initial presentation with a depressive episode, and may go on for years without a correct diagnosis.139–141 Delayed diagnosis, especially in children and adolescents,142,143 has been linked with poorer outcomes, including longer episodes and higher rates of recurrence.144–147 Structural brain abnormalities seen on MRI have been detected in patients as early as the first episode, with some changes progressing, including reduced grey matter in the hippocampus, temporal lobe and fusiform gyrus.148,149
RNFL changes in bipolar disorder
More recently, OCT has been used to correlate these changes in the retina. Multiple studies have demonstrated statistically significant global thinning of the pRNFL in patients (Table 1).83,84,86,150 Compared with healthy controls, statistically significant global RNFL thinning was observed in euthymic (current ‘normal’ mood) BD patients.84 However, similar studies that age-matched controls have observed all but temporal thinning.83 Conversely, using age and sex-matched controls led to the observation of all but nasal thinning.85,150 Studies that measured antipsychotic use did not find a correlation between prescribed medications and RNFL thinning, hence patients presented with significant RNFL thinning regardless of antipsychotic use.84,150 In addition, duration of illness has been shown to negatively correlate with RNFL thickness and GCC volume in euthymic and psychotic patients, suggesting a progressive neurodegenerative process.83,84
Multiple studies have observed lower IPL and GCL volume in BD patients,84,85,150 and a significant negative correlation between severity and duration of illness.84 One study observed a significant compensatory thickening of the INL in the BD group,150 contrary to previous BD studies, but similar to reported findings in Parkinson disease.151 Therefore, the absence of more observations of total retinal thickness change in BD may be a result of masking of thinning by simultaneous thickening of other layers.150 Newer, swept-source OCT able to image both the retina and choroid has been used to examine patients with BD, demonstrating significant thinning in the central macula, as well as in the inner temporal, nasal and inferior zones (Table 2).86 However, no changes in choroidal thickness were observed.
ERG changes in bipolar disorder
Patients at high genetic risk for BD (n = 20) and schizophrenia (n = 9) (one parent with BD or schizophrenia) have demonstrated diminished rod b-wave amplitude and delayed latency compared with age and sex-matched healthy controls, but no statistical difference in cones.152 A more recent and larger follow up study comparing high-risk children with age- and sex-matched healthy controls also observed diminished rod b-wave amplitude and delayed latency but also found delayed cone b-wave latency.153 These results suggest that retinal rod response abnormalities may serve as a biomarker for the risk of developing BD.
Future possibilities in bipolar disorder
Few studies have assessed the structural and functional changes in the retina associated with BD directly. Growing evidence from OCT studies suggests neurodegeneration in BD can be characterized by RNFL, GCL and IPL thinning with increasing duration of disease.83–86 The pathophysiological changes that underpin these observations remain unclear, hence more research is needed to support these findings. A potential explanation could be that low retinal dopamine, as suggested by reduced b-wave amplitude, may play a part in hypo-arousal states (anhedonia, melancholia) as suggested in seasonal affective disorder (SAD).154,155 Still, small study size, chronic medication use and early age of onset are just a few of the barriers to establishing OCT, ERG and other similar measures as valid biomarkers to show degeneration and follow progression of BD and other psychiatric disorders.
Major depression
Major depression (MD) is a neuropsychiatric disorder that can affect individuals across a life span. MD is marked by anhedonia, low mood and cognitive disturbances.156 According to WHO estimates, depression is the leading cause of disability worldwide.157 It is estimated that over 322 million people suffer from depression, 4.4% of the world’s population. A number of risk factors have been identified, including female gender, high stress, family history, childhood experiences, personality traits and genetics.158–161 It has been observed that healthy patients who self-report visual impairment without visual acuity loss have almost double the odds of depression compared with visual acuity loss alone, after controlling for other risk factors.162 This finding could either be due to changes in the brain–vision axis, or other psychological influences.
The role of neuroinflammation has recently been implicated in the aetiology of MD, as evidenced by activation of the immune system seen in depressed patients and the strong relationship observed between depression and autoimmune diseases.163–165 By one estimate, up to 50% of autoimmune patients exhibit impaired quality of life or depressive symptoms.166 Moreover, increasing evidence has pointed to the importance of neuroinflammation in AD pathogenesis,167–170 and history of MD as a risk factor for AD.171–175 Likewise, MRI and postmortem studies have shown decreased grey matter volume and glial density in the prefrontal and cingulate cortices and decreased hippocampal volume in patients with depression, regions of the brain involved in the emotional and cognitive characteristics of depression.176–180 In MRI studies, decreased volumes in the prefrontal cortex, hippocampus and basal ganglia have been found bilaterally.180 It is also important to note that the shape of the hippocampus has been found to be abnormal in MD patients without volume deficits through high-dimensional mapping.181 Based on these findings, several studies have been conducted to investigate the relationship between these changes and functional and structural retinal changes related to MD.
ERG findings in major depression
ERG changes have been described in several studies of MD patients. The largest of these studies (n = 200) comparing the cone and rod ERGs between MD patients and healthy controls observed prolonged b-waves at the cone level and reduced rods/cone a-waves in medicated and unmedicated MD patients compared with controls, suggesting that these irregularities are not modifiable by medication.182 In contrast, another smaller (n = 40) study assessing the response to duloxetine in MD patients and healthy controls found that treatment-responsive MD patients had significantly higher baseline rod b-wave amplitudes compared with nonresponders and healthy controls; however, no statistical difference between all groups was found at 12 weeks.183 Their findings suggest that rod b-wave amplitude alterations could be state-dependent or potentially predict response to antidepressants in subtypes of depression.
Significant reductions in pattern electroretinogram (PERG) amplitude have been observed in MD patients compared with controls; unmedicated and medicated MD patients displayed significantly lower retinal contrast gain compared with controls. In addition, studies have found a strong correlation between contrast gain and depression severity.184 In those patients who achieved remission, MD patients with low baseline contrast gains normalised on par with controls, whereas patients not achieving remission continued to show reduced RGC function, providing evidence that retinal contrast gains may be state-dependent and therefore useful as a prognostic biomarker.185 Moreover, as mentioned previously and similar to BD, reduced b-wave amplitude may be indicative of low retinal dopamine, contributing to hypo-arousal as seen in SAD.154,155
Wide retinal venules
There is strong evidence from longitudinal studies that patients with MD are at increased risk of developing cardiovascular disease.186–192 In light of this risk, a large (n = 865) cross-sectional study based on the cohorts from the Brisbane Longitudinal Twin Study and the Twin Eye Study in Tasmania examined whether an association existed between depression and anxiety with retinal vessel calibre. In the adolescents and young adults who participated, depression and anxiety symptoms were associated with wider retinal venules, even after adjusting for other cardiovascular risk factors.193
OCT in major depression
In vivo visualization of the retina via OCT in MD patients has been explored by several studies, with mixed findings (Table 1). Significantly reduced GCL, IPL and global and temporal superior RNFL thickness have been observed in recurrent MD patients compared with first episode patients, and in all MD patients compared with healthy controls.194 However, most of the evidence comparing MD patients with healthy controls to date found no statistically significant difference in OCT measures although differences within MD groups were more profound (Table 1).87,88,195 Duration and severity of disease was observed to correlate negatively with GCL and IPL volumes and positively with global RNFL thickness; however, the evidence is weak for all measures possibly owing to the majority of MD patients taking psychotropic medication.87,194 Finally, the total retinal volume of the left eye has been shown to be greater than the right eye in MD patients, indicating a possible hemisphere-specific lateralisation of cognitive processing.195 This idea has been compounded by positron emission tomography (PET) studies showing increased orbitofrontal and prefrontal activity in healthy volunteers during temporary states of sadness,196,197 with MD patients more often having altered left prefrontal functioning.198–201
Future possibilities in major depression
Compared with other diseases included in this review, there is a dearth of evidence defining the structural and functional changes in the retina in MD. More studies are required to confirm the presence or absence of retinal changes between MD patients and healthy controls, severity of symptoms, duration of illness, timing of depressive episodes, effect of antidepressant use and how they may relate to future neurodegeneration. The lack of differences between MD patients and controls seen in studies may be a real absence of effect, or alternatively due to small study numbers, insufficient resolution of OCT or other methodological issues. For example, retinal thickness has been found to fluctuate according to diurnal variations in healthy patients, yet no study reviewed here reported timing of examinations, which could explain the lack of difference seen between MD patients and the control group, if not standardized.202–205 As suggested by the monoamine deficiency hypothesis, the pathophysiology of MD may result from deficiencies in norepinephrine, serotonin and dopamine.206–208 Therefore, if retinal dopamine, released by amacrine cells in the INL, is diminished, subsequent input to retinal ganglion cells is reduced and could lead to atrophy in these layers as noted on OCT.209 Therefore, OCT may be an important tool to track neurodegeneration in patients with MD, yet few studies have investigated its role longitudinally, possibly due to the statistically insignificant findings thus far.194 Advancements in OCT imaging technology assure high-resolution images that will, in the future, allow any structural changes to be detected with greater sensitivity, in particular the GCL.210
Conclusion
The studies reviewed propose that various changes in structure and function of the retina reflect changes in the brain and could thus serve as important biomarkers in psychiatric disorders. In schizophrenia, the hyper- and hypoactivity of NMDA glutamatergic receptor found at the fovea could potentially be associated with functional changes, but more studies are needed, observing retinal changes over time. It is well accepted that dopamine abnormalities, which also may be linked to positive and negative symptoms, impair visual function. Additionally, OCT has revealed retinal thinning and reduced macular volume and thickness in schizophrenia; however, it is unclear if these findings correlate with duration and severity of symptoms. Moreover, it is unknown how RNFL thinning relates to atrophy of specific regions of the brain in schizophrenia and MD.78 In particular, if occipital atrophy does occur, knowing when it occurs in relation to RNFL thinning could help explain why some studies have found no difference in RNFL thickness between patients with schizophrenia and controls despite visual symptomology.82 Lessons may be learned from a similar finding of absent retinal thinning with visual disturbances in anti-NMDA receptor encephalitis, pointing, instead, to potential dysfunction in the anterior and posterior visual pathways and thus demonstrating deeper cortical and subcortical processing impairments in the brain.211 It also must be noted that the majority of studies reviewed reported exclusion of patients with concomitant ocular diseases; however, it is less clear whether diseases that could inevitably affect ocular structure and function, such as diabetes, were excluded or controlled for.
In BD, OCT has demonstrated pRNFL thinning, and lower IPL and GCL with increasing duration of disease, thus proposing a potential method to aid in early diagnosis of this disease. Moreover, ERG has allowed for observation of diminished rod b-wave amplitude and delayed cone b-wave latency in bipolar patients with high genetic risks. On the other hand, in major depression, prolonged b-waves at the cone level were found, suggesting that ERG could serve as a valuable tool to evaluate antidepressant response and differentiate between bipolar disorder and depression during diagnosis. Despite many structural and functional changes in the retina of patients with MD, there is a gap in understanding how these biomarkers can aid practice at the clinical level. In all these conditions, if a long-term biomarker (over decades) might be found in the retina, it is unlikely to replace functional and behavioural outcomes, as these are what are assumed to cause the disability in these psychiatric illnesses as opposed to poor vision. However, it may serve as a surrogate endpoint.
Major advances in retinal imaging in recent years, specifically with OCT, have enabled the discovery of these potential biomarkers. The results from these studies demonstrate how retinal biomarkers could help robust clinical diagnostic methods and the monitoring of disease progression. Since much of the existing research is limited by cross-sectional designs, further studies utilising more robust methods, such as cohorts, with large and sufficiently powered sample sizes are key to developing a more comprehensive understanding the relationship between OCT measures and psychiatric disorders and establishing retinal biomarkers that are reliable enough to diagnose psychiatric conditions early on, potentially predicting disease onset and severity before symptoms manifest.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Maria Francesca Cordeiro  https://orcid.org/0000-0001-8663-6525
https://orcid.org/0000-0001-8663-6525
Contributor Information
Melanie T. Almonte, Western Eye Hospital, Imperial College Healthcare NHS Trust (ICHNT), London, UK Imperial College Ophthalmic Research Group (ICORG), Imperial College London, UK.
Pamela Capellàn, Weill Cornell Medicine, New York, NY, USA.
Timothy E. Yap, Western Eye Hospital, Imperial College Healthcare NHS Trust (ICHNT), London, UK Imperial College Ophthalmic Research Group (ICORG), Imperial College London, UK.
Maria Francesca Cordeiro, Glaucoma and Retinal Neurodegeneration Group, Department of Visual Neuroscience, UCL Institute of Ophthalmology 11-43 Bath Street, London EC1V 9EL, UK; Western Eye Hospital, Imperial College Healthcare NHS Trust (ICHNT), London, UK; Imperial College Ophthalmic Research Group (ICORG), Imperial College London, UK.
References
- 1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013; 382: 1575–1586. [DOI] [PubMed] [Google Scholar]
- 2. Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry 2016; 3: 171–178. [DOI] [PubMed] [Google Scholar]
- 3. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol 2014; 43: 476–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ginn S, Horder J. “One in four” with a mental health problem: the anatomy of a statistic. BMJ 2012; 344: e1302. [DOI] [PubMed] [Google Scholar]
- 5. Lancon C, Martinelli M, Michel P, et al. Psychiatric comorbidities and quality of life in adult individuals with high potential: relationships with self-esteem. Presse Med 2015; 44: e177–e184. [DOI] [PubMed] [Google Scholar]
- 6. Chen CM, Lee IC, Su YY, et al. The longitudinal relationship between mental health disorders and chronic disease for older adults: a population-based study. Int J Geriatr Psychiatry 2017; 32: 1017–1026. [DOI] [PubMed] [Google Scholar]
- 7. First MB, Bhat V, Adler D, et al. How do clinicians actually use the Diagnostic and Statistical Manual of Mental Disorders in clinical practice and why we need to know more. J Nerv Ment Dis 2014; 202: 841–844. [DOI] [PubMed] [Google Scholar]
- 8. Cederlund M, Hagberg B, Gillberg C. Asperger syndrome in adolescent and young adult males. Interview, self- and parent assessment of social, emotional, and cognitive problems. Res Dev Disabil 2010; 31: 287–298. [DOI] [PubMed] [Google Scholar]
- 9. Fan Z, Wu Y, Shen J, et al. Schizophrenia and the risk of cardiovascular diseases: a meta-analysis of thirteen cohort studies. J Psychiatr Res 2013; 47: 1549–1556. [DOI] [PubMed] [Google Scholar]
- 10. Lowe B, Unutzer J, Callahan CM, et al. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care 2004; 42: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 11. Wittkampf K, van Ravesteijn H, Baas K, et al. The accuracy of Patient Health Questionnaire-9 in detecting depression and measuring depression severity in high-risk groups in primary care. Gen Hosp Psychiatry 2009; 31: 451–459. [DOI] [PubMed] [Google Scholar]
- 12. Kjaergaard M, Arfwedson Wang CE, Waterloo K, et al. A study of the psychometric properties of the Beck Depression Inventory-II, the Montgomery and Asberg Depression Rating Scale, and the Hospital Anxiety and Depression Scale in a sample from a healthy population. Scand J Psychol 2014; 55: 83–89. [DOI] [PubMed] [Google Scholar]
- 13. Takeuchi H, Fervaha G, Remington G. Reliability of a patient-reported outcome measure in schizophrenia: Results from back-to-back self-ratings. Psychiatry Res 2016; 244: 415–419. [DOI] [PubMed] [Google Scholar]
- 14. Demirci O, Calhoun VD. Functional magnetic resonance imaging – implications for detection of schizophrenia. Eur Neurol Rev 2009; 4: 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zimmerman M, Galione JN, Ruggero CJ, et al. Are screening scales for bipolar disorder good enough to be used in clinical practice? Compr Psychiatry 2011; 52: 600–606. [DOI] [PubMed] [Google Scholar]
- 16. Svendsen AM, Kessing LV, Munkholm K, et al. Is there an association between subjective and objective measures of cognitive function in patients with affective disorders? Nord J Psychiatry 2012; 66: 248–253. [DOI] [PubMed] [Google Scholar]
- 17. Silverstein SM, Rosen R. Schizophrenia and the eye. Schizophr Res Cogn 2015; 2: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doustar J, Torbati T, Black KL, et al. Optical coherence tomography in Alzheimer’s disease and other neurodegenerative diseases. Front Neurol 2017; 8: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol 2014; 98(Suppl. 2): 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joe P, Ahmad M, Riley G, et al. A pilot study assessing retinal pathology in psychosis using optical coherence tomography: choroidal and macular thickness. Psychiatry Res 2018; 263: 158–161. [DOI] [PubMed] [Google Scholar]
- 21. Pan J, Zhou Y, Xiang Y, et al. Retinal nerve fiber layer thickness changes in Schizophrenia: a meta-analysis of case-control studies. Psychiatry Res 2018; 270: 786–791. [DOI] [PubMed] [Google Scholar]
- 22. Oberwahrenbrock T, Traber GL, Lukas S, et al. Multicenter reliability of semiautomatic retinal layer segmentation using OCT. Neurol Neuroimmunol Neuroinflamm 2018; 5: e449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhugra D. The global prevalence of schizophrenia. PLoS Med 2005; 2: e151; quiz e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lora A, Kohn R, Levav I, et al. Service availability and utilization and treatment gap for schizophrenic disorders: a survey in 50 low- and middle-income countries. Bull World Health Organ 2012; 90: 47–54, 54A–54B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheniaux E, Landeira-Fernandez J, Versiani M. The diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder and unipolar depression: interrater reliability and congruence between DSM-IV and ICD-10. Psychopathology 2009; 42: 293–298. [DOI] [PubMed] [Google Scholar]
- 26. Chan MK, Cooper JD, Bahn S. Commercialisation of biomarker tests for mental illnesses: advances and obstacles. Trends Biotechnol 2015; 33: 712–723. [DOI] [PubMed] [Google Scholar]
- 27. Yolken RH, Dickerson FB, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunol 2009; 31: 706–715. [DOI] [PubMed] [Google Scholar]
- 28. Arango C, Fraguas D, Parellada M. Differential neurodevelopmental trajectories in patients with early-onset bipolar and schizophrenia disorders. Schizophr Bull 2014; 40(Suppl. 2): S138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quinones MP, Kaddurah-Daouk R. Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol Dis 2009; 35: 165–176. [DOI] [PubMed] [Google Scholar]
- 30. Haijma SV, Van Haren N, Cahn W, et al. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull 2013; 39: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim GW, Kim YH, Jeong GW. Whole brain volume changes and its correlation with clinical symptom severity in patients with schizophrenia: a DARTEL-based VBM study. PLoS One 2017; 12: e0177251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larsen TK, Melle I, Auestad B, et al. Early detection of psychosis: positive effects on 5-year outcome. Psychol Med 2011; 41: 1461–1469. [DOI] [PubMed] [Google Scholar]
- 33. Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry 2017; 16: 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weickert CS, Weickert TW, Pillai A, et al. Biomarkers in schizophrenia: a brief conceptual consideration. Dis Markers 2013; 35: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keri S, Antal A, Szekeres G, et al. Spatiotemporal visual processing in schizophrenia. J Neuropsychiatry Clin Neurosci 2002; 14: 190–196. [DOI] [PubMed] [Google Scholar]
- 36. Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol 1998; 107: 49–62. [DOI] [PubMed] [Google Scholar]
- 37. Klosterkotter J, Hellmich M, Steinmeyer EM, et al. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry 2001; 58: 158–164. [DOI] [PubMed] [Google Scholar]
- 38. Nieman D, Becker H, van de Fliert R, et al. Antisaccade task performance in patients at ultra high risk for developing psychosis. Schizophr Res 2007; 95: 54–60. [DOI] [PubMed] [Google Scholar]
- 39. Kelemen O, Kiss I, Benedek G, et al. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: the potential impact of GABA concentration in the visual cortex. Prog Neuropsychopharmacol Biol Psychiatry 2013; 47: 13–19. [DOI] [PubMed] [Google Scholar]
- 40. Phillipson OT, Harris JP. Perceptual changes in schizophrenia: a questionnaire survey. Psychol Med 1985; 15: 859–866. [DOI] [PubMed] [Google Scholar]
- 41. Kiss I, Fabian A, Benedek G, et al. When doors of perception open: visual contrast sensitivity in never-medicated, first-episode schizophrenia. J Abnorm Psychol 2010; 119: 586–593. [DOI] [PubMed] [Google Scholar]
- 42. Schubert EW, Henriksson KM, McNeil TF. A prospective study of offspring of women with psychosis: visual dysfunction in early childhood predicts schizophrenia-spectrum disorders in adulthood. Acta Psychiatr Scand 2005; 112: 385–393. [DOI] [PubMed] [Google Scholar]
- 43. Hayes JF, Picot S, Osborn DPJ, et al. Visual acuity in late adolescence and future psychosis risk in a cohort of 1 million men. Schizophr Bull. Epub ahead of print 12 June 2018. DOI: 10.1093/schbul/sby084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schiffman J, Maeda JA, Hayashi K, et al. Premorbid childhood ocular alignment abnormalities and adult schizophrenia-spectrum disorder. Schizophr Res 2006; 81: 253–260. [DOI] [PubMed] [Google Scholar]
- 45. Kimhy D, Corcoran C, Harkavy-Friedman JM, et al. Visual form perception: a comparison of individuals at high risk for psychosis, recent onset schizophrenia and chronic schizophrenia. Schizophr Res 2007; 97: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adams SA, Nasrallah HA. Multiple retinal anomalies in schizophrenia. Schizophr Res 2018; 195: 3–12. [DOI] [PubMed] [Google Scholar]
- 47. Viertio S, Laitinen A, Perala J, et al. Visual impairment in persons with psychotic disorder. Soc Psychiatry Psychiatr Epidemiol 2007; 42: 902–908. [DOI] [PubMed] [Google Scholar]
- 48. Caldito NG, Saidha S, Sotirchos ES, et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: a longitudinal study. Brain 2018; 141: 3115–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siger M, Dziegielewski K, Jasek L, et al. Optical coherence tomography in multiple sclerosis: thickness of the retinal nerve fiber layer as a potential measure of axonal loss and brain atrophy. J Neurol 2008; 255: 1555–1560. [DOI] [PubMed] [Google Scholar]
- 50. Paolillo A, Pozzilli C, Gasperini C, et al. Brain atrophy in relapsing-remitting multiple sclerosis: relationship with ‘black holes’, disease duration and clinical disability. J Neurol Sci 2000; 174: 85–91. [DOI] [PubMed] [Google Scholar]
- 51. Double KL, Halliday GM, Kril JJ, et al. Topography of brain atrophy during normal aging and Alzheimer’s disease. Neurobiol Aging 1996; 17: 513–521. [DOI] [PubMed] [Google Scholar]
- 52. Ferrari L, Huang SC, Magnani G, et al. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer’s disease. J Alzheimers Dis 2017; 56: 1101–1107. [DOI] [PubMed] [Google Scholar]
- 53. Liu D, Zhang L, Li Z, et al. Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol 2015; 15: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schwartz TL, Sachdeva S, Stahl SM. Glutamate neurocircuitry: theoretical underpinnings in schizophrenia. Front Pharmacol 2012; 3: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci 2013; 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lakhan SE, Caro M, Hadzimichalis N. NMDA receptor activity in neuropsychiatric disorders. Front Psychiatry 2013; 4: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 2001; 158: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 58. Deutsch SI, Rosse RB, Schwartz BL, et al. A revised excitotoxic hypothesis of schizophrenia: therapeutic implications. Clin Neuropharmacol 2001; 24: 43–49. [DOI] [PubMed] [Google Scholar]
- 59. Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res 1997; 37: 3483–3493. [DOI] [PubMed] [Google Scholar]
- 60. Shen Y, Liu XL, Yang XL. N-methyl-D-aspartate receptors in the retina. Mol Neurobiol 2006; 34: 163–179. [DOI] [PubMed] [Google Scholar]
- 61. Samani NN, Proudlock FA, Siram V, et al. Retinal layer abnormalities as biomarkers of schizophrenia. Schizophr Bull 2018; 44: 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee WW, Tajunisah I, Sharmilla K, et al. Retinal nerve fiber layer structure abnormalities in schizophrenia and its relationship to disease state: evidence from optical coherence tomography. Invest Ophthalmol Vis Sci 2013; 54: 7785–7792. [DOI] [PubMed] [Google Scholar]
- 63. Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1988; 1: 179–186. [DOI] [PubMed] [Google Scholar]
- 64. Brisch R, Saniotis A, Wolf R, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry 2014; 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brandies R, Yehuda S. The possible role of retinal dopaminergic system in visual performance. Neurosci Biobehav Rev 2008; 32: 611–656. [DOI] [PubMed] [Google Scholar]
- 66. Djamgoz MB, Hankins MW, Hirano J, et al. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision Res 1997; 37: 3509–3529. [DOI] [PubMed] [Google Scholar]
- 67. Lavoie J, Maziade M, Hébert M. The brain through the retina: The flash electroretinogram as a tool to investigate psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 2014; 48: 129–134. [DOI] [PubMed] [Google Scholar]
- 68. Demmin DL, Davis Q, Roche M, et al. Electroretinographic anomalies in schizophrenia. J Abnorm Psychol 2018; 127: 417–428. [DOI] [PubMed] [Google Scholar]
- 69. Moghimi P, Torres Jimenez N, McLoon LK, et al. Electoretinographic evidence of retinal ganglion cell-dependent function in schizophrenia. Schizophr Res. Epub ahead of print 12 October 2019. DOI: 10.1016/j.schres.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hébert M, Merette C, Gagné AM, et al. The electroretinogram may differentiate schizophrenia from bipolar disorder. Biol Psychiatry 2020. 87: 263–270. [DOI] [PubMed] [Google Scholar]
- 71. Hébert M, Merette C, Paccalet T, et al. Light evoked potentials measured by electroretinogram may tap into the neurodevelopmental roots of schizophrenia. Schizophr Res 2015; 162: 294–295. [DOI] [PubMed] [Google Scholar]
- 72. Celik M, Kalenderoglu A, Sevgi Karadag A, et al. Decreases in ganglion cell layer and inner plexiform layer volumes correlate better with disease severity in schizophrenia patients than retinal nerve fiber layer thickness: findings from spectral optic coherence tomography. Eur Psychiatry 2016; 32: 9–15. [DOI] [PubMed] [Google Scholar]
- 73. Ascaso FJ, Cruz N, Modrego PJ, et al. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J Neurol 2014; 261: 1522–1530. [DOI] [PubMed] [Google Scholar]
- 74. Cabezon L, Ascaso F, Ramiro P, et al. Optical coherence tomography: a window into the brain of schizophrenic patients. Acta Ophthalmologica 2012. 90: 1755. [Google Scholar]
- 75. Ascaso FJ, Rodriguez-Jimenez R, Cabezon L, et al. Retinal nerve fiber layer and macular thickness in patients with schizophrenia: influence of recent illness episodes. Psychiatry Res 2015; 229: 230–236. [DOI] [PubMed] [Google Scholar]
- 76. Kazakos CT, Karageorgiou V. Retinal changes in schizophrenia: a systematic review and meta-analysis based on individual participant data. Schizophr Bull 2020; 46: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. La Morgia C, Di Vito L, Carelli V, et al. Patterns of retinal ganglion cell damage in neurodegenerative disorders: parvocellular vs magnocellular degeneration in optical coherence tomography studies. Front Neurol 2017; 8: 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Silverstein SM, Paterno D, Cherneski L, et al. Optical coherence tomography indices of structural retinal pathology in schizophrenia. Psychol Med 2018; 48: 2023–2033. [DOI] [PubMed] [Google Scholar]
- 79. Topcu-Yilmaz P, Aydin M, Cetin Ilhan B. Evaluation of retinal nerve fiber layer, macular, and choroidal thickness in schizophrenia: spectral optic coherence tomography findings. Psychiat Clin Psych 2019; 29: 28–33. [Google Scholar]
- 80. Ascaso FJ, Laura C, Quintanilla MÁ, et al. Retinal nerve fiber layer thickness measured by optical coherence tomography in patients with schizophrenia: a short report. Eur J Psychiat 2010; 24: 227–235. [Google Scholar]
- 81. Yilmaz U, Kucuk E, Ulgen A, et al. Retinal nerve fiber layer and macular thickness measurement in patients with schizophrenia. Eur J Ophthalmol 2016; 26: 375–378. [DOI] [PubMed] [Google Scholar]
- 82. Chu EM, Kolappan M, Barnes TR, et al. A window into the brain: an in vivo study of the retina in schizophrenia using optical coherence tomography. Psychiatry Res 2012; 203: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mehraban A, Samimi SM, Entezari M, et al. Peripapillary retinal nerve fiber layer thickness in bipolar disorder. Graefes Arch Clin Exp Ophthalmol 2016; 254: 365–371. [DOI] [PubMed] [Google Scholar]
- 84. Kalenderoglu A, Sevgi-Karadag A, Celik M, et al. Can the retinal ganglion cell layer (GCL) volume be a new marker to detect neurodegeneration in bipolar disorder? Compr Psychiatry 2016; 67: 66–72. [DOI] [PubMed] [Google Scholar]
- 85. Khalil MA, Saleh AA, Gohar SM, et al. Optical coherence tomography findings in patients with bipolar disorder. J Affect Disord 2017; 218: 115–122. [DOI] [PubMed] [Google Scholar]
- 86. Polo V, Satue M, Gavin A, et al. Ability of swept source OCT to detect retinal changes in patients with bipolar disorder. Eye (Lond) 2019. 33: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yildiz M, Alim S, Batmaz S, et al. Duration of the depressive episode is correlated with ganglion cell inner plexifrom layer and nasal retinal fiber layer thicknesses: optical coherence tomography findings in major depression. Psychiatry Res Neuroimaging 2016; 251: 60–66. [DOI] [PubMed] [Google Scholar]
- 88. Sönmez I, Kosger F, Aykan U. Retinal nerve fiber layer thickness measurement by spectral-domain optical coherence tomography in patients with major depressive disorder. Noro Psikiyatr Ars 2017; 54: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bresee LC, Majumdar SR, Patten SB, et al. Prevalence of cardiovascular risk factors and disease in people with schizophrenia: a population-based study. Schizophr Res 2010; 117: 75–82. [DOI] [PubMed] [Google Scholar]
- 90. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J Gen Intern Med 2006; 21: 1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry 2013; 170: 324–333. [DOI] [PubMed] [Google Scholar]
- 92. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J 2005; 150: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 93. Lahti M, Tiihonen J, Wildgust H, et al. Cardiovascular morbidity, mortality and pharmacotherapy in patients with schizophrenia. Psychol Med 2012; 42: 2275–2285. [DOI] [PubMed] [Google Scholar]
- 94. Lin HC, Hsiao FH, Pfeiffer S, et al. An increased risk of stroke among young schizophrenia patients. Schizophr Res 2008; 101: 234–241. [DOI] [PubMed] [Google Scholar]
- 95. Malhotra N, Grover S, Chakrabarti S, et al. Metabolic syndrome in schizophrenia. Indian J Psychol Med 2013; 35: 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. De Hert M, Schreurs V, Vancampfort D, et al. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry 2009; 8: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Teixeira PJ, Rocha FL. The prevalence of metabolic syndrome among psychiatric inpatients in Brazil. Braz J Psychiatry 2007; 29: 330–336. [DOI] [PubMed] [Google Scholar]
- 98. Kato MM, Currier MB, Gomez CM, et al. Prevalence of metabolic syndrome in Hispanic and non-Hispanic patients with schizophrenia. Prim Care Companion J Clin Psychiatry 2004; 6: 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kumra S, Oberstar JV, Sikich L, et al. Efficacy and tolerability of second-generation antipsychotics in children and adolescents with schizophrenia. Schizophr Bull 2008; 34: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Correll CU. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J Clin Psychiatry 2008; 69(Suppl. 4): 26–36. [PubMed] [Google Scholar]
- 101. Meier MH, Shalev I, Moffitt TE, et al. Microvascular abnormality in schizophrenia as shown by retinal imaging. Am J Psychiatry 2013; 170: 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Meier MH, Gillespie NA, Hansell NK, et al. Retinal microvessels reflect familial vulnerability to psychotic symptoms: a comparison of twins discordant for psychotic symptoms and controls. Schizophr Res 2015; 164: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Meier M, Byrne E, Martin N, et al. 7.4 phenotypic and genetic associations between schizophrenia and retinal vessel diameter. Schizophr Bull 2018; 44: S12 DOI: 10.1093/schbul/sby014.026. [DOI] [Google Scholar]
- 104. Moises HW, Wollschlager D, Binder H. Functional genomics indicate that schizophrenia may be an adult vascular-ischemic disorder. Transl Psychiatry 2015; 5: e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Whitford V, O‘Driscoll GA, Titone D. Reading deficits in schizophrenia and their relationship to developmental dyslexia: a review. Schizophr Res 2018; 193: 11–22. [DOI] [PubMed] [Google Scholar]
- 106. Khosravani N, Goodarzi MA. Patients with schizophrenia show deficits on spatial frequency doubling. Vision Res 2013; 93: 49–53. [DOI] [PubMed] [Google Scholar]
- 107. Gracitelli CP, Vaz de, Lima FB, Bressan RA, et al. Visual field loss in schizophrenia: evaluation of magnocellular pathway dysfunction in schizophrenic patients and their parents. Clin Ophthalmol 2013; 7: 1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lima FB, Gracitelli CP, Paranhos Junior A, et al. Evaluation of magnocellular pathway abnormalities in schizophrenia: a frequency doubling technology study and clinical implications. Arq Bras Oftalmol 2013; 76: 85–89. [DOI] [PubMed] [Google Scholar]
- 109. Holzman PS, Proctor LR, Levy DL, et al. Eye-tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry 1974; 31: 143–151. [DOI] [PubMed] [Google Scholar]
- 110. Levy DL, Sereno AB, Gooding DC, et al. Eye tracking dysfunction in schizophrenia: characterization and pathophysiology. Curr Top Behav Neurosci 2010; 4: 311–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Slaghuis WL, Bowling AC, French RV. Smooth-pursuit eye movement and directional motion-contrast sensitivity in schizophrenia. Exp Brain Res 2005; 166: 89–101. [DOI] [PubMed] [Google Scholar]
- 112. Keri S, Kelemen O, Janka Z, et al. Visual-perceptual dysfunctions are possible endophenotypes of schizophrenia: evidence from the psychophysical investigation of magnocellular and parvocellular pathways. Neuropsychology 2005; 19: 649–656. [DOI] [PubMed] [Google Scholar]
- 113. Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry 2005; 18: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Anderson AJ, Johnson CA. Frequency-doubling technology perimetry. Ophthalmol Clin North Am 2003; 16: 213–225. [DOI] [PubMed] [Google Scholar]
- 115. Bedwell JS, Miller LS, Brown JM, et al. Functional magnetic resonance imaging examination of the magnocellular visual pathway in nonpsychotic relatives of persons with schizophrenia. Schizophr Res 2004; 71: 509–510. [DOI] [PubMed] [Google Scholar]
- 116. Braus DF, Weber-Fahr W, Tost H, et al. Sensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging study. Arch Gen Psychiatry 2002; 59: 696-701. [DOI] [PubMed] [Google Scholar]
- 117. Martinez A, Hillyard SA, Bickel S, et al. Consequences of magnocellular dysfunction on processing attended information in schizophrenia. Cereb Cortex 2012; 22: 1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Skottun BC, Skoyles JR. Contrast sensitivity and magnocellular functioning in schizophrenia. Vision Research 2007; 47: 2923–2933. [DOI] [PubMed] [Google Scholar]
- 119. Richards W, Felton TB. Spatial frequency doubling: retinal or central? Vision Res 1973; 13: 2129–2137. [DOI] [PubMed] [Google Scholar]
- 120. White AJ, Sun H, Swanson WH, et al. An examination of physiological mechanisms underlying the frequency-doubling illusion. Invest Ophthalmol Vis Sci 2002; 43: 3590–3599. [PubMed] [Google Scholar]
- 121. Jung KI, Kim EK, Park CK. Usefulness of frequency doubling technology perimetry 24-2 in glaucoma with parafoveal scotoma. Medicine (Baltimore) 2017; 96: e6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lim JKH, Li Q-X, He Z, et al. The eye as a biomarker for Alzheimer’s disease. Front Neurosci 2016; 10 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vita A, De Peri L, Deste G, et al. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry 2012; 2: e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Muller N, Weidinger E, Leitner B, et al. The role of inflammation in schizophrenia. Front Neurosci 2015; 9: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Haupt DW. Differential metabolic effects of antipsychotic treatments. Eur Neuropsychopharmacol 2006; 16(Suppl. 3): S149–S155. [DOI] [PubMed] [Google Scholar]
- 126. Tourjman V, Kouassi E, Koue ME, et al. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res 2013; 151: 43–47. [DOI] [PubMed] [Google Scholar]
- 127. Lindstrom E, Wieselgren IM, von Knorring L. Interrater reliability of the structured clinical interview for the positive and negative syndrome scale for schizophrenia. Acta Psychiatr Scand 1994; 89: 192–195. [DOI] [PubMed] [Google Scholar]
- 128. Andreasen NC. The scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl 1989; 155: 49–52. [PubMed] [Google Scholar]
- 129. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007; 4: 28–37. [PMC free article] [PubMed] [Google Scholar]
- 130. Jaaro-Peled H, Ayhan Y, Pletnikov MV, et al. Review of pathological hallmarks of schizophrenia: comparison of genetic models with patients and nongenetic models. Schizophr Bull 2010; 36: 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br J Pharmacol 2011; 164: 1162–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Carpenter WT, Koenig JI. The evolution of drug development in schizophrenia: past issues and future opportunities. Neuropsychopharmacology 2008; 33: 2061–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bodis-Wollner I. Visual deficits related to dopamine deficiency in experimental animals and Parkinson’s disease patients. Trends Neurosci 1990; 13: 296–302. [DOI] [PubMed] [Google Scholar]
- 134. Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med 2009; 39: 1763–1777. [DOI] [PubMed] [Google Scholar]
- 135. Mudo G, Belluardo N, Fuxe K. Nicotinic receptor agonists as neuroprotective/neurotrophic drugs.Progress in molecular mechanisms. J Neural Transm (Vienna) 2007; 114: 135–147. [DOI] [PubMed] [Google Scholar]
- 136. Yokoyama N, Sasaki H, Mori Y, et al. Additive effect of cigarette smoking on gray matter abnormalities in schizophrenia. Schizophr Bull 2018; 44: 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Moschos MM, Nitoda E, Laios K, et al. The impact of chronic tobacco smoking on retinal and choroidal thickness in Greek population. Oxid Med Cell Longev 2016; 2016: 2905789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry 2011; 68: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Akiskal HS, Bourgeois ML, Angst J, et al. Re-evaluating the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. J Affect Disord 2000; 59(Suppl. 1): S5–S30. [DOI] [PubMed] [Google Scholar]
- 140. Smith DJ, Griffiths E, Kelly M, et al. Unrecognised bipolar disorder in primary care patients with depression. Br J Psychiatry 2011; 199: 49–56. [DOI] [PubMed] [Google Scholar]
- 141. Hughes T, Cardno A, West R, et al. Unrecognised bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract 2016; 66: e71–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chengappa KN, Kupfer DJ, Frank E, et al. Relationship of birth cohort and early age at onset of illness in a bipolar disorder case registry. Am J Psychiatry 2003; 160: 1636–1642. [DOI] [PubMed] [Google Scholar]
- 143. Geller B, Tillman R, Craney JL, et al. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry 2004; 61: 459–467. [DOI] [PubMed] [Google Scholar]
- 144. Perlis RH, Miyahara S, Marangell LB, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry 2004; 55: 875–881. [DOI] [PubMed] [Google Scholar]
- 145. Strober M, Schmidt-Lackner S, Freeman R, et al. Recovery and relapse in adolescents with bipolar affective illness: a five-year naturalistic, prospective follow-up. J Am Acad Child Adolesc Psychiatry 1995; 34: 724–731. [DOI] [PubMed] [Google Scholar]
- 146. Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry 2006; 63: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Geller B, Craney JL, Bolhofner K, et al. Two-year prospective follow-up of children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry 2002; 159: 927–933. [DOI] [PubMed] [Google Scholar]
- 148. Moorhead TW, McKirdy J, Sussmann JE, et al. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry 2007; 62: 894–900. [DOI] [PubMed] [Google Scholar]
- 149. Vita A, De Peri L, Sacchetti E. Gray matter, white matter, brain, and intracranial volumes in first-episode bipolar disorder: a meta-analysis of magnetic resonance imaging studies. Bipolar Disord 2009; 11: 807–814. [DOI] [PubMed] [Google Scholar]
- 150. Garcia-Martin E, Gavin A, Garcia-Campayo J, et al. Visual function and retinal changes in patients with bipolar disorder. Retina 2019; 39: 2012–2021. [DOI] [PubMed] [Google Scholar]
- 151. Garcia-Martin E, Larrosa JM, Polo V, et al. Distribution of retinal layer atrophy in patients with Parkinson disease and association with disease severity and duration. Am J Ophthalmol 2014; 157: 470–478.e472. [DOI] [PubMed] [Google Scholar]
- 152. Hebert M, Gagne AM, Paradis ME, et al. Retinal response to light in young nonaffected offspring at high genetic risk of neuropsychiatric brain disorders. Biol Psychiatry 2010; 67: 270–274. [DOI] [PubMed] [Google Scholar]
- 153. Maziade M, Paccalet T, Gagné A-M, et al. 7.2 electroretinographic abnormalities seen in patients affected by schizophrenia or bipolar disorder are detectable early in children born to an affected parent: implications for the staging of risk status in childhood-adolesence. Schizophr Bull 2018; 44: S11. [Google Scholar]
- 154. Levitan RD. The chronobiology and neurobiology of winter seasonal affective disorder. Dialogues Clin Neurosci 2007; 9: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 2007; 64: 327–337. [DOI] [PubMed] [Google Scholar]
- 156. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997; 349: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 157. Friedrich MJ. Depression is the leading cause of disability around the world. JAMA 2017; 317: 1517. [DOI] [PubMed] [Google Scholar]
- 158. Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry 2004; 161: 631–636. [DOI] [PubMed] [Google Scholar]
- 159. Haavik J, Blau N, Thony B. Mutations in human monoamine-related neurotransmitter pathway genes. Hum Mutat 2008; 29: 891–902. [DOI] [PubMed] [Google Scholar]
- 160. Jabbi M, Korf J, Ormel J, et al. Investigating the molecular basis of major depressive disorder etiology: a functional convergent genetic approach. Ann N Y Acad Sci 2008; 1148: 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience 2009; 164: 300–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Zhang X, Bullard KM, Cotch MF, et al. Association between depression and functional vision loss in persons 20 years of age or older in the United States, NHANES 2005-2008. JAMA Ophthalmol 2013; 131: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry 2007; 12: 988–1000. [DOI] [PubMed] [Google Scholar]
- 164. Euesden J, Danese A, Lewis CM, et al. A bidirectional relationship between depression and the autoimmune disorders - New perspectives from the National Child Development Study. PLoS One 2017; 12: e0173015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bruce TO. Comorbid depression in rheumatoid arthritis: pathophysiology and clinical implications. Curr Psychiatry Rep 2008; 10: 258–264. [DOI] [PubMed] [Google Scholar]
- 166. Pryce CR, Fontana A. Depression in autoimmune diseases. Curr Top Behav Neurosci 2017; 31: 139–154. [DOI] [PubMed] [Google Scholar]
- 167. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015; 14: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ardura-Fabregat A, Boddeke E, Boza-Serrano A, et al. Targeting neuroinflammation to treat Alzheimer’s disease. CNS Drugs 2017; 31: 1057–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ferreira ST, Clarke JR, Bomfim TR, et al. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement 2014; 10: S76–S83. [DOI] [PubMed] [Google Scholar]
- 170. Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med 2010; 362: 329–344. [DOI] [PubMed] [Google Scholar]
- 171. Geerlings MI, den Heijer T, Koudstaal PJ, et al. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology 2008; 70: 1258–1264. [DOI] [PubMed] [Google Scholar]
- 172. Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012; 69: 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol 2003; 60: 753–759. [DOI] [PubMed] [Google Scholar]
- 174. Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 2006; 63: 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Speck CE, Kukull WA, Brenner DE, et al. History of depression as a risk factor for Alzheimer’s disease. Epidemiology 1995; 6: 366–369. [DOI] [PubMed] [Google Scholar]
- 176. Sanacora G, Banasr M. From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry 2013; 73: 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci 2007; 257: 250–260. [DOI] [PubMed] [Google Scholar]
- 178. Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Curr Opin Psychiatry 2006; 19: 25–33. [DOI] [PubMed] [Google Scholar]
- 179. Shah PJ, Ebmeier KP, Glabus MF, et al. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry 1998; 172: 527–532. [DOI] [PubMed] [Google Scholar]
- 180. Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 2003; 54: 338–352. [DOI] [PubMed] [Google Scholar]
- 181. Posener JA, Wang L, Price JL, et al. High-dimensional mapping of the hippocampus in depression. Am J Psychiatry 2003; 160: 83–89. [DOI] [PubMed] [Google Scholar]
- 182. Hebert M, Merette C, Paccalet T, et al. Electroretinographic anomalies in medicated and drug free patients with major depression: tagging the developmental roots of major psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 2017; 75: 10–15. [DOI] [PubMed] [Google Scholar]
- 183. Fornaro M, Bandini F, Ogliastro C, et al. Electroretinographic assessment in major depressed patients receiving duloxetine: might differences between responders and non-responders indicate a differential biological background? J Affect Disord 2011; 135: 154–159. [DOI] [PubMed] [Google Scholar]
- 184. Bubl E, Kern E, Ebert D, et al. Seeing gray when feeling blue? Depression can be measured in the eye of the diseased. Biol Psychiatry 2010; 68: 205–208. [DOI] [PubMed] [Google Scholar]
- 185. Bubl E, Ebert D, Kern E, et al. Effect of antidepressive therapy on retinal contrast processing in depressive disorder. Br J Psychiatry 2012; 201: 151–158. [DOI] [PubMed] [Google Scholar]
- 186. Anda R, Williamson D, Jones D, et al. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology 1993; 4: 285–294. [DOI] [PubMed] [Google Scholar]
- 187. Ford DE, Mead LA, Chang PP, et al. Depression is a risk factor for coronary artery disease in men: the precursors study. Arch Intern Med 1998; 158: 1422–1426. [DOI] [PubMed] [Google Scholar]
- 188. Pratt LA, Ford DE, Crum RM, et al. Depression, psychotropic medication, and risk of myocardial infarction. Prospective data from the Baltimore ECA follow-up. Circulation 1996; 94: 3123–3129. [DOI] [PubMed] [Google Scholar]
- 189. Glassman AH, Shapiro PA. Depression and the course of coronary artery disease. Am J Psychiatry 1998; 155: 4–11. [DOI] [PubMed] [Google Scholar]
- 190. Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak–the link between depression and cardiovascular disease. Nat Rev Cardiol 2012; 9: 526–539. [DOI] [PubMed] [Google Scholar]
- 191. Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull 2005; 131: 260–300. [DOI] [PubMed] [Google Scholar]
- 192. Jakobsen AH, Foldager L, Parker G, et al. Quantifying links between acute myocardial infarction and depression, anxiety and schizophrenia using case register databases. J Affect Disord 2008; 109: 177–181. [DOI] [PubMed] [Google Scholar]
- 193. Meier MH, Gillespie NA, Hansell NK, et al. Associations between depression and anxiety symptoms and retinal vessel caliber in adolescents and young adults. Psychosom Med 2014; 76: 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Kalenderoglu A, Celik M, Sevgi-Karadag A, et al. Optic coherence tomography shows inflammation and degeneration in major depressive disorder patients correlated with disease severity. J Affect Disord 2016; 204: 159–165. [DOI] [PubMed] [Google Scholar]
- 195. Schonfeldt-Lecuona C, Schmidt A, Kregel T, et al. Retinal changes in patients with major depressive disorder - A controlled optical coherence tomography study. J Affect Disord 2018; 227: 665–671. [DOI] [PubMed] [Google Scholar]
- 196. George MS, Ketter TA, Parekh PI, et al. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry 1995; 152: 341–351. [DOI] [PubMed] [Google Scholar]
- 197. Pardo JV, Pardo PJ, Raichle ME. Neural correlates of self-induced dysphoria. Am J Psychiatry 1993; 150: 713–719. [DOI] [PubMed] [Google Scholar]
- 198. Baxter LR, Jr, Schwartz JM, Phelps ME, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46: 243–250. [DOI] [PubMed] [Google Scholar]
- 199. Bench CJ, Friston KJ, Brown RG, et al. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med 1993; 23: 579–590. [DOI] [PubMed] [Google Scholar]
- 200. Kimbrell TA, Little JT, Dunn RT, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry 1999; 46: 1603–1613. [DOI] [PubMed] [Google Scholar]
- 201. Martinot JL, Hardy P, Feline A, et al. Left prefrontal glucose hypometabolism in the depressed state: a confirmation. Am J Psychiatry 1990; 147: 1313–1317. [DOI] [PubMed] [Google Scholar]
- 202. Ashraf H, Nowroozzadeh MH. Diurnal variation of retinal thickness in healthy subjects. Optom Vis Sci 2014; 91: 615–623. [DOI] [PubMed] [Google Scholar]
- 203. Read SA, Collins MJ, Alonso-Caneiro D. Diurnal variation of retinal thickness with spectral domain OCT. Optom Vis Sci 2012; 89: 611–619. [DOI] [PubMed] [Google Scholar]
- 204. Jo YJ, Heo DW, Shin YI, et al. Diurnal variation of retina thickness measured with time domain and spectral domain optical coherence tomography in healthy subjects. Invest Ophthalmol Vis Sci 2011; 52: 6497–6500. [DOI] [PubMed] [Google Scholar]
- 205. Ferro Desideri L, Barra F, Skhiri MI, et al. Methodological concerns on retinal thickness evaluation by spectral domain optical coherence tomography in patients with major depressive disorder. J Affect Disord 2018; 238: 226–227. [DOI] [PubMed] [Google Scholar]
- 206. Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45: 54–63. [DOI] [PubMed] [Google Scholar]
- 207. aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ 2009; 180: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Thase ME. Molecules that mediate mood. N Engl J Med 2007; 357: 2400–2402. [DOI] [PubMed] [Google Scholar]
- 209. Tian T, Zhu XH, Liu YH. Potential role of retina as a biomarker for progression of Parkinson’s disease. Int J Ophthalmol 2011; 4: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Salas M, Drexler W, Levecq X, et al. Multi-modal adaptive optics system including fundus photography and optical coherence tomography for the clinical setting. Biomed Opt Express 2016; 7: 1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Brandt AU, Oberwahrenbrock T, Mikolajczak J, et al. Visual dysfunction, but not retinal thinning, following anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm 2016; 3: e198. [DOI] [PMC free article] [PubMed] [Google Scholar]