Abstract
The most common cause of pulmonary artery filling defects on computed tomography pulmonary angiography or magnetic resonance imaging is pulmonary thromboembolism, but not infrequently, the presentation of this finding lacks specificity. Given that the morbidity and mortality associated with pulmonary thromboembolism is high, proper diagnosis of the condition is essential. Unusual or more rarely encountered etiologies must be considered when clinical manifestations and imaging findings are inconsistent. With this review, our purpose is to describe possible causes of pulmonary arterial filling defects. We aim to provide clinicians with a comprehensive list of differential diagnoses to facilitate a measured approach to the assessment of pulmonary arterial filling defects on computed tomography pulmonary angiography or magnetic resonance imaging.
Keywords: pulmonary artery filling defect, computed tomography pulmonary angiography, magnetic resonance imaging
Given the fact that pulmonary thromboembolism (PTE) is a relatively common pulmonary arterial disorder, the importance of quick, accurate diagnosis of the condition is paramount. Computed tomography pulmonary angiography (CTPA) has become the primary method used for the diagnosis of PTE in patients, largely replacing the previous method of choice, pulmonary angiography. Pulmonary arterial filling defects on CTPA is a quite important imaging finding to diagnose PTE with a sensitivity of 83–100% and a specificity of 89–96%;1,2 however, other clinical conditions may present with similar intraluminal filling defects on CTPA, truly mimicking PTE and leading to inappropriate diagnosis and possibly delayed intervention. The primary purpose of this paper is to describe the diseases that could cause pulmonary artery filling defects on CTPA and magnetic resonance imaging (MRI), to make clinicians aware of the various manifestations of these defects in the critical evaluation of patients suspected of having PTE. The intraluminal filling defects to be discussed include: PTE, nonthrombotic pulmonary embolism (NTPE), mimickers of PTE including primary pulmonary arterial neoplasm, pulmonary vascular involvement of IgG4-related disease (IgG4-RD), Takayasu's arteritis (TA), Behcet's disease, and Hughes–Stovin syndrome (HSS), as well as pulmonary arterial streak artifact (Table 1).
Table 1.
Diseases and differential diagnosis of pulmonary arterial filling defect sign.
| Categories | Diseases | CT | MRI | 18F FDG PET | Clinical information | |
|---|---|---|---|---|---|---|
| PTE | Acute PTE | Preserved caliber of the vessel; central or eccentric filling defect in “Polo mint” sign or “railway track” sign | Anticoagulant and thrombolytic therapy is effective | |||
| Chronic PTE | Vessel narrowing/complete amputation; intimal irregularities; webs/bands | Mild hypointensity on fat-suppressed T2WI without enhancement on contrasted images | A history of acute PE or deep vein thrombosis | |||
| NTPE | Tumor embolism | Malignant embolism | Central or eccentric filling defect in “Polo mint” sign or “railway track” sign; tumor enhancement of the filling defect | High uptake | The history of neoplasm; no resolution and even progresses at follow-up examination despite anticoagulant or thrombolytic therapy | |
| Leiomyoma embolism | The fill defect originating from the uterus and extending to the inferior vena cava, right heart, and pulmonary artery | Uterine fibroids or uterine fibroid surgery history | ||||
| Angiomyolipoma embolism | The fill defect in fat intensity with contrasted enhancement | Mild uptake | Renal angiomyolipoma | |||
| Hydatid cyst embolism | Filling defect with preserved caliber of the vessel even mild dilatation | The multi-cystic nature in hyperintensity of the filling defect on T2WI | Hydatid disease history | |||
| Inorganic particulate embolism | High attenuation in pulmonary artery in non-contrast chest CT | |||||
| Mimickers of PE | Pulmonary arterial malignancy | Pulmonary arterial sarcoma | The proximal margin of the filling defect with the “lobulated sign” or the “tongue sign”/the grape-like appearance of the distal PA with heterogeneous enhancement | Hyperintensity on fat-suppressed T2WI and DWI; hypointensity on Apparent Diffusion Coefficient (ADC) map. heterogeneous enhancement on contrasted images | Uneven high uptake | No resolution and progresses at follow-up examination despite anticoagulant or thrombolytic therapy |
| Pulmonary arterial benign tumor | Pulmonary arterial myxoma | Hyperintensity on T2WI and fat-saturated sequence. More heterogeneous enhancement on late gadolinium enhancement sequences | ||||
| Pulmonary arterial lipoma | Fat intensity in pulmonary artery | High intensity in T1WI and T2WI; low intensity in fat-saturated sequence | Negative uptake | No resolution at follow-up examination despite anticoagulant or thrombolytic therapy | ||
| Pulmonary arterial IgG4-related disease | Massive filling defects without enhancement or pulmonary artery aneurysm on CTPA | Weak uptake | Most cases had more than one organ affected, mostly with significantly increased serum IgG4 levels | |||
| Takayasu's arteritis | Vessel narrowing/complete amputation in pulmonary artery and aorta and branches, thickened and enhanced arterial wall in “double ring sign” | Hypointensity on fat-suppressed T2WI with enhancement on gadolinium enhancement sequence in double ring sign | High uptake | |||
| Behcet's disease/ Hughes–Stovin syndrome | Filling defect in pulmonary artery aneurysm/vessel narrowing/complete amputation/thrombosis of major veins | vasculitis and recurrent ulcers of the oral and genital mucosa, with relapsing uveitis | ||||
| PA streak artifact | filling defect in early phase contrast-enhanced imaging disappears in the late phase | |||||
CT: computed tomography; MRI: magnetic resonance imaging; PTE: pulmonary thromboembolism; T2WI: T2-weighted image; PE: pulmonary embolism; NTPE: nonthrombotic pulmonary embolism; CTPA: computed tomography pulmonary angiography.
Pulmonary thromboembolism
Acute pulmonary thromboembolism (APTE) is the third most common cardiovascular condition, after coronary artery disease and stroke.3 Since fresh thrombus consists of red cells and platelets in a fibrin mesh, the typical findings of APTE on CTPA is the filling defect leading to partial stenosis or complete obstruction of lumen. The “Polo mint” sign4 or “railway track” sign (Fig. 1) is described when the fresh thrombus is located in the center of the lumen and surrounded by contrast agent. When the fresh thrombus attaches to the pulmonary arterial wall, the filling defects formed acute angles with the vessel wall (Fig. 1), resulting in an off-centered stenosis.
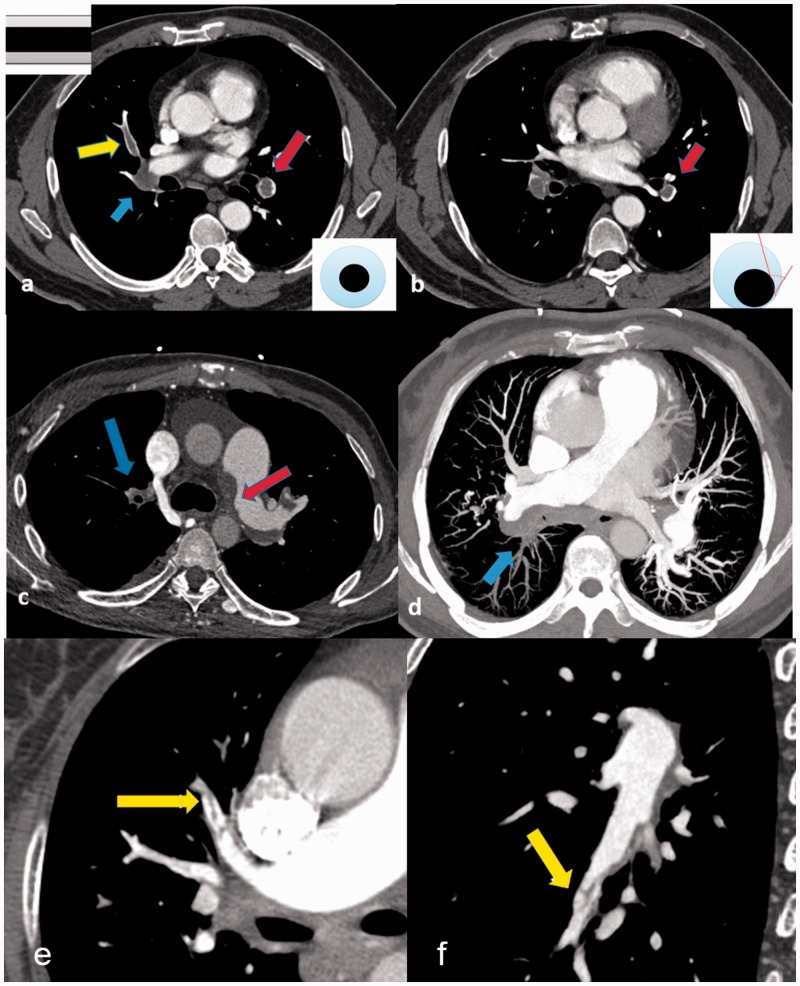
Fig. 1.
CT features of PTE: (a) acute PTE (transversal plane): filling defect of left lower pulmonary artery in “Polo mint” sign (red arrow) and filling defect of right middle pulmonary artery in “railway track” sign (yellow arrow), right lower lobe pulmonary artery occlusion (blue arrow); (b) acute PTE (transversal plane): the off-centered filling defect formed acute angles with the vessel wall (red arrow); (c) chronic PTE (transversal plane): the eccentric filling defect forming obtuse angle with the left pulmonary arterial wall (red arrow) and pouch defect of right upper pulmonary artery (blue arrow); (d) chronic PTE (maximum intensity projection): the eccentric filling defect forming obtuse angle with the right pulmonary arterial wall and pouch defect of right lower pulmonary artery (blue arrow); (e) chronic PTE (transversal plane): band-like filling defect of anterior superior lobe pulmonary artery (yellow arrow); and (f) chronic PTE (transversal plane): web-like filling defect of right lower lobe outer basal segment pulmonary artery (yellow arrow).
Chronic pulmonary thromboembolism (CPTE) results from incomplete resolution of thrombi. Residual organized fibrotic clot remains tightly attached to the thickened pulmonary arterial intima, occluding pulmonary vessels, and leading to a complex restructuring process in the pulmonary arteries, increasing pulmonary vascular resistance, subsequently causing pulmonary hypertension.5 The key finding on CTPA denoting CPTE (Fig. 1) is the presence of an eccentric filling defect forming obtuse angles with the arterial wall. Other findings include webs, bands, or obstructed thread-like arteries, abrupt cutoffs, narrowing arteries, and post-stenotic dilatation. Occasionally, calcifications can be observed in the arterial intima or calcified emboli.6 The typical finding of CPTE on MRI is the filling defect in mild hypointensity on fat-suppressed T2-weighted Image (T2WI) without significant contrasted enhancement which is significantly different with pulmonary artery sarcoma (PAS).7
Nonthrombotic pulmonary embolism
NTPE is defined as pulmonary arterial embolization with nonthrombotic emboli, including tumor embolism, hydatid cyst embolism, or other inorganic particulate embolism. Its pathogenesis is more complex than PTE. Given the unusual clinical signs and often atypical radiologic features of NTPE, its diagnosis is challenging.
Pulmonary arterial tumor embolism (PATE) refers to the embolization of massive tumor tissue in the pulmonary artery (PA). Most of them are malignant tumor embolism which has been reported in patients with hepatic, gastric, breast, renal cell carcinomas, osteogenic sarcoma, or lymphoma.8,9 Some unique cases were diagnosed as PATE from right heart myxoma9,10 and leiomyoma embolism.11,12 Massive PATE could simulate a typical APTE, with the low-attenuation filling defects of the PA in “polo mint” sign, “railway track” sign, or complete occlusion on CTPA (Fig. 2). The differentiation of PATE from APTE on CTPA is not easy in patients with malignancies, since it has been reported that these patients had a four-fold to six-fold risk of developing PTE.13 The lack of a sufficient reduction in size or even progress, despite adequate thrombolytic therapy, should raise suspicion for PATE in patients with a history of malignancy. High uptake on 18F fluro deoxyglucose positron emission tomography (18 F-FDG PET)/CT strongly suggests PATE; however, notably, negative uptake cannot exclude PATE.14 For these patients, a biopsy of the filling defects in PA is necessary.
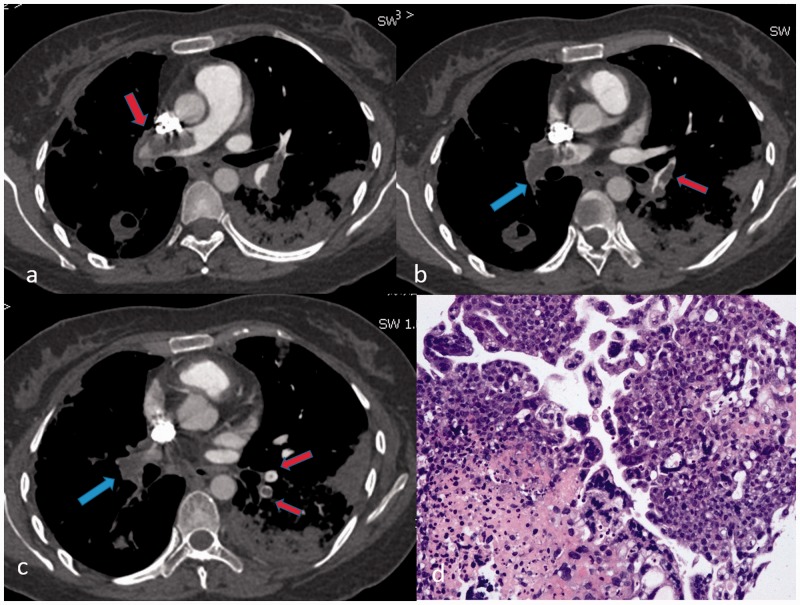
Fig. 2.
Pulmonary choriocarcinoma embolism on CTPA (transversal plane) in a 34-year-old female patient with choriocarcinoma. (a) Filling defect of bilateral pulmonary artery in railway track sign (red arrow); (b) abrupt cutoffs of right lower pulmonary artery (blue arrow) and railway track sign in left lung tongue pulmonary artery (red arrow); (c) pouch defect of right lower pulmonary artery (blue arrow) and polo mint sign of basal pulmonary artery in the left lower lobe (red arrow); (d) biopsy of left lower lobe by Zeek thrombus aspiration catheter and photomicrograph (hematoxylin–eosin stain; original magnification, × 200) demonstrates syncytiotrophoblast and cytotrophoblast.
Pulmonary arterial leiomyoma embolism arises from either a uterine leiomyoma or the smooth muscle layers of the uterine veins.11,12 The tumor extends primarily through the uterine veins, sometimes reaching the inferior vena cava, the right cardiac chambers, and then embolizing PA. The characteristic finding of intravenous leiomyomatosis on CTPA is a long filling defect extending into these same vascular structures (Supplement 1). Pelvis contrasted CT may indicate the presence of the fill defect originating from the uterus and extending to the inferior vena cava. In such cases, prompt surgical intervention is mandatory.1,2 Recently, pulmonary arterial angiomyolipoma embolism from renal angiomyolipoma has been found. The typical CT findings include the filling defect of PA in fat intensity, fat emboli in renal vein, as well as renal angiomyolipoma (Supplement 2).
Pulmonary arterial hydatid cyst embolism is one extremely rare clinical entity of NTPE.15–17 Organisms that reach the gastrointestinal system go to the liver via the portal vein and then to the right heart and to the lung via the PA. The diagnosis of pulmonary arterial hydatid cyst embolism is dependent on both medical history and characteristic MRI findings. CTPA shows the filling defects in the lumen of the PA, in contrast, the multi-cystic nature in hyperintensity of the filling defect on T2WI strongly suggest pulmonary hydatid cyst embolism (Fig. 3).
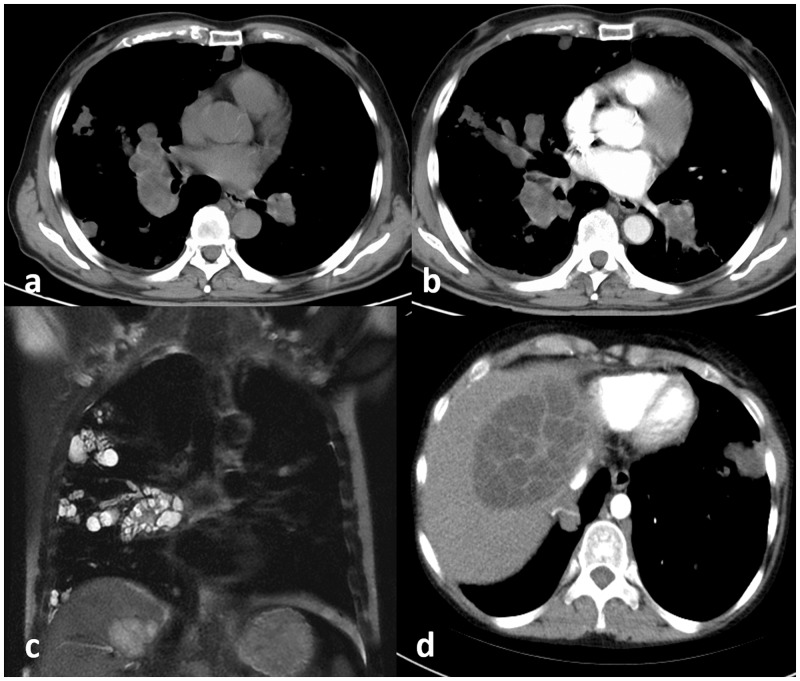
Fig. 3.
Pulmonary arterial hydatid cyst embolism in a 50-year-old patient with hepatic hydatid. (a) Axial non-contrasted CT shows hypotensive nodules in right lung and aneurysmal expansion of the bilateral lower pulmonary artery; (b) axial CTPA shows multiple cysts fills in bilateral pulmonary artery; (c) coronal T2WI shows hyperintense multiple cystic nodules in the right lung and lower pulmonary arteries; and (d) axial liver contrasted CT reveals typically hydatid liver cyst.
Pulmonary arterial cement embolism (PACE) is one of the inorganic particulate embolism, which occurs due to embolization into the bloodstream of polymethyl-methacrylate (cement) during surgical procedures like vertebroplasty or kyphoplasty.18–20 High focal pressure may facilitate the entry of cement into the venous system, but the occurrence of PACE is independent of the volume of injected cement. Kim et al.20 reported an incidence of 23.0% after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. In patients with vertebroplasty or kyphoplasty, a high-attenuation branching linear opacities filling the distal PA branches and along the spine on non-contrast chest CT strongly helps with the diagnosis of PCE (Supplement 3). Moreover, other iatrogenic embolism in high attenuation in the pulmonary arteries, such as prostate seeds, IVC filter legs, catheter fragments, CardioMEMS devices, and embolization material from a neuro procedure also have been reported.18,19
Primary pulmonary arterial neoplasm
Primary pulmonary arterial neoplasms are very uncommon. The majority are pulmonary artery sarcoma (PAS) with a poor prognosis. PAS is a rare malignancy arising from the mesenchymal cells of the PA. The primary manifestation of PAS on CTPA also is the filling defect that extremely resembles APE.21 Always, an unresolved APE after effective anticoagulation leads to the suspicion of PAS. There are more specific findings on CTPA that strongly suggest PAS, including a filling defect involving the entire main PA or one of its principal branches, the proximal margin of the filling defect with the “lobulated sign” or the “tongue sign” (Fig. 4) and the grape-like appearance of the distal PA7 and the filling defect with heterogeneous enhancement. The grape-like appearance of the dilated distal PA and extra-arterial invasion are recognized as the most specific findings for PAS. Moreover, 18F FDG PET/CT or MRI is often required as the non-invasive way to assess PAS. Strong uptake on 18F FDG PET/CT was observed in most PAS cases; however, weak or negative uptake could not exclude PAS.22,23 Liu et al.7 suggested that the filling defect with uneven hyperintensity on fat-suppressed T2-weighted Imaging (T2WI) and Diffusion Weighted Imaging (DWI) as well as heterogeneous enhancement on gadolinium-enhanced sequences were the characteristics of PAS on MRI (Supplement 4). Occasionally, APE could mimic PAS with some uncommon features on CTPA. MRI is recommended as an alternative to CTPA following short-term thrombolytic therapy or adequate anticoagulation treatment to avoid too much radiation exposure.
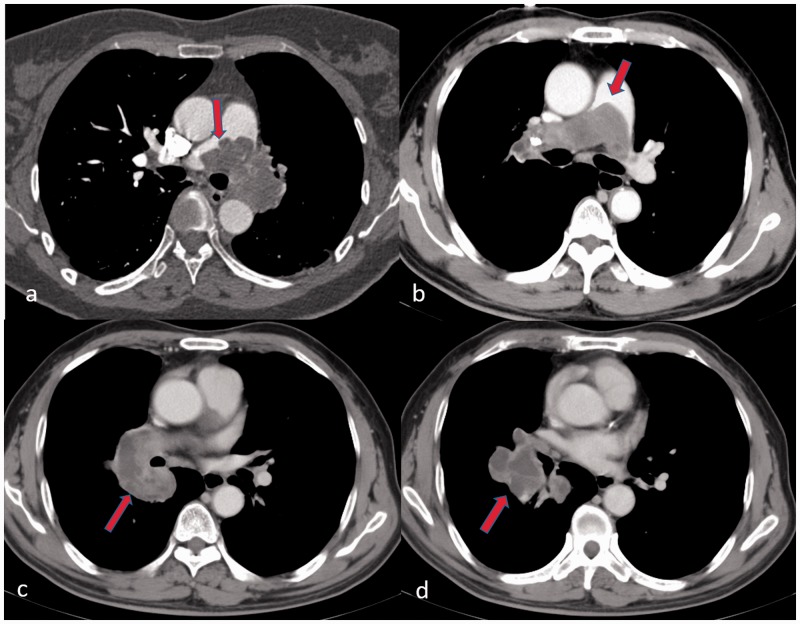
Fig. 4.
Imaging findings of PAS on CTPA (transversal plane): (a) the proximal margin of filling defect in lobular sign (red arrow); (b) a tongue sign (red arrow) of filling defect in main pulmonary artery; (c) heterogeneous enhancement (red arrow) of filling defect in right lower pulmonary artery; and (d) aneurysmal dilation and massive filling defect (red arrow) of the basal pulmonary artery is one specific finding of PAS.
Pulmonary arterial benign tumor is extremely rare. Less than five cases of primary pulmonary arterial myxoma have been reported.24,25 On CTPA, PA myxoma shows an irregular appearance mimicking APE. On MRI, the filling defect in hypointensity on T1-weighted Imaging (T1WI) and hyperintensity on T2WI and fat-saturated sequence are seen in the main PA. The myxoma shows more heterogeneous enhancement on late gadolinium enhancement sequences which is consistent with cardiac myxoma; however, differential diagnosis of PAS and pulmonary arterial myxoma is very challenging. Pulmonary arterial lipoma is a rare benign tumor that also shows the filling defect on CTPA. The key finding that differentiates primary pulmonary lipoma from PTE is fat intensity on CT and MRI.26
IgG4-RD of PA
IgG4-RD is an immune-mediated chronic fibrotic inflammation which can affect virtually any organ. However, IgG4-RD of PA were extremely reported.27,28 The prominent findings of PA IgG4-RD includes massive filling defects without enhancement or PA aneurysm on CTPA. Lesions with weak standard uptake value intake on PET were initially suspected as APE or PTUE. However, most cases had more than one organ affected, mostly with elevated IgG4 serum concentrations.27,28 Definite identification of IgG4-related pulmonary vascular disease always requires an intrathoracic surgical biopsy.
Takayasu's arteritis
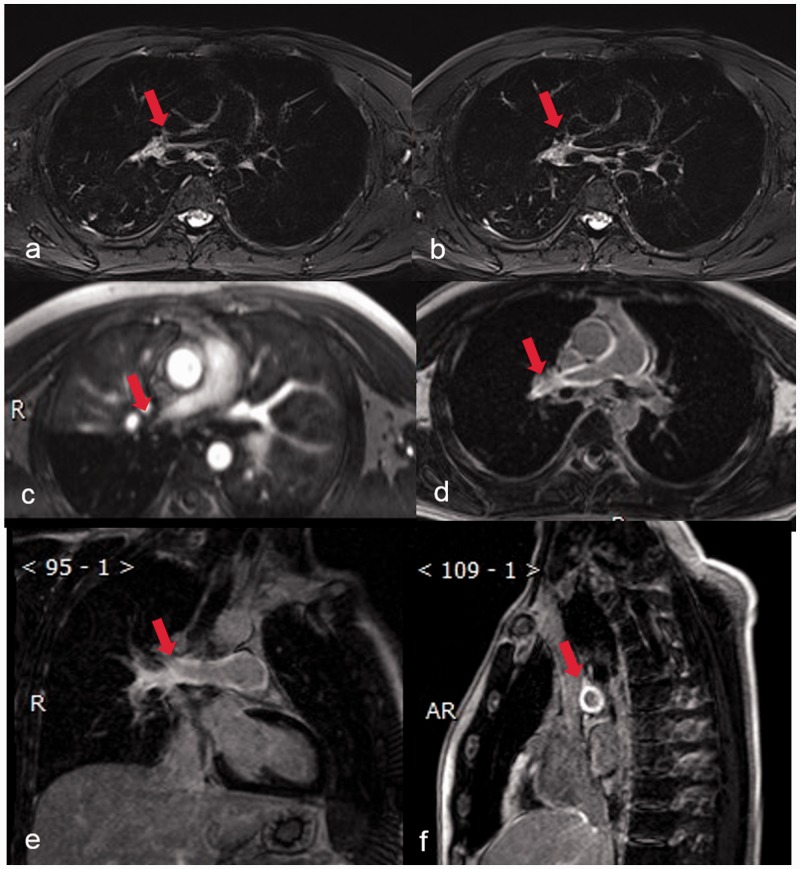
TA is an idiopathic inflammatory disease that primarily affects large vessels such as the aorta and its major branches while young women are predominantly affected. Studies describe there being pulmonary arterial involvement in approximately 63.3% of cases.29 In the rare case of reported isolated PA involvement, vessel stenosis or complete amputation of PA mimics the CPTE.30,31 On the post-enhanced CTA images, it exhibits a “double ring sign” while in the late phase (occlusive stage), arterial stenosis, occlusion, or aneurysmal dilatation may occur, associated with the mural thickening.32 MRI is the alternate radiological modality that can be conducted without radiation exposure for the assessment of suspected TA.33 Concentric wall thickening in high-intensity areas and enhancement of the arterial wall strongly suggests active inflammation (Fig. 5).34,35
Fig. 5.
MRI of a 33-year-old female patient with TA. (a and b) axial fat-suppressed T2WI shows right pulmonary artery stenosis with concentric mural thickening (red arrow); (c) axial contrast-enhanced MRA showed right pulmonary artery stenosis and lower lobe pulmonary artery occlusion (red arrow); (d) axial delayed contrast-enhanced MRI showed delay enhancement in right pulmonary arterial wall (red arrow); (e) delayed contrast-enhanced MRI in oblique coronal plane shows contrasted-enhancement in right pulmonary arterial wall (red arrow); and (f) delayed contrast-enhanced MRI in oblique sagittal plane shows concentric enhancement in right pulmonary arterial wall (red arrow).
Behcet's disease and Hughes-Stovin Syndrome
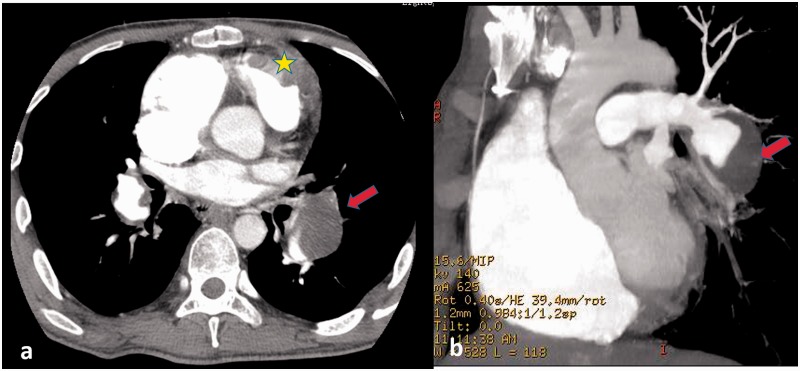
Behcet's disease is an idiopathic syndrome characterized by vasculitis and recurrent ulcers of the oral and genital mucosa, with relapsing uveitis. Vascular involvement occurs in 5–30% of Behcet's disease cases.36 On CTA, aneurysms are the most common finding, and these generally involve the pulmonary arteries but can also occur anywhere in the systemic circulation. PA aneurysm is the most common finding with a prevalence of 1–10% and tends to be multiple and bilateral.37 The pulmonary thrombosis of the aneurysm forms an in situ partial or complete filling defect (Fig. 6), and stenosis may also be found in the involved PA. Notably, PA aneurysms have a poor prognosis. Thrombosis of major veins such as the superior vena cava is a common finding in patients with or without PA aneurysms.
Fig. 6.
Behcet's disease on CTPA in a 24-year-old man. (a) Pulmonary aneurysm of bilateral lower lobes with massive filling defect in the left lower lobe (red arrow) and right ventricular thrombosis in situ (yellow star) and (b) maximum intensity projection of CTPA (oblique coronal plane) show the filling defect in pulmonary aneurysm of the left lower lobe (red arrow).
Hughes-stovin syndrome (HSS) is a rare disorder whose cause is unknown.38 The condition is also characterized by multiple PA and/or bronchial artery aneurysms as well as deep vein thrombosis, but the condition can be distinguished from Behcet's disease by the absence of mucocutaneous findings.39 HSS has been variably described as “the cardiovascular manifestation of Behcet's disease,” “incomplete Behcet's,” and “a rare case of Behcet's disease” in the literature.38
Pulmonary arterial streak artifact
Lung diseases such as tuberculosis, bronchiectasis, and some conditions such as pulmonary vein stenosis after radiofrequency ablation, systemic artery-PA shunt, and pulmonary hypertension may affect the hemodynamics of the PA and cause “streak artifact,” which could mimic a PA filling defect. Using the dual-phase scan protocol, a filling defect in early phase contrast-enhanced imaging disappears in the late phase strongly suggests a pulmonary arterial streak artifact (Supplement 5).
Conclusions
PA filling defects on CTPA or MRI may be observed in a spectrum of pathologic processes other than PTE. Definitive diagnosis may require correlation of the imaging findings with the clinical, laboratory, or even the histopathological results. To allow for appropriate therapeutic management, awareness of the various disease entities presenting as PA filling defects or stenosis and knowledge of the entire spectrum of their imaging features are essential.
Supplemental Material
Supplemental material, PUL910687 Supplemental Material for The filling defect of pulmonary artery, an imaging finding what we should know by Min Liu, Xin Cao Tao, Zhenguo Zhai, Zhanhong Ma, Li Zhu and Jie Luo in Pulmonary Circulation
Guarantor
M.L. and Z.Z. are the guarantor of this research and take responsibility for the integrity of this work.
Contributorship
All authors contributed to manuscript preparation and revisions and provided final approval of the version for publication.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work is supported by National Natural Science Foundation of China (81871328), Beijing Nature Science Foundation (7182149), China Medical Science and Technology College Youth Medical Talent Award Program (2018RC320013), Beijing Science and Technology Commission Pharmaceutical and Technology Innovation Project (Z181100001918034), and Beijing University of Chemical Technology-China-Japan Friendship Hospital Research Project (PYBA1807).
ORCID iD
Supplemental material
Supplemental material for this article is available online.
References
- 1.Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000; 217: 447–455. [DOI] [PubMed] [Google Scholar]
- 2.Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006; 354: 2317–2327. [DOI] [PubMed] [Google Scholar]
- 3.Giuntini C, Di Ricco G, Marini C, et al. Pulmonary embolism: epidemiology. Chest 1995; 107: 3S–9S. [DOI] [PubMed] [Google Scholar]
- 4.Wittram C, Maher MM, Yoo AJ, et al. CT angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. Radiographics 2004; 24: 1219–1238. [DOI] [PubMed] [Google Scholar]
- 5.Mahmud E, Madani MM, Kim NH, et al. Chronic thromboembolic pulmonary hypertension: evolving therapeutic approaches for operable and inoperable disease. J Am Coll Cardiol 2018; 71: 2468–2486. [DOI] [PubMed] [Google Scholar]
- 6.Castaner E, Gallardo X, Ballesteros E, et al. CT diagnosis of chronic pulmonary thromboembolism. Radiographics 2009; 29: 31–50. discussion 50–33. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Luo C, Wang Y, et al. Multiparametric MRI in differentiating pulmonary artery sarcoma and pulmonary thromboembolism: a preliminary experience. Diagn Interv Radiol 2017; 23: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez Baztarrica G, Nieva N, Gariglio L, et al. Images in cardiovascular medicine. Primary cardiac lymphoma: a rare case of pulmonary tumor embolism. Circulation 2010; 121: 2249–2250. [DOI] [PubMed] [Google Scholar]
- 9.Jorens PG, Van Marck E, Snoeckx A, et al. Nonthrombotic pulmonary embolism. Eur Respir J 2009; 34: 452–474. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Tripathy MP, Mohanty BB, et al. Sporadic multicentric right atrial and right ventricular myxoma presenting as acute pulmonary thromboembolism. Heart Views 2016; 17: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terada T. Vascular leiomyoma of the lung arising from pulmonary artery. Int J Clin Exp Pathol 2013; 6: 97–99. [PMC free article] [PubMed] [Google Scholar]
- 12.Song BG, Park YH, Kang GH, et al. Intravenous leiomyomatosis with intracardiac extension. Asian Cardiovasc Thorac Ann 2011; 19: 179. [DOI] [PubMed] [Google Scholar]
- 13.Bierry G, Holl N, Kellner F, et al. Venous thromboembolism and occult malignancy: simultaneous detection during pulmonary CT angiography with CT venography. AJR Am J Roentgenol 2008; 191: 885–889. [DOI] [PubMed] [Google Scholar]
- 14.Schmid S, Ohlschlegel C, Nagel W, et al. Pulmonary embolism in a patient with primary renal synovial sarcoma: the important differential diagnosis of tumor embolism and its therapeutic implications. Case Rep Oncol 2013; 6: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akgun V, Battal B, Karaman B, et al. Pulmonary artery embolism due to a ruptured hepatic hydatid cyst: clinical and radiologic imaging findings. Emerg Radiol 2011; 18: 437–439. [DOI] [PubMed] [Google Scholar]
- 16.Poyraz N, Demirbas S, Korkmaz C, et al. Pulmonary embolism originating from a hepatic hydatid cyst ruptured into the inferior vena vava: CT and MRI findings. Case Rep Radiol 2016; 2016: 3589812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savas G, Cosgun MS, Karabiyik U, et al. A rare cause of pulmonary embolism: hydatid cyst. Am J Respir Crit Care Med 2017; 196: 386–387. [DOI] [PubMed] [Google Scholar]
- 18.Unal E, Balci S, Atceken Z, et al. Nonthrombotic pulmonary artery embolism: imaging findings and review of the literature. AJR Am J Roentgenol 2017; 208: 505–516. [DOI] [PubMed] [Google Scholar]
- 19.Rudkovskaia AA, Bandyopadhyay D. Intraluminal arterial filling defects misdiagnosed as pulmonary emboli: what else could they be?. Clin Chest Med 2018; 39: 505–513. [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Lee JW, Park KW, et al. Pulmonary cement embolism after percutaneous vertebroplasty in osteoporotic vertebral compression fractures: incidence, characteristics, and risk factors. Radiology 2009; 251: 250–259. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez A, Sauler M, Mitchell JM, et al. Unresolved pulmonary embolism leading to a diagnosis of pulmonary artery sarcoma. Heart Lung 2014; 43: 574–576. [DOI] [PubMed] [Google Scholar]
- 22.Bandyopadhyay D, Panchabhai TS, Bajaj NS, et al. Primary pulmonary artery sarcoma: a close associate of pulmonary embolism-20-year observational analysis. J Thorac Dis 2016; 8: 2592–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hmelik S, Dobrenic M, Huic D. F-18 FDG PET/CT in pulmonary artery sarcoma: clinical vignette. Nucl Med Rev Cent East Eur 2018; 21: 48–49. [DOI] [PubMed] [Google Scholar]
- 24.Pereira R, Santos N, Cascarejo J, et al. Primary myxoma of the pulmonary artery. Clinical report. Rev Port Cir Cardiotorac Vasc 2009; 16: 135–137. [PubMed] [Google Scholar]
- 25.Mai XL, Fan HJ, Li BX, et al. Primary pulmonary artery myxoma: a rare case. Clin Imaging 2013; 37: 159–162. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Tao X, Xie W, et al. Primary pulmonary artery lipoma mimicking pulmonary thromboembolism. Am J Respir Crit Care Med 2018; 198: e111–e113. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Shao L, Ruan W, et al. Pulmonary vascular involvement of IgG4-related disease: case series with a PRISMA-compliant systemic review. Medicine (Baltimore) 2019; 98: e14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng H, Zhao S, Yue Y, et al. IgG4-related disease of pulmonary artery causing pulmonary hypertension. Medicine (Baltimore) 2018; 97: e10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu FP, Luo S, Wang ZJ, et al. Takayasu arteritis: imaging spectrum at multidetector CT angiography. Br J Radiol 2012; 85: e1282–e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucuk M, Oncel CR, Ucar M, et al. Pulmonary endarterectomy for large vessel pulmonary arteritis mimicking chronic thromboembolic disease. Arch Rheumatol 2016; 31: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riancho-Zarrabeitia L, Zurbano F, Gomez-Roman J, et al. Isolated pulmonary vasculitis: case report and literature review. Semin Arthritis Rheum 2015; 44: 514–517. [DOI] [PubMed] [Google Scholar]
- 32.Hur JH, Chun EJ, Kwag HJ, et al. CT features of vasculitides based on the 2012 international Chapel Hill Consensus Conference revised classification. Korean J Radiol 2017; 18: 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsunaga N, Hayashi K, Sakamoto I, et al. Takayasu arteritis: MR manifestations and diagnosis of acute and chronic phase. J Magn Reson Imaging 1998; 8: 406–414. [DOI] [PubMed] [Google Scholar]
- 34.Castaner E, Alguersuari A, Gallardo X, et al. When to suspect pulmonary vasculitis: radiologic and clinical clues. Radiographics 2010; 30: 33–53. [DOI] [PubMed] [Google Scholar]
- 35.Liu M, Liu W, Li H, et al. Evaluation of Takayasu arteritis with delayed contrast-enhanced MR imaging by a free-breathing 3D IR turbo FLASH. Medicine (Baltimore) 2017; 96: e9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owlia MB, Mehrpoor G. Behcet's disease: new concepts in cardiovascular involvements and future direction for treatment. ISRN Pharmacol 2012; 2012: 760484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marzban M, Mandegar MH, Karimi A, et al. Cardiac and great vessel involvement in “Behcet's disease”. J Card Surg 2008; 23: 765–768. [DOI] [PubMed] [Google Scholar]
- 38.Khalid U, Saleem T. Hughes–Stovin syndrome. Orphanet J Rare Dis 2011; 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ketchum ES, Zamanian RT, Fleischmann D. CT angiography of pulmonary artery aneurysms in Hughes–Stovin syndrome. AJR Am J Roentgenol 2005; 185: 330–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL910687 Supplemental Material for The filling defect of pulmonary artery, an imaging finding what we should know by Min Liu, Xin Cao Tao, Zhenguo Zhai, Zhanhong Ma, Li Zhu and Jie Luo in Pulmonary Circulation