Abstract
Background:
Human glypican-3 (hGPC3) is a protein highly expressed in hepatocellular carcinoma (HCC) but limited in normal tissues, making it an ideal target for immunotherapy. The adoptive transfer of hGPC3-specific chimeric antigen receptor T (CAR-T) cells for HCC treatment has been conducted in clinical trials. Due to the rigid construction, conventional CAR-T cells have some intrinsic limitations, like uncontrollable overactivation and inducing severe cytokine release syndrome.
Methods:
We redesigned the hGPC3-specific CAR by splitting the traditional CAR into two parts. By using coculturing assays and a xenograft mouse model, the in vitro and in vivo cytotoxicity and cytokine release of the split anti-hGPC3 CAR-T cells were evaluated against various HCC cell lines and compared with conventional CAR-T cells.
Results:
In vitro data demonstrated that split anti-hGPC3 CAR-T cells could recognize and lyse hGPC3+ HepG2 and Huh7 cells in a dose-dependent manner. Impressively, split anti-hGPC3 CAR-T cells produced and released a significantly lower amount of proinflammatory cytokines, including IFN-γ, TNF-α, IL-6, and GM-CSF, than conventional CAR-T cells. When injected into immunodeficient mice inoculated subcutaneously with HepG2 cells, our split anti-hGPC3 CAR-T cells could suppress HCC tumor growth, but released significantly lower levels of cytokines than conventional CAR-T cells.
Conclusions:
We describe here for the first time the use of split anti-hGPC3 CAR-T cells to treat HCC; split anti-hGPC3 CAR-T cells could suppress tumor growth and reduce cytokine release, and represent a more versatile and safer alternative to conventional CAR-T cells treatment.
Keywords: adoptive cellular therapy, CAR-T cell therapy, cytokine release syndrome, glypican-3, hepatocellular carcinoma, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is the fifth most commonly occurring cancer worldwide.1 In 2012, there were 782,000 new cases of HCC globally, 83% of which occurred in less developed regions.2 Although surgery is currently the most effective treatment for HCC, tumor recurrence rate is very high after tumor resection, and the age-standardized 5-year relative survival rate of HCC is only 10.1%.3 Owing to the difficulty of early diagnosis, most HCC patients are diagnosed at an advanced stage at the initial visit, and lose the opportunity for curative treatment such as hepatectomy or ablation, making HCC the second leading cause of cancer-related death in adult males due to the lack of effective therapies.4 Sorafenib and Lenvatinib, the two clinically approved targeted drugs for first-line treatment of patients with unresectable HCC, could extend the overall survival by only 2–3 months.5,6 Thus, there is an urgent need for new methods of treating HCC.
Reports have shown that activation of the host immune system produces significant antitumor effects.7 On the other hand, by releasing immune checkpoints that inhibit antitumor responses, a variety of cancer patients can achieve unprecedented long-term tumor response.8 For example, blocking the immune checkpoints by anticytotoxic T lymphocyte-associated protein 4 (CTLA-4) or programmed cell death 1 (PD-1) antibodies, either alone or in combination, unleashes the patient’s immune response and generates therapeutic effects in a variety of cancers,9 including HCC.10 Although this is promising, the main premise of inducing antitumor effects by immune checkpoint blockade is the preexistence of tumor-specific infiltrating T cells, which are not always available.11 Moreover, the release of nonspecific T cells by immune checkpoint blockade may result in autoimmune destruction.12 In this regard, immunotherapy targeting tumor-specific or tumor-associated antigens is less likely to cause autoimmune diseases. Human glypican-3 (hGPC3) is a member of the heparan sulfate (HS) proteoglycan family, and is attached to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor.13 A number of studies have shown that hGPC3 is highly expressed in HCC, but its expression in normal tissues is limited, making it an attractive target for immunotherapy of HCC.14
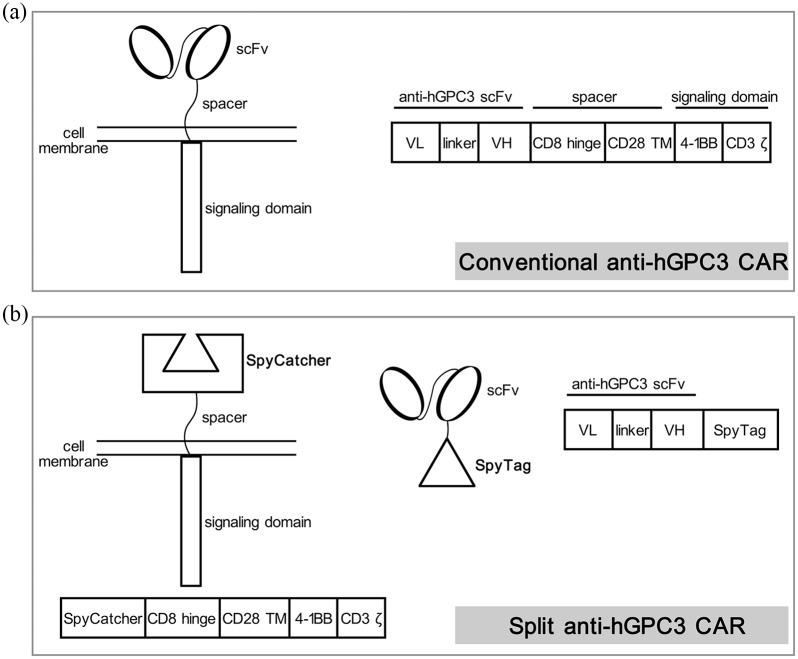
The chimeric antigen receptor (CAR) is a synthetic antigen receptor that redirects T cell specificity and function. Patient-derived CAR-T cell therapy is a promising approach for cancer immunotherapy, and has achieved tremendous success in hematological malignancies.15 The results from mouse models and patient-derived xenografts (PDX) of HCC indicated that CAR-T cells targeting hGPC3 could effectively eliminate hGPC3 positive tumors, providing an encouraging candidate for HCC treatment.16,17 To date, a dozen clinical trials using hGPC3-targeting CAR-T cells to treat HCC have been registered and carried out.18 However, conventional CARs have a rigid structure and are typically composed of a fixed antigen-specific single-chain variable fragment (scFv) and intracellular signaling domains (Figure 1a). When conventional CAR-T cells were activated by encountering the antigen, the expanded progeny cells retained all the features of parental cells and could be activated, proliferate, and produce lots of cytokines. For example, CAR-T cells expanded (up to 7000 times) rapidly after transfer into patients, resulting in severe tumor lysis syndrome (TLS) and fatal cytokine release syndrome (CRS).19 Therefore, there is an urgent need for a better system to improve the safety of CAR-T cell therapy.
Figure 1.
Schematic diagrams of (a) conventional and (b) split anti-hGPC3 CAR. The sequence of anti-hGPC3 scFv is from a frequently used hGPC3-specific mouse monoclonal antibody GC33. According to previous reports, SpyTag (13 aa) can form a rapid covalent bond with SpyCatcher (116 aa).
CAR, chimeric antigen receptor; hGPC3, human glypican-3; scFv, single-chain variable fragment; linker, (G4S)3; TM, transmembrane region; VH, variable region of antibody heavy chain; VL, variable region of antibody light chain.
Here, we describe a novel, split anti-hGPC3 CAR-T system composed of two components (Figure 1b): the anti-hGPC3 scFv-SpyTag consists of an anti-hGPC3 scFv linked to a 13 amino acid peptide SpyTag,20 and the signaling molecule SpyCatcher-CAR consists of extracellular SpyCatcher linked to the intracellular domains of 4-1BB and CD3ζ. SpyCatcher binds SpyTag specifically and covalently.20 The in vitro and in vivo cytotoxicity and cytokine release results demonstrated that our split anti-hGPC3 CAR-T cells can control the growth of HCC with decreased cytokine release compared with conventional CAR-T cells. This novel split anti-hGPC3 CAR system represents a more versatile and safer application for HCC treatment without compromising CAR-T cell efficacy.
Methods
Ethics statement
All animal experiments were approved by The Institutional Laboratory Animal Care and Use Committee at Southern Medical University, Guangzhou, P.R. China (IACUC 81671570). All experiments involving human specimens were conducted within the guidelines of the 1975 Declaration of Helsinki, and were approved by the Ethical Committee of Nanfang Hospital, Guangzhou, P.R. China (approval number NFEC-2015-140). Written informed consent that covered the introduction and purpose of the study, potential risks and discomforts, confidentiality, voluntary participation, and authorization was obtained from all healthy donors.
Cell lines and culture media
Human embryonic kidney 293T cells, human HCC HepG2 cells were obtained from American Type Culture Collection (ATCC). Human HCC Huh7 cells were purchased from Sangon Biotech (#E680517, Shanghai, China). All three cell lines were cultured in DMEM medium, high glucose (#E600003, BBI Life Science, Shanghai, China) supplemented with 10% fetal bovine serum (FBS) (#10099141C, Gibco, Waltham, MA, USA), 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin (Sangon Biotech).
Split anti-hGPC3 CAR construct design
The split anti-hGPC3 CAR system is composed of two parts: the antigen-recognition part anti-hGPC3 scFv-SpyTag and the signaling part SpyCatcher-CAR expressed on T cells. The scFv sequence is from a mouse anti-hGPC3 monoclonal antibody GC33.21 We constructed anti-hGPC3 scFv-SpyTag by attaching a 13 amino acid SpyTag to the carboxy-terminal of anti-hGPC3 scFv and constructed SpyCatcher-CAR by replacing the scFv domain of conventional CAR with a SpyCatcher motif (Figure 1b). The amino acid sequences of all constructs used in this study are listed in Figure S1 and the proteins of SpyCatcher and anti-hGPC3 scFv with or without a SpyTag were expressed and purified by ChinaPeptides (Shanghai, China).
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) were performed to verify the binding of anti-hGPC3 scFv with recombinant hGPC3 protein (ACROBiosystems, #GP3-H5258, Bejing, China) and the binding of SpyCatcher with anti-hGPC3 scFv-SpyTag. Briefly, 96-well microtiter plates (#FEP101896, JET BIOFIL, Guangzhou, China) were coated with 5 μg/ml hGPC3 or SpyCatcher protein in 100 μl phosphate-buffered saline (PBS) at 4°C overnight and blocked with 5% skimmed milk at 37°C for 2 h. After washing four times with PBS containing 0.1% Tween 20 (PBST), a series of diluted anti-hGPC3 scFv or anti-hGPC3 scFv-SpyTag was added and incubated at 37°C for 1 h. After washing, horseradish peroxidase (HRP)-labeled goat anti-mouse IgG F(ab’)2 fragment (#115-035-006, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) was added and incubated at 37°C for 30 min. After final washing, TMB substrate solution (#N301, Thermo Scientific, Waltham, MA, USA) was added and then stopped with 2 M sulfuric acid. Absorbance at 450nm was measured using a microplate reader (Bio-Rad 550, Hercules, CA, USA).
Immunofluorescence assay
Cells were seeded into 96-well cell-culture plates (#TCP011024, JET BIOFIL) with 1 × 105 cells/0.1 ml/well, After 24 h culture, the supernatants were discarded and the cells were rinsed twice with 100 μl of sterile PBS, then 100 μl of 2 μg/ml anti-hGPC3 scFv-SpyTag diluted with PBS containing 2% FBS was added to each well and incubated at room temperature (RT) for 15 min with gentle shaking. After washing with PBS, 100 μl of 1.5 μg/ml fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG F(ab’)2 fragment (#115-095-072, Jackson ImmunoResearch Laboratories) was added and incubated at RT for 15 min. After the final wash, 100 μl of sterile PBS was added to each well and the results were observed; images were captured with an Olympus inverted fluorescence microscope (Olympus, Tokyo, Japan).
Western blot
Total proteins were extracted from different cell lines using radioimmunoprecipitation assay (RIPA) reagents supplemented with protease and phosphatase inhibitors (Sangon Biotech, # C500005). The concentrations of extracted proteins were determined by a BCA Protein Assay Kit (ThermoFisher Scientific, # 23225) according to the manufacturer’s protocol. Extracted proteins (50 μg) were separated by 10% SDS-PAGE and transferred onto 0.45 μm polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skimmed milk powder in PBST for 2 h at RT. The membranes were then incubated with 2 μg/ml anti-hGPC3 scFv-SpyTag diluted in PBST containing 2% milk for 1.5 h at RT. After washing with PBST, the membranes were incubated with HRP-labeled goat anti-mouse IgG F(ab’)2 fragment for 1 h at RT. Blots were imaged at different exposure times using the ECL chemiluminescence reagents (ThermoFisher Scientific, #32209). The expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was also detected as an internal control by using anti-GAPDH antibody (#2118, CST, Danvers, MA, USA) and HRP-labeled goat anti-rabbit IgG H&L (#ab6721, Abcam, Cambridge, UK).
Lentiviral vector production
To produce replication-incomplete lentivirus, 293T cells were seeded at 6 × 106 cells/100-mm dish in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS and grown for 24 h. The conventional anti-hGPC3 CAR or SpyCatcher CAR expression vector was mixed with the viral packaging plasmids (#A43237, Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol, and were cotransfected into 293T cells at 15 μg total plasmids/dish using polyethylenimine (PEI) transfection reagent (#24765-1, Polysciences, Warrington, PA, USA). After 60 h, the culture supernatants were harvested and filtered through a 0.45 μm filter, then concentrated through ultracentrifugation or Lenti-X™ Concentrator (#631232, Clontech, Mountain View, CA, USA) according to the manufacturer’s description. Concentrated lentiviruses were stored in a –80°C refrigerator before use.
T cell transduction and expansion
Human peripheral blood mononuclear cells (PBMCs) were isolated from the whole blood of healthy donors by Ficoll-Paque (#17144002, GE Healthcare, Chicago, IL, USA) density gradient centrifugation. Human Naïve Pan T cells were isolated by magnetic negative selection (#17961, STEMCELL, Vancouver, BD, Canada). T cells were cultured in RPMI 1640 complete medium supplemented with 100 units/mL IL-2 (#200-02, Peprotech, Rocky Hill, NJ, USA) and activated for 2 days with immunoCult™ Human CD3/CD28 T Cell Activator (STEMCELL, #10971). The day before transduction, non-T-cell treated six-well plates were coated with RetroNectin (Clontech, #T100B) following the supplier’s protocol. On the transduction day, RetroNectin was removed, and the concentrated lentiviruses were added to each well and centrifuged for 90 min at 2000 × g. Then, viral supernatant was removed and 3 ml of activated human T cells at 1 million/ml in fresh RPMI 1640 medium was added to each well followed by spinning at 1000 × g for 30 min. The same transduction procedure was repeated on the following day. To expand T cells, the culture medium was refreshed, and cell density was adjusted to 1 million/ml every 2–3 days.
Flow cytometry
The transduction efficiencies of conventional anti-hGPC3 CAR-T cells and SpyCatcher CAR-T cells were detected by flow cytometry (FCM) staining with FITC-labeled goat anti-mouse IgGF(ab’)2 fragment and FITC-labeled SpyTag, respectively. The following antibodies were used to determine the phenotype of CAR-T cells: APC anti-hCD3 (#300312, BioLegend, San Diego, CA), PE anti-hCD8 (BioLegend, #301008)
Cytokine release and cytotoxicity assays
Different target cells (hGPC3+ HepG2 and Huh7 cells, hGPC3- 293T cells) were seeded into 96-well plates at 2 × 104 cells/0.1 ml/well; 16 h later, conventional anti-hGPC3 CAR-T or SpyCatcher CAR-T cells were added to each well at a series of different effector:target (E:T) ratio with or without the addition of anti-hGPC3 scFv-SpyTag. Supernatants were collected 24 h later, and cytokine release, including IFN-γ, IL-2, and IL-6, were measured by corresponding ELISA kit (BioLegend, #430101, #431801 and #430501). Cytotoxicities were determined by measuring lactate dehydrogenase (LDH) activity in the supernatant using a CytoTox kit (#G1780, Promega, Madison, WI, USA) following the manufacturer’s instructions.
Intracellular staining of IFN-γ
CAR-T cells were cocultured with various target cells as mentioned above for 12 h, followed by the addition of Golgi-Stop (BioLegend, #420701), and incubated for an additional 4 h to prevent cytokine secretion. Cells were harvested and stained for IFN-γ intracellularly as described previously.22
RNA extraction and qRT-PCR
CAR-T cells were cocultured with HepG2 cells in a 24-well plate at 3:1 E: T ratio for 24 h. CAR-T cells were purified using Ficoll-Paque gradient centrifugation followed by RNA extraction using a RNeasy Mini Kit (# 74104, Qiagen, Hilden, Germany) and reverse-transcribed to complementary DNA (cDNA) using the SuperScript™ First-Strand Synthesis kit (Invitrogen, #11904018) as instructed by the manufacturer. qPCR was performed to determine the gene expression level using SYBR Green Master Mix (#A25742, Applied Biosystems, Foster City, CA, USA) on a 7900HT fast real-time PCR system (Applied Biosystems). The levels of target genes were normalized to the endogenous control GAPDH. The primers used in the experiment are listed in Table S1.
Xenograft models of HCC and adoptive cell transfer
HepG2 tumor xenografts were established as we recently reported.23 Briefly, 6- to 8-week old female nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice (Charles River Laboratories, Beijing, China), housed in a specific-pathogen-free facility and handled in laminar flow hoods, were inoculated subcutaneously with 5 × 106 HepG2 cells on the right flank on day 0 and randomly assigned to different groups (four mice/group), followed by tail vein injection of 10 × 106 CAR-T cells or mock T cells on day 7. In some groups, different amounts of anti-hGPC3 scFv-SpyTag were injected intraperitoneally (i.p.) to each mouse every day from day 7 to day 16. Tumor growth was measured every 2 days with a caliper, and tumor sizes were calculated using the formula V = 1/2 (length × width2). Cytokines in the peripheral blood of mice were detected by ELISA. At the end of the experiment, all mice were euthanized by cervical dislocation.
Statistical analysis
All statistical tests were performed in GraphPad Prism 5 (GraphPad software, La Jolla, CA, USA). Comparisons between different groups were performed using Student’s t test (two-tailed). For all tests, a two-sided p < 0.05 was considered significant, error bars represent standard deviation (SD).
Results
Anti-hGPC3 scFv-SpyTag binds SpyCatcher with high affinity and recognizes HCC cell lines in vitro
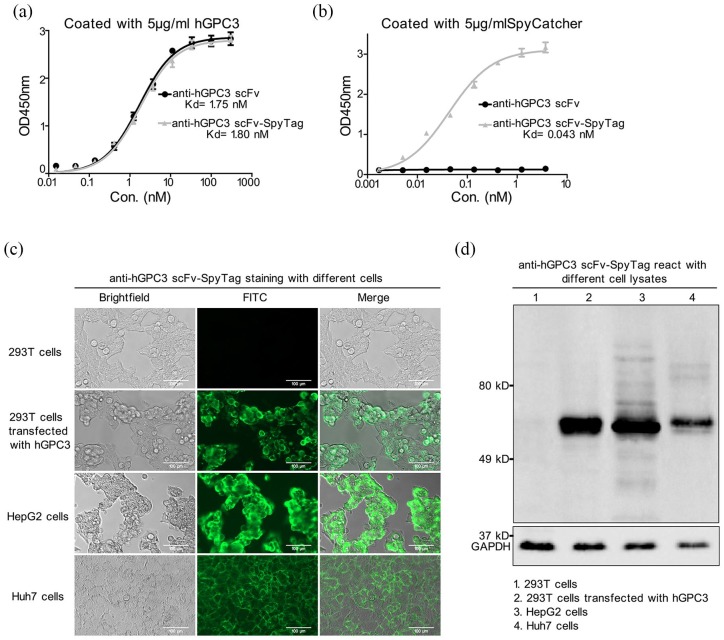
To improve the safety of CAR-T cells in the treatment of HCC, we first designed a split anti-hGPC3 CAR system composed of two parts (Figure 1b). It was reported recently that SpyTag, a 13-amino-acid peptide, could form a rapid covalent bond to its protein-ligand SpyCatcher consisting of 116 amino acids.24 Inspired by this, we introduced SpyTag and SpyCatcher to our Split anti-hGPC3 CAR system and constructed anti-hGPC3 scFv-SpyTag and SpyCatcher CAR-T cells. To verify the binding of anti-hGPC3 scFv-SpyTag with hGPC3 and SpyCatcher, we performed an indirect ELISA by coating with recombinant hGPC3 and SpyCatcher. We found that both anti-hGPC3 scFv and anti-hGPC3 scFv-SpyTag could bind to hGPC3 with comparable affinity (Kd = 1.75 nM and Kd = 1.80 nM, respectively, Figure 2a), indicating that SpyTag tethered to the C-terminal did not affect the binding activity of anti-hGPC3 scFv. We also found anti-hGPC3 scFv-SpyTag bond to SpyCatcher with high affinity (Kd = 0.043 nM), whereas anti-hGPC3 scFv did not (Figure 2b). Moreover, anti-hGPC3 scFv-SpyTag reacted with hGPC3+ HepG2 and Huh7 cells, but not hGPC3− 293T cells, and also reacted with hGPC3 transfected 293T cells (Figure 2c, d), suggesting that the reaction was hGPC3-specific. Together, these data suggest that anti-hGPC3 scFv-SpyTag binds SpyCatcher with high affinity, and specifically recognizes hGPC3 expressed on HCC cell lines.
Figure 2.
Anti-hGPC3 scFv-SpyTag specifically recognizes tumor-associated antigen hGPC3 in vitro. A 5-μg/ml aloquot of recombinant hGPC3 or SpyCatcher protein was coated onto 96-well ELISA plates, a series of diluted antihGPC3 scFv with or without a SpyTag was added, and HRP-labeled goat anti-mouse IgG F(ab’)2 fragment was used as the secondary antibody. All experiments were carried out in triplicate wells and repeated twice; the data were pooled and plotted as mean ± SD. (a) SpyTag hardly affects the binding of antihGPC3 scFv with hGPC3. (b) Anti-hGPC3 scFv cannot bind to SpyCatcher, whereas anti-hGPC3 scFv- SpyTag binds to SpyCatcher with high affinity. The Kd values were calculated by GraphPad Prism software. (c) Anti-hGPC3 scFv-SpyTag recognizes native hGPC3 expressed on HCC cell lines. Different cells were cultured in 96-well plates and incubated with 2 μg/ml anti-hGPC3 scFv-SpyTag and detected by FITC-labeled goat anti-mouse IgG F(ab’)2. Scale bar, 100 μm. (d) Total proteins were extracted from different cell lines, separated by 10% SDS-PAGE and transferred onto 0.45 μm PVDF membranes. The binding specificity of Anti-hGPC3 scFv-SpyTag was detected by Western blot.
FITC, fluorescein isothiocyanate; HCC, hepatocellular carcinoma; hGPC3, human glypican-3; HRP, horseradish peroxide; PVDF, polyvinylidene fluoride; SD, standard deviation.
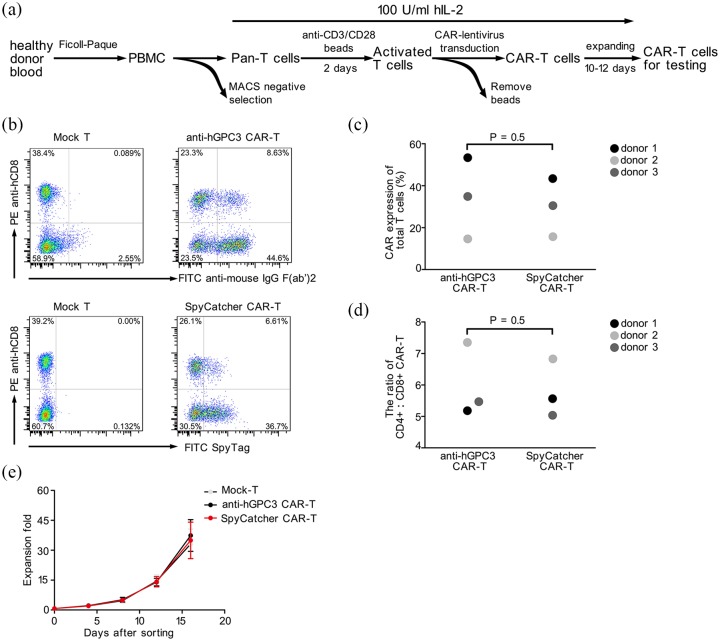
Anti-hGPC3 and SpyCatcher CAR-T cells were generated successfully from healthy donors
Next, we generated CAR-T cells from three healthy donors according to the protocol of our previous work with slight modification (Figure 3a).23 The sequences of anti-hGPC3 and SpyCatcher CAR are listed in Figure S1. The results showed that SpyCatcher CAR-T cells had CAR expression levels comparable with those of conventional anti-hGPC3 CAR-T cells (Figure 3b, c). Further analysis indicated that the transduction efficiency of CAR varies dramatically between different donors, with donor 1 having the highest (about 50%) and donor 2 the lowest (about 15%) transduction efficiency (Figure 3c). Besides, the ratio of CD4+: CD8+ CAR-T cells and proliferative potential in vitro were also similar between anti-hGPC3 and SpyCatcher CAR-T cells (Figure 3d, e).
Figure 3.
The preparation and characteristics of split and conventional anti-hGPC3 CAR-T cells. (a) The process of generating CAR-T cells. Primary human T cells from three healthy adult donors were transduced with CAR lentivirus and examined 10–12 days after transduction by flow cytometry. (b) Representative dot plots showing the transduction efficiency of CAR-T cells. All cells were gated on CD3+ T cells. Mock T represents T cells that underwent the same activation and expansion treatment without lentivirus transduction. (c, d) SpyCatcher CAR-T cells exhibited CAR expression levels and CD4/CD8 ratio comparable with those of conventional antihGPC3 CAR-T cells. (e) The expansion folds of total cells of donor 1 were monitored by counting the cell number using a hemocytometer every 4 days during the whole expansion period. Error bars represent SD.
CAR-T, chimeric antigen receptor T; hGPC3, human glypican-3; MACS, magnetic-activated cell sorting; PBMC, peripheral blood mononuclear cells; SD, standard deviation.
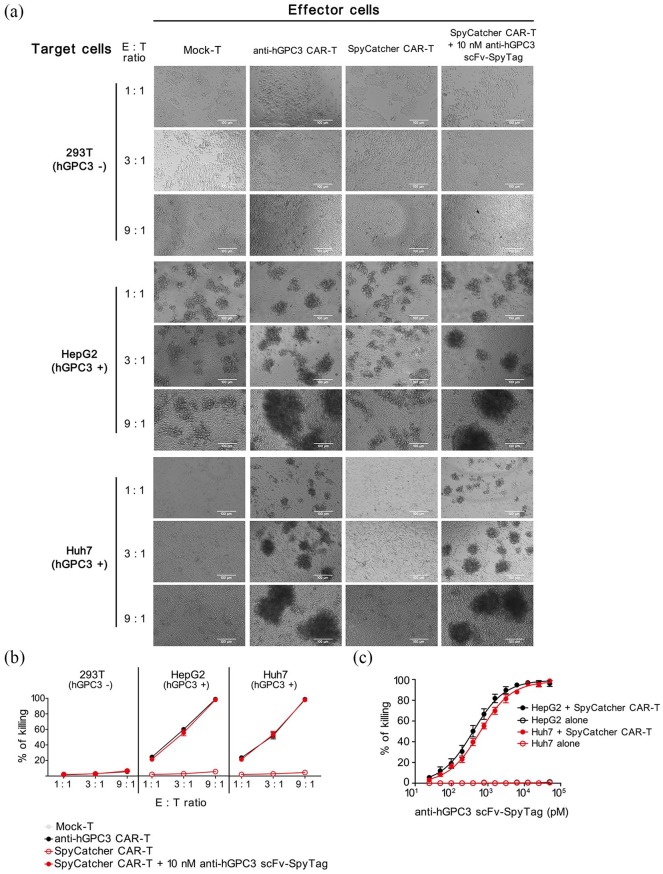
Split Anti-hGPC3 CAR-T cells specifically recognize and kill hGPC3+ HCC cell lines in vitro
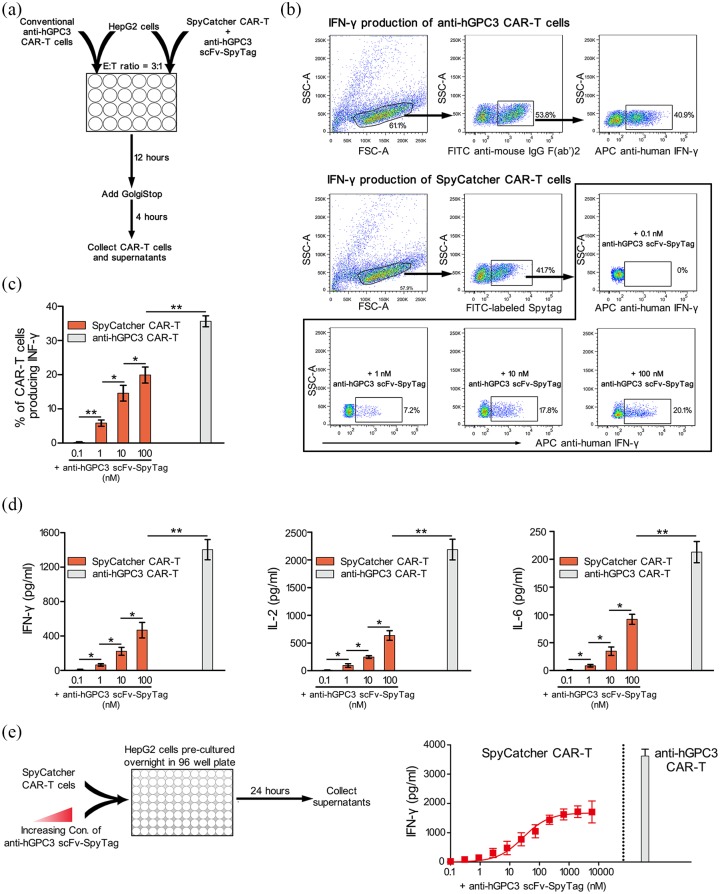
To identify whether our split anti-hGPC3 CAR-T system could recognize and kill HCC cells, we cocultured Mock-T, conventional anti-hGPC3 CAR-T, and SpyCatcher CAR-T cells with hGPC3− 293T cells and hGPC3+ HepG2, Huh7 cells at different E:T ratios. We found that the conventional anti-hGPC3 CAR-T cells could recognize and encircle HepG2 and Huh7 cells to form clusters, whereas mock-T and SpyCatcher CAR-T cells alone did not form obvious clusters, and could not kill any of the three target cell lines. However, in the presence of 10 nM anti-hGPC3 scFv-SpyTag, SpyCatcher CAR-T cells could recognize and kill hGPC3+ HepG2 and Huh7 cells, but not hGPC3− 293T cells (Figure 4a, b), suggesting that the killing is hGPC3 specific. To exclude the possibility that anti-hGPC3 scFv-SpyTag alone could induce target cell death, we cocultured three target cell lines with anti-hGPC3 scFv-SpyTag at various concentrations. The results confirmed that anti-hGPC3 scFv-SpyTag alone is insufficient to kill target cells (Figure S2), suggesting both SpyCatcher CAR-T cells and anti-hGPC3 scFv-SpyTag are indispensable in our split system to mediate cytotoxicity against hGPC3+ HCC cells. We also found that, upon addition of 10 nM anti-hGPC3 scFv-SpyTag, SpyCatcher CAR-T cells possess cytotoxicities against HepG2 and Huh7 cells comparable with those of conventional anti-hGPC3 CAR-T cells, and the cytotoxicities correlated positively with E:T ratio (Figure 4a, b). To study the kinetics of cytotoxicity of our split anti-hGPC3 CAR-T system, we cocultured SpyCatcher CAR-T cells with HepG2 and Huh7 cells with the addition of a series of diluted anti-hGPC3 scFv-SpyTag. The results showed that the cytotoxicity relies on the addition of anti-hGPC3 scFv-SpyTag and is dose dependent. SpyCatcher CAR-T cells killed nearly 20% and over 90% target cells with the addition of 0.1 nM and 10 nM anti-hGPC3 scFv-SpyTag, respectively (Figure 4c). Taken together, the data suggest that our split anti-hGPC3 CAR-T cells have killing effects against HCC cells comparable with those of conventional CAR-T cells in vitro, and the potency can be adjusted by controlling the addition of anti-hGPC3 scFv-SpyTag.
Figure 4.
Split anti-hGPC3 CAR-T cells have potent and specific cytotoxicity against hGPC3+ HCC cell lines. 293T, HepG2, and Huh7 cells were cocultured with Mock-T, conventional anti-hGPC3 CAR-T, and SpyCatcher CAR-T cells with or without 10 nM anti-hGPC3 scFv-SpyTag in 96-well plates at different E:T ratios for 24 h. (a) Pictures were taken under an inverted optical microscope at 200x magnification field of view. Scale bar, 100 μm. (b) Culture supernatants were collected, and the killing effect of each well was calculated using a LDH-based cytotoxicity assay kit according to the manufacturer’s instruction. (c) HepG2 and Huh7 cells were cultured alone or cocultured with SpyCatcher CAR-T cells at an E:T ratio of 9:1 followed by the addition of a serial diluted anti-hGPC3 scFv-SpyTag. After 24 h coculture, supernatants were collected and cytotoxicities determined by LDH-based assays. Experiments were repeated twice with three replicates each. Repeated-measures ANOVA was used to compare different groups.
ANOVA, analysis of variance; CAR-T, chimeric antigen receptor T; E:T, effector:target; HCC, hepatocellular carcinoma; hGPC3, human glypican-3; LDH, lactose dehydrogenase; SD, standard deviation.
Split anti-hGPC3 CAR-T cells significantly reduce cytokine release in vitro
To evaluate the cytokine production by split and conventional anti-hGPC3 CAR-T cells activated by tumor cells, we cocultured CAR-T cells with hGPC3+ HepG2 cells in a 24-well plate, and a series of ten-fold diluted anti-hGPC3 scFv-SpyTag was added to the SpyCatcher CAR-T group (Figure 5a). We measured the cytokine production of CAR-T cells by intracellular staining and found that ~40% of conventional anti-hGPC3 CAR-T cells could be stimulated by tumor cells to produce IFN-γ, which was significantly higher than SpyCatcher CAR-T cells (Figure 5b, c). With the addition of 10 nM anti-hGPC3 scFv-SpyTag, SpyCatcher CAR-T cells showed cytotoxicity against HepG2 cells comparable with that of conventional anti-hGPC3 CAR-T cells (Figure 4a, b), yet only ~15% of SpyCatcher CAR-T cells produced IFN-γ (Figure 5b, c). Even at the highest concentration (100 nM), ~20% SpyCatcher CAR-T cells produced IFN-γ. Next, the cytokines released into the supernatant were detected by ELISA kits and the results confirmed that our split anti-hGPC3 CAR-T cells produced significantly lower amounts of cytokines (including IFN-γ, IL-2, and IL-6) as compared with conventional CAR-T cells (Figure 5d), which was further verified by the results of cytokines mRNA expression levels (Figure S3). The kinetics of IFN-γ, IL-2, and IL-6 release also showed that the cytokine release of our split anti-hGPC3 CAR-T cells is dependent on the dose of anti-hGPC3 scFv-SpyTag (Figures 5e and S4). Critically, as the concentration of anti-hGPC3 scFv-SpyTag exceeds ~1000 nM, the cytokine release of our split CAR-T system will reach its peak, which is still significantly lower than that of conventional anti-hGPC3 CAR-T cells, and no longer increases. Taken together, the data suggest that the cytokine release is largely and significantly decreased in our split anti-hGPC3 CAR-T system, and can be tuned precisely by manipulating the concentration of anti-hGPC3 scFv-SpyTag.
Figure 5.
Split anti-hGPC3 CAR-T cells significantly reduce cytokine release compared with conventional CAR-T cells. (a) Schematic diagram of the experimental process. CAR-T cells generated from healthy donors (n = 3) were cocultured with HepG2 cells in 24-well plates at a 3:1 E:T ratio for 16 h; GolgiStop was added for the last 4 h to block release of cytokines. CAR-T cells were collected and analyzed by intracellular staining. Supernatants were also collected and cytokines were measured by ELISA. For each donor, the experiment was repeated twice. (b) Representative dot plots showing the gating strategy and the IFN-γ+ CAR-T cells. (c) The proportion of IFN-γ-producing CAR-T cells. Data were pooled and plotted as mean ± SD. (d) The concentrations of cytokines in the coculture supernatants were measured by ELISA. Data were pooled and plotted as mean ± SD. All comparisons were analyzed by two-tailed Student’s t test. *p < 0.05, **p < 0.01. (e) Spycatcher CAR-T cells (with the addition of a serial diluted anti-hGPC3 scFv- SpyTag) and conventional anti-hGPC3 CAR-T cells were cocultured with HepG2 cells in 96-well plates at a 3:1 E:T ratio for 24 h (left). Cytokines in the supernatants were measured by ELISA. Data of IFN-γ concentration in different groups were pooled and plotted as mean ± SD.
CAR-T, chimeric antigen receptor T; ELISA, enzyme-linked immunosorbent assay; E:T, effector:target; HCC, hepatocellular carcinoma; hGPC3, human glypican-3; IFN, interferon; SD, standard deviation.
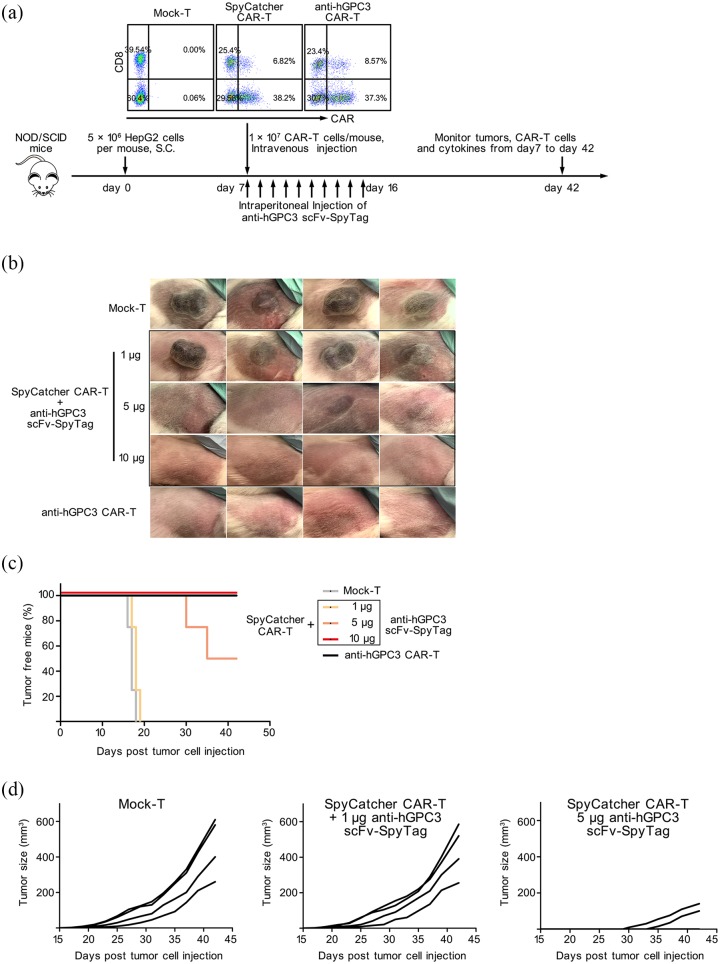
Split and conventional anti-hGPC3 CAR-T cells are equally efficacious in a xenograft mouse model
To evaluate the antitumor effect of our split anti-hGPC3 CAR-T cells in vivo, immunodeficient NOD/SCID mice bearing 7 day’s subcutaneous HepG2 tumors were adoptively transferred with human CAR-T cells and tumor growth was monitored. Mice transferred with SpyCatcher CAR-T cells were further divided into three groups and received 1 μg, 5 μg, and 10 μg anti-hGPC3 scFv-SpyTag per day, respectively, from day 7 to day 16 (Figure 6a). We found that adoptive transfer of conventional anti-hGPC3 CAR-T cells or SpyCatcher CAR-T cells with the repeated injection of 10 μg anti-hGPC3 scFv-SpyTag 100% (4/4) suppressed HepG2 tumor outgrowth in NOD/SCID mice (Figure 6b), whereas in the 1 μg group and mock-T treated group, all four mice formed tumors before day 20 (Figure 6b, c) that grew continually (Figure 6d). In the 5 μg group, although 50% (2/4) of the mice developed a tumor (Figure 6b), tumor formation was delayed, visible only at day 30 and day 35, respectively, and tumors grew slowly (Figure 6c, d).
Figure 6.
Adoptive transfer of split anti-hGPC3 CAR-T cells suppresses HepG2 tumor growth in immunodeficient NOD/SCID mice. (a) NOD/SCID mice bearing 7-day HepG2 tumors were randomly assigned to five groups (four mice/group) and adoptively transferred with 10 million CAR-T cells. The mice of three SpyCatcher CAR-T treated groups were injected i.p. with 10 doses of anti-hGPC3 scFv- SpyTag from day 7 to day 16. Proportions of CAR+ cells within the transferred cells were shown. Tumor outgrowth, CAR-T cells, and cytokines in the peripheral blood were monitored. (b) Pictures of HepG2 tumors at day 42 after inoculation. (c, d) The outgrowth and volume of each tumor are presented.
CAR-T, chimeric antigen receptor T; hGPC3, human glypican-3; i.p., intraperitoneally; NOD/SCID, nonobese diabetic/severe combined immunodeficiency.
Next, we performed the established tumor model to see if our Split CAR-T system could eliminate existing massive tumors. At day 43 post tumor cell injection, two mice in the Mock-T group bearing tumors larger than 500 mm3 were injected intravenously (i.v.) with 1 × 107 CAR-T cells. Anti-hGPC3 scFv-SpyTag was injected i.p. to each mouse at 10 μg/mouse from day 43 to day 46, and 50 μg/mouse from day 47 to day 52. The results showed that the tumors grew continuously, and exceeded 1800 mm3 at day 60 (Figure S5). Taken together, these in vivo antitumor effect data are consistent with the in vitro data and suggest that, with the addition of sufficient anti-hGPC3 scFv-SpyTag, our split CAR-T system has anti-HepG2 tumor capacity comparable with that of conventional anti-hGPC3 CAR-T cells, and our split CAR-T system alone cannot eliminate established huge tumors.
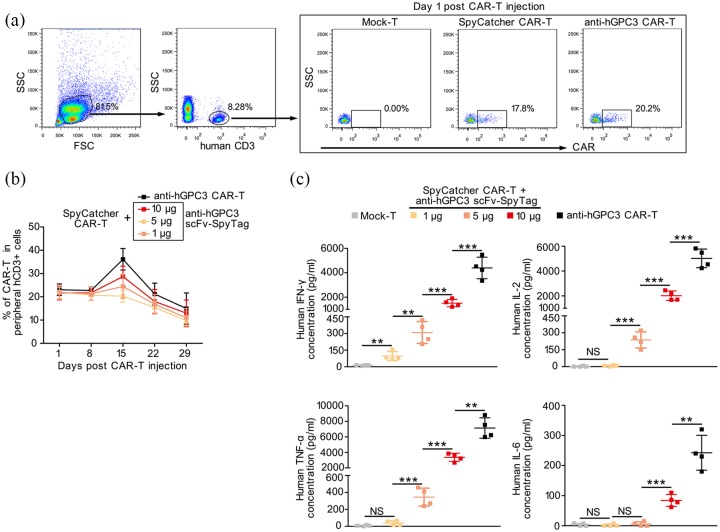
Split Anti-hGPC3 CAR-T cells release lower levels of cytokines in vivo
Next, we monitored weekly the persistence and expansion of adoptively transferred CAR-T cells and the concentration of human cytokines in the peripheral blood of NOD/SCID mice. We found that, pre-transfer, the proportiona of CAR+ cells within SpyCatcher CAR-T and conventional anti-hGPC3 CAR-T cells were 45.02% and 45.87%, respectively (Figure 6a), whereas, 24 h post-transfer, the percentages of peripheral CAR+ cells declined to approximately half of that of pre-transfer CAR-T cells within all groups of mice (Figure 7a, b). Interestingly, there was a significant increase in CAR+ cells among the conventional anti-hGPC3 CAR-T cells on day 15 post-transfer. By contrast, among the mice that transferred with SpyCatcher CAR-T cells, there was no increase of CAR+ cells in 1 μg anti-hGPC3 scFv-SpyTag treated group, and only a slight increase in 5 μg and 10 μg anti-hGPC3 scFv-SpyTag-treated groups on day 15 post-transfer. After day 15, the proportion of CAR+ cells in peripheral blood from all four groups of mice decreased continuously (Figure 7b). We also measured the concentration of cytokines in peripheral blood 24 h post CAR-T cells transfer, and the results indicated that our split anti-hGPC3 CAR-T cells produced significantly lower levels of cytokines, including human IFN-γ, IL-2, TNF-α, and IL-6 (Figure 7c), which was consistent with the results in vitro.
Figure 7.
Adoptive transfer of split anti-hGPC3 CAR-T cells releases significantly decreased cytokines compared with conventional CAR-T cells in xenograft mouse models. The experimental process was described in Figure 6. Peripheral blood was obtained weekly from each mouse after adoptive transfer; CAR-T cells and cytokines were monitored. (a) Representative dot plots showing the gating strategy and percentage of CAR-T cells in total human CD3+ T cells. (b) Summary of kinetics of the proportion of peripheral CART cells/CD3+ T cells in each group. Data are presented as mean ± SD. (c) At 24 h post-CAR-T cells transfer, the concentrations of cytokines in peripheral blood from each mouse were measured by ELISA; the data were pooled and plotted as mean ± SD. All comparisons were analyzed by two-tailed Student’s t test. **p < 0.01, ***p < 0.001.
CAR-T, chimeric antigen receptor T; ELISA, enzyme-linked immunosorbent assay; hGPC3, human glypican-3; IFN, interferon; NS, not significant; SD, standard deviation.
Discussion
CAR-T cell therapy has emerged as a promising immunotherapy for patients with hematological malignancies and has been approved in 2017 by the US Food and Drug Administration (FDA) for the treatment of B cell malignancies in pediatric and adult patients.25 Multiple studies using CAR-T cells to treat solid tumors, including HCC, are in progress.26–30 However, the inability to control the CAR-T cell activation level has resulted in treatment-related toxicities.31 So, there is an urgent need for a new CAR-T system that can precisely control T cell activation with improved flexibility to make CAR-T cell therapy safer.
Approximately 70–80% of HCC tumor cells express hGPC3, and its level is associated with poor patient survival rate, making it an ideal target for CAR-T cell therapy.32 Here, we reported a novel split hGPC3-targeting CAR-T system composed of soluble anti-hGPC3 scFv-SpyTag and SpyCatcher CAR-T cells with enhanced controllability and safety. As CRS is one of the most deadly toxicities for patients treated with CAR-T cell therapy,33,34 it is crucial to reduce cytokine release and, at the same time, maintain the cancer-cytotoxicity by CAR-T cells. By introducing an optimal concentration of anti-hGPC3 scFv-SpyTag, our split CAR-T cells exhibit anti-HCC efficacy comparable to that of conventional CAR-T cells but induce reduced levels of cytokine release in vitro and in vivo. In our in vitro studies, we were able to titrate the cytotoxicity (Figure 4c) and cytokine secretion (Figures 5e and S4) of CAR-T cells by adjusting the doses of added anti-hGPC3 scFv-SpyTag. Watanabe and colleagues reported recently that CAR-T cells could efficiently lyse target cells that express even the lowest density of antigen CD20 (~200 molecules/cell), but that cytokine production and T cell proliferation required a higher density of CD20, nearly 5000 molecules/cell,33 indicating that the threshold of tumor antigen density required to trigger cytokine release is much higher than that required to trigger tumor cell lysis by CAR-T cells. Therefore, by precisely controlling the density of binding molecule pairs between CAR-T cells and tumor cells, it is possible to reduce cytokine release and maintain the target cell cytolytic activity of CAR-T cells. Due to the fixed design of traditional CAR, it is not feasible to regulate the binding intensity between CAR molecule and tumor antigen without re-engineering the T cells. By contrast, our split anti-hGPC3 CAR-T system design makes it easy to control the binding intensity between CAR molecule and tumor antigen by manipulating the concentration of anti-hGPC3 scFv-SpyTag added. Actually, all the concentrations of anti-hGPC3 scFv-SpyTag we tested in the experiment, even as high as 5000 nM, induced significantly lower IFN-γ, IL-2, and IL-6 production than conventional CAR-T cells (Figures 5e and S4). Moreover, by optimizing the concentration of anti-hGPC3 scFv-SpyTag added to the system, SpyCatcher CAR-T cells could lyse the HCC cell line hepG2 cells in a manner comparable with that of conventional anti-hGPC3 CAR-T cells, together with a dramatic decrease in cytokine production.
A remarkable advantage of our split anti-hGPC3 CAR-T system is the super-high affinity between anti-hGPC3 scFv-SpyTag and SpyCatcher CAR (4.3 × 10−11 M, Figure 2b). While several other split systems that use switchable biomolecule-labeled antibodies to redirect the specificity of CAR-T cells have been reported, none have demonstrated comparable affinity with our system.34–36 Due to this unique feature, we can introduce a lower amount of engineered SpyCatcher CAR -T cells and soluble anti-hGPC3 scFv-SpyTag than other split systems used to treat cancer patients with the similar response, which will reduce treatment-related side effects and lower treatment costs. Additionally, conventional CAR-T cells utilize scFv as the antigen-recognition domain, yet scFv proteins are known to often aggregate and form multimeric species.37 The aggregation of scFv typically results in antigen-independent tonic signaling due to spontaneous clustering of CAR molecules, leading to early exhaustion of CAR-T cells, which limits antitumor efficacy.38 Instead of scFv, our split anti-hGPC3 CAR-T system uses a 12 kD SpyCatcher protein fragment as the extracellular domain of CAR, and, to date, there are no reports of the auto-aggregation of SpyCatcher. Although not investigated here, we anticipate that SpyCatcher CAR-T cells will simply induce antigen-independent tonic signaling.
Numerous studies have indicated that incorporation of additional costimulatory signals into CAR-T cells are necessary to avoid T cell anergy, sustain their expansion, and allow full activation.39 CD28 and 4-1BB are the two major costimulatory receptors employed by most CAR-T clinical trials. It has been reported that 4-1BB costimulation ameliorates T cell exhaustion, whereas CD28 costimulation augments T cell exhaustion induced by tonic signaling of CAR.40 Therefore, we introduced the intracellular fragment of a costimulatory receptor 4-1BB as part of the signaling domain of SpyCatcher CAR. In the presence of anti-hGPC3 scFv-SpyTag and HCC target cells, SpyCatcher CAR-T cells were able to synthesize and secrete a great deal of IL-2 (Figure 5d). IL-2, in turn, stimulates CAR-T cell proliferation in an autocrine and paracrine manner. Our SpyCatcher CAR-T cells might persist over several years in patients, as already observed for conventional CAR-T cells,41 and could be reactivated as a universal weapon against HCC relapse or other newly occurred malignant diseases at any time, merely by infusing a target antigen-specific scFv-SpyTag. For example, scFvs targeting distinct epitopes of hGPC3, or with different affinities, can be incorporated into our system to optimize the treatment of HCC, and scFvs targeting HCC-associated antigen other than hGPC3, for example, α-fetoprotein (AFP), can be used to treat hGPC3-loss HCC relapse. Although AFP is an intracellular and secreted tumor-associated antigen, CAR-T cells generated from TCR-like antibodies that bind to AFP peptide-MHC complex specifically can still regress both HepG2 and AFP-expressing SK-HEP-1 tumors in SCID-Beige mice.42
There are some intrinsic limitations of our split anti-hGPC3 CAR-T system. As the key elements of the system, both the SpyTag and SpyCatcher sequences are derived from Streptococcus pyogenes, which may have the potential to induce immune response and reduce the antitumor effects of our split CAR-T system. In our study, the sequence of anti-hGPC3 scFv is from mouse monoclonal antibody GC33, and immunogenicity is indeed an issue requiring serious consideration. Humanization of GC33 should be a feasible solution. Recently, a phase I clinical study indicated that fully humanized GC33 was well tolerated in advanced HCC patients.43 hGPC3 is expressed not only in HCC but also in other important tissues like placenta, ovary, mammary gland, lung, enteric ganglia, and kidney.44 Our split anti-hGPC3 CAR-T system could not mitigate the on-target/off-tumor toxicity. Another limitation is our split system alone could not eliminate established huge tumors (Figure S5) and combinatorial therapy with other approaches such as chemotherapy or immune checkpoint inhibitors, for example, anti-PD1/PD-L1, may be needed to improve the treatment effect.The extracellular spacer domain of CAR is important to CAR-T cells anti-tumor activity.45 Considering our SpyCatcher CAR design used the same fixed spacer domain as conventional CAR, further investigation would be needed to evaluate the effect of these variables on the SpyCatcher CAR-T cell activity. The short serum half-life of scFv-SpyTag could also be problematic, as SpyCatcher CAR-T cells require scFv-SpyTag to maintain activity in vivo. Protein engineering approaches (e.g. PEGylation, Fc fusion) may be needed to increase the half-life of scFv-SpyTag if necessary.
In summary, we have reported here for the first time the construction and use of a novel split hGPC3-targeting CAR-T system to treat HCC. The results of in vitro and xenograft mouse models demonstrated that our split CAR-T system possesses therapeutic effects comparable with those of conventional anti-hGPC3 CAR-T cells, and that the system has robust flexibility and reduced side effects, especially CRS. Our split anti-hGPC3 CAR-T system based on the covalent binding between SpyTag and SpyCatcher represents a promising new model in HCC cell therapy that has the potential for improved safety and versatility compared with conventional CAR-T therapies.
Supplemental Material
Supplemental material, figure_S5 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Supplemental material, fig_S1 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Supplemental material, fig_S2 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Supplemental material, fig_S3 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Supplemental material, fig_S4 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Supplemental material, Table_S1 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Acknowledgments
Authors Xuan Liu, Jianyun Wen, and Honglei Yi contributed equally to this work. We thank Professor Jinlin Hou for supporting this work.
Footnotes
Author contributions: XTJ and XDW designed the research; XL, JYW, HLY, XRH, YY and GFY performed the research; XTJ and XL analyzed the data; XTJ wrote the paper; XTJ and XDW performed all statistical analysis; all authors reviewed the paper.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Natural Science Foundation of Guangdong Province, China (grant number 2018A030313113), Guangdong Province Science and Technology Plan Projects, China (grant number 2016A020215102), Science and Technology Program of Guangzhou, China (grant number 201607010241), Medical Scientific Research Foundation of Guangdong Province, China (grant number A2019147), and Dean’s Foundation of Nanfang Hospital (grant number 2018C013).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xuan Liu, Department of Pediatrics, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Jianyun Wen, Department of Pediatrics, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Honglei Yi, Department of Orthopedics, General Hospital of Southern Theater Command, Guangzhou, China.
Xiaorui Hou, Department of Immunology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China.
Yue Yin, Department of Immunology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China.
Guofu Ye, State Key Laboratory of Organ Failure Research, Guangdong Provincial Key Laboratory of Viral Hepatitis Research, Department of Infectious Diseases, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Xuedong Wu, Department of Pediatrics, Nanfang Hospital, Southern Medical University, Tonghe Road, Guangzhou, 510515, China.
Xiaotao Jiang, Department of Immunology, School of Basic Medical Sciences, Southern Medical University, Shatai Road, Guangzhou, 510515, China; State Key Laboratory of Organ Failure Research, Guangdong Provincial Key Laboratory of Viral Hepatitis Research, Department of Infectious Diseases, Nanfang Hospital, Southern Medical University, Guangzhou, China; Guangdong Provincial Key Laboratory of Proteomic, Guangzhou, China.
References
- 1. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67: 358–380. [DOI] [PubMed] [Google Scholar]
- 2. Choo SP, Tan WL, Goh BKP, et al. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016; 122: 3430–3446. [DOI] [PubMed] [Google Scholar]
- 3. Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003–2005: a population-based study. Int J Cancer 2015; 136: 1921–1930. [DOI] [PubMed] [Google Scholar]
- 4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 7. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest 2015; 125: 3335–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359: 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016; 13: 473–486. [DOI] [PubMed] [Google Scholar]
- 10. Kudo M. Immune checkpoint blockade in hepatocellular carcinoma: 2017 update. Liver Cancer 2016; 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan Q, Chen Z, Wang C, et al. Toward biomaterials for enhancing immune checkpoint blockade therapy. Adv Funct Mater 2018; 28: 1802540. [Google Scholar]
- 12. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–168. [DOI] [PubMed] [Google Scholar]
- 13. Zhou F, Shang W, Yu X, et al. Glypican-3: a promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med Res Rev 2018; 38: 741–767. [DOI] [PubMed] [Google Scholar]
- 14. Haruyama Y, Kataoka H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma. World J Gastroenterol 2016; 22: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med 2018; 379: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao H, Li K, Tu H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 2014; 20: 6418–6428. [DOI] [PubMed] [Google Scholar]
- 17. Jiang Z, Jiang X, Chen S, et al. Anti-GPC3-CAR T cells suppress the growth of tumor cells in patient-derived xenografts of hepatocellular carcinoma. Front Immunol 2016; 7: 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang R, Zhang Z, Liu Z, et al. Adoptive cell transfer therapy for hepatocellular carcinoma. Front Med 2019; 13: 3–11. [DOI] [PubMed] [Google Scholar]
- 19. Pettitt D, Arshad Z, Smith J, et al. CAR-T Cells: a systematic review and mixed methods analysis of the clinical trial landscape. Mol Ther 2018; 26: 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li L, Fierer JO, Rapoport TA, et al. Structural analysis and optimization of the covalent association between SpyCatcher and a peptide Tag. J Mol Biol 2014; 426: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishiguro T, Sugimoto M, Kinoshita Y, et al. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res 2008; 68: 9832–9838. [DOI] [PubMed] [Google Scholar]
- 22. Jiang X, Zhang M, Lai Q, et al. Restored circulating invariant NKT cells are associated with viral control in patients with chronic hepatitis B. PLoS One 2011; 6: e28871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu W, Peng Y, Wang L, et al. Identification of α-fetoprotein-specific T-cell receptors for hepatocellular carcinoma immunotherapy. Hepatology 2018; 68: 574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zakeri B, Fierer JO, Celik E, et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci U S A 2012; 109: E690–E697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guedan S, Ruella M, June CH. Emerging cellular therapies for cancer. Annu Rev Immunol 2019; 37: 145–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoseini SS, Cheung NV. Immunotherapy of hepatocellular carcinoma using chimeric antigen receptors and bispecific antibodies. Cancer Lett 2017; 399: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov 2016; 6: 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Posey AD, Schwab RD, Boesteanu AC, et al. Engineered CAR T cells targeting the cancer-associated Tn-Glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 2016; 44: 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kakarla S, Chow KKH, Mata M, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther 2013; 21: 1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahmed N, Salsman VS, Kew Y, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res 2010; 16: 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang X, Xu J, Liu M, et al. Adoptive CD8+ T cell therapy against cancer: challenges and opportunities. Cancer Lett 2019; 462: 23–32. [DOI] [PubMed] [Google Scholar]
- 32. Shirakawa H, Suzuki H, Shimomura M, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 2009; 100: 1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watanabe K, Terakura S, Martens AC, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 ζ chimeric antigen receptor-modified effector CD8+ T cells. J Immunol 2015; 194: 911–920. [DOI] [PubMed] [Google Scholar]
- 34. Ma JSY, Kim JY, Kazane SA, et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc Natl Acad Sci U S A 2016; 113: E450–E458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viaud S, Ma JSY, Hardy IR, et al. Switchable control over in vivo CAR T expansion, B cell depletion, and induction of memory. Proc Natl Acad Sci U S A 2018; 115: E10898–E10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kudo K, Imai C, Lorenzini P, et al. T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer Res 2014; 74: 93–103. [DOI] [PubMed] [Google Scholar]
- 37. Whitlow M, Filpula D, Rollence ML, et al. Multivalent Fvs: characterization of single-chain Fv oligomers and preparation of a bispecific Fv. Protein Eng Des Sel 1994; 7: 1017–1026. [DOI] [PubMed] [Google Scholar]
- 38. Long AH, Haso WM, Shern JF, et al. 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 2015; 21: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. D’Aloia MM, Zizzari IG, Sacchetti B, et al. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis 2018; 9: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schultz L, Mackall C. Driving CAR T cell translation forward. Sci Transl Med 2019; 11: pii: eaaw2127. [DOI] [PubMed] [Google Scholar]
- 41. Scholler J, Brady TL, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012; 4: 132ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu H, Xu Y, Xiang J, et al. Targeting alpha-fetoprotein (AFP)-MHC complex with CAR T-cell therapy for liver cancer. Clin Cancer Res 2017; 23: 478–488. [DOI] [PubMed] [Google Scholar]
- 43. Zhu AX, Gold PJ, El-Khoueiry AB, et al. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2013; 19: 920–928. [DOI] [PubMed] [Google Scholar]
- 44. Kim H, Xu GL, Borczuk AC, et al. The heparan sulfate proteoglycan GPC3 is a potential lung tumor suppressor. Am J Respir Cell Mol Biol 2003; 29: 694–701. [DOI] [PubMed] [Google Scholar]
- 45. Hudecek M, Sommermeyer D, Kosasih PL, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res 2015; 3: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, figure_S5 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Supplemental material, fig_S1 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Supplemental material, fig_S2 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Supplemental material, fig_S3 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Supplemental material, fig_S4 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology
Supplemental material, Table_S1 for Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release by Xuan Liu, Jianyun Wen, Honglei Yi, Xiaorui Hou, Yue Yin, Guofu Ye, Xuedong Wu and Xiaotao Jiang in Therapeutic Advances in Medical Oncology