Abstract
Background
Attention deficit hyperactivity disorder (ADHD) is one of the most common childhood mental health disorders. Stimulant drugs as the most commonly used treatment and first-line therapy for ADHD have side effects. One of the newest approaches to select the best choices and optimize dosages of medications is personalized medicine.
Methods
This historical cohort study was carried out on the data taken from the period of 2008 to 2015. Eligible subjects were included in the study randomly. We used mixed-effects logistic regression models to personalize the dosage of Methylphenidate (MPH) in ADHD. The patients’ heterogeneity was considered using subject-specific random effects, which are treated as the realizations of a stochastic process. To recommend a personalized dosage for a new patient, a two-step procedure was proposed. In the first step, we obtained estimates for population parameters. In the second step, the dosage of the drug for a new patient was updated at each follow-up.
Results
Of the 221 children enrolled in the study, 169 (76.5%) were male and 52 (23.5%) were females. The overall mean age at the beginning of the study is 82.5 (± 26.5) months. In multivariable mixed logit model, three variables (severity of ADHD, time duration receiving MPH, and dosage of MPH) had a significant relationship with improvement. Based on this model the personalized dosage of MPH was obtained.
Conclusions
To determine the dosage of MPH for a new patient, the more the severity of baseline is, the more of an initial dose is required. To recommend the dose in the next times, first, the estimation of random coefficient should be updated. The optimum dose increased when the severity of ADHD increased. Also, the results show that the optimum dose of MPH as one proceeds through the period of treatment will decreased.
Keywords: Precision medicine, Tailor medicine, Attention deficit disorder with hyperactivity, Methylphenidate, Mixed-effect models, Longitudinal data
Background
Attention deficit hyperactivity disorder (ADHD) is one of the most common childhood mental health disorders [1]. The estimated prevalence of ADHD in Iranian children and adolescents is ranging between 2.8 and 19.9% [2] and ranging from 5 to 12% in school-aged children worldwide [3].
The etiology of ADHD is multi-factorial [4]. It is a combination of genetic [5, 6] and environmental (e.g., exposure to alcohol or lead, prenatal maternal smoking, prematurity, pregnancy complications, and low birth weight) [7, 8] factors. ADHD symptoms (hyperactivity, impulsiveness, and a developmental lack of attention) could cause significant damage in school tasks [9] and in the functions of daily activities [10]. In most children with ADHD, symptoms continue into adolescence and adulthood; it leads to social, occupational and personal dysfunctions [11]; therefore, early diagnosis and appropriate treatment could be beneficial [12]. ADHD often accompany other mental and behavioral disorders [9].
Stimulant drugs as the most commonly used treatment and first-line therapy for ADHD have side effects including abdominal pain, nausea, loss of appetite, nervousness, insomnia, compulsive behaviors and movement disorders [13]. Unclear long-term benefits due to undesirable side effects of psychopharmacological treatments, caused scientific society to search for alternative approaches to its treatment [14].
One of the newest approaches to select the best choices and optimize dosages of medications is personalized medicine (PM). PM tries to enable patients to receive an earlier diagnosis and optimal treatments with the least complications and the lowest costs [15]. In PM care, the genetic profile and other information of patients including concurrent medication, allergies, comorbidity, etc., are considered to establish the patient’s unique characteristics to tailor the best diagnosis and treatment [15–17].
This study evaluates the relationship between dosage of methylphenidate (MPH) and other important factors with response (improvement) in ADHD patients under the framework of a mixed-effect logit model; then proposes an optimal dose on the basis of the individualized factors of each patient.
Methods
Study design
This historical cohort study was carried out on the data taken from the period of 2008 to 2015 on Children with a diagnosis of ADHD who were admitted in the psychiatric clinic as a referral center and a main pediatrics hospital in Iran (the Children’s Medical Center in Tehran). The Ethics Committee of the Tehran University of Medical Sciences approved this study. The researchers followed the principles of the Helsinki Declaration.
Participants
The children with the primary diagnosis of ADHD (based on DSM-IVTR [18] and DSM-V [19]) and the following criteria were entered to the study
Being within the age range of 3 to 13 years,
Filling the questionnaire for scaling the severity of ADHD (Conners’ Parent Rating Scale-revised Short Form (CPRS-R:S)),
Having at least one follow-up visit.
The exclusion criteria were as follows:
The main diagnosis was another disorder other than ADHD,
The children had just one visit (without any follow up),
Other drugs instead of MPH were prescribed,
The children didn’t respond to the treatment up to the last available follow up.(i.e. the MPH have not had any effect on them during the study time)
Data collection
Of about 5000 available records, based on eligibility criteria, patients’ records were assessed randomly. 40% of the reviewed records did not satisfy inclusion criteria. The sampling was completed when the sample size reached 221.
Study variables
Gender, birth weight, the age of the first diagnosis, severity of ADHD at baseline, weight per visit, type of comorbidity (if present), time intervals of visits (comparing with the first visit), dose of MPH, consumption of risperidone and fluoxetine were recorded as the basic data of the participants.
To Evaluate and score the severity of ADHD, a reliable Persian version (Cronbach alpha = 0.73) [20] of Conners’ Parent Rating Scale-revised Short Form (CPRS-R:S) was used. This scale consisted of 27 items rated from 0 (never) to 3 (very often) [21]. The mean score of at least 1.5 (i.e. crude total score greater than 40) was considered as ADHD. In each follow-up, based on this scale, diagnosis of ADHD was considered as a binary outcome variable (0 = meeting the criteria of ADHD (score > 40), 1 = not meeting the criteria of ADHD (score ≤40)). It should be declared that not meeting the criteria of ADHD (score ≤40) is the therapeutic target.
A milligram per kilogram scale was used to document the dosage of MPH.
Statistical analysis
The basic characteristics, for quantitative variables, were summarized by mean (±standard deviation), and for qualitative variables, by frequency (percentage). The comparison of the explanatory variables between the two genders was assessed by t-test and nonparametric tests of Mann-Whitney U and chi-square. The significance level was set to 5% for all tests. The statistical analysis was carried out using R version 3.5.1 (package mle4) and STATA version 14.
Multilevel mixed-effect logit model
We applied a generalized linear mixed model with the binomial response and a logit link,
| 1 |
for i = 1,…, N and j = 1,…, ni, where Yij indicates the binary response variable, the subscript i denoted the study subject. Here p0 will symbolize a target value for P(Yij = 1). Dij is the drug dosage administered for i-th subject and j-th time point, and d is the corresponding fixed coefficient. In this model Xij is the vector of covariates corresponding to fixed-effects parameter vector β, β0 is a fixed intercept and bi is a random-effect coefficient for the subject i. we assume it has N(0, σ2).
The vector of covariates includes gender, age of the onset of ADHD, severity of disease at baseline, birth weight, weight in follow-ups, time interval (the time passed after the commencement of treatment), taking risperidone, taking fluoxetine, being accompanied with affective disorders (mood and bipolar disorders), anxiety disorders (generalized anxiety disorder (GAD), social anxiety disorder (SAD), obsessive-compulsive disorder (OCD), phobia, and anxiety disorder), oppositional defiant disorder (ODD) and, other comorbidities (mental retardation (MR), learning disorder (LD), stutter, Tic, and major depressive disorder (MDD)). The adjusted regression coefficients (β = ln(OR)) and their 95% confidence intervals (CIs) were calculated in Table 3.
Table 3.
Final multivariable random effect model
| Covariates | Coefficient | (95% CI) | P-Value | |
|---|---|---|---|---|
| Fixed effects | Intercept | 1.9377 | (0.9286, 2.9469) | < 0.001* |
| Time interval (month) | 0.0800 | (0.0640,0.0959) | < 0.001* | |
| Severity | −0.0512 | (−0.0720,-0.030) | < 0.001* | |
| Log Dose MPH, (mg per kg) | 0.8610 | (0.5069, 1.2149) | < 0.001* | |
| Random intercept | Variance estimation | (95% CI) | ||
| 1.452 | (1.2931, 1.6108) | |||
*Significant at 0.05
To recommend drug dosage for a new patient, a two-step procedure was proposed.
In the first step, we obtained estimates for population parameters , β0 and .
In the second step, for the new patient k the estimation for k was updated at each time as below.
At time t1, set k,0 = 0 and make the initial dose
| 2 |
where the p0 is calculated using by predicting P(Yij = 1). In order to predict P(Yij = 1), we used all available responses, and then the 90th percentile of these probabilities was determined.
-
2.
For time tn (n > 1), based on the values of the covariates of the previous time of this individual the estimate was obtained, the proposed dose for time tn was obtained from the following equation
| 3 |
in which bi is predicted by the adaptive Gauss-Hermite approximation to the log-likelihood.
Results
Evaluating 298 records, the sample size of 221 was achieved that means 77 records didn’t meet the eligibility criteria.
This 77 records were excluded because CPRS-R:S was not correctly completed in 22 records, the main complaint was not ADHD in 19 records (five ODD, three MR, three communication disorder, two GAD, two LD, one SAD, one autism, one MDD, and one tic), other drugs were prescribed instead of MPH for 16 records, 14 records did not have any follow up, and for 6 records the age was less than 3 years old.
Of the 221 children enrolled in the study, 169 (76.47%) were male and 52 (23.53%) were female. The basic characteristics of patients between two sexes are not significantly different. The basic characteristics of these individuals are shown in Table 1.
Table 1.
The basic characteristics of children with ADHD
| Variable | Overall (n = 221) | Female (n = 52) | Male (n = 169) | p-value |
|---|---|---|---|---|
| Age at baseline (month; mean ± SD) | 82.53 ± 26.47 | 85.65 ± 28.22 | 81.57 ± 25.92 | 0.332 |
| Birth weight (kg; mean ± SD) | 3.09 ± 0.56 | 3.07 ± 0.57 | 3.09 ± 0.56 | 0.815 |
| Low birth weighta, n (%) | 36 (16.3%) | 7 (13.5%) | 29 (17.2%) | 0.528 |
| Weight at baseline (kg; mean ± SD) | 30.95 ± 5.62 | 25.65 ± 9.66 | 24.97 ± 7.28 | 0.191 |
| Period of treatment (month; mean ± SD) | 22.42 ± 15.19 | 20.13 ± 14.46 | 23.12 ± 15.38 | 0.216 |
| Severity of ADHD at baseline (mean ± SD) | 51.38 ± 10.82 | 48.96 ± 9.03 | 52.12 ± 11.23 | 0.065 |
| Number of follow-ups (mean ± SD) | 4.94 ± 2.83 | 4.69 ± 2.65 | 5.01 ± 2.89 | 0.478 |
aChildren with a birth weight of lower than 2500 g were considered as having a low birth weight
In this study, six children had attention deficit disorder (ADD) (two females and four males) and 143 children had comorbidities with ADHD (55 anxiety disorders, 43 ODD, 6 affective disorders, and 39 had other comorbidities). These comorbidities were found to be as follows: anxiety disorder (GAD: 33 (14.93%), SAD: 18 (8.14%), Phobia: 6 (2.71%), OCD: 8 (3.62%), Anxiety: 7 (3.17%)); affective disorder (Mood: 5 (2.26%), Bipolar: 1 (0.45%)); Mental retardation: 4 (1.81%); Learning disorder: 20 (9.05%); TIC: 11 (4.98%); MDD: 3 (1.36%); Stuttery: 1 (0.45%); ODD: 43 (19.45%).
The overall improvement rate was calculated to be 62.70%, and no significant difference (p = 0.251) was observed between males (61.63%) and females (65.77%).
In the univariable mixed-logit analysis, four variables had significant relations with improvement (not meeting criteria of ADHD) including the weight of the patients in each follow-up, the severity of ADHD at the baseline, time interval of receiving the MPH, and dosage of MPH. These variables were imported to the multivariable mixed-logit model. As a result of the analysis based on this model, three variables (except weight) had a significant relationship with improvement. (Table 2).
Table 2.
Association between explanatory variables and improvement of ADHD
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Fixed effects | Covariates | OR (95% CI) | P-Value | OR (95% CI) | P-Value |
| Sex (Male) | 0.8358 (0.5684, 1.2289) | 0.361 | – | – | |
| Age (month) | 0.9991 (0.9829, 1.0053) | 0.795 | – | – | |
| Weight (kg) | 1.0270 (1.0084, 1.0459) | 0.004* | – | – | |
| Birthweight (kg) | 0.8265 (0.6149, 1.1109) | 0.212 | – | – | |
| Time interval (month) | 1.0844 (1.0677, 1.1014) | < 0.001* | 1.0833 (1.0661,1.1007) | < 0.001* | |
| Severity a | 0.9597 (0.9462, 0.9734) | < 0.001* | 0.9500 (0.9305,0.9699) | < 0.001* | |
| Log Dose MPH, (mg per kg) | 2.2766 (1.6855,3.0750) | < 0.001* | 2.3653 (1.6602,3.3699) | < 0.001* | |
| Taking risperidone | 0.9035 (0.6720, 1.2150) | 0.503 | – | – | |
| Taking Fluoxetine | 0.7398 (0.4524, 1.2097) | 0.227 | – | – | |
| ODD | 0.6842 (0.4648, 1.0070) | 0.064 | – | – | |
| Mood disorder b | 0.6846 (0.2952,1.5873) | 0.379 | – | – | |
| Anxiety disorder c | 1.9619 (0.7667,1.5672) | 0.614 | – | – | |
| Other comorbidities d | 0.9692 (0.8631,1.0883) | 0.587 | – | – | |
| Random intercept | – | Variance estimation | (95% CI) | ||
| – | 1.452 | (1.2931, 1.6108) | |||
a The OR and its CI is computed for the centered value(i.e. severity- 51.3817). b Mood Disorder is the combination of Mood and bipolar disorders. c Anxiety disorder is a combination of GAD, SAD, OCD, Phobia, and Anxiety disorder. d Other comorbidities are included MR, LD, stutter, Tic, and MDD. *Significant at 0.05
In order to calculate the optimal dose, we use the coefficient of the final multivariable random effect model (Table 3).
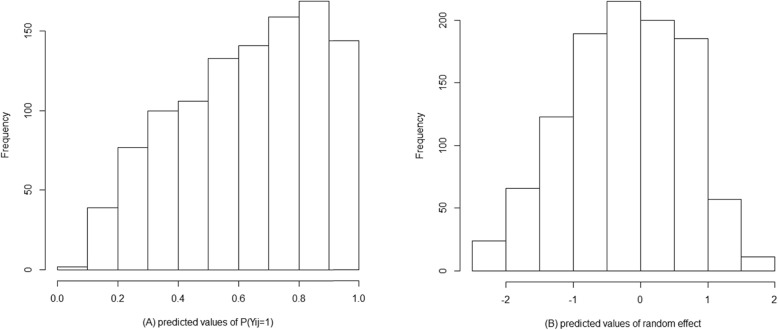
The target value for P(Yij = 1) is p0 =0.9866. The histogram of predicted values of P(Yij = 1) and random effect are shown in Fig. 1. To determining the dosage of MPH for a new patient (k) at the first visit, the initial dose can be calculated as follows:
| 4 |
Fig. 1.
The histogram of (a) the predicted value for P(Yij = 1) at the last visit and (b) the predicted value for the random effect ()
Where 4.2990 is the logit of the predicted value p0, 1.9377 is the estimate of fixed intercept, and 51.3817 is the mean of the severity of ADHD at baseline. The diagram of this equation is shown in Fig. 2.
Fig. 2.

The optimum initial dosage of MPH in ADHD, based of severity of baseline (CPRS-R:S) The figure represents average patients
Also at the n-th time point (follow-up), the dosage can be calculated based on the following formula:
| 5 |
Where p0 is the target probability of improvement, severity is the severity of ADHD at baseline (based on CPRS-R:S) and time is the number of months on treatment. The diagram of the dosage recommendation for different time points is shown in Fig. 3. In order to illustrate the above relationship, the optimum dosage is obtained for some hypothetical data (Table 4). Furthermore, the computer code for predicting bi and for computing the optimal dosage is available in Additional file 1.
Fig. 3.

The optimum dosage of MPH in ADHD, based on severity of baseline for different time points. This figure corresponds to the average patients
Table 4.
Recommended dosage for hypothetical patients
| Patient ID | weight | Severity of baseline | Time (month) | Baseline response | Dichotomized responses | Recommended dose (mg/kg)a | |
|---|---|---|---|---|---|---|---|
| 1 | 24 | 56 | 1 | 45 | 0 | − 0.1647 | 0.1638 |
| 1 | 24 | 56 | 2 | 42 | 0,0 | −0.1651 | 0.1247 |
| 1 | 24 | 56 | 4 | 39 | 0,0,1 | −0.1690 | 0.0871 |
| 2 | 23 | 78 | 1 | 67 | 0 | −0.1497 | 0.6023 |
| 2 | 23 | 78 | 2 | 65 | 0,0 | −0.1449 | 0.4781 |
| 2 | 23 | 78 | 5 | 39 | 0,0,1 | −0.1456 | 0.0773 |
| 2 | 23 | 78 | 6 | 30 | 0,0,1,1 | −0.1439 | 0.0412 |
a: the recommended dose is predicted based on the formula (5)
Discussion
In this study, the clinical severity at baseline, logarithm of dosage of MPH, and time duration of receiving the MPH were associated with improvement of ADHD.
The dosage of MPH in logarithmic scale had a meaningful association with improving from ADHD, in which the chance of improvement increased 2.36 times as one unit adding in the dosage of MPH in the logarithm scale.
According to multivariable random effect logistic model (Table 2), the odds ratio of severity is 0.9500, therefore with increasing one unit in severity, the chance of improvement decreased. Also, the odds ratio of time is 1.0833, therefore increasing one month receiving MPH, the chance of improvement increased.
As ADHD can cause social, emotional and economic failure and also increased mortality [22], pharmacological treatment in many cases is inevitable. On the other hand MPH as the most common drug, has an important complication, so trying to prescribe the optimum dose for each patient is a considerable achievement.
In this study, although the rate of males and females was not the same, as the demographic data were not different, we didn’t make subgroups of males and females to analyze the data.
We used generalized linear mixed models (GLMMs) method to personalize the dosage of MPH in ADHD. Generalized linear models (GLMs) are used to investigate and analyze the relationship between clinical, demographic, and genetic covariates on the response variable such as patient recovery. But these models aggregately study the relationships and characteristics of individuals. To investigate the relationships of the response with the unique characteristics of the studied subjects, another family of statistical models, usually called GLMMs (or random-effects (REs) linear models) is used. Using these models can be a valuable tool and a comprehensive conceptual framework for the development of personalized medicine [23].
The fact that GLMMs have the concepts that allow explaining patient populations as a whole (the fixed effects) and, simultaneously, concepts that allow describing patients as individuals (the REs) suggests that these models include the key ideas for providing PM with a precise statistical language [24, 25]. Thus, the variation of random components are not only due to a mathematical artifact control for patients’ heterogeneity but also the consequence of actual variation in the biological and environmental factors making humans develop as individuals [26]. Hence, both biological and statistical evidence supports the development of a methodological tool for PM based on GLMMs.
In this study we proposed a drug dosage individualization procedure than could be considered as an extension of the individualization algorithm proposed by Diaz et al. for continuous responses to dichotomous responses [25, 27].
Based on the result of this study, in order to determine the dosage of MPH for a new patient (initial dose), the greater the severity of baseline is, the higher the initial dosage is required.
To recommend the dosage in the next times, first, due to the information of the patient the random coefficient should be updated then the dosage should be calculated for him/her. According to the results of this study, in patients with the same severity of disease and same response to the treatment, with increasing the period of receiving MPH, the optimum dose of MPH decreased on the log scale by the rate of 0.08 mg/kg. Furthermore, the more the severity of ADHD is, the higher of the optimum dose is needed, i.e. considering that response to the treatment is similar among patients, at the same time the optimum dose of MPH increased by the rate of 0.06 mg/kg as the severity of ADHD based on CPRS-R:S increased. As seen in Fig. 1, for an average patient, the optimal dose with higher degree of severity is increased. Also, the optimal dose of MPH decreased when the time duration of receiving MPH increased.
To best of our knowledge, few studies on factors affecting treatment in ADHD have been published. In a Cochrane systematic review by Osland et al. in 2018, mentioned 3 studies that evaluated the MPH in ADHD [28]. In a cross-over trial, children were randomized to three weeks each of MPH, dextroamphetamine, and placebo. MPH significantly decreased hyperactivity at all doses [29]. In this study, they did not propose any optimum dose of MPH. Gadow KD et al. mentioned that treatment with 3 doses (0.1, 0.3 and 0.5 mg/kg) of MPH resulted in best improvement of ADHD with no significant differences between 0.1-mg/kg and 0.3-mg/kg dose on any of dependent measures [30].
In another study evaluating the treatment of ADHD in children with tic A, The dosage of average 25.9 mg/d of MPH was proposed for ADHD [31]. Therefore it seems that no study had been done to evaluate the effective factors to estimate the best dosage.
The strengths of this study include its conduct with a large and diverse sample of children with the main diagnosis of ADHD and the mean follow up period of 22.42 month. Also, we used the CPRS-R:S version of it because of its reliability and validity in the Persian version [20]. Furthermore, we used GLMM to individualize the dosage of MPH. Since participants were randomly recruited in this study across a referral hospital; therefore samples may not represent the population of ADHD children, hence it is proposed that a multicenter study could be done to better generalizability of the findings. However, full consideration of all factors that may have an impact on the improvement of ADHD, such as those related to the social economic status of children’s family, environmental factors like possible stresses that may be encountered by any patient, was outside the scope of this paper and hence these may represent confounding variables. Assessing factors associated with improvement for each comorbidity separately was also outside the scope of this paper. Nevertheless, the results of this study provide an individualized dosage for each patient due to factors affecting their improvement.
Conclusions
In this paper, we propose a two-step procedure to make personalized dosage recommendations. The key idea of this method is to utilize subject-specific random effects from longitudinal responses specifying unique individual information that could contribute to post-treatment outcomes. In order to determine the dosage of MPH for a new patient, the greater the severity of baseline is, the higher the initial dose is required. Furthermore, in the next visits, to recommend the dose, the estimation of the random coefficients should be updated. The optimum dose increased when the severity of ADHD increased. Also, the results show that the optimum dose of MPH as one proceeds through the period of treatment, will decrease.
Supplementary information
Acknowledgments
The authors would like to express their thanks to the staff of pediatric psychological clinic of the Children’s Medical Center in Tehran, Iran. Also, their appreciation and gratitude go to Dr. Mohammad Ali Shams for reviewing the manuscript and revising the language of this article.
Abbreviations
- ADD
Attention deficit disorder
- ADHD
Attention deficit hyperactivity disorder
- CPRS-R:S
Conners’ Parent Rating Scale-revised Short Form
- CRS
Conners’ Rating Scales
- GAD
Generalized anxiety disorder
- GLM
Generalized linear models
- GLMM
Generalized linear mixed models
- LD
Learning disorder
- MDD
Major depressive disorder
- MPH
Methylphenidate
- MR
Mental retard
- OCD
Obsessive-compulsive disorder
- ODD
Oppositional defiant disorder
- PM
Personalized medicine
- Re
Random-effect
- SAD
Social anxiety disorder
Authors’ contributions
HS: acquisition and interpretation of data, study concept and design, study management and drafting of the manuscript. MH and MY: study concept and design, acquisition and interpretation of data and critical revision of the manuscript. AF, JM, and SAM: study concept and design, interpretation of data and critical revision of the manuscript. The authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from any agency in the public, commercial, or not-for-profit sector.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Tehran University of Medical Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mehdi Yaseri, Email: m.yaseri@gmail.com.
Mostafa Hoseini, Email: mhossein110@yahoo.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12874-020-00934-y.
References
- 1.Rowland AS, Lesesne CA, Abramowitz AJ. The epidemiology of attention-deficit/hyperactivity disorder (ADHD): a public health view. Ment Retard Dev Disabil Res Rev. 2002;8(3):162–170. doi: 10.1002/mrdd.10036. [DOI] [PubMed] [Google Scholar]
- 2.Shooshtary MH, Chimeh N, Najafi M, Mohamadi MR, Yousefi-Nouraie R, Rahimi-Mvaghar A. The prevalence of attention deficit hyperactivity disorder in Iran: a systematic review. Iran J Psychiatry. 2010;5(3):88. [PMC free article] [PubMed] [Google Scholar]
- 3.Raman SR, Man KK, Bahmanyar S, Berard A, Bilder S, Boukhris T, et al. Trends in attention-deficit hyperactivity disorder medication use: a retrospective observational study using population-based databases. Lancet Psychiatry. 2018;5(10):824–835. doi: 10.1016/S2215-0366(18)30293-1. [DOI] [PubMed] [Google Scholar]
- 4.Antony A. Study of factors influencing treatment adherence in childhood attention deficit hyperactivity disorder in a tertiary healthcare facility. Indian J Psychol Med. 2016;38(1):20. doi: 10.4103/0253-7176.175094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baht M, Grizenko N, Ben-Amor L, Joober R. Obstetric complications in children with attention deficit/hyperactivity disorder and learning disability. McGill J Med. 2005;8(2):109. [Google Scholar]
- 7.Millichap JG. Etiologic classification of attention-deficit/hyperactivity disorder. Pediatrics. 2008;121(2):e358–ee65. doi: 10.1542/peds.2007-1332. [DOI] [PubMed] [Google Scholar]
- 8.Cerrillo-Urbina AJ, García-Hermoso A, Martínez-Vizcaíno V, Pardo-Guijarro MJ, Ruiz-Hermosa A, Sánchez-López M. Prevalence of probable attention-deficit/hyperactivity disorder symptoms: result from a Spanish sample of children. BMC Pediatr. 2018;18(1):111. doi: 10.1186/s12887-018-1083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer TJ. ADHD and comorbidity in childhood. J Clin Psychiatry. 2006;67:27–31. doi: 10.4088/JCP.v67n0312. [DOI] [PubMed] [Google Scholar]
- 10.Courtabessis E, Pupier F, Surig L, Picot M-C, Nogué E, Macioce V, et al. Clinical factors associated with decision to recommend methylphenidate treatment for children with ADHD in France. Eur Child Adolescent Psychiatry. 2018;27(3):367–376. doi: 10.1007/s00787-017-1061-4. [DOI] [PubMed] [Google Scholar]
- 11.Resnick RJ. Attention deficit hyperactivity disorder in teens and adults: they don't all outgrow it. J Clin Psychol. 2005;61(5):529–533. doi: 10.1002/jclp.20117. [DOI] [PubMed] [Google Scholar]
- 12.Polanczyk G, De Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatr. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 13.Kim B-N, Kim Y-N, Cheong U-S, Kim J-W, Hwang J-W, Shin M-S, et al. Switching from methylphenidate-immediate release (MPH-IR) to methylphenidate-OROS (OROS-MPH): a multi-center, open-label study in Korea. Clin Psychopharmacol Neurosci. 2011;9(1):29. doi: 10.9758/cpn.2011.9.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cagigal César, Silva Tánia, Jesus Mariana, Silva Carla. Does Diet Affect the Symptoms of ADHD? Current Pharmaceutical Biotechnology. 2019;20(2):130–136. doi: 10.2174/1389201019666180925140733. [DOI] [PubMed] [Google Scholar]
- 15.Vogenberg FR, Barash CI, Pursel M. Personalized medicine: part 1: evolution and development into theranostics. Pharm Ther. 2010;35(10):560. [PMC free article] [PubMed] [Google Scholar]
- 16.Guidi GC, Lippi G. Will “personalized medicine” need personalized laboratory approach? Clin Chim Acta. 2009;400(1–2):25–29. doi: 10.1016/j.cca.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Alemi F, Erdman H, Griva I, Evans CH. Improved statistical methods are needed to advance personalized medicine. Open Transl Med J. 2009;1:16. doi: 10.2174/1876399500901010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GUZE SAMUEL B. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) American Journal of Psychiatry. 1995;152(8):1228–1228. doi: 10.1176/ajp.152.8.1228. [DOI] [Google Scholar]
- 19.Association AP. Diagnostic and statistical manual of mental disorders, 5th edition (DSM-5). Am Psychiatric Assoc. 10.1176/appi.books.9780890425596.
- 20.Shahabian A, Shahim S, Bashash L, Yousefi F. Psychometry, factor analysis, and reliability of Conner rating scale for children 6-11 in shiraz: parents short form. Q J Psychol Stud. 2007;3(3):97–120. [Google Scholar]
- 21.Conners C. Conners' rating scales-revised. North Tonawanda, New York: Multi-Health Systems. Inc; 1997. [Google Scholar]
- 22.Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, Pedersen MG. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190–2196. doi: 10.1016/S0140-6736(14)61684-6. [DOI] [PubMed] [Google Scholar]
- 23.Diaz FJ. Measuring the individual benefit of a medical or behavioral treatment using generalized linear mixed-effects models. Stat Med. 2016;35(23):4077–4092. doi: 10.1002/sim.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz FJ, Yeh H-W, de Leon J. Role of statistical random-effects linear models in personalized medicine. Current Pharmacogenomics and Personalized Medicine (Formerly Current Pharmacogenomics) 2012;10(1):22–32. doi: 10.2174/1875692111201010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz FJ, Cogollo MR, Spina E, Santoro V, Rendon DM, de Leon J. Drug dosage individualization based on a random-effects linear model. J Biopharm Stat. 2012;22(3):463–484. doi: 10.1080/10543406.2010.547264. [DOI] [PubMed] [Google Scholar]
- 26.Diaz FJ, Santoro V, Spina E, Cogollo M, Rivera T, Botts S, et al. Estimating the size of the effects of co-medications on plasma clozapine concentrations using a model that controls for clozapine doses and confounding variables. Pharmacopsychiatry. 2008;41(03):81–91. doi: 10.1055/s-2007-1004591. [DOI] [PubMed] [Google Scholar]
- 27.Diaz FJ, Rivera TE, Josiassen RC, Jd L. Individualizing drug dosage by using a random intercept linear model. Stat Med. 2007;26(9):2052–2073. doi: 10.1002/sim.2636. [DOI] [PubMed] [Google Scholar]
- 28.Osland ST, Steeves TD, Pringsheim T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2018;6:CD007990. doi: 10.1002/14651858.CD007990.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castellanos FX, Giedd JN, Elia J, Marsh WL, Ritchie GF, Hamburger SD, et al. Controlled stimulant treatment of ADHD and comorbid Tourette's syndrome: effects of stimulant and dose. J Am Acad Child Adolesc Psychiatry. 1997;36(5):589–596. doi: 10.1097/00004583-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 30.GADOW KENNETH D., NOLAN EDITH, SPRAFKIN JOYCE, SVERD JEFFREY. School Observations of Children with Attention-Deficit Hyperactivity Disorder and Comorbid Tic Disorder. Journal of Developmental & Behavioral Pediatrics. 1995;16(3):167–176. doi: 10.1097/00004703-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Tourette's Syndrome Study G Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58(4):527–536. doi: 10.1212/WNL.58.4.527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.