Abstract
We investigated sleep in therock hyrax, Procavia capensis, a social mammal that typically lives in colonies on rocky outcrops throughout most parts of Southern Africa. The sleep of 5 wild-captured, adult rock hyraxes was recorded continuously for 72 h using telemetric relay of signals and allowing unimpeded movement. In addition to waking, slow wave sleep (SWS) and an unambiguous rapid eye movement (REM) state, a sleep state termed somnus innominatus (SI), characterized by low-voltage, high-frequency electroencephalogram, an electromyogram that stayed at the same amplitude as the preceding SWS episode and a mostly regular heart rate, were identified. If SI can be considered a form of low-voltage non-REM, the implication would be that the rock hyrax exhibits the lowest amount of REM recorded for any terrestrial mammal studied to date. Conversely, if SI is a form of REM sleep, it would lead to the classification of a novel subdivision of this state; however, further investigation would be required. The hyraxes spent on average 15.89 h (66.2%) of the time awake, 6.02 h (25.1%) in SWS, 43 min (3%) in SI and 6 min (0.4%) in REM. The unambiguous REM sleep amounts were on average less than 6 min/day. The most common state transition pathway in these animals was found to be wake → SWS → wake. No significant differences were noted with regard to total sleep time, number of episodes and episode duration for all states between the light and dark periods. Thus, prior classification of the rock hyrax as strongly diurnal does not appear to hold under controlled laboratory conditions.
Key Words: Rapid eye movement, Non-rapid eye movement, Slow wave sleep, Evolution, Hyracoidea, Afrotheria
Introduction
Sleep is typically defined as a homeostatically regulated, easily reversible state of sustained quiescence accompanied by reduced sensory responsiveness. Mammalian sleep has been physiologically divided into non-rapid eye movement sleep (NREM), occupied mostly by slow wave sleep (SWS) and rapid eye movement sleep (REM) [Nicolau et al., 2000; Zepelin et al., 2005; Cirelli and Tononi, 2008; Lesku et al., 2008; Siegel, 2008]. No consensus has been reached as to the functions of REM and NREM, but one avenue towards understanding these functions is an examination of how sleep amounts vary across species with differing physiological and ecological specializations [Tobler, 2005; Zepelin et al., 2005; Siegel, 2008; Horne, 2009; Rial et al., 2010].
A recent review [McNamara et al., 2008] detailed that sleep studies have been undertaken for approximately 127 different mammalian species representing 46 families from 17 different orders. They concluded that on average mammals sleep for approximately 12 h, with REM occupying approximately 4.08 h (17%) of total sleep time (TST); however, this is a generalization as TST varies significantly among different mammalian species. In addition to this variation in sleep times, some mammals show some unusual forms of sleep – cetaceans exhibit unihemispheric SWS [Lyamin et al., 2008c], the platypus, Ornithorhynchus anatinus, ferret, Mustela putorius furo, armadillo, Chaetophractus villosus, and opossums, Didelphis marsupialis and Lutreolina crassicaudata, show unusually high amounts of REM sleep [Siegel, 2005b], the fur seal, Callorhinus ursinus, has very little REM sleep while in the water compared to when they are on land [Lyamin et al., 2008a, b], and the platypus, which has up to 8 h/day of REM sleep, lacks cortical desynchronization during REM [Siegel et al., 1999].
The rock hyrax, Procavia capensis, belongs to the order Hyracoidea, which in turn is part of the clade Afrotheria [Tabuce et al., 2008]. For members of the Afrotheria, sleep has been recorded behaviorally in captive Asian, Elephas maximus, and African elephants, Loxodonta africana, with TST in these animals being 4–6.5 and 3.3 h, respectively [Kurt, 1960; Tobler, 1992]. Electroencephalogram (EEG) recordings of a fully aquatic Amazonian manatee, Trichechus inungius, showed that this animal spent 6.48 h (27%) of the recorded time in SWS, which could be unihemispheric in nature, and 14.4 min (1%) in paradoxical or REM sleep [Mukhametov et al., 1992]; however, the study was performed on a single animal and the sleep recording commenced directly after surgery and lasted only a few days. Within the Hyracoidea, Snyder [1974] reports a TST of 4.9 h for Procavia johnstoni (rock hyrax), 5.7 h for Heterohyrax brucei (bush hyrax) and 4.9 h for Dendrohyrax validus (tree hyrax); however, only an abstract has been published for the aforementioned study and detailed evidence has not been provided to support the reported data. Reports of sleep in other Afrotherian species are not known to the authors. In the present study some physiologically measurable parameters of sleep – EEG, electromyogram (EMG) and electrocardiogram (ECG) – and their accompanying behavior were recorded using telemetry in the rock hyrax, P. capensis, a previously unstudied hyrax species.
Materials and Methods
A total of 5 adult rock hyraxes, with body masses ranging between 1.74 and 4.3 kg (table 1) [body mass was used to identify adult individuals based on data provided in Skinner and Chimimba, 2005], were used in the present study. Permits from the Limpopo and Gauteng Provincial Governments were obtained for the capture and transport of the animals from the wild. All animals were treated and used according to the guidelines of the University of the Witwatersrand Animal Ethics Committee, which parallel those of the NIH for the care and use of animals in scientific experimentation. The animals were captured at random from wild populations and thereafter allowed to acclimatize for a period of 1 month to the recording enclosures that had a 12:12 lighting schedule (light intensity 420 lx, measured with a digital lux meter) with temperature maintained between 19 and 21°C. Each animal was implanted with a telemetric recording devise (Data Sciences International) that allowed for the recording of EEG, EMG and ECG without cables or restraint. The enclosure in which recording occurred was 1.8 × 1.5 m with a painted concrete surface that was covered with straw. The height of the chamber was approximately 1.5 m and steel mesh was placed over the top of the enclosure to prevent the animals from escaping. A wooden box (90 × 90 × 30 cm) with a Perspex roof and two entrances was placed inside the chamber and food (combinations of cucumber, tomato, sweet potato, pumpkin, apples and rabbit pellets as a source of roughage) and fresh water were supplied daily. Behavior was recorded with a low light CCD digital camera connected to a DVD recorder.
Table 1.
Total times of each defined state based on the 5-second scoring method
| Animal ID | Body mass, kg | Brain mass, g | Sex | Total wake time* | TST* | Total SWS* | Total SI* | Total REM* |
|---|---|---|---|---|---|---|---|---|
| H107 | 3.1 | 19.6 | female | 71.6 | 25.6 | 23.5 | 1.6 | 0.5 |
| H03 | 3.14 | 27.1 | male | 69 | 28.9 | 27.4 | 1.4 | 0.1 |
| H05 | 4.3 | 20.4 | male | 73.4 | 20.4 | 16.7 | 3.4 | 0.3 |
| H06 | 1.74 | 19.5 | male | 60.4 | 35 | 29 | 4.7 | 1.3 |
| H07 | 2.05 | 17.9 | male | 56.4 | 32.8 | 28.7 | 4.1 | 0.04 |
| Species mean | 2.87 | 20.9 | 66.2 | 28.5 | 25.1 | 3 | 0.4 | |
Values are percentages of 24 h for each individual animal as well as the species mean.
Surgical Procedure
After acclimatization, surgical implantation of the telemetric recoding device was performed. The animals were weighed before surgery and anesthetized with weight-appropriate doses of a 2:1 mixture of ketamine and xylazine (Anaket-V and Chanazine 2% Injection, Bayer HealthCare). The head and neck, left thoracic (two 2 × 1 cm) and abdominal (10 × 10 cm) regions were shaved and cleaned with CHX chlorhexidine disinfectant (0.5% chlorhexidine digluconate in 75% alcohol, Kyron Laboratories Pty Ltd.) before surgery commenced. These areas correspond to the regions where the EEG, EMG and ECG electrodes and telemeter would be implanted. The animal was placed on a heat blanket in order to maintain a constant body temperature throughout the surgery and the head was placed in a stereotaxic frame to prevent movement and allow for the accurate placement of the EEG and EMG electrodes. During the surgical procedure the animal was kept under a constant state of anesthesia by means of isoflurane ventilation (1–2% in an oxygen/70% nitrous oxide mixture, Isofor inhalation anaesthetic, Safe Line Pharmaceuticals Pty Ltd.). The animal's heart rate, body temperature and percentage oxygen saturation were monitored.
Under aseptic conditions, a midsagittal incision was made over the skull and the skin and temporal muscle were reflected to expose the part of the skull overlying the motor cortex. Using a dental drill, three 2-mm-diameter holes were made in the cranial vault to expose the underlying dura mater. The first hole was drilled anterior to the olfactory bulbs for the placement of the indifferent electrode, while two holes were drilled approximately 5 mm apart just lateral to the sagittal sinus over the left motor cortex for the placement of the stainless steel recording electrodes (gauge of electrode 0.457 mm, silastic outside diameter 0.9 mm and inside diameter 0.508 mm, PhysioTel® Multiplus Transmitter, Data Sciences International). The electrodes were placed in such a manner that the tips rested firmly on the surface of the cortex but did not pierce the dura mater, and were secured in place with dental cement. Two of the stainless steel EMG electrodes (1.5 cm apart) were sutured into the dorsal nuchal musculature, while two ECG electrodes (3 cm apart) were sutured into the subdermal tissue overlying the left thoracic region. A subcutaneous pocket was created (10 × 10 cm) over the left abdominal region for the implantation of the telemetry unit. All skin incisions were sutured following implantation. After surgery was complete, the animal was given an intramuscular analgesic (0.1 ml Tamgestic, Schering-Plough, mixed with 0.9 ml sterile water, 1 ml mixture/kg) and returned to the recording enclosure. Recovery was monitored every half hour until it could be established that the animal was able to move freely and eat and drink normally.
Sleep Recording
After the surgical procedure the animal was allowed a recovery period of 1 week before the recording of sleep commenced. The animals were housed in the same enclosure (i.e. the enclosure they were acclimatized to prior to surgery and experimentation) within a sound-attenuating room for recovery as well as recording. A receiver was mounted and secured to one wall of the enclosure while a low light CCD digital camera was mounted above the enclosure. The telemetric recording system (Data Sciences International, DSI, PhysioTel Multiplus Transmitter, model TL10M3-D70-EEE implant – this particular model provided the strongest signal in our recording enclosure, smaller transmitters were unable to transmit a signal to the receiver as the distance over which it had to operate was too long) consisted of a DEM multiplex interface to which the receiver was connected. The signal from the implanted transmitter (round, 13 cm2 with stainless steel electrodes, weight 37 g, volume 25 ml, 3 channels) detected by the receiver was relayed to the input amplifier of the Data Sciences computer system, after which it was digitally recorded (in DSI format) for analysis. After the recording was completed, data digitally saved in the DSI format was converted to text format and these files were in turn converted into the appropriate format needed for recognition and analysis by the Spike 2 computer program (version 4.2, Cambridge Electronic Design). Sleep (physiologically measurable parameters and the DVD-recorded associated behavior) was recorded continuously for a period of 72 h. The animals were disturbed only once a day for approximately 5 min at the same time during each of the recording days for feeding.
Data Analysis
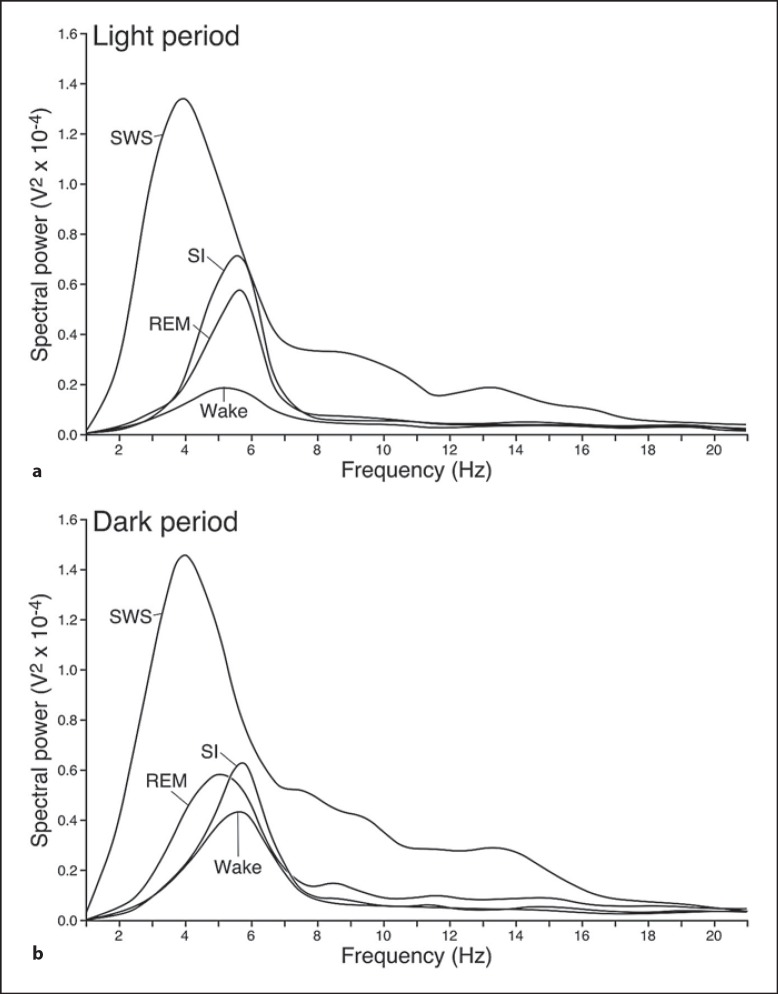
Version 4.2.2 of the Spike 2 software (Cambridge Electronic Designs, UC) was used in order to convert the recorded data into the appropriate format, i.e. Spike 2 data format, for offline analysis. The EEG data was scored in 5-second epochs as: (1) waking, characterized by low-voltage, high-frequency EEG and high-voltage EMG; (2) SWS, characterized by high-voltage, low-frequency EEG and EMG lower in amplitude than waking; (3) REM, characterized by low-voltage, high-frequency EEG, an almost atonic EMG and irregular ECG, or (4) somnus innominatus (SI), characterized by a low-voltage, high-frequency EEG, EMG amplitude characteristic of SWS and a regular ECG (fig. 1, 2, 3, 4). An epoch was only assigned to a particular state if it occupied at least 50% of the epoch. The power spectrum for each of the defined states was calculated with the aid of the Spike 2 computer program (Hanning window, FFT number 512, sampling frequency 500 Hz, segment length 1.024 s) for both the light and dark period (fig. 4). The data obtained from the 5-second epoch scoring was analyzed to determine the modal state per minute to generate the data for the 1-min epoch scoring.
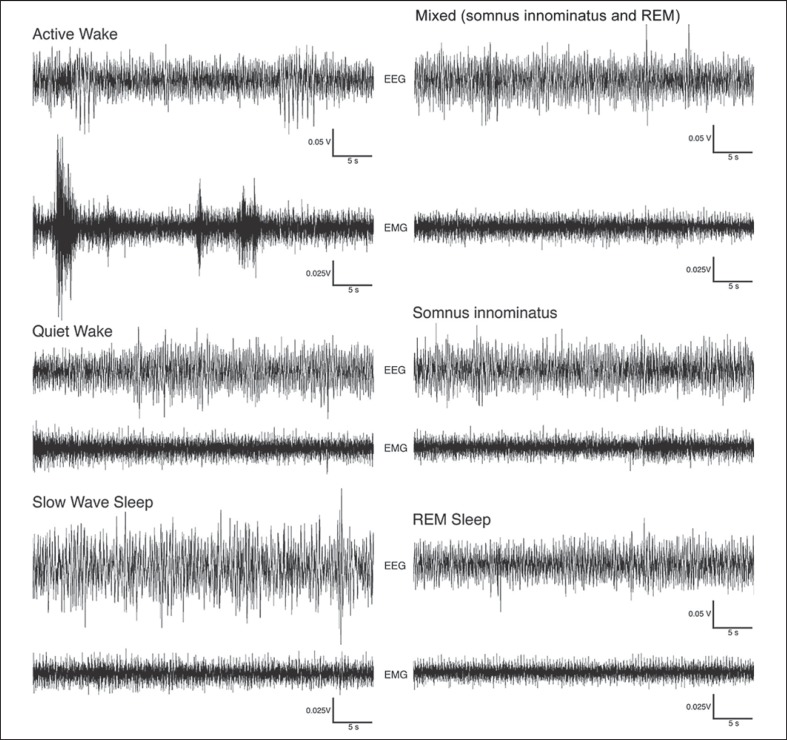
Fig. 1.
Examples of EEG and EMG polygraphs demonstrating 2-min episodes of active wake, quiet wake, SWS, SI and REM states in the rock hyrax. Waking episodes were characterized by a low-voltage, high-frequency EEG. The EMG for active waking exhibited higher voltages compared to the quiet waking state, and high-voltage spikes that likely correspond with movements were evident during this state. SWS was characterized by a high-voltage, low-frequency EEG and an EMG that was lower in amplitude than during the waking states. The EEG for both SI and REM resembled that of waking; however, during SI the EMG remained at the same amplitude as the preceding SWS episode, while during REM the EMG was reduced in amplitude.
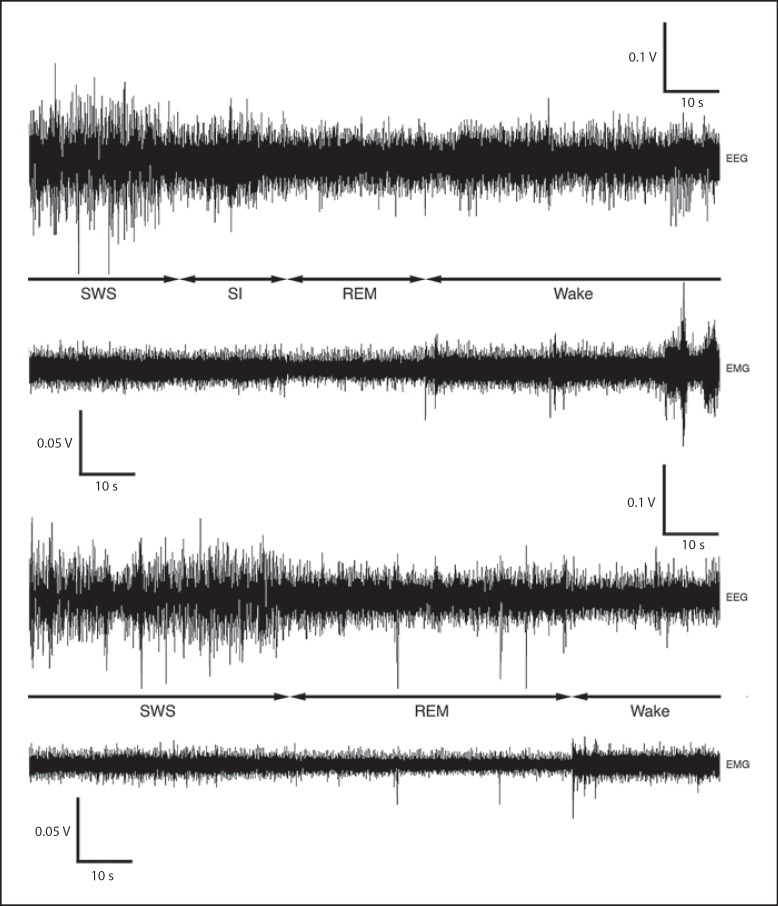
Fig. 2.
EEG and EMG polygraphs demonstrating the transitions that occur between the different sleep states in the rock hyrax. The first set of polygraphs is an example of SWS transitioning to SI, which then leads to REM followed by waking. Note that the EMG during SI remains at the same amplitude as during the preceding SWS episode, and that when SI transitions to REM the EMG reduces in amplitude. The second set of polygraphs demonstrates transition from SWS to REM followed by waking.
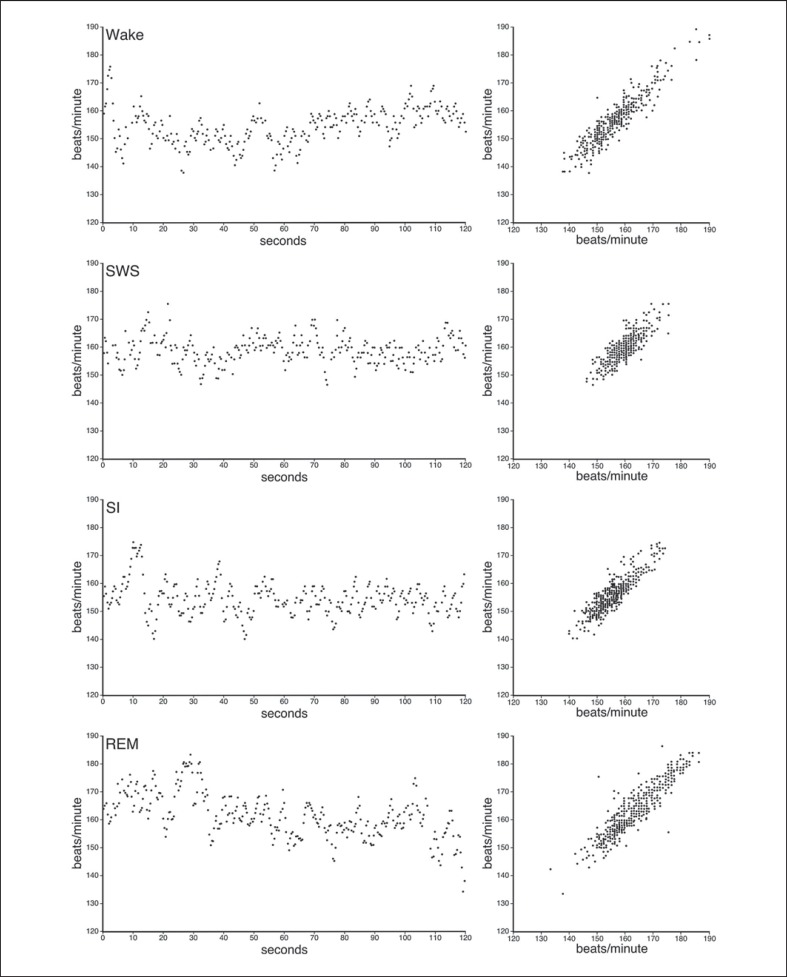
Fig. 3.
Plots representing the IHR during each of the sleep states and waking. The IHR rate during waking was irregular and more widely scattered compared to SWS, which exhibited a more regular pattern. The IHR of SI resembled that of waking while REM was even more irregular and widely distributed than waking. The left-hand column shows the IHR for an individual animal over a 2-min period in each of the states, while the graphs in the right-hand column represent compiled data from 3 individuals (2 min from each individual) for IHR plotted against the subsequent IHR.
Fig. 4.
The spectral power and associated frequency band characteristics of waking, SWS, SI and REM in the rock hyrax during both the light (a) and the dark (b) periods.
Behavior was scored in 1-min epochs as: (1) immobile – animal was completely immobile for >30 s; (2) quiet waking – animal was immobile and only moving its head or made minor movements in the same place for >30 s; (3) active waking – animal was actively moving around for >30 s (this state included exploratory and grooming behavior), or (4) eating/drinking – animal was eating and/or drinking for >30 s (fig. 5).
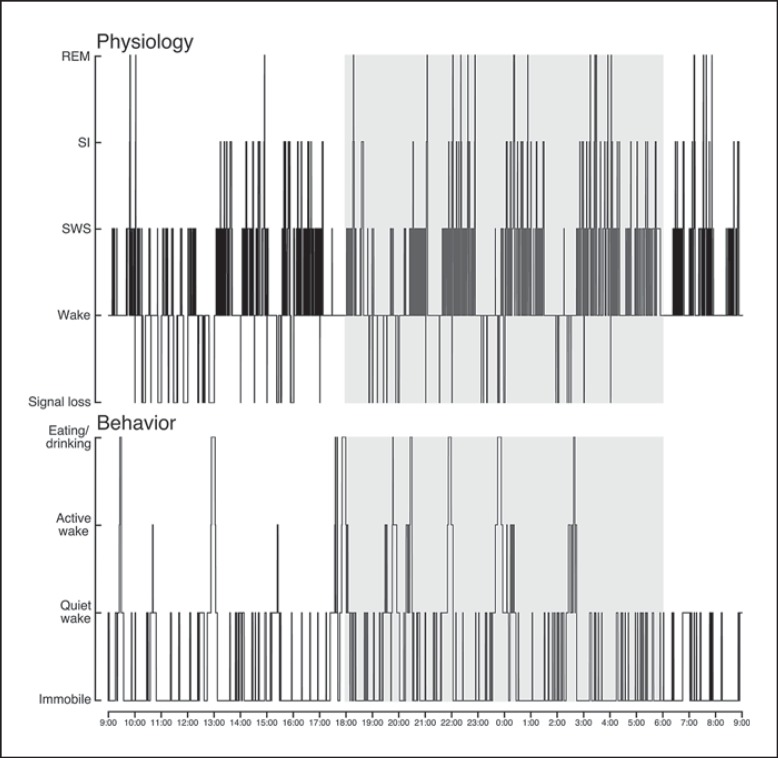
Fig. 5.
Hypnograms showing the physiological and correlated behavioral state transitions occurring over a 24-hour period for one hyrax (H06) starting at 9 a.m. The shaded area represents the dark period. The polycyclic nature of sleep is visible in the physiological hypnogram, with SWS equally represented during both the light and dark periods. The animal also appeared to be more active and was eating more during the dark periods compared to the light periods for the 24 h represented here.
Data was tested for normality prior to statistical analysis (Shapiro-Wilk's W test for normality, p > 0.05). As all data turned out to be normally distributed, there was no need to transform any data. A t test for dependent variables was used in all statistical analyses and a statistically significant difference was obtained in cases with p < 0.05. Statistical tests were performed to determine whether significant differences existed between the 5-second and 1-min scoring methods during the 24 h, light and dark periods, respectively, with regard to the total state times, number of episodes and episode duration for each of the physiologically defined states. Dependent t tests were also used to determine whether statistically significant differences existed between SWA (slow wave activity) during all states and during SWS, and whether a difference existed between these variables during the light and dark periods. The dependent t test was again used to determine whether a statistically significant difference existed between the light and dark period for each of the behaviorally defined states. Microsoft Excel and Statistica version 10 computer programs were used in the analysis of the data.
Results
Certain physiologically measurable parameters of sleep (EEG, EMG, ECG), as well as their associated behaviors, were recorded in a total of 5 rock hyraxes, P. capensis, continuously for a period of 72 h. The species mean per day for waking amounted to 15.89 h (66.2%), SWS 6.02 h (25.1%), SI 43 min (3%) and REM 6 min (0.4%). No statistically significant differences (t test for dependent variables, d.f. = 4, p > 0.05) in the distribution of these states between the light and dark periods were noted with regard to the percentage of time occupied by each state, the number of episodes per state or episode duration (table 1; fig. 5, 6). Waking most often transitioned to SWS and vice versa in these animals, making these the most common state transition pathways. There was a 5.1% probability that SWS was followed by an episode of SI and a 0.05% chance that it was followed by an REM episode. SI most often transitioned to waking, but was also followed by REM. In most cases waking followed REM, but REM also transitioned to SI. Thus, the three most common state transitions were waking → SWS → waking; waking → SWS → SI → waking, and waking → SWS → SI → REM → waking (fig. 2, 7).
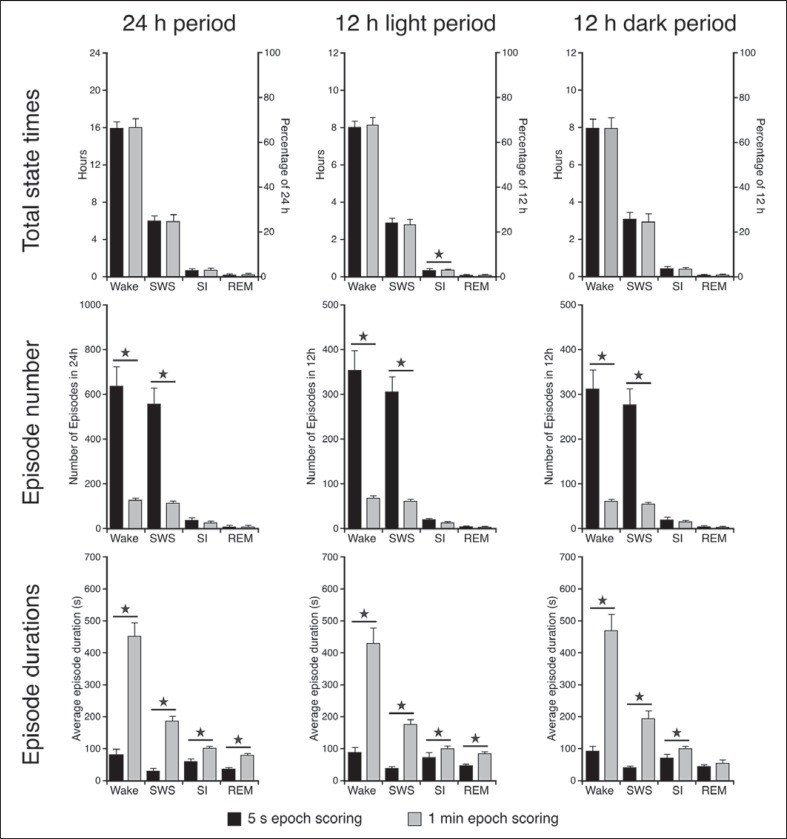
Fig. 6.
Histograms depicting the species means for the total amount of time spent in each of the physiological defined states as well as the average number of episodes and episode duration for 24 h (left row of graphs), the light period (middle row of graphs) and the dark period (right row of graphs) for both the scoring methods. Statistically significant differences between the two scoring methods are indicated by a star and the small bars represent standard error bars (t test for dependent variables, d.f. = 4, p < 0.05, please refer to the ‘Results’ section for respective t and p values).
Fig. 7.
State transition probabilities in the rock hyrax during 24 h based on the data of all 5 animals studied. In most cases SWS was followed by waking, thus making this the most common sleep pathway observed in the rock hyrax. The next most common pathway would be when SWS is followed by SI which then predominantly transitions to waking; however, in some cases it also transitions to REM, which in turn is most commonly followed by waking.
The instantaneous heart rate (IHR) during waking and SWS was on average around 157.5 bpm (standard error (SER) 0.85) and 158.1 bpm (SER 0.59), respectively (fig. 3). REM in rock hyrax was ambiguous and did not exhibit the typical characteristics of this stage as defined by other authors [Desiraju et al., 1966; Elgar et al., 1988; Tobler, 1995; Siegel, 2005b; Steriade, 2005; Horne, 2009]. Only 3 out of the 5 hyraxes studied exhibited muscle twitches associated with REM; however, these twitches did not occur during all REM episodes and in the hyraxes that exhibited these twitches it was only observed a maximum of five times throughout the entire 72-hour recording period. A loss of muscle tone was evident in all of the hyraxes examined whereby the animal would be immobile and lying with its head on the ground and then slowly falling to one side before repositioning itself. Head drooping was also noted in cases when the animal's head was not initially placed on the ground. An additional sleep state was also identified in the rock hyrax and termed SI due to the difficulty in determining whether this was an NREM or REM state (see ‘Discussion’ section). IHR during REM and SI was on average around 165.4 bpm (SER 1.17) and 148.1 bpm (SER 1.34), respectively (fig. 3).
Physiological Data
Time Budgets (Number of Epochs)
Results obtained from the 5-second scoring method revealed that these animals spent on average 15.89 h (66.2%) in waking, 6.02 h (25.1%) in SWS, 43 min (3%) in SI and 6 min (0.4%) in REM during 24 h. The 1-min epoch scoring method showed similar results to the 5-second scoring method (waking 16 h, 66.7%; SWS 6 h, 24.8%; SI 45 min, 3.1%; REM 6 min, 0.4%) and no statistically significant difference was noted between the two scoring methods for the 24-hour period (t test for dependent variables, d.f. = 4, p > 0.05). During the light period, waking amounted to 7.98 h (66.5%, 5 s) and 8.08 h (67.3%, 1 min), SWS to 3 h (25%, 5 s) and 2.96 h (24.7%, 1 min), SI to 20.2 min (2.8%, 5 s) and 21.6 min (3%, 1 min), and REM to 3 min (0.4%, 5 s and 1 min). During the dark period, waking amounted to 7.93 h (66.1%, 5 s and 1 min), SWS to 3.04 h (25.4%, 5 s) and 2.93 h (24.4%, 1 min), SI to 23.8 min (3.3%, 5 s and 1 min), and REM to 3.6 min (0.5%, 5 s) and 2.9 min (0.4%, 1 min) (fig. 6). No statistically significant difference was noted between the two scoring methods for the light and dark periods (t test for dependent variables, d.f. = 4, p > 0.05) for each of the physiologically defined states; however, during the light period a statistically significant difference between the two scoring methods exist for SI (1 min > 5 s, t test for dependent variables, d.f. = 4, t = −3.662, p = 0.0215) (fig. 6).
Number of Episodes
The average number of waking, SWS, SI and REM episodes in 24 h for the 5-second epoch scoring method amounted to 638, 559, 37 and 8, respectively, whereas for the 1-min epoch scoring method it amounted to 129, 116, 27 and 4, respectively. A statistically significant difference was noted between the two scoring methods with regard to the number of waking and SWS episodes for the 24 h (waking t = 6.057, p = 0.0038; SWS t = 6.844, p = 0.0024), light (waking t = 5.90, p = 0.0041; SWS t = 6.684, p = 0.0026) and dark (waking t = 5.679, p = 0.0122; SWS t = 6.229, p = 0.0034) periods (t test for dependent variables used in all cases, d.f. = 4, p < 0.05). A greater number of episodes were observed for these states during the 5-second compared to the 1-min scoring method (fig. 6).
Duration of Episodes
The average duration per state over 24 h amounted to 90 s for waking, 39 s for SWS, 70 s for SI and 46 s for REM for the 5-second scoring methods, whereas for the 1-min scoring method it amounted to 448, 185, 99 and 78 s, respectively (fig. 6). A statistically significant difference between the two scoring methods for all states (except REM during the dark period) was noted for the 24 h (waking t = −8.157, p = 0.0012; SWS t = −10.103, p = 0.0005; SI t = −5.687, p = 0.0047; REM t = −5.63, p = 0.0049), light (waking t = −7.502, p = 0.0017; SWS t = −12.868, p = 0.0002; SI t = −3.922, p = 0.0172; REM t = −5.007, p = 0.0075) and dark (waking t = −8.035, p = 0.0013; SWS t = −8.181, p = 0.0012; SI t = −6.79, p = 0.0025) periods (1 min > 5 s, t test for dependent variables used in all cases, d.f. = 4, p < 0.05) (fig. 6).
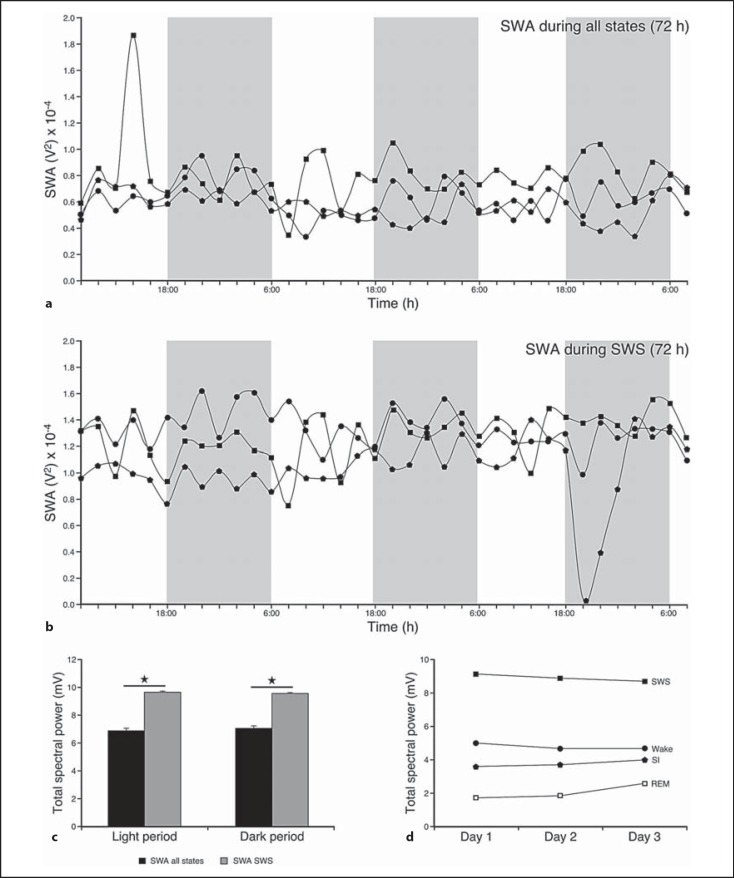
Slow Wave Activity
SWA based on 2-hour intervals was calculated for all states (SWA-all) and for SWS in all 5 animals subjected to experimentation (for clarity, fig. 8a and b only represents data for 3 animals). The results revealed that the SWA was highest during SWS and showed a gradual reduction in intensity from waking to SI to REM. SWA for both SWA-all and SWA during SWS remained relatively constant throughout the 3 days of recording. A statistically significant difference was noted when the SWA during SWS was compared to SWA-all. SWA during SWS was greater than SWA-all over 24 h (t = −5.039, p = 0.0073), as well as during both dark (t = −5.687, p = 0.0047) and light (t = −4.484, p = 0.01096) periods (t test for dependent variables used in all cases, d.f. = 4, p < 0.05; fig. 8).
Fig. 8.
SWA (based on 2-hour intervals) for the 72-hour recording period during all states (a) and during SWS (b). SWA remained fairly constant during all 3 recording days and a statistically significant difference between SWA during SWS and SWA during all states was noted (SWA during SWS > SWA-all, t test for dependent variables, d.f. = 4, p < 0.05; please refer to the ‘Results’ section for respective t and p values). c Histogram illustrating the average spectral power for the 72-hour recording period for all states and during SWS during the light and dark period. For both SWA during all states and SWS there were no differences between the light and dark periods. However, when SWA during all states and SWA during SWS were compared for the light and dark periods, respectively, statistically significant differences were observed, with SWA during SWS being greater than SWA during all states during both the light and dark periods (t test for dependent variables, d.f. = 4, p < 0.05; please refer to the ‘Results’ sections for respective t and p values). d Line graph showing the average SWA during wake and sleep stages. SWA was the greatest during SWS and this feature was consistent throughout the recording period. Data obtained from all 5 animals were analyzed; however, for clarity graphs a and b represent 3 animals. Statistically significant results are indicated by a star.
Behavioral Data
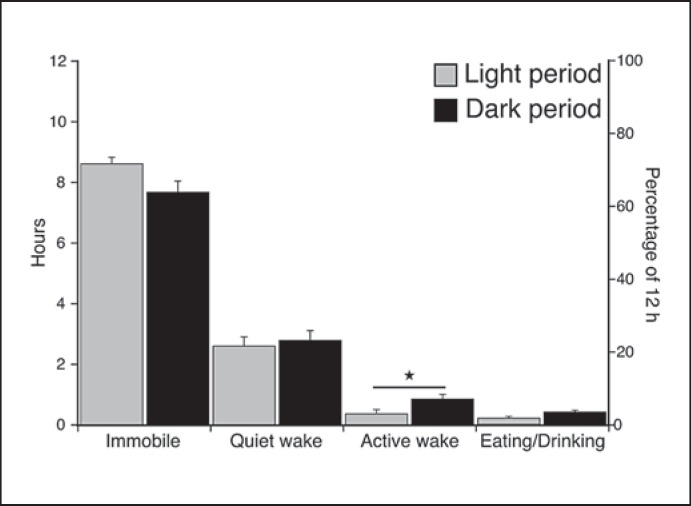
Behavior was recorded simultaneously with the physiological recordings and scored in 1-min epochs as immobile, quiet waking, active waking (which included exploratory and grooming behavior) or eating/drinking. On average over 24 h, 16.3 h (67.9%) were spent in a state of immobility, 5.5 h (22.9%) in quiet waking, 1.3 h (5.4%) in active waking and 42 min (2.9%) were spent eating/drinking. During the light and dark periods the animals were immobile for 8.6 h (71.7%) and 7.7 h (64.2%), in quiet waking for 2.7 h (22.5%) and 2.8 h (23.3%), in active waking for 24 min (3.3%) and 54 min (7.5%), and eating/drinking for 12 min (1.7%) and 30 min (4.2%), respectively. A t test for dependent variables, d.f. = 4, was used to determine whether a statistically significant difference existed between the light and dark periods with regard to the amount of time occupied by each of the states (p < 0.05). The results showed no statistically significant difference between the light and dark periods for each of the states except active waking (t = −3.297, p = 0.0458), which lasted on average 30 min longer during the dark period. Although not statistically significant, eating/drinking behavior was on average 17 min longer during the dark period (fig. 9).
Fig. 9.
Histogram illustrating the species mean for the percentage of time occupied by each behavioral state for the 72-hour recording period during the light and dark period, respectively. There is no statistically significant difference in the distribution of immobility, quiet waking and eating/drinking behaviors between the light and dark periods; however, a statistically significant difference was noted with regard to active waking. More active waking was noted during the dark compared to the light period (t test for dependent variables, d.f. = 4, p < 0.05; please refer to the ‘Results’ section for respective t and p values). Although not statistically significant, there was also a tendency to more eating/drinking behavior during the dark period. Statistically significant results are indicated by a star.
Discussion
The aim of the present study was to describe sleep in the rock hyrax, P. capensis. On average over 24 h these animals spent approximately 15.89 h in a state of waking, 6.02 h in SWS, 43 min in SI and 6 min in REM. The polygraphic aspects of waking, SWS and REM in the rock hyrax were characteristic of what has been described for these physiological states in many mammals [Desiraju et al., 1966; Elgar et al., 1988; Tobler, 1995; Siegel, 2005a; Steriade, 2005; Horne, 2009]. An additional state termed SI, which has not been described in other mammals studied to date, was characterized by a desynchronized EEG with amplitude between that of SWS and REM, an EMG with amplitude similar to that seen for SWS, and a mostly regular ECG. When the 5-second and 1-min epoch scoring techniques were compared, no statistically significant difference was noted with regard to TST and waking times for the 24 h or light and dark periods for all states except SI during the light period where it was found to be greater for the 1-min scoring method. A statistically significant difference was noted with regard to the number of episodes (only waking and SWS) and episode duration (all states except REM during the dark period) for the 24 h, light and dark periods (fig. 6). Thus, when drawing conclusions regarding total times over the 24-hour period for the different states, either the 5-second or 1-min epoch data will provide the same answers, but when determining the number of episodes and episode duration it appears to be more biologically relevant to use the 1-min epoch scoring method.
SI – A Type of NREM or REM?
As described above, a sleep state termed SI was observed in the rock hyrax. At present, with the data we currently have, it is difficult to determine whether this sleep state, which was found to exhibit the same physiological parameters in all hyraxes studied, is a type of NREM or REM. It is possible that SI, as defined in this study, could be considered a form of low-voltage slow wave sleep, a type of NREM. According to Bergmann et al. [1987], low-voltage slow wave sleep is characterized by behavioral quiescence, desynchronized EEG, EMG that is at the same amplitude as during SWS and theta activity that resembles waking and is lower than during REM. In the rock hyrax SI exhibited some of these characteristics such as behavioral quiescence and desynchronized EEG and EMG similar to SWS. In addition to this, the ECG was not as irregular as seen during unambiguous REM sleep in the hyrax. Thus, the data can be interpreted in such a way as to consider SI a type of low-voltage NREM similar in structure to low-voltage slow wave sleep as defined by Bergmann et al. [1987] for the rat.
Despite this, it is also possible that SI is a type of REM. The desynchronized EEG, ECG less regular in comparison to SWS, behavioral quiescence, greater amount of theta activity and that SI always followed an SWS episode, all indicate that SI could be considered a type of REM. Clearly this is not what would be described as typical mammalian REM sleep, but REM sleep in certain mammalian species, such as the platypus which has REM with cortical synchronization [Siegel et al., 1999], the rat, Rattus norvegicus, and rabbit, Oryctolagus cuniculus, which are not always atonic in REM [e.g. Pivik et al., 1981; Gottesman, 1992], and Tupaia for which EMG has a higher amplitude in REM than in SWS [Berger and Walker, 1972], does not always fit the standard criteria determined in studies of typical laboratory animals and humans. Thus, with the currently available data SI might also be interpreted as a form of REM sleep.
While we do not currently have the data to resolve this issue, future studies of the rock hyrax, measuring such variables as hippocampal theta activity and electro-oculogram, as well as single unit recordings from the sleep-related brainstem nuclei could resolve whether SI in the rock hyrax is a form of NREM or REM. Whatever the result of such future studies, the implications of SI being a type of NREM or REM are of interest. If SI is a type of NREM, then, like many other mammals, the rock hyrax would have more than one form of NREM; however, this would mean that the rock hyrax exhibits the lowest amount of REM sleep of any terrestrial mammal studied to date, approximately 6 min/day. This is an interesting possibility and may make the hyrax an interesting animal model for the study of the function of REM sleep due to its possible paucity in this species. With the alternative theory that SI is a type of REM state the hyrax would exhibit more than one type of REM, which would also be unusual, and NREM of the hyrax would consist purely of SWS. This again makes the hyrax an interesting model animal for the study of REM function, as if SI is a type of REM, then perhaps the potential benefits of REM sleep for organismal function can be derived without the expression of the full range of typical REM sleep polygraphic features. However, it is also possible that SI could merely be a ‘transitional’ sleep stage exhibiting some characteristics of both NREM and REM sleep. This is clearly an area that needs further investigation.
Comparison to Previous Sleep Studies in Hyraxes
A previous abstract by Snyder [1974] reported sleep in three other species of hyrax, Procavia johnstoni, Heterohyrax brucei and Dendrohyrax validus. Snyder reported that TST for P. johnstoni was 4.9 h (SWS contributing 4.66 h and REM 0.24 h), for H. brucei was 5.7 h (5.24 h consisting of SWS and 0.45 h REM) and for D. validus was 4.9 h (SWS contributing 4.66 h and REM 0.24 h). There was no statistically significant difference with regard to TST between light and dark periods for P. johnstoni and H. brucei, but a light/dark difference in TST was noted for D. validus, which was the only solitary nocturnal species studied [Kingdon, 1971; Campbell and Tobler, 1984; Elgar et al., 1988]; however, as stated previously only an abstract was published for the TST reported for these species of hyrax and no spectral power analysis was performed on the data to corroborate these findings. Our results showed a 1.9- and 1.1-hour increase in TST when compared to P. johnstoni and H. brucei, respectively. Total REM (composed of both SI and REM) time in our study was also considerably greater compared to the reported values stated previously in these two species (P. capensis 50 min/day, P. johnstoni 14.4 min/day, H. brucei 27 min/day); however, if SI is not considered to be a form of REM, total REM time in our study would be significantly less than the reported values for these other species of hyrax (table 2). No statistically significant difference was noted between the light and dark periods with regard to TST in the current study as well as in the Snyder [1974] study (P. johnstoni and H. brucei only), underlining the polycyclic nature of sleep in the Hyracoidea.
Table 2.
Comparison between TST for four different species of hyrax
| P. capensis | P. johnstoni | H. brucei | D. validus | |
|---|---|---|---|---|
| Waking/active, h | 15.9 | 12.5 | 12.6 | 13.7 |
| SWS, h | 6.0 | 4.9 | 5.7 | 4.9 |
| Transitional sleep, h | – | 6.1 | 4.8 | 4.9 |
| SI, min | 43.2 | – | – | – |
| REM, min | 5.8 | 33.1 | 54.7 | 30.2 |
The data represented for P. capensis have been obtained from the current study, whereas the data represented for P. johnstoni, H. brucei and D. validus have been obtained from a study by Snyder [1974]. A greater amount of time is spent in a state of waking and SWS in P. capensis compared to the other 3 species represented here. If SI can be considered a form of REM in P. capensis, then the total amount of time spent in this particular state will fall in between the values reported for P. johnstoni and H. brucei. However, if SI cannot be considered a form of REM in P. capensis but rather a form of low-voltage SWS, the implication would be that P. capensis has the lowest amount of REM recorded for this species as well as for any terrestrial mammal studied to date.
Comparison to Sleep in Other Afrotheria, Xenarthra and Other Mammals
Hyraxes belong to the superorder Afrotheria, which includes the African and Asian elephants (Proboscidea), the manatee and dugong (Sirenia), aardvark (Tubulidentata), golden moles, tenrecs (Afrosoricida), and sengis (Macroscelidea). Observational studies have reported that TST in the Asian and African elephants amounts to approximately 3.8 h [Kurt, 1960; Campbell and Tobler, 1984], with sleep mainly occurring during the dark periods in the early morning hours [Wyatt and Eltringham, 1974]. Moss [1982] reported that wild African elephants, L. africana, sleep a total of 3–5 h at night, whereas captive Asian elephants, E. maximus, slept for approximately 4–6.5 h [Tobler, 1992; Wilson et al., 2006]. An electrophysiological study on an Amazonian manatee, T. inungius, revealed that this animal spent approximately 6.5 h (27%) in SWS and 14.4 min (1%) in REM sleep over a 24-hour period and that 25% of SWS was occupied by interhemispheric asymmetry [Mukhametov et al., 1992]; however, it must be noted that the TST reported was based on the study of a single animal and the recording of sleep was done directly after surgery, thus the TST obtained may not be representative of this species. As for the insectivoran-like afrotheres, TST in the tenrecs has been reported to be 15.6 h, with SWS contributing 13.26 h and REM 2.34 h [Campbell and Tobler, 1984; Elgar et al., 1988; Snyder et al., 1972].
It appears, as is clear from the aforementioned examples, that sleep in the Afrotherians is just as diverse and unusual as the composition of this group, and this sleep diversity could possibly be attributed to the different lifestyles and behaviors of each of its members [Siegel, 2009]. For example, some members are diurnal (e.g. elephants, manatee, rock hyrax, sengis), whereas others are nocturnal (e.g. tenrecs, golden moles, aardvark). Some member's offspring are precocial (e.g. rock hyrax), whereas others are altricial (e.g. tenrecs). The degree of sociality also differs greatly between the different Afrotherians, with elephants and rock hyraxes being social mammals, the manatee weakly social but essentially solitary [Hartman, 1979; Nowak, 1999], whereas the sengis live in monogamous pairs. The above list of similarities and differences would appear to suggest that a combination of phylogenetic constraints and adaptation to environmental factors would need to be invoked to fully explain the observed results.
Sleep has also been studied both behaviorally and electrophysiologically in the Afrotherian sister group Xenarthra [armadillos, sloths and anteaters; Hallström et al., 2007]. TST in the giant armadillo, Priodontes giganteus, has been reported to be 18.1 h [Affanni, 1972] and in the nine-banded armadillo, Dasypus novemcinctus, 17.4 h [Van Twyver and Allison, 1974]. For both species no difference was observed with regard to the distribution of TST between the light and dark periods [Campbell and Tobler, 1984]. Behavioral studies conducted on the two-toed sloth, Choloepus hoffmanni, showed that TST in these animals amounted to 16.4 h [Sunquist and Montgomery, 1973]. The brown-throated three-toed sloth, Bradypypus variegatus, was found to have TST of 15.84 h when it was recorded in captivity [Campbell and Tobler, 1984; de Moura Filho et al., 1983] and a TST of 9.63 h when recorded in the wild [Rattenborg et al., 2008]. The rock hyrax and three-toed sloth are both herbivores, whereas the giant armadillo and tenrec are insectivorous or carnivorous. When body mass is compared to the number of hours of sleep per day, the tenrec and giant armadillo sleep for a larger part of the day compared to the rock hyrax and three-toed sloth. An analysis of all reported sleep times found TST in carnivores are significantly greater than that of herbivores [Siegel, 2005a].
Certain physiological parameters such as body and brain mass, metabolic rate and state of birth have been correlated to TST in mammals; however, no consensus can be reached regarding these correlations as the different studies have opposing views. For example, when just comparing body mass, mammals with the same dietary preference that fall within the same body mass range have different TST, the rock hyrax with an average body mass of 4 kg has a TST of approximately 7 h each day, whereas the three-toed sloth, which has an average body mass of 3.5–4.5 kg, sleeps for approximately 10 h/day in the wild and 16 h in captivity [Siegel, 2005a; Rattenborg et al., 2008]. It has also been shown that risk of predation as well as relative exposure of the sleeping site correlates negatively with TST [Capellini et al., 2008]. Rock hyraxes have been reported to have a danger factor of approximately 0.5 [Allison and Cicchetti, 1976] and are species that are typically heavily predated, even though they sleep in relatively unexposed sites. Considering the evidence it is thus possible that ethological rather than physiological factors are responsible for the variations seen in TST between different mammalian species.
The current data also pose some interesting questions. The results showed that these animals were awake on average for approximately 16 h/day, and the behavioral data suggested that active waking and eating was more predominant during the dark periods as opposed to the light periods. Thus, the hyraxes are not strongly diurnal or nocturnal, but can best be characterized as polycyclic without a strong circadian difference in activity or sleep patterns. In the wild rock hyraxes are rarely seen out of their dens during the night; so could the bursts of nocturnal activity that have been observed in our study be replicated when these animals are in ‘hiding’ during the night in the wild, and what would the relevance of this activity be when these animals sleep in a social setting? It is also possible that the activity patterns observed in these animals in the current study could be attributed to the standardized laboratory conditions (i.e. constant ambient temperature, 12-hour light/dark cycle as well as light intensity). In the wild these animals are social, they do not always experience 12 h of light followed by 12 h of dark, they are not always exposed to the same light intensity and the ambient temperature can vary greatly between day and night. It has also been shown that rock hyraxes have poor thermoregulative abilities and are often seen basking in the sun and typically sleep huddled up in groups [Olds and Shoshani, 1982], a possible mechanism for conserving body heat. It is therefore possible that the biology of the species can account for differences observed with regard to the phasing of sleep and activity budgets seen in the current study and that the standardized conditions might just represent an unusual condition that will only be experienced for a limited amount of time in the wild. Further studies on rock hyraxes in the wild would be required to determine to what extent the standardized laboratory conditions alter the natural phasing of sleep and activity budgets in these animals.
Acknowledgements
The current study was supported by funding from the National Research Foundation of South Africa to P.R.M. We gratefully acknowledge the assistance of the members of the Central Animal Services of the University of the Witwatersrand throughout this study.
References
- Affanni JM. Observations on the sleep of some South American marsupials and edentates. In: Chase M, The Sleeping Brain, editor. Los Angeles, Brain Research Institute, University of California. 1972. pp. pp 21–23. [Google Scholar]
- Allison T, Cicchetti PV. Sleep in mammals: ecological and constitutional correlates. Science. 1976;194:732–734. doi: 10.1126/science.982039. [DOI] [PubMed] [Google Scholar]
- Berger RJ, Walker JM. A polygraphic study of sleep in the tree shrew (Tupaia glis) Brain Behav Evol. 1972;5:54–69. doi: 10.1159/000123737. [DOI] [PubMed] [Google Scholar]
- Bergmann BM, Winter JB, Rosenberg RS, Rechtschaffen A. NREM sleep with low-voltage EEG in the rat. Sleep. 1987;10:1–11. doi: 10.1093/sleep/10.1.1. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:296–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- Capellini I, Barton RA, McNamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution. 2008;62:1764–1776. doi: 10.1111/j.1558-5646.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura Filho AG, Huggins SE, Lines SG. Sleep and waking in the three-toed sloth Bradypus tridactylus. Comp Biochem Physiol A. 1983;76:345–355. doi: 10.1016/0300-9629(83)90336-5. [DOI] [PubMed] [Google Scholar]
- Desiraju T, Anand BK, Singh B. Electrographic studies on the nature of sleep and wakefulness. Physiol Behav. 1966;1:285–291. [Google Scholar]
- Elgar MA, Pagel MD, Harvey PH. Sleep in mammals. Anim Behav. 1988;36:1407–1419. [Google Scholar]
- Gottesman C. Detection of seven sleep-waking stages in the rat. Neurosci Biobehav Rev. 1992;16:31–38. doi: 10.1016/s0149-7634(05)80048-x. [DOI] [PubMed] [Google Scholar]
- Hallström BM, Kullberg M, Nilsson MA, Janke A. Phylogenomic data analyses provide evidence that Xenarthra and Afrotheria are sister groups. Mol Biol Evol. 2007;24:2059–2068. doi: 10.1093/molbev/msm136. [DOI] [PubMed] [Google Scholar]
- Hartman DS. Ecology and Behavior of the Manatee (Trichechus manatus) in Florida. Pittsburgh, American Society of Mammalogists. 1979 [Google Scholar]
- Horne J. REM sleep, energy balance and ‘optimal foraging’. Neurosci Behav Rev. 2009;33:466–474. doi: 10.1016/j.neubiorev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Kingdon J. East African Mammals – An Atlas of Evolution in Africa. London, Academic Press. 1971 [Google Scholar]
- Kurt F. Le sommeil des éléphants. Mammalia. 1960;24:259–272. [Google Scholar]
- Lesku JA, Bark RJ, Martinez-Gonzalez D, Rattenborg NC, Amlaner CJ, Lima SL. Predator-induced plasticity in sleep architecture in wild-caught Norway rats (Rattus norvegicus) Behav Brain Res. 2008;189:298–305. doi: 10.1016/j.bbr.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Lyamin OI, Kosenko PO, Lapierre JL, Mukhametov LM, Siegel JM. Fur seals display a strong drive for bilateral slow-wave sleep while on land. J Neurosci. 2008a;28:12614–12621. doi: 10.1523/JNEUROSCI.2306-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamin OI, Lapierre JL, Kosenko PO, Mukhametov LM, Siegel JM. Electroencephalogram asymmetry and spectral power during sleep in the northern fur seal. J Sleep Res. 2008b;17:154–165. doi: 10.1111/j.1365-2869.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, Siegel JM. Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav Rev. 2008c;32:1451–1484. doi: 10.1016/j.neubiorev.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara P, Capellini I, Harris E, Nunn CL, Barton RA, Preston B. The phylogeny of sleep database: a new resource for sleep scientists. Open Sleep J. 2008;1:11–14. doi: 10.2174/1874620900801010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. Portraits in the Wild. Chicago, University of Chicago Press. 1982 [Google Scholar]
- Mukhametov LM, Lyamin OI, Chetyrbok IS, Vassilyev AA, Diaz RP. Sleep in an Amazonian manatee, Trichechus inunguis. Experientia. 1992;48:417–419. doi: 10.1007/BF01923447. [DOI] [PubMed] [Google Scholar]
- Nicolau MC, Akaârir M, Gamundí A, Gonzáles J, Rial RV. Why we sleep: the evolutionary pathway to the mammalian sleep. Prog Neurobiol. 2000;62:379–406. doi: 10.1016/s0301-0082(00)00013-7. [DOI] [PubMed] [Google Scholar]
- Nowak RM. Walker's Mammals of the World. Baltimore, Johns Hopkins. (ed 6) 1999;2 [Google Scholar]
- Olds N, Shoshani J. Procavia capensis. Mammalian Species. 1982;171:1–7. [Google Scholar]
- Pivik RT, Sircar S, Braun C. Nuchal muscle tonus during sleep, wakefulness and tonic immobility in the rabbit. Physiol Behav. 1981;26:13–20. doi: 10.1016/0031-9384(81)90072-x. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC, Voirin B, Vyssotski AL, Kays RW, Spoelstra K, Kuemmeth F, Heidrich W, Wikelski M. Sleeping outside the box: electroencephalographic measures of sleep in sloths inhabiting a rainforest. Biol Lett. 2008;4:402–405. doi: 10.1098/rsbl.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial RV, Akaârir M, Gamundí A, Nicolau C, Garau C, Aparicio S, Tejada S, Gené L, González J, De Vera LM, Coenen AM, Baraceló P, Esteban S. Evolution of wakefulness, sleep and hibernation: from reptiles to mammals. Neurosci Biobehav Rev. 2010;34:1144–1160. doi: 10.1016/j.neubiorev.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Clues to the function of mammalian sleep. Nature. 2005a;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, REM Sleep . Principles and Practices of Sleep Medicine. In: Kryger MH, Roth R, Dement WC, editors. New York, Saunders. ed 4 2005b. [Google Scholar]
- Siegel JM. Do all animals sleep? Trends Neurosci. 2008;31:208–213. doi: 10.1016/j.tins.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Sleep viewed as a state of adaptive inactivity. Nat Rev Neurosci. 2009;10:747–753. doi: 10.1038/nrn2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Manger PR, Nienhuis R, Fahringer HM, Pettigrew JD. Sleep in the platypus. Neuroscience. 1999;91:391–400. doi: 10.1016/s0306-4522(98)00588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner JD, Chimimba CT. Cape Town, Cambridge University Press. ed 3 2005. The Mammals of the Southern African Subregion. [Google Scholar]
- Snyder F. Sleep-waking patterns of Hyracoidea. Sleep Res. 1974;3:87. [Google Scholar]
- Snyder F, Bugbee N, Douthitt T. Telemetric studies of 24-hour sleep-waking patterns in some primitive mammals. Psychophysiology. 1972;9:122. [Google Scholar]
- Steriade M. Brain electrical activity and sensory processing during waking and sleeping states. In: Kryger MH, Roth R, Dement WC, Principles and Practices of Sleep Medicine, editors. New York, Saunders. ed 4 2005. [Google Scholar]
- Sunquist M, Montgomery G. Activity patterns and rates of movement of two-toed and three-toed sloths, Choloepus hoffmani and Bradypus infuscatus. J Mammal. 1973;54:946–954. [PubMed] [Google Scholar]
- Tabuce R, Asher RJ, Lehmann T. Afrotherian mammals: a review of current data. Mammalia. 2008;72:2–14. [Google Scholar]
- Tobler I. Behavioral sleep in the Asian elephant in captivity. Sleep. 1992;15:1–12. [PubMed] [Google Scholar]
- Tobler I. Is sleep fundamentally different between mammalian species? Behav Brain Res. 1995;69:35–41. doi: 10.1016/0166-4328(95)00025-o. [DOI] [PubMed] [Google Scholar]
- Tobler I, Phylogeny of sleep regulation . Principles and Practices of Sleep Medicine. In: Kryger MH, Roth R, Dement WC, editors. New York, Saunders. ed 4 2005. [Google Scholar]
- Van Twyver H, Allison T. Sleep in the armadillo Dasypus novemcinctus at moderate and low ambient temperatures. Brain Behav Evol. 1974;9:107–120. doi: 10.1159/000123659. [DOI] [PubMed] [Google Scholar]
- Wilson ML, Bashaw MJ, Fountain K, Kieschnick S, Maple TL. Nocturnal behavior in a group of female African elephants. Zoo Biol. 2006;25:173–186. [Google Scholar]
- Wyatt JR, Eltringham SK. The daily activity of the elephant in the Rwenzori National Park, Uganda. East Afr Wildl J. 1974;12:213–289. [Google Scholar]
- Zepelin H, Siegel JM, Tobler I, Mammalian sleep Kryger MH, Roth R, Dement WC. New York, Saunders. ed 4 2005. Principles and Practices of Sleep Medicine. [Google Scholar]