Abstract
Multiple sclerosis (MS) is an immune-mediated disease of the central nervous system characterized by focal or diffuse inflammation, demyelination, axonal loss and neurodegeneration. Brain atrophy can be seen in the earliest stages of MS, progresses faster compared to healthy adults, and is a reliable predictor of future physical and cognitive disability. In addition, it is widely accepted to be a valid, sensitive and reproducible measure of neurodegeneration in MS. Reducing the rate of brain atrophy has only recently been incorporated as a critical endpoint into the clinical trials of new or emerging disease modifying drugs (DMDs) in MS. With the advent of easily accessible neuroimaging softwares along with the accumulating evidence, clinicians may be able to use brain atrophy measures in their everyday clinical practice to monitor disease course and response to DMDs. In this review, we will describe the different mechanisms contributing to brain atrophy, their clinical relevance on disease presentation and course and the effect of current or emergent DMDs on brain atrophy and neuroprotection.
Keywords: Multiple sclerosis, Bran, Atrophy, Neurodegeneration, Axon, Inflammation, Neuroprotection, Drugs
Introduction
Multiple sclerosis (MS) is an immune-mediated disease that affects the entire central nervous system (CNS) [1–3]. Magnetic resonance imaging (MRI) lesions are well-scattered at white matter (WM) and grey matter (GM) [4], while normal-appearing brain tissue in MRI also seems to be affected in pathological studies [4]. Brain atrophy, the gradual loss of brain volume, is quite extensive in MS, nearly 0.5–1.35% per year, far off the limits of normal aging [5, 6]. It arises early in the course of the disease, accelerates with disease progression [7–12] but is attenuated by disease-modifying drugs [13].
There has been increasing interest in measuring tissue loss in CNS, as it represents the net effect of all destructive pathogenic processes during the disease course [14–17]. It is worth recalling that neurons occupy almost half (46%) of the tissue volume, myelin is 24%, and glial and other cells almost 30% [5]. GM [4] holds much less myelin than WM (about one tenth), while neurons comprise its most abundant component [18]. Relative to glial cells, oligodendrocytes outweigh the number of astrocytes, microglia and oligodendrocyte progenitor cells, although the exact percentage is still unknown [19, 20].
Atrophy in MS is often considered to be the result of extensive axonal transection and demyelination [21–23]. The contribution of neuroglia may be less clear; reactive gliosis has the potential to mask considerable tissue loss in WM lesions [24, 25]. Measurement of brain atrophy is also considerably influenced by the amount of tissue fluids [26], which is increased by active inflammation and vasogenic edema in WM plaques, and decreased during treatment with agents with strong anti-inflammatory properties (pseudoatrophy effect) [14, 26].
Transient volume changes could also be attributed to idiosyncrasic and technical factors [14]. Dehydration may affect functional integrity of neuroglial cells, while decreased protein levels mainly affect synaptic densities [26]. Unlike demyelination, water volume fluctuations and transient biological factors, neuroaxonal damage is irreversible in CNS, and atrophy is primarily considered to reflect this neurodegenerative component in MS [27–30]. Finally, the atrophy rates may also be influenced to some extent by the genetic makeup of a person; Human leukocyte antigen (HLA) genotypes considered as ‘high risk for MS’, namely DRB1 and DQB1, have been associated with significantly lower WM and GM volumes, alongside with higher mean annualized percentage of brain volume change (PBVC) compared with medium and low risk HLA genotypes independent from patients clinical features (age, gender, disease course) or the DMTs used [31].
Pathogenesis of brain atrophy
The time trajectory of brain atrophy
Focal tissue loss in WM plaques is undoubtedly a major contributor to brain atrophy. However, the correlation between demyelination foci and whole brain atrophy is still a matter of debate [16]. Some studies have found a strong association [32, 33], while others have not [25, 34–36], suggesting that separate pathologic processes may also contribute to tissue destruction.
Chard et al. [37] in a longitudinal 14-year study found that atrophy is more related to early rather than late focal lesion volumes. Inflammation may be an important contributor to global tissue loss in early disease stages (i.e. in clinically isolated syndrome). As the disease progresses, additional mechanisms emerge that are, at least partly, independent from WM injury, such as microglia activation, meningeal inflammation, iron deposition, oxidative stress and diffuse axonal damage in normal appearing white matter (NAWM). The lack of a significant relationship between white matter fraction (WMF) and T2 lesion load [34, 38] further support this hypothesis. Biopsy studies also confirm that the atrophy may proceed even in the absence of inflammation [39, 40].
Regional atrophy studies may also be helpful. Indeed, the volume loss of deep GM structures may be present in the early stages of the disease and it is strongly correlated with the disease course [41]. In MS, brain atrophy may develop in different CNS structures and varies depending on the clinical disease phenotypes; ventricular enlargement is more prominent in relapsing–remitting MS [RRMS], whereas cortical atrophy seems to be more important in the progressive forms of the disease [42].
All things considered, it has been suggested that the pathogenic trajectory of brain atrophy changes with disease progression; from primarily inflammatory to less inflammatory and primarily neurodegenerative in the late stages of the disease [43, 44].
Pathogenesis of acute demyelination and axonal injury
In the initial stages of MS, many different components of the adaptive and the innate immunity induce demyelination and neuronal loss [43]. The activation of auto-reactive CD4+ T lymphocytes in the peripheral immune system is necessary for their migration across the blood–brain-barrier (BBB) and into the CNS. After myelin destruction, T cells are in situ reactivated by antigens within myelin debris and their clonal expansion results in multifocal demyelinating plaques [45]. Peripheral B lymphocytes are involved in the antigen presentation and initial stimulation of CD4 T cells. Also, they are an essential source of pro- and anti-inflammatory cytokines (IL-6 among others) promoting every autoimmunity response (driven by Th1, Th2, Th 17 cells) driving MS. In addition, the presence of chemokines (CXCL13) and survival factors (BAFF and APRIL) in the CSF of patients with MS, promotes the formation of meningeal follicle like structures, in progressive phases but also in early RRMS [46]. T cells and B cells may, therefore, play an equally important role in the immunopathology of MS [47].
Axonal destruction is quite extensive (up to 60–80%) in all active WM lesions [9, 12, 48] and the extend of axonal loss is related to the number of immune cells within the plaques [49]. Activated immune cells (T and B cells) and microglia/macrophages release a number of pro-inflammatory cytokines (e.g. TNFa, INFγ), proteolyticenzymes (e.g. perforin, granzymes) and free radicals (e.g. nitric oxide, glutamate) that can directly damage axons [50]. Additionally, axons may die secondarily, due to the loss of pre- and post-synaptic signals (i.e. dying-back and Wallerian axonal degeneration) in regions far from the lesion site [43].
Active MS lesions are characterized by profound heterogeneity regarding their demyelination pattern [51], which is persistent over time [52]. The most commonly observed patterns are pattern II, which is a complement- and antibody-mediated demyelination, and pattern III, in which the initial event in lesion formation is a brief yet exorbitant oligodendrocyte injury [53]. In other patients with RRMS, new lesions are associated with T cells, and activated microglia only. Pathologic heterogeneity across individuals in demyelination may imply different stimuli in the initial inflammation or different vulnerability to tissue loss across individuals [54].
In WM lesions, inflammation and brain edema, demyelination, axonal loss, gliosis, and remyelination, all happen simultaneously [35, 55]. Brain edema which increases brain volume might bias atrophy measurements, but it resolves in the first few weeks after lesion formation. Notably, CNS has the capacity to use a great number of compensatory mechanisms (i.e. remyelination, redistribution of sodium channels, expression of neurotrophic factors etc.) to re-establish lost functioning to demyelinated foci [48].
To conclude, tissue loss due to inflammation and demyelination maybe partly reversible in RRMS [56, 57], while tissue loss and axonal damage due to mechanisms other than inflammation is irreversible, and remains the major component of brain atrophy especially in the progressive disease stages.
Mechanisms of late axonal loss (Fig. 1)
Fig. 1.
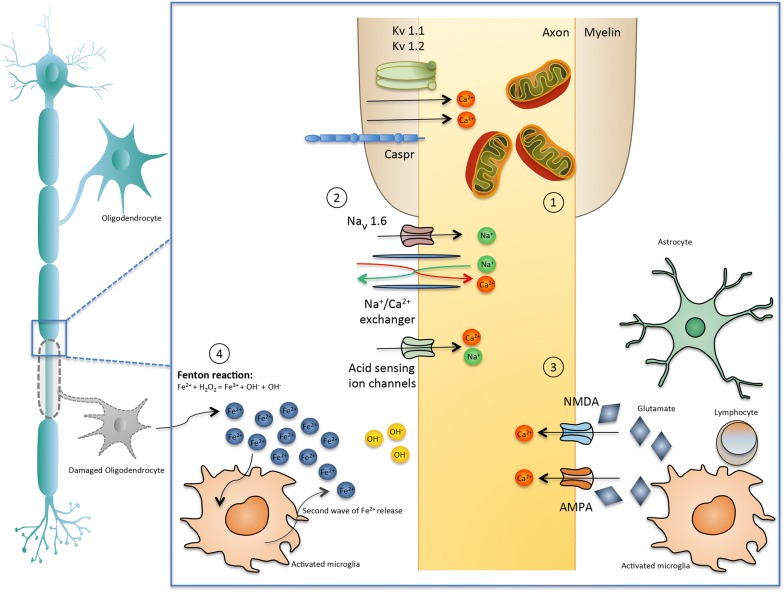
Mechanisms of late axonal loss. Molecular and cellular mechanisms driving neurodegeneration and atrophy. Key elements are considered to be: (1) Mitochondria Dysfunction: Inflammation in acute demyelinating lesions lead to respiratory protein complexes inhibition, mitochondrial injury and dysfunction, release of apoptosis-inducing factors and mitochondrial DNA deletions. In chronic inactive plaques, ionic imbalance, high energy demands and clonal expansion of defective mitochondria further impair oxidative damage. These mitochondrial alterations of functional impairment and structural damage lead to histotoxic hypoxia and energy failure and consequently to neurodegeneration. [146] Upregulation of sodium channels, acid sensing ion channels and expression of maladaptive isoforms (Nav1.6 channels), paranodal (Caspr) and juxtparanodal (Kv1.2) protein lead to high energy demands, intra-axonal calcium accumulation, and subsequent axonal degeneration. (3) Glutamate Excitotoxicity: Increased glutamate production by activated microglial cells and lymphocytes, and impaired clearance by resident cells such as astrocytes lead to higher lever of glutamate. High levels of glutamate lead to over-activation of N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (which are permeable for calcium and sodium ions) and subsequent calcium overload and oligodendocyte and neuron cell death. (4) Iron release: In MS lesions free iron [Fe2+] is released in the extracellular space leading to production of highly reactive hydroxyl molecules (OH−) by the Fenton reaction. Further, iron is released by activated glial cells, which become dystrophic and disintegrate, leading to a second wave of Fe2+ release
While the destruction of CNS myelin is associated with clinical relapses, acute or late axonal loss is considered to be the main cause of permanent clinical disability in MS [49]. Axons are more vulnerable to acute injury by inflammatory mediators, due to their shape and structure, compared to cell bodies or dendrites [43], while thin axons (< 2.5 μm in diameter) are mainly affected [24, 58]. Neurofilament light chain (NfL) protein is only expressed in neurons. It is an essential component of the axonal cytoskeleton, and reflects the axonal integrity and the stability of neurons. Under conditions of acute axonal transection, NfL are released and can be found as a result, in the cerebrospinal fluid (CSF) and blood of patients with MS. Of note, ultra high versus low blood NfL levels have been associated with MRI related (increased number of gadolinium enhancing or T2 lesion load, whole brain atrophy) and clinical measures (number of relapses, disability worsening) of disease activity and evolution and may, therefore, have prognostic value for patients and clinicians [59].
Transected axons and ovoids are abundant in MS lesions [9, 27] but, abnormalities have also been reported in chronic inactive plaques, in normal appearing white matter (NAWM), and cortical areas, in which inflammation is less prominent [48, 57]. Therefore, additional mechanisms of axonal loss coexist with disease progression. It should be noted that these mechanisms have been postulated for both acute and late axonal loss (i.e. “late” signifying the absence of apparent inflammation):
Ion overload
Several ion channels show compensatory changes a few weeks after demyelination [60] a process that eventually promotes energy deficiency, and neurodegeneration. Aberrant expression of sodium channels, acid sensing ion channels, increased expression of maladaptive isoforms (i.e. Nav1.6 channels) [61], paranodal (Caspr) and juxtparanodal (Kv1.2) protein alterations [62] have also been detected in WM lesions, in NAWM, and GM. Alternation in the expression of these ion channels lead to intra-axonal calcium accumulation, and subsequent axonal degeneration and atrophy, particularly in secondary progressive MS [49].
Mitochondria dysfunction
There has been increasing interest in the role of mitochondrial injury in MS demyelination and axonal destruction. In acute inflammatory lesions mitochondrial nicotinamide adenine dinucleotide-hydrogen (NADH) oxidase [63] and complex IV defects (COX I) have been described, in axons, oligodendrocytes, and astrocytes [58]. In chronic inactive plaques, ionic imbalance and high energy demands result to swollen and dysfunctional mitochondria [64, 65], a phenomenon in which is partially reversed in remyelinating axons [66]. There are also additional mtDNA deletions in GM structures of patients with SPMS [67]. Furthermore, the respiration deficient neurons were diffusely distributed in the subcortical WM resulting in axonal loss in the absence of demyelination or inflammation. In oligodendrocytes, mitochondrial damage results in cell death and demyelination. Progenitor cells are also impaired, regarding their capacity to differentiate and produce myelin [48]. Plus, genetic defects in mitochondrial genes potentiate MS lesions [68]. From what can be deducted, mitochondrial dysfunction, in neurons and glia, is recognized as an important cause of atrophy and degeneration in MS and in other primarily neurodegenerative deceases such as Alzheimer’s disease and Parkinson’s disease [65, 69].
Iron dysregulation
Iron [Fe] loading accumulates with age and in patients with MS, it can further increase oxidative tissue loss. In the CNS, iron is mainly stored in oligodendrocytes, binding with ferritin. Under conditions of oxidative stress, such as MS lesions, when oligodendrocytes are destroyed, free iron [Fe2+] is released in the extracellular space and becomes an additional source of reactive oxygen species (Fenton reaction: Fe2+ + H2O2 = Fe3+ + OH. + OH−) [48]. Further, iron is released by activated glial cells, which become dystrophic and disintegrate, leading to a second wave of Fe2+release.
Diffuse T2hypointenselesions, which represent increased iron deposition [70] are commonly found in patients with MS in cortical and deep GM areas (i.e. thalamus, basal ganglia, dentate nucleus [71–73] and WM plaques [74]. Notably, T2 hypointensity has been associated with brain atrophy and early axonal loss [73]. Furthermore, in progressive MS, there is a significant decrease in iron levels in NAWM [75]. Iron is important for myelin synthesis and neurogenesis, and iron depletion in normal appearing tissue, may further promote diffuse axonal loss and CNS atrophy.
Glutamate excitotoxicity
Several lines of evidence suggest that glutamate could also mediate injury to myelin, oligodendrocytes and neurons in the autoimmune experimental encephalomyelitis (EAE) model and in MS [76]. Glutamate levels are elevated in CSF [77], in the centre of active plaques, on the borders of chronic active lesions [78], and in NAWM [79].
There are two factors intertwining for glutamate accumulation: increased glutamate production by activated microglial cells and lymphocytes, and impaired clearance by resident cells such as astrocytes. High levels of glutamate lead to the over-activation of N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) [80] receptors (permeable for calcium and sodium ions) and subsequent calcium overload and oligodendocyte and neuron cell death.
Clinical correlates of brain atrophy
Clinical symptoms and signs do not usually correlate with changes seen on conventional MRI measures (the “clinical-MRI paradox”) [81, 82]. Whole brain atrophy, on the other hand, has a significant imaging association with physical disability as measured by Expanded Disability Status Scale (EDSS) score [83–88]. In a longitudinal study, whole brain (WB) and cortical atrophy as well as other MRI related metrics such as the enlargement of ventricular CSF spaces have been associated with disability progression over a 10 year follow up [89]. Furthermore, brain volume changes during the first year after disease onset, estimated by PBVC, were the best predictor of future neurologic impairment [90] regardless of the intermediate relapse rate [91]. Increased brain volume loss (BVL) has been correlated disability progression, independent from the number of previous relapses or the T2 lesion load in RRMS [92].
In a similar vein, when patients with clinically definite MS were compared to patients with clinically isolated syndrome (CIS), at baseline, all brain volume metrics, except for cortical GM, were significantly lower in the MS cohort. Over a mean follow-up period of about 3 years, the annual PBVC values were significantly lower in CIS patients when compared to the MS cohort [93]. Neuropsychological impairment, affecting mental speed processing, episodic memory, executive functions and attention, may be present in up to 50% of patients with MS [94] and has been found to occur early in the disease course [95]. Changes of brain parenchymal fraction (BPF) have been shown to predict cognitive impairment over 2 years in patients with early MS [96]. Cortical atrophy was the best predictor of poor cognitive functioning, even when mild impairment was detected. Poorcognitive functioning has been associated with significant cortical thinning [97], especially in the fronto-parietal cortical and subcortical regions [98]. Pravatà et al. [98] specifically reported that the thinning of the right precuneus and high T2 lesion load were the best predictors of cognitive impairment. Strong correlations have also been reported between cognitive impairment and thalamic atrophy [80, 98, 99]. Not surprisingly, patients with brain atrophy and higher education or high “cognitive reserve” are relatively protected against cognitive decline [100].
Other clinical aspects of CNS atrophy include mood and personality disorders (i.e. euphoria, disinhibition, aggression, major depressive disorder) [101] autonomic dysfunction and sexual disorders [85]. Fatigue has been reported to be associated with GM atrophy in frontal regions [102] and depressed patients were found to present selective cortical thinning in the fronto-temporal regions, while the frontal thinning was found to be the best predictor for depression in MS patients [98].
Taken together, this growing body of evidence suggests that brain atrophy is a valid and sensitive measure of disease burden and progression in MS patients and may effectively be used in routine clinical practice and treatment trials.
Effect of disease modifying treatments (Tables 1, 2 and 3)
Table 1.
First line therapies and their effect on brain volume loss (BVL)
| References | DMT and trial design | Clinical trial | Baseline/MRI cohorts | Type of MS | Main effect on brain atrophy |
|---|---|---|---|---|---|
| Rudick et al. [83, 87] | IFN β-1a i.m. 30 mcg weekly vs placebo | Phase III MS.C.RG 2 years | 140 IFN β-1a n = 68, placebo n = 72 | RRMS | Percent change inbrain parenchymal fraction was lower in IFN β-1a treated patients compared to placebo, during the second year of treatment (p = 0.03) but not the first (p = 0.71) |
| Fisher et al. [104] | IFN β-1a i.m. 30 mcg weekly vs placebo | Retrospective analysis of Phase III MS.C.RG 2 years | 131 IFN β-1a n = 62, placebo n = 69 | RRMS | IFN β-1a significantly preserved GM [4] atrophy (p = 0.03) and whole brain atrophy (p = 0.04) during the second year of treatment, but not WM atrophy (at any point) |
| Fillipi et al. [106] | IFN β-1a s.c. 22 μg weekly vs placebo | Phase III ETOMS 2 years | 262 IFN β-1a n = 131, placebo n = 132 | CIS | Significant reductions in PBVC the IFN β-1a treated arm (p = 0.0031) compared to placebo, from baseline to second year |
| De Stefano et al. [173] | IFN β-1a s.c. 44mcg TIW vs placebo | double-blind and rater-blind phase IMROVE 40 weeks | 180 (double-blind phase) IFN β-1a n = 120, placebo n = 60 | RRMS | Non-significant differences in mean [102] PBVC between treatment groups (placebo: − 0.24% [0.48%]; IFN β-1a: − 0.22% [0.54%]; p = 0.76) at week 16 (end of double-blind phase) |
| De Stefano et al. [105] | IFN β-1a s.c. 44 mg TIW vs once a week vs placebo | Phase III REFLEX 2 years | 517 IFN β-1a TIW n = 171, IFN β-1a, once a week n = 175, placebo n = 171 | CIS | No differences in BVL (from baseline to 2 years) in patients receiving once or three times a week IFNβ-1a vs placebo. The greatest loss was in the TIW IFN β-1a group compared with the once a week IFN β-1a and placebo groups |
| Kappos et al. [108] | IFN β-1a s.c. 44 or 22 mcg TIW vs placebo (2 years); then open label (4 years); long term follow up (2 years) | Phase III PRISMS ~ 8 years | 382 44 mcg sc TIW n = 136, 22 mcg sc TIW n = 123, placebo n = 123 | RRMS | Non-significant differences in median BPV (from baseline to long term follow-up and each study period therein) for all treatment arms |
| Hardmeier et al. [174] | IFN β-1a i.m. 30 μg or 60 μg | Retrospective of The European IFNb-1a Dose- Comparison Study 3 years | annual MRI cohort n = 386, frequent MRI cohort n = 138 | RRMS | The greatest BVL took place during the first 4 months of therapy in frequent MRI cohort (from baseline to month 4, p < 0.05). Non-significant reduction in the brain atrophy in the 2nd and 3rd year of treatment |
| Molyneux et al. [175] | INF β-1b s.c. 8 MIU every other day vs placebo | Phase III 3 years | 92 INF β-1b n = 48, placebo n = 44 | SPMS | Not significant effect of treatment with INF β-1b on cerebral volume loss (p = 0.343, from baseline to 3 years) compared with placebo. |
| Kappos et al. [176, 177] | INF β-1βs.c. 250 μg every other day (early vs delayed treatment) | Extension study of the Phase III BENEFIT trial (3 and 5 years follow up) | Follow-up phase n = 418 early treatment n = 261, delayed treatment n = 157 5-year completers n = 358 early treatment n = 235, delayed treatment n = 123 | CIS | Marginal, non-significant differences between early and delayed treatment (p = 0.15, from baseline to 3 years, p = 0.121 from baseline to 5 years) |
| Calabresi et al. [111] | Peginterferon b-1a s.c. 125 μg Q2 W vs Q4 W vs placebo | Phase III ADVANCE 1 year, then open label | 1512 PEG-IFN β-1a 125 μg Q2 W n = 512, PEG-IFN β-1a 125 μg Q4 W n = 500, placebo n = 500 | RRMS |
Core study: During the first 6 months of treatment there was a significant “pseudoatrophy” effect (PEG-IFN β-1a 125 μg Q2 W vs placebo, p = 0.0170) Baseline to year 1: Νo significant differences on whole brain volume between groups (Q2Wvs placebo p = 0.0841; Q4 W vs placebo p = 0.3747) |
| Arnold et al. (F2069, 1rst EAN Congress 2015) [112] | Peginterferon b-1a s.c. 125 μg Q2 W vs Q4 W | Extension study of Phase III ADVANCE 2 years | At week 96/569 PEG-IFN β-1a 125 μg Q2 W n = 384, PEG-IFN β-1a 125 μg Q4 W n = 185 (delayed treatment) | RRMS | From week 24 to week 96, the delayed treatment PEG-IFN β-1a 125 μg Q4 W n = 185 demonstrated a significantly greater decrease in whole brain volume compared with the Q2 W group (p = 0.0034) |
| Sorensen et al. [110] | INF β-1a s.c. 44 μg plus Methylprednisolone orally 200 mg or placeboorally | Phase III NORMI.M.S 2 years | 110 INF β-1a and oral methylprednisolone n = 54, IFN β-1a and placebo n = 56 | RRMS | Mean changes in normalized brain parenchymal volume favored pulsed treatment with oral methylprednisolone combined with INF β-1a vs INFβ-1a monotherapy, but the benefit was not significant (p = 0.25) between baseline and week 96 |
| Ravnborg et al. [109] | INF β-1a i.m. 30 μg once weekly plus Methylprednisoloneorally 500 mg daily (3 consecutive days per month for 3–4 years) or placebo | Phase III MECOMBIN 3 years | 338 INF β-1a plus placebo n = 167, INF β-1a plus methylprednisolone n = 171 | RRMS | The study showed no effect on brain parenchymal volume (p = 0.58) or change in normalized brain volume (p = 0.52) |
| Comi et al. [114] | GAs.c. 20 mg daily vs Placebo | Phase III PreCISe 2 years | 481 GA n = 243, Placebo n = 238 | CIS | No significant difference in percentage change from baseline to last observed value in brain volume between the treatment groups (− 0.33% in GA vs − 0.38% in placebo) |
| Comi et al. [115] | GAs.c. 20 mgdailyvs placebo | open-label, extension phase of Phase III PreCISe 2 years | 409 early-treatment group n = 198, placebo (delayed-treatment) n = 211 | CIS | Significant reduction of BVL in early versus delayed treatment onset (28% reduction, p = 0.0209) |
| Ge et al. [116] | GAs.c. 20 mg daily vs placebo | Phase III The US GA study 2 years | 27 GA treated n = 14, placebo n = 13 | RRMS | GA significantly reduced the rate of BVL (77% reduction) in the 2-year treatment period (p = 0.007) compared with placebo |
| Rovaris et al. [119] | GA s.c. 20 mg daily vs placebo for 9 months, then GA open-label | Phase III European/ Canadian GA trial 18 months | 227 GA n = 113, placebo n = 114 | RRMS |
During the double-blind, placebo-controlled phase of the study, GA treatment did not have any measurable impact on the absolute or percentage change of BV (from baseline to 9 months, p = 0.88) In the subsequent open-label phase, early GA treatment showed a 40% reduction in the rate of brain atrophy (from 9th to 18th month) |
| Rovaris et al. [118] | GA s.c. 20 mg daily | Extension of the Phase III European/ Canadian GA trial 5 years | 142 Early treatment n = 73 Delayed treatment n = 69 | RRMS | Baseline to 5 years: Non-significant differences in median PBVC in early vs delayed treatment groups. |
| Comi et al. [113] | GA s.c. 20 mg vs 40 mg (dose comparison) | Phase III FORTE 1 year | 1155 GA 20 mg n = 586, GA 40 mg n = 569 | RRMS | PBVCs were similar in both groups (p = 0.423). Higher dose of GA did not have a clear-cut impact on brain volume loss. Slower atrophy rates, compared with the Eur/Canadian GA trial |
| Khan et al. [178] | GA s.c. 40 mg TIW vs placebo | Phase III GALA 1 year | 1263 GA 40 mg TIW n = 840, placebo n = 423 | RRMS | The percentage change in brain volume (from baseline to 1 year) was not statistically different between treatment arms (− 0.706 with GA vs − 0.645 with placebo; p = 0.2058) |
| Lublin et al. [179] | INF β-1a i.m. 30 mg weekly, GA s.c. 20 mg daily | Phase III CombiRx 3 years | 790 IFN + GA n = 388, IFN n = 187, GA n = 215 | RRMS | Combination treatment was not superior to either INF β-1a or GA agents alone (CSF volume change from baseline to month 36; IFN β-1a + GA vs IFN, p = 0.008, INF β-1a vs GA p = 0.48). Whole brain tissue loss was reflected by the change in normalized CSF from baseline |
| O’Connor et al. [180] | INF β- 1b s.c. 250 μg or 500 μg, every other day or GA s.c. 20 mg daily | Phase III BEYOND 2 years | 2244 IFN β-1b 500 μg n = 899, IFN β-1b 250 μg n = 897, GA n = 448 | RRMS |
Non-significant differences between treatment groups High dose INF β-1b was not superior to the standard dose (500 μg IFN β-1b vs 250 μg IFN β-1b p = 0.74). Both doses of IFN β-1b had similar measurable brain volume (BV) effect as compared with GA (500 μg IFN β-1b vs GA p = 0.33; 250 μg IFN β-1b vs GA p = 0.46). During year 1, patients under IFN β-1b had a significantly greater reduction in mean brain volume than did patients treated with GA (250 μg IFN β-1b vs GA p = 0.02; 500 μg IFN β-1b vs GA p = 0.007) |
| Arnold et al. [136] | DMF orally 240 mg BID vs TID vs placebo | Phase III DEFINE 2 years | 540 DMF BID n = 176, DMF TID n = 184, Placebo n = 180 | RRMS | Significant results for the DMF BID versus placebo on brain atrophy, from either baseline or 6 months to second year (baseline to 2 years p = 0.0449, 6 months to 2 years p = 0.0214). Non-statistically results for the DMF TID dose group |
| Miller et al. [137] | DMF orally 240 mg BID vs TID vs GA 20 mg once daily vs placebo | Phase III CONFIRM 2 years | 681 DMF BID n = 169, DMF TID n = 170, GA n = 175, placebo n = 167 | RRMS | At 2 years, PBVC favored DMF BID, but not TID or GA, compared to placebo (BID vs placebo; p = 0.0645; TID vs placebo; p = 0.2636; GA vs placebo p = 0.8802) |
| Kappos et al. (P7.243, AAN) [181] | DMF orally 240 mg BID vs TID vs placebo | 8 year follow-up study of Phase III ENDORSE Ongoing | year 1/464 DMF BID n = 197, GA n = 88, placebo n = 179 | RRMS | There was no significant effect in brain volume loss for the placebo/DMF and the GA/DMF groups relative to the group treated continuously with DMF BID (BID/BID group) (median PVC, from baseline to 5 years, BID/BID vs placebo/DMF p = 0.1165, BID/BID vs GA/DMF p = 0.3436) |
| Miller et al. [129] | Teriflunomide orally 7 or 14 mg once-daily vs placebo | Phase III TOPIC 4 years | 614 Teriflunomide 14 mg n = 214, Teriflunomide 7 mg n = 203, placebo n = 197 | CIS | No significant differences were recorded for brain atrophy (SIENA). (Mean change from baseline at week 108 vs placebo, 14 mg p = 0.4495; 7 mg p = 0.4462) |
| O’Connor et al. [130] | Teriflunomide orally 7 or 14 mg once-daily vs placebo | Phase III TEMSO 2 years | 1086 Teriflunomide 14 mg n = 358, Teriflunomide 7 mg n = 365, placebo n = 363 | RRMS | No effect on relative BPF change among the study groups (from baseline to 2 years: Teriflunomide 7 mg vs. placebo p = 0.19; Teriflunomide 14 mg vs. placebo p = 0.35) |
| Wolinsky et al. [131] | Teriflunomide orally 7 or 14 mg once-daily vs placebo | Post hoc analysis of Phase III TEMSO 108 weeks | 1088 Teriflunomide 14 mg n = 359, Teriflunomide 7 mg n = 366, placebo n = 363 | RRMS | There was a significant decrease in WM fraction (from baseline to108 weeks) for both doses of Teriflunomide (WMF change 14 mg vs placebo p = 0.0002; 7 mg vs placebo p = 0.0609) |
| Radue et al. (P3-089 AAN 2016) [134] | Teriflunomide orally 7 or 14 mg once-daily vs placebo | Post hoc analysis of Phase III TEMSO and TOWER 9 years | 969 808 baseline and week 48, 709 baseline and week 108 | RRMS | Significant gain in brain volume loss, by using an alternative method (SIENA). Median PVC, from baseline to first year, Teriflunomide 14 mg vs placebo p = 0.0001; Teriflunomide 7 mg vs placebo p = 0.0011; from baseline to second year: Teriflunomide 14 mg vs placebo p = 0.0001; Teriflunomide 7 mg vs placebo p = 0.0019) |
| Sprenger et al. P3.047 [132] | Teriflunomide orally 14 mg once-daily vs placebo | Post hoc analysis of Phase III TEMSO and TOWER 2 years | 969 808 first year, 709 s year | RRMS | Teriflunomide resulted in lower atrophy rate in patients with and without disability progression vs placebo. Without disability progression: Median PBVC, from baseline to first year, Teriflunomide 14 mg vs placebo (22%) p = 0.0128 and from baseline to second year (23%) p = 0.0129 With disability progression: Median PBVC, from baseline to first year, Teriflunomide 14 mg vs placebo (69%) p = 0.0037 and from baseline to second year (44%) p = 0.0043 |
| Wuerfel et al. P3.052 [135] | Teriflunomide orally 14 mg once-daily vs placebo | Post hoc analysis of Phase III of TEMSO | Year one cohort. 0–2 in previous 2 year: Teriflunomide 14 mg n = 191, placebo n = 197 2–3 in previous 2 year Teriflunomide 14 mg n = 195, placebo n = 198 | RRMS |
Significant impact on median PBVC regardless of the level of disease activity (prior relapse rate) Patients with few prior relapses (0–2 in previous 2 years): Baseline to year 1: Teriflunomide 14 mg vs placebo, relative change in percentage brain volume 40% p = 0.0001. Year 1to year 2: relative change 36%, p = 0.0001. This finding was confirmed in patients with a greater number of relapses (2–3 in previous 2 years): p = 0.0018 at year 1 and p = 0.0067 at year 2 |
| Freedman et al. (P734 ETCRIMS 2016) [133] | Teriflunomide orally 7 or 14 mg once-daily vs placebo | Subgroup analysis of Phase III TEMSO | 971 treatment-naïve n = 704, 1 Prior DMT n = 208, ≥2 Prior DMTs n = 57 | RMS | Positive results on median PBVC regardless of treatment history. PVC change from baseline to year 1, Teriflunomide 14 mg No prior DMT vs placebo p = 0.0025; baseline to year 2: p = 0.0109; Teriflunomide 14 mg prior DMT vs placebo p = 0.0119, baseline to year 2: p = 0.0109. PVC change from baseline to year 1: Terilunomide 7 mg No prior DMT vs placebo p = 0.0002; baseline to year e: p = 0.0089. Teriflunomide 7 mg prior DMT vs placebo p = 0.0119, baseline to year 2: p = 0.0109 |
mg: milligrams; mcg: micrograms; μg: micrograms; vs: versus; PBVC: percentage of brain volume change; BPF: brain parenchymal fraction; BPV: brain parenchymal volume; SIENA: structural imaging evaluation using normalization of atrophy; i.m.: intramuscular; s.c: subcutaneous; i.v.: intravenous; ΤΙW: three times weekly; SD: standard deviation;Q2W: once every 2 weeks; Q4W: once every 4 weeks; BID: twice daily; TID: thrice daily; DMT: disease modifying therapies; CSF: cerebrospinal fluid; PVC: percentage volume change
Table 2.
Second line therapies and their effect on brain volume loss (BVL)
| References | DMT and trial design | Clinical trial | Baseline/MRI cohorts | Type of MS | Main effect on brain atrophy |
|---|---|---|---|---|---|
| Kappos et al. [122] | Fingolimod orally 0.5 mg or 1.25 mg once daily vs placebo for 2 years, then FTY open-label | Phase III FREEDOMS 2 years | 1272 Fingolimod 1.25 mg n = 429, Fingolimod 0.5 mg n = 425, placebo n = 418 | RRMS | Significant reductions in the rate of brain volume loss were detected as early as 6 months for the 12 mg Fingolimod treatment group (PBVC values from baseline to 6 months, 1.25 mg Fingolimodvs placebo p = 0.003; 0.5 mg Fingolimodvs placebo p = 0.006) and remained significant at 24 months (P < 0.001 in all comparisons) |
| Kappos et al. [124] | Fingolimod orally 0.5 mg or 1.25 mg once daily (FTY open label) | Extension of Phase III FREEDOMS 2 years | 920 | RRMS |
Significantly lower atrophy rates in the continuous Fingolimod groups relative to the combined switch group, over 4 years (Continuous Fingolimod 0.5 mg p = 0.0013; Continuous Fingolimod 1.25 mg p = 0.001) Patients who switched to Fingolimod 0.5 mg during the extension study experienced significant improvements in rates of brain volume decline (Placebo—Fingolimod 0.5 mg p = 0.0084, months 24–48 vs months 0–24) |
| Cohen et al. [121] | Fingolimod orally 1.25 or 0.5 mgonce daily vs INF β-1a i.m. 30 μg (1 year, then open-label) | Phase III TRANSFORMS 1 year | 1280 Fingolimod 1.25 mg n = 420, Fingolimod 0.5 mg n = 429, INF β-1a N = 431 | RRMS | Compared to i.m. INF β-1a, patients treated with Fingolimod presented less brain volume loss, over 1 year (all p < 0.001) |
| Khatri et al. [125] | Fingolimod orally 1.25 or 0.5 mg once daily | Extension of Phase III TRANSFORMS 2 years | 799 INF β-1a to 0.5 mg Fingolimod n = 124, INF β-1a to 1.25 mg Fingolimod n = 130. Continuous 0.5 mg Fingolimod n = 290, Continuous 1.25 mg Fingolimod n = 255 | RRMS | Patients switching from INF β-1a to Fingolimod (either 1.25 or 0.5 mg) reduced their brain volume decrease (PBVC: months 13–24 vs months 0–12, p = 0.006 for the INF β-1a to 0.5 mg Fingolimod switch group p = 0·007 for the INF β-1a to 1.25 mg FTY720 switch group. No further gain in BVL for patients on continuous Fingolimod treatment (p values non-significant at months 13–24 vs months 0–12) |
| Calabresi et al. [120] | Fingolimod orally 1.25 or 0.5 mg once daily vs placebo | Phase III FREEDOMS II 1 year | 1083 Fingolimod 1.25 mg n = 370, Fingolimod 0.5 mg n = 358, placebo n = 355 | RRMS | Patients given Fingolimod had decreased brain volume loss compared with those given placebo from baseline to months 6 (Fingolimod 1.25 mg vs placebo, p = <0.0001; Fingolimod 0.50 mg vs placebo, p = 0.012) 12 (Fingolimod 1.25 mg vs placebo, p = <0.0001; Fingolimod 0.50 mg vs placebo, p = 0.004) and 24 (Fingolimod 1.25 mg vs placebo, p = <0.0001; Fingolimod 0.50 mg vs placebo, p = 0.013). (Total treatment effect on PBVC vs placebo p < 0·0001 and p = 0·0002 respectively) |
| Cohen et al. [123] | Fingolimod orally 1.25 or 0.5 mg once daily IFN β-1a i.m. 30 μg once a week | Extension of Phase III TRANSFORMS 4.5 years | Fingolimod 0.5 mg n = 356, IFN β-1a-switch Fingolimod 0.5 mg n = 167, Fingolimod 1.25 mg n = 330, IFN β-1a switch fingolimod 1.25 mg n = 174 | RRMS | Non-significant long term benefit on mean PBVC (from baseline to 4.5 years): continuous-fingolimodvs IFN β-1a-switch group −1.01% (−0.8) vs −0.96% (−0.8); p = 0.937. The PBVC from baseline to month 12 was reduced significantly by fingolimod compare to IFN β-1a (p < 0.0001) and the low rate was maintained through the study completion |
| Lublin et al. [126] | Fingolimod orally 0.5 mg once daily vs placebo | Phase III INFORMS 3 years | 714 Fingolimod 0.5 mg n = 293, placebo n = 421 | PPMS | In patients with primary progressive MS, percentage change in brain volume did not differ between Fingolimod and placebo groups (p = 0.673) |
| Miller et al. [139] | Natalizumab i.v. 300 mg every 4 weeks vs placebo | Phase III AFFIRM 2 years | 942 Natalizumab n = 627, placebo n = 315 | RRMS | Overall, not significant effect of treatment with Natalizumabvs placebo (mean percentage change in BPF, 0.80% vs 0.82%, p = 0.822, from baseline to 2 years). During the first year, natalizumab-treated patients presented greater BVL compared to placebo (0.56% vs 0.40%, p = 0.002) but the rate of brain atrophy was significantly less in natalizumab-treated patients over the second year of treatment (0.24% vs 0.43% p = 0.004) |
| Radue et al. [140] | (IFN β-1a i.m.30 μg + Natalizumab i.v.300 mg every 4 weeks) vs IFN β-1a i.m. 30 μg + placebo once weekly | Phase III SENTINEL 2 years | 1171 IFN β-1a + natalizumab n = 589, IFN β-1a + placebo n = 582 | RRMS | From baseline to second year, no significant differences were reported between the 2 treatment arms regarding change in BPF (p = 0.926). During the first year, there was a significant reduction in BPF in the Natalizumab treated arm (p = 0.058), but lower rates during the 2nd year of treatment (– 0.31% versus – 0.40%; p = 0.020) |
mg: milligrams; mcg: micrograms; μg: micrograms; vs: versus; PBVC: percentage of brain volume change; BPF: brain parenchymal fraction; BPV: brain parenchymal volume; SIENA: structural imaging evaluation using normalization of atrophy; i.m.: intramuscular; s.c: subcutaneous; i.v.: intravenous; ΤΙW: three times weekly; SD: standard deviation;Q2W: once every 2 weeks; Q4W: once every 4 weeks; BID: twice daily; TID: thrice daily; DMT: disease modifying therapies; CSF: cerebrospinal fluid; PVC: percentage volume change
Table 3.
New or emerging therapies and their effect on brain volume loss (BVL)
| References | DMT and trial design | Clinical trial | Baseline/MRI cohorts | Type of MS | Main effect on brain atrophy |
|---|---|---|---|---|---|
| Comi et al. [157] | Laquinimod orally 0.6 mg once daily vs placebo | Phase III ALLEGRO 2 years | 1106 Laquinimod n = 550, placebo n = 556 | RRMS | Laquinimod had a significant effect on reducing brain volume loss vs placebo (p < 0.001, from baseline to 2 years) |
| Vollmer et al. [158] | Laquinimod orally 0.6 mg once daily vs IFN β-1a i.m. 30 μg once weekly vs oral placebo | Phase III BRAVO 1 year | 1331 Laquinimod n = 434, IFN β-1a i.m. n = 4 47, placebo n = 450 | RRMS | Robust effects on reducing brain atrophy are replicated for Laquinimod (p < 0.001, from baseline to year 1), whereas IFN β-1a showed no benefit at all (non-significant. increased BVL 11% vs placebo, p = 0.14) |
| Cohen et al. [147] | Alemtuzumab i.v. 12 mg (once per day for 5 days at baseline and once per day for 3 days at 12 months) vs INF β-1a s.c. 44 μg TIW | Phase III CARE-MS I 2 years | 563 Alemtuzumab n = 376, INF β-1a n = 187 | RRMS | Median change in brain parenchymal fraction was less in Alemtuzumab (− 0.867%) was compared with INF β-1a (1.488%), p < 0.001) |
| Coles et al. [148] | Alemtuzumab i.v. 12 mg once per day vs 24 mg once per day (once per day for 5 days at baseline and for 3 days at 12 months) vs INF β-1a s.c. 44 μg TIW | Phase III CARE-MS II 2 years | 628 Alemtuzumab 12 mg n = 426, INF β-1a n = 202 | RRMS | Compared to 44 μgsc IFN β-1a (− 0.810%), alemtuzumab-treated (− 0.615%) patients showed less reduction in median parenchymal brain fraction during the first year of the trial (p = 0.01) |
| Traboulsee et al. P1181 ECTRI.M.S [6] | Alemtuzumab i.v. 12 mg once daily received 2 annual courses (on 5 consecutive days at baseline and on 3 consecutive days 12 months later). Patients could receive additional treatment with alemtuzumab (12 mg on 3 consecutive days ≥ 1 year after the most recent course) during the extension study | Extension of Phase III CARE-MS I, CARE-MS II 4 years | 93% of CARE-MS I n = 325, 88% of CARE-MS II n = 393 | RRMS | Durable MRI positive outcomes (i.e. sustained low brain atrophy rates, in the absence of continuous treatment with Alemtuzumab or other DMTs during the follow up period) |
| Coles et al. [150] | Alemtuzumab i.v.(12 mg on 3 consecutive days) Alemtuzumab-treated patients who completed CARE-MS II could enroll in the extension and receive, at the investigator’s discretion, additional alemtuzumab courses (12 mg on 3 consecutive days) ≥ weeks after the most recent course, if they had evidence of MS disease activity. Patients who received s.c. IFN-b-1a for 2 years in the core study could also enroll in the extension and switch to alemtuzumab treatment; results for these patients will be reported separately | CARE-MS II 5 years follow-up | Most alemtuzumab-treated patients (92.9%) who completed CARE-MS II entered the extension; 59.8% received no alemtuzumab retreatment | RRMS | Median yearly BVL remained low in the extension (years 1–5: − 0.48%, − 0.22%, − 0.10%, − 0.19%, − 0.07%). Yearly BVL rate continued to decrease in year 3 compared with the core study, remaining low in years 4 and 5. Median BPF change from baseline to year 5 was − 0.855% |
| Arnold et al. (P558, ECTRI.M.S 2015) [151] | Daclizumab s.c 150 mg every 4 weeks vs INF β-1ai.m. 30mcg once weekly | Post hoc of Phase III DECIDE 2 years | 1806 Daclizumab n = 899, INF β-1a n = 907 | RRMS | Daclizumab showed a significant effect in limiting the rate of brain atrophy vs IFN β-1a, between baseline and week 96 (p < 0.0001), week 0 and week 24 (p = 0.0325) and between week 24 and week 96 (p < 0.0001) |
| Montalban et al. [155] | Ocrelizumab i.v. 600 mg (two 300 mg infusions 14 days apart) vs placebo | Phase III ORATORIO | 732 Ocrelizumab 600 mg, n = 488, placebo n = 244 | PPMS | Ocrelizumab reduced the rate of whole brain volume loss from week 24 to week 120 by 17.5%120 (p = 0.0206) compared with placebo |
| Arnold et al. [154] | Ocrelizumab i.v 600 mg.every 24 weeks vs INF β-1as.c. 44 mcg TIW | Phase III OPERA I 96 weeks | 821 Ocrelizumab n = 410, IFN β-1a n = 411 | RMS | Ocrelizumab reduced brain volume loss compared with INF β-1a. (p < 0.001 from baseline to 96th week and p = 0.0042 from 24th to 96th week) |
| Arnold et al. [154] | Ocrelizumab i.v 600 mg.every 24 weeks vs INF β-1as.c. 44 mcg TIW | Phase III OPERA II | 835 Ocrelizumab n = 417, IFN β-1a n = 418 | RMS | Ocrelizumab reduced brain volume loss compared with INF β-1a. [p = 0.001 from baseline to 96th week and p = 0.09 (non-significant) from 24th to 96th week] |
| De Stefano et al. [162] | Cladribine3.5 mg/kg or Cladribine5.25 mg/kgvs placebo | Phase III CLARITY | 1025 Cladribine 3.5 mg/kg n = 336, Cladribine 5.25 mg/kg n = 351, placebo n = 338 | RMS | Patients treated with cladribine had significantly less annualized brain atrophy over 2 years compared with patients receiving placebo. At 18 months, patients treated with cladribine had 20% reduction in brain atrophy compared with patients receiving placeboIn patients under cladribine tablets 3.5 mg/kg (− 0.56% ± 0.68, p = 0.010) and 5.25 mg/kg (− 0.57% ± 0.72, p = 0.019), the annualized PBVC was reduced compared with placebo (− 0.70% ± 0.79) |
mg: milligrams; mcg: micrograms; μg: micrograms; vs: versus; PBVC: percentage of brain volume change; BPF: brain parenchymal fraction; BPV: brain parenchymal volume; SIENA: structural imaging evaluation using normalization of atrophy; i.m.: intramuscular; s.c: subcutaneous; i.v.: intravenous; ΤΙW: three times weekly; SD: standard deviation;Q2W: once every 2 weeks; Q4W: once every 4 weeks; BID: twice daily; TID: thrice daily; DMT: disease modifying therapies; CSF: cerebrospinal fluid; PVC: percentage volume change
Approved DMTs and brain volume outcomes
The need of agents to control the inflammatory process in multiple sclerosis pathology is obvious, but the need for medications to halt brain atrophy progression and neurodegeneration is also evident. Currently approved treatments for MS differ in their effects on brain atrophy [103] (Table 1 for the first line therapies, Table 2 for the second line therapies and Table 3 for the emerging therapies).
In general, studies of traditional injectable treatments have not exerted robust beneficial effects in the rate of brain atrophy. Intramuscular IFN-β-1a produced lower rates of brain volume loss (BVL) when compared to placebo during the second year of treatment in relapsing–remitting MS patients (− 0.23% vs − 0.51%; p = 0.03) [83, 104]. However, the subcutaneous (sc) IFN-β-1a produced inconsistent results in both CIS and RRMS patients [105–108]. BV data for intramuscular INF-β-1a in CIS patients and for subcutaneous INF-β-1b in relapsing MS patients has not been made available to date. The addition of monthly oral methylprednisolone pulses to subcutaneous interferon beta-1a treatment provided no further gain in normalized BV change in two published trials against placebo [109, 110]. The approved long-acting pegylated interferon beta-1a has only shown limited and inconclusive evidence for a beneficial effect on BV change in RRMS [111, 112]. A possible delayed effect in reducing brain atrophy has been reported for Glatiramer acetate [GA] [113–119]. In the PReCISe clinical trial, GA failed to show an immediate effect on brain volume outcomes versus placebo (− 0.38% vs 0.33%), but the subsequent open label phase of the trial showed a clear–cut benefit on PBCV for the early treatment group, when compared to patients with delayed treatment onset (40% reduction, p = 0.0209) [114, 115]. In relapsing–remitting MS, data from the extension phase of the European/Canadian GA trial come back as negative [118].
Available oral therapies (Fingolimod, Teriflunomide, Dimethyl fumarate) have shown various effects on BV decline. Fingolimod has been reported consistentin reducing median PBVC by approximately 30 to 45% versus placebo or IFNβ-1a, in its three phase III clinical trials [120–122] and their extensions [123–125]. Of note, this reduction was observed as early as 6 months after treatment onset [120, 122]. In the extension phase of the TRANFORMS trial, patients switching from intramuscular (i.m.) INF β1a to FTY720 slowed their median PBVC, and patients continuing on FTY720 sustained low atrophy rates, over the following 4.5 years of therapy [123]. However, no similar effects were reproduced in patients with the primary progressive form of MS, a finding that that could have otherwise strengthened the evidence for a direct action of fingolimod on brain cellular components [126]. Finally, further condoning the aforementioned observations, in a study by Yousuf et al. [127], cortical GM, alongside T2 lesion volume, remained stable in the cohort treated with fingolimod, as compared to the untreated group, where it decreased and increased respectively, in the first 2 years of treatment.
Regarding Teriflunomide [128], brain volume outcomes have been reported for clinically isolated syndrome and relapsing- remitting MS in the TOPIC and TEMSO clinical trials respectively. Both doses of 7 mg or 14 mg failed to show a clear effect on slowing BVL when compared to placebo [129, 130]. However, when tissue specific volume changes were examined a significant reduction in the rate of WM loss was detected for the 14 mg teriflunomide treatment arm versus placebo [131]. Similar results have recently been reported in 4 retrospective analyses of TOWER and TEMSO trials when an alternative method of brain loss evaluation was implemented [132–135].
Dimethyl fumarate (DMF/BG12) showed a 21% reduction in BVL compared to placebo in the DEFINE study (the 240 mg twice daily regimen only) [136] and produced only marginal but beneficial effects in BVL reduction in the CONFIRM study [137]. A recent pilot study of 20 patients with RRMS showed a protective effect of DMF treatment in whole brain atrophy (PBVC: − 0.37 ± 0.49% vs. − 1.04 ± 0.67%, p = 0.005) and putamen atrophy (− 0.06 ± 0.22 vs. − 0.32 ± 0.28 ml, p = 0.02), but no effect on other subcortical volumes or total GM atrophy [138].
Natalizumab, a monoclonal antibody against the cell adhesion molecule a 4-integrin, in two pivotal clinical trials was found to increase the rate of BVL in the first year of treatment and then significantly reduced it when compared to the placebo in the second year [139, 140]. Post–marketing observational studies confirmed that most of the BVL occurring while on Natalizumabtherapy takes place during the first months of therapy, and that it primarily involves WM volume changes [141, 142]. One trial has shown superiority of Natalizumab over conventional MS therapies (IFN-β and GA) and placebo regarding cortical atrophy [143]. Recently, treatment with Natalizumabdid not affect the loss of brain volume compared to placebo in secondary progressive MS patients (ASCENT) [144]. Τhe study by Arpín et al. [145] also suggests a neuroprotective effect of Natalizumab, after the measurement and comparison of the corpus calosum index, and the absence of brain atrophy in several patients under treatment during the follow up.
Alemtuzumab, a monoclonal antibody against cells that express the CD52, has demonstrated greater MRI and clinical improvement in comparison to IFNb-1a in its three pivotal studies in active relapsing MS patients [146–148]. Additionally, most patients remained free of disability and MRI progression, for the following 6 years of the initial treatment [6]. Brain atrophy measures showed that brain parenchymal fraction was smaller in Alemtuzumab compared to the INF β-1a treatment arm either in treatment naïve patients [149] or in participants who had relapsed on prior therapy [147–149]. Extension studies showed sustained low brain atrophy rates, in the absence of continuous treatment with Alemtuzumab or other DMTs during the follow up period [149]. The CARE-MS II 5-year follow-up study (2017) provided class III evidence that Alemtuzumab slows brain atrophy; the annual BVL rate continued to drop during the third year and remained low through the fourth and fifth year as well [150].
The immune-modulatory agent Daclizumab in a 3-year post hoc analysis of 899 RRMS patients was compared to IFN beta-1a on brain volume change. Median annualised PBVC was significantly reduced in the DAC treatment group during both the first and the second year of treatment (baseline—24th weeks: − 0.674 vs − 0.745; 24th–96th weeks: − 0.511 vs − 0.549; all p < 0.0001) in comparison to INF β treatment [151], a finding which was consistent with previous longitudinal data [151–153].
Ocrelizumab is a humanized mAb designed to target CD20+ B cells. MRI outcomes hint towards a positive effect on BVL and clinical disability progression. Treatment with Ocrelizumab has significantly slowed brain atrophy rates in comparison to INF-β1a (baseline to 96 weeks: 23.5% p < 0.0001 in OPERA 1 and 23.8% p < 0.0001 in OPERA 2) along with clinical disability [154]. Ocrelizumab reduced the rate of whole BVL in PPMS from week 24 to week 120 by 17.5%120 (p = 0.0206) compared with placebo (ORATORIO) [155].
Emerging DMTs and their effect on PBVC
Several new agents are currently undergoing clinical development, including immuno-modulatory, neuroprotective or remyelinating compounds.
Laquinimod, a linomide derivative, has also shown promising results on PVC rates in RRMS, most probably as a result of reduced astrocytic activation within the CNS [156]. In the ALLEGRO clinical trial, after adjusting for baseline active inflammation, laquinimod markedly reduced BVL as compared to the placebo [157]. Positive effects on PBVC are replicated in one active comparator trial [BRAVO] versus im IFN-β-1a [158, 159]. At present, the agent is further investigated in RRMS [CONCERTO] and PPMS patients [ARPEGGIO].
Cladribine, an antiproliferative agent that takes effect by interfering with DNA synthesis, has shown significant effects in terms of relapse rate and disability progression [160, 161]. Data from CLARITY study suggested that at 18 months, patients treated with cladribine had 20%reduction in brain atrophy compared with patients receiving placebo [162]. However, further studies are needed, in order to cladribine’s effect on brain atrophy rates, be fully elucidated [161, 163, 164].
Conclusions
MS is an evolving disease, now considered of both inflammatory and neurodegenerative nature [165–168]. Axonal injury and loss accounting for brain atrophy may be either acute (i.e. due to inflammation) or chronic/late due to pathogenic mechanisms primed by the preceding inflammation and later perpetuating with disease progression [169–171]. Brain atrophy occurs as early as CIS, progresses faster than it does in healthy adults, and is the best predictor of future disability, physical and cognitive [166, 172]. It is widely accepted to be a valid, sensitive and reproducible measure of neuroprotection in MS research studies and therapeutic trials.
There is now a variety of approved DMDs, with secondary neuroprotective properties, and an even greater number of novel compounds, in various stages of development and investigation. A firm belief remains that for a therapy to be effective in delaying the disease progression, its impact on axon and neuronal survival needs to be monitored. Conventional MRI findings (T1-hypotensive or T2 hypertensive lesion load) have already shown their limits for monitoring the disease burden and progression in MS patients. Newly introduced sophisticated imaging methods hold promise for the future of the clinical surveillance of the disease. Trials incorporating brain atrophy in their endpoints are providing accumulating evidence that rises substantial hopes for treating neurodegeneration in the near future.
Acknowledgements
Not applicable
Authors’ contributions
AA, ED, AA, MS, VS, GMH were involved in the conception of the study. AA, ED, AA, MS, VS, GMH, ZT, AMA, IN, CB, GT, GD, NG, DPB were involved in the acquisition of the data and study design. AA, ED, AA, MS, VS, GMH, ZT, AMA, IN, CB, GT, GD, NG, DPB were involved in the writing of the article. AA, ED, AA, MS, VS, GMH, ZT, AMA, IN, CB, GT, GD, NG, DPB critically revised the manuscript. All authors read and approved the final manuscript.
Funding
The author(s) received no specific funding for this work.
Ethics approval and consent to participate
Compliance with ethical standards. Human and animal rights: This article does not contain any studies with animals performed by any of the authors. This article does not require informed consent due to the lack of human participants.
Consent for publication
This article does not require informed consent due to the lack of human participants.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Athina Andravizou and Efthimios Dardiotis shared first authorship
References
- 1.Vasileiadis GK, Dardiotis E, Mavropoulos A, Tsouris Z, Tsimourtou V, Bogdanos DP, Sakkas LI, Hadjigeorgiou GM. Regulatory B and T lymphocytes in multiple sclerosis: friends or foes? Auto Immun Highlights. 2018;9(1):9. doi: 10.1007/s13317-018-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitrovič M, Patsopoulos NA, Beecham AH, Dankowski T, Goris A, Dubois B, D’hooghe MB, Lemmens R, Van Damme P, Søndergaard HB, Sellebjerg F. Low-frequency and rare-coding variation contributes to multiple sclerosis risk. Cell. 2018;175(6):1679–1687.e1677. doi: 10.1016/j.cell.2018.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadjigeorgiou GM, Kountra PM, Koutsis G, Tsimourtou V, Siokas V, Dardioti M, Rikos D, Marogianni C, Aloizou AM, Karadima G, Ralli S, Grigoriadis N, Bogdanos D, Panas M, Dardiotis E. Replication study of GWAS risk loci in Greek multiple sclerosis patients. Neurol Sci. 2018 doi: 10.1007/s10072-018-3617-6. [DOI] [PubMed] [Google Scholar]
- 4.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128(Pt 11):2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 5.Lycklama A, Nijeholt GJ. Reduction of brain volume in MS. MRI and pathology findings. J Neurol Sci. 2005;233(1–2):199–202. doi: 10.1016/j.jns.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, Sormani MP, Thalheim C, Traboulsee A, Vollmer T. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9:S5–S48. doi: 10.1016/j.msard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese M, Atzori M, Bernardi V, Morra A, Romualdi C, Rinaldi L, McAuliffe MJ, Barachino L, Perini P, Fischl B, Battistin L, Gallo P. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J Neurol. 2007;254(9):1212–1220. doi: 10.1007/s00415-006-0503-6. [DOI] [PubMed] [Google Scholar]
- 8.Paolillo A, Pozzilli C, Gasperini C, Giugni E, Mainero C, Giuliani S, Tomassini V, Millefiorini E, Bastianello S. Brain atrophy in relapsing-remitting multiple sclerosis: relationship with ‘black holes’, disease duration and clinical disability. J Neurol Sci. 2000;174(2):85–91. doi: 10.1016/S0022-510X(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120(Pt 3):393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- 10.Bruck W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005;252(Suppl 5):v3–v9. doi: 10.1007/s00415-005-5002-7. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen C, Hagemeier J, Myhr KM, Nyland H, Lode K, Bergsland N, Ramasamy DP, Dalaker TO, Larsen JP, Farbu E, Zivadinov R. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85(10):1109–1115. doi: 10.1136/jnnp-2013-306906. [DOI] [PubMed] [Google Scholar]
- 12.Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64(3):255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- 13.Tsivgoulis G, Katsanos AH, Grigoriadis N, Hadjigeorgiou GM, Heliopoulos I, Papathanasopoulos P, Dardiotis E, Kilidireas C, Voumvourakis K. The effect of disease-modifying therapies on brain atrophy in patients with clinically isolated syndrome: a systematic review and meta-analysis. Ther Adv Neurol Disord. 2015;8(5):193–202. doi: 10.1177/1756285615600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5(2):158–170. doi: 10.1016/s1474-4422(06)70349-0. [DOI] [PubMed] [Google Scholar]
- 15.Pelletier D, Garrison K, Henry R. Measurement of whole-brain atrophy in multiple sclerosis. J Neuroimaging. 2004;14(3 Suppl):11s–19s. doi: 10.1177/1051228404266264. [DOI] [PubMed] [Google Scholar]
- 16.Riley C, Azevedo C, Bailey M, Pelletier D. Clinical applications of imaging disease burden in multiple sclerosis: MRI and advanced imaging techniques. Expert Rev Neurother. 2012;12(3):323–333. doi: 10.1586/ern.11.196. [DOI] [PubMed] [Google Scholar]
- 17.Ghione E, Bergsland N, Dwyer MG, Hagemeier J, Jakimovski D, Paunkoski I, Ramasamy DP, Silva D, Carl E, Hojnacki D, Kolb C, Weinstock-Guttman B, Zivadinov R. Brain atrophy is associated with disability progression in patients with MS followed in a clinical routine. AJNR Am J Neuroradiol. 2018;39(12):2237–2242. doi: 10.3174/ajnr.A5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chard D, Miller D. Grey matter pathology in clinically early multiple sclerosis: evidence from magnetic resonance imaging. J Neurol Sci. 2009;282(1–2):5–11. doi: 10.1016/j.jns.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29(11):1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 20.von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol. 2016;524(18):3865–3895. doi: 10.1002/cne.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippi M, Charil A, Rovaris M, Absinta M, Rocca MA. Insights from magnetic resonance imaging. Handb Clin Neurol. 2014;122:115–149. doi: 10.1016/b978-0-444-52001-2.00006-6. [DOI] [PubMed] [Google Scholar]
- 22.Chu R, Kim G, Tauhid S, Khalid F, Healy BC, Bakshi R. Whole brain and deep gray matter atrophy detection over 5 years with 3T MRI in multiple sclerosis using a variety of automated segmentation pipelines. PLoS ONE. 2018;13(11):e0206939. doi: 10.1371/journal.pone.0206939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amiri H, de Sitter A, Bendfeldt K, Battaglini M, Gandini Wheeler-Kingshott CAM, Calabrese M, Geurts JJG, Rocca MA, Sastre-Garriga J, Enzinger C, de Stefano N, Filippi M, Rovira A, Barkhof F, Vrenken H. Urgent challenges in quantification and interpretation of brain grey matter atrophy in individual MS patients using MRI. Neuroimage Clin. 2018;19:466–475. doi: 10.1016/j.nicl.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller DH, Barkhof F, Frank JA, Parker GJ, Thompson AJ. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain. 2002;125(Pt 8):1676–1695. doi: 10.1093/brain/awf177. [DOI] [PubMed] [Google Scholar]
- 25.Anderson VM, Fox NC, Miller DH. Magnetic resonance imaging measures of brain atrophy in multiple sclerosis. J Magn Reson Imaging. 2006;23(5):605–618. doi: 10.1002/jmri.20550. [DOI] [PubMed] [Google Scholar]
- 26.Zivadinov R, Reder AT, Filippi M, Minagar A, Stuve O, Lassmann H, Racke MK, Dwyer MG, Frohman EM, Khan O. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology. 2008;71(2):136–144. doi: 10.1212/01.wnl.0000316810.01120.05. [DOI] [PubMed] [Google Scholar]
- 27.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(22 Suppl 3):S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- 28.Neacsu V, Jasperse B, Korteweg T, Knol DL, Valsasina P, Filippi M, Barkhof F, Rovaris M, Vrenken H. Agreement between different input image types in brain atrophy measurement in multiple sclerosis using SIENAX and SIENA. J Magn Reson Imaging. 2008;28(3):559–565. doi: 10.1002/jmri.21501. [DOI] [PubMed] [Google Scholar]
- 29.Saidha S, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD, Prince JL, Pham D, Roy S, Van Zijl P. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78(5):801–813. doi: 10.1002/ana.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruberte E, Sinnecker T, Amann M, Gaetano L, Naegelin Y, Penner IK, Kuhle J, Derfuss T, Kappos L, Granziera C, Wuerfel J, Yaldizli O. Central slab versus whole brain to measure brain atrophy in multiple sclerosis. Eur Neurol. 2019;80(3–4):207–214. doi: 10.1159/000495798. [DOI] [PubMed] [Google Scholar]
- 31.Lorefice L, Fenu G, Sardu C, Frau J, Coghe G, Costa G, Schirru L, Secci MA, Sechi V, Barracciu MA, Marrosu MG, Cocco E. Multiple sclerosis and HLA genotypes: a possible influence on brain atrophy. Mult Scler. 2017 doi: 10.1177/1352458517739989. [DOI] [PubMed] [Google Scholar]
- 32.Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346(3):158–164. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 33.Tedeschi G, Lavorgna L, Russo P, Prinster A, Dinacci D, Savettieri G, Quattrone A, Livrea P, Messina C, Reggio A, Bresciamorra V, Orefice G, Paciello M, Brunetti A, Coniglio G, Bonavita S, Di Costanzo A, Bellacosa A, Valentino P, Quarantelli M, Patti F, Salemi G, Cammarata E, Simone IL, Salvatore M, Bonavita V, Alfano B. Brain atrophy and lesion load in a large population of patients with multiple sclerosis. Neurology. 2005;65(2):280–285. doi: 10.1212/01.wnl.0000168837.87351.1f. [DOI] [PubMed] [Google Scholar]
- 34.Dalton CM, Chard DT, Davies GR, Miszkiel KA, Altmann DR, Fernando K, Plant GT, Thompson AJ, Miller DH. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain. 2004;127(Pt 5):1101–1107. doi: 10.1093/brain/awh126. [DOI] [PubMed] [Google Scholar]
- 35.Meier DS, Weiner HL, Khoury SJ, Guttmann CR. Magnetic resonance imaging surrogates of multiple sclerosis pathology and their relationship to central nervous system atrophy. J Neuroimaging. 2004;14(3 Suppl):46s–53s. doi: 10.1177/1051228404266268. [DOI] [PubMed] [Google Scholar]
- 36.Pelletier D, Nelson SJ, Oh J, Antel JP, Kita M, Zamvil SS, Goodkin DE. MRI lesion volume heterogeneity in primary progressive MS in relation with axonal damage and brain atrophy. J Neurol Neurosurg Psychiatry. 2003;74(7):950–952. doi: 10.1136/jnnp.74.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chard DT, Brex PA, Ciccarelli O, Griffin CM, Parker GJ, Dalton C, Altmann DR, Thompson AJ, Miller DH. The longitudinal relation between brain lesion load and atrophy in multiple sclerosis: a 14 year follow up study. J Neurol Neurosurg Psychiatry. 2003;74(11):1551–1554. doi: 10.1136/jnnp.74.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125(Pt 2):327–337. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- 39.Hochmeister S, Grundtner R, Bauer J, Engelhardt B, Lyck R, Gordon G, Korosec T, Kutzelnigg A, Berger JJ, Bradl M, Bittner RE, Lassmann H. Dysferlin is a new marker for leaky brain blood vessels in multiple sclerosis. J Neuropathol Exp Neurol. 2006;65(9):855–865. doi: 10.1097/01.jnen.0000235119.52311.16. [DOI] [PubMed] [Google Scholar]
- 40.Trapp BD, Vignos M, Dudman J, Chang A, Fisher E, Staugaitis SM, Battapady H, Mork S, Ontaneda D, Jones SE. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study. Lancet Neurol. 2018;17(10):870–884. doi: 10.1016/S1474-4422(18)30245-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eshaghi A, Prados F, Brownlee WJ, Altmann DR, Tur C, Cardoso MJ, De Angelis F, van de Pavert SH, Cawley N, De Stefano N, Stromillo ML, Battaglini M, Ruggieri S, Gasperini C, Filippi M, Rocca MA, Rovira A, Sastre-Garriga J, Vrenken H, Leurs CE, Killestein J, Pirpamer L, Enzinger C, Ourselin S, Wheeler-Kingshott C, Chard D, Thompson AJ, Alexander DC, Barkhof F, Ciccarelli O. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol. 2018;83(2):210–222. doi: 10.1002/ana.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagani E, Rocca MA, Gallo A, Rovaris M, Martinelli V, Comi G, Filippi M. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am J Neuroradiol. 2005;26(2):341–346. [PMC free article] [PubMed] [Google Scholar]
- 43.Minagar A, Toledo EG, Alexander JS, Kelley RE. Pathogenesis of brain and spinal cord atrophy in multiple sclerosis. J Neuroimaging. 2004;14(3 Suppl):5s–10s. doi: 10.1177/1051228404266263. [DOI] [PubMed] [Google Scholar]
- 44.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 45.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. doi: 10.1056/nejm200009283431307. [DOI] [PubMed] [Google Scholar]
- 46.Popescu BFG, Lucchinetti CF. Meningeal and cortical grey matter pathology in multiple sclerosis. BMC Neurol. 2012;12(1):11. doi: 10.1186/1471-2377-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Häusser-Kinzel S, Weber MS. The role of B cells and antibodies in multiple sclerosis, neuromyelitis optica, and related disorders. Front Immunol. 2019;10:201. doi: 10.3389/fimmu.2019.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 49.Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol. 2011;93(1):1–12. doi: 10.1016/j.pneurobio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellwardt E, Zipp F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp Neurol. 2014;262(Pt A):8–17. doi: 10.1016/j.expneurol.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47(6):707–717. doi: 10.1002/1531-8249(200006)47:6<707::AID-ANA3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 52.Metz I, Weigand SD, Popescu BF, Frischer JM, Parisi JE, Guo Y, Lassmann H, Bruck W, Lucchinetti CF. Pathologic heterogeneity persists in early active multiple sclerosis lesions. Ann Neurol. 2014;75(5):728–738. doi: 10.1002/ana.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55(4):458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 54.Enzinger C, Ropele S, Smith S, Strasser-Fuchs S, Poltrum B, Schmidt H, Matthews PM, Fazekas F. Accelerated evolution of brain atrophy and “black holes” in MS patients with APOE-epsilon 4. Ann Neurol. 2004;55(4):563–569. doi: 10.1002/ana.20027. [DOI] [PubMed] [Google Scholar]
- 55.Koudriavtseva T, Mainero C. Neuroinflammation, neurodegeneration and regeneration in multiple sclerosis: intercorrelated manifestations of the immune response. Neural Regen Res. 2016;11(11):1727. doi: 10.4103/1673-5374.194804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filippi M, Rocca MA. MR imaging of multiple sclerosis. Radiology. 2011;259(3):659–681. doi: 10.1148/radiol.11101362. [DOI] [PubMed] [Google Scholar]
- 57.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 58.Mahad D, Ziabreva I, Lassmann H, Turnbull D. Mitochondrial defects in acute multiple sclerosis lesions. Brain. 2008;131(Pt 7):1722–1735. doi: 10.1093/brain/awn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuhle J, Kropshofer H, Haering DA, Kundu U, Meinert R, Barro C, Dahlke F, Tomic D, Leppert D, Kappos L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007–e1015. doi: 10.1212/wnl.0000000000007032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lubetzki C, Stankoff B. Demyelination in multiple sclerosis. Handb Clin Neurol. 2014;122:89–99. doi: 10.1016/b978-0-444-52001-2.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waxman SG, Craner MJ, Black JA. Na + channel expression along axons in multiple sclerosis and its models. Trends Pharmacol Sci. 2004;25(11):584–591. doi: 10.1016/j.tips.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Desmazieres A, Sol-Foulon N, Lubetzki C. Changes at the nodal and perinodal axonal domains: a basis for multiple sclerosis pathology? Mult Scler. 2012;18(2):133–137. doi: 10.1177/1352458511434370. [DOI] [PubMed] [Google Scholar]
- 63.Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, van Horssen J, Lassmann H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135(Pt 3):886–899. doi: 10.1093/brain/aws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell GR, Mahad DJ. Mitochondria as crucial players in demyelinated axons: lessons from neuropathology and experimental demyelination. Autoimmune Dis. 2011;2011:262847. doi: 10.4061/2011/262847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witte ME, Mahad DJ, Lassmann H, van Horssen J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol Med. 2014;20(3):179–187. doi: 10.1016/j.molmed.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 66.Zambonin JL, Zhao C, Ohno N, Campbell GR, Engeham S, Ziabreva I, Schwarz N, Lee SE, Frischer JM, Turnbull DM, Trapp BD, Lassmann H, Franklin RJ, Mahad DJ. Increased mitochondrial content in remyelinated axons: implications for multiple sclerosis. Brain. 2011;134(Pt 7):1901–1913. doi: 10.1093/brain/awr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campbell GR, Ziabreva I, Reeve AK, Krishnan KJ, Reynolds R, Howell O, Lassmann H, Turnbull DM, Mahad DJ. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann Neurol. 2011;69(3):481–492. doi: 10.1002/ana.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mao P, Reddy PH. Is multiple sclerosis a mitochondrial disease? Biochimica et biophysica acta 1802. Biochim Biophys Acta. 2010;1:66–79. doi: 10.1016/j.bbadis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carvalho KS. Mitochondrial dysfunction in demyelinating diseases. Semin Pediatr Neurol. 2013;20(3):194–201. doi: 10.1016/j.spen.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Filippi M, Rocca MA, Barkhof F, Bruck W, Chen JT, Comi G, DeLuca G, De Stefano N, Erickson BJ, Evangelou N, Fazekas F, Geurts JJ, Lucchinetti C, Miller DH, Pelletier D, Popescu BF, Lassmann H. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012;11(4):349–360. doi: 10.1016/s1474-4422(12)70003-0. [DOI] [PubMed] [Google Scholar]
- 71.Bakshi R, Shaikh ZA, Janardhan V. MRI T2 shortening (‘black T2’) in multiple sclerosis: frequency, location, and clinical correlation. NeuroReport. 2000;11(1):15–21. doi: 10.1097/00001756-200001170-00004. [DOI] [PubMed] [Google Scholar]
- 72.Bermel RA, Puli SR, Rudick RA, Weinstock-Guttman B, Fisher E, Munschauer FE, 3rd, Bakshi R. Prediction of longitudinal brain atrophy in multiple sclerosis by gray matter magnetic resonance imaging T2 hypointensity. Arch Neurol. 2005;62(9):1371–1376. doi: 10.1001/archneur.62.9.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ceccarelli A, Rocca MA, Perego E, Moiola L, Ghezzi A, Martinelli V, Comi G, Filippi M. Deep grey matter T2 hypo-intensity in patients with paediatric multiple sclerosis. Mult Scler. 2011;17(6):702–707. doi: 10.1177/1352458510395645. [DOI] [PubMed] [Google Scholar]
- 74.Stankiewicz JM, Neema M, Ceccarelli A. Iron and multiple sclerosis. Neurobiology of aging 35. Supplement. 2014;2:S51–S58. doi: 10.1016/j.neurobiolaging.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 75.Stephenson E, Nathoo N, Mahjoub Y, Dunn JF, Yong VW. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat Rev Neurol. 2014;10(8):459–468. doi: 10.1038/nrneurol.2014.118. [DOI] [PubMed] [Google Scholar]
- 76.Stojanovic IR, Kostic M, Ljubisavljevic S. The role of glutamate and its receptors in multiple sclerosis. J Neural Transm. 2014;121(8):945–955. doi: 10.1007/s00702-014-1188-0. [DOI] [PubMed] [Google Scholar]
- 77.Sarchielli P, Greco L, Floridi A, Floridi A, Gallai V. Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Arch Neurol. 2003;60(8):1082–1088. doi: 10.1001/archneur.60.8.1082. [DOI] [PubMed] [Google Scholar]
- 78.Geurts JJ, Wolswijk G, Bo L, van der Valk P, Polman CH, Troost D, Aronica E. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain. 2003;126(Pt 8):1755–1766. doi: 10.1093/brain/awg179. [DOI] [PubMed] [Google Scholar]
- 79.Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128(Pt 5):1016–1025. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- 80.Papathanasiou A, Messinis L, Zampakis P, Panagiotakis G, Gourzis P, Georgiou V, Papathanasopoulos P. Thalamic atrophy predicts cognitive impairment in relapsing remitting multiple sclerosis. Effect on instrumental activities of daily living and employment status. J Neurol Sci. 2015;358(1–2):236–242. doi: 10.1016/j.jns.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol. 2002;15(3):239–245. doi: 10.1097/00019052-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Healy BC, Buckle GJ, Ali EN, Egorova S, Khalid F, Tauhid S, Glanz BI, Chitnis T, Guttmann CR, Weiner HL. Characterizing clinical and MRI dissociation in patients with multiple sclerosis. J Neuroimaging. 2017;27(5):481–485. doi: 10.1111/jon.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudick RA, Fisher E, Lee JC, Duda JT, Simon J. Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon beta-1a. Mult Scler. 2000;6(6):365–372. doi: 10.1177/135245850000600601. [DOI] [PubMed] [Google Scholar]
- 84.Fisher E, Rudick RA, Simon JH, Cutter G, Baier M, Lee JC, Miller D, Weinstock-Guttman B, Mass MK, Dougherty DS, Simonian NA. Eight-year follow-up study of brain atrophy in patients with MS. Neurology. 2002;59(9):1412–1420. doi: 10.1212/01.WNL.0000036271.49066.06. [DOI] [PubMed] [Google Scholar]
- 85.Zivadinov R, Bakshi R. Central nervous system atrophy and clinical status in multiple sclerosis. J Neuroimaging. 2004;14(3 Suppl):27s–35s. doi: 10.1177/1051228404266266. [DOI] [PubMed] [Google Scholar]
- 86.Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol. 2014;75(1):43–49. doi: 10.1002/ana.24018. [DOI] [PubMed] [Google Scholar]
- 87.Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology. 1999;53(8):1698–1704. doi: 10.1212/WNL.53.8.1698. [DOI] [PubMed] [Google Scholar]
- 88.Rocca MA, Comi G, Filippi M. The role of T1-weighted derived measures of neurodegeneration for assessing disability progression in multiple sclerosis. Front Neurol. 2017;8:433. doi: 10.3389/fneur.2017.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zivadinov R, Uher T, Hagemeier J, Vaneckova M, Ramasamy DP, Tyblova M, Bergsland N, Seidl Z, Dwyer MG, Krasensky J, Havrdova E, Horakova D. A serial 10-year follow-up study of brain atrophy and disability progression in RRMS patients. Mult Scler. 2016;22(13):1709–1718. doi: 10.1177/1352458516629769. [DOI] [PubMed] [Google Scholar]
- 90.Rojas JI, Patrucco L, Besada C, Bengolea L, Cristiano E. Brain atrophy at onset and physical disability in multiple sclerosis. Arq Neuropsiquiatr. 2012;70(10):765–768. doi: 10.1590/S0004-282X2012001000003. [DOI] [PubMed] [Google Scholar]
- 91.Samann PG, Knop M, Golgor E, Messler S, Czisch M, Weber F. Brain volume and diffusion markers as predictors of disability and short-term disease evolution in multiple sclerosis. AJNR Am J Neuroradiol. 2012;33(7):1356–1362. doi: 10.3174/ajnr.A2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fragoso YD, Wille PR, Abreu M, Brooks JBB, Dias RM, Duarte JA, Farage L, Finkelsztejn A, Frohlich AC, Goncalves MVM, Guedes BVS, Medeiros L, Oliveira RA, Ribas FD, da Rocha FCG, Santos GAC, Scorcine C, da Silveira GL, Spedo CT, Tauil CB, Varela JS, Vieira VLF. Correlation of clinical findings and brain volume data in multiple sclerosis. J Clin Neurosci. 2017;44:155–157. doi: 10.1016/j.jocn.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 93.Pichler A, Khalil M, Langkammer C, Pinter D, Bachmaier G, Ropele S, Fuchs S, Enzinger C, Fazekas F. Combined analysis of global and compartmental brain volume changes in early multiple sclerosis in clinical practice. Mult Scler. 2016;22(3):340–346. doi: 10.1177/1352458515593405. [DOI] [PubMed] [Google Scholar]
- 94.Bakirtzis C, Ioannidis P, Messinis L, Nasios G, Konstantinopoulou E, Papathanasopoulos P, Grigoriadis N. The rationale for monitoring cognitive function in multiple sclerosis: practical issues for clinicians. Open Neurol J. 2018;12:31–40. doi: 10.2174/1874205x01812010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anhoque CF, Biccas Neto L, Domingues SCA, Teixeira AL, Domingues RB. Cognitive impairment in patients with clinically isolated syndrome. Dement Neuropsychol. 2012;6:266–269. doi: 10.1590/S1980-57642012DN06040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zivadinov R, Sepcic J, Nasuelli D, De Masi R, Bragadin LM, Tommasi MA, Zambito-Marsala S, Moretti R, Bratina A, Ukmar M, Pozzi-Mucelli RS, Grop A, Cazzato G, Zorzon M. A longitudinal study of brain atrophy and cognitive disturbances in the early phase of relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;70(6):773–780. doi: 10.1136/jnnp.70.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Calabrese M, Rinaldi F, Mattisi I, Grossi P, Favaretto A, Atzori M, Bernardi V, Barachino L, Romualdi C, Rinaldi L, Perini P, Gallo P. Widespread cortical thinning characterizes patients with MS with mild cognitive impairment. Neurology. 2010;74(4):321–328. doi: 10.1212/WNL.0b013e3181cbcd03. [DOI] [PubMed] [Google Scholar]
- 98.Pravata E, Rocca MA, Valsasina P, Riccitelli GC, Gobbi C, Comi G, Falini A, Filippi M. Gray matter trophism, cognitive impairment, and depression in patients with multiple sclerosis. Mult Scler. 2017;23(14):1864–1874. doi: 10.1177/1352458517692886. [DOI] [PubMed] [Google Scholar]
- 99.Houtchens MK, Benedict RH, Killiany R, Sharma J, Jaisani Z, Singh B, Weinstock-Guttman B, Guttmann CR, Bakshi R. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69(12):1213–1223. doi: 10.1212/01.wnl.0000276992.17011.b5. [DOI] [PubMed] [Google Scholar]
- 100.Sumowski JF, Chiaravalloti N, Wylie G, Deluca J. Cognitive reserve moderates the negative effect of brain atrophy on cognitive efficiency in multiple sclerosis. J Int Neuropsychol Soc. 2009;15(4):606–612. doi: 10.1017/s1355617709090912. [DOI] [PubMed] [Google Scholar]
- 101.Benedict RH, Wahlig E, Bakshi R, Fishman I, Munschauer F, Zivadinov R, Weinstock-Guttman B. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1–2):29–34. doi: 10.1016/j.jns.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 102.Sepulcre J, Masdeu JC, Goni J, Arrondo G, Velez de Mendizabal N, Bejarano B, Villoslada P. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler. 2009;15(3):337–344. doi: 10.1177/1352458508098373. [DOI] [PubMed] [Google Scholar]
- 103.Luessi F, Siffrin V, Zipp F. Neurodegeneration in multiple sclerosis: novel treatment strategies. Expert Rev Neurother. 2012;12(9):1061–1076. doi: 10.1586/ern.12.59. [DOI] [PubMed] [Google Scholar]
- 104.Fisher E, Nakamura K, Lee JC, You X, Sperling B, Rudick RA. Effect of intramuscular interferon beta-1a on gray matter atrophy in relapsing-remitting multiple sclerosis: a retrospective analysis. Mult Scler. 2016;22(5):668–676. doi: 10.1177/1352458515599072. [DOI] [PubMed] [Google Scholar]
- 105.De Stefano N, Comi G, Kappos L, Freedman MS, Polman CH, Uitdehaag BM, Hennessy B, Casset-Semanaz F, Lehr L, Stubinski B, Jack DL, Barkhof F. Efficacy of subcutaneous interferon beta-1a on MRI outcomes in a randomised controlled trial of patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry. 2014;85(6):647–653. doi: 10.1136/jnnp-2013-306289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Filippi M, Rovaris M, Inglese M, Barkhof F, De Stefano N, Smith S, Comi G. Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9444):1489–1496. doi: 10.1016/s0140-6736(04)17271-1. [DOI] [PubMed] [Google Scholar]
- 107.Jones CK, Riddehough A, Li DKB, Zhao G, Paty DW, Group. atPS MRI cerebral atrophy in relapsing-remitting MS: results from the PRISMS trial. Neurology. 2001;56(suppl 3):379 (abstr). [Google Scholar]
- 108.Kappos L, Traboulsee A, Constantinescu C, Eralinna JP, Forrestal F, Jongen P, Pollard J, Sandberg-Wollheim M, Sindic C, Stubinski B, Uitdehaag B, Li D. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology. 2006;67(6):944–953. doi: 10.1212/01.wnl.0000237994.95410.ce. [DOI] [PubMed] [Google Scholar]
- 109.Ravnborg M, Sorensen PS, Andersson M, Celius EG, Jongen PJ, Elovaara I, Bartholome E, Constantinescu CS, Beer K, Garde E, Sperling B. Methylprednisolone in combination with interferon beta-1a for relapsing-remitting multiple sclerosis (MECOMBIN study): a multicentre, double-blind, randomised, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9(7):672–680. doi: 10.1016/s1474-4422(10)70132-0. [DOI] [PubMed] [Google Scholar]
- 110.Sorensen PS, Mellgren SI, Svenningsson A, Elovaara I, Frederiksen JL, Beiske AG, Myhr KM, Sogaard LV, Olsen IC, Sandberg-Wollheim M. NORdic trial of oral Methylprednisolone as add-on therapy to Interferon beta-1a for treatment of relapsing-remitting Multiple Sclerosis (NORMIMS study): a randomised, placebo-controlled trial. Lancet Neurol. 2009;8(6):519–529. doi: 10.1016/s1474-4422(09)70085-7. [DOI] [PubMed] [Google Scholar]
- 111.Calabresi PA, Kieseier BC, Arnold DL, Balcer LJ, Boyko A, Pelletier J, Liu S, Zhu Y, Seddighzadeh A, Hung S, Deykin A. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol. 2014;13(7):657–665. doi: 10.1016/s1474-4422(14)70068-7. [DOI] [PubMed] [Google Scholar]
- 112.Arnold DL, Kieseier BC, You X, Liu S, Sperling B, Hung S. Effects of Peginterferon Beta-1a on brain volume loss and magnetisation transfer ratio in patients with relapsing-remitting multiple sclerosis: 2-year advance results flash poster 2069. Eur J Neurol. 2015;22:614. doi: 10.1111/ene.12808. [DOI] [Google Scholar]
- 113.Comi G, Cohen JA, Arnold DL, Wynn D, Filippi M. Phase III dose-comparison study of glatiramer acetate for multiple sclerosis. Ann Neurol. 2011;69(1):75–82. doi: 10.1002/ana.22316. [DOI] [PubMed] [Google Scholar]
- 114.Comi G, Martinelli V, Rodegher M, Moiola L, Bajenaru O, Carra A, Elovaara I, Fazekas F, Hartung HP, Hillert J, King J, Komoly S, Lubetzki C, Montalban X, Myhr KM, Ravnborg M, Rieckmann P, Wynn D, Young C, Filippi M. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374(9700):1503–1511. doi: 10.1016/s0140-6736(09)61259-9. [DOI] [PubMed] [Google Scholar]
- 115.Comi G, Martinelli V, Rodegher M, Moiola L, Leocani L, Bajenaru O, Carra A, Elovaara I, Fazekas F, Hartung HP, Hillert J, King J, Komoly S, Lubetzki C, Montalban X, Myhr KM, Preziosa P, Ravnborg M, Rieckmann P, Rocca MA, Wynn D, Young C, Filippi M. Effects of early treatment with glatiramer acetate in patients with clinically isolated syndrome. Mult Scler. 2013;19(8):1074–1083. doi: 10.1177/1352458512469695. [DOI] [PubMed] [Google Scholar]
- 116.Ge Y, Grossman RI, Udupa JK, Fulton J, Constantinescu CS, Gonzales-Scarano F, Babb JS, Mannon LJ, Kolson DL, Cohen JA. Glatiramer acetate (Copaxone) treatment in relapsing-remitting MS: quantitative MR assessment. Neurology. 2000;54(4):813–817. doi: 10.1212/WNL.54.4.813. [DOI] [PubMed] [Google Scholar]
- 117.Khan O, Shen Y, Caon C, Bao F, Ching W, Reznar M, Buccheister A, Hu J, Latif Z, Tselis A, Lisak R. Axonal metabolic recovery and potential neuroprotective effect of glatiramer acetate in relapsing-remitting multiple sclerosis. Mult Scler. 2005;11(6):646–651. doi: 10.1191/1352458505ms1234oa. [DOI] [PubMed] [Google Scholar]
- 118.Rovaris M, Comi G, Rocca MA, Valsasina P, Ladkani D, Pieri E, Weiss S, Shifroni G, Wolinsky JS, Filippi M. Long-term follow-up of patients treated with glatiramer acetate: a multicentre, multinational extension of the European/Canadian double-blind, placebo-controlled, MRI-monitored trial. Mult Scler. 2007;13(4):502–508. doi: 10.1177/1352458506070704. [DOI] [PubMed] [Google Scholar]
- 119.Rovaris M, Comi G, Rocca MA, Wolinsky JS, Filippi M. Short-term brain volume change in relapsing-remitting multiple sclerosis: effect of glatiramer acetate and implications. Brain. 2001;124(Pt 9):1803–1812. doi: 10.1093/brain/124.9.1803. [DOI] [PubMed] [Google Scholar]
- 120.Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, Vollmer T, Agius MA, Kappos L, Stites T, Li B, Cappiello L, von Rosenstiel P, Lublin FD. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–556. doi: 10.1016/s1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 121.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 122.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 123.Cohen JA, Khatri B, Barkhof F, Comi G, Hartung HP, Montalban X, Pelletier J, Stites T, Ritter S, von Rosenstiel P, Tomic D, Kappos L. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. J Neurol Neurosurg Psychiatry. 2016;87(5):468–475. doi: 10.1136/jnnp-2015-310597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kappos L, O’Connor P, Radue EW, Polman C, Hohlfeld R, Selmaj K, Ritter S, Schlosshauer R, von Rosenstiel P, Zhang-Auberson L, Francis G. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology. 2015;84(15):1582–1591. doi: 10.1212/wnl.0000000000001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khatri B, Barkhof F, Comi G, Hartung HP, Kappos L, Montalban X, Pelletier J, Stites T, Wu S, Holdbrook F, Zhang-Auberson L, Francis G, Cohen JA. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011;10(6):520–529. doi: 10.1016/s1474-4422(11)70099-0. [DOI] [PubMed] [Google Scholar]
- 126.Lublin F, Miller DH, Freedman MS, Cree BA, Wolinsky JS, Weiner H, Lubetzki C, Hartung HP, Montalban X, Uitdehaag BM, Merschhemke M, Li B, Putzki N, Liu FC, Haring DA, Kappos L. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075–1084. doi: 10.1016/s0140-6736(15)01314-8. [DOI] [PubMed] [Google Scholar]
- 127.Yousuf F, Dupuy SL, Tauhid S, Chu R, Kim G, Tummala S, Khalid F, Weiner HL, Chitnis T, Healy BC, Bakshi R. A two-year study using cerebral gray matter volume to assess the response to fingolimod therapy in multiple sclerosis. J Neurol Sci. 2017;383:221–229. doi: 10.1016/j.jns.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 128.Khalil M, Enzinger C, Langkammer C, Petrovic K, Loitfelder M, Tscherner M, Jehna M, Bachmaier G, Wallner-Blazek M, Ropele S. Cognitive impairment in relation to MRI metrics in patients with clinically isolated syndrome. Mult Scler J. 2011;17(2):173–180. doi: 10.1177/1352458510384009. [DOI] [PubMed] [Google Scholar]
- 129.Miller AE, Wolinsky JS, Kappos L, Comi G, Freedman MS, Olsson TP, Bauer D, Benamor M, Truffinet P, O’Connor PW. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(10):977–986. doi: 10.1016/s1474-4422(14)70191-7. [DOI] [PubMed] [Google Scholar]
- 130.O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, Benzerdjeb H, Truffinet P, Wang L, Miller A, Freedman MS. Randomized trial of Oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–1303. doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- 131.Wolinsky JS, Narayana PA, Nelson F, Datta S, O’Connor P, Confavreux C, Comi G, Kappos L, Olsson TP, Truffinet P, Wang L, Miller A, Freedman MS. Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler. 2013;19(10):1310–1319. doi: 10.1177/1352458513475723. [DOI] [PubMed] [Google Scholar]
- 132.Sprenger T, Kappos L, Radue E-W, Gaetano L, Mueller-Lenke N, Wuerfel J, Thangavelu K, Panzara M, Cavalier S, Wolinsky J. Teriflunomide significantly slows brain volume loss in MS patients irrespective of disability progression (P3.047). Neurology 2016;86 (16 Supplement). https://n.neurology.org/content/86/16_Supplement/P3.047.
- 133.Freedman MS, T Sprenger T, Radue E-W, Wuerfel J, Miller AE, Thangavelu K, Panzara MA, Cavalier S, Kappos L. Teriflunomide is effective in reducing brain volume loss in previously treated patients: a subgroup analysis of TEMSO SIENA data (P734). ECTRIMS 2016; 2016. https://onlinelibrary.ectrims-congress.eu/ectrims/2016/32nd/146574/mark.s.freedman.teriflunomide.is.effective.in.reducing.brain.volume.loss.in.html?f=p6m3e1031o14010.
- 134.Radue E-W, Sprenger T, Gaetano L, Mueller-Lenke N, Wuerfel J, Thangavelu K, Cavalier S, Kappos L. Teriflunomide slows brain volume loss in relapsing MS: A SIENA analysis of the TEMSO MRI dataset (P3.089). Neurology 2016;86 (16 Supplement). https://n.neurology.org/content/86/16_Supplement/P3.089.
- 135.Wuerfel J, Kappos L, Radue E-W, Gaetano L, Mueller-Lenke N, Thangavelu K, Panzara M, Cavalier S, Wolinsky J, Sprenger T. Teriflunomide slows brain volume loss: subgroup analysis of the SIENA TEMSO MRI dataset (P3.052). Neurology 2016;86 (16 Supplement). https://n.neurology.org/content/86/16_Supplement/P3.052.
- 136.Arnold DL, Gold R, Kappos L, Bar-Or A, Giovannoni G, Selmaj K, Yang M, Zhang R, Stephan M, Sheikh SI, Dawson KT. Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study. J Neurol. 2014;261(9):1794–1802. doi: 10.1007/s00415-014-7412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miller DH, Fox RJ, Phillips JT, Hutchinson M, Havrdova E, Kita M, Wheeler-Kingshott CAM, Tozer DJ, MacManus DG, Yousry TA, Goodsell M, Yang M, Zhang R, Viglietta V, Dawson KT, For the Csi Effects of delayed-release dimethyl fumarate on MRI measures in the phase 3 CONFIRM study. Neurology. 2015;84(11):1145–1152. doi: 10.1212/WNL.0000000000001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dupuy SL, Tauhid S, Hurwitz S, Chu R, Yousuf F, Bakshi R. The effect of dimethyl fumarate on cerebral gray matter atrophy in multiple sclerosis. Neurol Ther. 2016 doi: 10.1007/s40120-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miller DH, Soon D, Fernando KT, MacManus DG, Barker GJ, Yousry TA, Fisher E, O’Connor PW, Phillips JT, Polman CH, Kappos L, Hutchinson M, Havrdova E, Lublin FD, Giovannoni G, Wajgt A, Rudick R, Lynn F, Panzara MA, Sandrock AW. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68(17):1390–1401. doi: 10.1212/01.wnl.0000260064.77700.fd. [DOI] [PubMed] [Google Scholar]
- 140.Radue EW, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Rudick RA, Lublin FD, Weinstock-Guttman B, Wynn DR, Fisher E, Papadopoulou A, Lynn F, Panzara MA, Sandrock AW. Natalizumab plus interferon beta-1a reduces lesion formation in relapsing multiple sclerosis. J Neurol Sci. 2010;292(1–2):28–35. doi: 10.1016/j.jns.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 141.Portaccio E, Stromillo ML, Goretti B, Hakiki B, Giorgio A, Rossi F, De Leucio A, De Stefano N, Amato MP. Natalizumab may reduce cognitive changes and brain atrophy rate in relapsing-remitting multiple sclerosis—a prospective, non-randomized pilot study. Eur J Neurol. 2013;20(6):986–990. doi: 10.1111/j.1468-1331.2012.03882.x. [DOI] [PubMed] [Google Scholar]
- 142.Vidal-Jordana A, Sastre-Garriga J, Perez-Miralles F, Tur C, Tintore M, Horga A, Auger C, Rio J, Nos C, Edo MC, Arevalo MJ, Castillo J, Rovira A, Montalban X. Early brain pseudoatrophy while on natalizumab therapy is due to white matter volume changes. Mult Scler. 2013;19(9):1175–1181. doi: 10.1177/1352458512473190. [DOI] [PubMed] [Google Scholar]
- 143.Rinaldi F, Calabrese M, Seppi D, Puthenparampil M, Perini P, Gallo P. Natalizumab strongly suppresses cortical pathology in relapsing-remitting multiple sclerosis. Mult Scler. 2012;18(12):1760–1767. doi: 10.1177/1352458512447704. [DOI] [PubMed] [Google Scholar]
- 144.Kapoor R, Ho P-R, Campbell N, Chang I, Deykin A, Forrestal F, Lucas N, Yu B, Arnold DL, Freedman MS. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405–415. doi: 10.1016/S1474-4422(18)30069-3. [DOI] [PubMed] [Google Scholar]
- 145.Arpin EC, Sobrino TG, Vivero CD, del Campo Amigo Jorrin M, Regal AR, Gonzalez JP, Bouzas ML. Changes in brain atrophy indices in patients with relapsing-remitting multiple sclerosis treated with natalizumab. Neurodegener Dis Manag. 2016;6(1):5–12. doi: 10.2217/nmt.15.53. [DOI] [PubMed] [Google Scholar]
- 146.Investigators TCT Alemtuzumab vs interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786–1801. doi: 10.1056/nejmoa0802670. [DOI] [PubMed] [Google Scholar]
- 147.Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Fisher E, Brinar VV, Giovannoni G, Stojanovic M, Ertik BI, Lake SL, Margolin DH, Panzara MA, Compston DA. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–1828. doi: 10.1016/s0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- 148.Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Miller T, Fisher E, Sandbrink R, Lake SL, Margolin DH, Oyuela P, Panzara MA, Compston DA. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–1839. doi: 10.1016/s0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- 149.Traboulsee A, Barnett M, Comi G, LaGanke C, Pelletier D, Rovira A, Schippling S, Margolin DH, Thangavelu K, Nakamura K, Arnold DL, Investigators obotC-MIaC-MI. Alemtuzumab durably slows brain volume loss over 6 years in the absence of continuous treatment in patients with active RRMS who were treatment-naive (CARE-MS I) or had an inadequate response to prior therapy (CARE-MS II) (P1181). ECTRIMS 2016; 2016.
- 150.Coles AJ, Cohen JA, Fox EJ, Giovannoni G, Hartung HP, Havrdova E, Schippling S, Selmaj KW, Traboulsee A, Compston DAS, Margolin DH, Thangavelu K, Chirieac MC, Jody D, Xenopoulos P, Hogan RJ, Panzara MA, Arnold DL. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology. 2017;89(11):1117–1126. doi: 10.1212/wnl.0000000000004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arnold DL, Kappos L, Khan O, Gauthier SA, Greenberg S, Ma W, Wang P, Elkins J, Sabatella G. Reduction in brain volume loss in patients receiving daclizumab HYP versus intramuscular interferon beta-1a: results of the DECIDE study (P558). ECTRIMS 2015; 2015.
- 152.Borges IT, Shea CD, Ohayon J, Jones BC, Stone RD, Ostuni J, Shiee N, McFarland H, Bielekova B, Reich DS. The effect of daclizumab on brain atrophy in relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2013;2(2):133–140. doi: 10.1016/j.msard.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue EW, Stefoski D, Robinson R, Riester K, Rana J, Elkins J, O’Neill G. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9884):2167–2175. doi: 10.1016/s0140-6736(12)62190-4. [DOI] [PubMed] [Google Scholar]
- 154.Arnold D, Bar-Or A, Comi G, Hartung H-P, Hauser S, Kappos L, Lublin F, Selmaj K, Traboulsee A, Klingelschmitt G, Masterman D, Fontoura P, Chin P, Garren H, Wolinsky J. Effect of ocrelizumab on MRI inflammatory and neurodegenerative markers of disease in patients with relapsing multiple sclerosis: analysis of the phase III, double-blind, double-dummy, interferon beta-1a-controlled OPERA I and OPERA II studies (S49.002). Neurology 2016;86 (16 Supplement). https://n.neurology.org/content/86/16_Supplement/S49.002.
- 155.Montalban X, Hemmer B, Rammohan K, Giovannoni G, De Seze J, Bar-Or A, Arnold D, Sauter A, Masterman D, Fontoura P, Garren H, Chin P, Wolinsky J. Efficacy and safety of ocrelizumab in primary progressive multiple sclerosis: results of the phase III double-blind, placebo-controlled ORATORIO study (S49.001). Neurology 2016;86 (16 Supplement). https://onlinelibrary.ectrims-congress.eu/ectrims/2015/31st/116701/xavier.montalban.efficacy.and.safety.of.ocrelizumab.in.primary.progressive.html?f=m3.
- 156.Bruck W, Pfortner R, Pham T, Zhang J, Hayardeny L, Piryatinsky V, Hanisch UK, Regen T, van Rossum D, Brakelmann L, Hagemeier K, Kuhlmann T, Stadelmann C, John GR, Kramann N, Wegner C. Reduced astrocytic NF-kappaB activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol. 2012;124(3):411–424. doi: 10.1007/s00401-012-1009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Comi G, Jeffery D, Kappos L, Montalban X, Boyko A, Rocca MA, Filippi M. Placebo-Controlled Trial of Oral Laquinimod for Multiple Sclerosis. N Engl J Med. 2012;366(11):1000–1009. doi: 10.1056/NEJMoa1104318. [DOI] [PubMed] [Google Scholar]
- 158.Vollmer TL, Sorensen PS, Selmaj K, Zipp F, Havrdova E, Cohen JA, Sasson N, Gilgun-Sherki Y, Arnold DL. A randomized placebo-controlled phase III trial of oral laquinimod for multiple sclerosis. J Neurol. 2014;261(4):773–783. doi: 10.1007/s00415-014-7264-4. [DOI] [PubMed] [Google Scholar]
- 159.Alroughani R, Deleu D, El Salem K, Al-Hashel J, Alexander KJ, Abdelrazek MA, Aljishi A, Alkhaboori J, Al Azri F, Al Zadjali N. A regional consensus recommendation on brain atrophy as an outcome measure in multiple sclerosis. BMC Neurol. 2016;16(1):240. doi: 10.1186/s12883-016-0762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Filippi M, Rovaris M, Iannucci G, Mennea S, Sormani MP, Comi G. Whole brain volume changes in patients with progressive MS treated with cladribine. Neurology. 2000;55(11):1714–1718. doi: 10.1212/WNL.55.11.1714. [DOI] [PubMed] [Google Scholar]
- 161.Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Sørensen PS, Vermersch P, Chang P, Hamlett A, Musch B, Greenberg SJ. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- 162.De Stefano N, Giorgio A, Battaglini M, De Leucio A, Hicking C, Dangond F, Giovannoni G, Sormani MP. Reduced brain atrophy rates are associated with lower risk of disability progression in patients with relapsing multiple sclerosis treated with cladribine tablets. Mult Scler. 2018;24(2):222–226. doi: 10.1177/1352458517690269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.De Stefano N, Giorgio A, et al. Response to ‘Does cladribine have an impact on brain atrophy in people with relapsing remitting multiple sclerosis?’ by Schiffmann et al. Mult Scler. 2018;24(10):1388–1389. doi: 10.1177/1352458517748476. [DOI] [PubMed] [Google Scholar]
- 164.Schiffmann I, Scheiderbauer J, Riemann-Lorenz K, Heesen C. Does cladribine have an impact on brain atrophy in people with relapsing remitting multiple sclerosis? Mult Scler. 2018;24(10):1387–1388. doi: 10.1177/1352458517749895. [DOI] [PubMed] [Google Scholar]
- 165.Dardiotis E, Arseniou S, Sokratous M, Tsouris Z, Siokas V, Mentis AA, Michalopoulou A, Andravizou A, Dastamani M, Paterakis K, Bogdanos D, Brotis A. Vitamin B12, folate, and homocysteine levels and multiple sclerosis: a meta-analysis. Mult Scler Relat Disord. 2017;17:190–197. doi: 10.1016/j.msard.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 166.Dardiotis E, Nousia A, Siokas V, Tsouris Z, Andravizou A, Mentis AA, Florou D, Messinis L, Nasios G. Efficacy of computer-based cognitive training in neuropsychological performance of patients with multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord. 2018;20:58–66. doi: 10.1016/j.msard.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 167.Sokratous M, Dardiotis E, Bellou E, Tsouris Z, Michalopoulou A, Dardioti M, Siokas V, Rikos D, Tsatsakis A, Kovatsi L, Bogdanos DP, Hadjigeorgiou GM. CpG island methylation patterns in relapsing-remitting multiple sclerosis. J Mol Neurosci. 2018;64(3):478–484. doi: 10.1007/s12031-018-1046-x. [DOI] [PubMed] [Google Scholar]
- 168.Dardiotis E, Tsouris Z, Aslanidou P, Aloizou AM, Sokratous M, Provatas A, Siokas V, Deretzi G, Hadjigeorgiou GM. Body mass index in patients with multiple sclerosis: a meta-analysis. Neurol Res. 2019 doi: 10.1080/01616412.2019.1622873. [DOI] [PubMed] [Google Scholar]
- 169.Sokratous M, Dardiotis E, Tsouris Z, Bellou E, Michalopoulou A, Siokas V, Arseniou S, Stamati T, Tsivgoulis G, Bogdanos D, Hadjigeorgiou GM. Deciphering the role of DNA methylation in multiple sclerosis: emerging issues. Auto Immun Highlights. 2016;7(1):12. doi: 10.1007/s13317-016-0084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Efthymiou G, Dardiotis E, Liaskos C, Marou E, Tsimourtou V, Rigopoulou EI, Scheper T, Daponte A, Meyer W, Sakkas LI, Hadjigeorgiou G, Bogdanos DP. Immune responses against Helicobacter pylori-specific antigens differentiate relapsing remitting from secondary progressive multiple sclerosis. Sci Rep. 2017;7(1):7929. doi: 10.1038/s41598-017-07801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dardiotis E, Panayiotou E, Siokas V, Aloizou A-M, Christodoulou K, Hadjisavvas A, Pantzaris M, Grigoriadis N, Hadjigeorgiou GM, Kyriakides T. Gene variants of adhesion molecules predispose to MS: a case-control study. Neurol Genet. 2019;5(1):e304. doi: 10.1212/nxg.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dardiotis E, Panayiotou E, Provatas A, Christodoulou K, Hadjisavvas A, Antoniades A, Lourbopoulos A, Pantzaris M, Grigoriadis N, Hadjigeorgiou GM, Kyriakides T. Gene variants of adhesion molecules act as modifiers of disease severity in MS. Neurol Neuroimmunol Neuroinflamm. 2017;4(4):e350. doi: 10.1212/nxi.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.De Stefano N, Sormani MP, Stubinski B, Blevins G, Drulovic JS, Issard D, Shotekov P, Gasperini C. Efficacy and safety of subcutaneous interferon beta-1a in relapsing-remitting multiple sclerosis: further outcomes from the IMPROVE study. J Neurol Sci. 2012;312(1–2):97–101. doi: 10.1016/j.jns.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 174.Hardmeier M, Wagenpfeil S, Freitag P, Fisher E, Rudick RA, Kooijmans M, Clanet M, Radue EW, Kappos L. Rate of brain atrophy in relapsing MS decreases during treatment with IFNbeta-1a. Neurology. 2005;64(2):236–240. doi: 10.1212/01.wnl.0000149516.30155.b8. [DOI] [PubMed] [Google Scholar]
- 175.Molyneux PD, Kappos L, Polman C, Pozzilli C, Barkhof F, Filippi M, Yousry T, Hahn D, Wagner K, Ghazi M, Beckmann K, Dahlke F, Losseff N, Barker GJ, Thompson AJ, Miller DH. The effect of interferon beta-1b treatment on MRI measures of cerebral atrophy in secondary progressive multiple sclerosis. European Study Group on Interferon beta-1b in secondary progressive multiple sclerosis. Brain. 2000;123(Pt 11):2256–2263. doi: 10.1093/brain/123.11.2256. [DOI] [PubMed] [Google Scholar]
- 176.Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, Montalban X, Barkhof F, Radu EW, Bauer L, Dahms S, Lanius V, Pohl C, Sandbrink R. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet. 2007;370(9585):389–397. doi: 10.1016/s0140-6736(07)61194-5. [DOI] [PubMed] [Google Scholar]
- 177.Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, Montalban X, Barkhof F, Radu EW, Metzig C, Bauer L, Lanius V, Sandbrink R, Pohl C. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 2009;8(11):987–997. doi: 10.1016/s1474-4422(09)70237-6. [DOI] [PubMed] [Google Scholar]
- 178.Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R. Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Ann Neurol. 2013;73(6):705–713. doi: 10.1002/ana.23938. [DOI] [PubMed] [Google Scholar]
- 179.Lublin FD, Cofield SS, Cutter GR, Conwit R, Narayana PA, Nelson F, Salter AR, Gustafson T, Wolinsky JS. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol. 2013;73(3):327–340. doi: 10.1002/ana.23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.O’Connor P, Filippi M, Arnason B, Comi G, Cook S, Goodin D, Hartung HP, Jeffery D, Kappos L, Boateng F, Filippov V, Groth M, Knappertz V, Kraus C, Sandbrink R, Pohl C, Bogumil T, O’Connor P, Filippi M, Arnason B, Cook S, Goodin D, Hartung HP, Kappos L, Jeffery D, Comi G. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009;8(10):889–897. doi: 10.1016/s1474-4422(09)70226-1. [DOI] [PubMed] [Google Scholar]
- 181.Kappos L, Fox R, Gold R, Arnold D, Potts J, Zhang A, Kurukulasuriya N. Sustained low rate of brain volume loss under long-term delayed-release dimethyl fumarate treatment in relapsing-remitting multiple sclerosis patients: results from the ENDORSE study (P7.243). Neurology 2015;84 (14 Supplement). https://n.neurology.org/content/84/14_Supplement/P7.243.