Abstract
Background & Aims:
Celiac disease can develop at any age, but outcomes of adults with positive results from serologic tests for tissue transglutaminase antibodies (tTGA) without endoscopic determination of celiac disease (called celiac autoimmunity) have not been thoroughly evaluated. We investigated the proportion of adults with celiac autoimmunity at a community medical center and their progression to celiac disease.
Methods:
We analyzed waste blood samples from a community clinic from 15,551 adults for tTGA and, if titers were above 2 U/mL, for endomysial antibody. The blood samples had been collected at 2 time points (median interval of 8.8 years), from 2006 through 2017. We collected data from the clinic on diagnoses of celiac disease based on duodenal biopsy analysis.
Results:
Of the serum samples collected at the first timepoint, 15,398 were negative for tTGA and were 153 positive for tTGA (>4 U/mL). Based on medical records, 6 subjects received a diagnosis of celiac disease, for a cumulative incidence of celiac disease diagnosis of 0.06% (95% CI, 0.01%–0.11%). Forty-nine subjects with a negative result from the first serologic test for tTGA (0.32%) had a positive result from the second test. Among the 153 adults who were tTGA positive at the first time point, 31 (20%) had a subsequent diagnosis of celiac disease, 81 (53%) remained positive for tTGA without a clinical diagnosis of celiac disease, and 41 (27%) tested negative for tTGA at the second time point. Higher initial tTGA titers, female sex, and a history of hypothyroidism and autoimmune disease were associated with increased risks of subsequent diagnosis of celiac disease. Interestingly, adults whose first blood sample was positive but second blood sample was negative for tTGA were older, had lower than average initial tTGA titers, and had a higher mean body mass index than adults whose blood samples were positive for tTGA at both time points or adults later diagnosed with celiac disease.
Conclusions:
In an analysis of serum samples collected from a community clinic an average of 8.8 years apart, we found that fewer than 1% of adults with negative results from an initial test for tTGA have a positive result in a second test. Of adults with positive results from the test for tTGA, only 20% are later diagnosed with celiac disease—the remaining individuals maintain persistent increases in tTGA without diagnoses of celiac disease or have negative results from second tests.
Keywords: de novo disease, epidemiology, serology, pre-clinical phase
Introduction
Celiac disease (CeD) is considered a lifelong treatable but irreversible disorder, affecting approximately 1% of the population. Studies of birth cohorts have described that there is a high rate of seroconversion in childhood and to a lesser extent of developing disease.1–6 However, few studies for the development of CeD have been performed in adults.7–9 Moreover, studies on the factors that cause or trigger CeD has centered on those that affect infants (e.g., feeding patterns, weaning, infections), but little attention has been paid to the frequency and factors that could lead to the new onset of CeD in adults. Only a few studies of relatively small sample size7–9 have shown that CeD autoimmunity can occur in adults. Clinically, it is important to know the subsequent risk of CeD in an adult with a negative result of a CeD screening test.
Positive CeD serology is generally considered to be highly predictive of CeD. However, some studies in children have found that CeD autoimmunity may be transient, even though the child remains on a gluten-containing diet.10–13 A few studies that have small sample sizes have evaluated the natural history of adults with positive CeD serology.7, 9A study by Biagi et al9 showed a subset of seropositive adults did not develop mucosal damage, and instead maintained a normal duodenal mucosa for many years of continuous exposure to gluten. Thus, the natural history of people with positive CeD serology is largely unknown in adults, and further systematic approaches are warranted to find what factors and which groups are at high risk for CeD.
Therefore, our aim was to evaluate the risk of the development of de novo CeD autoimmunity and, further, to study the fates of adults with positive CeD serology by retesting a large community-based sample of persons who had been previously tested for CeD serology 5 to 10 years prior. Additionally we investigated factors associated with clinical diagnosis of CeD among the retested cohort.
Materials and Methods
In this prospective cohort study, waste blood samples from residents of a community were tested for CeD autoimmunity at two time points. This community has been repeatedly studied to investigate the epidemiologic data for many medical interests, including CeD.14–17 In our prior community-based studies,16, 17 serum samples of adult residents of Olmsted County, who were not diagnosed with CeD, were tested for tTGA and, if tTGA titers were above 2 U/mL, then subsequently tested for EMA to identify CeD autoimmunity. In this follow up study, a total of 15,551 subjects were retested for CeD serology between 2011 and 2017 over a median of 8.8 years (IQR, 7.1–16.4, a minimum of 4-year interval between the initial and second testing). The comparisons of demographic characteristics between those who had a second testing (retesting) and without the second testing were summarized in the supplemental table 1. Retesting subjects were older, more female, and higher tTGA titers, compared to subjects who were tested at only initial testing (supplemental table 1). All samples were stripped of patient identifiers, and CeD serology testing was performed with no knowledge of the patients’ clinical data. The comparison Our study was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center, Rochester, Minnesota.
Laboratory Tests for CeD
The human recombinant tTGA-based enzyme-linked immunosorbent assay (Inova Diagnostics) was used to measure titers of tTGA.18, 19 If tTGA titers were 2.0 U/mL or more, then sequential testing for the presence of IgA specific for endomysium (EMA) was performed with indirect immunofluorescence using the reticulin component of the endomysium of the smooth muscle in monkey esophagus tissue (Inova Diagnostics).18 For this study, CeD serologic status was categorized as follows: tTGA titers <4.0 U/mL (negative) and tTGA ≥ 4 U/mL (positive), with tTGA titers ≥ 40 U/mL (strong positive). The chosen cutoff was 40 U/mL, because it is 10 times the upper normal limit, which cutoff is used in the ESPGHAN guideline20.
Data Collection
Associated autoimmune diseases prior to first serum collection date were electronically captured with diagnosis codes on the entire cohort. Furthermore, our previous work included reviewing medical records of all subjects with initially positive tTGA in a blinded fashion within a matched subset of tTGA negative controls17. The records contained inpatient, outpatient, and emergency department data to obtain information related to CeD, such as history of autoimmune diseases, diet history, and other conditions. Autoimmune diseases included thyroid disease, systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, type 1 diabetes, and multiple sclerosis. We used this abstracted data to describe the history of the initially positive subjects in this retested cohort.
Subsequent clinical diagnosis of CeD was determined using the same data sources in our prior studies to estimate the incidence rate of CeD among the residents of Olmsted County16, 17, 21. Briefly, diagnosis and procedure codes for CeD through the Rochester Epidemiology Project, which collaborates with most providers in the county and provides a unique records linkage system, were used to find potential CeD patients. CeD diagnoses of these patients were confirmed by the manual review of the electronic medical records, through CeD serology and intestinal histology. Diagnosis of CeD was defined by positive CeD serology and small bowel villous atrophy or increased intraepithelial lymphocytes.
In accordance with Minnesota law, patients who declined to provide authorization for the use of their medical records for research purposes were not included in this study.
Measurement of Alkylresorcinols (AR)
Serum AR concentrations, which can be used as a biomarker for gluten-containing diet in prior investigations,22, 23 were measured using the normal-phase liquid chromatography-tandem mass spectrometry (LC-MS/MS), as described elsewhere24 with some modifications for instrumentation. Briefly, 100 μL of serum was loaded on HybridSPE Plus phospholipid removal 96-well plates (Sigma-Aldrich, St Louis, MO, USA) and recovered with acetone. After evaporating and re-suspending the eluted samples in heptane-ethanol (95:5 v/v), extracts were transferred to chromatographic vials and by LC-MS/MS (QTRAP 6500+; AB Sciex, Framingham, USA). AR homologues from C17 to C25 were measured, and data are reported as a total AR concentration (sum of all homologues).
Statistical Analyses
Descriptive statistics used to summarize the baseline data included number (percentage) for categorical data and mean (± standard deviation [SD]) or median (interquartile range [IQR]) for continuous data as appropriate. The Kaplan-Meier product-limit method was used to estimate the cumulative incidence of CeD per 1,000 person-years of follow-up. Cox proportional hazards models were used to assess the demographic features and the development of CeD diagnosis and are described with hazard ratios (95% confidence intervals). Associations with de novo CeD autoimmunity were tested with logistic regression adjusting for the time interval between serum collections. Characteristics present at initial collection time of subjects with initially positive CeD serology were compared with Chi-Square and ANOVA (Kruskal-Wallis test where appropriate) to determine associations with their retest status. Retest status of the positives was categorized as positive serology, negative serology, or clinically diagnosed with CD over follow up (as we would expect the majority of these to seroconvert to negative if following a gluten-free diet). SAS 9.4M5 software was used for all analyses. A significance level of 0.05 was used.
Results
Study population
The mean age of 15,551 re-tested adults in this study was 47.0 years (standard deviation ±13.8) at the time of initial sampling, and 58% were female. The median interval between the initial test and retesting was 8.9 years (IQR, 7.1–16.4). Table 1 lists the clinical characteristics of the retested study population according to their initial tTGA results. Based on the initial tTGA results, 15,398 were negative while 153 adults were positive (Figure 1). Thirty-three subjects were strong positive (≥ 40 U/mL), and all of these subjects had positive EMA results. Of the 120 adults with positive tTGA (4 ≤ tTGA<40), 77% (n=92) were positive EMA. Interestingly, 8 of 112 adults with a tTGA titer between 2 and 4 U/mL, which was considered negative tTGA but was eligible for EMA testing, showed positive EMA results. Subjects with tTGA < 2 U/mL were not tested for EMA. Figure 1 shows the study population according to results of tTGA at the initial and second testing.
Table 1.
Clinical Characteristics of Study Population Retested for tTGA
| Initial tTGA Value, U/mL | ||||
|---|---|---|---|---|
| Characteristic | <4 (n=15,398) | 4 ≤ tTGA <40 (n= 120) | ≥40 (n= 33) | P Value |
| Age at initial testing, mean (SD), y | 47.0 (13.8) | 44.1 (12.3) | 41.9 (12.9) | 0.007a |
| Female sex | 8,966 (58.2%) | 68 (56.7%) | 20 (60.6%) | 0.9b |
| Median follow-up interval (IQR), y | 8.9 (7.1–16.4) | 8.2 (6.7–10.1) | 8.2 (7.1–8.9) | 0.004c |
| Initial EMA positive, % | 8 (0.1%)d | 92 (76.7%) | 33 (100%) | <0.001b |
| tTGA at follow-up, U/mL | <0.001b | |||
| <4 | 15,349 (99.7%) | 59 (49.2%) | 8 (24.2%) | |
| 4 ≤ tTGA<40 | 46 (0.3%) | 50 (41.7%) | 10 (30.3%) | |
| ≥ 40 | 3 (0.0%) | 11 (9.2%) | 15 (45.5%) | |
Abbreviations: CeD, celiac disease; EMA, endomysial antibodies; IQR, interquartile range; NA, not applicable; tTGA, tissue transglutaminase antibody.
Analysis of variance F-test.
χ2 test.
Kruskal-Wallis test.
Only 112 adults with negative tTGA, whose tTGA values were between 2 and 4 U/mL, were tested for EMA.
Figure 1.
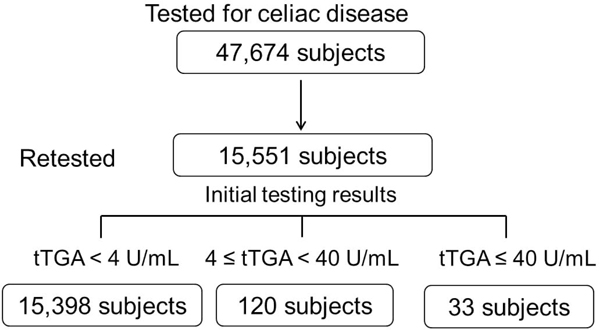
Flow chart of the study population according to tissue transglutaminase-IgA (tTGA) results
Subsequent CeD diagnosis
Figure 2 shows the cumulative incidence of a clinical diagnosis of CeD within 10 years from the original blood sample drawn, according to the initial results of tTGA testing. The cumulative incidence of a clinical diagnosis was significantly higher in adults with a strongly positive tTGA titer (≥40 U/mL, 30.4% [95% CI, 11.4%−45.3%]) and those with a positive tTGA titer (4 ≤ tTGA < 40 U/mL, 22.0% [95% CI, 12.1%−30.8%]) than in adults with initial negative tTGA (0.06% [95% CI, 0.01%−0.11%]).
Figure 2.
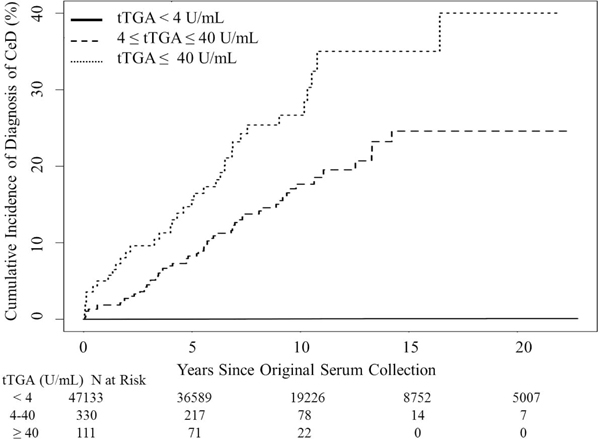
Cumulative Incidence of Subsequent Celiac Disease (CeD) Diagnosis According to Initial tTGA titers in the Community Cohort. tTGA, tissue transglutaminase antibody (U/mL).
Table 2 shows the hazard ratios for subsequent clinical diagnosis of CeD according to characteristics found prior to their initial serum collection. Female adults had an increased cumulative incidence of CeD (0.37%; 95% CI, 0.23%−0.51%) compared with men (0.17%; 95% CI, 0.04%−0.30%). Interestingly, history of hypothyroidism (HR 2.4, 95% CI, 1.11–5.36) and the presence of autoimmune disorders (HR 2.54, 95% CI, 1.31–4.93) were associated with increased risk for being subsequently diagnosed with CeD.
Table 2.
Cumulative Incidence Rates and Relative Hazard of Subsequent Clinical Diagnosis of CeD within 10 Years in a Community based Cohort
| Characteristic | Events, n | Cumulative Incidence Rate, % (95% CI) | Hazard Ratio (95% CI)a | P Value |
|---|---|---|---|---|
| Female (n=9,054) | 29 | 0.37 (0.23–0.51) | 3.02 (1.32–6.88) | 0.009 |
| Male (n=6,497) | 7 | 0.50 (0.26–0.73) | Reference | |
| Hypothyroidism (n=1,594) | 8 | 0.59 (0.17–1.01) | 2.44 (1.11–5.36) | 0.026 |
| No Hypothyroidism (n=13,957) | 28 | 0.25 (0.16–0.35) | Reference | |
| Autoimmune disorder (n=3,440) | 15 | 0.55 (0.26–0.85) | 2.54 (1.31–4.93) | 0.006 |
| No Autoimmune disorder (n=12,111) | 21 | 0.21 (0.12–0.31) | Reference | |
| Initial CeD serology: tTGA, U/mL | ||||
| tTGA < 4 (n=15,398) | 6 | 0.06 (0.01–0.11) | Reference | |
| 4 ≤ tTGA < 40 (n=120) | 21 | 22.01 (12.12–30.79) | 520.18 (209.73–1290.14) | <0.001 |
| tTGA ≥ 40 (n=33) | 9 | 30.35 (11.36–45.28) | 865.99 (307.15–2441.57) | <0.001 |
Abbreviations: CeD, Celiac Disease; tTGA, tissue transglutaminase antibody.
Findings Among Initially Negative tTGA Subjects
Of the 15,398 adults who were initially negative for tTGA, 49 (0.32%) subsequently had positive tTGA in the second testing. Moreover, 22 (0.14%) subjects were EMA positive in the second serum samples. Only six subjects were subsequently diagnosed with CeD within 10 years, resulting in an estimation that the cumulative incidence of CeD was only 0.06% (95% CI, 0.01%−0.011%). The characteristics of these 6 patients were summarized in the supplementary table 2. Among eight adults whose initial tTGA was between 2 and 4 U/mL with EMA positivity, half of them became EMA negative (n=4) on second testing while the other four remained EMA positive. None of these eight adults were subsequently diagnosed with CeD. Table 3 shows the frequency and odds raito of de novo CeD autoimmunity that was based on positive EMA among subjects with initially negative tTGA. Among subjects with initially negative tTGA, female sex, hypothyroidism, and a history of autoimmune disease were not associated with increased odds for de novo CeD autoimmunity in adults.
Table 3.
Frequency and Odds Ratio of de novo CeD Autoimmunity Among Initial tTGA Negative Subjects in a Community based Cohort
| Characteristic | Frequency (%) | Odds Ratio (95% CI)a | P Value |
|---|---|---|---|
| Female (n=8,966) | 13 (0.14%) | 1.15 (0.48–2.78) | 0.757 |
| Male (n=6,432) | 8 (0.12%) | Reference | |
| Hypothyroidism (n=1,573) | 2 (0.13%) | 0.95 (0.22–4.07) | 0.941 |
| No Hypothyroidism (n=13,825) | 19 (0.14%) | Reference | |
| Autoimmune disorder (n=3,397) | 4 (0.12 %) |
0.82 (0.28–2.45) | 0.728 |
| No Autoimmune disorder (n=12,001) |
17 (0.14%) | Reference |
Abbreviations: CeD, Celiac Disease; tTGA, tissue transglutaminase antibody.
Findings Among Initially Positive tTGA Subjects
Among 153 adults with initially positive tTGA, 56% (n=86) remained positive for tTGA at the second testing, and the remaining 44% (n=67) normalized for tTGA (Figure 1). Regarding subsequent diagnosis of CeD, 20% (n=31) initially tTGA positive adults were subsequently diagnosed with CeD after the initial testing (Figure 1). Table 4 shows the characteristics of three groups within the 153 adults with positive tTGA at the initial testing: 1) adults with a subsequent diagnosis of CeD 2) adults with positive tTGA titers at the second testing, ‘persistently positive’, and 3) adults with negative tTGA titers at the second testing, ‘normalized’. The changes of tTGA titers between the initial and second testing according to three groups were depicted in the supplemental figure 1.
Table 4.
Comparison of Characteristics of Retested Adults with Initial Positive tTGA according to Subsequent Statusa
| Characteristic | Normalized tTGA (n=41) | Persistently positive tTGA (n=81) | Subsequent diagnosis of CeD (N=31) | P Value |
|---|---|---|---|---|
| Age at the initial testing, mean (SD), y | 46.8 (14.7) | 44.6 (10.5) | 36.8 (11.6) | 0.002a |
| Female sex | 23 (56.1%) | 39 (48.1%) | 26 (83.9%) | 0.003b |
| BMI (kg/m2), median (IQR) | ||||
| At initial testing, (n=86) | 32.4 (27.2–36.4) | 29.6 (25.9–36.4) | 24.4 (22.4–29.2) | 0.025c |
| At second testing, (n=110) | 28.6 (21.1–31.8) | 30.2 (25.8–35.9) | 24.7 (22.0–32.5) | 0.116c |
| Median follow-up interval (IQR), y | 9.1 (7.1–18.0) | 8.1 (6.7–9.2) | 7.7 (6.6–9.1) | 0.022c |
| Median tTGA at initial testing (IQR), U/mL |
8.7 (5.8–17.6) | 19.2 (9.7–42.5) | 15.5 (9.6–49.3) | <0.001c |
| Initial EMA positive | 29 (70.7%) | 69 (85.2%) | 27 (87.1%) | 0.102b |
| Presence of CeD related symptoms | 8 (19.5%) | 16 (20.0%) | 26 (83.9%) | <0.001b |
| Presence of autoimmune disease | 14 (34.1%) | 25 (31.3%) | 17 (54.8%) | 0.063b |
Abbreviations: BMI, body mass index; CeD, celiac disease; IQR, interquartile range; tTGA, tissue transglutaminase antibody.
Analysis of variance F-test.
χ2 test.
Kruskal-Wallis test.
Excluding subjects with subsequent diagnosis of CeD, 66% (n=81) remained positive for tTGA, and 34% (n=41) turned to negative for tTGA at the second testing. Among the persistently positive tTGA adults, about half (n=38) had increased titers of tTGA in the second testing. In the 41 adults whose tTGA results became negative without any CeD diagnosis, none were following a gluten-free diet. Additionally, 71% (29/41) whose tTGA converted to negative at the second testing were EMA positive at the initial testing.
When comparing the three groups, adults with normalized tTGA at the second testing had longer duration of follow-up and lower initial tTGA titers compared to adults with persistent positive tTGA or with subsequent diagnosis of CeD (Table 4). Adults with persistent positive tTGA or with a subsequent diagnosis of CeD were younger than those with normalized tTGA. Female sex and presence of CeD related symptoms were more common in adults with subsequent diagnosis of CeD than adults with normalized tTGA. Interestingly, adults with a subsequent diagnosis of CeD had a lower body mass index at the time of initial testing than other groups (P=.025). The history of autoimmune diseases was not different amongst the three groups.
Changes of ARs concentrations according to tTG-IgA status
ARs concentrations were measured in three groups. Figure 3 showed the changes of ARs concentration among three groups between initial and second serum samples. AR concentrations were similar in the initial serum samples among three groups. AR concentrations of patients, who were subsequently diagnosed with CeD, significantly decreased in the second serum samples (164.2 ± 58.0 nmol/L to 26.9 ± 4.0 nmol/L) (p=.03). However, AR concentrations of those with normalized tTGA were similar between initial (107.5 ± 23.6 nmol/L) and second (97.8 ± 39.6) serum samples (p=.33). In addition, in the group of persistently positive, ARs concentrations were not significantly changed between initial and second samples (78.3 ± 16.7 nmol/L to 99.4 ± 15.5 nmol/L) (p=.36).
Figure 3.
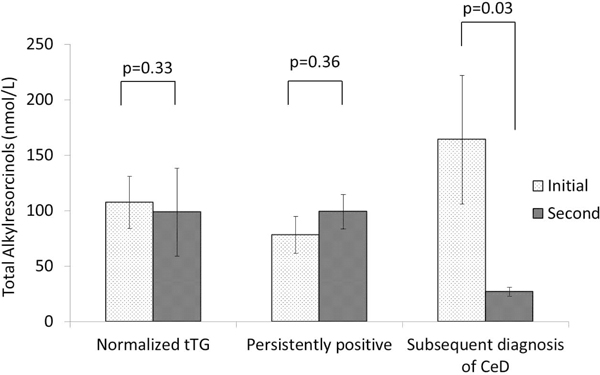
Total alkylresorcinol (AR) concentrations of initial and second serum samples among three groups. * indicates mean (S.E.).
Discussion
The natural history of adults with negative or positive CeD serology has not been well studied. This large community-based study explores the longitudinal consequences of adults with negative or positive CeD serology (tTGA) while maintaining a gluten-containing diet. We show how those with positive CeD serology can progress to clinically obvious disease, remain silent, or even normalize their serology status. We found 3 main results. First, among adult with initial negative tTGA, the incidence rate of de novo adult CeD, defined as new incident cases of CeD over 10-years of follow up, was 0.06%, and the rate of de novo CeD autoimmunity, defined as tTGA positive on second serum collection after a minimum of 4 years, is 0.32% (49 of 15,398 initially seronegative individuals subsequently became seropositive). These demonstrate that CeD can occur in adulthood though the risk is very low suggesting that retesting a person with initial negative CeD serologic results after 10 years is unlikely to detect de novo CeD in adults. Second, while about half of adults with initially positive tTGA results had persistently positive results without receiving a diagnosis of CeD, 26% spontaneously normalized without any apparent indication. Third, adults with a high tTGA titer had a high risk for a subsequent diagnosis of CeD. In addition, female sex, the presence of hypothyroidism, and a history of autoimmune disease were significant predictors for a subsequent diagnosis of CeD in seropositive individuals.
Historically, CeD had long been considered a pediatric disease with most patients presenting with severe malabsorption at an early age.25, 26 Recently, epidemiologic features of CeD have markedly changed, and it has become a more common disease, especially in adults.17, 21 The rate of diagnosis has dramatically increased in recent years; intriguingly, in more than half of patients the disease still remains undetected and a diagnosis is often greatly delayed.27–29 The development of an abnormal immune response in CeD is assumed to happen in early life after introduction of gluten into the diet, and most studies evaluating the development of autoimmunity have been performed in children1, 9, 10, 12, 30. Indeed, only a few studies with a limited number of cases have shown that CeD autoimmunity occurs in adults.7, 9 Catassi et al7 in the CLUE cohort from Maryland, tested serum samples from 1974 and 1989 for 3,504 adults who had negative CeD serologic results, and 0.26% (n=9) had seroconversion (negative to positive) over a 15-year period. Our large study is of adults with diseases and conditions from a community that has a long history of epidemiology studies14–17. We found that the subsequent risk of clinical CeD is much greater in those who are seropositive, even though our data demonstrates that de novo CeD autoimmunity can occur in adults with a prior sero-negative result. It may be unnecessary to retest adults who have negative serologic results unless they have high risk factors such as a family history of CeD or autoimmune disease.
Another interesting finding in this study is the subset of adults who initially had positive-CeD serologic results that spontaneously became negative while they continuously consumed gluten-containing foods. Several studies have had similar findings, but all were limited to children10, 11, 31. Van Koppen et al.31 conducted a 10-year follow-up study of children with CeD and found that 1 of 6 asymptomatic children with seropositive results for CeD had spontaneous seroconversion (going from positive to negative). Another group who studied pediatric CeD found transient or fluctuating CeD serological results: of the 46 patients who had positive serologic results for CeD at one point, 46% had a subsequent loss of anti–tTGA at follow-up testing and 13% had even fluctuations of tTGA levels oscillating between positive and negative levels in multiple follow-up testings2, 11. In another study, Castellaneta et al13 also found that serum anti-tTG levels became negative in 20% of children with type 1 diabetes mellitus despite gluten consumption. In adults, we found that 26% with initially positive tTGA levels had spontaneous normalization of CeD serologic results at the follow-up test without any evidence of following a gluten-free diet. In particular, low-tTGA titers at the initial test were significantly associated with the spontaneous normalization of CeD titers. As we recently proposed in a review on the potential courses of CeD autoimmunity32, it is conceivable that a subset of adults with seropositive results does not progress toward symptomatic CeD but spontaneously becomes negative for CeD despite consuming gluten-containing foods. Serum alkylresorcinols, objective biomarkers of gluten intake, suggest that those with normalised tTG and who were persistently positive did not change their intake of gluten containing cereals over time, while those who were diagnosed with CeD made dietary adjustments to reduce intake of gluten containing foods. The reason for the spontaneous conversion of CeD serological result is unknown. But it does suggest that, at least at the early stage, CeD autoimmunity can exist as a reversible condition, during which we can intervene to reverse or re-establish self-tolerance of gluten. Thus, more studies are needed to determine which people with seropositive results would benefit from detection and treatment.
We found only 20% of subjects with initial positive tTGA were subsequently diagnosed CeD over a median of 8.8 years, which is relatively low. Some studies suggested the current case finding strategies may not be effective to detect patients with undiagnosed CeD.33, 34 Several cross-sectional studies have shown that CeD is associated with autoimmune disease, including type 1 diabetes mellitus and hypothyroidism.17, 35, 36 Their findings highlight the increased risk of autoimmune disease in patients with CeD. Our study, based on longitudinal follow-up, found an increased incidence of progression to clinical CeD in seropositive patients with hypothyroidism or autoimmune disease. This finding is an indication that a subset of patients with CeD tend to have a general increase in autoimmunity. One possible explanation of the association between CeD and autoimmune diseases is a common genetic predisposition. HLA-DQ2 haplotypes are associated with type 1 diabetes mellitus and some of the autoimmune thyroid diseases37–39. Thus, screening for CeD should be considered in patients with autoimmune disease, especially those with autoimmune thyroid disease. Another factor that may be involved in the increased subsequent diagnosis of CeD in patients with autoimmune diseases is physicians increased awareness of CeD which could prompt increased CeD testing in patients with autoimmune disease. Female gender was associated with increased risk for subsequent diagnosis of CeD. However, interestingly, the proportion of tTGA positivity was not different between men and women in the initial testing. Overall, these findings suggested females are more likely to be diagnosed with CD despite of having a similar prevalence of CeD autoimmunity between men and women.
Our study does have some limitations. First, the retesting of samples was based on the availability of a serum sample in the waste blood bank after a clinically indicated blood test. As the samples available for retesting were obtained from patients who were accessing health care, selection bias may be inherent and may have induced overestimation of the new-onset rate. There was a slight excess of older and female patients in the retested cohort (supplemental table 1) Second, the persons who had seroconversion may previously have had a high negative or indeterminate serum status, and this would suggest the necessity of follow-up in subset of high negative or an equivocal range of tTGA to detect CeD. Third, the occurrence of new cases of CeD may have been due to a time (secular) effect; that is, since the prior test, some pervasive change in the environment may have affected the rate of CeD occurrence - even though this is unlikely over such a short period. Additionally we did not have access to DNA to allow for determination of genetic susceptibility to CeD, though the genotypes at risk are prevalent in our community.40 Third, the cumulative incidence of clinically diagnosed CeD in seronegative persons from Olmsted County was significantly lower than the rate of new-onset CeD; a factor indicating that retesting of screened negative population may not be an efficient way to detect new cases of CeD. Lastly, since total IgA levels were not assessed in the current study, the prevalence of CeD autoimmunity could be underestimated.
In conclusion, CeD immunity can occur de novo in adulthood but at a low rate. As we found new-onset CeD was rare and systematic retesting may not be necessary (within 10 years) in persons with previously negative celiac serologic results. In addition, a subset of adults with positive CeD serology can have spontaneous normalization of CeD serologic results for no apparent reason, particularly those with low initial titers of tTGA.
Supplementary Material
ACKNOWLEDGEMENT
The authors acknowledge the expert assistance of Tricia L. Brantner and Immunodermatology Lab of Mayo Clinic Rochester for conducting the celiac serological testing. Also, the authors wish to thank Linda H. Michalowski and Courtney N. Hoover for the assistance in the preparation and submission of the manuscript.
Funding:Funding for this study was provided by a grant from the National Institutes of Health (R01DK057892), by a grant from the National Institute on Aging of the National Institutes of Health (Rochester Epidemiology Project Award Number R01AG034676), and by Mayo Foundation for Medical Education and Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Dr. Joseph A. Murray has received grant support from the National Institutes of Health, ImmunogenX, COUR, ImmunsanT, and Allakos. He served on the advisory board of Celimmune, LLC; was a consultant to Intrexon, Bioniz, Lilly, Amgen, Innovate. Sonomaceuticals, LLC, GlaxoSmithKline, Genentech, Inc., and Glenmark Pharmaceuticals Ltd.; and has equity options in Torax Medical, and royalties from Evolo. The remaining authors have no conflicts to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Maki M, Mustalahti K, Kokkonen J, et al. Prevalence of Celiac disease among children in Finland. N Engl J Med 2003;348:2517–24. [DOI] [PubMed] [Google Scholar]
- 2.Liu E, Dong F, Baron AE, et al. High Incidence of Celiac Disease in a Long-term Study of Adolescents With Susceptibility Genotypes. Gastroenterology 2017;152:1329–1336 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA 2005;293:2343–51. [DOI] [PubMed] [Google Scholar]
- 4.Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 2006;101:2333–40. [DOI] [PubMed] [Google Scholar]
- 5.Lionetti E, Castellaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 2014;371:1295–303. [DOI] [PubMed] [Google Scholar]
- 6.Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 2014;371:1304–15. [DOI] [PubMed] [Google Scholar]
- 7.Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med 2010;42:530–8. [DOI] [PubMed] [Google Scholar]
- 8.Vilppula A, Kaukinen K, Luostarinen L, et al. Increasing prevalence and high incidence of celiac disease in elderly people: a population-based study. BMC Gastroenterol 2009;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biagi F, Trotta L, Alfano C, et al. Prevalence and natural history of potential celiac disease in adult patients. Scand J Gastroenterol 2013;48:537–42. [DOI] [PubMed] [Google Scholar]
- 10.Liu E, Lee HS, Aronsson CA, et al. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med 2014;371:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu E, Bao F, Barriga K, et al. Fluctuating transglutaminase autoantibodies are related to histologic features of celiac disease. Clin Gastroenterol Hepatol 2003;1:356–62. [DOI] [PubMed] [Google Scholar]
- 12.Auricchio R, Tosco A, Piccolo E, et al. Potential celiac children: 9-year follow-up on a gluten-containing diet. Am J Gastroenterol 2014;109:913–21. [DOI] [PubMed] [Google Scholar]
- 13.Castellaneta S, Piccinno E, Oliva M, et al. High rate of spontaneous normalization of celiac serology in a cohort of 446 children with type 1 diabetes: a prospective study. Diabetes Care 2015;38:760–6. [DOI] [PubMed] [Google Scholar]
- 14.Choung RS, Rubio-Tapia A, Lahr BD, et al. Evidence Against Routine Testing of Patients With Functional Gastrointestinal Disorders for Celiac Disease: A Population-based Study. Clin Gastroenterol Hepatol 2015;13:1937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 2009;137:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfrey JD, Brantner TL, Brinjikji W, et al. Morbidity and mortality among older individuals with undiagnosed celiac disease. Gastroenterology 2010;139:763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choung RS, Larson SA, Khaleghi S, et al. Prevalence and Morbidity of Undiagnosed Celiac Disease From a Community-Based Study. Gastroenterology 2017;152:830–839 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulkanen S, Halttunen T, Laurila K, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 1998;115:1322–8. [DOI] [PubMed] [Google Scholar]
- 19.Choung RS, Ditah IC, Nadeau AM, et al. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am J Gastroenterol 2015;110:455–61. [DOI] [PubMed] [Google Scholar]
- 20.Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–60. [DOI] [PubMed] [Google Scholar]
- 21.Ludvigsson JF, Rubio-Tapia A, van Dyke CT, et al. Increasing incidence of celiac disease in a North American population. Am J Gastroenterol 2013;108:818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choung RS, Murray JA, Marietta EV, et al. Serum alkylresorcinols as biomarkers of dietary gluten exposure in coeliac disease. Aliment Pharmacol Ther 2017;45:643–652. [DOI] [PubMed] [Google Scholar]
- 23.Lind MV, Madsen ML, Rumessen JJ, et al. Plasma Alkylresorcinols Reflect Gluten Intake and Distinguish between Gluten-Rich and Gluten-Poor Diets in a Population at Risk of Metabolic Syndrome. J Nutr 2016;146:1991–1998. [DOI] [PubMed] [Google Scholar]
- 24.Ross AB, Svelander C, Savolainen OI, et al. A high-throughput method for liquid chromatography-tandem mass spectrometry determination of plasma alkylresorcinols, biomarkers of whole grain wheat and rye intake. Anal Biochem 2016;499:1–7. [DOI] [PubMed] [Google Scholar]
- 25.Jackson AD. Forty years ago. Coeliac disease. Arch Dis Child 1990;65:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paveley WF. From Aretaeus to Crosby: a history of coeliac disease. BMJ 1988;297:1646–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paez MA, Gramelspacher AM, Sinacore J, et al. Delay in Diagnosis of Celiac Disease in Patients Without Gastrointestinal Complaints. Am J Med 2017;130:1318–1323. [DOI] [PubMed] [Google Scholar]
- 28.Norstrom F, Lindholm L, Sandstrom O, et al. Delay to celiac disease diagnosis and its implications for health-related quality of life. BMC Gastroenterol 2011;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green PHR, Stavropoulos SN, Panagi SG, et al. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol 2001;96:126–31. [DOI] [PubMed] [Google Scholar]
- 30.Olsson C, Hernell O, Hornell A, et al. Difference in celiac disease risk between Swedish birth cohorts suggests an opportunity for primary prevention. Pediatrics 2008;122:528–34. [DOI] [PubMed] [Google Scholar]
- 31.van Koppen EJ, Schweizer JJ, Csizmadia CG, et al. Long-term health and quality-of-life consequences of mass screening for childhood celiac disease: a 10-year follow-up study. Pediatrics 2009;123:e582–8. [DOI] [PubMed] [Google Scholar]
- 32.Choung RS, Murray JA. The US Preventive Services Task Force Recommendation on Screening for Asymptomatic Celiac Disease: A Dearth of Evidence. JAMA 2017;317:1221–1223. [DOI] [PubMed] [Google Scholar]
- 33.Hujoel IA, Van Dyke CT, Brantner T, et al. Natural history and clinical detection of undiagnosed coeliac disease in a North American community. Aliment Pharmacol Ther 2018;47:1358–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen A, Sandstrom O, Carlsson A, et al. Usefulness of symptoms to screen for celiac disease. Pediatrics 2014;133:211–8. [DOI] [PubMed] [Google Scholar]
- 35.Cosnes J, Cellier C, Viola S, et al. Incidence of autoimmune diseases in celiac disease: protective effect of the gluten-free diet. Clin Gastroenterol Hepatol 2008;6:753–8. [DOI] [PubMed] [Google Scholar]
- 36.Elfstrom P, Montgomery SM, Kampe O, et al. Risk of thyroid disease in individuals with celiac disease. J Clin Endocrinol Metab 2008;93:3915–21. [DOI] [PubMed] [Google Scholar]
- 37.Ch’ng CL, Jones MK, Kingham JG. Celiac disease and autoimmune thyroid disease. Clin Med Res 2007;5:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ergur AT, Ocal G, Berberoglu M, et al. Celiac disease and autoimmune thyroid disease in children with type 1 diabetes mellitus: clinical and HLA-genotyping results. J Clin Res Pediatr Endocrinol 2010;2:151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurien M, Mollazadegan K, Sanders DS, et al. Celiac Disease Increases Risk of Thyroid Disease in Patients With Type 1 Diabetes: A Nationwide Cohort Study. Diabetes Care 2016;39:371–5. [DOI] [PubMed] [Google Scholar]
- 40.Murray JA, Moore SB, Van Dyke CT, et al. HLA DQ gene dosage and risk and severity of celiac disease. Clin Gastroenterol Hepatol 2007;5:1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


